Abstract
Surgery of the ear and the lateral skull base is a fascinating, yet challenging field in otorhinolaryngology. A thorough knowledge of the associated complications and pitfalls is indispensable for the surgeon, not only to provide the best possible care to his patients, but also to further improve his surgical skills.
Following a summary about general aspects in pre-, intra-and postoperative care of patients with disorders of the ear/lateral skull base, this article covers the most common pitfalls and complications in stapes surgery, cochlear implantation and surgery of vestibular schwannomas and jugulotympanal paragangliomas. Based on these exemplary procedures, basic “dos and don’ts” of skull base surgery are explained, which the reader can easily transfer to other disorders. Special emphasis is laid on functional aspects, such as hearing, balance and facial nerve function. Furthermore, the topics of infection, bleeding, skull base defects, quality of life and indication for revision surgery are discussed.
An open communication about complications and pitfalls in ear/lateral skull base surgery among surgeons is a prerequisite for the further advancement of this fascinating field in ENT surgery. This article is meant to be a contribution to this process.
1 Introduction
Risks and complications are an integral part of every surgical procedure. Beside teaching surgical techniques, the surgeon should always strive to share risks and possible complications with his colleagues and his fellows. This process requires both the surgeon’s ability and his willingness to inform his colleagues about pitfalls and complications he experienced himself. The authors feel especially obliged to all surgeons who share their personal knowledge in this field with others.
Our surgical profession requires a faithful cooperation with our colleagues, leaving room for discussions about possible risks and pitfalls. If a surgeon experiences a complication, his view gets narrowed by his own personal involvement. In such cases, an honest and open communication with fellow surgeons may have a disburdening effect and help the surgeon to overcome his own restricted view. In addition to the positive impact on the individual patient’s treatment, further patients and surgeons will profit from this dialogue in the future. However, many surgeons are cautious to communicate complications, because they are afraid of being sued for medical malpractice. Therefore, it is of utmost importance to differentiate inherent risks and complications of a surgical procedure from cases of medical negligence in order to keep this fruitful and indispensable dialogue alive.
This review covers selected topics of ear and lateral skull base surgery including the facial nerve. Lessons from exemplary surgical procedures can be transferred to further pathologies by the reader. The text focuses on pitfalls and complications, while inevitable consequences of surgery (“sequelae”) and failure to cure are usually not considered – although it is sometimes difficult to differentiate between the former and the latter. While trying to provide an up-to-date account of the risks and complications in ear and lateral skull base surgery, the authors are aware of the fact that this chapter will have to be rewritten in the future. This endeavour will require an ongoing open discussion among ear surgeons worldwide. We hope that this paper will contribute to this process.
2 General preoperative aspects of ear surgery
An adequate preoperative diagnostic work-up is an indispensable prerequisite for every surgical procedure of the ear and the lateral skull base. The following chapter provides a summary of how the adequate consideration of specific pre-operative aspects can help the surgeon to identify intraoperative risks and thus avoid intra- and postoperative complications.
2.1 Otological and audiological examination
Indication for ear surgery is based on three pillars: (i) the patient’s history, (ii) otomicroscopy and (iii) audiometry. Interpretation of audiometric tests has been reported to be a common source of mistakes in this context [1]. Therefore, it is essential to compare the results of pure-tone and speech audiometry to each other and to correlate them with the otoscopic findings. For instance, the expected air-bone gap in pure-tone audiometry can be estimated from the size of a tympanic membrane perforation [1]. If otoscopic and audiometric findings do not fit together in such cases, either (i) the results of the hearing tests have to be judged critically and/or (ii) the surgeon needs to consider a further middle ear pathology in addition to the perforation of the tympanic membrane.
2.2 Vestibular examination
The ear surgeon should always be aware of the fact that middle and inner ear disorders are often associated with vestibular dysfunction. Therefore, neurotological history-taking and a symptom-based clinical examination should be an integral part of the diagnostic work-up before every surgical procedure of the ear/lateral skull base (reviewed e.g. by [2]). Additional laboratory tests can be employed depending on the clinical findings (reviewed e.g. by [3]). In this context, it should be stressed that vestibular symptoms do not always correlate with vestibular signs. For instance, around one third of patients with profound sensorineural hearing loss (SNHL) report about subjective vestibular problems [4], [5], whereas twice as many show pathological vestibular signs in caloric testing and/or vestibular evoked myogenic potentials (VEMPs) [6].
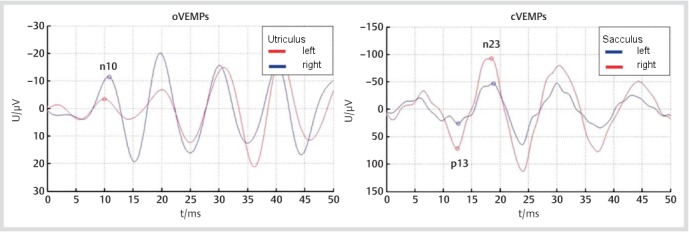
If a surgical procedure involves a possible or mandatory opening of the inner ear (e.g. cholesteatoma or cochlear implant surgery) the preoperative neurotological examination is important for two main reasons. First, it is essential for the detection of pre-operative vestibular dysfunction and thus offers the possibility to counsel the patient with respect to the vestibular findings. Second, it allows the ear surgeon to determine the indication and the extent of the surgical procedure according to the patient’s vestibular function. If a patient with a cholesteatoma shows additional symptoms/signs of a labyrinthine fistula, for instance, then the surgeon will have to remove the cholesteatoma and close the fistula (see chapter 3.5.5). In any case, the ear surgeon should always critically ask himself whether the ear pathology, which is to be treated by the planned surgical procedure, offers a sufficient explanation for the vestibular problems of the patient. In this context it should be noted that it is mandatory to check vestibular function of both ears before every surgical procedure of the ear/lateral skull base, as illustrated by case 1 and Figure 1 (Fig. 1).
Figure 1. Recording of ocular and cervical vestibular evoked myogenic potentials (VEMPs) from the patient described in case 1. Asymmetry of n10 and p13-n23 amplitudes indicates hypofunction of the left utriculus and sacculus, which fits with the clinical diagnosis of left-sided vestibular neuritis.
Case 1
A previously healthy 63-year-old female patient presented to her otorhinolaryngologist with a first episode of prolonged spinning vertigo, which was present at rest and increased transiently with every movement. Furthermore, the patient described a long-standing hearing loss on the right ear without acute exacerbation. Otomicroscopy of the right ear revealed an epitympanic retraction pocket/erosion along with otorrhea, indicative of a cholesteatoma. No abnormal findings were observed during otomicroscopy of the left side. Examination with Frenzel’s glasses showed a right-beating spontaneous nystagmus (SN). A cholesteatoma-related labyrinthine fistula of the right ear was suspected, and the patient was referred for immediate surgery of the right ear.
Neurotological examination confirmed a spontaneous, head-shaking and positional nystagmus (in all body positions) beating to the right ear. However, the vestibulo-ocular reflex (VOR) was abnormal on the left and normal on the right side, indicating a left-sided acute peripheral-vestibular syndrome. The clinical diagnosis was supported by the recording of ocular and cervical vestibular evoked myogenic potentials (o-/cVEMPs; Figure 1 (Fig. 1)). Furthermore, vertigo could not be triggered by sneezing, coughing and lifting heavy weight, and no fistula signs were present on neurotological examination, speaking against the pre-sence of a labyrinthine fistula (for details see chapter 4.3). In accordance with these clinical findings, no bony defect of the labyrinth was detected by high-resolution computed tomography (HRCT) of the temporal bone.
In summary, the patient’s vertigo was due to a left-sided vestibular neuritis and not the right-sided cholesteatoma! The patient, who was treated with i.v./oral methylprednisolone and vestibular training, recovered quickly. Tympanoplasty of the right ear for cholesteatoma was performed six weeks later, after vestibular compensation of the vestibular neuritis had been completed. No labyrinthine fistula was visible during cholesteatoma surgery.
If caloric testing is precluded by tympanic membrane/middle ear pathology, VEMPs and/or the vestibulo-ocular reflex (VOR) offer alternative approaches to obtain mono-aural information about vestibular function (see case 1). In case an acoustic stimulus is not able to evoke a sufficient VEMP signal due to conductive hearing loss on the test ear, vibration at the midline of the forehead at the hairline (a region called Fz) can be employed as a stimulus instead.
The importance of preoperative vestibular testing is also evident from cochlear implant (CI) surgery (see chapter 5.2.2.2). Here, preoperative detection of asymmetric vestibular function is one major aspect of the examination. Among other considerations, it is recommended to insert the electrode into the inner ear with the weaker vestibular function in order to minimize the risk of postoperative vertigo and balance problems [7]. In particular, the neurotological examination deserves special attention before deciding for cochlear implant surgery of the second ear. Although bilateral vestibular failure following cochlear implantation is rare, the surgeon must consider this possible complication due to its debilitating consequences for the patient [8], [9].
Furthermore, the preoperative neurotological examination is also able to identify adverse factors for postoperative central vestibular compensation (summarized by [2]). In cases of an inevitable vestibular lesion as a sequela of ear/lateral skull base surgery (e.g. in vestibular schwannoma surgery), the patient should also be informed about the possibility of vestibular prehabilitation (for details see chapter 5.3.5). This concept is based on (i) the slow induction of a vestibular loss on the affected ear before the operation and (ii) the decoupling of vestibular deficit and surgical procedure. Thus, central vestibular compensation commences before the complete unilateral vestibular loss during surgery, which reduces postoperative vertigo and accelerates the patient’s recovery (see case 2 and [10]).
Case 2
A previously healthy 60-year-old female patient presented to our Neurotology Clinic with a right-sided intracanalicular vestibular schwannoma (VS). Audiometric testing revealed profound sensorineural hearing loss of the right ear. The patient reported no vestibular symptoms apart from occasional imbalance triggered by quick movements. Caloric testing revealed residual excitability of the right horizontal semicircular canal (Figure 2 (Fig. 2) a). Ocular vestibular evoked myogenic potentials (oVEMPs) showed symmetric n10 amplitudes (Figure 2 (Fig. 2) b). In summary, neurotological examination confirmed residual right-sided vestibular function. Consequently, VS surgery was expected to result in postoperative vertigo. After the different treatment modalities had been explained to the patient, she decided for translabyrinthine surgery following vestibular prehabilitation (for details see chapter 5.3.5). The patient received a total of three gentamicin injections (12 mg each) into the right middle ear. After each of the injections, the patient experienced mild imbalance lasting for some days. The next injection was delayed until the vestibular symptoms had completely subsided. In parallel, a customized vestibular rehabilitation programme was performed with the patient.
Figure 2. Vestibular evaluation before (a, b) and after (c, d) intratympanic application of gentamicin during vestibular prehabilitation before vestibular schwannoma surgery (case 2). a.) Caloric testing (x-axis: time [min], y-axis: slow phase velocity [°/sec]): right-sided hypofunction. b.) n10 amplitudes of ocular vestibular evoked myogenic potentials (oVEMPs) indicate symmetric utricular function before gentamicin treatment. c.) Loss of right-sided caloric excitability following three applications of intratympanic gentamicin. d.) The marked asymmetry of oVEMP n10 amplitudes indicates gentamicin-induced loss of utricular function on the right side.
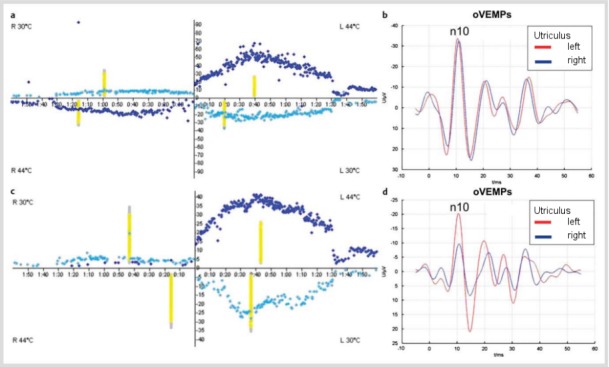
A near-total loss of right-sided vestibular function was confirmed by caloric irrigation and VEMPs after the third gentamicin injection (Figure 2 (Fig. 2) c, d). Translabyrinthine VS surgery was performed after vestibular compensation had been completed. The patient did not experience any subjective disequilibrium after the operation and was able to walk around without assistance from the first postoperative day onwards. A very subtle initial left-beating nystagmus subsided within a few days.
Finally, comparison of pre- and postoperative neurotological findings enables the surgeon to obtain information about subclinical vestibular effects of the procedure, which helps him to reflect and refine his surgical techniques.
2.3 Facial nerve
The facial nerve is of paramount importance to the ear surgeon, as its function has far-reaching implications on the patient’s quality of life [11], [12], [13], [14]. In particular, incomplete eyelid closure, facial droop and problems with eating, drinking and speaking constitute a heavy burden for the affected patient [15]. Therefore, preservation of facial nerve function is a major aim in ear and lateral skull base surgery. The ear surgeon should always be aware of the fact that the various techniques of facial nerve reconstruction will usually not be able to restore a facial nerve function grade I or II according to the House-Brackmann (H-B) grading system [16], [17].
Facial nerve function should always be examined before ear and lateral skull base surgery, as preoperative dysfunction has been shown to be associated with a higher risk of postoperative facial nerve palsy, e.g. in vestibular schwannoma surgery [18]. In this context, it is important to mention that slight defects of facial nerve function may easily be missed during clinical examination. Therefore, electrophysiological evaluation of the motor branches and assessment of facial nerve secretory/sensory function (e.g. tear production, taste) should be considered as well. With regard to the common intraoperative exposure of the chorda tympani, the ear surgeon should pay equal attention to facial nerve motor and sensory function.
2.4 Imaging
The role of preoperative imaging in ear surgery has been discussed controversially. In most cases, the clinically suspected diagnosis can be confirmed and treated adequately during the operation. In case the intraoperative evaluation does not support the suspected diagnosis, the surgical procedure can be stopped in order to perform further diagnostic steps.
When considering preoperative imaging of the ear, the surgeon should ask himself the following three questions: Does the selected imaging technique have an impact on the diagnosis? Will it change the surgical procedure? Will it help to reduce the risk of possible complications? A general recommendation for preoperative imaging should be viewed with caution, especially if it is performed mainly for medicolegal reasons [19].
As far as computed tomography (CT) is concerned, the surgeon has to balance the expected gain of information against the radiation exposure of the eye. Furthermore, the cost-benefit ratio should be considered. On the other hand, CT imaging provides the surgeon with detailed information about the individual patient’s temporal bone anatomy. For instance, a series of 178 temporal bone scans revealed a high-riding jugular bulb in 32%, an anterior sigmoid sinus in 34%, a low-lying dura in 26% and an aberrant internal carotid artery (ICA) in 0.02% of patients [20].
In case of a high-riding jugular bulb, the surgeon should carefully check the CT scan for the presence of bony defects in the hypotympanon, which are associated with an increased risk of intraoperative venous bleeding. In addition, jugular bulb abnormalities (e.g. high-riding jugular bulb, jugular bulb diverticula) have been shown to erode bony structures of the inner ear, such as the vestibular aqueduct, the facial nerve canal and the posterior semicircular canal (SCC), which may lead to hearing loss, tinnitus and vertigo [21].
Usually, high-resolution CT (HRCT) is used to detect small bony erosions of the skull base and the semicircular canals. Contrast agents are only required in selected cases, e.g. inflammatory disease or suspected neoplasia [22]. In summary, indications for preoperative CT imaging of the temporal bone include: (i) presence of cholesteatoma, tumours or inflammatory disorders (e.g. mastoiditis, facial nerve palsy), (ii) a history of meningitis/trauma, (iii) planned surgery of the only hearing ear/cochlear implant surgery, (iv) vertigo, SNHL and (v) suspicion of malformation.
A possible connection between the latter two deserves the ear surgeon’s special attention: malformations of the inner ear have to be expected in 20% of patients with profound congenital SNHL [23], [24]. HRCT is able to detect abnormalities of the inner ear, which are associated with an increased risk of perilymph gusher, such as a common cavity/incomplete partition (with or without enlargement of the vestibular aqueduct) or SCC dysplasia [25]. Reconstructed three-dimensional (3D) CT images have been reported to be helpful in planning ear surgery for patients with an abnormal course of the facial nerve [26]. In this context, it should be noted that malformations of the cochlea and the ossicles have been observed in 7 out of 972 patients with facial nerve abnormalities [27].
Usually, both HRCT of the temporal bone and magnetic resonance imaging (MRI) of the brain are performed before cochlear implantation in order to detect the following pathologies: inner ear malformations (up to 20% in pediatric patients), labyrinthine fistulae, ossification of the cochlea, abnormalities of the 8th cranial nerve/central auditory pathway (e.g. vestibular schwannoma, brain stem lesions). This information helps the surgeon to plan cochlear implant surgery depending on the underlying pathology (e.g. choice of the electrode, surgical approach to the cochlea) [28], [29], [30].
Furthermore, HRCT of the temporal bone is required in the preoperative assessment of patients with advanced otosclerosis accompanied by severe to profound SNHL. Here, decision-making (cochlear implant, stapes surgery or hearing aid) is based on both audiometric findings and the presence of otosclerotic foci in the cochlea (grade 1: fenestral lesions only, grade 2A: retrofenestral lesions with halo/double ring effect, grade 2B: narrowed basal turn of the cochlea, grade 2C: 2A + 2B, grade 3: diffuse involvement of the cochlea) [31].
MRI before middle ear surgery is recommended only in selected cases. For instance, it helps to determine tumour extension of paragangliomas (chapter 5.4) or malignancies of the external auditory canal (EAC). If facial nerve palsy does not recover within 6 weeks, MRI is recommended in order to rule out an underlying tumour [32]. Furthermore, MRI offers the possibility to assess the cochlea, the auditory nerve, the cerebellopontine angle (CPA) and the brain before cochlear implant surgery.
Finally, the synopsis of HRCT and MRI findings is required for the proper diagnosis of special temporal bone pathologies. For instance, cholesterol granulomas of the petrous apex are characterized by (i) osteolytic lesions with a smooth margin in CT images and (ii) a high signal intensity with a characteristic dark halo of haemosiderin in T2-weighted MRI scans [22]. However, MRI alone is not sufficient in the preoperative imaging of petrous bone disease. For instance, HRCT is required for petrous bone cholesteatoma in order to detect possible bony erosions of the inner ear [33] and to determine the optimal surgical approach.
2.5 Informed consent
Beside general surgical aspects, preoperative counselling of the patient must cover specific risks of ear surgery, e.g. a possible injury of the chorda tympani. Here, the impact of an intraoperative lesion on the patient’s quality of life is determined by the preoperative function of the nerve (e.g. preoperative dysfunction in cholesteatoma versus normal function in otosclerosis; for details see chapter 3.5.8). Therefore, the surgeon will have to adjust preoperative counselling of the patient according to the underlying ear pathology and the specific risks of the operation.
If the primary aim of ear surgery is improvement of hearing, the surgeon has to mention alternative therapeutic approaches, such as hearing aids [34]. In case of external/middle ear malformations, the patient should be informed about bone-anchored hearing aids as an alternative to reconstructive surgery. Furthermore, active middle ear implants should be considered if previous middle ear operations were not successful in closing an air-bone gap. If available, pre-operative radiological findings can be used in order to explain the most important surgical steps to the patient.
3 General intraoperative aspects of ear surgery
Despite the close anatomical relationship between ossicles, inner ear, facial nerve, vessels and brain, complications in ear surgery are quite rare [1]. Beside general problems such as bleeding and impaired wound healing, ear-specific complications such as hearing loss/deafness, vertigo/dizziness, tinnitus, facial nerve palsy and disturbance of taste have to be considered [34]. Other authors report hearing loss/insufficient improvement of hearing, blunting, recurrence of cholesteatoma, tympanic membrane perforations and otorrhea (especially when creating an open mastoid cavity) as most common complications after ear surgery – however not all of these are complications sensu stricto [1]. The following chapter outlines general aspects of ear surgery independent of the underlying pathology. Typical pitfalls and complications of exemplary procedures in ear and lateral skull base surgery will be covered in chapter 5.
3.1 Anaesthesiological aspects
Surgery of the ear/lateral skull base is performed in patients from early infancy to old age (e.g. cholesteatoma surgery, cochlear implantation). 6.5% of intra-/postoperative complications (n=123) were reported for cochlear implantations in patients <18 years (mainly respiratory problems like stridor/laryngospasm) [35]. In case of delayed awakening after skull base surgery, intracranial complications and accumulation of anaesthetics (e.g. remifentanil) should be considered as possible differential diagnoses [36].
Due to the ongoing demographic changes in industrialized countries, the ear surgeon will increasingly often be faced with the question whether or not to perform ear surgery in senior patients. Decision making in these patients require an even closer cooperation between ear surgeons and anaesthesiologists in synopsis with the underlying ear pathology. At this point, the authors can only mention this issue without going further into detail.
3.2 Exposure of the operating field
Surgery of the ear/lateral skull base is generally performed with an operating microscope. Optimal exposure of the operating field is an indispensable prerequisite for successful surgery. The ear surgeon may have to widen the EAC or remove overhanging bone in order to achieve an optimal illumination of the entire operating field and to perform the surgical procedure with the various straight and angled instruments. Insufficient exposure of the operating field is regarded as the most common cause for complications in ear surgery [37]. This issue also needs to be considered when using endoscopes during the operation [1].
3.3 Nerve monitoring
Preservation of facial nerve function is of outstanding importance in ear and lateral skull base surgery, as facial nerve palsy has debilitating functional and aesthetic consequences for the patient. During surgery, facial nerve function is most commonly observed by means of electromyographic (EMG) monitoring. Furthermore, the auditory nerve is controlled via the recording of auditory brainstem responses (ABRs) during procedures at the internal auditory canal (IAC)/CPA (for details see chapter 5.3.2).
3.4 Intraoperative radiography/navigation
Intraoperative radiography allows the ear surgeon to control individual surgical steps and to adjust the surgical procedure accordingly. For instance, intraoperative HRCT or rotational tomography are recommended in cochlear implant surgery for i.) checking the intracochlear position of the electrode and ii.) detecting a possible dislocation of the electrode in cases of inner ear malformation [29], [38], [39]. Generally, these radiological techniques can also be applied postoperatively (see chapter 5.2.1.2). Furthermore, high-resolution radiography is able to localize the electrode in the scala tympani or the scala vestibuli and to detect a possible dislocation from one scale to another [40]. This information is of great help for the surgeon in refining his surgical technique [29].
Navigation is particularly applied during cochlear implant surgery in patients with inner ear malformations [41], [42]. Without going into further detail at this point, the authors would like to draw the ear surgeon’s attention to current and future developments in the field of intraoperative imaging and navigation.
3.5 General risks and complications of ear surgery
3.5.1 Ossicular chain dislocation
Dislocation of the malleus is a rare event due to its stabilization by both the tympanic membrane and the malleal ligaments. However, the ear surgeon should always be cautious about a possible dislocation of the incus. If the incus is subluxed accidentally during the operation, the ear surgeon has to decide whether to reposition or to remove the incus in combination with ossicular chain reconstruction [43]. Repositioning bears the risk of incus ankylosis resulting in fixation of the ossicular chain and conductive hearing loss [34]. On the other hand, the possibilities and limits of ossicular chain reconstruction in terms of hearing outcome have to be considered.
Iatrogenic stapes luxation along with an opening of the vestibulum may occur during preparation of cholesteatoma matrix from the stapes. While dislocation of a stapes with an intact annular ligament requires a force of 30 to 40 g [44], a possible hypermobile stapes due to a weakened annular ligament has to be considered during cholesteatoma surgery [34]. Preparation in the dorso-ventral direction uses the stabilizing force of the stapedial tendon and thus may help to prevent a (sub-)luxation of the stapes. In case of a subluxation, the stapes can usually be repositioned in combination with sealing of the stapes footplate. If the stapes has to be removed following dislocation, it is recommended to seal the oval window and treat the patient with i.v. ceftriaxone and methylprednisolone (1,000 mg QD for two days) [45]. In addition, sealing of the oval window is recommended if the stapes footplate has been fractured during surgery [34]. In such cases, some authors have suggested to perform ossicular chain reconstruction in a second procedure.
3.5.2 Drill-generated acoustic trauma
Two pathomechanisms have been proposed for drill-generated acoustic trauma (AT) in ear surgery: direct contact of the burr with either (i) the ossicles, which may result in sound pressure levels (SPLs) of up to 130 dB [46] or (ii) the still-intact endosteal membrane of the cochlea [47]. Based on further animal studies, it has been recommended to avoid touching the endosteal membrane with the burr in order to reduce the risk of drill-induced AT [48].
Therefore, the ear surgeon should consider alternative techniques (e.g. House Curette), when removing bony parts in close vicinity to the ossicles and the cochlear endosteum. In addition, it should be noted that type and size of the burr have an impact on the noise level as well. In general, diamond burrs cause 5 to 11 dB less noise than cutting burrs. Furthermore, the noise level correlates with the size of the burr [49]. For instance, a 4-mm cutting burr was associated with noise trauma in an animal study, while a 0.5-mm cutting burr was not [50]. The ear surgeon should always be aware of the fact that even disarticulation of the incustapedial joint does not protect the inner ear against drill-induced AT caused by a direct contact between the burr and the incus, as has been shown in a guinea pig study [51].
Bone conduction of drill-associated noise to the inner ear as a cause for (temporary) SNHL following ear surgery has been discussed controversially in the literature. A temporary threshold shift in the middle and high frequencies within the first 48 hours after mastoidectomy was reported in a study of 25 patients [52]. This observation is supported by an animal study with n=10 guinea pigs, which were exposed to drill-associated noise for 55 min, which had been recorded during human mastoidectomy. ABRs revealed a temporary threshold shift between 2 and 32 kHz immediately after surgery, which resolved within 3 weeks postoperatively [53]. On the contrary, a prospective study in 40 patients did not detect any statistically significant hearing loss in the first 24 hours following mastoidectomy [54].
Furthermore, it is of interest for the ear surgeon whether extensive drilling (e.g. during translabyrinthine vestibular schwannoma surgery) may induce a noise trauma in the contralateral ear via bone conduction. In a study of 50 patients, no permanent SNHL was reported for the contralateral ear 3 months following resection of a vestibular schwannoma [55]. Reduced amplitudes of distortion-product otoacoustic emissions (DPOAEs), but no threshold shifts were detected in 2 out of 12 patients following mastoidectomy/vestibular schwannoma surgery [56]. On the contrary, SNHL of the contralateral ear after mastoidectomy was observed in a study with 55 subjects (temporary SNHL: n=31; permananet SNHL: n=10) [57]. Although drill-induced AT of the contralateral ear remains a rare clinical observation, it cannot be completely excluded. In summary, the ear surgeon should always keep the risk of drill-induced AT in mind and consider patient-specific factors, e.g. individual vulnerability or preoperative damage of the inner ear.
Systemic and local application of corticosteroids is the mainstay of protecting the inner ear against noise trauma. Protective effects against drill-induced SNHL have been confirmed in animal studies with (i) application of dexamethason into the scala tympani via an osmotic mini-pump [58] and (ii) local application of methylprednisolone to the round window membrane [59]. However, corticosteroids were not able to prevent AT caused by drill-induced injury of the incus in an animal study [60].
3.5.3 Sensorineural hearing loss in middle ear surgery
Permanent SNHL following ear surgery has been reported in 1.2 to 4.5% of cases [54], [61]. Ossicular manipulation bears a low risk of low-frequency hearing loss [62], [63]. A retrospective study of 393 middle ear surgical procedures (chronic otitis media: n=125, cholesteatoma: n=164, tympanosclerosis: n=44, otosclerosis: n=60) did not detect a statistically significant difference between pre- and postoperative sensorineural hearing thresholds [64].
3.5.4 Opening of the inner ear
Opening of the inner ear during surgery may be harmful for the cochlea and the vestibular organ. In this context, cholesteatoma surgery deserves special attention, as the cholesteatoma may affect the inner ear on the level of the round window, the oval window and the lateral SCC. Whenever possible, it is recommended to separate the perimatrix of the cholesteatoma from the membranous labyrinth without opening the latter. In order to achieve this aim, the surgeon should perform the preparation steps slowly and avoid direct suction at the labyrinth in any case. Furthermore, protection of the inner ear with corticosteroids and antibiotics should be considered [65].
If the inner ear has been opened accidentally during ear surgery, it is of utmost importance to seal the defect tightly with autogenous tissue in order to prevent inflammation and/or loss of function. In case of insufficient view, direct suction of the open labyrinth is strictly contraindicated. The surgeon should rather use irrigation and place the suction at a certain distance to the labyrinth in order to improve vision in the operating field without jeopardizing inner ear function.
In case the ear surgeon intends to combine sealing of an open oval window with ossicular chain reconstruction in one procedure, ear cartilage can be used. For instance, it has been recommended to insert a T-shaped piece of cartilage into the oval window niche before sealing the oval window with fascia [65]. By entering into the perilymph space, the vertical part of the T is able to transduce sound waves to the cochlea, whereas the transverse part prevents the cartilage from entering into the vestibulum too deeply.
3.5.5 Semicircular canal injury
During cholesteatoma surgery, the ear surgeon has to be very careful when removing cholesteatoma matrix from the lateral SCC, which is the most common site for a labyrinthine fistula under these circumstances [66]. Usually, the defect is created while the perimatrix, which has already eroded the bony wall of the canal, is lifted from the delicate membranous labyrinth. If a patient with cholesteatoma shows clinical symptoms/signs of a labyrinthine fistula before surgery (summarized by [2]), a preoperative HRCT may help to detect and localize the bony defect [67], [68]. The prevalence of cholesteatoma-related fistulae has been reported with 5.8% [68], 7% [63] and 7.5% [69] in the literature. Accidental drill-induced injury of the lateral SCC in non-choleasteatomatous disease is a rare event.
The question whether cholesteatoma perimatrix covering a labyrinthine fistula should be removed has been discussed controversially. It has been suggested to remove the perimatrix completely for small fistulae (otic capsule defect <2 mm), while it should not be detached from larger defects [70]. Furthermore, the perimatrix should only be lifted if it is not firmly attached to the membraneous labyrinth. In case the fistula affects (i) the anterior or the posterior SCC, (ii) more than one SCC, (iii) the vestibulum and/or (iv) the cochlea, it is generally recommended to leave the perimatrix in situ, as removal has been associated with a 50% risk of profound SNHL in these situations [71]. On the contrary, other authors hold the view that cholesteatoma perimatrix has to be removed completely in any case and granulation tissue should be followed into the labyrinth in order not to miss a possible cholesteatoma behind the granulations [72].
Preservation of preoperative hearing thresholds was reported in 80% of 22 patients and 70% of 27 patients following complete removal of cholesteatoma perimatrix from a labyrinthine fistula [68], [69], while another study observed postoperative hearing deterioration in one of 16 patients [73]. Following removal of the cholesteatoma from the fistula, the resulting bony defect is sealed with bone dust and fibrin glue/blood together with an i.v. shot of corticosteroids in order to protect inner ear function [45]. Temporal fascia, vein wall, cartilage and hydroxyapatite have been described as further suitable materials in the literature.
In case the labyrinthine fistula affects the membraneous labyrinth as well, occlusion of the affected SCC has been proposed. 22 patients who were treated with this method did not show any postoperative deterioration of hearing [74].
3.5.6 Anatomical variations and anomalies of the facial nerve
Dehiscence of the fallopian canal is a common finding in middle ear surgery. A prevalence of 55% / 56% was observed in two temporal bone studies (n=535/n=1000), the average size of the dehiscence was 0.7 to 0.9 mm [75], [76]. Further analyses reported bony defects of the facial canal in 8.9% [77], 11.4% [78], 17.1% [79], 25% [80] and 57% [81] of cases.
The tympanic part of the fallopian canal next to the oval window is the most common site for dehiscence [82], [83]. The two temporal bone studies mentioned above [75], [76] detected 83%/73.5% of bony defects in this area. The surgeon should always keep in mind that fallopian canal dehiscence is often not visible during surgery: for instance, a discrepancy between the prevalence of a dehiscent facial canal in histopathological and clinical studies has been reported (up to 74% versus 7–11%) [84]. In case of a large defect, the tympanic part of the facial nerve may even herniate into the middle ear and mimick a tumour [85].
Furthermore, the (dehiscent) nerve may cross the oval window, look like a thickened chorda tympani or show a duplication [82], [86]. A bifurcation of the facial nerve was observed in three out of 500 temporal bones [87]. Anatomical variations of the facial nerve deserve particular attention when performing ear surgery in infants: due to the superficial location of the stylomastoid foramen at this age, the skin incision alone may suffice to cause permanent damage to the facial nerve [34].
The ear surgeon should always keep in mind that one malformation is often associated with further abnormalities. Therefore, anatomical variations and anomalies of the facial nerve should especially be suspected in patients with known malformations of the temporal bone [82], [88], [89]. For instance, facial nerve abnormalities have been reported in 20% of patients with congenital malformations of the ear [90]. Anatomical variations of the facial nerve were observed in 15 out of 66 cases with minor temporal bone malformations and in 21 out of 62 cases with major temporal bone malformations [91]. Another study reported 22 facial nerve abnormalities in 50 major aural malformations [92]. A lack of bony cover (25 cases) and an aberrant course of the facial nerve (13 cases) were detected in 71 major aural malformations [93].
Figure 3 (Fig. 3) [82] summarizes the most common anatomical variants and anomalies of the facial nerve within the middle ear, including:
Figure 3. Anatomical variants and anomalies of the facial nerve (overview, taken from [82]). a.) normal course, b.) subtotal obstruction of the oval window by an overhanging facial nerve, c.) lack of bony cover in the tympanic segment, d.) dehiscent nerve crossing the oval window, e.) herniation of the dehiscent nerve into the middle ear and obstruction of the oval window, f.) elongation of the second genu resulting in a sharp angle between tympanic and mastoidal segment of the nerve, g.) duplication of the tympanic portion, h.) lateralization of the mastoid portion, i.) anterior displacement of the mastoid portion.
