Abstract
APRIL (A Proliferation-Inducing Ligand, TNFSF13) is a member of the tumor necrosis factor superfamily that regulates lymphocyte survival and activation and has been implicated in tumorigenesis and autoimmune diseases. Here we report the expression and first known activity of APRIL in the nervous system. APRIL and one of its receptors, BCMA (B-Cell Maturation Antigen, TNFRSF17), are expressed by hippocampal pyramidal cells of fetal and postnatal mice. In culture, these neurons secreted APRIL, and function-blocking antibodies to either APRIL or BCMA reduced axonal elongation. Recombinant APRIL enhanced axonal elongation, but did not influence dendrite elongation. The effect of APRIL on axon elongation was inhibited by anti-BCMA and the expression of a signaling-defective BCMA mutant in these neurons, suggesting that the axon growth-promoting effect of APRIL is mediated by BCMA. APRIL promoted phosphorylation and activation of ERK1, ERK2 and Akt and serine phosphorylation and inactivation of GSK-3β in cultured hippocampal pyramidal cells. Inhibition of MEK1/MEK2 (activators of ERK1/ERK2), PI3-kinase (activator of Akt) or Akt inhibited the axon growth-promoting action of APRIL, as did pharmacological activation of GSK-3β and the expression of a constitutively active form of GSK-3β. These findings suggest that APRIL promotes axon elongation by a mechanism that depends both on ERK signaling and PI3-kinase/Akt/GSK-3β signaling.
Keywords: Hippocampal pyramidal cell, Axon, Tumor necrosis factor superfamily, Tumor necrosis factor receptor superfamily
Introduction
The 19 members of the tumor necrosis factor superfamily (TNFSF) are best understood for their many critical functions in the development and function of the immune system, but are increasingly recognized as having diverse roles in other tissues and organs (Hehlgans and Pfeffer, 2005). These cytokines are type II transmembrane glycoproteins that are generally active both as membrane-bound ligands and as soluble ligands following cleavage from the cell membrane by the action of specific metalloproteinases. TNFSF ligands bind to one or more members of the TNF receptor superfamily (TNFRSF) (Grivennikov et al., 2006).
Recent work has shown that several TNFSF proteins are able to modulate neurite growth from developing neurons. For example, in certain neurons, soluble FasL and GITRL enhance neurite growth (Desbarats et al., 2003; O'Keeffe et al., 2008; Zuliani et al., 2006) whereas soluble LIGHT, TNF and RANKL reduce neurite growth (Gavalda et al., 2009; Gutierrez et al., 2008, 2013; Neumann et al., 2002). Reverse signaling via membrane integrated TNF has also recently been shown to promote sympathetic axon growth and tissue innervation (Kisiswa et al., 2013).
APRIL (A Proliferation-Inducing Ligand, TNFSF13) is a member of the TNFSF that was first characterized for its ability to promote tumor growth (Hahne et al., 1998), but is best characterized for its role in regulating lymphocyte survival and activation (Schneider, 2005). APRIL has also been implicated in the etiology and/or maintenance of a variety of autoimmune diseases (Dillon et al., 2006). In addition to its synthesis by a variety of cells of the immune system (Craxton et al., 2003; Litinskiy et al., 2002; Schwaller et al., 2007), APRIL has also been detected in adipocytes, keratinocytes and osteoclasts (Alexaki et al., 2009; Hemingway et al., 2011; Pelekanou et al., 2008). APRIL is unusual among the TNFSF in not being expressed at the cell surface as a membrane-anchored protein, but is processed in the Golgi apparatus by a furin-convertase enzyme to generate a biologically active, secreted protein (Lopez-Fraga et al., 2001).
APRIL binds two TNFRSF members, BCMA (B-Cell Maturation Antigen, TNFRSF17) and TACI (Transmembrane Activator and Cyclophilin Ligand, TNFRSF13B) (Bossen and Schneider, 2006), and can also bind to the proteoglycan syndecan-1 (Hendriks et al., 2005; Ingold et al., 2005). A closely related TNFSF member, BAFF (B-cell activating factor, TNFSF13B) also binds to BCMA and TACI and to another TNFRSF member BAFFR (BAFF receptor, TNFRSF13C) (Vincent et al., 2013). However, whereas APRIL binds strongly to BCMA and moderately to TACI, BAFF binds weakly to BCMA and strongly to TACI and BAFFR (Day et al., 2005).
Expression of APRIL in the nervous system has not previously been reported and APRIL has no known function in the developing or mature nervous system. As part of our ongoing investigation of the potential roles of members of the TNFSF in neural development, an expression screen revealed the presence of APRIL in the developing hippocampus, which was the starting point of the studies reported here. We focused on the potential effect of APRIL on the development of hippocampal pyramidal neurons, not least because these neurons are easily isolated and studied in vitro and are among the most extensively characterized models for investigating the differentiation and growth of axons and dendrites in the developing mammalian central nervous system (Bradke and Dotti, 2000; Dotti et al., 1988; Kaech and Banker, 2006; Kaech et al., 2012; Spruston, 2008). These large excitatory neurons receive very large numbers of excitatory and inhibitory synaptic inputs and project to neurons within and beyond the hippocampus (Piskorowski and Chevaleyre, 2012). In rodents, they are generated during embryonic development and extend axons and elaborate dendrites during late fetal and early postnatal development (Danglot et al., 2006). We find that APRIL selectively enhances the growth of axons from these neurons by BCMA-dependent activation of the ERK and PI3-kinase/Akt signaling pathways. This is the first reported activity for APRIL in the nervous system.
Results
APRIL and BCMA are expressed in the developing hippocampus
We began our investigation of the potential functions of APRIL in neural development by defining when and where APRIL is expressed in the developing hippocampus and whether either of its two receptors is expressed. We used qPCR to quantify the relative levels of APRIL, BCMA and TACI mRNAs in whole hippocampi dissected from E18, P0, P5 and P10 mice. Both APRIL and BCMA mRNAs were clearly detected throughout this period (Fig. 1A and B), but TACI mRNA was barely detectable (not shown). Whereas the level of BCMA mRNA increased significantly over this period (P < 0.0001, E18 versus P10, one-way ANOVA), there was no significant change in the level of APRIL mRNA (P > 0.05, E18 versus P10, one-way ANOVA).
Fig. 1.
Expression of APRIL and BCMA in the developing hippocampus. Graphs of the levels of APRIL mRNA (A) and BCMA mRNA (B) relative to reference mRNAs for GAPDH and SDHA in total RNA extracted from E18, P0, P5 and P10 hippocampi (mean ± s.e.m., n = 4 separate sets of hippocampal tissue at each age). Representative Western blots of lysates of E18, P0, P5, and P10 hippocampi probed for GAPDH together with either APRIL (C) or BCMA (D). Lysates from spleen tissue were probed as positive control.
Western analysis revealed bands corresponding to the pro and mature forms of APRIL (Fig. 1C) and BCMA (Fig. 1D) in lysates of hippocampi dissected from E18, P0, P5 and P10 mice. Bands of the same sizes were present in lysates of adult spleen used as positive control tissue (Aggarwal, 2003). In accordance with the qPCR data, TACI protein was barely detectable in lysates of cultured hippocampal neurons (not shown).
