Abstract
Background and objectives
Elevated total serum alkaline phosphatase levels have been associated with higher mortality in the general population, CKD patients, and hemodialysis patients. However, in peritoneal dialysis patients, this association has received little attention. The aim of this study was to evaluate the association between alkaline phosphatase and all-cause and cardiovascular mortality in peritoneal dialysis patients.
Design, setting, participants, & measurements
In this single center retrospective cohort study, 1021 incident peritoneal dialysis patients from January 1, 2006, to December 31, 2010 with baseline serum alkaline phosphatase values were enrolled. Collected baseline data included demographic characteristics and clinical and laboratory measurements. All patients were followed until December 31, 2012. The associations of total serum alkaline phosphatase levels with all-cause and cardiovascular mortality were assessed using multivariable-adjusted Cox models.
Results
Of 1021 patients, mean age was 47.5 (±15.5) years, 59.1% of patients were men, and 22.8% of patients were diabetic. The median serum alkaline phosphatase level was 64 U/L (interquartile range=52–82 U/L). During a median 31-month (interquartile range=19–45 months) follow-up period, 203 patients died, of which 109 deaths were caused by cardiovascular disease. After adjusting for demographics, comorbid conditions, liver function, and bone metabolism parameters, the highest alkaline phosphatase quartile was significantly associated with a hazard ratio for all-cause mortality of 1.70 (95% confidence interval, 1.06 to 2.74, P=0.03) and a hazard ratio for cardiovascular mortality of 1.94 (95% confidence interval, 1.02 to 3.72, P=0.04). Each 10 U/L higher baseline alkaline phosphatase level was associated with 4% (95% confidence interval, 1.00 to 1.08, P=0.04) and 7% (95% confidence interval, 1.02 to 1.11, P=0.003) higher risk of all-cause and cardiovascular mortality, respectively.
Conclusion
Higher total serum alkaline phosphatase levels at the commencement of peritoneal dialysis were independently associated with all-cause and cardiovascular mortality in peritoneal dialysis patients.
Introduction
Alkaline phosphatase (ALP) is a hydrolytic enzyme responsible for removing phosphate groups from many types of molecules. As a commonly collected laboratory measure in CKD patients, ALP is usually used as a biomarker of high-turnover bone disease. Emerging evidence suggests that ALP might be not only a marker of bone metabolism but also, a pathogenic factor in the general population and CKD patients associated with higher risk of mortality. Data from the National Health and Nutrition Examination Survey (NHANES) database show a graded independent association between higher ALP levels and mortality in the general population (1). In nondialysis-dependent CKD patients, higher ALP levels were associated with higher risk of ESRD and all-cause mortality (2–4). Similarly, in maintenance hemodialysis (HD) patients, elevated ALP levels were also associated with higher risk of mortality, independent of liver function and bone metabolism parameters (5–7).
Recently, Pelletier et al. (8) found that bone microarchitecture is more severely affected in patients on HD than patients receiving peritoneal dialysis (PD) using high-resolution quantitative computed tomography. Additionally, a cross-sectional study in China found that mineral and bone disorder (MBD) in PD patients is not as serious as in HD patients (9). However, whether a similar association between ALP and mortality also exists in PD patients is unknown. We hypothesized that higher total serum ALP levels are associated independently with higher risks of all-cause and cardiovascular mortality in PD patients. Therefore, we conducted this longitudinal cohort study to evaluate the relationship between ALP level and all-cause and cardiovascular mortality in PD patients followed up at our PD center.
Materials and Methods
Study Population and Data Collection
We studied all incident patients who used PD as their first RRT modality and were followed up at the PD center of The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China from January 1, 2006, to December 31, 2010. Inclusion criteria were age≥18 years at the start of PD and survival for at least 90 days from the first PD therapy. The patients who were catheterized in other hospitals, transferred from permanent HD, or failed renal transplantation were excluded in this study. The study was conducted in compliance with the ethical principles of the Helsinki Declaration (http://www.wma.net/en/30publications/10policies/b3/index.html) and approved by the Human Ethics Committees of Sun Yat-sen University. As a part of a larger cohort study, written informed consent was obtained from all participants.