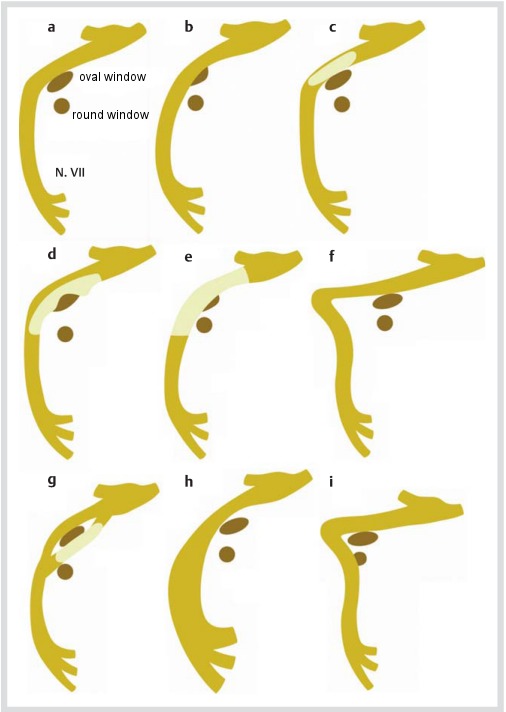
an overhanging (dehiscent) nerve with (sub-)total obstruction of the oval window (Figure 3 (Fig. 3) b, c, d, e)
elongation of the second genu resulting in a sharp angle between tympanic and mastoidal portion of the facial nerve (Figure 3 (Fig. 3) f)
duplication in the tympanic segment of the nerve (Figure 3 (Fig. 3) g)
lateralization/anterior displacement of the mastoidal portion (Fig. 3 (Fig. 3) h, i)
absence of the chorda tympani.
This knowledge requires diligent preparation along the course of the facial nerve during ear surgery in order to prevent injuries, especially in the tympanic part, where bony defects of the fallopian canal are very common. The presence of additional temporal bone malformations may help to prevent iatrogenic injury, as it raises the ear surgeon’s awareness about a possible anomaly of the facial nerve. Therefore, the risk of accidental iatrogenic facial nerve injury has been estimated to be higher in minor as compared to major middle ear malformations [91]. If an abnormal facial nerve is suspected, the ear surgeon has to treat every nervous tissue within the middle ear as if it was the facial nerve – until proven otherwise. In such cases, the detection of small vessels on the surface of a structure may help to identify the facial nerve. Furthermore, the surgeon should use the common landmarks of middle ear surgery (e.g. lateral SCC, oval and round window, cochleariform process) when looking for the facial nerve [34].
In addition, intraoperative facial nerve EMG may help the surgeon to identify the facial nerve in case of an anomaly. In general, the surgeon should use intraoperative EMG whenever he feels insecure about the course of the facial nerve – even if this means he has to interrupt the surgical procedure in order to build up the monitoring. Finally, the surgeon should pay special attention to a possibly elongated second genu between the tympanic and mastoidal segment of the facial nerve in order to prevent iatrogenic nerve injury while performing a mastoidectomy.
3.5.7 Facial nerve palsy in middle ear surgery
The incidence of facial nerve palsy due to ear surgery has been estimated to lie between 1:600 and 1:1200 [94]. In 70% of cases, iatrogenic injuries have been located to the pyramidal segment of the facial nerve (between tympanic and mastoidal segment) at the second genu (directly inferior to the lateral SCC). However, it should be noted that 20% of cases were observed after tympano-ossiculoplasty (particularly in tympanosclerosis) [1].
The ear surgeon should keep in mind that the clinical appearance may be misleading in the first days following an intraoperative lesion of the facial nerve. For instance, eyelid closure may still appear to be complete within the first three days after surgery due to the effects of gravity. Therefore, it is recommended to test eye closure in the supine position in order to recognize facial nerve palsy as early as possible.
If the surgeon notices a minor lesion of the facial nerve during surgery, a postoperative control of facial nerve function is justified. In case the nerve has been cut completely or has been injured over a long distance, primary reanastomosis or interposition grafting (using the greater auricular nerve in most cases) should be performed in the same session, depending on the type and extent on the lesion. The natural course of the facial nerve should be preserved. The interposition graft should be placed within the fallopian canal, sutures are usually not required. The surgeon should ensure a good contact between the nerve endings avoiding tissue, fibrin glue and blood within the anastomosis. Resection of 1 to 2 mm of epineurium has been suggested in order to prevent scarring between the nerve endings [34].
If facial nerve palsy is noticed immediately after the operation, the ear surgeon should also consider possible side effects of local anaesthetics as a differential diagnosis. Transient facial nerve palsy within the first two to three postoperative hours has been reported in this context [34], [95]. However, persistent postoperative facial nerve palsy requires further electrophysiological and radiological assessment in order to decide whether revision surgery is useful and necessary. In particular, the ear surgeon has to consider the possibility of an accidental iatrogenic injury, which was not noticed during the surgical procedure. In such cases, evaluation of the bony facial canal by HRCT may help to detect the site of the lesion. MRI, however, is not suited for judging the integrity and function of the facial nerve within the middle ear [1].
Even if the facial nerve is fully functional within the first postoperative days, patients may develop delayed facial nerve palsy, which is considered to be caused by virus reactivation [96]. Minor intraoperative trauma to the facial nerve has been suggested to trigger reactivation of the herpes simplex or varicella zoster virus resulting in the clinical picture of Ramsay Hunt syndrome [97], [98].
3.5.8 Chorda tympani
Chorda tympani symptoms (particularly taste alterations and dry mouth) following middle ear surgery are expected in 15 to 22% of patients [99], [100]. Persistent dysgeusia was reported in 2.7 to 12% of cases if the nerve had been preserved, and in 5.3 to 43% of cases if the chorda tympani had been disrupted [101], [102], [103], [104]. At first sight, these values seem to contradict common clinical observations.
Ear surgeons often make the experience that postoperative taste disturbance occurs more frequently after intraoperative stretching of the chorda tympani than after a complete transection of the nerve [101], [105], [106], [107], [108], [109]. This observation has been documented in the literature quite early: for instance, only three out of 45 patients reported ageusia following radical mastoidectomy with disruption of the chorda tympani [110]. Likewise, only 5% of patients (n=113) complained of persistent taste disturbance following transection of the chorda tympani during ear surgery. Furthermore, metallic taste and paraesthesia of the tongue (e.g. tingling) have been described in this situation [107], [111].
However, these general observations have to be differentiated according to the underlying ear pathology. The ear surgeon should always keep in mind that preoperative dysgeusia is more common in chronic inflammatory disease (e.g. cholesteatoma) than in non-inflammatory disorders of the middle ear (e.g. otosclerosis) [105], [112]. Therefore, a patient, whose chorda tympani has been disrupted during cholesteatoma surgery, will less likely notice a postoperative change in taste perception than a patient with a chorda tympani lesion due to stapes surgery. This aspect should also be explained to the patient during preoperative counselling (see also chapter 2.5). Accordingly, a prospective study on 45 patients reported a higher rate of postoperative taste disturbance after stapes versus cholesteatoma surgery [111].
Beside a possible preoperative chorda tympani dysfunction, neurophysiological aspects have to be considered in this context as well. For instance, unilateral disruption of the chorda tympani causes loss of contralateral inhibition as well, which enables the contralateral nerve to compensate for the loss of taste to a certain degree. Therefore, the patient often experiences less severe postoperative disturbance of taste than expected [113], [114], [115].
Intraoperative manipulations of the chorda tympani have to be carried out with utmost care. Suctioning, stretching and desiccation of the nerve fibres may cause loss of function [116]. Therefore, it has been recommended to moisten the chorda tympani regularly with wet pieces of Gelfoam once it has been detached from the tympanic membrane [1]. However it should be mentioned at this point that it is difficult for the surgeon to estimate the potential traumatic effect of individual manipulations during the operation.
Likewise, it is hard to predict the potential for recovery of postoperative chorda tympani symptoms. The underlying ear pathology (e.g. preoperative dysfunction due to inflammatory disease) and the extent of the surgical trauma are regarded to be the most important factors in this context [107]. Furthermore, the recovery rate of taste disturbance after ear surgery has been reported to be higher in younger than in older patients [117].
3.5.9 Skull base
The dura is an important landmark and can help to identify the lateral skull base in ear surgery. Small bleedings from the dura can usually be managed with bipolar cautery. If the superior petrous sinus has been opened, plugging with oxidized cellulose may be necessary. In general, the principles of endonasal duraplasty can also be applied to the lateral skull base [118]. Cartilage has been recommended to prevent a prolapse of brain tissue into the tympanic cavity [34].
3.5.10 Bleeding
Profuse intraoperative bleeding should always be avoided, as it compromises the ear surgeon’s view within the operating field. Common strategies include: (i) infiltration of the EAC/postauricular fold with vasoconstrictive agents before surgery, (ii) local application of Gelfoam pelltes soaked with vasoconstrictors and (iii) preoperative conservative treatment of inflammatory middle ear disease. Severe venous and arterial bleedings are rare events in ear surgery. Venous bleeding may be caused by an accidental injury of emissary veins or the sigmoid sinus/jugular bulb. In case of the emissary veins, a diamond burr without irrigation can be used to stop the bleeding. Minor defects in the sigmoid sinus wall/jugular bulb can be covered with oxidized cellulose or collagen fleece soaked in fibrin sealant. Major lacerations (e.g. injury of the jugular bulb) may require packing of the sigmoid sinus and/or the tympanic cavity. If the bleeding cannot be managed this way, the ear surgeon has to (i) obliterate the sigmoid sinus, the superior and inferior petrous sinus and (ii) ligate the jugular vein – a procedure known from jugular paraganglioma surgery (for details see chapter 5.4.5). An anterior sigmoid sinus and a high-riding dehiscent jugular bulb are at particular high risk for intraoperative injury. In the latter case, a simple myringotomy may suffice to cause severe venous bleeding within the hypotympanon [1], [34].
In analogy, a dehiscent ICA or an ICA aneurysm might be opened inadvertently during myringotomy [119], [120]. In case of an ICA haemorrhage during ear surgery, emergency measures include packing of the middle ear and stabilization of the patient’s circulation. As known from anterior skull base surgery, muscle is a suitable tissue for closing ICA defects [121]. After the severe bleeding has been stopped, a meticulous examination of the artery is mandatory. In this context, an angiographic evaluation has to be performed in order to check for a possible ICA dissection or aneurysm [34].
In general, a dehiscent ICA is a rare finding in middle ear surgery. However, the ear surgeon has to be aware of the fact that pulsation of the artery – which is weak or even absent in the endocranial space – may be missing within the middle ear as well [43]. Furthermore, a persistent stapedial artery may be a source for bleeding. As this artery may contribute to the brain’s blood supply, an angiographic evaluation has been recommended before closing it [122], [123].
4 General postoperative aspects of ear surgery
The ear surgeon should never negate a postoperative complication. Recognizing an adverse event is an indispensable prerequisite for its successful treatment.
4.1 Postoperative electromyographic evaluation
EMG of the facial nerve is required to differentiate between neuropraxia, axonotmesis and neurotmesis in case of a postoperative facial nerve palsy [124]. Complete recovery is usually observed for neuropraxia (axons and nerve sheath intact), while complete or faulty regeneration is possible in axonotmesis (disrupted axon, but intact nerve sheath). If a satisfactory recovery of function has not occurred within 12 months, surgical rehabilitation of the paralyzed faced should be considered. On the other hand, early revision surgery is indicated in case of neurotmesis (disrupted axon and nerve sheath) [34], [125].
4.2 Postoperative radiography
If the position of the cochlear implant electrode has not been determined during surgery (see chapter 3.4), the radiographic control is performed postoperatively. Usually, MRI examinations up to 1.5 Tesla are possible in CI users. If necessary, the magnet can be removed temporarily for certain types of implants [29]. In any case, the manufacturer’s specifications have to be observed.
Postoperative CT imaging is recommended in lateral skull base surgery in order to detect intracranial complications (e.g. haematoma). However, it has to be considered that these do not have to be present immediately after surgery, but may evolve within the first postoperative days. Therefore, additional CT controls have to be performed if the patient develops the respective clinical symptoms and signs [126], [127].
Postoperative radiography in skull base surgery is especially important if the operating field has been obliterated during surgery, which precludes a direct postoperative inspection. In these cases, HRCT and MRI findings often complement each other. Therefore, both imaging techniques have been recommended in the follow-up after resection of petrous bone cholesteatoma (once a year for at least five years) [33].
Moreover, HRCT and MRI imaging should be performed three months after surgery for temporal bone neoplasia. HRCT is required to document the bony anatomy after surgery. Often, MRI alone is sufficient for the subsequent annual imaging controls in these cases.
4.3 Balance
Postoperative labyrinthitis may affect the cochlea and/or the labyrinth. In general, the vestibular symptoms of labyrinthitis resemble those of vestibular neuritis. The patient displays a horizontal (rotatory) spontaneous nystagmus (SN) beating towards (i.) the affected ear in case of vestibular excitation and (ii) the contralateral ear in case of vestibular hypofunction. Conservative therapy includes (i) systemic administration of corticosteroids and antibiotics with a high penetrability to the inner ear (e.g. ceftriaxone) and (ii) vestibular rehabilitation training [128]. Depending on the degree of nausea and vomiting, i.v. fluid/electrolytes and antiemetic drugs should also be considered.
Revision surgery is required if a SN towards the contralateral ear and/or acute SNHL are observed in the early postoperative period. The surgeon should remove all foreign bodies (e.g. middle ear prostheses) in these cases and check for a defect of the otic capsule (labyrinthine fistula). Furthermore, sealing of the oval and round window has been recommended [34].
A labyrinthine fistula between middle and inner ear (e.g. at the horizontal SCC, round/oval window) is characterized by short bouts of spinning vertigo during pressure changes in the cerebrospinal fluid (CSF) – and thus the perilymph. The attacks can be triggered e.g. by sneezing, coughing, pushing and lifting heavy weight. If these symptoms are present, the ear surgeon has to check for fistula signs, such as nystagmus provoked by (i) pressure changes in the EAC (e.g. by applying a Politzer air bag) or (ii) transition from sitting to lying supine and vice versa. The fistula has to be identified and sealed (e.g. with connective tissue and/or bone dust). During surgery, i.v. corticosteroids and ceftriaxone should be administered in order to protect inner ear function [2], [3].
Furthermore, benign paroxysmal positional vertigo (BPPV) may be observed following ear surgery. Vibration due to the drilling procedure has been suggested to cause detachment of the otoliths from the utricular macula [129]. The special case of BPPV after stapes and cochlear implant surgery will be dealt with in chapters 5.1.4 and 5.2.2.2. Postoperative BPPV is treated with the same diagnostic and therapeutic maneuvers [2], [130], [131], [132], [133], [134], [135] as the idiopathic variant.
In case of a permanent vestibular deficit following ear surgery, the patient should receive vestibular rehabilitation training in order to (i) promote central vestibular compensation and (ii) induce somatosensory/visual substitution [136]. Vestibular exercises have been shown to improve central compensation following a unilateral loss of vestibular function [137].
Vestibular signs and symptoms do not always occur in the early postoperative period, but may also evolve after a symptom-free interval, e.g. following cochlear implant surgery (see also chapter 5.2.2.2). In these cases, it may be difficult to decide whether the balance problems are due to the surgery or an independent cause. In any case, it is advisable for the ear surgeon to have access to the whole spectrum of neurotological diagnostics and therapy in order to provide the optimal care for patients with vestibular problems following surgery of the ear/lateral skull base.
4.4 Periorbital/ocular complications
Postoperative complications affecting the eye were reported in 92 out of 2,318 patients following tympanoplasty/mastoidectomy [138]. These included: transient blurred vision (n=63), mild periorbital edema (n=24), severe periorbital edema (n=4) and periorbital edema with ecchymosis on the eyelids (n=1). While blurred vision and edema usually recovered within two to three days, it took six weeks until the edema with ecchymosis had dispappeared completely. Furthermore, periorbital edema [139] and edema anterior/anterosuperior to the auricle [140] were reported in cochlear implant studies.
The following etiologies of periorbital complications in ear/lateral skull base surgery have been proposed: (i) preauricular incision with harvesting of temporal fascia resulting in a compromised venous/lymphatic drainage of the eyelids, (ii) compression by an overly tight wound dressing, (iii) drilling causing capillary injuries in the periorbital tissue (particularly in elderly patients with fragile capillaries) and (iv) presence of rhinosinusitis [138], [139], [140]. Furthermore, a haematogenous spread of bacteria should also be considered as a possible reason for edema/erythema of the eyelids following ear surgery. In this context it is worth mentioning that one study detected bacteria in 8.4% of venous blood samples obtained immediately after tympanoplasty/mastoidectomy [141].
Corneal abrasion, which is the most common cause for postoperative blurred vision, may be caused by (i) surgical drapes/foreign bodies contacting the eye accidentally during surgery and/or (iii) reduced tear production due to anticholinergic agents administered during anaesthesia [138]. Keeping in mind that an injury of the cornea may result in irreversible blindness [142], the ear surgeon should always be very cautious when placing surgical drapes around the eye.
4.5 What to do in case of a complication
The surgeon’s attitude in case of an intra-/postoperative complication is of outstanding importance for the affected patient. Moreover, an honest and professional management of complications may help to prevent legal disputes [1]. Above all, it is essential to maintain a faithful relationship between the surgeon and his patient. In order to achieve this aim, the following suggestions should be considered: the surgeon should (i) look after the patient himself, (ii) explain honestly to the patient what has happened and (iii) treat the complication in a way the patient can understand. Sharing information with the patient’s relatives – after obtaining the patient’s permission to do so – may also help to stabilize the situation. If no personal communication with the patient is possible after a severe complication, the surgeon should try get into contact with the patient’s family as soon as possible.
5 Selected surgical procedures
The following chapter covers typical pitfalls and complications of exemplary procedures in ear and lateral skull base surgery, aiming to provide the reader with a basic knowledge of common principles, which he can easily transfer to other pathologies as well. In detail, stapes surgery was chosen as an example for middle ear surgery plus opening of the inner ear (chapter 5.1), while cochlear implant surgery represents the approach of mastoidectomy/posterior tympanotomy (chapter 5.2). The typical risks and complications of surgical procedures in the internal auditory canal/cerebellopontine angle are illustrated by the example of vestibular schwannoma (VS) surgery (chapter 5.3). Finally, an overview of jugular paraganglioma surgery provides an insight into the pitfalls of petrous bone surgery (chapter 5.4).
5.1 Stapedotomy/stapedectomy
Hearing can be improved by stapes surgery in the majority of patients with otosclerosis. In general, complications are rare. However, the ear surgeon must keep in mind that the need to open the labyrinth gives rise to additional risks and complications which are not observed in other surgical procedures of the middle ear (e.g. tympanoplasty). The most common complications of stapes surgery – which may have far-reaching consequences for the patient – include: SNHL, vertigo/dizziness, perilymph fistula, tympanic membrane perforation, transient facial nerve palsy, chorda tympani symptoms and inflammatory complications [143], [144].
5.1.1 Conductive hearing loss after stapes surgery
Recurrent or persistent conductive hearing loss has been reported to be the most common complication after stapes surgery (2.7% of cases). Stapes revision is generally recommended if the air-bone gap exceeds 20 dB [145]. According to the literature, 5–10% of primary stapes operations require revision surgery [146].
HRCT of the temporal bone is a useful tool in the decision-making process concerning stapes revision [147], especially if conductive hearing loss persists after primary surgery. In these cases, HRCT is able to detect additional pathologies of the temporal bone which may account for the persistent hearing loss, e.g. the rare finding of a jugular bulb diverticulum with a bony dehiscence towards the vestibular aqueduct [148] or a dehiscence of the superior SCC (superior canal dehiscence syndrome = SCDS; for a detailed explanation see end of this chapter) [149].
A retrospective analysis of 201 stapes revisions in 175 patients described the following intraoperative findings for patients with conductive hearing loss after primary surgery: prosthesis lateralization due to collagen contracture of the oval window neomembrane (53%), partial or complete incus necrosis (33%), reossification of the stapes footplate (31%) and loosening of the loop on the incus (9%) [146]. A prospective study with 279 stapes revisions in 260 patients [150] identified the following causes for conductive hearing loss:
The stapes piston had lost contact to the vestibulum in 81% of patients. Postoperative migration of the prosthesis due to collagen contracture of the oval window neomembrane was suggested as the main underlying cause. Furthermore, these contractions may also reduce mobility of the stapes piston. A prosthesis, which was too short to reach the oval window neomembrane, was only found in 5% of patients.
A fixed footplate was detected in 13% of cases. 60% showed partial incus erosion (>50% of incus neck diameter), while 31% presented with (near-)total incus erosion.
Incus dislocation (4%) was a rare observation. Fixation of the incus (2%) and the malleus (4%) usually occurred in the epitympanon.
A perilymph fistula was detected in 5% of patients. In 2%, the piston was found to protrude too deeply into the vestibulum.
Based on these observations, several recommendations have been made in order to avoid postoperative contracture of the oval window neomembrane with subsequent prosthesis migration: (i) mucosal trauma in the oval window niche should be kept at a minimum, (ii) only thin layers of connective tissue should be used for sealing the oval window fenestration, (iii) the CO2 laser should be applied to create the opening in the footplate in order to increase precision and minimize trauma; in these cases, clotted blood is often sufficient for a tight sealing of the fenestration [150]. While small amounts of blood are safe, the surgeon should keep in mind that larger amounts may lead to haemosiderin deposits in the labyrinth resulting in hearing loss and vertigo [151]. In case of a small fenestration, additional sealing is often not necessary [152]. Prosthesis lateralization may occur both after stapedectomy and after partial removal of the footplate. On the contrary, migration of the stapes piston following stapedotomy is supposed to be prevented by the small diameter of the oval window fenestration [153].
Accidental incus dislocation may occur during fixation of the prosthesis loop, especially if the stapes superstructure has already been removed. Therefore, it has been recommended to introduce and fix the piston while the incustapedial joint is still intact [153].
Persistent conductive hearing loss after primary stapes surgery may also be due to malleus fixation. Both the malleus and the stapes are fixed in the embryonic middle ear. During further development, resorption of the bone leads to formation of the anterior malleal ligament (malleus) and the anular ligament (stapes). If this step is missing (atavism), both the malleus and the stapes remain fixed [154]. In addition, tympanosclerosis has to be considered as an underlying cause for malleus and/or incus fixation. Therefore, the ear surgeon always has to check the mobility of the entire ossicular chain before performing stapes surgery. If fixations are present, these have to be resolved – or a malleovestibulopexy has to be performed.
Necrosis of the incus process corresponding to the pressure points of the piston has been described as one of the most common reasons for stapes revision surgery. If the new prosthesis cannot be attached to the proximal part of the incus, malleovestibulopexy is an alternative.
When judging hearing results after stapes surgery, time is a very important factor: for instance, one study showed that low-frequency hearing thresholds three weeks after stapedotomy were better for a 0.6-mm as compared to a 0.4-mm piston. However, this difference had disappeared in the three-month and one-year follow-up [155].
Although hearing outcome after stapes revision is generally supposed to be inferior to the results of primary surgery, the beneficial effects of revision surgery should not be underestimated. For instance, a study with 201 stapes revisions in 175 patients reported an improvement of the air-bone gap in 88% of cases [146]. The risk of profound SNHL following stapes revision has been estimated to be twice as high (2.2%) as compared to primary surgery [156]. Likewise, profound SNHL has been reported after an average of 1.7% (range: 0–14%) and 1.8% of stapes revisions (range 0–14%) in reviews of the literature. In this context, it should be noted that the result of 14% in one study with 35 patients is exceptionally high when compared to the rest of the studies [146], [157].
If the preoperative air-bone gap persists after stapes surgery, a bony dehiscence of the superior SCC should be considered as a differential diagnosis [158], [159], [160], [161], [162]. Temporal bone studies determined an anatomical prevalence of 0.4 and 1.6% for a dehiscent superior SCC [163], [164]. However, only a minority of the affected individuals presents with the characteristic clinical symptoms and signs of SCDS, which are due to the presence of a “third window” in the inner ear (between superior SCC and endocranial space), including: (i) pressure- and noise-induced attacks of spinning vertigo lasting for seconds, (ii) an air-bone gap and (iii) autophony in the affected ear. Although vestibular symptoms are the clinical hallmark of SCDS, the ear surgeon should keep in mind that 8% of symptomatic patients display an exclusively audiological manifestation of the disease [161]. Several cases were reported in the literature, where SCDS was discovered only after the air-bone gap had not improved following stapes surgery.
Furthermore, the ear surgeon has to consider that the symptoms of SCDS may be masked by fixation of the stapes footplate, if both otosclerosis and SCDS are present in one ear. In these cases, fenestration of the oval window during stapedotomy may trigger the symptoms of a previously “silent” SCDS [149]. If the surgeon misinterprets postoperative vertigo and persistent conductive hearing loss as complications of stapes surgery, the diagnosis of SCDS is missed again [162].
In addition, the rare association between otosclerosis and SCDS illustrates nicely that postoperative symptoms do not necessarily have to be complications, but may also be caused by an independent pathology. Therefore, a diligent evaluation of the patient’s neurotological symptoms and signs before and after a surgical procedure are of paramount importance in ear surgery.
5.1.2 Opening of the vestibulum
The stapes footplate should not be removed in total before creating an additional opening of the vestibulum (e.g. when fracturing the stapes crura). Otherwise, the sudden increase of pressure in the perilymph space may cause damage to the inner ear. In case of such an adverse event, a high-dose systemic treatment with corticosteroids has been recommended [165].
A floating footplate was observed in 1.2% of 420 primary stapedotomies [95]. In these cases, the authors recommended to create an additional opening at the promontory before removing the footplate with a micro hook. Furthermore, perforation of the stapes footplate while the stapes superstructure is still intact has been proposed to avoid a floating footplate [95]. This approach reduces the risk for incus dislocation as well (see also chapter 5.1.1). A floating footplate was not observed when a CO2 laser was applied for perforation of the stapes footplate in a study with 188 stapedotomies [166].
If a tiny part of the footplate falls into the vestibulum, it may gently be removed with a micro hook. However, the surgeon should not “dig” for bony parts of the footplate, which have disappeared in the labyrinth [165]. If laser systems (e.g. CO2 laser) are used, the surgeon has to check the parameters of the system (e.g. pulse mode, duration, energy and diameter of the laser beam) in order to avoid thermal trauma of the inner ear [166].
5.1.3 Sensorineural hearing loss after stapes surgery
SNHL following stapes surgery has been observed in 6.6% of cases and usually affects the high frequencies (5.7%) [167]. Profound SNHL, which is considered to be the most severe complication of stapes surgery, has been in reported in 1% of cases [156], [165]. Even for very experienced surgeons, this risk has been estimated with 0.5% [168] and 0.6% [169]. Therefore, preoperative counselling of the patient before stapes surgery has to include information about hearing aids as an alternative approach for improving the patient’s hearing thresholds [170].
Although it is difficult to identify the underlying reason for profound SNHL following stapes surgery, some factors are known today. For instance, Gelfoam should not be used to seal the fenestration of the footplate because (i) it has been associated with an increased incidence of perilymph fistulae [171], [172], [173], [174] and (ii) formaldehyde, which is used for sterilization of Gelfoam, is an ototoxic agent. It has been shown that 20 to 60 µg of formaldehyde suffice to cause irreversible damage to the organ of Corti [175]. Tissue for sealing the oval window fenestration should not contain large amounts of local anaesthetics, as these may induce postoperative vertigo and SNHL [152]. In general, the risk for inner ear trauma with SNHL is considered to be lower for stapedotomy as compared to stapedectomy [167].
5.1.4 Balance
Intraoperative opening of the vestibulum during stapes surgery carries the risk of inducing vestibular dysfunction. In this context, the surgeon should keep in mind that vestibular signs and symptoms do not necessarily show a perfect correlation [176], [177]. The few studies on balance disorders after stapes surgery are difficult to compare to each other due to different surgical techniques and postoperative follow-up periods. Furthermore, it has to be noticed that vestibular symptoms and signs may well have been present before stapes surgery [176].
Early vestibular symptoms (e.g. vertigo, tilting/floating sensation, dizziness) after stapes surgery have been reported by 52% [177] and 82% [178] of patients in recent studies. Usually, a complete resolution of symptoms is observed within the first postoperative week. Only 3% of patients still described a sensation of vertigo one month after stapedotomy [176]. On the contrary, 30% of patients experienced vestibular symptoms three months after stapedectomy in previous studies [179].
Vestibular symptoms include (i) spinning and non-spinning vertigo (e.g. rocking, swaying, tilting, bobbing, bouncing) and (ii) dizziness/lightheadedness [177]. The ear surgeon should be able to distinguish between the four following vestibular syndromes following stapes surgery: vestibular excitation, vestibular hypofunction, BPPV and perilymph fistula (for details see chapter 4.3 and [2]). Postoperative vestibular signs include: (i) nystagmus (spontaneous, head-shaking, positional/positioning, pressure-induced), (ii) a tilted subjective visual horizontal/vertical, (iii) asymmetric VEMPs and/or caloric responses and (iv) abnormal vestibulospinal function/posturography findings.
In case of vestibular excitation after stapes surgery, the nystagmus beats towards the operated ear, whereas a nystagmus to the contralateal side is observed for vestibular hypofunction [177], [176]. Furthermore, a reduced caloric response on the side of the operated ear indicates a vestibular deficit [180]. Tilting of the subjective visual horizontal away from the operated side has been interpreted as a sign for increased activity of the otolith organs following surgical manipulation of the vestibulum [181]. Furthermore, computed dynamic posturography detected a transient functional vestibular deficit following stapes surgery [178].
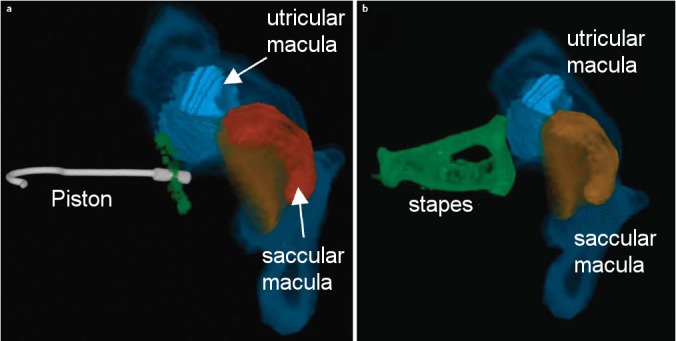
The anatomical relationship between stapes footplate, utriculus and sacculus is the key for understanding the pathomechanism of vestibular dysfunction after stapes surgery. Recent micro-CT studies have confirmed that the closest distance between stapes footplate and vestibular epithelium is located in the posterosuperior utricular macula (distance: 0.61 mm on average; see Figure 4 (Fig. 4)) [182]. Therefore, the utriculus is particularly at risk, when the stapes prosthesis protudes too deeply into the vestibulum, which may either cause (i) chronic vestibular excitation [181] or (ii) a permanent vestibular deficit due to damage of the vestibular epithelium. Furthermore, persistent vestibular hypofunction following stapes surgery may be due to labyrinthitis and/or loss of perilymph [177], [178], [183].
Figure 4. Three-dimensional reconstruction (3D) of micro-CT images from human temporal bone illustrating the spatial relationship between stapes footplate/piston, utriclus and sacculus (previously unpublished figures, with kind permission of Ian Curthoys and Payal Mukherjee, University of Sydney, Australia). a.) view of a stapes piston (insertion depth: 0.25 mm) in relation to the stapes footplate (green), utricular macula (light blue) and saccular macula (orange). The membranous structures of utriculus and sacculus are shown in dark blue and red, respectively. b.) 3D reconstruction of stapes (green), utricular (light blue) and saccular macula (orange). Both figures show that the posterosuperior utricular macula is located closest to the stapes footplate (for details see [182]).
Postoperative BPPV, which has been described in 6.1% [184] and 8.5% [185] of cases, may result from (i) bony fragments of the stapes footplates falling into the perilymph space or (ii) detachment of otoliths from the utricular membrane due to drill-associated vibration. At this point, it should be noted that the development of BPPV symptoms may be delayed after stapes surgery [184], [185].
Perilymph fistula presenting with pressure-induced vertigo attacks an/or fluctuating SNHL may be caused by (i) a dehiscent seal of the oval window fenestration or (ii) prosthesis migration resulting in tearing of the oval window neomembrane [176]. The surgeon should be aware of the fact that a perilymph fistula may occur with a latency of 12 to 15 years after stapes surgery [186].
In case a patient complains of vertigo/dizziness in the early postoperative period, the ear surgeon should check for the presence of nystagmus and perform the Weber tuning fork test. In any case, the pressure of the packing in the ear canal should be relaxed. BPPV is treated with the well-known liberatory/repositioning maneuvers (see also chapter 4.3). Conservative treatment of vestibular excitation/hypofunction and suspected perilymph fistula includes systemic administration of corticosteroids and antibiotics with a high penetrability to the inner ear (e.g. ceftriaxone). If symptoms persist despite these conservative measures, surgical revision has to be performed immediately [187].
HRCT has a high diagnostic value in the decision-making process for stapes revision due to postoperative vertigo. For instance, a dislocation of the piston into the vestibulum, a pneumolabyrinth indicating a perilymph fistula, newly formed otosclerotic foci, fibrous adhesions and defects of the stapes prosthesis are visible on CT images [188]. In summary, the synopsis of neurotological signs/symptoms and CT findings helps the surgeon to decide for the optimal treatment of vertigo following stapes surgery (conservative/surgical) in each individual case [1]. However, it should be kept in mind that the insertion depth of the stapes piston is often overestimated by CT and rotational tomography [189].
In a series of 175 patients, stapes revision was performed because of postoperative vertigo in 16 cases (8%) [146]. Intraoperative findings included: an overly long piston, excessive mobility of the piston due to incus displacement and irritation of the utricular macula by the piston [146].
If stapes surgery of the second ear is planned, it should be taken into consideration that vestibular dysfunction following surgery of the first ear may well be present, but completely asymptomatic. Therefore, a complete neurotological examination (including caloric irrigation, VOR and VEMPs) should be performed before every stapes surgery of the second ear, particularly in order to judge the potential for vestibular compensation in case of postoperative vestibular hypofunction.