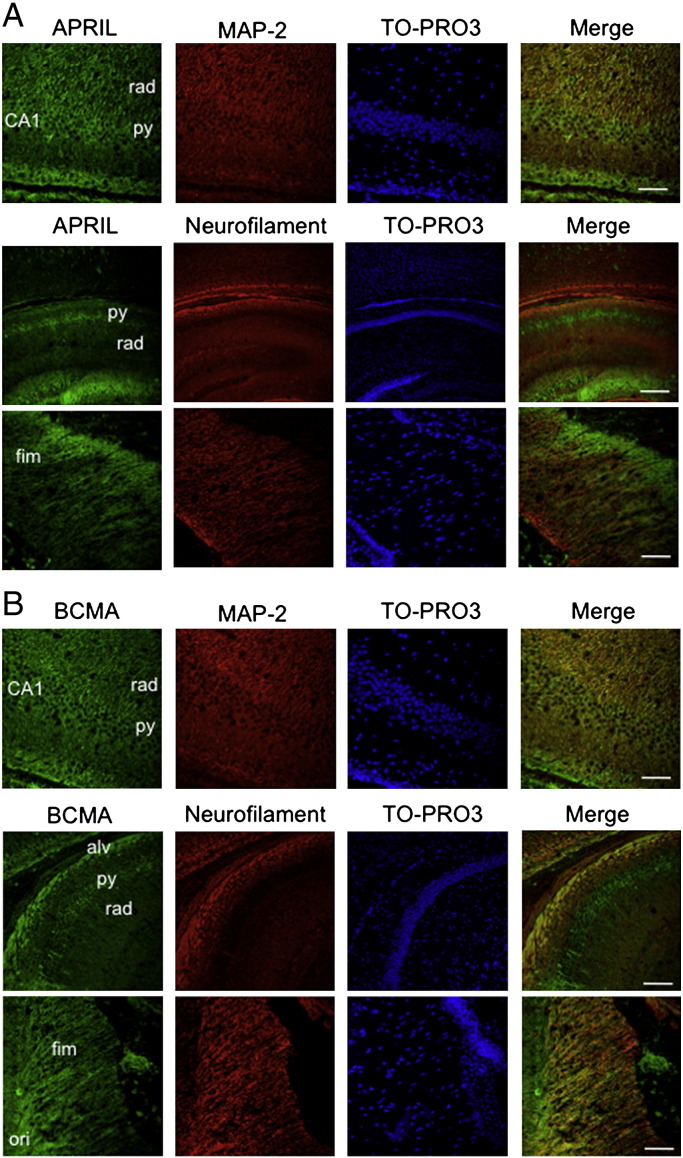
To clarify which kind of cells express APRIL and BCMA in the developing hippocampus and their cellular distribution, we localized these proteins by immunohistochemistry in hippocampal sections using the same specific antibodies that recognized these proteins in the above Western analysis. The sections were triple stained with the nuclear marker TO-PRO®-3 Iodide, either the dendrite marker anti-MAP2 or the axon marker anti-neurofilament and either anti-APRIL (Fig. 2A) or anti-BCMA (Fig. 2B). In sections of the hippocampus at P10, prominent APRIL and BCMA labeling was evident in the pyramidal cell layers of the CA1, CA2 and CA3 fields. High power images of CA1 show labeling of the cell bodies of the pyramidal cells and particularly prominent staining in the stratum radiatum, which consists mostly of the dendrites of pyramidal cells that are double labeled with anti-MAP2 antibodies. Sections through the fimbria and stratum oriens clearly revealed anti-BCMA labeled fibers that are doubled with anti-neurofilament antibodies and correspond in part to pyramidal cell axons that project to the subiculum and lateral entorhinal cortex. Very similar patterns for APRIL and BCMA were observed in hippocampal sections at E18, P0 and P5 (not shown). Sections were unlabeled by secondary antibodies alone and were unstained by anti-TACI antibodies (not shown).
Fig. 2.
Localization of APRIL and BCMA in the developing hippocampus. Frozen sections of P10 hippocampus triple labeled with TO-PRO®-3 Iodide, anti-MAP-2 or anti-200 kD neurofilament and either (A) anti-APRIL (B) or anti-BCMA. For each set, the upper panels show a higher power image of CA1 (scale bars = 25 μm), the middle panels show lower power images of the CA regions (scale bars = 200 μm) and the lower panel show a higher power image of the fimbria (scale bars = 25 μm). Abbreviations: rad, stratum radiatum; py, stratum pyramidale; fim, fimbria; alv, alveus; ori, stratum oriens.
APRIL promotes the growth of axons but not dendrites from cultured pyramidal cells
To investigate the potential role of APRIL in hippocampal neuron development, we established dissociated cultures from E18 mouse hippocampi, a stage at which the predominant neuron type in these cultures is the pyramidal cell. In agreement with others (Kaech and Banker, 2006; Kaech et al., 2012), the overwhelming majority of cells in these cultures are neurons (98.52% ± 0.22 of the cells are stained with anti-MAP-2 and only 1.48% ± 0.22 of the cells stain with anti-GFAP, which recognizes glial cells).
We examined the effect of APRIL on axon growth and dendrite growth separately. To investigate whether APRIL affects axon growth, the neurons were transfected with a green fluorescent protein expression plasmid 2 days after plating and were treated with APRIL for 18 h prior to fixation and immunostaining for GFP. The axons of APRIL-treated neurons were clearly longer than those in control cultures (Fig. 3A, B and C). Measurement of axon length revealed highly significant increases in length in cultures supplemented with APRIL compared with control cultures (Fig. 3B) at APRIL concentrations of 10 ng/ml and greater. Based on this dose response analysis, we used 100 ng/ml APRIL in subsequent experiments. Neurons corresponding to the 100th, 75th, 50th and 25th percentiles in terms of axon length for control and APRIL-treated cultures are illustrated in Fig. 3C. In addition to enhancing axonal extension from pyramidal cells in dissociated cultures, APRIL also clearly increased the density and extent or neurite outgrowth from E18 CA1 hippocampal explants after 3 days in culture (Fig. 3D).
Fig. 3.
APRIL selectively enhances axonal growth from cultured hippocampal pyramidal neurons. (A–C) Axonal growth from E18 hippocampal pyramidal neurons after 3 days in vitro. The neurons were transfected 2 days after plating with a plasmid expressing GFP and were treated with APRIL for the last 18 h of culture prior to fixation and immunostaining for GFP. (A) Representative neurons incubated without APRIL (Control) or treated with 100 ng/ml APRIL. Scale bar = 100 μm. (B) Axon length in control cultures and cultures treated with APRIL at the concentrations indicated. (C) Camera lucida drawings of control neurons and neurons treated with 100 ng/ml APRIL. The neurons illustrated correspond to the 100th, 75th, 50th and 25th percentiles in terms of total neurite length for each condition. (D) Representative E18 CA1 explants incubated with and without APRIL (100 ng/ml) for 3 days before labeling the emergent neurite with the fluorescent vital dye calcein AM. Scale bar = 200 μm. (E–F) Dendrite growth from E18 hippocampal pyramidal neurons after 7 days in vitro. The neurons were transfected with pGFP 6 days after plating and were incubated with and without APRIL for the last 18 h of culture prior to fixation and immunostaining for GFP. (E) Representative neurons incubated without APRIL (Control) or treated with 100 ng/ml APRIL. Scale bar = 50 μm. (F) Bar chart of the percentage of dendrites longer than 50 μm in control cultures and cultures treated with APRIL at the concentrations indicated. The data shown in B and F represent the mean ± s.e.m. of data compiled from > 150 neurons per condition from at least three separate experiments (*** indicates P < 0.0001, statistical comparison with control, Mann–Whitney U test).
Initial axon growth from pyramidal neurons was unaffected by APRIL. There were no significant differences in axon length between control cultures and those treated with APRIL at the time of plating after 24 h of incubation (57.96 ± 1.75 μm versus 56.74 ± 2.12 μm, mean ± sem) and 48 h of incubation (134.80 ± 10.42 μm versus 135.12 ± 11.01 μm, mean ± sem). This initial lack of dependence on APRIL is akin to the initial independence of axon extension from newly differentiated PNS neurons to neurotrophins (Davies, 1989).
After 7 days in culture, MAP-2-positive dendrites are well-developed (Kaech and Banker, 2006; Kaech et al., 2012). To investigate whether APRIL affects dendrite growth, neurons were transfected with pGFP 6 days after plating, treated with APRIL over a range of concentrations and immunostained for GFP 18 h later. The dendrite arbors appeared similar in APRIL-treated and control cultures (Fig. 3E). Quantification of the percentage of dendrites longer than 50 μm revealed that APRIL at concentrations ranging from 10 to 1000 ng/ml caused no significant increases in dendrite elongation (Fig. 3F). Taken together, these in vitro studies suggest that APRIL is able to selectively enhance axon growth, but not dendrite growth, from developing hippocampal pyramidal cells.
BCMA is required for APRIL-promoted axon growth
Although BCMA is clearly expressed by pyramidal cells and TACI is barely detectable, we carried out experiments to formally demonstrate that the effects of APRIL on axon growth are mediated by BCMA by manipulating the function of this receptor in hippocampal neurons cultured with and without APRIL. To do this, we transfected 2-day cultures of E18 neurons with a plasmid vector in which separate promoters drive the expression of GFP and full-length or truncated BCMA proteins. After transfection, the medium was changed to a medium with or without APRIL and the cultures were fixed and immunostained for GFP 18 h later. The extent of axon growth from neurons overexpressing full-length BCMA and grown in the absence of APRIL was comparable to that from neurons treated with APRIL, and the extent of axon growth from BCMA-overexpressing neurons was not further enhanced by APRIL (Fig. 4A and B). The extent of axon growth from neurons expressing a signaling-defective BCMA mutant that is truncated at the 83 residue in the C-terminal (BCMA ΔC83) (Yang et al., 2005) was significantly reduced compared with control cultures, and the expression of truncated BCMA prevented APRIL from promoting axonal elongation. These results suggest that the axon growth-enhancing effect of APRIL is mediated by BCMA.