All patients were followed up until cessation of PD, death, or December 31, 2012. Baseline demographic data included age, sex, primary cause of ESRD, and presence of diabetes and cardiovascular disease (CVD). Clinical and biochemical data at the initiation of PD included body mass index, BP, medication use, hemoglobin, serum albumin, serum creatinine, BUN, total cholesterol, triglycerides, corrected serum calcium, phosphorus, intact parathyroid hormone (iPTH), aspartate aminotransferase (AST), alanine transaminase (ALT), ALP, and total bilirubin. All baseline data were obtained during the first 1–3 months of PD. The ALP values around 6 months after initiation of PD were also collected. Baseline residual renal function was assessed by eGFR using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. Cardiovascular death, which was defined as death caused by coronary events, arrhythmias, sudden cardiac death, congestive heart failure, or cerebrovascular events (10), was determined by the PD follow-up panel composed of PD primary nurses and professors. The comorbidity score was determined according to the Charlson Comorbidity Index, which is one of the most commonly used comorbidity models (11). We adapted the guideline of the Kidney Disease Outcomes Quality Initiative for the evaluation and treatment of MBD in the PD patients (12), and in recent years, we have been managing our patients for their MBD in accordance with the guideline of the Kidney Disease: Improving Global Outcomes (13).
Statistical Analyses
Patients with measured ALP values were classified into quartiles (Qs): Q1≤52 U/L; Q2=53–64 U/L; Q3=65–81 U/L; Q4≥82 U/L. Participant characteristics were calculated by Qs of ALP. Results were expressed as frequencies and percentages for categorical variables, means and SDs for normally distributed continuous variables, and medians and interquartile ranges for continuous variables not normally distributed. Chi-squared, one-way ANOVA, or Kruskal–Wallis tests were used to test for differences in categorical or continuous factors among different categories of ALP. The correlations between ALP Q and inflammation, liver function, and bone metabolism parameters were assessed by Spearman rank correlation analysis. Survival times were estimated from Kaplan–Meier curves, and differences in survival probabilities among groups were assessed using the log-rank test. The association between serum ALP levels and all-cause and cardiovascular mortality was examined in Cox proportional hazards models. The censored data included switching to HD, renal transplantation, moving to another center, declining additional treatment, loss to follow-up, or still at our PD center on December 31, 2012. The relationships between ALP level and outcomes were evaluated by both continuous ALP and ALP Qs. Unadjusted associations were first examined followed by adjustments for age, sex, BP, 24-hour urine output, comorbidity score, hemoglobin, and neutrophil to lymphocyte ratio (N/L) as well as serum albumin, ALT, AST, and medication use, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, vitamin D analogs, and phosphate binders. Next, corrected serum calcium, phosphorus, and iPTH levels were added to examine whether the association of ALP with mortality was independent of bone metabolism parameters. Covariates with P<0.05 in the univariate Cox analyses or thought to be related to ALP level were chosen for multivariate Cox proportional hazards regression. The results were expressed as the hazard ratio (HR) and 95% confidence interval (95% CI). All descriptive and multivariate analyses were conducted using SPSS version 16.0 (SPSS, Inc., Chicago, IL). A value of P<0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
In total, 1193 incident PD patients were catheterized at our PD center, of whom 14 patients were younger than 18 years, 7 patients were transferred from failed renal transplantation, 36 patients were transferred from permanent HD, and 62 patients were on PD less than 3 months. The remaining 1074 patients were enrolled in this study. Of 1074 patients, 1021 patients had ALP measured at baseline and were eligible for the present analysis (Figure 1). The mean (±SD) age was 47.5±15.5 years; 59.1% of patients were men, and 22.8% of patients were diabetic. The primary cause of ESRD was chronic GN (60.3%) followed by diabetic nephropathy (22.1%) and hypertension (6.6%). Almost all of the patients received continuous ambulatory PD treatment, except two patients who used automated PD. Conventional PD solutions (Dianeal 1.5%, 2.5%, or 4.25% dextrose; Baxter Healthcare, Guangzhou, China), Y sets, and twin bag systems were used in all PD patients. During the management of MBD of our patients, vitamin D analogs were prescribed for 53.4% of patients, and calcium-containing phosphate binders were prescribed for 60.1% of patients. Very few patients used sevelamer carbonate (4.6%) and lanthanum carbonate (1.2%). Before June 1, 2012, we prescribed both conventional PD solution (Ca2+ concentration=1.75 mmol/L) and physiologic calcium peritoneal dialysate (Ca2+ concentration=1.25 mmol/L) for our PD patients according to the patients’ bone metabolism condition. Since that time, however, only physiologic calcium peritoneal dialysate is available for all patients in our center.
