Abstract
The traditional route for teaching cardiac anatomy involves didactic instruction, cadaver dissections, and familiarization with the main structure and relationships of the cardiac chambers, valves, and vasculature. In contemporary cardiac electrophysiology, however, a very different view of anatomy is required including details rarely appreciated with a general overview. In this review, we discuss the critical advances in cardiac electrophysiology that were possible only because of understanding detailed anatomic relationships. While we briefly discuss the clinical relevance, we explain in depth the necessary structural information for the student of clinical anatomy. Interspersed through the text are boxes that highlight and summarize the critical pieces of knowledge to be borne in mind while studying the fascinating structural anatomy of the human heart.
Keywords: Ventricular tachycardia, outflow tract, cardiac anatomy, catheter ablation, complications
Introduction
Ventricular arrhythmias affect patients with both structurally normal and abnormal hearts. Unlike those that occur in the settings of diseased myocardium, idiopathic ventricular arrhythmias usually originate from specific regions in the heart, including the ventricular outflow tracts, endocavitary structures, and the infrahisian conduction system (Ouyang FF et al., 2002; Haissaguerre M et al., 2002a; Cavalcanti JS et al, 2003; Yamauchi Y et al., 2005; Nogami A et al., 2005; Kumagai K et al., 2008; Doppalapudi H et al, 2008; Srivathsan KS et al., 2008; Abouezzeddine O et al., 2010; Yamada T et al., 2010; Betensky BP et al., 2011; Chen J et al., 2012; Hlivak P et al., 2012). Since most of these arrhythmias can be safely and effectively treated by catheter ablation, it has been increasingly offered as the first-line treatment or as an alternative to pharmacotherapy for those who have refractory symptoms or depressed left ventricular function secondary to tachyarrhythmias. Detailed knowledge of the myocardial substrates related to ventricular arrhythmogenesis is a critical component to decide when and how to ablate these arrhythmias. The current review discusses cardiac anatomy relevant to ventricular arrhythmias, with an emphasis on how it is related to surface electrocardiographic manifestation.
Ventricular Outflow Tracts
Approximately two-thirds of idiopathic ventricular arrhythmias originate from the ventricular outflow tracts, of which 70% to 80% comes from the right side (Ouyang FF et al., 2002; Lin D et al., 2008). More recently, ventricular arrhythmias arising from the left ventricular outflow tract and aortic sinuses were also recognized.(Ouyang FF et al., 2002; Cavalcanti JS et al, 2003; Yamauchi Y et al., 2005; Kumagai K et al., 2008; Srivathsan KS et al., 2008; Yamada T et al., 2010; Betensky BP et al., 2011; Chen J et al., 2012; Hlivak P et al., 2012). Although they are the most common targets for catheter ablations of ventricular arrhythmias, it is difficult to fully appreciate the complex anatomic relationships between ventricular outflow tracts and the neighboring structures. In the following discussion, several key concepts describing these anatomic relationships will be highlighted. For consistency and accuracy of nomenclature, we will utilize nomenclatures employed by Ho (Ho SY, 2009) to achieve uniform accurateness and clarity in describing the working portions of the aortic root throughout the article.
1. Right ventricular outflow tract is not on the right side of the heart
During embryonic development, outflow tract septation is accompanied by rotation of the muscular wall in a counterclockwise fashion to align the aorta with the left ventricle, and the pulmonary trunk to the right ventricle (McGuire MA et al., 1996). The resultant geometry of the right ventricular outflow tract is a free-standing muscular structure that can be thought of as a tube wrapping around the left ventricular outflow tract and the aortic root (Figure 1) (Merrick AF et al., 2000). At its lowest portion, the right ventricular outflow tract is continuous with the tricuspid annulus on the interventricular septum. The main body of the right ventricular outflow tract then crosses the left ventricular outflow anteriorly, with the pulmonary valve and the subpulmonary infundibulum becoming leftward to it. As a result, locations of the septum vary along the right ventricular outflow tract. For most parts of the it, the posterior wall of the muscular infundibulum is in continuity with the left ventricular outflow tract and the aortic root, whereas more distally, the septum becomes rightward. However, it should be noted that the true interventricular septum only extends up to the most proximal part of the right ventricular outflow tract. It is therefore more appropriate to refer to the four quadrants of the right ventricular outflow tract as the right, left, anterior, and posterior walls (Asirvatham SJ, 2009).