Fig. 4.
BCMA mediates APRIL-promoted axonal outgrowth. (A) Representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with plasmids expressing either GFP alone or GFP together with either wild-type BCMA or truncated BCMA (BCMA ΔC86). After transfection, the neurons were cultured for a further 18 h with and without APRIL (100 ng/ml) before GFP immunostaining. Scale bar = 100 μm. (B) Bar chart of axonal length under the experimental conditions illustrated in A. (C) Bar chart of axonal length of neurons cultured in the presence or absence of 3 μg/ml of anti-BCMA or 1.5 μg/ml of anti-APRIL with or without APRIL (100 ng/ml). The mean ± s.e.m. of data obtained from > 150 neurons per condition compiled from at least three separate experiments are shown (*** indicates P < 0.0001, statistical comparison with control, Mann–Whitney U test).
The significant reduction in axon elongation from pyramidal neurons expressing BCMA ΔC83 could be due to an effect of BCMA on axon growth independently of APRIL. However, the immunocytochemical demonstration of APRIL in pyramidal cells raised the possibility that these cells secrete APRIL in culture which could activate BCMA. To test this possibility, we used ELISA to estimate the level of APRIL in culture medium conditioned by pyramidal cells. After 3 days, the concentration of APRIL in the medium was 1.55 ± 0.20 ng/ml (mean ± s.e.m., n = 3 separate culture experiments), suggesting that these cells secrete APRIL in culture.
To test whether BCMA receptor activation by secreted APRIL affects the extent of axon growth, we compared the axon length in cultures treated with function-blocking antibodies to either anti-BCMA or anti-APRIL or IgG obtained from the same species in which the antibodies were raised. In these experiments, E18 hippocampal pyramidal cells were initially cultured for 2 days prior to transfection with pGFP and were treated with either of the function-blocking antibodies or control IgG in the presence or absence of APRIL for 18 h prior to fixation and immunostaining for GFP. Anti-BCMA treatment caused a highly significant reduction in axon length compared with IgG-treated cultures (Fig. 4C), and whereas anti-APRIL reduced axon length, this reduction did not quite reach significance (P = 0.068). However, in cultures in which anti-APRIL was added at the time of plating, axon length was significantly shorter than in the corresponding IgG-treated control cultures (828.7 ± 48.8 m versus 980.5 ± 54.3 m, mean ± s.e.m., n = 150 neurons per condition from 3 separate experiments, p < 0.05). The anti-BCMA and anti-APRIL antibodies completely inhibited the effects of exogenous APRIL on axonal growth, indeed axon length in these cultures was significantly less than in IgG-treated cultures (Fig. 4C). Taken together, these results suggest that APRIL secreted by hippocampal pyramidal cells significantly enhanced axonal growth from these neurons in culture.
APRIL enhances axonal growth by activating ERK1 and ERK2
To elucidate the molecular mechanism underlying the enhancement of axon growth by APRIL, we explored common links in intracellular signaling between the control of neurite growth and APRIL signaling in the immune system. The MEK/ERK pathway has been shown to be activated by APRIL in myeloma cells (Moreaux et al., 2004) and adipose-derived stem cells (Zonca et al., 2012) and by NGF in PC12 cells and sympathetic neurons where it contributes to the neurite growth response to neurotrophins (Atwal et al., 2000; Gomez and Cohen, 1991; Goold and Gordon-Weeks, 2005; O'Keeffe et al., 2008; Thompson et al., 2004).
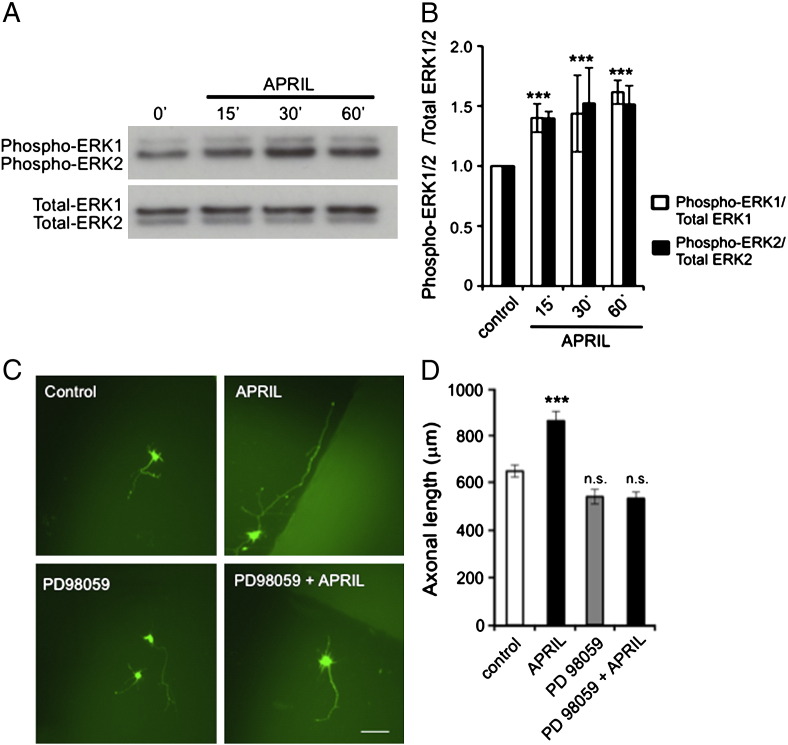
To investigate whether MEK/ERK signaling in the MAP kinase cascade contributes to APRIL-promoted axon elongation from hippocampal pyramidal cells, we first tested whether APRIL activates ERK1 and ERK2 in these neurons. In these experiments, we initially cultured E18 pyramidal cells for 3 days before treating them with APRIL. This treatment caused rapid increases in the levels of phospho-ERK1 and phospho-ERK2 within 15 min that remained elevated for at least 60 min (Fig. 5A and B), demonstrating that APRIL causes rapid ERK1/ERK2 activation in the pyramidal cells.
Fig. 5.
APRIL promotes axonal growth by activating ERK1 and ERK2. (A) Representative Western blots probed for phospho-ERK1/ERK2 and total ERK1/ERK2 of lysates of E18 hippocampal neurons of treated with 100 ng/ml APRIL for the times indicated after first culturing the neurons for 3 days. (B) Densitometric quantification of levels of phospho-ERK1/phospho-ERK2 relative to total ERK1/total ERK2 from 3 separate Western blot experiments (mean ± s.e.m., *** indicates P < 0.0001, statistical comparison with control, Mann–Whitney U test). (C) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with a plasmid expressing GFP and were treated with 10 μM of PD98059 2 h prior to incubation for 18 h with and without APRIL (100 ng/ml) before GFP immunostaining. Separate cultures were treated with APRIL after transfection and control cultures received neither PD98059 nor APRIL. Scale bar = 100 μm. (D) Bar chart of the axonal growth under the experimental conditions illustrated in C. The mean ± s.e.m. of data obtained from > 150 neurons per condition compiled from at least three separate experiments are shown (*** indicates P < 0.0001 and n.s., non-significant, statistical comparison with control, Mann–Whitney U test).
To ascertain if activation of ERK1 and ERK2 contributes to APRIL-promoted axon growth, we examined whether PD98059, a selective inhibitor of MEK1 and MEK2, the kinases that phosphorylate and activate ERK1 and ERK2 (Touyz et al., 2002), could prevent the APRIL-promoted axon growth. In these experiments, the neurons were transfected with a GFP plasmid 2 days after plating and were then pretreated for 2 h with PD98059 before adding APRIL and quantifying axon length 18 h later. Axons were significantly longer in cultures treated with APRIL alone compared with control cultures, and whereas axon length in cultures that received PD98059 alone was not significantly different from that in control cultures, PD98059 pretreatment prevented the axon growth-enhancing effect of APRIL (Fig. 5C and D). This suggests that MEK/ERK signaling plays a crucial role in mediating the effect of APRIL on axon growth from developing pyramidal cells.
APRIL enhances axonal growth by activating PI-3 kinase/Akt signaling
Another common link in intracellular signaling between neurite growth and APRIL signaling in the immune system is the phosphatidylinositol 3-kinase (PI-3 kinase)/Akt (also known as protein kinase B, PKB) pathway. Class I PI-3 kinases are activated by several kinds of cell surface receptors, including receptor tyrosine kinases, such as the Trk family of neurotrophin receptors. The subsequent production of phosphatidylinositol 3,4,5 triphosphates activates the serine/threonine Akt kinases, which contribute to the neurite growth-promoting effects of neurotrophins (Atwal et al., 2000; Cosker and Eickholt, 2007; Kuruvilla et al., 2000; Read and Gorman, 2009). APRIL activates PI-3 kinase in myeloma cells (Moreaux et al., 2004), B-cell lymphoma (Gupta et al., 2009) and human adipose-derived stem cells (Zonca et al., 2012).