Figure 1.
The flow chart shows how patients were selected for the present study. ALP, alkaline phosphatase; HD, hemodialysis; PD, peritoneal dialysis.
ALP Qs
Baseline serum ALP levels ranged from 4.2 to 447 U/L (median=64 U/L, interquartile range=52–82 U/L, mean=73 U/L), and 108 (10.6%) patients had ALP levels outside the normal range in our laboratory (0–110 U/L). Baseline characteristics of the patients by Qs of serum ALP levels are shown in Table 1. Patients with higher ALP levels were older, were more likely to be diabetic, had higher comorbidity score, had higher N/L, ALT, AST, and iPTH levels, and had lower BP, serum creatinine, and calcium levels (P<0.05) (Table 1). There were no significant differences among groups in body mass index, eGFR, medication use, hemoglobin, albumin, and serum phosphorus levels (Table 1). Spearman rank correlation analyses indicated that serum ALP levels positively correlated with C-reactive protein (CRP), N/L, ALT, AST, and iPTH levels and negatively correlated with calcium and phosphorus level (P<0.01) (Table 2). In a multivariate adjusted logistic regression model, higher age, lower calcium level, lower phosphorus level, and higher iPTH level were associated with higher ALP level (≥82 U/L; P<0.05).
Table 1.
Baseline characteristics of individuals stratified by quartiles of baseline serum ALP level
| Variable | Serum ALP (U/L) | P Value | |||
|---|---|---|---|---|---|
| ≤52 (n=271) | 53–64 (n=243) | 65–81 (n=253) | ≥82 (n=254) | ||
| Age (yr) | 44.7±14.9 | 45.1±15.8 | 48.3±14.9 | 52.0±15.3 | <0.001a |
| Men (%) | 158 (58.3) | 134 (55.1) | 169 (66.8) | 142 (55.9) | 0.03a |
| Body mass index (kg/m2) | 21.1±2.9 | 21.4±3.2 | 21.5±3.0 | 21.4±3.0 | 0.44 |
| Diabetes (%) | 46 (17.0) | 44 (18.1) | 71 (28.1) | 72 (28.3) | 0.001a |
| CVD (%) | 97 (35.8) | 76 (31.3) | 89 (35.2) | 105 (41.3) | 0.13 |
| Comorbidity score | 3.33±1.85 | 3.49±1.88 | 3.77±1.87 | 4.24±2.00 | <0.001a |
| 24-hr urine output (ml) | 1000 (500–1475) | 900 (540–1200) | 1000 (500–1500) | 925 (500–1400) | 0.47 |
| eGFR (ml/min per 1.73 m2) | 5.63 (4.23–7.44) | 5.65 (4.39–7.54) | 6.05 (4.62–8.08) | 5.99 (4.57–7.93) | 0.11 |
| Systolic pressure (mmHg) | 140±18 | 138±19 | 139±20 | 135±23 | 0.03a |
| Diastolic pressure (mmHg) | 86±14 | 86±14 | 84±14 | 82±16 | 0.02a |
| Hemoglobin (g/dl) | 8.96±2.38 | 9.27±2.29 | 9.04±2.13 | 9.26±2.35 | 0.32 |
| N/L | 2.69 (1.85–3.72) | 2.86 (2.03–4.07) | 2.97 (2.13–4.07) | 2.92 (2.23–4.32) | 0.03a |