5.1.5 Facial nerve palsy
The risk for transient/delayed facial nerve palsy following stapes surgery has been estimated with 0.07% in the literature [169]. Several authors have reported delayed facial nerve palsy after a free interval of some days after stapes surgery [190], [191], [192], [193]. Two large series with 2,307 and 2,152 stapedectomies described delayed facial nerve palsy in 0.22% [194] and 0.51% [195] of cases, respectively. Possible pathomechanisms include: (i) reactivation of a latent herpes simplex infection within the geniculate ganglion, (ii) intraoperative stretching of the chorda tympani, (iii) mechanical irritation of a dehiscent facial nerve and (iv) thermal injury caused by a potassium titanyl phosphate (KTP) laser [195], [196], [197] (see also chapter 3.5.7). Based on the virus reactivation theory, systemic treatment with acyclovir and corticosteroids has been recommended for delayed facial nerve palsies after stapes surgery. Although the nerve usually recovers completely [193], faulty regeneration with synkinesis and spasms) has been reported as well [190]. Identification of the facial nerve at the oval window may be very difficult during revision surgery due to misleading middle ear adhesions. Accordingly, facial nerve lesions have been reported in 3 to 20% of cases after stapes revision [150].
5.1.6 Chorda tympani
In general, taste disturbance is observed more frequently after stapes surgery as compared to middle ear surgery for inflammatory disease (see chapter 3.5.8). For instance, 80% of 126 patients reported taste alterations and a dry mouth following stapes surgery [101]. Moreover, taste disturbance was described in 95% of cases after the chorda tympani had been cut versus 52% if it had been preserved [102]. Six months after surgery, these numbers had dropped to 22% and 10%, respectively [198].
In general, preoperative counselling of the patient should always include the risk of chorda tympani dysfunction, as manipulation or even transection of the nerve might be necessary during stapes surgery. However, severe chorda tympani symptoms following disruption of the nerve are rare (<5%) – despite the high rate of subjective taste disorders in the early postoperative period [102].
5.1.7 Perilymph gusher
A perilymph gusher (extensive jet-like outflow of perilymph or CSF from of the vestibulum due to an open cochlear aqueduct) usually occurs unexpectedly during stapes surgery. Therefore, the surgeon should pay attention to possible stapes malformations (e.g. in the posterior crus), as these may herald an open cochlear aqueduct/perilymph gusher. When performing stapes surgery of the second ear, the surgeon has to keep in mind that uneventful surgery on the first side does not preclude a possible perilymph gusher in the second ear [199].
In case of the milder variant (“oozer”), it is often possible to introduce the stapes piston and seal the vestibulum tightly [156], [165]. In general, it has been recommended to cover the entire stapes footplate if increased perilymph flow is encountered during the operation [165]. If the stapes superstructure has been left in situ before performing the stapedotomy [37], [95], [155], it is possible to wedge a large piece of connective tissue between the footplate and the incus in order to seal the oval window fenestration tightly [165], [199]. In extreme cases, application of a lumbar drainage for three to four days may be of additional help to reduce CSF pressure [1], [156], [165].
5.1.8 Stapes surgery and otitis media
The ear surgeon has to decide when to perform stapes surgery in case of otosclerosis and concomitant otitis media. Surgery of the middle ear in presence of acute inflammatory disease is associated with an increased risk for labyrinthitis and bleeding, both of which jeopardize inner ear function [200]. Therefore it is generally recommended to postpone stapes surgery until the acute inflammation has resolved completely [201], [202]. In this context, it is worth mentioning that the risk for SNHL seems to be dependent on the nature of the middle ear pathogen/toxin. For instance, animal studies showed that opening of the inner ear after instillation of Pseudomonas aeruginosa exotoxin A into the middle ear may cause profound SNHL – an effect, which was not observed after infection of the middle ear with Streptococcus pneumoniae/Haemophilus influenzae or instillation of H. influenzae type b endotoxin [202].
5.1.9 Stapes surgery and meningitis
Bacterial labyrinthitis with subsequent meningitis is a rare, but severe complication of stapes surgery. For instance, one case of bacterial meningitis on the third postoperative day (POD 3) was reported [203]. Furthermore, two episodes of meningitis following stapedotomy were observed in one patient with a perilymph fistula plus inner ear malformation [204].
5.1.10 Bleeding
Manipulation of the mucosa around the stapes footplate is the most common cause for profuse intraoperative bleeding, which obscures the view of the operating field – particularly after the vestibulum has been opened. The surgeon has to be extremely careful when using suction near the fenestration of the oval window in such cases. While the inner ear can compensate for the loss of small amounts of perilymph, suctioning of larger amounts jeopardizes inner ear function. Therefore, it is recommended to apply Ringer’s solution or physiological saline to the vestibulum immediately in case of accidental suctioning of perilymph [194]. In order to avoid this complication, a combination of middle ear irrigation and suction can be used: placement of the suction tube at a certain distance to the oval window allows the surgeon to clear the view of the stapes footplate without challenging inner ear function (see also chapter 3.5.4).
In case of active otosclerosis (“Schwartze sign”) and inflammation, stapes surgery should be postponed [165]. Apart from possible inflammatory complications, the increased risk of bleeding with insufficient view of the stapes footplate has to be considered in this context (see chapter 5.1.8). An anteriorly displaced sigmoid sinus [143] or a persistent stapedial artery, which has to be expected in one of 1000 surgical procedures of the ear [205], are rare causes for bleeding in stapes surgery [143]. Recommendations for the management of a persistent stapedial artery range from (i) rerouting [206] and termination of the surgery if this is not possible [183] up to (ii) coagulation of the artery [207]. For further details see chapter 3.5.10.
5.1.11 Granulations
Granulation tissue of the middle/inner ear following stapes surgery has frequently been called “reparative granuloma”. Nowadays, application of this term is discouraged as (i) there is no histological evidence for granulomatous formation in this nonspecific granulation tissue and (ii) “reparative granuloma” can easily be confused with “giant cell reparative granuloma” (which usually involves the jaws!). Therefore, it has been suggested to use the term “stapes surgery induced granulation tissue (SSIG)” instead [208].
Middle/inner ear granulations following stapes surgery have been associated with SNHL [156]. For instance, granulation tissue was detected during stapes revision in three out of six patients with SNHL >85 dB following primary surgery (n=175 patients) [146]. Therefore, it is generally recommended to remove SSIG and seal the oval window niche subsequently. In order to avoid the development of granulations, the surgeon should be cautious not to introduce textile fibres along with the connective tissue used for sealing the oval window fenestration, as these may trigger a foreign body reaction with associated SNHL.
5.2 Cochlear implant surgery
Cochlear implant surgery is regarded to be a very safe and reliable procedure. Usually, complications respond to conservative treatment and/or circumscribed surgical procedures. Different classification schemes have been proposed for complications related to cochlear implantation. A commonly used system distinguishes between major complications, which are life-threatening or require surgical treatment including explantation, and minor events, which respond to conservative treatment [209], [210]. Other authors [211], [212] categorized the following as “major complications”: death; meningitis; surgery without reimplantation (including large scalp necrosis, severe wound infection, electrode displacement, tympanic membrane perforation, receiver repositioning and cholesteatoma). In this context, “minor complications” were defined as: transient facial nerve palsy; scalp haematoma; wound infection not requiring revision surgery; tinnitus, pain and facial stimulation, which could be alleviated by electrode deactivation. Moreover, complications have been classified according to the time of their occurrence: intra-/perioperative, early postoperative (<three months) and late postoperative (>three months) [212], [213], [214]. Finally, postoperative complications have been divided into flap-, patient- and implant-associated events [213].
The incidence of major complications associated with cochlear implant surgery has been estimated between 1.8 and 8.9% (minor complications: 3.5–27%) [210], [213], [214], [215], [216], [217], [218], [219], [220]. Other studies have described a total complication rate of 5.7–29% [209], [212], [217], [221], [222], [223], [224]. Early postoperative complications have been reported in 5% and late postoperative complications in 4.1% of cases [209]. The following text applies the classification scheme proposed by [209] and [210].
5.2.1 Major complications
5.2.1.1 Device failure
Device failure is considered to be the most common reason for reimplantation [221]. Failure rates of 1.7% and 2% have been reported in two large studies (n=720 and n=438, respectively) [209], [214]. No significant difference was observed for hearing performance before the device failure and after reimplantation, provided an equal number of electrode contacts was inserted into the cochlea during revision surgery [214].
The clinical picture of device failure is variable, which often makes it difficult to establish the correct diagnosis. The distinction between “hard” and “soft” device failures is based on their clinical presentation. In case of a “hard” device failure, (i) the patient has completely lost any kind of hearing impression on the implanted ear and (ii) telemetry fails to record any neural responses. On the contrary, a “soft” device failure is characterized by a deterioration of hearing performance, which cannot be attributed to any other cause. Fifteen hard and five soft device failures were reported in a series of 746 cochlear implantations [225].
Detection of a soft device failure is often a challenging task in clinical practice. At the end of the day, explantation of the old and reimplantation of a new device is the only possibility to confirm this diagnosis. If a soft device failure is suspected, a close cooperation between patient, cochlear implant team and manufacturer is essential in order to decide whether the hearing performance with the implant is still sufficient or whether a reimplantation should be performed.
If a technical defect of the device is confirmed after explantation, the diagnosis “soft device failure” has been proven. However, the situation may be less clear: sometimes, a technical defect cannot be detected in the explanted device. However, the patient’s hearing performance improves after reimplantation, which supports the diagnosis of device failure. In any case, it should be noted that cochlear implant devices cannot be compared to each other in a one-to-one fashion (e.g. slight differences in the intracochlear position of the electrode, new hardware/software, different modes of electrical stimulation).
A recent consensus document [226] recommends to consider the following aspects if soft device failure is suspected:
clinical symptoms (e.g. perceived decrement in performance/fluctuating performance, abnormal auditory and non-auditory sensations, reluctance to wear the speech processor)
medical aspects (e.g. electrode position, infection of the implantation site, delayed ossification of the cochlea, neurological disorders like stroke or demyelinating disease)
audiological findings (e.g. sudden or progressive deterioration of hearing performance, reduced sensitivity or tolerance to stimulation)
technical parameters of the device (e.g. reduced number of active channels, map deterioration, telemetry changes, abnormal results in the device integrity test).
The different definitions for device reliability and failure make it difficult for the clinician to compare individual cases to each other. In any case, a (suspected) device failure should be reported to the manufacturer in order to obtain an optimal overview of cochlear implant reliability. A European consensus document from 2005 [227] defined the term “device failure” as follows: “a device with characteristics outside the manufacturer’s specification resulting in a loss of clinical benefit”. If the problems resolve after reimplantation, a device failure should be considered and be reported to the manufacturer – even if testing of the device could not confirm a technical defect. On the contrary, a medical problem should be suspected if reimplantation of a new device fails to restore hearing function [227].
Detection of a device failure is particularly difficult in small children, as they are not able to communicate a performance decrement. Therefore, a close cooperation between family, cochlear implant team and manufacturer is essential in these situations in order to diagnose and treat a possible device failure as early as possible.
5.2.1.2 Electrode misplacement
Sites of improper electrode placement include: hypotympanic air cells (Figure 5 (Fig. 5)), vestibulum and IAC. The latter two have to be considered particularly in patients with cochlear malformations. Placement of the electrode array in the vestibulum was reported in four out of 720 cochlear implantations; one of these patients had a Mondini deformity, and three showed cochlear ossification following meningitis [225]. Difficulties with the insertion of the electrode array were reported in 21 of 550 cochlear implant procedures, these resulted in: damage (n=1), compression (n=2) and improper placement of the electrode (n=11) or failed insertion due to cochlear ossification (n=6) [215].
Figure 5. High-resolution computed tomography of the temporal bone (coronal reconstruction) showing misplacement of a cochlear implant electrode in a hypotympanic air cell (filled arrow). The open arrow points to the basal turn of the cochlea.
It is generally recommended to introduce the electrode into the scala tympani, however placement of the electrode within the scala vestibuli or migration of the electrode between the twoscales have also been observed [40]. The impact of electrode position on postoperative speech perception in CI users has been discussed controversially. While good hearing outcomes were reported for single cases of scala vestibuli insertion [228], a study with 43 patients revealed that speech perception one year after the implantation was dependent on the position of the electrode and declined in the following order: scala tympani > migration from scala tympani to scala vestibuli > scala vestibuli [229]. Furthermore, one study showed that the hearing performance six months after cochlear implantation correlates with the number of electrode contacts in the scala tympani [230]. Electrode migration from scala tympani to scala vestibuli has been discussed as a negative predictive factor for postoperative speech understanding [40].
Beside intraoperative telemetry measurements, intra- and postoperative radiography (X-ray and HRCT) help the surgeon to confirm electrode position. Beside HRCT, rotation tomography can be employed to visualize the intracochlear position of the electrode [29], [39]. In particular, the surgeon should be aware of a possible misplacement of the electrode in patients with cochlear malformation and ossification.
Finally, postoperative migration of an originally intracochlear electrode has to be considered in case of a delayed decrement in hearing performance. Therefore, the intra- or early postoperative radiographic evaluation is not sufficient to rule out a possible dislocation of the electrode, which has to be considered especially in patients with cochlear malformations [221]. In addition to the stimulation electrode, the position of the reference electrode needs to be checked: for instance, migration of the reference electrode to the EAC [221] or the dura mater [231] has been reported in the literature. Radiographic re-evaluation of the electrode position may help the surgeon in the decision-making process concerning deterioration of hearing performance in CI users.
5.2.1.3 Electrode exposure in an open mastoid cavity
If cochlear implant surgery is performed in a patient with an open mastoid cavity, a possible migration of the electrode into the cavity has to be considered [214]. Sufficient covering of the electrode has been recommended to avoid/treat this complication.
5.2.1.4 Magnet displacement
Magnet displacement, which is predominantly observed after head trauma in paediatric CI users, has been attributed to the relatively thin scalp and the pronounced convexity of the skull at this age [232], [233]. Usually, displacement of the magnet is diagnosed by palpation and/or plain skull radiography. One study described this complication in two out of 720 cochlear implantees. Treatment included repositioning of the magnet into the silicone pocket and subsequent covering with Lyodura [214].
5.2.1.5 Facial nerve stimulation/palsy
Undesired stimulation of the facial nerve, which is sometimes observed during hearing rehabilitation in CI users, may be caused by either (i) direct interaction between the electrode lead and the facial nerve in the area of the posterior tympanotomy or (ii) electrical co-stimulation of the facial nerve. While adjustment of the map and/or deactivation of selected electrodes is often sufficient to control facial co-stimulation, revision surgery is required in some cases. For instance, undesired stimulation of the facial nerve was reported in 13 out of 720 CI recipients. Revision surgery with an interposition of connective tissue between the nerve and the electrode lead was required in two of the patients [214].
Facial nerve palsy following cochlear implant surgery may be due to mechanical or thermal trauma during posterior tympanotomy. Thermal damage to the facial nerve, which was treated by decompression surgery, was reported in one out of 720 patients [214]. In general, facial nerve palsy after cochlear implant surgery is rare. In some studies, it has not been observed at all [209]. On the other hand, permanent facial nerve palsy was reported in five of 550 cochlear implant recipients (H-B grade III: n=4; grade V: n=1) [215]. Beside application of intraoperative monitoring, the ear surgeon should always be aware of possible facial nerve variants and anomalies within the mastoid, especially in patients with temporal bone malformations [234] (for details see chapter 3.5.6 and Figure 3 (Fig. 3)).
5.2.1.6 Cochlear implant surgery and otitis media/mastoiditis
Infections have been reported after 1.4–16.6% of cochlear implant surgeries [209]. An increased risk for acute otitis media (AOM) is expected for children within the first postoperative months. If AOM occurs within the first two months after surgery, an aggressive treatment with parenteral antibiotics has been recommended [235]. Oral antibiotics can be used to treat AOM occurring later than two months after surgery, provided none of the following findings is present: inner ear malformation or CSF leak, mastoiditis/meningitis or an implant model with a positioner (Advanced Bionics AB5100H and AB 5100H-11) [30]. Furthermore, myringotomy tubes can be applied in patients with cochlear implants and recurrent AOM without increasing the risk for subsequent meningitis [235]. Mastoiditis responding to antibiotic treatment was reported in six out of 550 cochlear implantees [215].
If conservative treatment fails, the device has to be removed. In this context, the detrimental impact of bacterial colonization and biofilm formation on the implant surface has to be considered [236], [237], [238] (see also chapter 5.2.1.9). For instance, one study (n=438 patients) reported recurrence of AOM/mastoiditis despite antibiotic treatment and surgical revision (mastoidectomy) in one paediatric patient. Following the second episode of infection, the device was explanted. The patient’s parents refused reimplantation later on [209].
Inflammatory disorders, such as chronic suppurative otitis media and retroauricular abscess formation, were the most common causes for explantation (n=11) in a series of 746 patients [225]. In general, the ear surgeon should consider to place a dummy into the cochlea if the implant has to be removed in order to maintain cochlear patency for a possible reimplantation.
A study with 550 consecutive cochlear implantations described tympanic membrane perforation/chronic suppurative otitis media (n=1) and development of cholesteatoma (n=6) as further complications in the middle ear. All seven patients underwent revision surgery [215].
5.2.1.7 Meningitis
Meningitis is a rare, but possibly fatal complication of cochlear implant surgery. Twenty-one cases of meningitis were observed after a total number of 24,488 cochlear implantations in North America [216]. One case of meningitis was described in a series with 550 cochlear implantations (0.2%) [215]. Furthermore, a CSF leak with associated meningitis was reported for one patient and a perilymph gusher without meningitis in 2.7% of cases (n=262) [239].
Risk factors for meningitis in cochlear implantees include: young age, implant type and presence of inner ear/temporal bone malformations [214]. Preoperative vaccination, tight sealing of the cochleostomy and an early diagnosis and treatment of suspected postoperative meningitis are of paramount importance for the successful management of this complication [216]. Vaccination against Streptococcus pneumoniae and Haemophilus influenzae type B is generally recommended before cochlear implant surgery [235], particularly in children [30].
In this context, it is worth mentioning that meningitis is not only a possible complication after, but also an indication for cochlear implant surgery. In the latter case, the underlying cause for the meningitis should be determined before cochlear implantation. Among others, bony dehiscence of the skull base and immunodeficiency syndromes should be considered as differential diagnoses. For instance, one child, who had received a cochlear implant for postmeningitic deafness, was diagnosed with X-linked agammaglobulinaemia after the operation. Finally, the device had to be explanted because of recurrent infections [240].
Choosing the right time for cochlear implantation in postmeningitic deafness is a decisive factor for successful hearing rehabilitation. In this context, it is important to know that labyrinthitis ossificans was detected by HRCT as early as four weeks after meningitis in a study with 95 children. Usually, a bilateral ossification of the inner ear has to be expected in this situation. Although cochlear osteoneogenesis is known to be progressive, the time course cannot be predicted for the individual patient. Therefore, bilateral cochlear implant surgery should be performed as early as possible in case of profound SNHL following meningitis [241].
5.2.1.8 Wound infection
Wound infections, which were observed for 4.2% of 733 CI recipients in one study, often respond to conservative treatment [242]. Early initiation of a broad-spectrum antibiotic treatment including agents with activity against methicillin-susceptible and methicillin-resistant Staphylococcus aureus has been recommended for children [30]. Later on, the antimicrobial therapy can be adjusted according to the antibiogram. Early diagnosis and immediate therapy are essential in case of wound infections following cochlear implant surgery. Delayed initiation of therapy may end up with revision surgery – or even explantation of the device [213], [243].
5.2.1.9 Flap necrosis/device extrusion
Necrosis of the skin/muscle flap covering the receiver of the implant may be due to an overly tight wound dressing, wound infection and/or excessive magnetic force between the coil and the implant. A consequent treatment of surgical site infections is essential to avoid necrosis. In case a necrosis has developed, careful debridement and reconstruction of the skin surface with a full-thickness skin graft or a rotational flap have been recommended. Single cases of device explantation due to flap necrosis have been reported in the literature. For instance, three out of 720 cochlear implant recipients experienced flap necrosis, which required explantation in two cases [214]. In a further study with 438 cochlear implantations, two cases of wound infection with Pseudomonas aeruginosa were observed, which resulted in explantation and reimplantation after eight and 12 months, respectively [209].
Skin flap irritation due to an overly long nylon thread, which had been used for fixation of the implant, was reported in one out of 720 patients. The inflammatory response subsided after shortening of the thread during revision surgery [214]. Eight out of 550 patients presented with wound infection and flap dehiscence [215]. In this context, it should be noted that the risk for soft tissue complications seems to be similar for children and adults [244].
Knowledge of the different types of skin incisions and flap designs is an indispensable prerequisite to avoid flap necrosis. In the early days of cochlear implant surgery, large skin flaps were created in order to avoid placement of the implant directly below the skin incision. In case the skin incision line crosses the implant, it is recommended to create a musculofascial flap, which completely covers the implant and the electrode lead. Extended skin flaps have been associated with an increased risk of scalp necrosis due to a compromised vascular supply of the skin [213]. The superficial temporal artery and the dermal plexus feed the area anterior to the auricle, while the posterior area is supplied by the retroauricular and occipital branches of the external carotid artery [215].
Delayed swelling/itching of the skin covering the implant may indicate a foreign body reaction, which may require explantation of the device in single cases. After an infection has been ruled out, a possible contact dermatitis to silicone should be considered as a differential diagnosis [245], [246]. Considering the knowledge on silicone implants from other subspecialities (e.g. breast implants), the ear surgeon should keep in mind that silicone allergy may manifest itself in the early or late postoperative period. For instance, latencies of up to ten years have been reported in the literature [247]. If a contact dermatitis to silicone is suspected in a cochlear implantee, allergy testing using the material kit provided by the manufacturer should be performed. In this context, it is important to know that different types of silicone are used in different types of implants. Accordingly, reimplantation with a different type of implant has been reported as a successful treatment of silicone allergy [209]. Furthermore, three patients were re-implanted with custom-made implants excluding the type of silicone causing the allergic reaction [248]. Finally, non-allergic foreign body reactions with the histopathological finding of polynuclear giant cells have been reported in CI recipients as well.
5.2.1.10 Haematoma
A haematoma requiring surgical revision is classified as a major complication. Haematomas are often caused by venous bleeding from the bone during preparation of the subperiostal plane. In case of a small skin incision, the bleeding source may be overlooked during the operation due to limited view. In these cases, illuminated retractors may help to gain an overview of the entire subperiostal pocket. One study reported surgical revision of a haematoma in three out of 720 cochlear implant recipients [214]. A subdural haematoma was observed in one patient following bipolar cautery of a prominent diploic vein in a study with 212 cochlear implantations [224].
5.2.2 Minor complications
5.2.2.1 Tinnitus
Development of tinnitus following cochlear implant surgery is rare: 0.7% (n=438) and 1.9% (n=262) of CI users reported tinnitus after surgery [209], [239].
5.2.2.2 Vertigo/dizziness
Although improvement of vestibular function following cochlear implantation has been reported [249], vestibular dysfunction is an important aspect in the postoperative care of cochlear implantees. As already mentioned in chapter 2.2, the physician has to distinguish between subjective vestibular symptoms and objective vestibular signs, which do not always correlate with each other perfectly [6].
Transient vertigo and dysequilibrium have been described as the most frequent minor complication after cochlear implant surgery [217]. The prevalence of postoperative vestibular symptoms has been reported to range between 0.33 to 75% [250], with values between 30 and 60% in most studies [5]. Ipsilateral caloric weakness following cochlear implantation has been observed in 16 to 50% of cases [4], [6], [250], [251], [252].
It is important to consider the different study designs and observation periods in order to judge these values adequately. Vertigo and dizziness may occur in the early postoperative period or after a latency of weeks to months [5], [253]. Moreover, various clinical presentations ranging from a single episode of vertigo up to recurrent vertigo attacks or persistent imbalance have to be distinguished [5].
A closer look at the underlying pathomechanisms helps to understand the different qualities and time courses of vestibular dysfunction following cochlear implantation. These models are based on both clinical observations and temporal bone studies [254], [255]. Due to the anatomy of the inner ear, the risk of intraoperative injury is highest for the sacculus, followed by the utriculus and the semicircular canals. Insertion of the electrode array into the cochlea may cause damage to the vestibular sensory epithelium [255]. Furthermore, a loss of perilymph may occur during cochleostomy. Both mechanisms are able to explain the clinical observation of an acute vestibular syndrome immediately after the operation.
BBPV, which is observed in 2.2 to 10% of patients following cochlear implantation [256], [257], may either be caused by (i) bone dust falling into the labyrinth or (ii) drill-associated dislocation of otoliths (see also chapters 4.3 and 5.1.4). Furthermore, activation of the implant has been discussed as a trigger for otolith displacement. Note that both adults and children may be affected by BPPV following cochlear implantation [258].
Post-mortem studies on temporal bones of cochlear implantees revealed various chronic changes of the vestibular organ (e.g. fibrosis of the vestibulum, osteogenesis, reactive neuroma), which were interpreted as sequelae of labyrinthitis, foreign body reaction or vascular lesion [255]. The time-course of these pathophysiological reactions may explain the delayed onset of vestibular symptoms in some CI users after an initial symptom-free interval in the early postoperative period. At this point it is worth mentioning that the chronic changes described above were only observed if the electrode had been inserted into the scala vestibuli. Therefore, the surgeon should aim to place the electrode within the scala tympani not only for audiological, but also for vestibular reasons. Furthermore, endolymph hydrops was detected in temporal bone studies following cochlear implantation, which is considered to be the histological correlate for the clinical observation of Menière-like vertigo attacks in CI recipients [254].
Some patients experience vertigo/disequilibrium when exposed to loud noise, provided that the implant is active. In these cases, vestibular co-stimulation by the cochlear implant has to be considered [259]. On the other hand, short pressure-induced attacks of vertigo (e.g. provoked by sneezing, coughing, lifting heavy weight) may be caused by a perilymph fistula due to an insufficient sealing of the cochleostomy (for further information see chapters 4.3 and 5.1.4).
In summary, the plethora of underlying causes for vestibular dysfunction in cochlear implantees requires a thorough neurotological work-up. Therapeutic options include: reposition maneuvers for BPPV, vestibular rehabilitation training for acute and chronic vestibular hypofunction [214] and tympanotomy in cases of a suspected perilymph fistula.
Soft surgery is generally recommended to reduce vestibular complications associated with cochlear implantation. In this context, several studies have compared postoperative vestibular function after anterior cochleostomy versus round window insertion of the electrode. A postoperative decrement of saccular and horizontal SCC function was observed more frequently following anterior cochleostomy (50% and 43%) as compared to round window insertion (13% and 9%) [260]. Furthermore, local application of methylprednisolone to the round window membrane during CI surgery has been recommended to reduce the incidence of postoperative vestibular symptoms [261].
Unfortunately, no preoperative predictors of postoperative vestibular function have been identified to date [262]. In particular, it must be noted that a chronic vestibular dysfunction may be present after cochlear implant surgery, even if the patient has no subjective symptoms or has recovered quickly from postoperative vertigo [263].
The ear surgeon should always keep in mind that vestibular problems may have a severe impact on the CI user’s quality of life. Vestibular re-evaluation is recommended before cochlear implant surgery of the second ear (see chapter 2.2 and 5.1.4) in order to balance the possible benefits of bilateral hearing against the risk of bilateral vestibulopathy. This aspect deserves special attention in the discussion about simultaneous bilateral cochlear implantation [9].
5.2.2.3 Transient facial nerve palsy
Delayed facial nerve palsy following cochlear implant surgery is treated with steroids and usually recovers well. For instance, two out of 720 patients displayed postoperative facial weakness, which resolved completely [214]. Another study reported 12 cases of transient facial nerve palsy and three cases of chorda tympani dysfunction in 550 CI recipients [215]. Transient chorda tympani symptoms were described by 5.7% of patients in a retrospective study with 505 implantations [217].
5.2.2.4 Tympanic membrane/posterior wall of the external auditory canal
Injuries of the tympanic membrane and the posterior wall of the EAC may occur during cochlear implant surgery. Usually they are treated during primary surgery. For instance, tympanic membrane perforation was observed in 10 patients, and injury of the posterior wall of the EAC was reported in 12 cases among 550 cochlear implantations [215].
5.2.2.5 Emphysema/haematoma
The ear surgeon should also pay attention to postoperative emphysema and haematoma, which may reach the lateral corner of the eye according to the extension of the temporal fascia. Revision surgery is rarely necessary in these cases. Wound swelling was reported in 13 of 438 cochlear implantations (3%) [209]. Scalp pneumatoceles triggered by Valsalva’s maneuver may occur early or months to years after cochlear implantation. Usually, they resolve by themselves, however they may be accompanied by infection or implant dysfunction [264].
5.3 Vestibular schwannoma
Treatment of vestibular schwannomas (VS) is determined by: tumour biology, long-term tumour control, functional outcome and quality of life. Due to the refinements in VS surgery, mortality rates are approaching zero [265], [266]. In summary, postoperative morbidity has been estimated to be <5% [267], [268], [269], [270]. Postoperative complications include: CSF leakage, cranial nerve (CN) dysfunction, meningitis, cerebral edema/contusion, intracranial haematoma, hemiparesis, hydrocephalus and wound infection [271]. Among these, CSF leakage (10–15% of cases) and dysfunction of CN VII and VIII are the most common problems.
5.3.1 Surgical approach
In general, three basic surgical approaches can be used for exposure of vestibular schwannomas: translabyrinthine (TL), middle fossa (MF) and retrosigmoid (RS). Complete microsurgical removal of the tumour has been reported in 97%, recurrence of disease has been observed in 9% of cases [271]. It is also possible to perform a deliberate near-total resection near the brainstem and the cranial nerves combined with postoperative radiosurgery in order to preserve cranial nerve function [272]. Small remnants of the tumour left on the cranial nerves often remain stable in size over time [273]. This aspect deserves the surgeon’s special attention in the decision-making process concerning totality of tumour removal versus postoperative complications.
A large meta-analysis (35 studies with 5,064 patients in total) analyzed the incidence of complications depending on the surgical approach, the most important results are summarized in Table 1 (Tab. 1) [274]. No statistically significant difference was observed for: residual tumour, tumour recurrence, mortality, non-CN neurological complications and dysfunction of cranial nerves other than CN VII and VIII [274].
Table 1. Complication rates of vestibular schwannoma surgery dependent on the selected approach [274].
The TL approach offers an excellent direct access to the IAC. Furthermore, it allows identification of the facial nerve and control over the dural blood supply of the tumour. Extradural retraction of the brain is usually not necessary. However, the high risk for CSF leaks is considered to be a major disadvantage [126]. However, it has to be considered that the TL approach is often applied for big tumours – which is an independent risk factor for CSF leakage [17], [275].
The RS approach requires retraction of the cerebellum and offers a good overview on the brainstem level. However, resection of bone at the dorsal border of the IAC and visualization of the IAC fundus are compromised due to the anatomical position of the labyrinth.
When using the MF approach, the surgeon has to keep in mind that the anatomical course of the facial nerve may compromise tumour exposure in the IAC. Furthermore, overview of the posterior fossa is limited. The surgeon has to be extremely cautious not to damage the facial nerve during tumour resection, particularly in case of schwannomas arising from the inferior vestibular nerve [276]. Although this approach offers the chance to preserve hearing, the surgeon has to be aware of the fact that the auditory nerve and its vascular supply are concealed by the tumour and the facial nerve most of the time during surgery – which may lead to injuries of these delicate structures causing subsequent hearing loss [277].
5.3.2 Intraoperative monitoring of cranial nerves
Maintenance of cranial nerve function is a major goal in VS surgery. As postoperative facial nerve function has a tremendous impact on the patient’s quality of life [278], monitoring of the facial nerve is of paramount importance. In recent years, monitoring of the auditory nerve has been implemented into VS surgery as well.
Morbidity was clearly reduced after introduction of intraoperative monitoring into VS surgery [279]. It allows the surgeon to adjust the treatment of VS in a way that allows optimal maintenance of CN function: (i) complete tumour resection, (ii) near-total resection or (iii) a combination of resection and postoperative radiosurgery [280], [281], [282].
EMG of the orbicularis oculi and orbicularis oris muscles is commonly applied for intraoperative monitoring of the facial nerve. It helps the surgeon to identify the nerve and control nerve function during surgery and at the end of the operation. Stimulation levels below 0.2 and above 0.5 mA are usually applied for identification of the nerve. If stimulation thresholds exceed 0.5 mA, the surgeon may expect further soft tissue/bone between the stimulator and the facial nerve [283]. In case of stimulation thresholds <0.2 mA, the nerve is already exposed or covered only by a very thin layer of tissue [284]. However, the surgeon must keep in mind that high stimulation thresholds may be false friends: if the nerve is adherent to the tumour, high levels may be required to obtain a neural answer, although the nerve is already very close to the stimulator [285].
The surgeon receives direct feedback from EMG monitoring in case he jeopardizes facial nerve function. This intraoperative “dialogue” allows the surgeon to reflect and refine his preparation techniques, which helps to reduce the risk of facial nerve injuries [286], [287], [288], [289], [290], [291]. Distinct EMG response patterns have been associated with stimulation, irritation and damage of the facial nerve. For instance, so-called “A trains” (high-frequency series of potentials lasting for seconds, amplitudes between 100–200 µV, sudden onset and termination), “B trains” (spikes and bursts) and “C trains” (irregular waves and amplitudes) have been distinguished (Figure 6 (Fig. 6)). Among these, “A trains” are of special importance for the surgeon, as they have been shown to predict postoperative facial nerve dysfunction [290]. An EMG response following proximal facial nerve stimulation at the end of surgery indicates that the continuity of the nerve has been preserved. However, postoperative facial nerve dysfunction has to be expected if stimulation thresholds have increased during surgery [283], [292], [293], [294], [295], [296], [297].
Figure 6. Characteristic traces of intraoperative facial nerve electromyography (EMG) during vestibular schwannoma surgery (simulated data according to [279]). a.) “A train”: high-frequency signals with sudden onset / end. b.) “B train” with spikes. c.) “C train” with continuous irregular EMG activity.
Despite the advantages of facial nerve monitoring, the surgeon should always keep in mind that this technique is not meant to replace anatomical knowledge and surgical ability. It increases the surgeon’s margin of safety, but does not always correlate perfectly with facial nerve function. For instance, bipolar cautery near the facial nerve may cause artifacts and interference. Furthermore, it should not be neglected that stimulation itself may damage the facial nerve, particularly during long-term application of high-amplitude stimuli [298], [299]. Animal studies have shown that intermittent stimulation and reduction of stimulus frequency reduce the risk of facial nerve damage, whereas stimulation levels seem to be of minor importance [298], [300], [301], [302], [303], [304]. In recent years, an infrared camera, which allows direct observation of the facial muscles during surgery, has been developed as an adjunct to EMG monitoring [15].