To test whether PI-3 kinase signaling contributes to APRIL-promoted axon growth, we first examined whether the selective PI-3 kinase inhibitor LY294002 (Vlahos et al., 1994) was able to prevent the APRIL-promoted axon elongation. In these experiments, the neurons were transfected with a GFP plasmid 2 days after plating and were pretreated for 2 h with LY294002 before adding APRIL to the culture medium and quantifying axon length 18 h later. Whereas axon length in cultures that received LY294002 alone was not significantly different from that in control cultures, LY294002 completely prevented the axon growth-enhancing effect of APRIL (Fig. 6A and B). To confirm the results of these pharmacological experiments, we transfected pyramidal cells with a construct that expresses both GFP and a dominant-negative form of the p85α regulatory subunit of PI-3 kinase that lacks the binding domain for the p110 catalytic subunit. The p85α mutant had a small, significant effect on axon extension from neurons not exposed to exogenous APRIL, and completely eliminated the axon growth-enhancing effect of exogenous APRIL (Fig. 6C and D). Taken together, these results suggest that PI3-kinase signaling is required for the effect of APRIL on axon growth.
Fig. 6.
APRIL-promoted axonal growth depends on PI-3 kinase activation. (A) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with a plasmid expressing GFP and were treated with 10 μM of LY294002 2 h prior to a further 18 h of incubation with and without APRIL (100 ng/ml) before GFP immunostaining. Separate cultures were treated with APRIL after transfection and control cultures received neither LY294002 nor APRIL. (B) Bar chart of the axonal growth under the experimental conditions illustrated in A. (C) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with plasmids expressing either GFP alone or GFP together with a dominant-negative form of p85α regulatory subunit of PI-3 kinase (p85α-DN). After transfection, the neurons were cultured for a further 18 h with and without APRIL (100 ng/ml) before GFP immunostaining. (D) Bar chart of the axonal growth under the experimental conditions illustrated in C. The mean ± s.e.m. of data obtained from > 150 neurons per condition compiled from at least three separate experiments are shown in B and D (** indicates P < 0.001, *** indicates P < 0.0001 and n.s., non-significant, statistical comparison with control, Mann–Whitney U test). Scale bars = 100 μm.
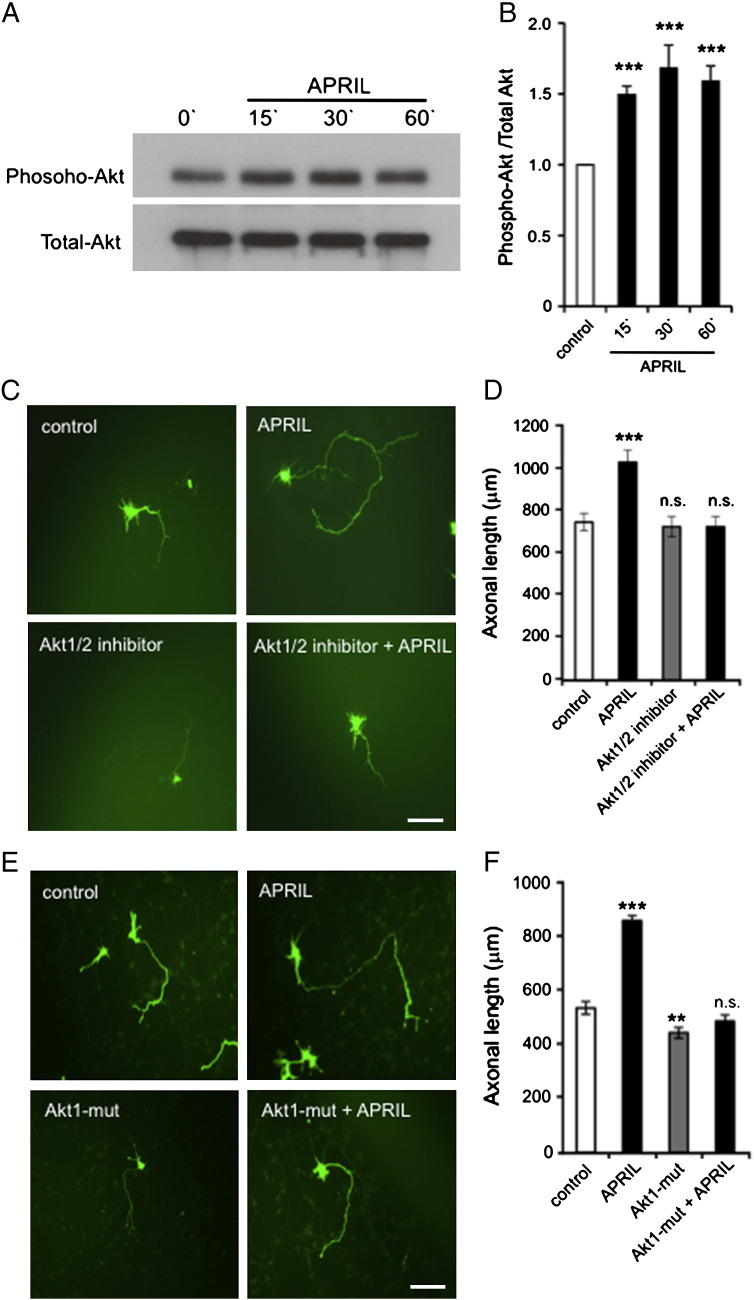
To determine the involvement of Akt signaling in APRIL-promoted axon growth, we first investigated whether APRIL treatment activates Akt. E18 pyramidal cells were initially cultured for 3 days prior to APRIL treatment. Western analysis showed that APRIL caused a rapid increase in the level phospho-Akt within 15 min, reaching a peak 30 min after APRIL addition. The level of phospho-Akt remained significantly elevated in APRIL-treated cultures for at least 60 min (Fig. 7A and B). To test whether Akt signaling contributes to APRIL-promoted axon growth, we first examined whether a selective dual Akt1/Akt2 kinase inhibitor (Zhao et al., 2008) was able to prevent the increase in axon elongation promoted by APRIL. E18 pyramidal cells were transfected with pGFP 2 days after plating and were pretreated for 2 h with the Akt1/Akt2 inhibitor before adding APRIL to the culture medium and quantifying the axon length 18 h later. Whereas axon length in cultures that received the Akt1/Akt2 inhibitor alone was not significantly different from that in control cultures, the Akt1/Akt2 inhibitor completely prevented the axon growth-enhancing effect of APRIL (Fig. 7C and D). As further confirmation of these results, we transfected pyramidal cells with a plasmid that expresses both GFP and an Akt construct with loss-of-function mutations in its active site. Expression of the Akt mutant had no significant effect on axon extension in the absence of exogenous APRIL, and completely eliminated the axon growth-enhancing effect of exogenous APRIL (Fig. 7E and F). Taken together, these results suggest that Akt signaling is required for the effect of APRIL on axon growth.
Fig. 7.
APRIL promotes axonal growth by activating Akt signaling. (A) Representative Western blot probed for phospho-Akt and total Akt in lysates of E18 hippocampal neurons of treated with 100 ng/ml APRIL for the times indicated after first culturing the neurons for 3 days. (B) Densitometric quantification of levels of phospho-Akt relative to total Akt from 3 separate Western blot experiments (mean ± s.e.m., *** indicates P < 0.0001, statistical comparison with control, Mann–Whitney U test). (C) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with a plasmid expressing GFP and were treated with 5 μM of the Akt1/2 inhibitor 2 h prior to a further 18 h incubation with and without APRIL (100 ng/ml) before GFP immunostaining. Separate cultures were treated with APRIL after transfection and control cultures received neither the inhibitor nor APRIL. (D) Bar chart of the axonal growth under the experimental conditions illustrated in C. (E) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro with plasmids expressing either GFP alone or GFP together with the Akt1 K179M T308A S473A mutant (Akt1-mut). After transfection, the neurons were cultured for a further 18 h with and without APRIL (100 ng/ml) before GFP immunostaining. (F) Bar chart of the axonal growth under the experimental conditions illustrated in E. The data shown in D and F are compiled from > 150 neurons per condition from at least three separate experiments (mean ± s.e.m., *** indicates P < 0.0001 and n.s. non-significant, statistical comparison with control, Mann–Whitney U test). Scale bars = 100 μm.