Figure 1.

Anatomical relationships of the ventricular outflow tract. The RVOT lies anterior to the left ventricular outflow tract. It arises above the tricuspid annulus and directs upward and leftward. As a result, the distal right ventricular outflow tract is leftward and cephalad to the left ventricular outflow tract. Also note that while the pulmonary valve lies horizontally, while the aortic root is tilted up on its left.
RVOT: right ventricular outflow tract; LVOT: left ventricular outflow tract.
2. All aortic sinuses are closely related to important cardiac structures
The tri-leaflet aortic valve is situated at the base of the heart. It lies inferior and rightward to the pulmonary valve and is tilted upward on its left at 30° (Figure 2) (Ho SY, 2009). The right coronary sinus is immediately posterior to the muscular infundibulum of the right ventricular outflow tract. On its left lies the left coronary sinus. As the aortic valve is tilted on its left, the left coronary sinus and its leaflet are at the highest position among the three. While the anterior left coronary sinus is adjacent to the posterior right ventricular outflow tract, the rest of it is in fibrous continuity with the mitral annulus, namely the aortomitral continuity. The posterior lobe of the left atrial appendage lies on the lateral side of this fibrous structure (Figure 3) (Asirvatham SJ, 2009; Tabatabaei N et al., 2009; Ho SY, 2009).
Figure 2.

Anatomical relationship of the aortic sinuses and the neighboring cardiac structures. The right coronary sinus lies immediately posterior to the mid right ventricular outflow tract. To its right it is closely related to the right atrial appendage and the superior vena cava-right atrial junction. As the aortic root is tilted up on the left, and the right ventricular outflow tract runs directs upward and leftward, the anterior portion of the left coronary sinus is in close proximity to the subpulmonic right ventricular outflow tract. The posterior lobe of the left atrial appendage is situated on the left side of the left coronary sinus. The non-coronary sinus is the most posteriorly located aortic sinus. It lies anterior to the mid interatrial septum and the adjacent right and left atrial walls, and is not anatomically related to any ventricular muscles. The membranous septum is located at the junction between the right and non-coronary sinuses. This is a consistent site where the His bundle penetrates the left ventricle.
RVOT: right ventricular outflow tract; R: right (coronary sinus); L: left (coronary sinus); N: non- (coronary sinus); SVC: superior vena cava.
Figure 3.

The aortomitral continuity separating the left ventricular inflow and outflow tracts. The aortomitral continuity is a fibrous structure extending from the left fibrous trigone at the posterior left coronary leaflet to the right fibrous trigone between the right and non-coronary leaflets. The membranous septum is located below the junction of the valvar attachments of the right and non-coronary leaflets, which is a consistent site where the bundle of His penetrates the left ventricle. The membranous septum and the right fibrous trigone are collectively known as the central fibrous body.
R: right (coronary leaflet); N: non- (coronary leaflet); L: left (coronary leaflet); MV: mitral valve; LBB: left bundle branch.
The non-coronary sinus seats between the right and left coronary sinuses and is immediately anterior to the central portion of the interatrial septum and the adjacent left and right atrial myocardium (Figure 2). The junction of the valvar attachments of the right and non-coronary leaflets is slightly above the junction of the valvar attachments of the septal and anterior leaflets of the tricuspid valve. Below this is the membranous septum, which is divided into the atrioventricular and interventricular components by the hinge-line of the tricuspid valvar attachment (Figure 4). The atrioventricular component of the membranous septum is a consistent location where the His bundle penetrates the left ventricle (Figure 5) (Anderson RH et al., 2002; Suleiman M et al., 2008; Asirvatham SJ, 2009; Anderson RH et al., 2013). The rest of the non-coronary sinus is also continuous with the aortomitral continuity (Figure 3). Because of that, the non-coronary sinus is the only aortic sinus that is not in direct contact with any ventricular myocardium (Asirvatham SJ, 2009; Tabatabaei N et al., 2009).
Figure 4.