When it comes to intraoperative monitoring of the auditory nerve, some pecularities have to be considered. First, the nerve is especially prone to mechanical trauma, as it has no perineurium. Second, its function may be affected by vascular injury within the internal auditory canal during tumour resection (e.g. vascular occlusion, rupture or spasm) [279]. Accordingly, vasoactive treatment following VS surgery has been associated with a significantly higher rate of hearing preservation [305].
ABRs are commonly applied for intraoperative monitoring of auditory function. Amplitudes and latencies of waves I, III and V are compared to preoperative values. It should be noted that the surgical preparation has to be interrupted during the measurement. Furthermore, it is difficult to make intrapoerative decisions based on ABR monitoring, as signal morphology is influenced by many different factors (e.g. anaesthetics, hypothermia, irrigation) [292], [306], [307], [308], [309], [310], [311], [312]. Electrocochleography and compound nerve action potentials are further techniques of auditory monitoring, however they only provide the surgeon with information about the peripheral auditory pathway [279].
5.3.3 Hearing preservation
Hearing preservation in VS surgery is largely dependent on tumour size. In particular, incorporation of the auditory nerve into the tumour has to be expected in 90% of cases for tumours >3 cm in diameter. Therefore, the surgeon should pursue maintenance of auditory function for smaller tumours only [273]. Good/serviceable hearing (class I/II hearing according to the Gardner-Robertson Hearing Scale) following microsurgical management of VS was reported in 50% of cases for the MF approach and in 31–33% for the RS approach [313], [314]. Furthermore, 37–73% of patients had postoperative hearing levels class A and B (according to the AAO-HNS hearing classification) following VS resection via the MF approach [276], [315], [316], [317], [318], [319], [320], [321], [322], [323]. Normal postoperative hearing was described in 57% of 28 patients who underwent VS resection using the (extended) MF approach [324]. For the RS approach, 27.3% of patients displayed normal pre- and postoperative hearing levels [325]. When comparing these results with each other, it has to be noticed that the RS approach is more commonly applied for larger tumours than the MF approach. Accordingly, hearing preservation in 48% of cases was reported for intracanalicular tumours treated with the RS approach [314]. The distance between tumour and IAC fundus is another decisive factor for hearing preservation: for distances <3 mm, the surgeon should not expect maintenance of hearing function following surgery.
In contrast to other treatment modalities (“wait and scan”, radiosurgery), hearing thresholds following VS surgery have been reported to be stable over time [316], [326], [327]. Consequently, surgical management offers the best chance to maintain long-term hearing in VS patients – given that the prerequisites mentioned above are fulfilled [276]. In case of postoperative hearing deterioration, a partial recovery is possible [328]. Single cases of hearing improvement following VS resection have also been reported [329].
In any case, the patient has to be informed about his individual chance of hearing preservation during preoperative counselling. Only with this information available, real “informed consent” is possible.
5.3.4 Tinnitus
Tinnitus before VS surgery was described in 29–70% of patients [18], [330], [331]. Newly diagnosed tinnitus following resection of the tumour was observed in 39% of cases, whereas postoperative improvement of tinnitus was reported in 33% [18].
5.3.5 Balance
Strictly speaking, vestibular dysfunction following dissection of the vestibular nerve in VS surgery is a sequela rather than a complication, which is reflected by the observation of vertigo in 80% of patients on POD 1 [18]. This number declines to 40% after one week and 9–30% after one year due to central vestibular compensation [18], [332].
The ear surgeon has to take care that this sequela does not turn into a complication for his patient by (i) performing a thorough neurotological examination before treatment of VS, (ii) choosing the right time for surgery and (iii) consequent treatment of pre- and postoperative balance problems. Vestibular dysfunction has a huge impact on the patient’s quality of life: e.g. 8–30% of patients have been reported to experience postoperative dysequilibrium as a disabling symptom [332] (for details see chapter 5.3.12). The following chapter outlines the basic principles in the pre- and postoperative care of the dizzy VS patient.
Neurotological history-taking and examination before VS surgery help to predict both the degree of postoperative vertigo and the speed of central compensation: the greater the preoperative peripheral-vestibular deficit on the side of the VS, the less vestibular function is lost during surgery and hence has to be compensated afterwards. Accordingly, patients without preoperative caloric response on the affected ear reported postoperative vertigo less frequently as compared to VS patients with maintained preoperative caloric response [18]. Furthermore, the ear surgeon should check for signs and symptoms of a central vestibular disorder, which may compromise postoperative vestibular compensation [333]. As for neurotological history-taking, the following aspects should be considered in this context: neurological disorders independent of VS (e.g. multiple sclerosis), ophthalmologic and/or orthopaedic problems, psychiatric comorbidity (particularly anxiety, depression) and medication acting on the CNS. Neurotological evaluation is an essential factor in the decision-making process concerning the optimal treatment modality of VS for each individual patient.
When talking about vestibular compensation following VS surgery, one has to distinguish between “physiological” and “functional” compensation: whereas the former can be measured by objective parameters (e.g. decrease of an initial SN, increasing nystagmus symmetry in rotational chair testing) [3], the latter is the prerequisite for the patient’s return to his preoperative level of activity. In this context, non-vestibular factors (e.g. somatic and psychiatric comorbidity) play an important role as well. The ear surgeon should also be aware of the fact that patients are particularly prone to developing depressive symptoms following VS surgery (for details see chapter 5.3.10), which in turn may compromise postoperative vestibular compensation [332]. On the other hand, several studies have demonstrated the positive effect of customized vestibular rehabilitation on both the subjective symptoms and objective signs of postoperative vestibular dysfunction following VS resection [136], [334].
Postoperative vestibular compensation is a multi-factor process and therefore difficult to “measure”. The so-called vestibular index, a scoring system including subjective and objective parameters, has been proposed by [3] in order to provide a long-term follow-up of vestibular function following VS surgery. In case of delayed postoperative compensation or deterioration of an initially good compensation, a thorough vestibular re-evaluation (including additional factors like comorbidities) is necessary in order to avoid the development of chronic vestibular dysfunction.
While vestibular rehabilitation therapy has been applied successfully for the treatment of postoperative vestibular dysfunction for many years, a new concept called “vestibular prehabilitation” has evolved in recent years [10], [335]. Patients with (residual) vestibular function on the side of the VS begin a vestibular training programme before the operation. After two weeks of training, three to four injections with gentamicin (12 mg each) are applied to the tympanic cavity of the affected side, inducing a slow progressive loss of vestibular function. Vestibular training is continued in parallel in order to promote vestibular compensation. Due to the combination of slow vestibular loss and parallel compensation, patients experience only mild subjective dysequilibrium – in contrast to the severe vertigo induced by the sudden loss of function caused by deafferentiation of a (partly) functional vestibular organ during VS surgery.
In case of vestibular prehabilitation, VS surgery is performed after (i) (near-)total loss of vestibular function on the affected side has been confirmed (Figure 2 (Fig. 2)) and (ii) the patient’s subjective vertigo symptoms have subsided (indicating successful vestibular compensation). Patients treated with vestibular prehabilitation report only mild disequilibrium following resection of the VS and are often able to walk around from POD 1 onwards (see case 2). Accordingly, reduced postoperative anteroposterior postural sway has been observed in patients who had been treated with vestibular prehabilitation as compared to the control group without this therapy [336].
Integration of vestibular prehabilitation into the treatment of VS is an important step towards an individualized symptom-based management of the disease aiming to provide the best possible postoperative quality of life for the patient.
5.3.6 Facial nerve
Independent of the surgical approach chosen, facial nerve continuity is maintained after VS surgery in >95% of cases. Accordingly, normal postoperative function/mild dysfunction of the facial nerve (H-B grade I/II) has been described in 61–90% of patients [313], [314]. For small vestibular schwannomas, postoperative functional integrity of the facial nerve has even been reported in up to 97% of cases [320].
The facial nerve is most vulnerable at its subarachnoidal portion right before it enters the internal auditory canal [337]. Transection of the nerve during preparation of the tumour was reported for one out of 67 patients [18]. In this context, the surgeon has to keep in mind that the facial nerve may be incorporated into the VS independent of tumour size – which increases the risk for postoperative facial nerve palsy [338]. In case the VS infiltrates the myelin sheath and the vessels of the facial nerve, it is recommended to leave tumour remnants in situ deliberately in order to preserve neural function.
Preoperative facial nerve palsy has been identified as an independent risk factor for an additional postoperative deterioration of function. For instance, functional integrity of the facial nerve was preserved in 91% (intracanalicular tumours), 35% (extrameatal tumour diameter: 1.5–3 cm) and 11% of patients (extrameatal tumour diameter >3 cm) on POD 1 provided that preoperative neural function was normal. On the other hand, a further deterioration of facial nerve function was observed in 82% of patients on POD 1, who had presented with preoperative facial nerve palsy [18]. A further study reported postoperative facial nerve palsy in 64% of those patients who had experienced preoperative chorda tympani symptoms [268]. Moreover, the surgeon has to keep in mind that facial nerve function may improve or deteriorate during the postoperative course [339]. Delayed postoperative facial nerve palsy may be caused by ischaemia, edema and viral reactivation [16]. Furthermore, it must be noticed that complete eyelid closure may still be possible due to gravity within the first three postoperative days despite an intraoperative lesion of the facial nerve (see also chapter 3.5.7). Therefore, a diligent postoperative follow-up of facial nerve function is essential after VS surgery. In addition, the surgeon should always check for the follow-up intervals when reading (and comparing) clinical studies about postoperative facial nerve function.
Cystic tumour consistency and previous (radio)surgery of VS are further factors, which render the facial nerve more susceptible to postoperative dysfunction [340]. Functional results have been reported to be more favourable following the resection of solid as compared to cystic VS [341], [342]. Preservation of facial nerve function is also less likely in patients with neurofibromatosis type 2 (NF2). For instance, two out of four patients with NF 2 displayed good postoperative facial nerve function (H-B grade I), while complete facial nerve palsy (grade VI) was observed for the other two [273].
Dysfunction of the intermediate nerve has attracted less attention. However, a dry eye caused by intraoperative irritation of the facial nerve may suffice to compromise the patient’s quality of life considerably. One study reported reduced/absent tear production in 162 out of 224 patients following VS resection [343]. A dry eye caused by incomplete eyelid closure and/or reduced tear production may require a life-long treatment with artificial tears and result in neurotrophic corneal ulcers or corneal perforation/iris prolapse. Accordingly, long-term application of ocular lubricants was necessary in 23 out of 62 patients following VS surgery, 20 patients required tarsorrhaphy, and three patients developed corneal perforations. A significant drop in visual acuity was observed in 12% of the patients after the operation [344].
In this context, it is important to mention that patients with postoperative loss of normal corneal sensation do not notice eye symptoms in the beginning. Therefore, an early ophthalmologic examination following VS surgery has been recommended. This suggestion has been confirmed by a prospective study about early complications and symptoms following CPA surgery. For instance, keratitis was observed in 30 out of 72 patients within the first eight postoperative days. Five patients with good postoperative facial nerve function (grade I) and seven patients with mild dysfunction (grade II) developed keratitis. Consequently, the possibility of reduced tear production should always be considered, even if postoperative facial nerve motor function seems to be normal [18].
5.3.7 Other cranial nerves
Cranial nerves other than CN VII and VIII have to be considered particularly during the resection of large vestibular schwannomas. One transient and three permanent CN VI palsies were reported following surgery of 56 large vestibular schwannomas (>3 cm). Transection of the nerve and intracranial haemorrhage were described as underlying reasons. Furthermore, one patient developed transient dysarthia, ataxia and dysfunction of the contralateral vagal nerve, which was attributed to brainstem shifting [273].
5.3.8 CSF leak/meningitis
A CSF leak has to be expected in 7 to 30% of patients following VS resection [18], [267], [268], [345], [346]. This complication, which has to be considered particularly for the TL approach, is a common cause for revision surgery. Dural defect reconstruction and obliteration of the mastoid cavity (e.g. with abdominal fat) help to prevent postoperative liquorrhea. The importance of these surgical steps is illustrated by the observation of a temporal lobe encephalocele following TL resection of a vestibular schwannoma [342].
In case of CSF leakage into the mastoid cells, the liquid accumulates in the tympanic cavity and commonly presents to the clinician as tympanic effusion or posterior rhinoliquorrhea (drainage via the eustachian tube). Further clinical manifestations of a CSF leak include subcutaneous CSF collection and CSF wound drainage. Therefore, the surgeon should always obliterate the mastoid cavity (and the antrum in case of a TL approach) beside performing a duraplasty. Furthermore, transnasal closure of the eustachian tube has been described as an option to manage rhinoliquorrhea [347], [348].
However, fat tissue may migrate into the subarachnoid CSF space/CPA and induce aseptic meningitis [349]. Disseminated fat particles can be visualized by MRI and do not necessarily cause complications. Usually, radiological and neurological controls are sufficient in the absence of clinical symptoms [350]. Apart from this special form of aseptic meningitis, chemical meningitis is rarely observed after CPA tumour surgery (<4% of cases) [351].
Bacterial meningitis following VS surgery, which has been reported in 0.14–8.2% of cases [267], [268], [269], is a severe and possibly fatal complication. Pneumococcal meningitis has been associated with a mortality rate of 20%, and even 60% for multidrug resistant strains [352].
In the light of these deleterious complications, it becomes clear that successful dural defect reconstruction following tumour resection is an issue of utmost importance in skull base surgery. The surgeon should always remember to design his surgical procedure in a way to allow not only optimal exposure and resection of the tumour, but also reconstruction of the skull base. Moreover, surgical draping should provide access to the tissues required for dural defect reconstruction (e.g. abdominal fat). Furthermore, a careful elevation of the dura around the bony margins of resection is recommended before opening the endocranial space in order to facilitate dural defect reconstruction at the end of surgery.
5.3.9 Bleeding/vascular injury
The sigmoid sinus may be injured during drilling of the mastoid bone. Circumscribed defects can be closed with oxidized cellulose. Extended lesions may require intra-/extraluminal packing of the sigmoid sinus. The surgeon should always check the preoperative CT/MRI scans for size, dominance and patency of the sigmoid sinus on both sides. Knowledge of the patient’s individual anatomy helps the surgeon to judge the importance of maintaining the patency of the sigmoid sinus.
In case of venous bleeding, a lesion of the superior petrous sinus has to be considered as well. In particular, the surgeon has to be aware of the fact that damage to the sigmoid/superior petrous sinus and temporal veins may result in venous insufficiency/thrombosis, which are a common cause for edema, ischaemia and infarction of the temporal lobe [126], [127]. Corresponding radiological signs were detected in 22% of patients (n=65) following VS resection via the TL approach [127]. Finally, a lesion of the superior petrous sinus has been considered as a possible underlying cause for the development of a subdural hygroma after translabyrinthine VS surgery [126]. These examples illustrate that a detailed knowledge of the patient’s venous anatomy is of paramount importance in VS/CPA surgery.
Delayed CPA haematomas were reported in association with postoperative anticoagulation. For instance, two patients, who received postoperative curative anticoagulation for suspected cardiac ischaemia and phlebitis respectively, developed CPA haematomas, which did not require revision surgery [18]. Beside the space-occupying effect, postoperative bleeding may also result in a secondary hydrocephalus. Finally, it should be taken into account that large tumours commonly release thromboplastin during surgery. Therefore, the ear surgeon should always observe the patient’s coagulation carefully during VS resection. This knowledge is particularly important, as intravascular coagulation is discussed as a possibly fatal complication of VS surgery [353].
Arterial injury during VS surgery may affect the anterior inferior cerebellar artery and the perforating branches of the basilar artery, resulting in the clinical picture of a brainstem infarct [345]. Moreover, the surgeon should keep in mind that the enlarged MF approach [354] may jeopardize blood supply of the ipsilateral eye, as the medial meningeal artery (which is transected during this approach) has to be considered as a possible feeder of the ophthalmic artery [355].
5.3.10 Affection of the brain
Cerebral contusion or infarction is observed in up to 10% of patients following extradural retraction of the brain during skull base surgery [13]. In particular, this risk has to be considered for the temporal lobe when applying the (enlarged) MF approach in VS surgery [354].
Numerous studies have focussed on temporal lobe pathologies following resection of VS using the MF approach. For instance, temporal lobe gliosis was detected by MRI in 31.25% [356] and 40.5% [357] of patients, respectively. A further MRI study reported temporal lobe gliosis (slight: n=11, moderate: n=9, severe: n=2) in 22 out of 32 patients one year after surgery [358]. Comparison of pre- and postoperative EEG measurements revealed de novo/progressive postoperative EEG changes in 86% of patients [359]. Moreover, postoperative mnestic deficits were detected in 60% of patients with temporal lobe gliosis, but only in 13 % of patients without gliosis [357]. Although 19 of 20 patients with temporal lobe gliosis did well in neuropsychological testing, one patient showed a functional deficit in the Boston Naming Test and the Berlin Amnesia Test [358]. A clinical study on 735 patients described temporal lobe contusion in two, an epileptic seizure in one and a transient neurological deficit in 42 patients (5.7%) [360]. Although epileptic seizures are rare in this context, patients in the UK are banned from driving in the first postoperative year after skull base surgery via a MF approach [361].
Depression following VS surgery is another important issue. Three studies reported postoperative depressive symptoms in 17–28.9% of patients [325], [362], [363]. Three out of 27 patients were depressive before resection of a VS (RS: n=24, TL: n=3), and nine more afterwards (particularly women). Three of these patients complained of persistent depression one year after surgery [364]. In this context, the surgeon should be particularly aware of the association between hearing loss, tinnitus and depression [365], [366]. Furthermore, vestibular rehabilitation after VS surgery may also be compromised by a postoperative depressive disorder (see chapter 5.3.5). A report on suicide in two patients stresses the outstanding importance of early diagnosis and adequate treatment of depression following VS surgery [364].
5.3.11 Abdominal haematoma
If abdominal fat has been applied for stabilization of dural defect reconstruction and obliteration of the mastoid cavity, the ear surgeon has to check for a possible postoperative abdominal haematoma. This aspect is worth mentioning, as the abdomen may easily slip out of the ear surgeon’s sight (and mind!). Subcutaneous abdominal haematomas were observed in three out of 72 patients (4%) following CPA surgery [18].
5.3.12 Complications of vestibular schwannoma surgery – the patient’s view
Postoperative quality of life is a very important issue in VS surgery. In one study with 386 patients, 155 (40.2%) reported disabling postoperative symptoms, including: hearing loss (10.1%), dysequilibrium (10.1%), facial nerve dysfunction (9.6%), tinnitus (5.2%), headache (3.1%) and eye problems (1.8%) [13]. In particular, it should be noted that patients with multiple afflictions rated facial weakness and eye symptoms as the most disabling problems [367].
The impact of these symptoms on the patient’s psychosocial function should not be underestimated. For instance, 7–38% of patients have been reported to be on long-term sick leave following resection of a vestibular schwannoma [362], [363], [368], [369]. Furthermore, patients after VS surgery scored lower in all eight domains of the SF-36 questionnaire as compared to a normal control population [13]. Different views regarding a possible influence of age and tumour size/localization on postoperative morbidity have been expressed in the literature, e.g. one study identified female gender, age >45 years and extrameatal localization of the tumour as negative predictors for postoperative quality of life [13].
In any case, the ear surgeon should always keep in mind that the patient’s perception of postoperative (dis-)abilities may differ a lot from his own view [370]. A study on hearing preservation after RS surgery of vestibular schwannomas illustrates this important observation: audiological evaluation confirmed residual hearing in ten patients, however only five of them reported this to be useful in everyday life [325].
5.3.13 Malignancy of vestibular schwannomas
Malignancies of the 8th cranial nerve are rare, and malignant transformation of a primarily benign VS is an even more unusual observation. A recent review of the literature described five cases of malignant transformation for vestibular schwannomas (three after radiosurgery and two without radiosurgery) [371].
Indications for tumour resection following radiosurgery of vestibular schwannomas include: (i) brainstem compression/secondary hydrocephalus due to tumour growth, surrounding edema, development of cysts and (ii) evolution of malignant disease [372]. For the latter, two entities have to be distinguished: (i) radiation-induced de novo secondary neoplasm (e.g. glioblastoma multiforme) and (ii) malignant transformation of the primarily benign VS [373]. Although these complications are rare, the ear surgeon has to keep them in mind, especially when informing the patient about the various treatment modalities for VS [373]. Due to the short follow-up periods reported in the literature, it is very hard to judge the “real” incidence of malignant transformation/radiation-induced malignancy for vestibular schwannomas: sarcomas are expected after an average latency of eight years following radiosurgery, gliomas after 14 years and meningeomas after 21 years [371].
5.4 Jugulotympanal paraganglioma
Jugulotympanal paragangliomas are the most common neoplasms of the middle ear and the temporal bone [374], [375]. The Fisch classification (class A–D with further subgroups) is based on tumour size and localization [376]. Treatment modalities include surgery, radiotherapy and “wait and scan”. Complete resection of jugulotympanal paragangliomas without cranial nerve lesion is usually possible for class A/B tumours. However, it may be very difficult to achieve complete tumour removal and preservation of cranial nerve function in case of advanced tumour stages [377].
Blood loss, ACI injury, CSF leakage and cranial nerve dysfunction are the most important complications in the resection of large jugulotympanal paragangliomas. The surgeon should always consider the growth rate of the tumour in the decision-making process concerning the extent of tumour resection: paragangliomas may grow very slowly, so that patients may be (almost) free of symptoms without therapy for over 40 years [378], [379].
5.4.1 Cranial nerves
Postoperative morbidity and disability are mainly determined by cranial nerve dysfunction [380], [381], [382]. In this context, (i) pulmonary aspiration (sometimes requiring tracheotomy to prevent pneumonia), (ii) dysphagia with subsequent malnutrition and (iii) facial nerve palsy causing eye symptoms are of particular importance.
In order to avoid intraoperative cranial nerve lesions, an understanding of the underlying pathomechanisms is essential. In this context, neural infiltration of jugulotympanal paragangliomas deserves special attention. For instance, one study reported an intraoperative impression of cranial nerve infiltration in 50% of patients, which was confirmed on the histopathological level for 29% (30 out of 102) of all cases [383]. A further study described the clinical observation of neural infiltration in five out of 41 patients [377]. If complete tumour resection is performed in these cases, a postoperative dysfunction of the respective cranial nerves has to be expected.
However, preoperative cranial nerve dysfunction does not necessarily have to be associated with neural infiltration of jugular paragangliomas [383]. Careful analysis of preoperative imaging is a useful tool in this context. Intradural tumour spread is known to occur via the medial wall of the jugular bulb. Therefore, an infiltration of the adjacent lower cranial nerves has to be expected if an intradural extension of the tumour is visible on preoperative imaging [33].
Preoperative cranial nerve dysfunction has been reported in 30–35% of patients [384], [385], a detailed description is provided in Table 2 (Tab. 2). Meticulous clinical evaluation of the cranial nerves before jugular paraganglioma surgery is essential in order to (i) document preoperative dysfunction and (ii) detect possible residual function. For instance, an additional intraoperative trauma to dysfunctional lower cranial nerves may result in postoperative decompensation of function (e.g. swallowing) [377].
Table 2. Prevalence of preoperative cranial nerve palsies in jugulotympanal paragangliomas.
Comparability of individual studies with respect to postoperative cranial nerve function is limited due to differences in (i) tumour stages, (ii) management of the facial nerve (e.g. re-routing) and (iii) the prevalence of (in-)complete preoperative cranial nerve palsies. Furthermore, a variable degree of neural infiltration is observed during paraganglioma surgery. Table 3 (Tab. 3) compares pre- and postoperative facial nerve function in 44 patients without neural infiltration of jugular paraganglioma [386]. All together, cranial nerve function is expected to be maintained in 75% of cases without neural infiltration [33]. Results for the individual cranial nerves in case of advanced jugular paragangliomas are given in brackets: IX (22.3–66.7%), X (54–81.8%), XI (61–74.3%) and XII (73–93%) [33], [387], [388], [389], [390].
Table 3. Pre- and postoperative facial nerve function in patients with jugulotympanal paragangliomas and no evidence of facial nerve infiltration [386].

Management of the facial nerve in advanced jugular paragangliomas often resembles navigation between Skylla and Charybdis. Anterior mobilization of the facial nerve was developed by Capps in order to achieve complete tumour removal [391]. Fisch recommended the anterior transposition of the nerve for a better control over the ICA [392], [393]. However, the surgeon has to be aware of the fact that mobilization of the facial nerve will compromise its blood supply [33]. Therefore, postoperative dysfunction (which may be transient or permanent) has to be expected in every case of facial nerve rerouting [381], [394], [395]. On the other hand, the surgeon is faced with the risk of incomplete tumour removal if he decides not to mobilize the facial nerve [396].
Facial nerve rerouting has been discussed controversially in the literature [397], [398], [399], and different surgical techniques (e.g. short/long anterior rerouting, posterior rerouting) have been proposed by various authors [391], [393], [399], [400], [401]. Some studies reported facial nerve function grade I and II (H-B) in 87–88% of patients and complete tumour removal in 80–82% [387], [402]. On the other hand, facial nerve function grade I/II was described in only 56.2% [381] and 64.7% [403] of cases following facial nerve rerouting by two other studies.
In any case, knowledge of facial nerve rerouting (including the different techniques and associated risks) is essential for the ear surgeon, because this technique can also be applied to other pathologies of the temproral bone, e.g. petrous bone cholesteatoma [33]. Moreover, jugular paraganglioma surgery teaches the ear surgeon valuable lessons about the management of facial nerve palsy in general – independent of the underlying pathology. In summary, intraoperative decompression is the option of choice in case of preoperative facial dysfunction with preserved continuity of the nerve [404]. Primary reanastomosis or interposition grafting are possible solutions if the nerve has been transected during surgery [33] (see also chapter 3.5.7). Finally, permanent postoperative facial nerve palsy can be treated by hypoglossal-facial anastomosis or various plastic-reconstructive techniques, such as muscle transpositions/free muscle flap grafts (dynamic) or lateral tarsorraphy (static) [405].
5.4.2 CSF leak
Resection of large jugular paragangliomas with intracranial extension requires a stable reconstruction of the dural defect, e.g. by means of a microvascular free flap supported with abdominal fat [406]. The clinical presentation of an insufficient duraplasty includes CSF leakage into the mastoid cells, the middle ear and the nasopharynx via the eustachian tube [337]. Two studies reported postoperative CSF leaks in three out of 42 [377] and three out of 53 patients [386], respectively. Two CSF leaks with associated meningitis were observed in 52 patients following resection of jugulotympanal paragangliomas [387]. Further studies reported postoperative CSF leaks in 11% [376], 4.5% [388] and 3.7% of cases [33].
CSF leaks following removal of jugular paragangliomas with intracranial extension often require revision surgery [386]. Extensive resection of bone and soft tissue, which is required for an optimal exposure of the tumour, often complicates subsequent dural defect reconstruction, resulting in postoperative CSF leakage [406]. Based on this experience, a two-stage surgical procedure has been recommended for jugular paragangliomas with intracranial extension. In particular, devascularization of the tumour during the first surgery results in decreased bleeding and improved view in the second procedure, thus facilitating both resection of the intradural tumour and dural defect reconstruction [33]. Again, this strategy may be transferred to the surgical management of other temporal bone pathologies as well [33].
5.4.3 Embolization
Beside autologous blood donation and intraoperative use of a cell saver, preoperative embolization has to be considered as an option to reduce intraoperative blood loss in jugular paraganglioma surgery. Nowadays, preoperative superselective embolization is commonly used to reduce operation time, minimize blood loss and reduce intraoperative complications in the treatment of hypervascular skull base tumours [407], [408]. However, before the age of superselective embolization, severe complications, such as (fatal) thromboembolic stroke, were observed in single cases. In recent years, refinement of embolization techniques and materials has helped to decrease the complication rate of preoperative embolization considerably [409].
The ear surgeon has to keep in mind that preoperative embolization may also compromise the arterial blood supply of the cranial nerves, resulting in cranial nerve palsy. In this context, “safe” and “dangerous” vessels have been defined [408]. Due to the rare occurrence of this complication, it is difficult to estimate the risk of embolization-associated cranial nerve palsy [410].
Recovery of function is possible in these cases. For instance, partial/complete recovery was reported in four cases of facial nerve palsy following embolization of a jugular paraganglioma with polyvinyl alcohol (PVA) particles [408], [411], [412]. Another study described embolization-induced cranial nerve dysfunction in three patients with jugular foramen vascular tumours (VII: n=2, X: n=2, XI: n=1, XII: n=1): partial recovery was observed for the facial nerve, and accessory nerve function recovered completely [410]. If cranial nerve dysfunction is observed after superselective embolization, the ear surgeon should also consider a possible compression of the nerve by tumour swelling, which requires surgical decompression (see chapter 5.4.1).
Knowledge of facial nerve vascularization is essential for understanding the pathomechanism of embolization-associated dysfunction. Usually, the tympanic and mastoidal sections of the facial nerve are supplied by the stylomastoid and medial meningeal arteries [413]. In 10% of cases, however, the stylomastoid artery is the only feeder of the facial nerve in the middle ear [412]. The stylomastoid artery, in turn, branches off the occipital artery in 60% and the postauricular artery in 40% of cases. If the tympanic and mastoidal portions of the facial nerve are exclusively fed by a stylomastoid artery branching off the occipital artery, embolization of the latter carries the risk of subsequent facial nerve palsy [410]. The lower cranial nerves are commonly supplied by branches of the ascendant pharyngeal artery, which explains the observation of embolization-induced dysfunction in this context [413].
Furthermore, the surgeon has to keep in mind that around 4% of head and neck paragangliomas release catecholamines, which may trigger a hypertensive crisis during embolization or tumour resection [414], as reported by [415]. Although 41% of paragangliomas have been estimated to be biochemically “silent” before intervention, the surgeon, the anaesthesiologist and the neuroradiologist should always be prepared for an excessive release of catecholamines during embolization/resection of jugular paragangliomas with all its consequences [414].
5.4.4 Internal carotid artery
The ICA is particularly prone to surgical damage during removal of jugular paragangliomas due to the close spatial relationship between artery and tumour. In a study with 42 jugular paraganglioma resections, ICA injury was treated by carotid artery angioplasty (n=3) or ligation of the artery (n=2) [377]. A further study (n= 34 surgical procedures) reported one case of iatrogenic ICA injury, which was successfully managed with a running suture [404].
When it comes to ICA involvement in skull base surgery, the ear surgeon has to be aware of the different degrees of adhesion between the pathology and the vessel. For instance, it is easier to dissect a petrous bone cholesteatoma from the adventitia of the ICA than a jugular paraganglioma. In any case, the ear surgeon has to ensure sufficient exposure of the artery during surgical management of any ICA-associated middle ear pathology in order to allow for instrumentation in case of an intraoperative vascular injury [33].
If preoperative diagnostics reveal extensive ICA involvement of jugular paraganglioma, trial balloon occlusion has been recommended in order to judge whether the ICA can be sacrificed safely. The finding of an insufficient cerebral blood supply during this test is an important information for the surgeon. On the other hand, the lack of a neurological deficit indicates – but does not prove – that closure of the ICA is possible without causing cerebral ischaemia. Therefore, it has been suggested to create a vascular reconstruction / vascular bypass in any case before sacrificing the ICA [388].
5.4.5 Sigmoid sinus/internal jugular vein
Resection of the tumour near the internal jugular vein, the jugular bulb and the sigmoid sinus is an important step in paraganglioma surgery. Wide exposure of these vessels is an indispensable prerequisite for the successful management of venous bleeding. In particular, the sigmoid sinus and the internal jugular vein should be accessible to the surgeon before tumour removal from the jugular bulb [33]. It is possible to leave a thin layer of bone on the sigmoid sinus deliberately in order to avoid drilling-induced injury. After drilling has been accomplished, the piece of bone can be removed gently with a micro hook.
Circumscribed lesions of the sigmoid sinus can be sealed with muscle patches or oxidized cellulose (see also chapters 3.5.10 and 5.3.9). The surgeon should always check the preoperative images for size, dominance and patency of the sigmoid sinus on both sides. If the sigmoid sinus is dominant on the side of the jugular paraganglioma, increased venous bleeding has to be expected in case of an injury [337]. Furthermore, the images should be analyzed for the anatomical variation of a unilateral sigmoid sinus [33]. In case of venous bleeding, intraluminal packing of the vessels is performed. Additional feeders of the jugular bulb beside the sigmoid sinus, e.g. the inferior petrous sinus, have to be noticed in this situation. If the jugular bulb is filled with tumour, the sigmoid sinus is often drained via the emissary veins. Therefore, venous drainage on the side of the tumour can be maintained in this situation by obliteration of the sigmoid sinus proximal to the emissary veins [33].
5.4.6 Further complications
The following complications (apart from cranial nerve dysfunction and ICA injury) were observed after the resection of 42 jugular paragangliomas: pneumonia (n=4), chronic imbalance (n=3), postoperative bleeding (n=2), pseudomembranous colitis (n=1), pulmonary edema with temporary respiratory failure (n=1), staphylococcal sepsis (n=1), trigeminal neuralgia (n=1), alcohol withdrawal delirium (n=1) and impaired wound healing (n=1) [377]. The incidence of pulmonary complications after paraganglioma surgery due to dysfunction of the lower cranial nerves should not be underestimated. For instance, pulmonary aspiration was reported in 4–10%, and pneumonia was observed in up to 6% of cases [33], [376], [387], [388]. One patient even died of postoperative pulmonary complications [404]. Mortality rates of 1% and 2.7% were described by other studies [376], [388]. Pulmonary embolism was found in 2% [376], [387] and 2.6% [388] of cases. Table 4 (Tab. 4) summarizes the incidence of the most common complications of paraganglioma surgery. A further study observed cranial nerve dysfunction (6–36%), pneumonia (6%), pulmonary aspiration (4%), wound infection (6%) and pulmonary embolism (1 case) [387].
Table 4. Complications following jugular paraganglioma resection.
Postoperative deterioration of hearing may be due to intraoperative (i) manipulation of the ossicular chain, (ii) obliteration of the middle ear or (iii) injury of the cochlea/the auditory nerve. Two studies described profound postoperative SNHL following jugular paraganglioma surgery in one patient [416] and two patients [404], respectively.