APRIL enhances axonal growth by inactivating glycogen synthase kinase-3β
Glycogen synthase kinase-3 (GSK-3) proteins are among the many substrates of Akt kinases, and have been implicated in the regulation of multiple aspects of neural development (Hur and Zhou, 2010), including the control of axon growth from developing sensory and hippocampal neurons (Kim et al., 2006; Zhou et al., 2004). The two isoforms of GSK-3, GSK-3α and GSK-3β, can be phosphorylated on serine-21 and serine-9, respectively, by Akt kinases (Cross et al., 1995). This results in inactivation of GSK-3, and several studies suggest that inactivated GSK-3 promotes axon elongation from cultured sensory and hippocampal neurons by regulating the molecular machinery that controls actin rearrangement and microtubule assembly (Kim et al., 2006; Kumar et al., 2009; Namekata et al., 2012; Yoshimura et al., 2005; Zhou et al., 2004). To determine if treating hippocampal neurons with APRIL leads to GSK-3 phosphorylation, we cultured E18 pyramidal cells for 3 days before treating them with APRIL. Western blot analysis revealed that APRIL caused a rapid increase in the phosphorylation of phospho-S9 GSK-3β that was sustained for at least 60 min (Fig. 8A and B), but caused no change in the level of phospho-S21 GSK-3α.
Fig. 8.
APRIL promotes axonal growth by activating GSK-3β signaling. (A) Representative Western blots probed for phospho-GSK-3α, phospho-GSK-3β and total GSK-3β in lysates of E18 hippocampal neurons treated with 100 ng/ml APRIL for the times indicated after first culturing the neurons for 3 days. (B) Densitometric quantification of levels of phospho-GSK-3β relative to total GSK-3β from 3 separate Western blot experiments (mean ± s.e.m., *** indicates P < 0.0001, statistical comparison with control, Mann–Whitney U test). (C) Images of representative E18 hippocampal pyramidal neurons that were transfected after 2 days in vitro plasmids expressing either GFP alone or GFP together with the GSK3β S9A mutant. After transfection, the neurons were cultured for a further 18 h with and without APRIL (100 ng/ml) before GFP immunostaining. (D) Bar chart of the axonal growth under the experimental conditions illustrated in C. The mean ± s.e.m. of data obtained from > 150 neurons per condition compiled from at least three separate experiments are shown in D (*** indicates P < 0.0001 and n.s., non-significant, statistical comparison with control, Mann–Whitney U test). Scale bars = 100 μm.
To evaluate the participation of GSK-3β in the axon growth-promoting effects of APRIL, we studied the effect of expressing a constitutively active form of GSK-3β in hippocampal neurons. Neurons that were transfected with a construct that expresses GFP together with a constitutively active GSK-3β protein (GSK-3β S9A) extended axons that were not significantly different in length from those transfected with a GFP-expressing construct. However, expression of the GSK3β S9A mutant completely eliminated the axon growth-enhancing effect of APRIL (Fig. 8C and D). These results suggest that the effect of APRIL on axon growth is mediated by GSK-3β inactivation.
Discussion
We have described and characterized the first known activity for APRIL in the nervous system. In culture, recombinant APRIL selectively enhanced axon elongation from neonatal hippocampal pyramidal cells, but did not affect the elongation of dendrites from these neurons. Several observations suggest that this effect of APRIL was mediated by one of its receptors, BCMA. First, BCMA mRNA and protein were clearly detectable in extracts of the developing hippocampus, and BCMA was localized to pyramidal cells by immunohistochemistry. In contrast, TACI, the alternative TNFRSF member through which APRIL signals (Bossen and Schneider, 2006), was barely detectable in the hippocampus. Second, function-blocking anti-BCMA antibodies inhibited the axon growth-promoting effects of recombinant APRIL. Third, expression of a truncated, non-functional BCMA mutant in hippocampal pyramidal cells inhibited the axon growth-promoting effects of APRIL. Fourth, overexpression of full-length BCMA promoted axon elongation just as effectively as recombinant APRIL. Finally, axon growth was not further enhanced by treating BCMA-overexpressing neurons with APRIL, suggesting that enhancing BCMA signaling independently of exogenous APRIL mimics the effect of APRIL treatment.
While BAFF also binds to BCMA (Day et al., 2005), several observations suggest that APRIL is a potentially relevant ligand for BCMA in the developing hippocampus. First, APRIL mRNA and protein were detectable in hippocampal extracts throughout the period of development pyramidal cells extend axons in vivo. Second, APRIL was secreted by hippocampal pyramidal cells in culture. Third, function-blocking anti-APRIL antibodies significantly reduced axon elongation from these neurons, at least when added to the medium at the time of plating, which suggests that biologically active APRIL is secreted in sufficient quantities by cultured hippocampal pyramidal cells to affect axon elongation.
Our immunohistochemical studies suggest that both APRIL and BCMA proteins are localized to pyramidal cells in the developing hippocampus. This raises the possibility that APRIL could act on these neurons by an autocrine and/or paracrine mechanism in vivo. Co-expression of APRIL and BCMA has been reported in mesenchymal cells of the placenta (Langat et al., 2008), and experimental evidence for APRIL/BCMA autocrine signaling has been obtained from studies of normal and malignant B cells (Chiu et al., 2007; He et al., 2004). Autocrine/paracrine signaling involving APRIL and BCMA could influence the development of hippocampal pyramidal cells in an analogous fashion to autocrine/paracrine signaling involving neurotrophins (Boukhaddaoui et al., 2001; Horch and Katz, 2002). It is possible that the level of expression of APRIL and BCMA in hippocampal pyramidal cells, and hence the extent of APRIL/BCMA signaling, could be regulated by factors acting on these neurons during development or by particular patterns of neural activity in these neurons. For these reasons, it will be informative to determine how the expression of APRIL and BCMA are regulated in hippocampal pyramidal cells during development, as this might provide a better understanding of how APRIL/BCMA signaling and its effects on axon growth are integrated with other aspects of hippocampal development. APRIL and BCMA are also expressed by other neurons in the hippocampus, and while there is a significant increase in the level of BCMA mRNA in the hippocampus unaccompanied by a parallel increase in APRIL mRNA levels during perinatal development, these levels represent the sum total of changes in multiple populations of neurons, some of which make afferent or efferent connections with neurons outside the hippocampus.
We have shown that recombinant APRIL caused rapid and robust phosphorylation and hence activation of ERK1, ERK2 and Akt in cultured hippocampal pyramidal neurons. These kinases have also been reported to be activated by APRIL in several other cell types (Gupta et al., 2009; Moreaux et al., 2004; Zonca et al., 2012). These kinases have also been implicated in regulating neurite growth from a variety of neurons and neuronal cell lines in response to neurotrophins (Atwal et al., 2000; Cosker and Eickholt, 2007; Gomez and Cohen, 1991; Goold and Gordon-Weeks, 2005; Hafner et al., 2012; Kuruvilla et al., 2000; O'Keeffe et al., 2008; Read and Gorman, 2009; Thompson et al., 2004). Importantly, we have shown that the axon growth-promoting effect of recombinant APRIL is abolished by pharmacological inhibition of either MEK1 and MEK2 (the kinases that lie upstream of ERK1 and ERK2 in the MAP kinase signaling pathway), PI-3 kinase (enzyme that generates phosphatidylinositol 3,4,5 triphosphates which in turn activate Akt) or Akt and by expression of a dominant-negative versions of Akt and the regulatory subunit PI-3 kinase. These findings suggest that both MAP kinase signaling and PI-3 kinase/Akt signaling are required for the axon growth-promoting action of APRIL.
Finally, we demonstrated that GSK-3β, one of Akt's many substrates, becomes rapidly phosphorylated on serine 9 in hippocampal pyramidal neurons following APRIL treatment. Phosphorylation on this residue results in inactivation of GSK-3 (Cross et al., 1995), and several studies suggest that inactivated GSK-3β promotes axon elongation from cultured sensory and hippocampal neurons by regulating the molecular machinery that controls actin rearrangement and microtubule assembly (Kim et al., 2006; Kumar et al., 2009; Namekata et al., 2012; Yoshimura et al., 2005; Zhou et al., 2004). Our demonstration that expression of a constitutively active mutant of GSK-3β prevents the axon growth-promoting action of APRIL suggests that this depends on GSK-3β inactivation.
APRIL and BCMA are potent activators of NF-κB family of transcription factors (Hatzoglou et al., 2000; Kern et al., 2004). NF-κB signaling either promotes or inhibits neurite growth depending on the mechanism of NF-κB activation and phosphorylation status of the p65 NF-κB subunit (Gutierrez and Davies, 2011). Although we have not explored whether APRIL affects NF-κB signaling in hippocampal pyramidal neurons, it will be interesting in future work to ascertain whether NF-κB signaling plays a role in modulating APRIL-promoted axon growth.