Gross specimen showing the position of the membranous septum (transilluminated) in between the right coronary and non-coronary leaflets. R: right (coronary leaflet); N: non- (coronary leaflet); Ao: Aorta; MS: membranous septum; LV: left ventricle.
Figure 5.

Gross specimen illustrating the location of the penetrating His bundle at the atrioventricular portion of the membranous septum.
RA – right atrium; RV – right ventricle; Ao – Aorta; AV – atrioventricular; LV – left ventricle.
3. Myocardium extends above the valvar attachment
As all three leaflets of the aortic valve attach to the outflow in semilunar fashions, instead of the traditional sense of a ring-like (annular) valvar attachment, the hinge-line of the attachment of the aortic valvar leaflets actually forms a crown-like structure with the tips right at the sinotubular junction and the nadirs within the left ventricular outflow tract. As a result, the histological ventriculo-arterial junction actually lies in between the tips and the nadirs of this structure and myocardial fibers are thus included in the aortic sinuses (Figure 6) (Anderson RH et al., 1991; Piazza N et al., 2008; Ho SY 2009). The myocardial extensions within the aortic sinuses are mostly asymmetrical and do not exceed more than a few millimeters above the basal valvar attachment (Asirvatham SJ, 2009; Gami AS et al., 2011). They are most commonly found at the right coronary sinus, followed by the left coronary sinus, but are rarely seen at the non-coronary sinus. This difference can be explained by the fact that only the right coronary sinus and the anterior portion of the left coronary sinus are in contact with ventricular myocardium (Asirvatham SJ 2009; Gami AS et al., 2011).
Figure 6.

Schematic diagram showing individual component of the aortic root. As each aortic valvar leaflet is attached to the left ventricular outflow in semilunar fashion, the valvar attachment to the outflow is in fact a crown-like structure rather than the traditional sense of an annulus (ring-like). The wall of the aortic root within the boundaries of each aortic valvar leaflet expands to form the corresponding aortic sinus. The aortic root is bounded at the top by the sinotubular junction, and at the bottom by the virtual ring formed by the basal attachments of the three aortic valvar leaflets. As the ventriculo-arterial junction is located in between these two rings, ventricular myocardial fibers are included in the aortic sinuses but never extend beyond the sinotubular junction.
Similarly, myocardial fibers are also found within the pulmonary sinuses. Unlike those in the aortic sinuses, myocardial extensions above the proximal pulmonary valvar attachment are usually symmetrical. This is consistent with the fact that all three pulmonary sinuses are continuous with myocardium of the right ventricular outflow tract (Gami AS et al., 2011; Cabrera JA et al., 2013).
4. Both coronary arteries and veins are closely related to the ventricular outflow tracts
The left coronary and right coronary sinuses are named as such because they are the usual origins of the left and right coronary arteries respectively, although variations exist in less than 1% of the patients in angiography studies (Topaz O et al., 1992; Garg N et al., 2000; Tuncer C et al., 2006; Chiu IS et al., 2012). Both arteries take off at 1 to 2 cm above the basal valvar attachment (Ho SY, 2009). Since the anterior portion of the left coronary sinus is immediately posterior to the right ventricular outflow tract, and that the pulmonary valve is higher than the aortic valve, the structure in closest proximity to the left main coronary artery is the subpulmonary infundibulum of the right ventricular outflow tract, rather than the left ventricular outflow tract (Figure 7) (Asirvatham SJ, 2009; Gami AS et al., 2011). The left main coronary artery gives off the left anterior descending and left circumflex arteries. The left anterior descending artery is directed anteriorly, courses down the left margin of the right ventricular outflow tract and runs along the anterior interventricular groove, while the left circumflex artery is directed posteriorly and runs under the left atrial appendage along the left atrioventricular groove (Asirvatham SJ 2009; Gami AS et al., 2011). As the proximal right ventricular outflow tract lies to the right of the heart, and the right coronary sinus is located at a more caudal position than the left coronary sinus, the right coronary artery is in also in close proximity to the proximal portion of the right ventricular outflow tract (Figure 7) (Asirvatham SJ, 2009).
Figure 7.

Relationship of the coronary arteries and veins to the ventricular outflow tracts.
RVOT: right ventricular outflow tract. SVC: superior vena cava.