| Albumin (g/dl) | 3.63±0.54 | 3.67±0.52 | 3.66±0.53 | 3.65±0.56 | 0.84 |
| Calcium (mg/dl) | 8.84±0.99 | 8.85±1.02 | 8.71±0.94 | 8.53±1.06 | 0.001a |
| Phosphorus (mg/dl) | 5.41±1.89 | 5.18±1.65 | 5.07±1.62 | 5.14±1.78 | 0.08 |
| iPTH (pg/ml) | 238 (123–442) | 238 (120–444) | 286 (146–442) | 342 (178–562) | <0.001a |
| Urea nitrogen (mg/dl) | 110 (85–155) | 113 (85–154) | 115 (86–155) | 115 (85–155) | 0.98 |
| Creatinine (mg/dl) | 8.9 (7.0–11.6) | 8.7 (6.8–11.2) | 8.4 (6.7–10.5) | 8.1 (6.4–10.4) | 0.01a |
| Total cholesterol (mg/dl) | 193±54 | 192±53 | 191±52 | 190±53 | 0.96 |
| Triglyceride (mg/dl) | 114 (82–166) | 124 (90–187) | 121 (86–165) | 117 (84–166) | 0.17 |
| ALT (U/L) | 14 (9–19) | 14 (10–22) | 15 (10–23) | 16 (11–25) | 0.003a |
| AST (U/L) | 17 (14–23) | 18 (14–23) | 18 (14–23) | 22 (15–30) | <0.001a |
| Total bilirubin (mg/dl) | 0.26 (0.20–0.32) | 0.26 (0.19–0.34) | 0.26 (0.20–0.35) | 0.26 (0.20–0.34) | 0.93 |
| Uric acid (mg/dl) | 7.53±1.81 | 7.69±1.84 | 7.54±1.76 | 7.54±1.99 | 0.74 |
| Vitamin D analog use (%) | 62 (22.9) | 55 (22.6) | 66 (26.1) | 63 (24.8) | 0.77 |
| Phosphate binder use (%) | 92 (33.9) | 88 (36.2) | 97 (38.3) | 100 (39.4) | 0.58 |
| ACEI/ARB use (%) | 123 (45.4) | 110 (45.3) | 122 (48.2) | 115 (45.3) | 0.89 |
| β-Blocker use (%) | 73 (26.9) | 67 (27.6) | 75 (29.6) | 66 (26.0) | 0.82 |
ALP, alkaline phosphatase; CVD, cardiovascular disease; N/L, neutrophil to lymphocyte ratio; iPTH, parathyroid hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P<0.05 is considered statistically significant.
Table 2.
Correlation between ALP quartile and parameters of inflammation, liver function, and bone metabolism
| ALP Quartile | CRP | Albumin | ALT | AST | Bilirubin | Calcium | Phosphorus | iPTH | |
|---|---|---|---|---|---|---|---|---|---|
| CRP | 0.13a | ||||||||
| Albumin | 0.01 | −0.12a | |||||||
| ALT | 0.12a | −0.09b | −0.01 | ||||||
| AST | 0.16a | −0.04 | −0.10a | 0.61a | |||||
| Bilirubin | 0.02 | −0.12a | 0.20a | 0.12a | 0.12a | ||||
| Calcium | −0.10a | −0.08b | 0.40a | −0.01 | −0.03 | 0.11a | |||
| Phosphorus | −0.08a | 0.11a | −0.06 | 0.03 | −0.03 | −0.01 | −0.31a | ||
| iPTH | 0.13a | <0.01 | 0.01 | 0.01 | −0.02 | −0.07 | −0.42a | 0.29a | |
| N/L | 0.09a | 0.14a | −0.01 | −0.02 | −0.02 | 0.03 | −0.07b | 0.04 | −0.01 |
CRP, C-reactive protein.
Correlation is significant at the 0.01 level (two-tailed).
Correlation is significant at the 0.05 level (two-tailed).