5.4.7 Quality of life
Complete rehabilitation after resection of a jugular paraganglioma may take one to two years [417]. Therefore, it is of utmost importance to consider the time interval between surgery and evaluation when comparing postoperative findings of different studies. A retrospective analysis of 36 patients after paraganglioma surgery described that 72% were back to work after 6 months, 98% returned to their previous occupation within one to two years after surgery. Likewise, normal social life was resumed after six months by 69% of the patients and by 97% after two years. On the other hand, nine patients (25%) never “felt the way they had before surgery”, and one patient (3%) never resumed work and did not return to normal social life [417].
5.4.8 Residual tumour
Due to the characteristic tumour spread mentioned above, complete removal of the tumour is not possible in a considerable number of patients with advanced jugular pargangliomas (stage C and D). For instance, residual tumour may be present at the bony margins of the paraganglioma. Therefore, it is recommended to resect the surrounding bone, until disease-free cortical bone can be observed under the operation microscope [33]. Moreover, the surgeon may decide to refrain from complete tumour resection in order to preserve the function of adjacent neurovascular structures. For instance, tumour remnants may be left on the cavernous sinus in order to preserve CN III, IV and VI function [33].
Due to the slow growth of the tumour, a long-term postoperative follow-up is mandatory in order to detect possible residual disease on CT and/or MRI. In single cases, angiography may be necessary to visualize tumour remnants. For instance, a study on 83 patients with jugular paragangliomas reported detection of residual tumour by all three diagnostic modalities in 24 patients, whereas angiography was necessary to establish the diagnosis in ten cases [418]. This observation was confirmed by a further study, where angiography was required in two out of eight patients to detect residual paraganglioma [377]. Angiography is particularly helpful, when residual tumour and scar formation on the level of the dura cannot be distinguished by MRI [419].
6 Concluding remarks
Surgery of the middle and inner ear, the temporal bone and the lateral skull base is a fascinating and multifaceted field in otorhinolaryngology. Basic principles can be applied to different pathologies. It is essential for the ear surgeon to know the specific pitfalls and complications of ear/lateral skull base surgery. This knowledge should be increased and shared continuously. Both the surgeon and the patient will profit from this process.
List of abbreviations
AAO-HNS: American Association of Otolaryngology - Head and Neck Surgery; ABR: auditory brainstem response; AOM: acute otitis media; AT: acoustic trauma; BPPV: benign paroxysmal positional vertigo; CI: cochlear implant; CN: cranial nerve; CPA: cerebellopontine angle; CSF: cerebrospinal fluid; CT: computed tomography; 3D: three-dimensional; DPOAEs: distortion-product otoacoustic emissions; EAC: external auditory canal; EMG: electromyography; H-B: House-Brackmann grading system of facial nerve function; HR: high-resolution; IAC: internal auditory canal; ICA: internal carotid artery; KTP: potassium titanyl phosphate; MF: middle fossa; NF2: neurofibromatosis type 2; POD: postoperative day; PVA: polyvinyl alcohol; QD: quaque die (= once a day); RS: retrosigmoid; SCC: semicircular canal; SCDS: superior canal dehiscence syndrome; SN: spontaneous nystagmus; SNHL: sensorineural hearing loss; SPL: sound-pressure level; SSIG: stapes surgery induced granulation tissue; TL: translabyrinthine; o-/cVEMPs: ocular/cervical vestibular evoked myogenic potentials; VOR: vestibule-ocular reflex; VS: vestibular schwannoma.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Linder TE, Lin F. Felsenbeinchirurgie. Komplikationen und unerwünschte Operationsfolgen. HNO. 2011;59:974–979. doi: 10.1007/s00106-011-2359-z. Available from: http://dx.doi.org/10.1007/s00106-011-2359-z. [DOI] [PubMed] [Google Scholar]
- 2.Dlugaiczyk J, Schick B. Klinische Differenzialdiagnose vestibulärer Symptome. CME Hals Nasen Ohrenheilkd. 2010;3:128–149. [Google Scholar]
- 3.Haid CT. Vestibularisprüfung und vestibuläre Erkrankungen. Berlin, Heidelberg, New York: Springer-Verlag; 1990. Available from: http://dx.doi.org/10.1007/978-3-662-10791-1. [DOI] [Google Scholar]
- 4.Enticott JC, Tari S, Koh SM, Dowell RC, O'Leary SJ. Cochlear implant and vestibular function. Otol Neurotol. 2006 Sep;27(6):824–830. doi: 10.1097/01.mao.0000227903.47483.a6. Available from: http://dx.doi.org/10.1097/01.mao.0000227903.47483.a6. [DOI] [PubMed] [Google Scholar]
- 5.Fina M, Skinner M, Goebel JA, Piccirillo JF, Neely JG, Black O. Vestibular dysfunction after cochlear implantation. Otol Neurotol. 2003 Mar;24(2):234–242. doi: 10.1097/00129492-200303000-00018. Available from: http://dx.doi.org/10.1097/00129492-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Krause E, Louza JP, Wechtenbruch J, Gürkov R. Influence of cochlear implantation on peripheral vestibular receptor function. Otolaryngol Head Neck Surg. 2010 Jun;142(6):809–813. doi: 10.1016/j.otohns.2010.01.017. Available from: http://dx.doi.org/10.1016/j.otohns.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Melvin TA, Della Santina CC, Carey JP, Migliaccio AA. The effects of cochlear implantation on vestibular function. Otol Neurotol. 2009 Jan;30(1):87–94. doi: 10.1097/MAO.0b013e31818d1cba. Available from: http://dx.doi.org/10.1097/MAO.0b013e31818d1cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santina CC. Vestibular function and cochlear implantation. In: Niparko JK, editor. Cochlear implants. Principles & Practices. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 187–190. [Google Scholar]
- 9.Wiener-Vacher S. Letter to the Editor. Int J Ped Otorhinolaryngol. 2010;74:105–106. doi: 10.1016/j.ijporl.2009.10.018. Available from: http://dx.doi.org/10.1016/j.ijporl.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Magnusson M, Kahlon B, Karlberg M, Lindberg S, Siesjö P. Preoperative vestibular ablation with gentamicin and vestibular 'prehab' enhance postoperative recovery after surgery for pontine angle tumours--first report. Acta Otolaryngol. 2007 Dec;127(12):1236–1240. doi: 10.1080/00016480701663433. Available from: http://dx.doi.org/10.1080/00016480701663433. [DOI] [PubMed] [Google Scholar]
- 11.Martin HC, Sethi J, Lang D, Neil-Dwyer G, Lutman ME, Yardley L. Patient-assessed outcomes after excision of acoustic neuroma: postoperative symptoms and quality of life. J Neurosurg. 2001 Feb;94(2):211–216. doi: 10.3171/jns.2001.94.2.0211. Available from: http://dx.doi.org/10.3171/jns.2001.94.2.0211. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen A. Acoustic tumors: with special reference to end-results and sparing of the facial nerve. Ann Surg. 1942 May;115(5):849–863. doi: 10.1097/00000658-194205000-00015. Available from: http://dx.doi.org/10.1097/00000658-194205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufarelli D, Meli A, Alesii A, De Angelis E, Badaracco C, Falcioni M, Sanna M. Quality of life after acoustic neuroma surgery. Otol Neurotol. 2006 Apr;27(3):403–409. doi: 10.1097/00129492-200604000-00018. Available from: http://dx.doi.org/10.1097/00129492-200604000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Youssef AS, Downes AE. Intraoperative neurophysiological monitoring in vestibular schwannoma surgery: advances and clinical implications. Neurosurg Focus. 2009 Oct;27(4):E9. doi: 10.3171/2009.8.FOCUS09144. Available from: http://dx.doi.org/10.3171/2009.8.FOCUS09144. [DOI] [PubMed] [Google Scholar]
- 15.Murphy EK. Use of an infrared camera to improve the outcome of facial nerve monitoring. Am J Electroneurodiagnostic Technol. 2008 Mar;48(1):38–47. [PubMed] [Google Scholar]
- 16.Brackmann DE, Cullen RD, Fisher LM. Facial nerve function after translabyrinthine vestibular schwannoma surgery. Otolaryngol Head Neck Surg. 2007 May;136(5):773–777. doi: 10.1016/j.otohns.2006.10.009. Available from: http://dx.doi.org/10.1016/j.otohns.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg. 2010 Apr;112(4):860–867. doi: 10.3171/2009.7.JNS0989. Available from: http://dx.doi.org/10.3171/2009.7.JNS0989. [DOI] [PubMed] [Google Scholar]
- 18.Lazard DS, Tosello M, Bozorg-Grayeli A, Vitte E, Bouccara D, Kalamarides M, Sterkers O. Early complications and symptoms of cerebellopontine angle tumor surgery: a prospective analysis. Eur Arch Otorhinolaryngol. 2011 Nov;268(11):1575–1582. doi: 10.1007/s00405-011-1539-5. Available from: http://dx.doi.org/10.1007/s00405-011-1539-5. [DOI] [PubMed] [Google Scholar]
- 19.Guyot JP. Where does essential reporting stop and where does "l'art pour l'art" begin while imaging the middle ear? ORL J Otorhinolaryngol Realt Spec. 2010;72:131–132. doi: 10.1159/000313442. Available from: http://dx.doi.org/10.1159/000313442. [DOI] [PubMed] [Google Scholar]
- 20.Karaca CT, Totos SZ, Noseri HK. Analysis of anatomic variations in temporal bone by radiology. Int Adv Otol. 2012;8:239–243. [Google Scholar]
- 21.Friedmann DR, Eubig J, Winata LS, Pramanik BK, Merchant AN, Lalwani AK. A clinical and histopathological study of jugular bulb abnormalities. Arch Otolaryngol Head Neck Surg. 2012;138:66–71. doi: 10.1001/archoto.2011.231. Available from: http://dx.doi.org/10.1001/archoto.2011.231. [DOI] [PubMed] [Google Scholar]
- 22.Kösling S, Brandt S, Neumann K. Bildgebung des Schläfenbeins. Radiologe. 2010;50:711–734. doi: 10.1007/s00117-010-2027-4. Available from: http://dx.doi.org/10.1007/s00117-010-2027-4. [DOI] [PubMed] [Google Scholar]
- 23.Jansen S. Malformation of the inner ear in deaf children. Acta Radiol. 1969;286:S1–S97. [PubMed] [Google Scholar]
- 24.Sennaroglu L, Sarac S, Ergin T. Surgical results of cochlear implantation in malformed cochlea. Otol Neurotol. 2006 Aug;27(5):615–623. doi: 10.1097/01.mao.0000224090.94882.b4. Available from: http://dx.doi.org/10.1097/01.mao.0000224090.94882.b4. [DOI] [PubMed] [Google Scholar]
- 25.Kontorinis G, Goetz F, Giourgas A, Lenarz T, Lanfermann H, Giesemann AM. Radiological diagnosis of incomplete partition type I versus type II: significance for cochlear implantation. Eur Radiol. 2012 Mar;22(3):525–532. doi: 10.1007/s00330-011-2301-5. Available from: http://dx.doi.org/10.1007/s00330-011-2301-5. [DOI] [PubMed] [Google Scholar]
- 26.Hara M, Takahashi H, Kanda Y. The usefulness of reconstructed 3D images in surgical planning for cochlear implantation in a malformed ear with an abnormal course of the facial nerve. Clin Exp Otorhinolaryngol. 2012;5(Suppl. 01):48–52. doi: 10.3342/ceo.2012.5.S1.S48. Available from: http://dx.doi.org/10.3342/ceo.2012.5.S1.S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song JJ, Park JH, Jang JH, Lee JH, Oh SH, Chang SO, Kim CS. Facial nerve aberrations encountered during cochlear implantation. Acta Otolaryngol. 2012 Jul;132(7):788–794. doi: 10.3109/00016489.2012.656765. Available from: http://dx.doi.org/10.3109/00016489.2012.656765. [DOI] [PubMed] [Google Scholar]
- 28.Aschendorff A, Laszig R, Maier W, Beck R, Schild C, Birkenhäger R, Wesarg T, Kröger S, Arndt S. Kochleaimplantat bei Innenohrfehlbildungen. HNO. 2009;57:533–541. doi: 10.1007/s00106-009-1936-x. Available from: http://dx.doi.org/10.1007/s00106-009-1936-x. [DOI] [PubMed] [Google Scholar]
- 29.Aschendorff A. Imaging bei Cochlear-Implant-Patienten. Laryngo Rhino Otol. 2011;90:S16–S21. doi: 10.1055/s-0030-1270448. Available from: http://dx.doi.org/10.1055/s-0030-1270448. [DOI] [PubMed] [Google Scholar]
- 30.AWMF online. Leitlinien der Deutschen Röntgengesellschaft. Radiologische Diagnostik im Kopf-Hals-Bereich. Schläfenbein. Nichttraumatische periphere Fazialisparese. [Google Scholar]
- 31.Rubin LG, Papsin B. Cochlear implants in children: surgical site infections and prevention and treatment of acute otitis media and meningitis. Pediatrics. 2010 Aug;126(2):381–391. doi: 10.1542/peds.2010-1427. Available from: http://dx.doi.org/10.1542/peds.2010-1427. [DOI] [PubMed] [Google Scholar]
- 32.Merkus P, van Loon MC, Smit CF, Smits C, de Cock AF, Hensen EF. Decision making in advanced otosclerosis: an evidence-based strategy. Laryngoscope. 2011 Sep;121(9):1935–1941. doi: 10.1002/lary.21904. Available from: http://dx.doi.org/10.1002/lary.21904. [DOI] [PubMed] [Google Scholar]
- 33.Sanna M, Pandya Y, Mancini F, Sequino G, Piccirillo E. Petrous bone cholesteatoma: classification, management and review of the literature. Audiol Neurotol. 2011;16:124–136. doi: 10.1159/000315900. Available from: http://dx.doi.org/10.1159/000315900. [DOI] [PubMed] [Google Scholar]
- 34.Schwager K. Akute Komplikationen in der Mittelohrchirurgie. Teil 1: Probleme während der Tympanoplastik - was tun? HNO. 2007;55:307–317. doi: 10.1007/s00106-006-1527-z. Available from: http://dx.doi.org/10.1007/s00106-006-1527-z. [DOI] [PubMed] [Google Scholar]
- 35.Yeh JS, Mooney KL, Gingrich K, Kim JT, Lalwani AK. Anesthetic complications in pediatric patients undergoing cochlear implantation. Laryngoscope. 2011 Oct;121(10):2240–2244. doi: 10.1002/lary.21924. Available from: http://dx.doi.org/10.1002/lary.21924. [DOI] [PubMed] [Google Scholar]
- 36.Wolfson A, Swamidoss C. Accidental subcutaneous remifentanil infusion as a cause of delayed awakening after craniotomy. Case Rep Anesthesiol. 2011;2011:919067. doi: 10.1155/2011/919067. Available from: http://dx.doi.org/10.1155/2011/919067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisch U. Tympanoplasty, Mastoidectomy and Stapes Surgery. Thieme-Verlag; 1994. [Google Scholar]
- 38.Yuan YY, Song YS, Chai CM, Shen WD, Han WJ, Liu J, Wang GJ, Dong TX, Han DY, Dai P. Intraoperative CT-guided cochlear implantation in congenital ear deformity. Acta Otolaryngol. 2012 Sep;132(9):951–958. doi: 10.3109/00016489.2012.674214. Available from: http://dx.doi.org/10.3109/00016489.2012.674214. [DOI] [PubMed] [Google Scholar]
- 39.Struffert T, Hertel V, Kyriakou Y, Krause J, Engelhorn T, Schick B, Iro H, Hornung J, Doerfler A. Imaging of cohlear implant electrode array with falt-detection CT and conventional multi-slice CT: comparison of image qualities and radiation dose. Acta Otolaryngol. 2010;130:443–452. doi: 10.3109/00016480903292700. Available from: http://dx.doi.org/10.3109/00016480903292700. [DOI] [PubMed] [Google Scholar]
- 40.Hertel V, Hornung J, Hoppe U, Zenk J, Iro H. Sprachverständnis nach Cochlea Implantation über Rundfenstermembraninsertion - Erlanger Erfahrungen. 82. Jahresversammlung der Deutschen Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie; 01.-05.06.2011; Freiburg i. Br.. Düsseldorf: German Medical Science GMS Publishing House; 2011. p. Doc11hnod357. Available from: http://dx.doi.org/10.3205/11hnod357. [DOI] [Google Scholar]
- 41.Aschendorff A, Maier W, Jaekel K, Wesarg T, Arndt S, Laszig R, Voss P, Metzger M, Schulze D. Radiologically assisted navigation in cochlear implantation for X-linked deafness malformation. Cochlear Implants Int. 2009;10(Suppl 1):14–18. doi: 10.1002/cii.379. Available from: http://dx.doi.org/10.1002/cii.379. [DOI] [PubMed] [Google Scholar]
- 42.Stelter K, Ledderose G, Hempel JM, Morhard DF, Flatz W, Krause E, Mueller J. Image guided navigation by intraoperative CT scan for cochlear implantation. Comput Aided Surg. 2012;17(3):153–160. doi: 10.3109/10929088.2012.668937. Available from: http://dx.doi.org/10.3109/10929088.2012.668937. [DOI] [PubMed] [Google Scholar]
- 43.Bellucci R. Iatrogenic surgical trauma in otology. J Laryngol Otol. 1983;8:13–17. [PubMed] [Google Scholar]
- 44.Hüttenbrink KB. Manipulating the mobile stapes during tympanoplasty: the risk of stapedial luxation. Laryngoscope. 1993 Jun;103(6):668–672. doi: 10.1288/00005537-199306000-00016. Available from: http://dx.doi.org/10.1288/00005537-199306000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Milewski C, Dornhoffer J, De Meester C. Möglichkeiten des Hörerhalts bei Labyrinth-Fisteln von unterschiedlichem Schweregrad. Laryngo Rhino Otol. 1995;74:408–412. doi: 10.1055/s-2007-997770. Available from: http://dx.doi.org/10.1055/s-2007-997770. [DOI] [PubMed] [Google Scholar]
- 46.Helms J. Acoustic trauma from the bone cutting burr. J Laryngol Otolo. 1976;90:1143–1149. doi: 10.1017/S0022215100083225. Available from: http://dx.doi.org/10.1017/S0022215100083225. [DOI] [PubMed] [Google Scholar]
- 47.Pau HW, Just T, Bornitz M, Lasurashvilli N, Zahnert T. Noise exposure of the inner ear during drilling a cochleostomy for cochlea implantation. Laryngoscope. 2007;117:535–540. doi: 10.1097/MLG.0b013e31802f4169. Available from: http://dx.doi.org/10.1097/MLG.0b013e31802f4169. [DOI] [PubMed] [Google Scholar]
- 48.Punke C, Zehlicke T, Sievert U, Pau HW. Akustisch-mechanisches Trauma während einer Cochleostomie. HNO. 2011;59:570–574. doi: 10.1007/s00106-010-2257-9. Available from: http://dx.doi.org/10.1007/s00106-010-2257-9. [DOI] [PubMed] [Google Scholar]
- 49.Kylén P, Stjernvall JE, Arlinger S. Variables affecting the drill-generated noise levels in ear surgery. Acta Otolaryngol. 1977 Sep-Oct;84(3-4):252–259. doi: 10.3109/00016487709123964. Available from: http://dx.doi.org/10.3109/00016487709123964. [DOI] [PubMed] [Google Scholar]
- 50.Paparella MM, Morizono T, Le CT, Mancini F, Sipilä P, Choo YB, Lidén G, Kim CS. Sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol. 1984 Nov-Dec;93(6 Pt 1):623–629. doi: 10.1177/000348948409300616. [DOI] [PubMed] [Google Scholar]
- 51.Gjuric M, Schneider W, Buhr W, Wolf SR, Wigand ME. Experimental sensorineural hearing loss following drill-induced ossicular chain injury. Acta Otolaryngol. 1997 Jul;117(4):497–500. doi: 10.3109/00016489709113427. Available from: http://dx.doi.org/10.3109/00016489709113427. [DOI] [PubMed] [Google Scholar]
- 52.Hedgewald M, Heitman R, Wiederhold ML, Cooper JC, Gates GA. High-frequency electrostimulation hearing after mastoidectomy. Otolaryngol Head Neck Surg. 1989;100:49–56. doi: 10.1177/019459988910000108. [DOI] [PubMed] [Google Scholar]
- 53.Suits GW, Brummett RE, Nunley J. Effect of otologic drill noise on ABR thresholds in a guinea pig model. Otolarnygol Head Neck Surg. 1993;109:660–667. doi: 10.1177/019459989310900405. [DOI] [PubMed] [Google Scholar]
- 54.Urquhart AC, McIntosh WA, Bodenstein NP. Drill-generated sensorineural hearing loss following mastoid surgery. Laryngoscope. 1992 Jun;102(6):689–692. doi: 10.1288/00005537-199206000-00016. Available from: http://dx.doi.org/10.1288/00005537-199206000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Tos M, Trojaborg N, Thomsen J. The contralateral ear after translabyrinthine removal of acoustic neuromas: is there a drill-noise generated hearing loss? J Laryngol Otol. 1989;103:845–849. doi: 10.1017/S0022215100110278. Available from: http://dx.doi.org/10.1017/S0022215100110278. [DOI] [PubMed] [Google Scholar]
- 56.da Cruz MJ, Fagan P, Atlas M, McNeill C. Drill-induced hearing loss in the nonoperated ear. Otolaryngol Head Neck Surg. 1997 Nov;117(5):555–558. doi: 10.1016/S0194-5998(97)70030-5. Available from: http://dx.doi.org/10.1016/S0194-5998(97)70030-5. [DOI] [PubMed] [Google Scholar]
- 57.Palva A, Sorri M. Can an operation of deaf ear be dangerous for hearing? Acta Otolaryngol Suppl. 1979;360:155–157. doi: 10.3109/00016487809123503. [DOI] [PubMed] [Google Scholar]
- 58.Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004 Oct;196(1-2):58–68. doi: 10.1016/j.heares.2004.06.003. Available from: http://dx.doi.org/10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 59.El-Hennawi DM, El-Deen MH, Abou-Halawa AS, Nadeem HS, Ahmed MR. Efficacy of intratympanic methylprednisolone acetate in treatment of drill-induced sensorineural hearing loss in guinea pigs. J Laryngol Otol. 2005 Jan;119(1):2–7. doi: 10.1258/0022215053222815. Available from: http://dx.doi.org/10.1258/0022215053222815. [DOI] [PubMed] [Google Scholar]
- 60.Schneider W, Gjuric M, Katalinic A, Buhr W, Wolf SR. The value of methylprednisolone in the treatment of an experimental sensorineural hearing loss following drill-induced ossicular chain injury: a randomized, blinded study in guinea-pigs. Acta Otolaryngol. 1998 Jan;118(1):52–55. doi: 10.1080/00016489850155125. Available from: http://dx.doi.org/10.1080/00016489850155125. [DOI] [PubMed] [Google Scholar]
- 61.Palva T, Kärjä J, Palva A. High-tone sensorineural losses following chronic ear surgery. Arch Otolaryngol. 1973 Sep;98(3):176–178. doi: 10.1001/archotol.1973.00780020184008. Available from: http://dx.doi.org/10.1001/archotol.1973.00780020184008. [DOI] [PubMed] [Google Scholar]
- 62.Kylén P, Arlinger SD, Bergholtz LM. Peroperative temporary thershold shift in ear surgery. An electrocochleographic study. Acta Otolaryngol. 1977;84:393–401. doi: 10.3109/00016487709123982. Available from: http://dx.doi.org/10.3109/00016487709123982. [DOI] [PubMed] [Google Scholar]
- 63.Kylén P, Arlinger S, Jerlvall L, Harder H. Ossicular manipulation in chronic ear surgery. An electrocochleographic study. Arch Otolaryngol. 1980 Oct;106(10):598–601. doi: 10.1001/archotol.1980.00790340006002. Available from: http://dx.doi.org/10.1001/archotol.1980.00790340006002. [DOI] [PubMed] [Google Scholar]
- 64.Schick B, Schick BT, Kochannek S, Starlinger V, Iro H. Temporäre sensorineurale Hörverluste nach Ohroperationen – eine retrospektive Analyse. Laryngo Rhino Otol. 2007;86:200–205. doi: 10.1055/s-2006-944750. Available from: http://dx.doi.org/10.1055/s-2006-944750. [DOI] [PubMed] [Google Scholar]
- 65.Helms J, Mlynski R, Phleps G. T-Knorpel-Tympanoplastik bei offenem ovalem Fenster. Laryngo Rhino Otol. 2011;90:462–463. doi: 10.1055/s-0031-1283296. Available from: http://dx.doi.org/10.1055/s-0031-1283296. [DOI] [PubMed] [Google Scholar]
- 66.Copeland BJ, Buchman CA. Management of labyrinthine fistulae in chronic ear surgery. Am J Otolaryngol. 2003 Jan-Feb;24(1):51–60. doi: 10.1053/ajot.2003.10. Available from: http://dx.doi.org/10.1053/ajot.2003.10. [DOI] [PubMed] [Google Scholar]
- 67.Fuse T, Tada Y, Aoyagi M, Sugai Y. CT detection of facial canal dehiscence and semicircular canal fistula: comparison with surgical findings. J Comput Assist Tomogr. 1996 Mar-Apr;20(2):221–224. doi: 10.1097/00004728-199603000-00009. Available from: http://dx.doi.org/10.1097/00004728-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Portier F, Lescanne E, Racy E, Nowak C, Lamblin B, Bobin S. Studies of labyrinthine cholesteatoma-related fistulas: report of 22 cases. J Otolarnygol. 2005;34:1–6. doi: 10.2310/7070.2005.00001. Available from: http://dx.doi.org/10.2310/7070.2005.00001. [DOI] [PubMed] [Google Scholar]
- 69.Soda-Merhy A, Betancourt-Suárez MA. Surgical treatment of labyrinthine fistula caused by cholesteatoma. Otolaryngol Head Neck Surg. 2000 May;122(5):739–742. doi: 10.1016/S0194-5998(00)70207-5. [DOI] [PubMed] [Google Scholar]
- 70.Vartiainen E. What is the best method of treatment for labyrinthine fistulae caused by cholesteatoma? Clin Otolaryngol Allied Sci. 1992 Jun;17(3):258–260. doi: 10.1111/j.1365-2273.1992.tb01839.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1992.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 71.Brackmann DE, Shelton C, Arriaga M. Otologic surgery. Saunders; 2001. [Google Scholar]
- 72.Helms J, Jahrsdoerfer RA. Kopf- und Halschirurgie. Band 2: Ohr. Stuttgart, New York: Thieme-Verlag; 1996. [Google Scholar]
- 73.Magliulo G, Celebrini A, Cuiuli G, Parrotto D. Surgical management of the labyrinthine fistula complicating chronic otitis media with or without cholesteatoma. J Otolaryngol Head Neck Surg. 2008 Apr;37(2):143–147. [PubMed] [Google Scholar]
- 74.Chen Z, Dongzhen, Wu Y, Shi H, Zhou H, Wang J, Miyoshi A, Yin S. Surgical treatment of labyrinthine fistula caused by cholesteatoma with semicircular canal occlusion. Acta Otolaryngol. 2010;130(1):75–78. doi: 10.3109/00016480902875083. Available from: http://dx.doi.org/10.3109/00016480902875083. [DOI] [PubMed] [Google Scholar]
- 75.Baxter AB. Dehiscence of the fallopian canal: an anatomical study. J Laryngol Otol. 1971;85:587–594. doi: 10.1017/S0022215100073849. Available from: http://dx.doi.org/10.1017/S0022215100073849. [DOI] [PubMed] [Google Scholar]
- 76.Moreano EH, Paparella MM, Zelterman D, Goycoolea MV. Prevalance of facial canal dehiscence und of persistent stapedial artery in the human middle ear: a report of 1 000 temporal bones. Laryngoscope. 1994;104:309–320. doi: 10.1288/00005537-199403000-00012. Available from: http://dx.doi.org/10.1288/00005537-199403000-00012. [DOI] [PubMed] [Google Scholar]
- 77.Bayazit YA, Ozer E, Kanlikama M. Gross dehiscence of the bone covering the facial nerve in the light of otological surgery. J Laryngol Otol. 2002 Oct;116(10):800–803. doi: 10.1258/00222150260293600. Available from: http://dx.doi.org/10.1258/00222150260293600. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Cao Y. Facial canal dehiscence: a report of 1,465 stapes operations. Ann Otol Rhinol Laryngol. 1996 Jun;105(6):467–471. doi: 10.1177/000348949610500609. [DOI] [PubMed] [Google Scholar]
- 79.Maru N, Cheita AC, Mogoanta CA, Prejoianu B. Intratemporal course of the facial nerve: morphological, topographic and morphometric features. Rom J Morph Embryo. 2010;51:243–248. [PubMed] [Google Scholar]
- 80.Beddard D, Saunders WH. Congenital defects in the fallopian canal. Laryngoscope. 1962 Jan;72:112–115. doi: 10.1288/00005537-196201000-00008. Available from: http://dx.doi.org/10.1288/00005537-196201000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Dietzel K. Über Dehiszenzen des Fazialiskanals. Z Laryngol Rhinol Otol. 1961;40:366–379. [PubMed] [Google Scholar]
- 82.Schwager K, Helms J. Anomalien des Nervus facialis im fehlgebildeten Felsenbein. Laryngo Rhino Otol. 1995;74:549–552. doi: 10.1055/s-2007-997799. Available from: http://dx.doi.org/10.1055/s-2007-997799. [DOI] [PubMed] [Google Scholar]
- 83.Spector JG, Ge X. Ossification patterns of the tympanic facial canal in the human fetus and neonate. Laryngoscope. 1993 Sep;103(9):1052–1065. doi: 10.1288/00005537-199309000-00018. Available from: http://dx.doi.org/10.1288/00005537-199309000-00018. [DOI] [PubMed] [Google Scholar]
- 84.Mutlu V, da Costo SS, Paparella MM, Schachern PA. Clinical-histopathological correlations of pitfalls in middle ear surgery. Eur Arch Otorhinolaryngol. 1998;255:189–194. doi: 10.1007/s004050050041. Available from: http://dx.doi.org/10.1007/s004050050041. [DOI] [PubMed] [Google Scholar]
- 85.Johnsson LG, Kingsley TC. Herniation of the facial nerve in the tympanic segment. Arch Otolaryngol. 1979;91:598–602. doi: 10.1001/archotol.1970.00770040828019. [DOI] [PubMed] [Google Scholar]
- 86.Nager GT, Proctor B. Anatomical variations and anomalies involving the facial canal. Ann Otol Rhinol Laryngol. 1982;91(Suppl):61–77. [PubMed] [Google Scholar]
- 87.Basek M. Anomalies of the facial nerve in normal temporal bones. Ann Otol Rhinol Laryngol. 1962 Jun;71:382–390. doi: 10.1177/000348946207100210. [DOI] [PubMed] [Google Scholar]
- 88.Helms J. Variations of the course of the facial nerve in the middle ear and mastoid. In: Samii D, Jannetta PJ, editors. The cranial nerves. Berlin, Heidelberg, New York: Springer; 1981. p. 391. Available from: http://dx.doi.org/10.1007/978-3-642-67980-3_49. [DOI] [Google Scholar]
- 89.Sedee GA. Facial nerve and dysplasia of the temporal bone. ORL J Otorhinolaryngol Relat Spec. 1973;35(3):222–228. doi: 10.1159/000275119. Available from: http://dx.doi.org/10.1159/000275119. [DOI] [PubMed] [Google Scholar]
- 90.Theissing J. Über operativ beobachtete Facialismissbildunge. [Facial nerve anomalies found at surgery (author's transl)]. HNO. 1975 Oct;23(10):310–312. (Ger). [PubMed] [Google Scholar]
- 91.Jahrsdoerfer RA. The facial nerve and the congenitally malformed middle ear. In: May M, editor. The facial nerve. Stuttgart, New York: Thieme; 1986. p. 631. [Google Scholar]
- 92.Crabtree JA. The facial nerve in congenital ear surgery. Otolaryngol Clin North Am. 1974 Jun;7(2):505–510. [PubMed] [Google Scholar]
- 93.Bellucci RJ. Congenital aural malformations: diagnosis and treatment. Otolaryngol Clin North Am. 1981 Feb;14(1):95–124. [PubMed] [Google Scholar]
- 94.Hawthorne M. Medical negligence in otology. In: Gleeson M, editor. Scott-Brown's otorhinolaryngology, head and neck surgery. 7. Auflage, Band 3, Teil 19 [Google Scholar]
- 95.Szymański M, Gołabek W, Morshed K, Siwiec H. The influence of the sequence of surgical steps on complications rate in stapedotomy. Otol Neurotol. 2007 Feb;28(2):152–156. doi: 10.1097/01.mao.0000247815.23948.89. Available from: http://dx.doi.org/10.1097/01.mao.0000247815.23948.89. [DOI] [PubMed] [Google Scholar]
- 96.Thorne MC, Dunham BP, Tom LW. Delayed facial paresis following tympanomastoid surgery in a pediatric patient. Ear Nose Throat. 2010;89:357–361. [PubMed] [Google Scholar]
- 97.Bonkowsky V, Kochanowski B, Strutz J, Pere P, Hosemann W, Arnold W. Delayed facial palsy following uneventful middle ear surgery: a herpes simplex virus type 1 reactivation? Ann Otol Rhinol Laryngol. 1998 Nov;107(11 Pt 1):901–905. doi: 10.1177/000348949810701101. [DOI] [PubMed] [Google Scholar]
- 98.Gyo K, Honda N. Delayed facial palsy after middle-ear surgery due to reactivation of varicella-zoster virus. J Laryngol Otol. 1999 Oct;113(10):914–915. doi: 10.1017/S0022215100145578. Available from: http://dx.doi.org/10.1017/S0022215100145578. [DOI] [PubMed] [Google Scholar]
- 99.McManus LJ, Stringer MD, Dawes PJ. Iatrogenic injury of the chorda tympani: a systematic review. J Laryngol Otol. 2012 Jan;126(1):8–14. doi: 10.1017/S0022215111002039. Available from: http://dx.doi.org/10.1017/S0022215111002039. [DOI] [PubMed] [Google Scholar]