The coronary venous system is also anatomically related to the ventricular outflow tracts. The anterior interventricular vein in the interventricular groove directs upward until it becomes the great cardiac vein, which runs down the left atrioventricular groove along with the left circumflex artery. The junction between the great cardiac vein and the anterior interventricular vein is thus closely associated with the subpulmonary infundibulum of the right ventricular outflow tract (Figure 7) (Asirvatham SJ, 2009; Lachman N et al., 2011).
5. Aortomitral continuity as an origin of ventricular arrhythmias
The aortomitral continuity is a fibrous curtain that connects the aortic and the mitral valve leaflets. It extends from the left fibrous trigone at the posterior portion of the left coronary leaflet, to the right fibrous trigone next to the membranous interventricular septum at the non-coronary leaflet (Figure 3) (Aiba T et al., 2001; Ho SY, 2009). Although this is a fibrous structure, ventricular arrhythmias have been shown to arise from this region, and radiofrequency ablation successfully eradicates the arrhythmia (Kumagai K, et al., 2008; Yamada T et al., 2010; Chen J et al., 2012). The reason for this remains unclear, but studies have shown that cells electrophysiologically resembling atrioventricular nodal cells are present in the aortomitral continuity, which may represent remnants of the conduction system during embryonic development which becomes arrhythmogenic later in life (Wit AL et al., 1973; McGuire MA et al., 1996; Kurosawa H et al., 1985; Gonzalez MD et al., 2004; Szili-Torok T et al., 2012; Anderson RH et al., 2013).
The Atrioventricular Conduction Axis
The atrioventricular conduction axis maintains electrical continuity through the heart's fibrous skeleton. The bundle of His is actually identified by its encasement in the central fibrous body and location within the membranous atrioventricular septum, transitioning both anatomically and histologically between the atrioventricular node and the bundle branches (Figure 4 & 5) (Anderson RH et al., 1975; Anderson RH et al., 2002; Anderson RH, 2009; Anderson RH et al., 2013).
The right bundle is a cord like structure (James TN et al., 1974) which runs superficially in the right ventricular endocardium in its upper third up to the level of the muscle of Lancisi (septal papillary muscle of the tricuspid valve). There, it courses deeper in the interventricular septum, then becomes superficial in its distal third to course within the ventricular trabeculations, usually travelling to the right ventricular free wall in the moderator band. The left bundle is a broad sheet-like structure that emerges beneath the non-coronary cusp of the aortic valve and gives rise to a thin anterior fascicle, a broader posterior fascicle and in approximately 60% of individuals a third fascicle termed the left median fascicle (Demoulin JC et al., 1973; Kulbertus HE, 1975). The anterior fascicle crosses the left ventricular outflow tract to the anterolateral wall and the region of the anterolateral papillary muscle, the posterior fascicle extends inferoposteriorly to insert near the base of the posterior papillary muscle and the free wall, and the median fascicle runs in the interventricular septum. This knowledge is fundamental to understanding the pathways, surface electrocardiographic manifestations and approach to ablation of ventricular arrhythmias that are related to the infrahisian conduction system (Nogami A, 2011).
Anatomical-electrocardiographic correlation
During recording of an electrocardiogram, the polarity of QRS complex in a lead reflects the direction of ventricular depolarization correspond to the location of that lead. As lead V1 is the most rightward and anterior chest lead (Figure 8), ventricular arrhythmias originating from the right ventricular outflow tract direct away from lead V1 and display negative QRS. At the same time, the electrical wavefronts also direct inferiorly leading to positive QRS vectors in the limb leads II, III and aVF (Figure 8). These give rise to the classical appearance of left bundle branch block morphology (i.e. negative QRS at lead V1) and inferior axis for ventricular arrhythmias arising from the right ventricular outflow tract (Figure 9). On the other hand, as most parts of the left ventricular outflow tract are located posterior and leftward to the right ventricular outflow tract, ventricular arrhythmias originating from the left ventricular outflow tract typically positive QRS in lead V1 (right bundle branch block morphology) and inferior axis (Figure 10).
Figure 8.

A. Limb lead positions for electrocardiographic recordings.
B. Precordial lead positions for electrocardiographic recordings.
Figure 9.