ALP, All-Cause Mortality, and CVD Mortality
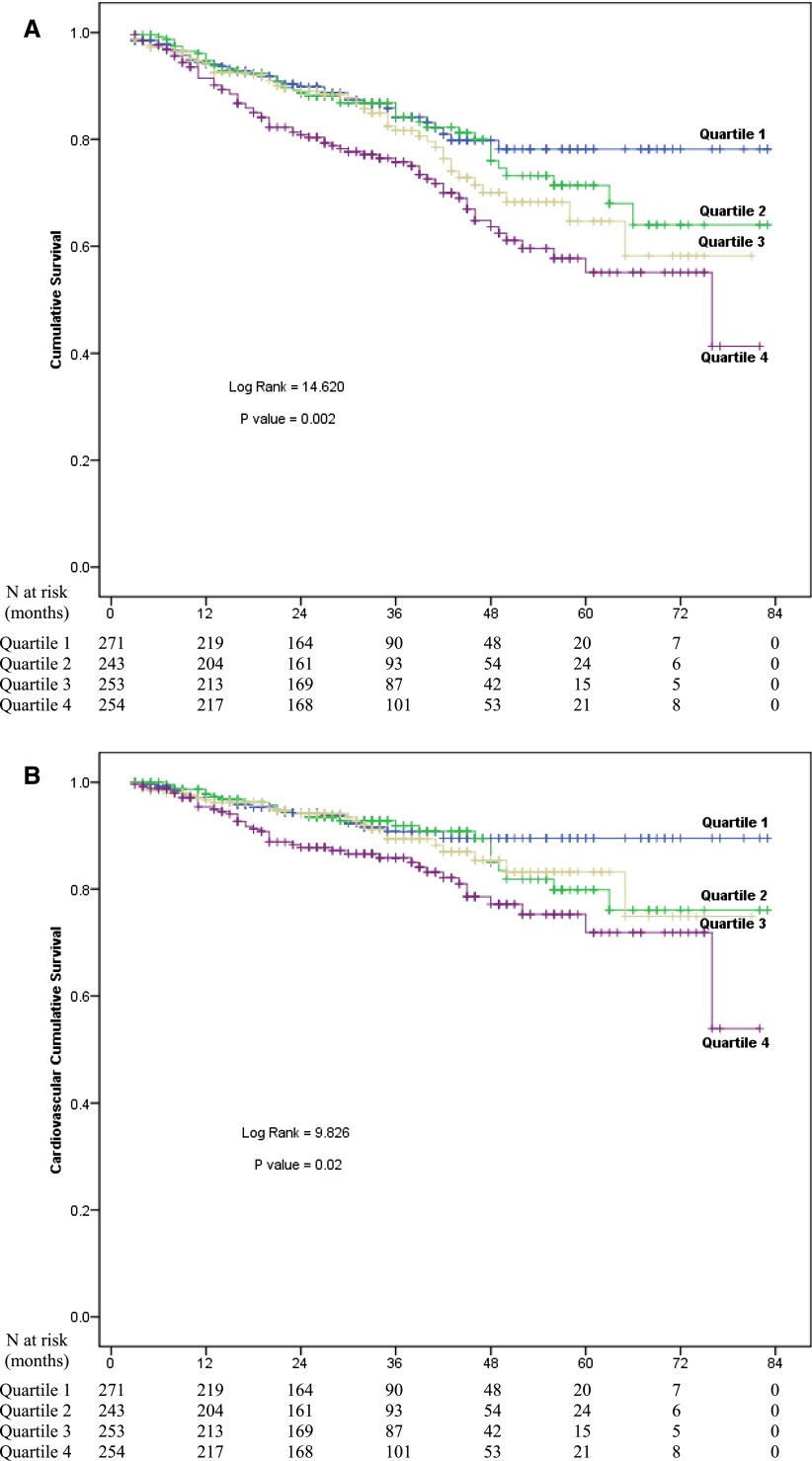
The median follow-up period was 31 months (interquartile range=19–45 months). By the end of this study, 203 (18.9%) patients had died, 172 (16.0%) patients had received kidney transplantation, 83 (7.7%) patients had transferred to HD, 58 (5.4%) patients had transferred to other PD centers, 34 (3.2%) patients had been lost to follow-up, and 12 (1.1%) patients had declined additional treatment; the remaining 512 (47.7%) patients were still followed at our PD center. Of 203 deaths, 109 (53.7%) deaths were caused by CVD, 35 (17.2%) deaths were caused by infectious disease, 7 (3.4%) deaths were caused by malignancy, 7 (3.4%) deaths were caused by cachexia, 17 (8.4%) deaths were caused by other reasons, and 28 (13.8%) deaths had an unknown reason. Kaplan–Meier estimates of all-cause and cardiovascular mortality for patients with different ALP levels are shown in Figure 2. At the end of 1, 3, and 5 years, all-cause mortality was 5.9%, 15.9%, and 21.8%, respectively, in the Q1 group; 5.3%, 15.8%, and 28.6%, respectively, in the Q2 group; 5.8%, 18.4%, and 35.3%, respectively, in the Q3 group; and 6.5%, 24.2%, and 44.9%, respectively, in the Q4 group. Compared with other three lower Qs, patient survival rate was significantly lower in the Q4 group (P=0.002) (Figure 2A). Cardiovascular mortality was 2.8%, 9.3%, and 10.5%, respectively, in the Q1 group; 2.2%, 8.1%, and 20.1%, respectively, in the Q2 group; 3.4%, 10.6%, and 16.8%, respectively, in the Q3 group; and 4.6%, 14.1%, and 28.1%, respectively, in the Q4 group. Similarly, patients in Q4 group had the lowest cardiovascular survival rate among the groups (P=0.02) (Figure 2B).