Our present demonstration that APRIL selectively promotes axon growth from developing hippocampal pyramidal neurons adds to the growing body of work implicating members of the TNFSF in regulating the growth and branching of neural processes during development (Desbarats et al., 2003; Gavalda et al., 2009; Gutierrez et al., 2008, 2013; Kisiswa et al., 2013; Neumann et al., 2002; O'Keeffe et al., 2008; Zuliani et al., 2006). It will be important in future studies to ascertain the physiological relevance of our findings by studying relevant aspects of the phenotype of mice possessing null mutations of the APRIL and BCMA genes. Also, it will be important to explore whether APRIL and BCMA play a role in regulating the growth of neural processes from neurons elsewhere in the developing nervous system.
Experimental methods
Animals
This study was conducted on tissues obtained from CD1 mice (Mus musculus). Breeding and housing were approved by the Cardiff University Ethical Review Board and was performed within the guidelines of the Home Office Animals (Scientific Procedures) Act, 1986.
Quantitative PCR
The levels of APRIL and BCMA mRNAs were quantified by RT-QPCR relative to a geometric mean of mRNAs for the house keeping enzymes glyceraldehyde phosphate dehydrogenase (GAPDH) and succinate dehydrogenase (SDHA). Total RNA was extracted from whole hippocampus with the RNeasy Mini Lipid extraction kit (Qiagen, Crawely, UK), and 5 μl was reverse transcribed for 1 h at 45 °C using the AffinityScript kit (Agilent, Berkshire, UK) in a 25 μl reaction according to the manufacturer's instructions. 2 μl of cDNA was amplified in a 20 μl reaction volume using Brilliant III ultrafast QPCR master mix reagents (Agilent, Berkshire, UK). QPCR products were detected using dual-labeled (FAM/BHQ1) hybridization probes specific to each of the cDNAs (MWG/Eurofins, Ebersberg, Germany). The PCR primers were: APRIL forward, 5′-CTG TCC TTC CTA GAT AAT G-3′ and reverse, 5′-CTA GTG ACA CTC TGA CAC-3′; BCMA forward, 5′-TGA CCA GTT CAG TGA AAG G-3′ and reverse, 5′-GGG TTC ATC TTC CTC AGC-3′; GAPDH forward, 5′-GAG AAA CCT GCC AAG TAT G-3′ and reverse, 5′-GGA GTT GCT GTT GAA GTC-3′; SDHA forward, 5′-GGA ACA CTC CAA AAA CAG-3′ and reverse, 5′-CCA CAG CAT CAA ATT CAT-3′. Dual-labeled probes were: APRIL, FAM-CAC CAA ATT CTC CTG AGG CT-BHQ1; BCMA, FAM-CGT ACA CGG TGC TCT GGA TCT TCT T-BHQ1; GAPDH, FAM-AGA CAA CCT GGT CCT CAG TGT-BHQ1; SDHA, FAM-CCT GCG GCT TTC ACT TCT CT-BHQ1. Forward and reverse primers were used at a concentration of 150 nM each and dual-labeled probes were used at a concentration of 300 nM. PCR was performed using the Mx3000P platform (Agilent, Berkshire, UK) using the following conditions: 45 cycles of 95 °C for 12 s and 60 °C for 35 s. Standard curves were generated in every 96-well plate, for each cDNA for every real time PCR run, by using serial three-fold dilutions of reverse transcribed adult mouse brain total RNA (Ambion, Paisley, UK). Three separate sets of dissections of the hippocampi were carried to harvest tissue at each of the following ages: E18, P0, P5 and P10.
Immunohistochemistry
Brains were fixed in 4% paraformaldehyde for 24 h and then cryoprotected in 30% sucrose before being frozen. OCT embedded tissues were snap frozen in isopentene (Sigma-Aldrich, Dorset, UK), cooled with dry ice and serially sectioned at 15 μm. Sections were mounted onto electrostatic charged slides (Leica Microsystems, Peterborough, UK), blocked with 5% goat serum and 2% bovine serum albumen containing 0.1% Triton X-100 (Sigma-Aldrich, Dorset, UK) in phosphate-buffered saline (PBS) for 1 h at room temperature, and then incubated for 18 h at 4 °C with primary antibodies dissolved in blocking buffer diluted 5 fold. Primary antibodies were: rabbit anti-APRIL (1:200, Abcam, Cambridge, UK, ab64967), rabbit anti-BCMA (1:200, Abcam, Cambridge, UK, ab5972), rabbit anti-TACI (1:200, Abcam, Cambridge, UK, ab79023), chicken anti-200 kD neurofilament Heavy (1:250, Abcam, Cambridge, UK, ab4680) and chicken anti-MAP2 (1:250, Abcam, Cambridge, UK, ab5392). After incubation, sections were subsequently washed three times in PBS, incubated for 1 h with biotin-conjugated anti-rabbit IgG (1:500, Vector Laboratories, Cambridgeshire, UK) and Alexa Fluor 546 conjugated goat anti-chicken IgG (1:500, Invitrogen, Paisley, UK). After a further incubation for 30 min with DyLight 488 conjugated streptavidin (1:500, Vector Laboratories, Cambridgeshire, UK) in blocking buffer diluted 5 fold, slices were washed with PBS three times and incubated for 10 min with TO-PRO®-3 Iodide staining (1:10,000 Invitrogen, Paisley, UK) for counterstaining off the nuclei. The slides were then washed three times with PBS (5 min per wash) and were mounted with VECTASHIELD® Mounting Media (Vector Laboratories, Cambridgeshire, UK). Images were obtained using a Zeiss Axioplan confocal microscope (Zeiss, Cambridge, UK). Negative controls (no primary antibody) were also set up.
Immunoblotting
For the immunoblotting either dissected hippocampi or neurons cultured at high density (200,000 cells/well) were lysed in ice-cold RIPA lysis buffer supplemented with proteinase and phosphatase inhibitor cocktail mix (Sigma-Aldrich, Dorset, UK) and insoluble debris was removed by centrifugation. Equal quantities of protein were separated on 10% SDS-PAGE gels and were transferred to PVDF membranes using the BioRad TransBlot (BioRad, Hertfordshire, UK). The blots were probed with rabbit anti-APRIL (1:200, Poway, CA, USA, Cat. No. 2415), rabbit anti-BCMA (1:200, Abcam, Cambridge, UK, ab5972), rabbit anti-TACI (1:200, Abcam, Cambridge, UK, ab79023), chicken anti-GAPDH (1:2000, Abcam, Cambridge, UK, ab83956), rabbit anti-Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (1:1000, Cell Signaling Technology, Danvers, MA, USA, 9101), rabbit anti-p44/42 MAPK (Erk1/2) (1:1000, Cell Signaling Technology, Danvers, MA, USA, 9102), rabbit anti-Phospho-Akt (Ser473) (1:1000, Cell Signaling Technology, Danvers, MA, USA, 4060), rabbit anti-Akt (1:1000, Cell Signaling Technology, Danvers, MA, USA, 4685), rabbit anti-phospho-GSK-3α/β (Ser21/9) (1:1000, Cell Signaling Technology, Danvers, MA, USA, 9331) and mouse anti-GSK-3 (1:1000, Cell Signaling Technology, Danvers, MA, USA, 9832). Binding of the primary antibodies was visualized with HRP-conjugated donkey anti-rabbit, anti-mouse or anti-chicken secondary antibodies (1:5000; rabbit W4011, mouse W4021, Promega, Southampton, UK, and 1:5000; chicken HRP Abcam, ab16349 Cambridge, UK,) and Chemiluminescence Luminol Reagent (Santa Cruz Biotechnology, CA, USA). Densitometry was carried out using Gel-Pro Analyzer 32 program (Media Cybernetics, USA).
ELISA (enzyme-linked immunosorbent assay)
Hippocampal cultures were plated in 3 wells of a 24-well plate with a density of 200,000 cells per well. After 3DIVs, the supernatant was collected, centrifuged to remove debris, and stored at − 80 °C until further use. Samples were quantified using an ELISA kit for APRIL protein (USCN Life Science Inc., rP91750Mu01, Houston, TX, USA) according to the manufacturer's instructions.