Classical electrocardiogram of right ventricular outflow tract tachycardia associated with left bundle branch block morphology and inferior axis.
LBBB: left bundle branch block.
Figure 10.
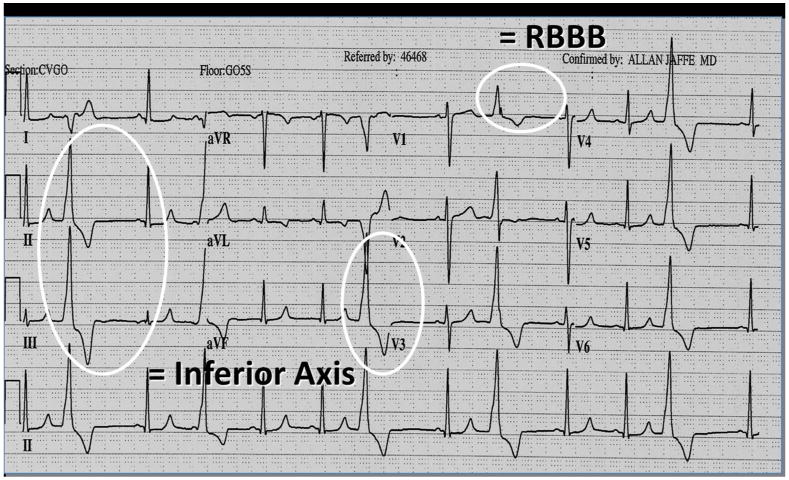
Classical electrocardiogram of left ventricular outflow tract premature ventricular complexes associated with right bundle branch block morphology and inferior axis.
RBBB: right bundle branch block.
There are exceptions to this rule. First, the electrical wavefronts of ventricular arrhythmias arising from the posterior infundibulum of the right ventricular outflow tract or the left-sided distal right ventricular outflow tract both direct towards lead V1 initially and thus show an initial positive R wave at lead V1 (Figure 11). Second, as the right coronary sinus is in close proximity with the posterior right ventricular outflow tract, similar QRS morphology may be seen with ventricular arrhythmias arising from this region (Huang et al., 2006; Zhang et al., 2009). In general, the more posterior and leftward is the arrhythmic origin, the more positive is the QRS vector in lead V1
Figure 11.

Non-sustained ventricular tachycardia originating from the paraHisian region. Note the left bundle branch block pattern, positive aVL and negative aVR. Because of medically refractory symptoms and tachycardia-mediated cardiomyopathy, patient agreed to proceed to ablation with the understanding that heart block may result. Procedure was performed which was complicated with permanent atrioventricular block requiring pacemaker implantation.
Similar principals hold for all other leads. For example, lead I is located at the most leftward portion of the heart (Figure 8). As a result, ventricular arrhythmias arising from near the pulmonary valve, which is on the left side of the heart, are characterized by QS complex in lead I, while those from the more proximal rightward portion of the right ventricular outflow tract are usually associated with initial R wave in lead I (Yamauchi Y et al., 2005; Asirvatham SJ, 2009). Another example is the electrocardiographic characterization of paraHisian ventricular arrhythmias. Since leads aVL and aVR are the left and right superior limb limbs respectively, ventricular arrhythmias originating from the high-lying right ventricular outflow tract are usually associated with QS complexes in these two leads (Figure 2). Nevertheless, since the His bundle is located at the most proximal and rightward part of the right ventricular outflow tract, the vector of aVL may become isoelectric or even slightly positive. When this electrocardiographic feature is present, one should reconsider the appropriateness of ablation procedures, as complete heart block may develop during ablation of a parahisian arrhythmic focus (Figure 11).
Summary
We have presented key anatomic facts on structure and relationships that every student of anatomy needs to know when appreciating the facilitatory role of understanding structure in allowing management of common ventricular arrhythmias that arise in the structurally normal heart.