Figure 2.
Survival curves for patients with different levels of serum ALP. Cumulative mortality curves for (A) all-cause mortality, and (B) cardiovascular mortality according to quartiles of ALP levels at baseline.
The association between baseline serum ALP levels and all-cause and cardiovascular mortality is shown in Table 3. There was significant association between ALP≥82 U/L (Q4) and all-cause and cardiovascular mortality, even after adjusting for liver enzymes and serum calcium, phosphorus, and iPTH (HR, 1.70; 95% CI, 1.06 to 2.74; P=0.03; HR, 1.94; 95% CI; 1.02 to 3.72; P=0.04, respectively). Association between baseline ALP level and all-cause and cardiovascular mortality was similar when we examined ALP as a continuous variable. In the multivariable adjusted model, each 10-U/L higher ALP level was associated with a 4% higher hazard (95% CI, 1.00 to 1.08; P=0.04) for all-cause death and a 7% higher hazard (95% CI, 1.02 to 1.11; P=0.003) for cardiovascular death. The follow-up ALP values in 702 patients around 6 months after PD initiation were available (median=64 U/L, interquartile range=52–78 U/L, mean=68 U/L) in time-depending analysis. During this period, 8.4% of patients used physiologic calcium peritoneal dialysate, which has been added in the multivariate adjusted model. After full adjustment, each 10-U/L higher ALP level was associated with an 8% higher risk (95% CI, 1.01 to 1.15; P=0.02) and an 8% higher risk (95% CI, 1.00 to 1.17; P=0.06) of all-cause and cardiovascular mortality, respectively (Table 3).
Table 3.
Associations between continuous and quartile of serum ALP and all-cause and cardiovascular mortality
| Model 1a | Model 2b,c | Model 3c,d | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All-cause mortality | ||||||
| Baseline continuous ALPe | 1.05 (1.02 to 1.08) | <0.001f | 1.03 (1.00 to 1.07) | 0.04f | 1.04 (1.00 to 1.08) | 0.04f |
| Follow-up continuous ALPe | 1.09 (1.04 to 1.13) | <0.001f | 1.08 (1.02 to 1.15) | 0.01f | 1.08 (1.01 to 1.15) | 0.02f |
| Baseline ALP quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Baseline ALP quartile 2 | 1.17 (0.75 to 1.81) | 0.50 | 1.18 (0.74 to 1.86) | 0.49 | 1.43 (0.85 to 2.41) | 0.18 |
| Baseline ALP quartile 3 | 1.38 (0.90 to 2.12) | 0.14 | 1.21 (0.77 to 1.89) | 0.42 | 1.40 (0.83 to 2.34) | 0.20 |
| Baseline ALP quartile 4 | 1.98 (1.33 to 2.93) | 0.001f | 1.67 (1.08 to 2.57) | 0.02f | 2.05 (1.24 to 3.41) | 0.01f |
| P for trend | <0.001f | 0.02f | 0.01f | |||
| Cardiovascular mortality | ||||||
| Baseline continuous ALPe | 1.07 (1.03 to 1.10) | <0.001f | 1.06 (1.02 to 1.10) | 0.003f | 1.07 (1.02 to 1.11) | 0.003f |
| Follow-up continuous ALPe | 1.08 (1.02 to 1.15) | 0.01f | 1.08 (1.00 to 1.16) | 0.05 | 1.08 (1.00 to 1.17) | 0.06 |
| Baseline ALP quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Baseline ALP quartile 2 | 1.30 (0.71 to 2.38) | 0.39 | 1.39 (0.74 to 2.61) | 0.31 | 1.61 (0.79 to 3.29) | 0.19 |
| Baseline ALP quartile 3 | 1.35 (0.74 to 2.47) | 0.33 | 1.19 (0.63 to 2.26) | 0.59 | 1.36 (0.66 to 2.80) | 0.41 |
| Baseline ALP quartile 4 | 2.17 (1.26 to 3.74) | 0.01f | 2.03 (1.12 to 3.67) | 0.02f | 2.40 (1.20 to 4.78) | 0.01f |
| P for trend | 0.004f | 0.03f | 0.02f | |||
HR, hazard ratio; 95% CI, 95% confidence interval.