- 100.Michael P, Raut V. Chorda tympani injury: operative findings and postoperative symptoms. Otolaryngol Head Neck Surg. 2007 Jun;136(6):978–981. doi: 10.1016/j.otohns.2006.12.022. Available from: http://dx.doi.org/10.1016/j.otohns.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 101.Bull TR. Taste and the chorda typani. J Laryngol Otol. 1965 Jun;79:479–493. doi: 10.1017/S0022215100063969. Available from: http://dx.doi.org/10.1017/S0022215100063969. [DOI] [PubMed] [Google Scholar]
- 102.Mahendran S, Hogg R, Robinson JM. To divide or manipulate the chorda tympani in stapedotomy. Eur Arch Otorhinolaryngol. 2005 Jun;262(6):482–487. doi: 10.1007/s00405-004-0854-5. Available from: http://dx.doi.org/10.1007/s00405-004-0854-5. [DOI] [PubMed] [Google Scholar]
- 103.Saito T, Shibamori Y, Manabe Y, Yamagishi T, Yamamoto T, Ohtsubo T, Saito H. Morphological and functional study of regenerated chorda tympani nerves in humans. Ann Otol Rhinol Laryngol. 2000 Aug;109(8 Pt 1):703–709. doi: 10.1177/000348940010900801. [DOI] [PubMed] [Google Scholar]
- 104.Yeo SB, Loy AH. Chorda tympani trauma--how much does it affect taste? Singapore Med J. 1997 Aug;38(8):329–331. [PubMed] [Google Scholar]
- 105.Gopalan P, Kumar M, Gupta D, Phillipps JJ. A study of chorda tympani nerve injury and related symptoms following middle-ear surgery. J Laryngol Otol. 2005 Mar;119(3):189–192. doi: 10.1258/0022215053561657. Available from: http://dx.doi.org/10.1258/0022215053561657. [DOI] [PubMed] [Google Scholar]
- 106.House HP. Early and late complications of stapes surgery. Arch Otolaryngol. 1963 Oct;78:606–613. doi: 10.1001/archotol.1963.00750020618022. Available from: http://dx.doi.org/10.1001/archotol.1963.00750020618022. [DOI] [PubMed] [Google Scholar]
- 107.Just T, Homoth J, Graumüller S, Pau HW. Schmeckstörungen und Erholung der Schmeckfunktion nach Mittelohroperationen. Laryngo Rhino Otol. 2003;82:494–500. doi: 10.1055/s-2003-40899. Available from: http://dx.doi.org/10.1055/s-2003-40899. [DOI] [PubMed] [Google Scholar]
- 108.Kveton JF, Bartoshuk LM. The effect of unilateral chorda tympani damage on taste. Laryngoscope. 1994 Jan;104(1 Pt 1):25–29. doi: 10.1288/00005537-199401000-00006. Available from: http://dx.doi.org/10.1288/00005537-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 109.Moon CN, Pullen EW. Effects of chorda tympani section during middle ear surgery. Laryngoscope. 1963;73:392–405. doi: 10.1288/00005537-196304000-00004. Available from: http://dx.doi.org/10.1288/00005537-196304000-00004. [DOI] [Google Scholar]
- 110.Krarup B. Taste fibres and the chorda tympani. Acta Otolaryngol Suppl. 1958;140:201–205. doi: 10.3109/00016485809124416. Available from: http://dx.doi.org/10.3109/00016485809124416. [DOI] [PubMed] [Google Scholar]
- 111.Clark MPA, O'Malley S. Chorda tympani nerve function after middle ear surgery. Otol Neurotol. 2007;28:335–340. doi: 10.1097/01.mao.0000247820.16325.f0. Available from: http://dx.doi.org/10.1097/01.mao.0000247820.16325.f0. [DOI] [PubMed] [Google Scholar]
- 112.Hüttenbrink KB. Störungen des Riech- und Schmecksinns. Ther Umsch. 1995;52:732–737. [PubMed] [Google Scholar]
- 113.Catalanotto FA, Bartoshuk LM, Östrum KM, Gent JF, Fast K. Effects of anesthesia of the facial nerve on taste. Chem Senses. 1993;18:461–470. doi: 10.1093/chemse/18.5.461. Available from: http://dx.doi.org/10.1093/chemse/18.5.461. [DOI] [Google Scholar]
- 114.Östrum KM, Catalanotto FA, Garcia J, Bartoshuk LM. Effects of oral sensory filed loss of taste scaling ability. Chem Senses. 1985;10:459. [Google Scholar]
- 115.Yamada Y, Tomita H. Influences on taste in the area of chorda tympani nerve after transtympanic injection of local anesthetic (4% lidocaine) Auris Nasus Larynx. 1989;16(Suppl 1):S41–S46. doi: 10.1016/S0385-8146(89)80028-8. Available from: http://dx.doi.org/10.1016/S0385-8146(89)80028-8. [DOI] [PubMed] [Google Scholar]
- 116.Helms J, Schwager K. Mittelohrmissbildungen. In: Helms J, editor. Oto-Rhino-Laryngologie in Klinik und Praxis, Band 1: Ohr. Stuttgart: Thieme; 1994. pp. 545–563. [Google Scholar]
- 117.Sone M, Sakagami M, Tsuji K, Mishiro Y. Younger patients have a higher rate of recovery of taste function after middle ear surgery. Arch Otolaryngol Head Neck Surg. 2001 Aug;127(8):967–969. doi: 10.1001/archotol.127.8.967. Available from: http://dx.doi.org/10.1001/archotol.127.8.967. [DOI] [PubMed] [Google Scholar]
- 118.Schick B, Ibing R, Brors D, Draf W. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001 Feb;110(2):142–147. doi: 10.1177/000348940111000209. [DOI] [PubMed] [Google Scholar]
- 119.Hittel JP, Mertens J. Aberranter Verlauf der A. carotis interna. Laryngorhinootologie. 2000;79:557–558. doi: 10.1055/s-2000-6940. Available from: http://dx.doi.org/10.1055/s-2000-6940. [DOI] [PubMed] [Google Scholar]
- 120.Delank KW, Stoll W, Schuierer G, Wassmann H. Die Aneurysmablutung als Komplikation der Parazentese. HNO. 1998;46:762–765. doi: 10.1007/s001060050308. Available from: http://dx.doi.org/10.1007/s001060050308. [DOI] [PubMed] [Google Scholar]
- 121.Valentine R, Boase S, Jervis-Bardy J, Dones Cabral JD, Robinson S, Wormald PJ. The efficacy of hemostatic techniques in the sheep model of carotid artery injury. Int Forum Allergy Rhinol. 2011 Mar-Apr;1(2):118–122. doi: 10.1002/alr.20033. Available from: http://dx.doi.org/10.1002/alr.20033. [DOI] [PubMed] [Google Scholar]
- 122.Draf W. Tumoren des Mittelohres. In: Helms J, editor. Oto-Rhino-Laryngologie in Klinik und Praxis. Band 1: Ohr. Stuttgart: Thieme; 1994. pp. 701–726. [Google Scholar]
- 123.Welling DB, Glasscock ME, 3rd, Tarasidis N. Management of carotid artery hemorrhage in middle ear surgery. Otolaryngol Head Neck Surg. 1993 Dec;109(6):996–999. doi: 10.1177/019459989310900604. [DOI] [PubMed] [Google Scholar]
- 124.Stennert E. Fazialisparesen. In: Helms J, editor. Oto-Rhino-Laryngologie in Klinik und Praxis. Band 1: Ohr. Stuttgart: Thieme; 1994. pp. 666–701. [Google Scholar]
- 125.Rudert H. Die Versorgung der intratemporalen Facialisläsionen. HNO. 1975;23:297–306. [PubMed] [Google Scholar]
- 126.Das K, Murali R, Lindstrom CJ, Couldwell WT. Symptomatic subdural hygroma and temporal lobe edema after translabyrinthine removal of acoustic neuroma. Skull Base. 2001 May;11(2):137–142. doi: 10.1055/s-2001-14434. Available from: http://dx.doi.org/10.1055/s-2001-14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Horowitz SW, Leonetti JP, Azar-Kia B, Anderson D. Postoperative radiographic findings following acoustic neuroma removal. Skull Base Surg. 1996;6(4):199–205. doi: 10.1055/s-2008-1058626. Available from: http://dx.doi.org/10.1055/s-2008-1058626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostrowski VB, Borjab DI. Otolith dysfunction and semicircular canal dysfunction. In: Jackler RK, Brackmann DE, editors. Neurotology. 2. Auflage. Philadelphia: Elsevier Mosby; 2005. pp. 241–255. Available from: http://dx.doi.org/10.1016/B978-0-323-01830-2.50020-1. [DOI] [Google Scholar]
- 129.Nigam A, Moffat DA, Varley EW. Benign paroxysmal positional vertigo resulting from surgical trauma. J Laryngol Otol. 1989 Feb;103(2):203–204. doi: 10.1017/S0022215100108448. Available from: http://dx.doi.org/10.1017/S0022215100108448. [DOI] [PubMed] [Google Scholar]
- 130.Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952 Jun;45(6):341–354. doi: 10.1177/003591575204500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dlugaiczyk J, Siebert S, Hecker DJ, Brase C, Schick B. Involvement of the anterior semicircular canal in posttraumatic benign paroxysmal positioning vertigo. Otol Neurotol. 2011 Oct;32(8):1285–1290. doi: 10.1097/MAO.0b013e31822e94d9. Available from: http://dx.doi.org/10.1097/MAO.0b013e31822e94d9. [DOI] [PubMed] [Google Scholar]
- 132.Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992 Sep;107(3):399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- 133.Fife TD, Iverson DJ, Lempert T, Furman JM, Baloh RW, Tusa RJ, Hain TC, Herdman S, Morrow MJ, Gronseth GS. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008 May;70(22):2067–2074. doi: 10.1212/01.wnl.0000313378.77444.ac. Available from: http://dx.doi.org/10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 134.Kim YK, Shin JE, Chung JW. The effect of canalith repositioning for anterior semicircular canal canalithiasis. ORL J Otorhinolaryngol Relat Spec. 2005;67(1):56–60. doi: 10.1159/000084336. Available from: http://dx.doi.org/10.1159/000084336. [DOI] [PubMed] [Google Scholar]
- 135.Lempert T, Tiel-Wilck K. A positional maneuver for treatment of horizontal-canal benign positional vertigo. Laryngoscope. 1996 Apr;106(4):476–478. doi: 10.1097/00005537-199604000-00015. Available from: http://dx.doi.org/10.1097/00005537-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 136.Herdman SJ, Whitney SL. Interventions for the patient with vestibular hypofunction. In: Herdman SJ, editor. Vestibular Rehabilitation. 3. Auflage. Philadelphia: F.A. Davis Company; 2007. pp. 309–337. [Google Scholar]
- 137.Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology. 1998 Sep;51(3):838–844. doi: 10.1212/WNL.51.3.838. Available from: http://dx.doi.org/10.1212/WNL.51.3.838. [DOI] [PubMed] [Google Scholar]
- 138.Lu X, Zhao Y, Lu D, An P, Zheng Y, Qin X, Wang L, Roberson JB. Rarely reported ocular complications following surgery to correct chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2012 Feb;269(2):407–411. doi: 10.1007/s00405-011-1646-3. Available from: http://dx.doi.org/10.1007/s00405-011-1646-3. [DOI] [PubMed] [Google Scholar]
- 139.Rudnick EF, Chu MW, Sismanis A, Dodson KM, Mitchell RB. Orbital sequelae of rhinosinusitis after cochlear implantation in children. Laryngoscope. 2006 Aug;116(8):1368–1371. doi: 10.1097/01.mlg.0000225381.36099.54. Available from: http://dx.doi.org/10.1097/01.mlg.0000225381.36099.54. [DOI] [PubMed] [Google Scholar]
- 140.Hoffman RA, Parisier SC, Roland JT., Jr In reference to Orbital sequelae of rhinosinusitis after cochlear implantation in children. Laryngoscope. 2007 Aug;117(8):1505. doi: 10.1097/MLG.0b013e318065aa42. Available from: http://dx.doi.org/10.1097/MLG.0b013e318065aa42. [DOI] [PubMed] [Google Scholar]
- 141.Keles, E, Kizirgil A, Kaygusuz I, Karlidag T, Yalçin S, Alpay HC, Demir YS. Bacteriemia during mastoidectomy and/or tympanoplasty. Otolaryngol Head Neck Surg. 2005 Sep;133(3):347–351. doi: 10.1016/j.otohns.2005.02.012. Available from: http://dx.doi.org/10.1016/j.otohns.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 142.Warner ME, Fronapfel PJ, Hebl JR, Herman DC, Warner DO, Decker P, Warner MA. Perioperative visual changes. Anesthesiology. 2002 Apr;96(4):855–859. doi: 10.1097/00000542-200204000-00012. Available from: http://dx.doi.org/10.1097/00000542-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 143.Ulug T, Basaran B, Minareci O, Aydin K. An unusual complication of stapes surgery: profuse bleeding from the anteriorly located sigmoid sinus. Eur Arch Otorhinolaryngol. 2004 Aug;261(7):397–399. doi: 10.1007/s00405-003-0655-2. Available from: http://dx.doi.org/10.1007/s00405-003-0655-2. [DOI] [PubMed] [Google Scholar]
- 144.Shea JJ., Jr Complications of the stapedectomy operation. Ann Otol Rhinol Laryngol. 1963 Dec;72:1108–1123. [PubMed] [Google Scholar]
- 145.Lesinski SG. Revision stapedectomy. Curr Opin Otolaryngol Head Neck Surg. 2003 Oct;11(5):347–354. doi: 10.1097/00020840-200310000-00007. Available from: http://dx.doi.org/10.1097/00020840-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 146.Schmid P, Häusler R. Revision stapedectomy: an analysis of 201 operations. Otol Neurotol. 2009 Dec;30(8):1092–1100. doi: 10.1097/MAO.0b013e3181b4ecb2. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181b4ecb2. [DOI] [PubMed] [Google Scholar]
- 147.Röösli C, Hoffmann A, Treumann T, Linder TE. Stellenwert der CT-Diagnostik vor Revisions-Stapedotomien. HNO. 2008;56:895–900. doi: 10.1007/s00106-008-1670-9. Available from: http://dx.doi.org/10.1007/s00106-008-1670-9. [DOI] [PubMed] [Google Scholar]
- 148.Van Rompaey V, Offeciers E, De Foer B, Somers T. Jugular bulb diverticulum dehiscence towards the vestibular aqueduct in a patient with otosclerosis. J Laryngol Otol. 2012;126:313–315. doi: 10.1017/S0022215111003100. Available from: http://dx.doi.org/10.1017/S0022215111003100. [DOI] [PubMed] [Google Scholar]
- 149.Hope A, Fagan P. Latent superior canal dehiscence syndrome unmasked by stapedotomy for otosclerosis. J Laryngol Otol. 2010 Apr;124(4):428–430. doi: 10.1017/S0022215109991654. Available from: http://dx.doi.org/10.1017/S0022215109991654. [DOI] [PubMed] [Google Scholar]
- 150.Lesinski SG. Causes of conductive hearing loss after stapedectomy or stapedotomy: a prospective study of 279 consecutive surgical revisions. Otol Neurotol. 2002 May;23(3):281–288. doi: 10.1097/00129492-200205000-00009. Available from: http://dx.doi.org/10.1097/00129492-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 151.Vibert D, Häusler R, Lövblad KO, Schroth G. Hearing loss and vertigo in superficial siderosis of the central nervous system. Am J Otolaryngol. 2004 Mar-Apr;25(2):142–149. doi: 10.1016/j.amjoto.2003.10.001. Available from: http://dx.doi.org/10.1016/j.amjoto.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 152.Incesulu A, Häusler R. Advantages and risks of various sealing procedures of the oval window: vein graft, adipose tissue, gelfoam, merogel. In: Arnold W, Häusler R, editors. Otosclerosis and stapes surgery. Basel: Karger; 2007. pp. 206–209. (Adv Otorhinolarnygol; 65). [DOI] [PubMed] [Google Scholar]
- 153.Fisch U. Stapedotomy versus stapedectomy. Otol Neurotol. 2009 Dec;30(8):1166–1167. doi: 10.1097/MAO.0b013e3181c17941. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181c17941. [DOI] [PubMed] [Google Scholar]
- 154.Schick B, Brors D, Prescher A, Draf W. Conductive hearing loss in Beckwith-Wiedemann syndrome. Int J Pediatr Otorhinolaryngol. 1999 May;48(2):175–179. doi: 10.1016/S0165-5876(99)00015-4. Available from: http://dx.doi.org/10.1016/S0165-5876(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 155.Fisch U. Stapedotomy versus stapedectomy. Am J Otol. 1982 Oct;4(2):112–117. [PubMed] [Google Scholar]
- 156.Minovi A, Probst G, Dazert S. Aktuelle Aspekte zur chirurgischen Therapie der Otosklerose. HNO. 2009;57:273–286. doi: 10.1007/s00106-009-1888-1. Available from: http://dx.doi.org/10.1007/s00106-009-1888-1. [DOI] [PubMed] [Google Scholar]
- 157.Battista RA, Wiet RJ, Joy J. Revision stapedectomy. Otolaryngol Clin North Am. 2006 Aug;39(4):677–97, v. doi: 10.1016/j.otc.2006.04.003. Available from: http://dx.doi.org/10.1016/j.otc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 158.Mikulec AA, McKenna MJ, Ramsey MJ, Rosowski JJ, Herrmann BS, Rauch SD, Curtin HD, Merchant SN. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004 Mar;25(2):121–129. doi: 10.1097/00129492-200403000-00007. Available from: http://dx.doi.org/10.1097/00129492-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 159.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998 Mar;124(3):249–258. doi: 10.1001/archotol.124.3.249. Available from: http://dx.doi.org/10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 160.Minor LB, Carey JP, Cremer PD, Lustig LR, Streubel SO, Ruckenstein MJ. Dehiscence of bone overlying the superior canal as a cause of apparent conductive hearing loss. Otol Neurotol. 2003 Mar;24(2):270–278. doi: 10.1097/00129492-200303000-00023. Available from: http://dx.doi.org/10.1097/00129492-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 161.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005 Oct;115(10):1717–1727. doi: 10.1097/01.mlg.0000178324.55729.b7. Available from: http://dx.doi.org/10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 162.Li PM, Bergeron C, Monfared A, Agrawal S, Blevins NH. Superior semicircular canal dehiscence diagnosed after failed stapedotomy for conductive hearing loss. Am J Otolaryngol. 2011 Sep-Oct;32(5):441–444. doi: 10.1016/j.amjoto.2010.07.016. Available from: http://dx.doi.org/10.1016/j.amjoto.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 163.Carey JP, Minor LB, Nager GT. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000 Feb;126(2):137–147. doi: 10.1001/archotol.126.2.137. Available from: http://dx.doi.org/10.1001/archotol.126.2.137. [DOI] [PubMed] [Google Scholar]
- 164.Tsunoda A, Terasaki O. Dehiscence of the bony roof of the superior semicircular canal in the middle cranial fossa. J Laryngol Otol. 2002 Jul;116(7):514–518. doi: 10.1258/002221502760132377. Available from: http://dx.doi.org/10.1258/002221502760132377. [DOI] [PubMed] [Google Scholar]
- 165.Schwager K. Akute Komplikationen in der Mittelohrchirurgie. Teil 2: Missgeschicke in der klassischen Stapeschirurgie und ihre Behebung. HNO. 2007;55:411–416. doi: 10.1007/s00106-006-1528-y. Available from: http://dx.doi.org/10.1007/s00106-006-1528-y. [DOI] [PubMed] [Google Scholar]
- 166.Jovanovic S, Schönfeld U, Fischer R, Döring M, Prapavat V, Müller G, Scherer H. Thermische Belastung des Innenohres bei der Laserstapedotomie. Teil I: Kontinuierlich strahlende Laser. [Thermal stress of the inner ear during laser stapedotomy. I: Continuous-wave laser]. HNO. 1995 Dec;43(12):702–709. (Ger). [PubMed] [Google Scholar]
- 167.Motta G, Ruosi M, Motta S. Stapedotomia vs stapedectomia. Valutazione comparative degli insuccessi e delle complicanze. [The small fenestra vs large stapedectomy: comparative evaluation of failures and complications]. Acta Otorhinolaryngol Ital. 1996 Apr;16(2 Suppl 53):28–35. (Ita). [PubMed] [Google Scholar]
- 168.Vincent R, Sperling NM, Oates J, Jindal M. Surgical findings and long-term hearing results in 3,050 stapedotomies for primary otosclerosis: a prospective study with the otology-neurotology database. Otol Neurotol. 2006 Dec;27(8 Suppl 2):S25–S47. doi: 10.1097/01.mao.0000235311.80066.df. Available from: http://dx.doi.org/10.1097/01.mao.0000235311.80066.df. [DOI] [PubMed] [Google Scholar]
- 169.Shea JJ., Jr Forty years of stapes surgery. Am J Otol. 1998 Jan;19(1):52–55. [PubMed] [Google Scholar]
- 170.Guyot JP, Sakbani K. Patients' lives following stapedectomy complications. In: Arnold W, Häusler R, editors. Otosclerosis and Stapes Surgery. Adv Otorhinolaryngol. Basel: Karger; 2007. pp. 348–352. (Adv Otorhinolaryngol; 65). [DOI] [PubMed] [Google Scholar]
- 171.Crabtree JA, Britton BH, Powers WH. An evaluation of revision stapes surgery. Laryngoscope. 1980 Feb;90(2):224–227. doi: 10.1288/00005537-198002000-00006. Available from: http://dx.doi.org/10.1288/00005537-198002000-00006. [DOI] [PubMed] [Google Scholar]
- 172.Derlacki EL. Revision stapes surgery: problems with some solutions. Laryngoscope. 1985 Sep;95(9 Pt 1):1047–1053. [PubMed] [Google Scholar]
- 173.Sheehy JL, Perkins JH. Stapedectomy: gelfoam compared with tissue grafts. Laryngoscope. 1976 Mar;86(3):436–444. doi: 10.1288/00005537-197603000-00013. Available from: http://dx.doi.org/10.1288/00005537-197603000-00013. [DOI] [PubMed] [Google Scholar]
- 174.Sheey JL, Nelson RA, House HP. Revision stapedectomy: a review of 258 cases. Laryngoscope. 1981;91:43–51. doi: 10.1288/00005537-198101000-00007. [DOI] [PubMed] [Google Scholar]
- 175.Shenoi PM. Ototoxicity of absorbable gelatin sponge. Proc R Soc Med. 1973 Feb;66(2):193–196. doi: 10.1177/003591577306600242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kujala J, Aalto H, Hirvonen TP. Video-oculography findings in patients with otosclerosis. Otol Neurotol. 2005 Nov;26(6):1134–1137. doi: 10.1097/01.mao.0000179525.40156.fa. Available from: http://dx.doi.org/10.1097/01.mao.0000179525.40156.fa. [DOI] [PubMed] [Google Scholar]
- 177.Kujala J, Aalto H, Hirvonen T. Video-oculography findings and vestibular symptoms on the day of stapes surgery. Eur Arch Otorhinolaryngol. 2010 Feb;267(2):187–190. doi: 10.1007/s00405-009-1024-6. Available from: http://dx.doi.org/10.1007/s00405-009-1024-6. [DOI] [PubMed] [Google Scholar]
- 178.Ozmen AO, Aksoy S, Ozmen S, Saraç S, Sennaroğlu L, Gürsel B. Balance after stapedotomy: analysis of balance with computerized dynamic posturography. Clin Otolaryngol. 2009 Jun;34(3):212–217. doi: 10.1111/j.1749-4486.2009.01915.x. Available from: http://dx.doi.org/10.1111/j.1749-4486.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 179.Fisch U. Vestibuläre Symptome vor und nach Stapedektomie. Acta Otolaryngol. 1965;60:515–530. doi: 10.3109/00016486509127035. Available from: http://dx.doi.org/10.3109/00016486509127035. [DOI] [PubMed] [Google Scholar]
- 180.Grayeli AB, Sterkers O, Toupet M. Audiovestibular function in patients with otosclerosis and balance disorders. Otol Neurotol. 2009 Dec;30(8):1085–1091. doi: 10.1097/MAO.0b013e3181b0fd5d. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181b0fd5d. [DOI] [PubMed] [Google Scholar]
- 181.Tribukait A, Bergenius J. The subjective visual horizontal after stapedotomy: evidence for an increased resting activity in otolithic afferents. Acta Otolaryngol. 1998 Jun;118(3):299–306. doi: 10.1080/00016489850183368. Available from: http://dx.doi.org/10.1080/00016489850183368. [DOI] [PubMed] [Google Scholar]
- 182.Mukherjee P, Uzun-Coruhlu H, Curthoys IS, Jones AS, Bradshaw AP, Pohl DV. Three-dimensional analysis of the vestibular end organs in relation to the stapes footplate and piston placement. Otol Neurotol. 2011 Apr;32(3):367–372. doi: 10.1097/MAO.0b013e3182096ddd. Available from: http://dx.doi.org/10.1097/MAO.0b013e3182096ddd. [DOI] [PubMed] [Google Scholar]
- 183.Häusler R. Advances in stapes surgery. Laryngorhinootologie. 2000;S2:95–139. doi: 10.1055/s-2000-15920. Available from: http://dx.doi.org/10.1055/s-2000-15920. [DOI] [Google Scholar]
- 184.Atacan E, Sennaroglu L, Genc A, Kaya S. Benign paroxysmal positional vertigo after stapedectomy. Laryngoscope. 2001 Jul;111(7):1257–1259. doi: 10.1097/00005537-200107000-00021. Available from: http://dx.doi.org/10.1097/00005537-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 185.Magliulo G, Gagliardi M, Cuiuli G, Celebrini A, Parrotto D, D'Amico R. Stapedotomy and postoperative benign paroxysmal positional vertigo. J Vestib Res. 2005;15:169–172. [PubMed] [Google Scholar]
- 186.Fusetti M, Eibenstein A, Sabato M, Piredda G, Caporale C. Su tre casi di fistola labirintica post-stapedectomia. [3 cases of post-stapedectomy labyrinthine fistula]. Acta Otorhinolaryngol Ital. 1997 Dec;17(6):436–443. (Ita). [PubMed] [Google Scholar]
- 187.Dazert S, Hildmann H. Stapes revision surgery. In: Hildmann H, Sudhoff H, editors. Middle ear surgery. Heidelberg: Springer; 2006. pp. 131–133. Available from: http://dx.doi.org/10.1007/978-3-540-47671-9_24. [DOI] [Google Scholar]
- 188.Woldag K, Meister EF, Kösling S. Diagnostik bei persistierenden Gleichgewichtsstörungen nach Operationen am Stapes. Laryngo Rhino Otol. 1995;74:403–407. doi: 10.1055/s-2007-997769. Available from: http://dx.doi.org/10.1055/s-2007-997769. [DOI] [PubMed] [Google Scholar]
- 189.Bozzato A, Struffert T, Hertel V, Iro H, Hornung J. Analysis of the accuracy of high-resolution computed tomography techniques for the measurement of stapes prostheses. Eur Radiol. 2010 Mar;20(3):566–571. doi: 10.1007/s00330-009-1582-4. Available from: http://dx.doi.org/10.1007/s00330-009-1582-4. [DOI] [PubMed] [Google Scholar]
- 190.Ginsberg IA. Cochlear reaction following stapedectomy. A comparison of two techniques. Arch Otolaryngol. 1968 Nov;88(5):481–490. doi: 10.1001/archotol.1968.00770010483006. Available from: http://dx.doi.org/10.1001/archotol.1968.00770010483006. [DOI] [PubMed] [Google Scholar]
- 191.Shea JJ. Thirty years of stapes surgery. J Laryngol Otol. 1988 Jan;102(1):14–19. doi: 10.1017/S0022215100103846. Available from: http://dx.doi.org/10.1017/S0022215100103846. [DOI] [PubMed] [Google Scholar]
- 192.Zohar Y, Laurian N. Facial palsy following stapedectomy: (a case report) J Laryngol Otol. 1985 Apr;99(4):387–388. doi: 10.1017/S0022215100096894. Available from: http://dx.doi.org/10.1017/S0022215100096894. [DOI] [PubMed] [Google Scholar]
- 193.Smith MCF, Simon P, Ramalingam KK. Delayed facial palsy following uncomplicated stapedectomy. J Laryngol Otol. 1990;104:611–612. doi: 10.1017/S0022215100113374. Available from: http://dx.doi.org/10.1017/S0022215100113374. [DOI] [PubMed] [Google Scholar]
- 194.Althaus SR, House HP. Delayed post-stapedectomy facial paralysis: a report of five cases. Laryngoscope. 1973 Aug;83(8):1234–1240. doi: 10.1288/00005537-197308000-00007. Available from: http://dx.doi.org/10.1288/00005537-197308000-00007. [DOI] [PubMed] [Google Scholar]
- 195.Shea JJ, Jr, Ge X. Delayed facial palsy after stapedectomy. Otol Neurotol. 2001 Jul;22(4):465–470. doi: 10.1097/00129492-200107000-00009. Available from: http://dx.doi.org/10.1097/00129492-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 196.Ng M, Maceri DR. Delayed facial paralysis after stapedotomy using KTP laser. Am J Otol. 1999 Jul;20(4):421–424. [PubMed] [Google Scholar]
- 197.Salvinelli F, Casale M, Vitaliana L, Greco F, Dianzani C, D'Ascanio L. Delayed peripheral facial palsy in the stapes surgery: can it be prevented? Am J Otolaryngol. 2004 Mar-Apr;25(2):105–108. doi: 10.1016/j.amjoto.2003.11.010. Available from: http://dx.doi.org/10.1016/j.amjoto.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 198.Yung M, Smith P, Hausler R, Martin C, Offeciers E, Pytel J, Skladzien J, Somers T, Ven de Heyning P. International Common Otology Database: taste disturbance after stapes surgery. Otol Neurotol. 2008 Aug;29(5):661–665. doi: 10.1097/MAO.0b013e3181778211. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181778211. [DOI] [PubMed] [Google Scholar]
- 199.Rebol J. The unilateral stapes gusher. Wien Klin Wochenschr. 2004;116(Suppl 2):90–92. [PubMed] [Google Scholar]
- 200.Bellucci RJ. Cochlear hearing loss in tympanoplasty. Otolaryngol Head Neck Surg. 1985 Aug;93(4):482–485. doi: 10.1177/019459988509300403. [DOI] [PubMed] [Google Scholar]
- 201.Lo TM, Zhang M, Young LJ, Antonelli PJ. Hearing loss with stapedotomy and treated otitis media. Otolaryngol Head and Neck Surg. 2006;134:674–679. doi: 10.1016/j.otohns.2005.11.008. Available from: http://dx.doi.org/10.1016/j.otohns.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 202.Antonelli PJ, Briggs RD, Gerhardt KJ. Hearing preservation with labyrinthine ablation in otitis media. Laryngoscope. 2000 May;110(5 Pt 1):779–786. doi: 10.1097/00005537-200005000-00008. Available from: http://dx.doi.org/10.1097/00005537-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 203.Nielsen TR, Thomsen J. Meningitis following stapedotomy: a rare and early complication. J Laryngol Otol. 2000 Oct;114(10):781–783. doi: 10.1258/0022215001903915. Available from: http://dx.doi.org/10.1258/0022215001903915. [DOI] [PubMed] [Google Scholar]
- 204.García AM, Pérez A, Viada J, Cadaval F. Meningoencefalitis post estapedotomía. Análisis de un caso clínico. [Repeated episodes of meningoencephalitis after a stapedotomy. Report of one case]. Rev Med Chil. 2004 Nov;132(11):1407–1411. [PubMed] [Google Scholar]
- 205.Marion M, Hinojosa R, Khan AA. Persistence of the stapedial artery: a histopathological study. Otolaryngol Head Neck Surg. 1985;93:298–312. doi: 10.1177/019459988509300304. [DOI] [PubMed] [Google Scholar]
- 206.Schuknecht HF. Otosclerosis Surgery. In: Nadol JB, Schuknecht HF, editors. Surgery of the ear and temporal bone. New York: Raven Press; 1993. [Google Scholar]
- 207.Sudhoff H, Hildmann H. Stapes surgery. In: Hildmann H, Sudhoff H, editors. Middle ear surgery. Heidelberg: Springer; 2006. pp. 112–119. Available from: http://dx.doi.org/10.1007/978-3-540-47671-9_22. [DOI] [Google Scholar]
- 208.Fenton JE, Turner J, Shirazi A, Fagan PA. Post-stapedectomy reparative granuloma: a misnomer. J Laryngol Otol. 1996 Feb;110(2):185–188. doi: 10.1017/S0022215100133134. Available from: http://dx.doi.org/10.1017/S0022215100133134. [DOI] [PubMed] [Google Scholar]
- 209.Ciorba A, Bovo R, Trevisi P, Rosignoli M, Aimoni C, Castiglione A, Martini A. Postoperative complications in cochlear implants: a retrospective analysis of 438 consecutive cases. Eur Arch Otorhinolaryngol. 2012 Jun;269(6):1599–1603. doi: 10.1007/s00405-011-1818-1. Available from: http://dx.doi.org/10.1007/s00405-011-1818-1. [DOI] [PubMed] [Google Scholar]
- 210.Cohen NL, Hoffman RA. Complications of cochlear implant surgery in adults and children. Ann Otol Rhinol Laryngol. 1991 Sep;100(9 Pt 1):708–711. doi: 10.1177/000348949110000903. [DOI] [PubMed] [Google Scholar]
- 211.Bhatia K, Gibbin KP, Nikolopoulos TP, O'Donoghue GM. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol. 2004;25:730–739. doi: 10.1097/00129492-200409000-00015. Available from: http://dx.doi.org/10.1097/00129492-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 212.Venail F, Sicard M, Piron JP, Levi A, Artieres F, Uziel A, Mondain M. Reliability and complications of 500 consecutive cochlear implantations. Arch Otolaryngol Head Neck Surg. 2008 Dec;134(12):1276–1281. doi: 10.1001/archoto.2008.504. Available from: http://dx.doi.org/10.1001/archoto.2008.504. [DOI] [PubMed] [Google Scholar]
- 213.Dutt SN, Ray J, Hadjihannas E, Cooper H, Donaldson I, Proops DW. Medical and surgical complications of the second 100 adult cochlear implant patients in Birmingham. J Laryngol Otol. 2005;119:759–764. doi: 10.1258/002221505774481291. Available from: http://dx.doi.org/10.1258/002221505774481291. [DOI] [PubMed] [Google Scholar]
- 214.Kim CS, Oh SH, Chang SO, Kim HM, Hur DG. Management of complications in cochlear implantation. Acta Oto Laryngol. 2008;128:408–414. doi: 10.1080/00016480701784973. Available from: http://dx.doi.org/10.1080/00016480701784973. [DOI] [PubMed] [Google Scholar]