Footnotes
Conflicts of Interest: None
Abbreviations: None
References
- Abouezzeddine O, Suleiman M, Buescher T, Kapa S, Friedman PA, Jahangir A, Mears JA, Ladewig DJ, Munger TM, Hammill SC, Packer DL, Asirvatham SJ. Relevance of endocavitary structures in ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21:245–254. doi: 10.1111/j.1540-8167.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- Aiba T, Suyama K, Aihara N, Taguchi A, Shimizu W, Kurita T, Kamakura S. The role of Purkinje and pre-Purkinje potentials in the reentrant circuit of verapamil-sensitive idiopathic LV tachycardia. Pacing Clin Electrophysiol. 2001;24:333–344. doi: 10.1046/j.1460-9592.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- Ainge G, Clarke CJ. Spontaneous myocardial concentration of Purkinje fiberlike cells in a Beagle dog. Toxicol Pathol. 2000;28:827–828. doi: 10.1177/019262330002800609. [DOI] [PubMed] [Google Scholar]
- Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109:1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- Aizawa Y, Naitoh N, Kitazawa H, Kusano Y, Uchiyama H, Washizuka T, Shibata A. Frequency of presumed reentry with an excitable gap in sustained ventricular tachycardia unassociated with coronary artery disease. Am J Cardiol. 1993;72:916–921. doi: 10.1016/0002-9149(93)91107-s. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Denker S, Lehmann MH, Mahmud R. Macro-reentry within the His Purkinje system. Pacing Clin Electrophysiol. 1983;6:1010–1028. doi: 10.1111/j.1540-8159.1983.tb04440.x. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Damato AN, Batsford WP, Ruskin JN, Ogunkelu JB, Vargas G. Demonstration of re-entry within the His-Purkinje system in man. Circulation. 1974;50:1150–1162. doi: 10.1161/01.cir.50.6.1150. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Becker AE, Brechenmacher C, Davies MJ, Rossi L. The human atrioventricular junctional area. A morphological study of the A-V node and bundle Eur J Cardiol. 1975;3:11–25. [PubMed] [Google Scholar]
- Anderson RH, Devine WA, Ho SY, Smith A, McKay R. The myth of the aortic annulus: The anatomy of the subaortic outflow tract. Ann Thorac Surg. 1991;52:640–646. doi: 10.1016/0003-4975(91)90966-t. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Ho SY. The morphology of the specialized atrioventricular junctional area: The evolution of understanding. PACE. 2002;25:957–966. doi: 10.1046/j.1460-9592.2002.00957.x. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Yanni J, Boyett MR, Chandler NJ, Dobrzynski H. The anatomy of the Cardiac Conduction System. Clinical Anatomy. 2009;22:99–113. doi: 10.1002/ca.20700. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Boyett MR, Dobrzynski H, Moorman AFM. The anatomy of the condution system: Implications for the clinical cardiologist. J of Cardiovasc Trans Res. 2013;6:187–196. doi: 10.1007/s12265-012-9433-0. [DOI] [PubMed] [Google Scholar]
- Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol. 2009;20:955–968. doi: 10.1111/j.1540-8167.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- Betensky BP, Park RE, Marchlinski FE, Hutchinson MD, Garcia FC, Dixit S, Callans DJ, Cooper JM, Bala R, Lin D, Riley MP, Gerstenfeld EP. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57:2255–2262. doi: 10.1016/j.jacc.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Cabrera JA. Damian anchez-Quintana: Cardiac anatomy: What the electrophysiologist needs to know. Heart. 2013;99:417–431. doi: 10.1136/heartjnl-2011-301154. [DOI] [PubMed] [Google Scholar]
- Chen J, Hoff PI, Rossvoll O, De Bortoli A, Solheim E, Sun L, Schuster P, Larsen T, Ohm OJ. Ventricular arrhythmias originating from the aortomitral continuity: An uncommon variant of left ventricular outflow tract tachycardia. Europace. 2012;14:388–395. doi: 10.1093/europace/eur318. [DOI] [PubMed] [Google Scholar]