Model 1: unadjusted.
Model 2: adjusted for age, sex, 24-hour urine output, BP, comorbidity score, hemoglobin, albumin, serum ALT, AST, N/L, and phosphate binders use.
Additionally adjusted for physiologic calcium peritoneal dialysate use while analyzing follow-up ALP.
Model 3: model 2 adjusted for corrected calcium, phosphorus, and iPTH.
Per 10 U/L higher ALP.
P<0.05 is considered statistically significant.
Because 60.3% of our patients had GN as the primary etiology, we examined the interaction between GN and ALP Qs on the outcomes of PD patients in multivariate adjusted model. However, we did not find any significant interaction between GN and ALP level on all-cause (P=0.91) and cardiovascular (P=0.94) mortality.
Discussion
In the present single center retrospective cohort study, we identified PD patient characteristics associated with total serum ALP levels and found that higher total serum ALP levels were incrementally associated with higher all-cause and cardiovascular mortality, even after adjustment for liver function and bone metabolism parameters. To our knowledge, this study is the first to show the association between serum ALP level and mortality in PD patients.
ALP is a hydrolytic enzyme that dephosphorylates various molecules and most effectively operates in an alkaline environment. Although ALP is expressed in a variety of tissue, its concentrations are highest in bone, liver, and the kidneys (14). Accordingly, serum levels of ALP are primarily used in clinical practice as a marker of hepatic or bony disease. Traditionally, ALP has only been considered a surrogate of bone metabolism in patients with CKD and ESRD. This study, along with other recently published studies, questions this widely held opinion (2–6). Elevated ALP levels have been associated with mortality in nondialysis-dependent CKD patients. Kovesdy et al. (3) reported that higher ALP level was associated with higher death risk in individuals with CKD stages 1–5. Beddhu et al. (2) reported that doubling baseline ALP level was associated with a 55% increase in all-cause mortality rate by a post hoc analysis from the African-American Study of Kidney Disease and Hypertension database. Another study including 28,678 patients with CKD stages 3 and 4 found that each 1-SD (42.7 U/L) higher ALP level was associated with 15% and 16% higher risk of ESRD and mortality, respectively, after multivariate adjustment (4).
Some studies have examined the association between ALP level and mortality in HD patients. Kalantar-Zadeh et al. (15) showed a higher risk of all-cause mortality associated with higher baseline and time-varying ALP levels in HD patients using the DaVita database. However, these analyses were not adjusted for serum calcium and phosphorus levels, which have been associated with higher mortality risk. Another analysis of the Dialysis Outcomes and Practice Patterns Study database showed that elevated serum ALP levels were associated with higher risk of hospitalization and death in HD patients, independent of serum calcium, phosphorus, and PTH levels, without including adjustment for liver function (5). Subsequently, using the DaVita and Hemodialysis Study databases, researchers found that high serum ALP levels in HD patients, adjusted for serum calcium, phosphorus, PTH, and liver enzymes, were indeed associated with higher mortality (6,7). However, little is known about PD patients. Our study revealed that total serum ALP levels were associated with higher all-cause and cardiovascular mortality in PD patients, independent of serum calcium, phosphorus, iPTH, and liver enzymes (AST and ALT). It was reported that bone mineral disease characteristics are different in PD and HD (9) and that bone microarchitecture is more severely affected in patients on HD than patients receiving PD (8); however, our study indicated that the association between ALP and mortality in PD patients is similar with the previous study in HD patients (6).