- 215.Brito R, Monteiro TA, Leal AF, Tsuji RK, Pinna MH, Bento RF. Surgical complications in 550 consecutive cochlear implantation. Braz J Otorhinolaryngol. 2012 Jun;78(3):80–85. doi: 10.1590/S1808-86942012000300014. Available from: http://dx.doi.org/10.1590/S1808-86942012000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cohen NL, Roland JT, Jr, Marrinan M. Meningitis in cochlear implant recipients: the North American experience. Otol Neurotol. 2004 May;25(3):275–281. doi: 10.1097/00129492-200405000-00013. Available from: http://dx.doi.org/10.1097/00129492-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 217.Hansen S, Anthonsen K, Stangerup SE, Jensen JH, Thomsen J, Cayé-Thomasen P. Unexpected findings and surgical complications in 505 consecutive cochlear implantations: a proposal for reporting consensus. Acta Otolaryngol. 2010 May;130(5):540–549. doi: 10.3109/00016480903358261. Available from: http://dx.doi.org/10.3109/00016480903358261. [DOI] [PubMed] [Google Scholar]
- 218.Kandogan T, Levent O, Gurol G. Complications of paediatric cochlear implantation: experience in Izmir. J Laryngol Otol. 2005 Aug;119(8):606–610. doi: 10.1258/0022215054516331. Available from: http://dx.doi.org/10.1258/0022215054516331. [DOI] [PubMed] [Google Scholar]
- 219.Stratigouleas ED, Perry BP, King SM, Syms CA., 3rd Complication rate of minimally invasive cochlear implantation. Otolaryngol Head Neck Surg. 2006 Sep;135(3):383–386. doi: 10.1016/j.otohns.2006.03.023. Available from: http://dx.doi.org/10.1016/j.otohns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 220.Telian SA, El-Kashlan HK, Arts HA. Minimizing wound complications in cochlear implant surgery. Am J Otol. 1999 May;20(3):331–334. [PubMed] [Google Scholar]
- 221.Orhan KS, Guldiken Y, Basaran B, Ulusan M, Polat B, Celik M, Deger K. Complications and their management following pediatric cochlear implantations. Int Adv Otol. 2012;8:244–252. [Google Scholar]
- 222.Pirzadeh A, Khorsandi M, Mohammadi MA, Pirzadeh A. Complications related to cochlear implants: experience in Tehran. J Pak Med Assoc. 2011 Jul;61(7):622–624. [PubMed] [Google Scholar]
- 223.Ramos A, Charlone R, de Miguel I, Valdivielso A, Cuyas JM, Pérez D, Vasallo JR. Complicaciones en la implantación coclear. [Complications in cochlear implantation]. Acta Otorrinolaringol Esp. 2006 Mar;57(3):122–125. doi: 10.1016/S0001-6519(06)78675-7. Available from: http://dx.doi.org/10.1016/S0001-6519(06)78675-7. [DOI] [PubMed] [Google Scholar]
- 224.Stamatiou GA, Kyrodimos E, Sismanis A. Complications of cochlea implantations in adults. Ann Otol Rhinol Laryngol. 2011;120:428–432. doi: 10.1177/000348941112000702. [DOI] [PubMed] [Google Scholar]
- 225.Masterson L, Kumar S, Kong JH, Briggs J, Donnelly N, Axon PR, Gray RF. Cochlear implant failures: lessons learned from a UK centre. J Laryngol Otol. 2012 Jan;126(1):15–21. doi: 10.1017/S0022215111002829. Available from: http://dx.doi.org/10.1017/S0022215111002829. [DOI] [PubMed] [Google Scholar]
- 226.Balkany TJ, Hodges AV, Buchman CA, Luxford WM, Pillsbury CH, Roland PS, Shallop JK, Backous DD, Franz D, Graham JM, Hirsch B, Luntz M, Niparko JK, Patrick J, Payne SL, Telischi FF, Tobey EA, Truy E, Staller S. Cochlear implant soft failures consensus development conference statement. Otol Neurotol. 2005 Jul;26(4):815–818. doi: 10.1097/01.mao.0000178150.44505.52. Available from: http://dx.doi.org/10.1097/01.mao.0000178150.44505.52. [DOI] [PubMed] [Google Scholar]
- 227.European consensus statement on cochlear implant failures and explantations. Otol Neurotol. 2005 Nov;26(6):1097–1099. doi: 10.1097/01.mao.0000194885.51647.bb. Available from: http://dx.doi.org/10.1097/01.mao.0000194885.51647.bb. [DOI] [PubMed] [Google Scholar]
- 228.Kiefer J, Weber A, Pfennigdorff T, von Ilberg C. Scala vestibuli insertion in cochlear implantation: a valuable alternative for cases with obstructed scala tympani. ORL J Otorhinolaryngol Relat Spec. 2000 Sep-Oct;62(5):251–256. doi: 10.1159/000027755. Available from: http://dx.doi.org/10.1159/000027755. [DOI] [PubMed] [Google Scholar]
- 229.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007 Apr;28(2 Suppl):75S–79S. doi: 10.1097/AUD.0b013e318031542e. Available from: http://dx.doi.org/10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 230.Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008 Oct;29(7):920–928. doi: 10.1097/MAO.0b013e318184f492. Available from: http://dx.doi.org/10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Arnoldner C, Helbig S, Wagenblast J, Gstoettner W. Cochlear implant reference electrode migration to dura mater. Otol Neurotol. 2009 Oct;30(7):1013–1014. doi: 10.1097/MAO.0b013e31818edf35. Available from: http://dx.doi.org/10.1097/MAO.0b013e31818edf35. [DOI] [PubMed] [Google Scholar]
- 232.Yohn DC, Maessen H, Morris DP. Cochlear implant magnet extrusion with subsequent surgical replacement and restoration of full implant use without the need for device explantation. Cochlear Implants Int. 2011 Nov;12(4):244–247. doi: 10.1179/1754762810Y.0000000004. Available from: http://dx.doi.org/10.1179/1754762810Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 233.Yun JM, Colburn MW, Antonelli PJ. Cochlear implant magnet displacement with minor head trauma. Otolaryngol Head Neck Surg. 2005 Aug;133(2):275–277. doi: 10.1016/j.otohns.2005.02.018. Available from: http://dx.doi.org/10.1016/j.otohns.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 234.Hoffman RA, Downey LL, Waltzman SB, Cohen NL. Cochlear implantation in children with cochlear malformations. Am J Otol. 1997 Mar;18(2):184–187. [PubMed] [Google Scholar]
- 235.Melton MF, Backous DD. Preventing complications in pediatric cochlear implantation. Curr Opin Otolaryngol Head Neck Surg. 2011 Oct;19(5):358–362. doi: 10.1097/MOO.0b013e32834a023b. Available from: http://dx.doi.org/10.1097/MOO.0b013e32834a023b. [DOI] [PubMed] [Google Scholar]
- 236.Loeffler KA, Johnson TA, Burne RA, Antonelli PJ. Biofilm fomration in an in vitro model of cochlear implants with removable magnets. Otolaryngol Head Neck Surg. 2007;136:583–588. doi: 10.1016/j.otohns.2006.11.005. Available from: http://dx.doi.org/10.1016/j.otohns.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 237.Macassey E, Dawes P. Biofilms and their role in otorhinolaryngological disease. J Laryngol Otol. 2008 Dec;122(12):1273–1278. doi: 10.1017/S0022215108002193. Available from: http://dx.doi.org/10.1017/S0022215108002193. [DOI] [PubMed] [Google Scholar]
- 238.Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007 Oct;15(5):347–351. doi: 10.1097/MOO.0b013e3282b97327. Available from: http://dx.doi.org/10.1097/MOO.0b013e3282b97327. [DOI] [PubMed] [Google Scholar]
- 239.Hou JH, Zhao SP, Ning F, Rao SQ, Han DY. Postoperative complications in patients with cochlear implants and impacts of nursing intervention. Acta Otolaryngol. 2010 Jun;130(6):687–695. doi: 10.3109/00016480903334445. Available from: http://dx.doi.org/10.3109/00016480903334445. [DOI] [PubMed] [Google Scholar]
- 240.Minoda R, Takahashi H, Miyamaru S, Masuda M, Miwa T, Sanuki T, Hirai T, Yumoto E. A postmeningitic cochlear implant patient who was postoperatively diagnosed as having X-linked agammaglobulinemia. Auris Nasus Larynx. 2012 Dec;39(6):638–640. doi: 10.1016/j.anl.2011.12.005. Available from: http://dx.doi.org/10.1016/j.anl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 241.Durisin M, Bartling S, Arnoldner C, Ende M, Prokein J, Lesinski-Schiedat A, Lanfermann H, Lenarz T, Stöver T. Cochlear osteoneogenesis after meningitis in cochlear implant patients: a retrospective analysis. Otol Neurotol. 2010 Sep;31(7):1072–1078. doi: 10.1097/MAO.0b013e3181e71310. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181e71310. [DOI] [PubMed] [Google Scholar]
- 242.Cunningham CD, 3rd, Slattery WH, 3rd, Luxford WM. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131:109–114. doi: 10.1016/j.otohns.2004.02.011. Available from: http://dx.doi.org/10.1016/j.otohns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 243.Vaid N, Vaid S, Manikoth M. Case report - biofilm infection of a cochlear implant. Cochlear Implants Int. 2013 Mar;14(2):117–120. doi: 10.1179/1754762811Y.0000000025. Available from: http://dx.doi.org/10.1179/1754762811Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 244.Das Purkayastha PK, Jewell S, James AL, Gordon K, Papsin B. Soft tissue complications after pediatric cochlear implantation in children younger than 12 months. Otol Neurotol. 2011 Jul;32(5):780–783. doi: 10.1097/MAO.0b013e318214ea88. Available from: http://dx.doi.org/10.1097/MAO.0b013e318214ea88. [DOI] [PubMed] [Google Scholar]
- 245.Kronenberg J, Wolf M, Migirov L, Shapira Y, Avien-Ronen S, Hildesheimer M. Foreign body reaction to cochlear implant. Otorhinolaryngol Nova. 2001;11:207–208. doi: 10.1159/000063002. Available from: http://dx.doi.org/10.1159/000063002. [DOI] [Google Scholar]
- 246.Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005 Oct;115(10):1760–1762. doi: 10.1097/01.mlg.0000172202.58968.41. Available from: http://dx.doi.org/10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- 247.Meyer DR, Bui HX, Carlson JA, Ratliff CD, Guevarra MC, DelRosario AD, Ross JS, Mihm M., Jr Silicon granulomas and dermatomyositis-like changes associated with chronic eyelid edema after silicone breast implant. Ophthal Plast Reconstr Surg. 1998 May;14(3):182–188. doi: 10.1097/00002341-199805000-00007. Available from: http://dx.doi.org/10.1097/00002341-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 248.Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB., Jr Silicone allergy: a new cause for cochlea extrusion and its management. Otol Neurotol. 2006;27:1078–1082. doi: 10.1097/01.mao.0000235378.64654.4d. Available from: http://dx.doi.org/10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- 249.Ribári O, Küstel M, Szirmai A, Répássy G. Cochlear implantation influences contralateral hearing and vestibular responsiveness. Acta Otolaryngol. 1999 Mar;119(2):225–228. doi: 10.1080/00016489950181710. Available from: http://dx.doi.org/10.1080/00016489950181710. [DOI] [PubMed] [Google Scholar]
- 250.Buchman CA, Joy J, Hodges A, Telischi FF, Balkany TJ. Vestibular effects of cochlear implantation. Laryngoscope. 2004 Oct;114(10 Pt 2 Suppl 103):1–22. doi: 10.1097/00005537-200410001-00001. Available from: http://dx.doi.org/10.1097/00005537-200410001-00001. [DOI] [PubMed] [Google Scholar]
- 251.Brey RH, Facer GW, Trine MB, Lynn SG, Peterson AM, Suman VJ. Vestibular effects associated with implantation of a multiple channel cochlear prosthesis. Am J Otol. 1995;16:424–430. [PubMed] [Google Scholar]
- 252.Klenzner T, Neumann M, Aschendorff A, Laszig R. Thermische Erregbarkeit des Vestibularorgans nach Cochlear-Implantation. Laryngo Rhino Otol. 2004;83:659–664. doi: 10.1055/s-2004-825678. Available from: http://dx.doi.org/10.1055/s-2004-825678. [DOI] [PubMed] [Google Scholar]
- 253.Shoman N, Ngo R, Archibald J, Pijl S, Chan S, Westerberg BD. Prevalence of new-onset vestibular symptoms following cochlear implantation. J Otolaryngol Head Neck Surg. 2008 Jun;37(3):388–394. [PubMed] [Google Scholar]
- 254.Handzel O, Burgess BJ, Nadol JB., Jr Histopathology of the peripheral vestibular system after cochlear implantation in the human. Otol Neurotol. 2006 Jan;27(1):57–64. doi: 10.1097/01.mao.0000188658.36327.8f. Available from: http://dx.doi.org/10.1097/01.mao.0000188658.36327.8f. [DOI] [PubMed] [Google Scholar]
- 255.Tien HC, Linthicum FH., Jr Histopathological changes in the vestibule after cochlear implantation. Otolaryngol Head Neck Surg. 2002;127:260–264. doi: 10.1067/mhn.2002.128555. Available from: http://dx.doi.org/10.1067/mhn.2002.128555. [DOI] [PubMed] [Google Scholar]
- 256.Limb CJ, Francis HF, Lustig LR, Niparko JK, Jammal H. Benign positional vertigo after cochlear implantation. Otolaryngol Head Neck Surg. 2005 May;132(5):741–745. doi: 10.1016/j.otohns.2005.01.004. Available from: http://dx.doi.org/10.1016/j.otohns.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 257.Viccaro M, Mancini P, La Gamma R, De Seta E, Covelli E, Filipo R. Positional vertigo and cochlear implantation. Otol Neurotol. 2007 Sep;28(6):764–767. doi: 10.1097/MAO.0b013e318064e8d4. Available from: http://dx.doi.org/10.1097/MAO.0b013e318064e8d4. [DOI] [PubMed] [Google Scholar]
- 258.Shetye A. Benign paroxysmal positional vertigo in a child: an infrequent complication following a fairground ride and post-cochlear implant surgery. Cochlear Implants Int. 2012;13:177–180. doi: 10.1179/1754762811Y.0000000011. Available from: http://dx.doi.org/10.1179/1754762811Y.0000000011. [DOI] [PubMed] [Google Scholar]
- 259.Coordes A, Basta D, Götze R, Scholz S, Seidl RO, Ernst A, Todt I. Sound-induced vertigo after cochlear implantation. Otol Neurotol. 2012 Apr;33(3):335–342. doi: 10.1097/MAO.0b013e318245cee3. Available from: http://dx.doi.org/10.1097/MAO.0b013e318245cee3. [DOI] [PubMed] [Google Scholar]
- 260.Todt I, Basta D, Ernst A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg. 2008 Jan;138(1):8–12. doi: 10.1016/j.otohns.2007.09.003. Available from: http://dx.doi.org/10.1016/j.otohns.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 261.Enticott JC, Eastwood HT, Briggs RJ, Dowell RC, O'Leary SJ. Methylprednisolone applied directly to the round window reduces dizziness after cochlear implantation: a randomized clinical trial. Audiol Neurotol. 2011;16:289–303. doi: 10.1159/000322137. Available from: http://dx.doi.org/10.1159/000322137. [DOI] [PubMed] [Google Scholar]
- 262.Krause E, Louza JP, Wechtenbruch J, Hempel JM, Rader T, Gürkov R. Incidence and quality of vertigo symptoms after cochlea implantation. J Laryngol Otol. 2009;123:278–282. doi: 10.1017/S002221510800296X. Available from: http://dx.doi.org/10.1017/S002221510800296X. [DOI] [PubMed] [Google Scholar]
- 263.Parmar A, Savage J, Wilkinson A, Hajioff D, Nunez DA, Robinson P. The role of vestibular caloric tests in cochlear implantation. Otolaryngol Head Neck Surg. 2012 Jul;147(1):127–131. doi: 10.1177/0194599812442059. Available from: http://dx.doi.org/10.1177/0194599812442059. [DOI] [PubMed] [Google Scholar]
- 264.Qui S, Gray RF, Kumar S, Axon P. Pneumocele after cochlear implantation. Cochlear Implants Int. 2012;13:188–192. doi: 10.1179/146701011X12962268235788. Available from: http://dx.doi.org/10.1179/146701011X12962268235788. [DOI] [PubMed] [Google Scholar]
- 265.Cohen NL, Lewis WS, Ransohoff J. Hearing preservation in cerebellopontine angle tumor surgery: the NYU experience 1974-1991. Am J Otol. 1993 Sep;14(5):423–433. doi: 10.1097/00129492-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 266.Tos M, Thomsen J, Harmsen A. Results of translabyrinthine removal of 300 acoustic neuromas related to tumour size. Acta Otolaryngol Suppl. 1988;452:38–51. doi: 10.3109/00016488809124993. Available from: http://dx.doi.org/10.3109/00016488809124993. [DOI] [PubMed] [Google Scholar]
- 267.Darrouzet V, Martel J, Enée V, Bébéar JP, Guérin J. Vestibular schwannoma surgery outcomes: our multidisciplinary experience in 400 cases over 17 years. Laryngoscope. 2004 Apr;114(4):681–688. doi: 10.1097/00005537-200404000-00016. Available from: http://dx.doi.org/10.1097/00005537-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 268.Samii M, Matthies C. Management of 1 000 vestibular schwannomas (acoustic neuromas): the facial nerve-preservation and restitution of function. Neurosurgery. 1997;40:684–694. doi: 10.1097/00006123-199704000-00006. Available from: http://dx.doi.org/10.1097/00006123-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 269.Sanna M, Taibah A, Russo A, Falcioni M, Agarwal M. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004 May;25(3):379–386. doi: 10.1097/00129492-200405000-00029. Available from: http://dx.doi.org/10.1097/00129492-200405000-00029. [DOI] [PubMed] [Google Scholar]
- 270.Sterkers JM, Morrison GA, Sterkers O, El-Dine MM. Preservation of facial, cochlear, and other nerve functions in acoustic neuroma treatment. Otolaryngol Head Neck Surg. 1994 Feb;110(2):146–155. doi: 10.1177/019459989411000202. [DOI] [PubMed] [Google Scholar]
- 271.Rutherford SA, King AT. Vestibular schwannoma management: What is the 'best' option? Br J Neurosurg. 2005 Aug;19(4):309–316. doi: 10.1080/02688690500305399. Available from: http://dx.doi.org/10.1080/02688690500305399. [DOI] [PubMed] [Google Scholar]
- 272.Porter RW. Comment to: Cranial nerve preservation in surgery for large acoustic neuromas. Skull Base. 2004;14:90–91. doi: 10.1055/s-2004-828700. Available from: http://dx.doi.org/10.1055/s-2004-828700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Roland JT, Jr, Fishman AJ, Golfinos JG, Cohen N, Alexiades G, Jackman AH. Cranial nerve preservation in surgery for large acoustic neuromas. Skull Base. 2004;14:85–91. doi: 10.1055/s-2004-828699. Available from: http://dx.doi.org/10.1055/s-2004-828699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ansari SF, Terry C, Cohen-Gadol AA. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus. 2012 Sep;33(3):E14. doi: 10.3171/2012.6.FOCUS12163. Available from: http://dx.doi.org/10.3171/2012.6.FOCUS12163. [DOI] [PubMed] [Google Scholar]
- 275.Mamikoglu B, Wiet RJ, Esquivel CR. Translabyrinthine approach for the management of large and giant vestibular schwannomas. Otol Neurotol. 2002 Mar;23(2):224–227. doi: 10.1097/00129492-200203000-00020. Available from: http://dx.doi.org/10.1097/00129492-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 276.DeMonte F, Gidley PW. Hearing preservation surgery for vestibular schwannoma: experience with the middle fossa approach. Neurosurg Focus. 2012 Sep;33(3):E10. doi: 10.3171/2012.7.FOCUS12172. Available from: http://dx.doi.org/10.3171/2012.7.FOCUS12172. [DOI] [PubMed] [Google Scholar]
- 277.Chen L, Chen LH, Ling F, Liu YS, Samii M, Samii A. Removal of vestibular schwannoma and facial nerve preservation using small suboccipital retrosigmoid craniotomy. Chin Med J. 2010 Feb;123(3):274–280. [PubMed] [Google Scholar]
- 278.Cheng S, Naidoo Y, da Cruz M, Dexter M. Quality of life in postoperative vestibular schwannoma patients. Laryngoscope. 2009 Nov;119(11):2252–2257. doi: 10.1002/lary.20217. Available from: http://dx.doi.org/10.1002/lary.20217. [DOI] [PubMed] [Google Scholar]
- 279.Oh T, Nagasawa DT, Fong BM, Trang A, Gopen Q, Parsa AT, Yang I. Intraoperative neuromonitoring techniques in the surgical management of acoustic neuromas. Neurosurg Focus. 2012 Sep;33(3):E6. doi: 10.3171/2012.6.FOCUS12194. Available from: http://dx.doi.org/10.3171/2012.6.FOCUS12194. [DOI] [PubMed] [Google Scholar]
- 280.Glasscock ME, 3rd, Hays JW, Minor LB, Haynes DS, Carrasco VN. Preservation of hearing in surgery for acoustic neuromas. J Neurosurg. 1993;78:864–870. doi: 10.3171/jns.1993.78.6.0864. Available from: http://dx.doi.org/10.3171/jns.1993.78.6.0864. [DOI] [PubMed] [Google Scholar]
- 281.Lassaletta L, Fontes L, Melcon E, Sarria MJ, Gavilan J. Hearing preservation with the retrosigmoid approach for vestibular schwannoma: myth or reality? Otolaryngol Head Neck Surg. 2003 Oct;129(4):397–401. doi: 10.1016/S0194-5998(03)00628-4. Available from: http://dx.doi.org/10.1016/S0194-5998(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 282.Yates PD, Jackler RK, Satar B, Pitts LH, Oghalai JS. Is it worthwhile to attempt hearing preservation in larger acoustic neuromas? Otol Neurotol. 2003 May;24(3):460–464. doi: 10.1097/00129492-200305000-00017. Available from: http://dx.doi.org/10.1097/00129492-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 283.Silverstein H, Smouha EE, Jones R. Routine intraoperative facial nerve monitoring during otologic surgery. Am J Otol. 1988 Jul;9(4):269–275. [PubMed] [Google Scholar]
- 284.Delgado TE, Bucheit WA, Rosenholtz HR, Chrissian S. Intraoperative monitoring of facila muscle evoked responses obtained by intracranial stimulation of the facila nerve: a more accurate technique for facila nerve dissection. Neurosurgery. 1979 May;4(5):418–421. doi: 10.1227/00006123-197905000-00007. Available from: http://dx.doi.org/10.1227/00006123-197905000-00007. [DOI] [PubMed] [Google Scholar]
- 285.Grayeli AB, Kalamarides M, Fraysse B, Deguine O, Favre G, Martin C, Mom T, Sterkers O. Comparison between intraoperative observations and electromyographic monitoring data for facial nerve outcome after vestibular schwannoma surgery. Acta Otolaryngol. 2005;125:1069–1074. doi: 10.1080/00016480510038608. Available from: http://dx.doi.org/10.1080/00016480510038608. [DOI] [PubMed] [Google Scholar]
- 286.Benecke JE, Jr, Calder HB, Chadwick G. Facial nerve monitoring during acoustic neuroma removal. Laryngoscope. 1987;97:697–700. doi: 10.1288/00005537-198706000-00009. Available from: http://dx.doi.org/10.1288/00005537-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 287.Ebersold MJ, Harner SG, Beatty CW, Harper CM, Jr, Quast LM. Current results of the retrosigmoid approach to acoustic neurinoma. J Neurosurg. 1992 Jun;76(6):901–909. doi: 10.3171/jns.1992.76.6.0901. Available from: http://dx.doi.org/10.3171/jns.1992.76.6.0901. [DOI] [PubMed] [Google Scholar]
- 288.Isaacson B, Kileny PR, El-Kashlan H, Gadre AK. Intraoperative monitoring and facial nerve outcomes after vestibular schwannoma resection. Otol Neurotol. 2003 Sep;24(5):812–817. doi: 10.1097/00129492-200309000-00020. Available from: http://dx.doi.org/10.1097/00129492-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 289.Møller AR, Jannetta PJ. Preservation of facial function during removal of acoustic neuromas. Use of monopolar constant-voltage stimulation and EMG. J Neurosurg. 1984 Oct;61(4):757–760. doi: 10.3171/jns.1984.61.4.0757. Available from: http://dx.doi.org/10.3171/jns.1984.61.4.0757. [DOI] [PubMed] [Google Scholar]
- 290.Romstöck J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebelopontine angle surgery. J Neurosurg. 2000;93:586–593. doi: 10.3171/jns.2000.93.4.0586. Available from: http://dx.doi.org/10.3171/jns.2000.93.4.0586. [DOI] [PubMed] [Google Scholar]
- 291.Silverstein H, Rosenberg SI, Flanzer J, Seidman MD. Intraoperative facial nerve monitoring in acoustic neuroma surgery. Am J Otol. 1993 Nov;14(6):524–532. [PubMed] [Google Scholar]
- 292.Kartush JM, Larouere MJ, Graham MD, Bouchard KR, Audet BV. Intraoperative cranial nerve monitoring during posterior skull base surgery. Skull Base Surg. 1991;1(2):85–92. doi: 10.1055/s-2008-1056986. Available from: http://dx.doi.org/10.1055/s-2008-1056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Marin P, Pouliot D, Fradet G. Facial nerve outcome with a preoperative stimulation threshold under 0.05 mA. Laryngoscope. 2011;121:2295–2298. doi: 10.1002/lary.22359. Available from: http://dx.doi.org/10.1002/lary.22359. [DOI] [PubMed] [Google Scholar]
- 294.Nissen AJ, Sikand A, Curto FS, Welsh JE, Gardi J. Value of intraoperative threshold stimulus in predicting postoperative facial nerve function after acoustic tumor resection. Am J Otol. 1997 Mar;18(2):249–251. [PubMed] [Google Scholar]
- 295.Selesnick SH, Carew JF, Victor JD, Heise CW, Levine J. Predictive value of facial nerve electrophysiologic stimulation thresholds in cerebellopontine-angle surgery. Laryngoscope. 1996 May;106(5 Pt 1):633–638. doi: 10.1097/00005537-199605000-00022. Available from: http://dx.doi.org/10.1097/00005537-199605000-00022. [DOI] [PubMed] [Google Scholar]
- 296.Silverstein H, Willcox TO, Jr, Rosenberg SI, Seidman MD. Prediction of facial nerve function following acoustic neuroma resection using intraoperative facial nerve stimulation. Laryngoscope. 1994 May;104(5 Pt 1):539–544. doi: 10.1002/lary.5541040506. Available from: http://dx.doi.org/10.1002/lary.5541040506. [DOI] [PubMed] [Google Scholar]
- 297.Zeitopuni AG, Hammerschlag PE, Cohen NL. Prognostic significance of intraoperative facial nerve stimulus thresholds. Am J Otol. 1997;18:494–497. [PubMed] [Google Scholar]
- 298.Randall DA, Wester DC, Hunsaker DH. Reliability of disposable intraoperative facial nerve stimulators. Laryngoscope. 1997 Feb;107(2):192–199. doi: 10.1097/00005537-199702000-00010. Available from: http://dx.doi.org/10.1097/00005537-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 299.Silverstein H, White DW. Continuous electrical stimulation as a help adjunct during intraoperative facial nerve monitoring. Skull Base Surg. 1991;1:127–131. doi: 10.1055/s-2008-1056993. Available from: http://dx.doi.org/10.1055/s-2008-1056993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Agnew WF, McCreery DB, Yuen TG, Bullara LA. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection of parameters. Ann Biomed Eng. 1989;17(1):39–60. doi: 10.1007/BF02364272. Available from: http://dx.doi.org/10.1007/BF02364272. [DOI] [PubMed] [Google Scholar]
- 301.Eisele DW, Wang SJ, Orloff LA. Electrophysiologic facial nerve monitoring during parotidectomy. Head Neck. 2010 Mar;32(3):399–405. doi: 10.1002/hed.21190. Available from: http://dx.doi.org/10.1002/hed.21190. [DOI] [PubMed] [Google Scholar]
- 302.Hughes GB, Bottomy MB, Dickins JR, Jackson CG, Sismanis A, Glasscock ME., 3rd A comparative study of neuropathologic changes following pulsed and direct current stimulation of the mouse sciatic nerve. Am J Otolaryngol. 1980 Nov;1(5):378–384. doi: 10.1016/S0196-0709(80)80018-4. Available from: http://dx.doi.org/10.1016/S0196-0709(80)80018-4. [DOI] [PubMed] [Google Scholar]
- 303.McCreery DB, Agnew WF, Yuen TG, Bullara LA. Relationship between stimulus amplitude, stimulus frequency and neural damage during electrical stimulation of sciatic nerve of cat. Med Biol Eng Comput. 1995 May;33(3 Spec No):426–429. doi: 10.1007/BF02510526. [DOI] [PubMed] [Google Scholar]
- 304.Sapmaz E, Kaygusuz I, Alpay HC, Akpolat N, Keles E, Karlidag T, Orhan I, Yalcin S. Histopathologic and functional effects of facial nerve following electrical stimulation. Eur Arch Otorhinolaryngol. 2010 Apr;267(4):607–612. doi: 10.1007/s00405-009-1107-4. Available from: http://dx.doi.org/10.1007/s00405-009-1107-4. [DOI] [PubMed] [Google Scholar]
- 305.Strauss C, Bischoff B, Neu M, Berg M, Fahlbusch R, Romstöck J. Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg. 2001 Nov;95(5):771–777. doi: 10.3171/jns.2001.95.5.0771. Available from: http://dx.doi.org/10.3171/jns.2001.95.5.0771. [DOI] [PubMed] [Google Scholar]
- 306.Dubois MY, Sato S, Chassy J, Macnamara TE. Effects of enflurane on brainstem auditory evoked responses in humans. Anesth Analg. 1982 Nov;61(11):898–902. doi: 10.1213/00000539-198211000-00002. Available from: http://dx.doi.org/10.1213/00000539-198211000-00002. [DOI] [PubMed] [Google Scholar]
- 307.Legatt AD. Mechanisms of intraoperative brainstem auditory evoked potential changes. J Clin Neurophysiol. 2002 Oct;19(5):396–408. doi: 10.1097/00004691-200210000-00003. Available from: http://dx.doi.org/10.1097/00004691-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 308.Lüder H. Surgical monitoring with auditory evoked potentials. J Clin Neurophysiol. 1988;5:261–285. doi: 10.1097/00004691-198807000-00003. Available from: http://dx.doi.org/10.1097/00004691-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 309.Manninen PH, Lam AM, Nicholas JF. The effects of isoflurane and isoflurane-nitrous oxide anesthesia on brainstem auditory evoked potentials in humans. Anesth Analg. 1985 Jan;64(1):43–47. doi: 10.1213/00000539-198501000-00009. Available from: http://dx.doi.org/10.1213/00000539-198501000-00009. [DOI] [PubMed] [Google Scholar]
- 310.Markand ON. Brainstem auditory evoked potentials. J Clin Neurophysiol. 1994 May;11(3):319–342. doi: 10.1097/00004691-199405000-00004. Available from: http://dx.doi.org/10.1097/00004691-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 311.Simon MV. Neurophysiologic intraoperative monitoring of the vestibulocochlear nerve. J Clin Neurophysiol. 2011 Dec;28(6):566–581. doi: 10.1097/WNP.0b013e31823da494. Available from: http://dx.doi.org/10.1097/WNP.0b013e31823da494. [DOI] [PubMed] [Google Scholar]
- 312.Stockard JJ, Sharbrough FW, Tinker JA. Effects of hypothermia on the human brainstem auditory response. Ann Neurol. 1978 Apr;3(4):368–370. doi: 10.1002/ana.410030416. Available from: http://dx.doi.org/10.1002/ana.410030416. [DOI] [PubMed] [Google Scholar]
- 313.Behr R, Schlake HP, Michel O, Wedekind C, Helms J, Stennert E, Roosen K, Klug N. Funktionserhaltende Chirurgie des Akustikusneurinoms – Ergebnisse interdisziplinärer Kooperation. In: Bootz F, Strauss G, editors. Die Chirurgie der lateralen Schädelbasis. Springer-Verlag; 2002. pp. 65–70. Available from: http://dx.doi.org/10.1007/978-3-642-56058-3_17. [DOI] [Google Scholar]
- 314.Schaller B. Die Chirurgie des Kleinhirnbrückenwinkels. Teil 2: Spezielle Bemerkungen. HNO. 2003;51:375–385. doi: 10.1007/s00106-002-0798-2. Available from: http://dx.doi.org/10.1007/s00106-002-0798-2. [DOI] [PubMed] [Google Scholar]
- 315.Arts HA, Telian SA, El-Kashlan H, Thompson BG. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using the middle cranial fossa approach. Otol Neurotol. 2006 Feb;27(2):234–241. doi: 10.1097/01.mao.0000185153.54457.16. Available from: http://dx.doi.org/10.1097/01.mao.0000185153.54457.16. [DOI] [PubMed] [Google Scholar]