- Chiu IS, Anderson RH. Can we better understand the known variations in coronary arterial anatomy? The Annals of thoracic surgery. 2012;94:1751–1760. doi: 10.1016/j.athoracsur.2012.05.133. [DOI] [PubMed] [Google Scholar]
- Demoulin JC, Kulbertus HE. Left hemiblocks revisited from the histopathological viewpoint. Am Heart J. 1973;86:712–713. doi: 10.1016/0002-8703(73)90354-2. [DOI] [PubMed] [Google Scholar]
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol. 2008;1:23–29. doi: 10.1161/CIRCEP.107.742940. [DOI] [PubMed] [Google Scholar]
- Gami AS, Noheria A, Lachman N, Edwards WD, Friedman PA, Talreja D, Hammill SC, Munger TM, Packer DL, Asirvatham SJ. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol. 2011;30:5–15. doi: 10.1007/s10840-010-9523-3. [DOI] [PubMed] [Google Scholar]
- Garg N, Tewari S, Kapoor A, Gupta DK, Sinha N. Primary congenital anomalies of the coronary arteries: A coronary: Arteriographic study. International journal of cardiology. 2000;74:39–46. doi: 10.1016/s0167-5273(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Habib A, Lachman N, Christensen KN, Asirvatham SJ. The anatomy of the coronary sinus venous system for the cardiac electrophysiologist. Europace. 2009;11(Suppl 5):v15–21. doi: 10.1093/europace/eup270. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Shah DC, Jais P, Shoda M, Kautzner J, Arentz T, Kalushe D, Kadish A, Griffith M, Gaita F, Yamane T, Garrigue S, Hocini M, Clementy J. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–678. doi: 10.1016/S0140-6736(02)07807-8. [DOI] [PubMed] [Google Scholar]
- Hlivak P, Peichl P, Cihak R, Wichterle D, Kautzner J. Catheter ablation of idiopathic ventricular tachycardia originating from myocardial extensions into a noncoronary aortic cusp. J Cardiovasc Electrophysiol. 2012;23:98–101. doi: 10.1111/j.1540-8167.2011.02152.x. [DOI] [PubMed] [Google Scholar]
- Ho SY. Structure and anatomy of the aortic root. Eur J Echocardiogr. 2009;10:i3–10. doi: 10.1093/ejechocard/jen243. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang X, Ouyang F, Antz M. Catheter ablation of anteroseptal accessory pathway in the non-coronary aortic sinus. Europace. 2006;8:1041–1044. doi: 10.1093/europace/eul122. [DOI] [PubMed] [Google Scholar]
- James TN, Sherf L, Urthaler F. Fine structure of the bundle-branches. Br Heart J. 1974;36:1–18. doi: 10.1136/hrt.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbertus HE. Concept of left hemiblocks revisited. A histopathological and experimental study. Adv Cardiol. 1975;14:126–135. doi: 10.1159/000397645. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Fukuda K, Wakayama Y, Sugai Y, Hirose M, Yamaguchi N, Takase K, Yamauchi Y, Takahashi A, Aonuma K, Shimokawa H. Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 2008;19:495–501. doi: 10.1111/j.1540-8167.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Lachman N, Syed FF, Habib A, Kapa S, Bisco SE, Venkatachalam KL, Asirvatham SJ. Correlative anatomy for the electrophysiologist, part II: cardiac ganglia, phrenic nerve, coronary venous system. J Cardiovasc Electrophysiol. 2011;22:104–110. doi: 10.1111/j.1540-8167.2010.01882.x. [DOI] [PubMed] [Google Scholar]
- Lin D, Ilkhanoff L, Gerstenfeld E, Dixit S, Beldner S, Bala R, Garcia F, Callans D, Marchlinski FE. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5:663–669. doi: 10.1016/j.hrthm.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Merrick AF, Yacoub MH, Ho SY, Anderson RH. Anatomy of the muscular subpulmonary infundibulum with regard to the Ross procedure. Ann Thorac Surg. 2000;69:556–561. doi: 10.1016/s0003-4975(99)01300-4. [DOI] [PubMed] [Google Scholar]
- McGuire MA, de Bakker JM, Vermeulen JT, Moorman AF, Loh P, Thibault B, Vermeulen JL, Becker AE, Janse MJ. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation. 1996;94:571–577. doi: 10.1161/01.cir.94.3.571. [DOI] [PubMed] [Google Scholar]