Neuron culture
Hippocampi were dissected from embryonic day 18 (E18) CD1 mouse fetuses, and were triturated to produce a single cell suspension following trypsin digestion (Worthington, Lakewood, USA) and DNase I treatment (Roche Applied Science, East Sussex, UK). The neurons were plated on plastic dishes coated with poly-l-lysine (Sigma-Aldrich, Dorset, UK) at a density of 50,000 cells/cm2 and were cultured in Neurobasal A (Invitrogen, Paisley, UK) supplemented with 2% B27 (Invitrogen, Paisley, UK), 0.5 mM GlutaMAX I (Invitrogen, Paisley, UK), 100 units/ml penicillin and 100 g/ml streptomycin (Gibco BRL, Crewe, UK). The cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Neuronal transfection
After 2 or 6 days in vitro, the neurons were transfected with expression vectors using lipofectamine 2000 (Invitrogen, Paisley, UK) according to the manufacturer's instructions with modifications. Briefly, 1.2 μg of DNA was mixed with 4 μl of lipofectamine. After 20 min, this mixture was added to the cultures. After 3 h, the cultures were then washed with culture medium, at which point recombinant human APRIL was added (R&D Systems, Abingdon, UK) and the neurons were maintained in culture for further 18 h. Chemical inhibitors (PD98059, Sigma-P215, LY294002 hydrochloride, Sigma-L9908 and Akt1/2 kinase inhibitor A6730, Sigma-Aldrich Company, Dorset, UK) were added 2 h before incubation of recombinant APRIL. Function-blocking antibodies (mouse anti-BCMA, MAB5931 and human anti-APRIL, MAB5860, R&D Systems, Abingdon, UK) were added after transfection.
Expression vectors and report constructs
The vector expressing copGFP (pCDH-CMV-MCS-EF1-copGFP) was obtained from Cambridge Bioscience (Cambridge, UK). Vectors expressing murine BCMA and BCMA ΔC83 were kindly provided by Adrian T. Ting (Yang et al., 2005). The BCMA mBCMA ΔC83 sequences were cloned into the HindIII and NotI sites of the vector pCDNA3.1. pCDH-CMV-mBCMA-EF1-copGFP and pCDH-CMV-mBCMA ΔC83-EF1-copGFP were made by excising the respective cDNA encoding sequences from pCDNA3.1 and inserting them into the NheI and NotI sites of the vector pCDH-CMV-MCS-EF1-copGFP.
To generate the construct that expresses GFP together with a dominant-negative form of the p85α regulatory subunit of PI-3 kinase, the sequence of p85α that lacks the binding domain for the p110 catalytic subunit (Addgene, Cambridge, MA, USA, plasmid 13432, deposited by C. R. Kahn, ref. Taniguchi et al., 2006) was cloned into the copGFP vector. To generate the construct that expresses GFP together with Akt1 that possesses loss-of-function mutations in its active site, the sequence of Akt1 that has the following substitutions K179M, T308A and S473A (Addgene, Cambridge, MA, USA, plasmid 9031, deposited by W. R. Sellers, ref. Ramaswamy et al., 1999) was cloned into the copGFP vector. To generate the construct that expresses GFP together with a constitutively active mutant of GSK-3β, the sequence of GSK-3β that has S9A substitution (Addgene, Cambridge, MA, USA, plasmid 14754, deposited by J. Woodgett, ref. Stambolic and Woodgett, 1994) was cloned into the copGFP vector.
Analysis of axon and dendrite growth
18 h after transfection, the neurons were fixed for 30 min in 4% paraformaldehyde, permeabilized for 15 min at room temperature with 0.5% Triton X-100 in PBS and blocked for 1 h with 10% goat serum in PBS containing 0.1% Triton X-100. The neurons were then incubated for 1 h at room temperature with primary antibody (anti-Cop/turbo GFP, 1:250, Origene Technologies, Rockville, MD, USA) in PBS containing 10% goat serum and 0.1% Triton X-100. After washing with PBS, the cultures were incubated with an appropriate secondary antibody (Alexa Fluor488 conjugated goat anti-mouse IgG, 1:500, Invitrogen, Paisley, UK) in PBS containing 10% goat serum and 0.1% Triton. Labeled neurons were visualized using an Axioplan confocal microscope (Zeiss). For every experimental condition in each experiment, images of at least 150 neurons were digitally acquired.
For the analysis of axonal length, hippocampal neurons were transfected with GFP-plasmids after 2 days in vitro and were fixed after 3 days in vitro in 4% paraformaldehyde for 30 min. Axonal length was measured using ImageJ software (for each axon, the “segmented line tool” was repeatedly used to define a line segment corresponding to the axon whose length was subsequently measured in the program). The analysis of dendrite morphology was performed as described previously (Salama-Cohen et al., 2005). Briefly, for each neuron, the number of dendrites that crossed a circle of radius of 50 μm centered at the cell soma was divided by the total number of primary dendrites and the quotient was expressed as a percentage. The mean and standard errors of these percentages are plotted.
Explant cultures
Explant cultures of the CA1 region of the hippocampus were established in a 3-dimensional matrix of Matrigel (BD Biosciences) along the lines described for collagen gels (Lumsden and Davies, 1983). A tissue chopper was used to obtain 350 μm thick hippocampal slices and CA1 was dissected from these slices using tungsten needles. A bed of Matrigel (BD Biosciences) was kept on ice prior to use to avoid polymerization. A bed of 20 μl of Matrigel was pipetted into wells of 4-well dishes, which were placed in a 37 °C incubator to polymerize before transferring the explants to these beds. A further 20 μl of Matrigel was pipetted on top of each explant. The dishes were then incubated at 37 °C for a further 15 min to polymerize the Matrigel, after which 2 ml of complete neurobasal medium was added to each dish. After 3 days of culture, the emergent neurites were labeled with the fluorescent vital dye calcein AM (Invitrogen), and the explants were imaged by confocal microscopy.
Statistical analysis
Pair-wise comparisons were made using Student's t-test (two tailed). For non-parametric multiple comparisons the Mann–Whitney U test was used.
Acknowledgments
We thank Dr. Adrian T. Ting for plasmids expressing BCMA and BCMA ΔC83. This work was supported by the Wellcome Trust (grant number 085984) to AMD and Proyecto de Excelencia of the Regional Government of Andalussia (grant number P10-CVI-6740) to AR. CO was a recipient of Fundacao para a Ciencia e a Tecnologia PhD grant. PC was a recipient of a ‘Sara Borrell’ Postdoctoral Fellowship from the National Institute of Health ‘Carlos III’, Spain (grant number CD08/00078).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Alexaki V.I., Notas G., Pelekanou V., Kampa M., Valkanou M., Theodoropoulos P., Stathopoulos E.N., Tsapis A., Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors, Fn14, BAFF-R, TACI, and BCMA, at different stages of normal and pathological adipose tissue development. J. Immunol. 2009;183:5948–5956. doi: 10.4049/jimmunol.0901186. [DOI] [PubMed] [Google Scholar]
- Atwal J.K., Massie B., Miller F.D., Kaplan D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Bossen C., Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Boukhaddaoui H., Sieso V., Scamps F., Valmier J. An activity-dependent neurotrophin-3 autocrine loop regulates the phenotype of developing hippocampal pyramidal neurons before target contact. J. Neurosci. 2001;21:8789–8797. doi: 10.1523/JNEUROSCI.21-22-08789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Dotti C.G. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Chiu A., Xu W., He B., Dillon S.R., Gross J.A., Sievers E., Qiao X., Santini P., Hyjek E., Lee J.W. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109:729–739. doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker K.E., Eickholt B.J. Phosphoinositide 3-kinase signalling events controlling axonal morphogenesis. Biochem. Soc. Trans. 2007;35:207–210. doi: 10.1042/BST0350207. [DOI] [PubMed] [Google Scholar]
- Craxton A., Magaletti D., Ryan E.J., Clark E.A. Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Danglot L., Triller A., Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- Davies A.M. Intrinsic differences in the growth rate of early nerve fibres related to target distance. Nature. 1989;337:553–555. doi: 10.1038/337553a0. [DOI] [PubMed] [Google Scholar]