- 316.Friedman RA, Kesser B, Brackmann DE, Fisher LM, Slattery WH, Hitselberger WE. Long-term hearing preservation after middle fossa removal of vestibular schwannoma. Otolaryngol Head Neck Surg. 2003 Dec;129(6):660–665. doi: 10.1016/j.otohns.2003.08.002. Available from: http://dx.doi.org/10.1016/j.otohns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 317.Hillman T, Chen DA, Arriaga MA, Quigley M. Facial nerve function and hearing preservation acoustic tumor surgery: does the approach matter? Otolaryngol Head Neck Surg. 2010 Jan;142(1):115–119. doi: 10.1016/j.otohns.2009.10.015. Available from: http://dx.doi.org/10.1016/j.otohns.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 318.Jacob A, Robinson LL, Jr, Bortman JS, Yu L, Dodson EE, Welling DB. Nerve of origin, tumor size, hearing preservation, and facial nerve outcome in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope. 2007;117:2087–2092. doi: 10.1097/MLG.0b013e3181453a07. Available from: http://dx.doi.org/10.1097/MLG.0b013e3181453a07. [DOI] [PubMed] [Google Scholar]
- 319.Kutz JW, Jr, Scoresby T, Isaacson B, Mickey BE, Madden CJ, Barnett SL, Coimbra C, Hynan LS, Roland PS. Hearing preservation using the middle fossa approach for the treatment of vestibular schwannoma. Neurosurgery. 2012;70:334–341. doi: 10.1227/NEU.0b013e31823110f1. Available from: http://dx.doi.org/10.1227/NEU.0b013e31823110f1. [DOI] [PubMed] [Google Scholar]
- 320.Wang YP, Hsu WC, Young YH. Vestibular evoked myogenic potentials in acute acoustic trauma. Otol Neurotol. 2006 Oct;27(7):956–961. doi: 10.1097/01.mao.0000231590.57348.4b. Available from: http://dx.doi.org/10.1097/01.mao.0000231590.57348.4b. [DOI] [PubMed] [Google Scholar]
- 321.Phillips DJ, Kobylarz EJ, De Peralta ET, Stieg PE, Selesnick SH. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010 Dec;31(9):1463–1468. [PubMed] [Google Scholar]
- 322.Sameshima T, Fukushima T, McElveen JT, Jr, Friedman AH. Critical assessment of operative approaches for hearing preservation in small acoustic neuroma surgery: retrosigmoid vs middle fossa approach. Neurosurgery. 2010 Sep;67(3):640–644. doi: 10.1227/01.NEU.0000374853.97891.FB. Available from: http://dx.doi.org/10.1227/01.NEU.0000374853.97891.FB. [DOI] [PubMed] [Google Scholar]
- 323.Satar B, Jackler RK, Oghalai J, Pitts LH, Yates PD. Risk-benefit analysis of using the middle fossa approach for acoustic neuromas with >10 mm cerebellopontine angle component. Laryngoscope. 2002 Aug;112(8 Pt 1):1500–1506. doi: 10.1097/00005537-200208000-00031. Available from: http://dx.doi.org/10.1097/00005537-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 324.Kanzaki J, Ogawa K, Inoue Y, Shiobara R. Hearing preservation surgery in acoustic neuroma patients with normal hearing. Skull Base Surg. 1997;7(3):109–113. doi: 10.1055/s-2008-1058601. Available from: http://dx.doi.org/10.1055/s-2008-1058601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kane NM, Kazanas S, Maw AR, Coakham HB, Torrens MJ, Morgan MH, Stranjalis G, Butler SR. Functional outcome in patients after excision of extracanalicular acoustic neuromas using the suboccipital approach. Ann R Coll Surg Engl. 1995 May;77(3):210–216. [PMC free article] [PubMed] [Google Scholar]
- 326.Hilton CW, Haines SJ, Agrawal A, Levine SC. Late failure rate of hearing preservation after middle fossa approach for resection of vestibular schwannoma. Otol Neurotol. 2011 Jan;32(1):132–135. doi: 10.1097/MAO.0b013e3182001c7d. Available from: http://dx.doi.org/10.1097/MAO.0b013e3182001c7d. [DOI] [PubMed] [Google Scholar]
- 327.Sughrue ME, Yang I, Aranda D, Kane AJ, Parsa AT. Hearing preservation rates after microsurgical resection of vestibular schwannoma. J Clin Neurosci. 2010 Sep;17(9):1126–1129. doi: 10.1016/j.jocn.2010.01.018. Available from: http://dx.doi.org/10.1016/j.jocn.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 328.Rudolf J, Freigang B. Hörvermögen vor, während und nach transtemporaler Akustikusneurinomextirpation. In: Bootz F, Strauuss G, editors. Die Chirurgie der lateralen Schädelbasis. Springer-Verlag; 2002. Springer-Verlag; 2002. pp. 71–75. Available from: http://dx.doi.org/10.1007/978-3-642-56058-3_18. [DOI] [Google Scholar]
- 329.Schick B, Iro H. Aktuelles Management bei Vestibularisschwannomen. In: Biesinger E, Iro H, editors. HNO Praxis heute 27. Springer-Verlag; 2007. pp. 87–97. [Google Scholar]
- 330.Baguley DM, Humphriss RL, Axon PR, Moffat DA. Change in tinnitus handicap after translabyrinthine vestibular schwannoma excision. Otol Neurotol. 2005 Sep;26(5):1061–1063. doi: 10.1097/01.mao.0000185043.54147.3a. Available from: http://dx.doi.org/10.1097/01.mao.0000185043.54147.3a. [DOI] [PubMed] [Google Scholar]
- 331.Kameda K, Shono T, Hashiguchi K, Yoshida F, Sasaki T. Effect of tumor removal on tinnitus in patients with vestibular schwannoma. J Neurosurg. 2010 Jan;112(1):152–157. doi: 10.3171/2009.3.JNS081053. Available from: http://dx.doi.org/10.3171/2009.3.JNS081053. [DOI] [PubMed] [Google Scholar]
- 332.Saman Y, Bamiou DE, Gleeson M. A contemporary review of balance dysfunction following vestibular schwannoma surgery. Laryngoscope. 2009 Nov;119(11):2085–2093. doi: 10.1002/lary.20648. Available from: http://dx.doi.org/10.1002/lary.20648. [DOI] [PubMed] [Google Scholar]
- 333.Driscoll CL, Lynn SG, Harner SG, Beatty CW, Atkinson EJ. Preoperative identification of patients at risk of developing persistent disequilibrium after acoustic neuroma removal. Am J Otol. 1998;19:491–495. [PubMed] [Google Scholar]
- 334.Vereeck L, Wuyts FL, Truijen S, De Valck C, Van de Heyning PH. The effect of early customized vestibular rehabilitation on balance after acoustic neuroma resection. Clin Rehabil. 2008 Aug;22(8):698–713. doi: 10.1177/0269215508089066. Available from: http://dx.doi.org/10.1177/0269215508089066. [DOI] [PubMed] [Google Scholar]
- 335.Magnusson M, Karlberg M, Tjernström F. PREHAB": vestibular prehabilitation to ameliorate the effect of a sudden vestibular loss. Neuro Rehabilitation. 2011;29:153–156. doi: 10.3233/NRE-2011-0689. [DOI] [PubMed] [Google Scholar]
- 336.Tjernström F, Fransson PA, Kahlon B, Karlberg M, Lindberg S, Siesjö P, Magnusson M. Vestibular PREHAB and gentamicin before schwannoma surgery may improve long-term postural function. J Neurol Neurosurg Psychiatr. 2009 Nov;80(11):1254–1260. doi: 10.1136/jnnp.2008.170878. Available from: http://dx.doi.org/10.1136/jnnp.2008.170878. [DOI] [PubMed] [Google Scholar]
- 337.Behari S, Tyagi I, Banerji D, Kumar V, Jaiswal AK, Phadke RV, Jain VK. Postauricular, transpetrous, presigmoid approach for extensive skull base tumors in the petroclival region: the successes and the travails. Acta Neurochir (Wien) 2010 Oct;152(10):1633–1645. doi: 10.1007/s00701-010-0701-y. Available from: http://dx.doi.org/10.1007/s00701-010-0701-y. [DOI] [PubMed] [Google Scholar]
- 338.Schaller B, Probst R, Gratzl O, Rem JA, Hauser R, Tolnay M. Different aspects of hearing preservation in surgery of vestibular schwannoma in women and men. Acta Neurochir (Wien) 1996;138(11):1275–1281. doi: 10.1007/BF01411055. Available from: http://dx.doi.org/10.1007/BF01411055. [DOI] [PubMed] [Google Scholar]
- 339.Magliulo G, D'Amico R, Di Cello P. Delayed facial palsy after vestibular schwannoma resection: clinical data and prognosis. J Otolaryngol. 2003 Dec;32(6):400–404. doi: 10.2310/7070.2003.13968. Available from: http://dx.doi.org/10.2310/7070.2003.13968. [DOI] [PubMed] [Google Scholar]
- 340.Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid appraoch in a series of 200 patients. J Neurosurg. 2006;105:527–535. doi: 10.3171/jns.2006.105.4.527. Available from: http://dx.doi.org/10.3171/jns.2006.105.4.527. [DOI] [PubMed] [Google Scholar]
- 341.Piccirillo E, Wiet MR, Flanagan S, Dispenza F, Giannuzzi A, Mancini F, Sanna M. Cystic vestibular schwannoma: classification, management, and facial nerve outcomes. Otol Neurotol. 2009 Sep;30(6):826–834. doi: 10.1097/MAO.0b013e3181b04e18. Available from: http://dx.doi.org/10.1097/MAO.0b013e3181b04e18. [DOI] [PubMed] [Google Scholar]
- 342.Yashar P, Zada G, Harris B, Giannotta SL. Extent of resection and early postoperative outcomes following removal of cystic vestibular schwannomas: surgical experience over a decade and review of the literature. Nurosurg Focus. 2012;33:E13. doi: 10.3171/2012.7.FOCUS12206. Available from: http://dx.doi.org/10.3171/2012.7.FOCUS12206. [DOI] [PubMed] [Google Scholar]
- 343.Irving RM, Viani L, Hardy DG, Baguley DM, Moffat DA. Nervus intermedius function after vestibular schwannoma removal: clinical features and pathophysiologocal mechanisms. Laryngoscope. 1995;105:809–813. doi: 10.1288/00005537-199508000-00007. Available from: http://dx.doi.org/10.1288/00005537-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 344.Mulhern MG, Aduriz-Lorenzo PM, Rawluk D, Viani L, Eustace P, Logan P. Ocular complications of acoustic neuroma surgery. Br J Ophthalmol. 1999 Dec;83(12):1389–1392. doi: 10.1136/bjo.83.12.1389. Available from: http://dx.doi.org/10.1136/bjo.83.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Farris P, Brown K, Vuitch F, Meyerhoff WL. Middle ear mucocele: an unusual complication of the tranlabyrinthine approach to acoustic neuroma. Skull Base Surg. 1997;7(4):207–210. doi: 10.1055/s-2008-1058597. Available from: http://dx.doi.org/10.1055/s-2008-1058597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Fishman AJ, Marrinan MS, Golfinos JG, Cohen NL, Roland JT., Jr Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope. 2004 Mar;114(3):501–505. doi: 10.1097/00005537-200403000-00022. Available from: http://dx.doi.org/10.1097/00005537-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 347.Friedman RA, Cullen RD, Ulis J, Brackmann DE. Management options of cerebrospinal fluid leaks after acoustic tumour removal. Neurosurgery. 2007;61:35–39. doi: 10.1227/01.neu.0000289709.87802.12. Available from: http://dx.doi.org/10.1227/01.neu.0000289709.87802.12. [DOI] [PubMed] [Google Scholar]
- 348.Selesnick SH, Liu JC, Jen A, Carew JF. Management options for cerebrospinal fluid leak after vestibular schwannoma surgery and introduction of an innovative treatment. Otol Neurotol. 2004 Jul;25(4):580–586. doi: 10.1097/00129492-200407000-00027. Available from: http://dx.doi.org/10.1097/00129492-200407000-00027. [DOI] [PubMed] [Google Scholar]
- 349.Reece AT, O'Reilly B, Teasdale E, Todd NV. Subarachnoid fat embolism complicating autologous fat grafting following translabyrinthine excision of acoustic neuroma. J Laryngol Otol. 1989 Sep;103(9):870–871. doi: 10.1017/S0022215100110345. Available from: http://dx.doi.org/10.1017/S0022215100110345. [DOI] [PubMed] [Google Scholar]
- 350.Carvalho GA, Cervio A, Matthies C, Samii M. Subarachnoid fat dissemination after resection of a cerebellopontine angle dysontogenic cyst: case report and review of the literature. Neurosurgery. 2000;47:760–763. doi: 10.1097/00006123-200009000-00047. [DOI] [PubMed] [Google Scholar]
- 351.Sanchez GB, Kaylie DM, O'Malley MR, Labadie RF, Jackson CG, Haynes DS. Chemical meningitis following cerebellopontine angle tumor surgery. Otolaryngol Head Neck Surg. 2008 Mar;138(3):368–373. doi: 10.1016/j.otohns.2007.10.038. Available from: http://dx.doi.org/10.1016/j.otohns.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 352.Hertel V, Schick B. Diagnostik und Therapie von frontobasalen Liquorfisteln. Laryngo Rhino Otol. 2012;91:585–597. doi: 10.1055/s-0032-1316382. Available from: http://dx.doi.org/10.1055/s-0032-1316382. [DOI] [PubMed] [Google Scholar]
- 353.Mattock C, Crockard A. Does intravascular coagulation contribute to the operative mortality for large acoustic neuromas? J Neurol Neurosurg Psych. 1986;49:699–701. doi: 10.1136/jnnp.49.6.699. Available from: http://dx.doi.org/10.1136/jnnp.49.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wigand ME, Haid T, Berg M, Rettinger G. The enlarged transtemporal approach to the cerebellopontine angle: technique and indications. Acta Otorhinolaryngol Ital. 1982;2:571–582. [Google Scholar]
- 355.Shimada K, Kaneko Y, Sato I, Ezure H, Murakami G. Classification of the ophthalmic artery that arises from the middle meningeal artery in Japanese adults. Okajimas Folia Anat Jpn. 1995 Aug;72(2-3):163–176. doi: 10.2535/ofaj1936.72.2-3_163. [DOI] [PubMed] [Google Scholar]
- 356.Brors D, Schäfers M, Bodmer D, Draf W, Kahle G, Schick B. Postoperative magnetic resonance imaging findings after transtemporal and translabyrinthine vesibular schwannoma resection. Laryngoscope. 2003;113:420–426. doi: 10.1097/00005537-200303000-00006. Available from: http://dx.doi.org/10.1097/00005537-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 357.Minovi A, Mangold R, Kollert M, Hofmann E, Draf W, Bockmühl U. Funktionelle Ergebnisse, Lebensqualität, kognitive und affective Konsequenzen nach transtemporaler Extirpation von Akustikusneurinomen. Laryngorhinootologie. 2005;84:915–920. doi: 10.1055/s-2005-870573. Available from: http://dx.doi.org/10.1055/s-2005-870573. [DOI] [PubMed] [Google Scholar]
- 358.Schick B, Greess H, Gill S, Pauli E, Iro H. Magnetic resonance imaging and neuropsychological testing after middle fossa vestibular schwannoma surgery. Otol Neurotol. 2007;29:39–45. doi: 10.1097/mao.0b013e31815c2ad7. Available from: http://dx.doi.org/10.1097/mao.0b013e31815c2ad7. [DOI] [PubMed] [Google Scholar]
- 359.Thomsen J, Stougaard M, Becker B, Tos M, Jennum P. Middle fossa approach in vestibular schwannoma surgery. Postoperative hearing preservation and EEG changes. Acta Otolaryngol. 2000 Jun;120(4):517–522. doi: 10.1080/000164800750046027. Available from: http://dx.doi.org/10.1080/000164800750046027. [DOI] [PubMed] [Google Scholar]
- 360.Gjurić M, Wigand ME, Wolf SR. Enlarged middle fossa vestibular schwannoma surgery: experience with 735 cases. Otol Neurotol. 2001 Mar;22(2):223–230. doi: 10.1097/00129492-200103000-00019. Available from: http://dx.doi.org/10.1097/00129492-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 361.Aggarwal R, Green KM, Ramsden RT. Epilepsy following middle-fossa extradural retraction: implications for driving. J Laryngol Otol. 2005 Nov;119(11):853–855. doi: 10.1258/002221505774783368. Available from: http://dx.doi.org/10.1258/002221505774783368. [DOI] [PubMed] [Google Scholar]
- 362.Jorgensen B, Pedersen CB. Medical and socio-economic status of patients operated on for acoustic neuroma. In: Tos M, Thomsen J, editors. Acoustic neuroma. Amsterdam/New York: Kugler Publications; 1992. pp. 881–886. [Google Scholar]
- 363.Parving A, Tos M, Thomsen J, Møller H, Buchwald C. Some aspects of life quality after surgery for acoustic neuroma. Arch Otolaryngol Head Neck Surg. 1992 Oct;118(10):1061–1064. doi: 10.1001/archotol.1992.01880100053013. Available from: http://dx.doi.org/10.1001/archotol.1992.01880100053013. [DOI] [PubMed] [Google Scholar]
- 364.Blomstedt GC, Katila H, Henriksson M, Ekholm A, Jääskeläinen JE, Pyykko I. Depression after surgery for acoustic neuroma. J Neurol Neurosurg Psychiatr. 1996 Oct;61(4):403–406. doi: 10.1136/jnnp.61.4.403. Available from: http://dx.doi.org/10.1136/jnnp.61.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Keitner GI, Ryan CE, Miller IW, Kohn R, Epstein NB. 12-month outcome of patients with major depression and comorbid psychiatric or medical illness (compound depression) Am J Psychiatry. 1991 Mar;148(3):345–350. doi: 10.1176/ajp.148.3.345. [DOI] [PubMed] [Google Scholar]
- 366.Mc Kenna L, Hallam RS, Hinchcliffe R. The prevalence of psychological disturbance in neurotology outpatients. Clin Otolaryngol Allied Sci. 1991;16:452–456. doi: 10.1111/j.1365-2273.1991.tb01038.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1991.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 367.Ryzenman JM, Pensak ML, Tew JM., Jr Patient perception of comorbid conditions after acoustic neuroma management: survey results from the acoustic neuroma association. Laryngoscope. 2004 May;114(5):814–820. doi: 10.1097/00005537-200405000-00005. Available from: http://dx.doi.org/10.1097/00005537-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 368.Mercke U, Magnusson M, Linderoth L, Harris S, Sundbarg G. Long-term effect of translabyrinthine acoustic neuroma surgery on work capacity. In: Tos M, Thomsen J, editors. Acoustic neuroma. Amsterdam/New York: Kugler Publications; 1992. pp. 877–880. [Google Scholar]
- 369.Wiegand DA, Fickel V. Acoustic neuroma - the patient's perspective: subjective assessment of symptoms, diagnosis, therapy, and outcome in 541 patients. Laryngoscope. 1989;99:179–187. doi: 10.1288/00005537-198902000-00010. [DOI] [PubMed] [Google Scholar]
- 370.Hardy DG, Moffat DA. Acoustic neuroma surgery: how much morbidity is swept under the carpet. 3rd European Congress of Surgery; 1993; London. [Google Scholar]
- 371.Demetriades AK, Saunders N, Rose P, Fisher C, Rowe J, Tranter R, Hardwidge C. Malignant transformation of acoustic neuroma/vestibular schwannoma 10 years after gamma knife stereotactic radiosurgery. Skull Base. 2010 Sep;20(5):381–387. doi: 10.1055/s-0030-1253576. Available from: http://dx.doi.org/10.1055/s-0030-1253576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kwon Y, Khang SK, Kim CJ, Lee DJ, Lee JK, Kwun BD. Radiologic and histopathologic changes after Gamma Knife radiosurgery for acoustic schwannoma. Stereotact Funct Neurosurg. 1999;72 Suppl 1:2–10. doi: 10.1159/000056433. Available from: http://dx.doi.org/10.1159/000056433. [DOI] [PubMed] [Google Scholar]
- 373.Balasubramaniam A, Shannon P, Hodaie M, Laperriere N, Michaels H, Guha A. Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: case report and review of the literature. Neuro-oncology. 2007 Oct;9(4):447–453. doi: 10.1215/15228517-2007-027. Available from: http://dx.doi.org/10.1215/15228517-2007-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sanna M, Fois P, Pasanisi E, Russo A, Bacciu A. Middle ear and mastoid glomus tumors (glomus tympanicum): an algorithm for the surgical management. Auris Nasus Larynx. 2010 Dec;37(6):661–668. doi: 10.1016/j.anl.2010.03.006. Available from: http://dx.doi.org/10.1016/j.anl.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 375.Weber PC, Patel S. Jugulotympanic paragangliomas. Otolaryngol Clin North Am. 2001 Dec;34(6):1231–40, x. doi: 10.1016/S0030-6665(05)70376-5. Available from: http://dx.doi.org/10.1016/S0030-6665(05)70376-5. [DOI] [PubMed] [Google Scholar]
- 376.Fisch U, Mattox D. Microsurgery of the skull base. Stuttgart, New York: Thieme Verlag; 1988. pp. 148–281. [Google Scholar]
- 377.Schick B, Draf W, Kahle G. Jugulotympanale Paragangliome: Therapiekonzepte in der Entwicklung. Laryngo Rhino Otol. 1998;77:434–443. doi: 10.1055/s-2007-997004. Available from: http://dx.doi.org/10.1055/s-2007-997004. [DOI] [PubMed] [Google Scholar]
- 378.Bickerstaff ER, Howell JS. The neurological importance of tumours of the glomus jugulare. Brain. 1953;76(4):576–593. doi: 10.1093/brain/76.4.576. Available from: http://dx.doi.org/10.1093/brain/76.4.576. [DOI] [PubMed] [Google Scholar]
- 379.Steinberg N, Holz WG. Glomus jugularis tumors. Arch Otolaryngol. 1965 Oct;82(4):387–394. doi: 10.1001/archotol.1965.00760010389009. Available from: http://dx.doi.org/10.1001/archotol.1965.00760010389009. [DOI] [PubMed] [Google Scholar]
- 380.Cece JA, Lawson W, Biller HF, Eden AR, Parisier SC. Complications in the management of large glomus jugulare tumors. Laryngoscope. 1993;97:152–157. doi: 10.1288/00005537-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 381.Poe DS, Jackson G, Glasscock ME, Johnson GD. Long-term results after lateral cranial base surgery. Laryngoscope. 1991 Apr;101(4 Pt 1):372–378. doi: 10.1002/lary.1991.101.4.372. [DOI] [PubMed] [Google Scholar]
- 382.Milewski C. Morphologie und Klinik der Paragangliome im Kopf-Hals-Bereich. HNO. 1993;41:526–531. [PubMed] [Google Scholar]
- 383.Makek M, Franklin DJ, Zhao JC, Fisch U. Neural infiltration of glomus temporale tumors. Amer J Otol. 1990;11:1–5. [PubMed] [Google Scholar]
- 384.Cole JM, Beiler D. Long-term results of treatment for glomus jugulare and glomus vagale tumors with radiotherapy. Laryngoscope. 1994 Dec;104(12):1461–1465. doi: 10.1288/00005537-199412000-00006. Available from: http://dx.doi.org/10.1288/00005537-199412000-00006. [DOI] [PubMed] [Google Scholar]
- 385.Spector GJ, Compagno J, Perez CA, Maisel RH, Ogura JH. Glomus jugulare tumors: effects of radiotherapy. Cancer. 1975 May;35(5):1316–1321. doi: 10.1002/1097-0142(197505)35:5<1316::AID-CNCR2820350511>3.0.CO;2-#. Available from: http://dx.doi.org/10.1002/1097-0142(197505)35:5<1316::AID-CNCR2820350511>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 386.Sanna M, Jain Y, De Donato G, Rohit, Lauda L, Taibah A. Management of jugular paragangliomas: the Gruppo Otologico experience. Otol Neurotol. 2004 Sep;25(5):797–804. doi: 10.1097/00129492-200409000-00025. Available from: http://dx.doi.org/10.1097/00129492-200409000-00025. [DOI] [PubMed] [Google Scholar]
- 387.Green JD, Jr, Brackmann DE, Nguyen CD, Arriaga MA, Telischi FF, De la Cruz A. Surgical management of previously untreated glomus jugulare tumors. Laryngoscope. 1994;104:917–921. doi: 10.1288/00005537-199408000-00001. Available from: http://dx.doi.org/10.1288/00005537-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 388.Jackson CG, McGrew BM, Forest JA, Netterville JL, Hampf CF, Glasscock ME., 3rd Lateral skull base surgery for glomus tumors: long-term control. Otol Neurotol. 2001 May;22(3):377–382. doi: 10.1097/00129492-200105000-00018. Available from: http://dx.doi.org/10.1097/00129492-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 389.Manolidis S, Jackson CG, Von Doersten PG, Pappas D, Glasscock ME. Lateral skull base surgery: the otology group experience. Skull Base Surg. 1997;7(3):129–137. doi: 10.1055/s-2008-1058604. Available from: http://dx.doi.org/10.1055/s-2008-1058604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Moe KS, Li D, Linder TE, Schmid S, Fisch U. An update on the surgical treatment of temporal bone paraganglioma. Skull Base Surg. 1999;9(3):185–194. doi: 10.1055/s-2008-1058145. Available from: http://dx.doi.org/10.1055/s-2008-1058145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.CAPPS FC. Glomus jugulare tumours of the middle ear. J Laryngol Otol. 1952 Jul;66(7):302–314. doi: 10.1017/S002221510004771X. Available from: http://dx.doi.org/10.1017/S002221510004771X. [DOI] [PubMed] [Google Scholar]
- 392.Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978 Nov;92(11):949–967. doi: 10.1017/S0022215100086382. Available from: http://dx.doi.org/10.1017/S0022215100086382. [DOI] [PubMed] [Google Scholar]
- 393.Fisch U. Current surgical treatment of intratemporal facial palsy. Clin Plast Surg. 1979 Jul;6(3):377–388. [PubMed] [Google Scholar]
- 394.Brackman D, Kinney S, Fu K. Glomus tumor: diagnosis and management. Head Neck Surg. 1987 May-Jun;9(5):306–311. doi: 10.1002/hed.2890090511. Available from: http://dx.doi.org/10.1002/hed.2890090511. [DOI] [PubMed] [Google Scholar]
- 395.Jackson CG, Glasscock ME, 3rd, Nissen AJ, Schwaber MK. Glomus tumor surgery: the approach, results, and problems. Otolaryngol Clin North Am. 1982 Nov;15(4):897–916. [PubMed] [Google Scholar]
- 396.Glasscock ME, 3rd, Harris PF, Newsome G. Glomus tumors: diagnosis and treatment. Laryngoscope. 1974;84:2006–2032. doi: 10.1002/lary.5540841116. [DOI] [PubMed] [Google Scholar]
- 397.Al-Mefty O, Teixeira A. Complex tumors of the glomus jugulare: criteria, treatment, and outcome. J Neurosurg. 2002 Dec;97(6):1356–1366. doi: 10.3171/jns.2002.97.6.1356. Available from: http://dx.doi.org/10.3171/jns.2002.97.6.1356. [DOI] [PubMed] [Google Scholar]
- 398.Brackmann DE. The facial nerve in the infratemporal approach. Otolaryngol Head Neck Surg. 1987 Jul;97(1):15–17. doi: 10.1177/019459988709700103. [DOI] [PubMed] [Google Scholar]
- 399.Leonetti JP, Brackmann DE, Prass RL. Improved preservation of facial nerve function in the infratemporal approach to the skull base. Otolaryngol Head Neck Surg. 1989 Jul;101(1):74–78. doi: 10.1177/019459988910100112. [DOI] [PubMed] [Google Scholar]
- 400.House WF, Hitselberger WE. The transcochlear approach to the skull base. Arch Otolaryngol. 1976 Jun;102(6):334–342. doi: 10.1001/archotol.1976.00780110046004. Available from: http://dx.doi.org/10.1001/archotol.1976.00780110046004. [DOI] [PubMed] [Google Scholar]
- 401.Liu JK, Sameshima T, Gottfried ON, Couldwell WT, Fukushima T. The combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach for resection of glomus jugulare tumors. Neurosurgery. 2006 Jul;59(1 Suppl 1):ONS115–ONS125. doi: 10.1227/01.NEU.0000220025.81500.8D. Available from: http://dx.doi.org/10.1227/01.NEU.0000220025.81500.8D. [DOI] [PubMed] [Google Scholar]
- 402.Fisch U. Infratemporal fossa approach for glomus tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1982 Sep-Oct;91(5 Pt 1):474–479. doi: 10.1177/000348948209100502. [DOI] [PubMed] [Google Scholar]
- 403.Russo A, Piccirillo E, De Donato G, Agarwal M, Sanna M. Anterior and Posterior Facial Nerve Rerouting: A Comparative Study. Skull Base. 2003 Aug;13(3):123–130. doi: 10.1055/s-2003-43322. Available from: http://dx.doi.org/10.1055/s-2003-43322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Borba LAB, Araújo JC, De Oliveira JG, Filho MG, Moro MS, Tirapelli LF, Colli BO. Surgical management of glomus jugulare tumors: a proposal for approach selection based on tumor relationships with the facial nerve. J Neurosurg. 2010;112:88–98. doi: 10.3171/2008.10.JNS08612. Available from: http://dx.doi.org/10.3171/2008.10.JNS08612. [DOI] [PubMed] [Google Scholar]
- 405.Draf W. Rehabilitation des gelähmten Gesichts. In: Naumann HH, Helsm J, Herberhold C, Jahrsdoerfer RA, Lastenbauer ER, Panje WR, Tardy ME Jr, editors. Kopf- und Hals-Chirurgie. Band 1: Gesicht, Nase und Gesichtsschädel, Teil II. 1995. pp. 831–886. [Google Scholar]
- 406.Jackson CG, Kaylie DM, Coppit G, Gardner EK. Glomus jugulare tumors with intracranial extension. Neurosurg Focus. 2004 Aug;17(2):E7. doi: 10.3171/foc.2004.17.2.7. Available from: http://dx.doi.org/10.3171/foc.2004.17.2.7. [DOI] [PubMed] [Google Scholar]
- 407.Murphy TP, Brackmann DE. Effects of preoperative embolization on glomus jugulare tumors. Laryngoscope. 1989 Dec;99(12):1244–1247. doi: 10.1288/00005537-198912000-00007. Available from: http://dx.doi.org/10.1288/00005537-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 408.Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986 Sep-Oct;7(5):943–952. [PMC free article] [PubMed] [Google Scholar]
- 409.Gruber A, Bavinzski G, Killer M, Richling B. Preoperative embolization of hypervascular skull base tumors. Minim Invasive Neurosurg. 2000 Jun;43(2):62–71. doi: 10.1055/s-2000-8321. Available from: http://dx.doi.org/10.1055/s-2000-8321. [DOI] [PubMed] [Google Scholar]
- 410.Gartrell BC, Hansen MR, Gantz BJ, Gluth MB, Mowry SE, Aagaard-Kienitz BL, Baskaya MK, Gubbels SP. Facial and lower cranial neuropathies after preoperative embolization of jugular foramen lesions with ethylene vinyl alcohol. Otol Neurotol. 2012 Sep;33(7):1270–1275. doi: 10.1097/MAO.0b013e31825f2365. Available from: http://dx.doi.org/10.1097/MAO.0b013e31825f2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Herdman RC, Gillespie JE, Ramsden RT. Facial palsy after glomus tumour embolization. J Laryngol Otol. 1993 Oct;107(10):963–966. doi: 10.1017/S0022215100124934. Available from: http://dx.doi.org/10.1017/S0022215100124934. [DOI] [PubMed] [Google Scholar]
- 412.Marangos NM, Schumacher M. Facial palsy after glomus jugulare tumor embolization. J Laryngol Otol. 1999;113:268–270. doi: 10.1017/S0022215100143762. Available from: http://dx.doi.org/10.1017/S0022215100143762. [DOI] [PubMed] [Google Scholar]
- 413.Ozanne A, Pereira V, Krings T, Toulgoat F, Lasjaunias P. Arterial vascularization of the cranial nerves. Neuroimaging Clin N Am. 2008 May;18(2):431–9, xii. doi: 10.1016/j.nic.2007.12.010. Available from: http://dx.doi.org/10.1016/j.nic.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 414.Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, Young WF Jr. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001 Nov;86(11):5210–5216. doi: 10.1210/jc.86.11.5210. Available from: http://dx.doi.org/10.1210/jc.86.11.5210. [DOI] [PubMed] [Google Scholar]
- 415.Graillon T, Fuentes S, Régis J, Metellus P, Brunel H, Roche PH, Dufour H. Multidisciplinary management of giant functional petrous bone paraganglioma. Acta Neurochir (Wien) 2011 Jan;153(1):85–89. doi: 10.1007/s00701-010-0818-z. Available from: http://dx.doi.org/10.1007/s00701-010-0818-z. [DOI] [PubMed] [Google Scholar]
- 416.Gold SR, Kamerer DB Jr. Preservation of conductive hearing in approaches to tumors of the jugular foramen. Otol Neurotol. 2006 Dec;27(8):1126–1130. doi: 10.1097/01.mao.0000235965.07733.3f. Available from: http://dx.doi.org/10.1097/01.mao.0000235965.07733.3f. [DOI] [PubMed] [Google Scholar]
- 417.Briner HH, Linder TE, Pauw B, Fisch U. Long-term results of surgery for temporal bone paragangliomas. Laryngoscope. 1999;109:577–583. doi: 10.1097/00005537-199904000-00011. Available from: http://dx.doi.org/10.1097/00005537-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 418.Danesi G, Mazzoni A, Pareschi R, Zappone C, Sanna M. Results of surgery for C-class glomus tumors of the temporal bone: treatment options. In: Mazzoni A, Sanna M, editors. Skull base surgery – Update. Amsterdam, New York: Kugler publications; 1995. pp. 301–304. [Google Scholar]
- 419.Anand VK, Leonetti JP, al-Mefty O. Neurovascular considerations in surgery of glomus tumors with intracranial extensions. Laryngoscope. 1993 Jul;103(7):722–728. doi: 10.1288/00005537-199307000-00003. Available from: http://dx.doi.org/10.1288/00005537-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 420.Woods CI, Strasnick B, Jackson CG. Surgery for glomus tumors: the Otology Group experience. Laryngoscope. 1993 Nov;103(11 Pt 2 Suppl 60):65–70. doi: 10.1002/lary.1993.103.s60.65. [DOI] [PubMed] [Google Scholar]
- 421.O'Leary MJ, Shelton C, Giddings NA, Kwartler J, Brackmann DE. Glomus tympanicum tumors: a clinical perspective. Laryngoscope. 1991 Oct;101(10):1038–1043. doi: 10.1288/00005537-199110000-00002. [DOI] [PubMed] [Google Scholar]