- Nogami A, Sugiyasu A, Kubota S, Kato K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart rhythm : the official journal of the Heart Rhythm Society. 2005;2:646–649. doi: 10.1016/j.hrthm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Nogami A. Purkinje-related arrhythmias Part I: Monomorphic ventricular tachycardias. PACE. 2011;34:624–650. doi: 10.1111/j.1540-8159.2011.03044.x. [DOI] [PubMed] [Google Scholar]
- Otomo K, Azegami K, Sasaki T, Kawabata M, Hirao K, Isobe M. Successful catheter ablation of focal left atrial tachycardia originating from the mitral annulus aorta junction. Int Heart J. 2006;47:461–468. doi: 10.1536/ihj.47.461. [DOI] [PubMed] [Google Scholar]
- Ouyang F, Fotuhi P, Ho SY, Hebe J, Volkmer M, Goya M, Burns M, Antz M, Ernst S, Cappato R, Kuck KH. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol. 2002;39:500–508. doi: 10.1016/s0735-1097(01)01767-3. [DOI] [PubMed] [Google Scholar]
- Piazza N, de Jaegere P, Schultz C, Backer AE, Surruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1:74–81. doi: 10.1161/CIRCINTERVENTIONS.108.780858. [DOI] [PubMed] [Google Scholar]
- Suleiman M, Asirvatham SJ. Ablation above the semilunar valves: When, why, and how? Part I. Heart Rhythm. 2008;5:1485–1492. doi: 10.1016/j.hrthm.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Srivathsan KS, Bunch TJ, Asirvatham SJ, Edwards WD, Friedman PA, Munger TM, Hammill SC, Cha YM, Brady PA, Jahangir A, Bradley DJ, Rea RF, Packer DL, Shen WK. Mechanisms and utility of discrete great arterial potentials in the ablation of outflow tract ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2008;1:30–38. doi: 10.1161/CIRCEP.107.750315. [DOI] [PubMed] [Google Scholar]
- Szili-Torok T, van Malderen S, de Groot N. ‘Born’ with a ‘dead’-end-tract resulting in arrhythmias in the aorto-mitral continuity: coincidence, causation, and ‘commensuration’. Europace. 2012;14:308–309. doi: 10.1093/europace/eur433. [DOI] [PubMed] [Google Scholar]
- Tabatabaei N, Asirvatham SJ. Supravalvular arrhythmia: identifying and ablating the substrate. Circ Arrhythm Electrophysiol. 2009;2:316–326. doi: 10.1161/CIRCEP.108.847962. [DOI] [PubMed] [Google Scholar]
- Topaz O, DeMarchena EJ, Perin E, Sommer LS, Mallon SM, Chahine RA. Anomalous coronary arteries: angiographic findings in 80 patients. Int J Cardiol. 1992;34:129–138. doi: 10.1016/0167-5273(92)90148-v. [DOI] [PubMed] [Google Scholar]
- Tuncer C, Batyraliev T, Yilmaz R, Gokce M, Eryonucu B, Koroglu S. Origin and distribution anomalies of the left anterior descending artery in 70,850 adult patients: Multicenter data collection. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2006;68:574–585. doi: 10.1002/ccd.20858. [DOI] [PubMed] [Google Scholar]
- Wit AL, Cranefield PF. Triggered activity in cardiac muscle fibers of the simian mitral valve. Circ Res. 1976;38:85–98. doi: 10.1161/01.res.38.2.85. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Aonuma K, Takahashi A, Sekiguchi Y, Hachiya H, Yokoyama Y, Kumagai K, Nogami A, Iesaka Y, Isobe M. Electrocardiographic characteristics of repetitive monomorphic right ventricular tachycardia originating near the His-bundle. J Cardiovasc Electrophysiol. 2005;16:1041–1048. doi: 10.1111/j.1540-8167.2005.40787.x. [DOI] [PubMed] [Google Scholar]
- Yamada T, McElderry HT, Doppalapudi H, Murakami Y, Yoshida Y, Yoshida N, Okada T, Tsuboi N, Inden Y, Murohara T, Epstein AE, Plumb VJ, Singh SP, Kay GN. Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. Journal of the American College of Cardiology. 2008;52:139–147. doi: 10.1016/j.jacc.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen M, Yang B, Ju W, Chen H, Yu J, Lau CP, Cao K, Tse HF. Electrocardiographic algorithm to identify the optimal target ablation site for idiopathic right ventricular outflow tract ventricular premature contraction. Europace. 2009;11:1214–1220. doi: 10.1093/europace/eup231. [DOI] [PubMed] [Google Scholar]