- Day E.S., Cachero T.G., Qian F., Sun Y., Wen D., Pelletier M., Hsu Y.M., Whitty A. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–1931. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- Desbarats J., Birge R.B., Mimouni-Rongy M., Weinstein D.E., Palerme J.S., Newell M.K. Fas engagement induces neurite growth through ERK activation and p35 upregulation.[see comment] Nat. Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- Dillon S.R., Gross J.A., Ansell S.M., Novak A.J. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- Dotti C.G., Sullivan C.A., Banker G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalda N., Gutierrez H., Davies A.M. Developmental regulation of sensory neurite growth by the tumor necrosis factor superfamily member LIGHT. J. Neurosci. 2009;29:1599–1607. doi: 10.1523/JNEUROSCI.3566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353:170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Goold R.G., Gordon-Weeks P.R. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol. Cell. Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Grivennikov S.I., Kuprash D.V., Liu Z.G., Nedospasov S.A. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms. Int. Rev. Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- Gupta M., Dillon S.R., Ziesmer S.C., Feldman A.L., Witzig T.E., Ansell S.M., Cerhan J.R., Novak A.J. A proliferation-inducing ligand mediates follicular lymphoma B-cell proliferation and cyclin D1 expression through phosphatidylinositol 3-kinase-regulated mammalian target of rapamycin activation. Blood. 2009;113:5206–5216. doi: 10.1182/blood-2008-09-179762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H., Davies A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-kappaB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H., O'Keeffe G.W., Gavalda N., Gallagher D., Davies A.M. Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. J. Neurosci. 2008;28:8246–8256. doi: 10.1523/JNEUROSCI.1941-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H., Kisiswa L., O'Keeffe G.W., Smithen M.J., Wyatt S., Davies A.M. Regulation of neurite growth by tumour necrosis superfamily member RANKL. Open Biol. 2013;3:120150. doi: 10.1098/rsob.120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner A., Obermajer N., Kos J. gamma-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. Biochem. J. 2012;443:439–450. doi: 10.1042/BJ20111351. [DOI] [PubMed] [Google Scholar]
- Hahne M., Kataoka T., Schroter M., Hofmann K., Irmler M., Bodmer J.L., Schneider P., Bornand T., Holler N., French L.E. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou A., Roussel J., Bourgeade M.F., Rogier E., Madry C., Inoue J., Devergne O., Tsapis A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- He B., Chadburn A., Jou E., Schattner E.J., Knowles D.M., Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J. Immunol. 2004;172:3268–3279. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- Hehlgans T., Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway F., Taylor R., Knowles H.J., Athanasou N.A. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48:938–944. doi: 10.1016/j.bone.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Hendriks J., Planelles L., de Jong-Odding J., Hardenberg G., Pals S.T., Hahne M., Spaargaren M., Medema J.P. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- Horch H.W., Katz L.C. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat. Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Hur E.M., Zhou F.Q. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold K., Zumsteg A., Tardivel A., Huard B., Steiner Q.G., Cachero T.G., Qiang F., Gorelik L., Kalled S.L., Acha-Orbea H. Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S., Banker G. Culturing hippocampal neurons. Nat. Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kaech S., Huang C.F., Banker G. General considerations for live imaging of developing hippocampal neurons in culture. Cold Spring Harb. Protoc. 2012;2012:312–318. doi: 10.1101/pdb.ip068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C., Cornuel J.F., Billard C., Tang R., Rouillard D., Stenou V., Defrance T., Ajchenbaum-Cymbalista F., Simonin P.Y., Feldblum S. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103:679–688. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Zhou F.Q., Zhou J., Yokota Y., Wang Y.M., Yoshimura T., Kaibuchi K., Woodgett J.R., Anton E.S., Snider W.D. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006;52:981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiswa L., Osório C., Erice C., Vizard T., Wyatt S., Davies A.M. TNFα reverse signaling promotes sympathetic axon growth and target innervation. Nat. Neurosci. 2013;16:865–873. doi: 10.1038/nn.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Lyle K.S., Gierke S., Matov A., Danuser G., Wittmann T. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 2009;184:895–908. doi: 10.1083/jcb.200901042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R., Ye H., Ginty D.D. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Langat D.L., Wheaton D.A., Platt J.S., Sifers T., Hunt J.S. Signaling pathways for B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) in human placenta. Am. J. Pathol. 2008;172:1303–1311. doi: 10.2353/ajpath.2008.071139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy M.B., Nardelli B., Hilbert D.M., He B., Schaffer A., Casali P., Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fraga M., Fernandez R., Albar J.P., Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A.G., Davies A.M. Earliest sensory nerve fibres are guided to peripheral targets by attractants other than nerve growth factor. Nature. 1983;306:786–788. doi: 10.1038/306786a0. [DOI] [PubMed] [Google Scholar]
- Moreaux J., Legouffe E., Jourdan E., Quittet P., Reme T., Lugagne C., Moine P., Rossi J.F., Klein B., Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekata K., Harada C., Guo X., Kimura A., Kittaka D., Watanabe H., Harada T. Dock3 stimulates axonal outgrowth via GSK-3beta-mediated microtubule assembly. J. Neurosci. 2012;32:264–274. doi: 10.1523/JNEUROSCI.4884-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H., Schweigreiter R., Yamashita T., Rosenkranz K., Wekerle H., Barde Y.A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J. Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe G.W., Gutierrez H., Pandolfi P.P., Riccardi C., Davies A.M. NGF-promoted axon growth and target innervation requires GITRL-GITR signaling. Nat. Neurosci. 2008;11:135–142. doi: 10.1038/nn2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelekanou V., Kampa M., Kafousi M., Darivianaki K., Sanidas E., Tsiftsis D.D., Stathopoulos E.N., Tsapis A., Castanas E. Expression of TNF-superfamily members BAFF and APRIL in breast cancer: immunohistochemical study in 52 invasive ductal breast carcinomas. BMC Cancer. 2008;8:76. doi: 10.1186/1471-2407-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski R.A., Chevaleyre V. Synaptic integration by different dendritic compartments of hippocampal CA1 and CA2 pyramidal neurons. Cell. Mol. Life Sci. 2012;69:75–88. doi: 10.1007/s00018-011-0769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S., Nakamura N., Vazquez F., Batt D.B., Perera S., Roberts T.M., Sellers W.R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D.E., Gorman A.M. Involvement of Akt in neurite outgrowth. Cell. Mol. Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama-Cohen P., Arevalo M.A., Meier J., Grantyn R., Rodriguez-Tebar A. NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of Notch. Mol. Biol. Cell. 2005;16:339–347. doi: 10.1091/mbc.E04-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr. Opin. Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Schwaller J., Schneider P., Mhawech-Fauceglia P., McKee T., Myit S., Matthes T., Tschopp J., Donze O., Le Gal F.A., Huard B. Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B-cell lymphoma aggressiveness. Blood. 2007;109:331–338. doi: 10.1182/blood-2006-02-001800. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stambolic V., Woodgett J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 1994;303:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi C.M., Tran T.T., Kondo T., Luo J., Ueki K., Cantley L.C., Kahn C.R. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Dolcet X., Hilton M., Tolcos M., Davies A.M. HGF promotes survival and growth of maturing sympathetic neurons by PI-3 kinase- and MAP kinase-dependent mechanisms. Mol. Cell. Neurosci. 2004;27:441–452. doi: 10.1016/j.mcn.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Deschepper C., Park J.B., He G., Chen X., Neves M.F., Virdis A., Schiffrin E.L. Inhibition of mitogen-activated protein/extracellular signal-regulated kinase improves endothelial function and attenuates Ang II-induced contractility of mesenteric resistance arteries from spontaneously hypertensive rats. J. Hypertens. 2002;20:1127–1134. doi: 10.1097/00004872-200206000-00024. [DOI] [PubMed] [Google Scholar]
- Vincent F.B., Saulep-Easton D., Figgett W.A., Fairfax K.A., Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–215. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Yang M., Hase H., Legarda-Addison D., Varughese L., Seed B., Ting A.T. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell-activating factor of the TNF family, induces antigen presentation in B cells. J. Immunol. 2005;175:2814–2824. doi: 10.4049/jimmunol.175.5.2814. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Robinson R.G., Barnett S.F., Defeo-Jones D., Jones R.E., Hartman G.D., Huber H.E., Duggan M.E., Lindsley C.W. Development of potent, allosteric dual Akt1 and Akt2 inhibitors with improved physical properties and cell activity. Bioorg. Med. Chem. Lett. 2008;18:49–53. doi: 10.1016/j.bmcl.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou F.Q., Zhou J., Dedhar S., Wu Y.H., Snider W.D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zonca M., Mancheno-Corvo P., DelaRosa O., Manes S., Buscher D., Lombardo E., Planelles L. APRIL and BAFF proteins increase proliferation of human adipose-derived stem cells through activation of Erk1/2 MAP kinase. Tissue Eng. A. 2012;18:852–859. doi: 10.1089/ten.TEA.2011.0316. [DOI] [PubMed] [Google Scholar]
- Zuliani C., Kleber S., Klussmann S., Wenger T., Kenzelmann M., Schreglmann N., Martinez A., del Rio J.A., Soriano E., Vodrazka P. Control of neuronal branching by the death receptor CD95 (Fas/Apo-1) Cell Death Differ. 2006;13:31–40. doi: 10.1038/sj.cdd.4401720. [DOI] [PubMed] [Google Scholar]