A possible explanation for the described association between total serum ALP level and mortality is that ALP is a marker of high-turnover bone disease, which has been linked to higher mortality (15,16). However, ALP may be more than a marker of bone turnover. There is mounting evidence that ALP can promote vascular calcification by hydrolyzing pyrophosphate in the arterial wall (14,17). In our study, the highest Q of ALP was associated with higher risk of cardiovascular death. In addition, our results showed that there was significantly positive relationship between the levels of ALP and iPTH. It is well known that both mineral bone metabolism parameters are associated with vascular calcification (18). However, in our study, serum ALP level negatively correlated with phosphorus level. The higher iPTH level and more frequent prescription of phosphate binders in the higher ALP group patients may be responsible for this result. Inhibitors of ALP were capable of reducing calcification in models of vascular calcification (19). Levamisole, a nonspecific inhibitor of ALP, could also reduce aortic calcification of uremic rat (17). Apart from vascular calcification, previous studies suggested that inflammation indicated by a higher level of CRP or the counts of white blood cells might be associated with elevated ALP and then participate in higher mortality (3,20,21). N/L is widely used as a marker of inflammation and a strong predictor for overall and cardiovascular mortality in PD patients (22). Our results also showed a positive correction between ALP and CRP as well as the N/L level (Table 2). Other plausible explanations for the observed association are decreased vitamin D levels, which are associated with mortality in CKD stage 5 patients (23), and insulin resistance (24). Thus, multiple potential mechanisms may contribute to the association between elevated ALP level and higher mortality. Based on the result that ALP may play a pathogenic role in uremia, leading to higher all-cause and cardiovascular mortality, ALP might be a potential therapeutic target in ESRD patients. Future studies are warranted to determine the potential role of inhibitor of ALP in ESRD patients.
There are some limitations in the present study. (1) It was a single center study, and therefore, center-specific effects cannot be excluded. (2) Its retrospective nature allows us to establish associations but not causal relationships. (3) We did not have bone-specific ALP available, which is a more sensitive and specific marker of bone metabolism than total ALP. However, few studies have shown that bone-specific ALP is a better predictor of death than total ALP in HD patients (25). A recent analysis using NHANES data did not show an association between bone-specific ALP level and mortality in the nondialysis-dependent CKD population (26). In addition, bone-specific ALP is not measured routinely, because the assay is not readily available and is expensive. Furthermore, bone-specific ALP immunoassays seem unable to distinguish the ALP isoenzyme optimally (27,28). (4) During the management of MBD of our patients, the vitamin D analogs and phosphate binders were used to acquire the targets recommended by the guidelines. These treatments may mitigate the mortality risk associated with ALP and need to be studied further. (5) Because of the restriction of sample size, we did not adjust all factors associated with higher mortality. Therefore, the effect of residual confounding cannot be eliminated completely.
In conclusion, we found an independent relationship between elevated total serum ALP level and higher risk of all-cause and cardiovascular mortality in PD patients. These findings, along with previous studies in this area, suggest that clinicians could use ALP as a risk assessment tool to identify PD patients with higher risk of mortality.
Disclosures
None.
Acknowledgments
We thank Jianxiong Lin, Chunyan Yi, and all nurses in our peritoneal dialysis center for their excellent data collection and Fenghua Xu and Qian Zhou for their assistance in statistical analyses.
This work was supported by Key Clinical Program of the Ministry of Health, China Grant 2010-439, Guangdong Natural Science Foundation of China Grant 9151008901000051, and National Basic Research Program of China Grant 2011CB504000.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N, Muntner P: Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 120: 1784–1792, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Beddhu S, Ma X, Baird B, Cheung AK, Greene T: Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 4: 1805–1810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Ureche V, Lu JL, Kalantar-Zadeh K: Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant 25: 3003–3011, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taliercio JJ, Schold JD, Simon JF, Arrigain S, Tang A, Saab G, Nally JV, Jr., Navaneethan SD: Prognostic importance of serum alkaline phosphatase in CKD stages 3-4 in a clinical population. Am J Kidney Dis 62: 703–710, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, Akiba T, Keen ML, Young EW, Port FK: High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int 74: 655–663, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, Van Wyck D, Kopple JD, Kalantar-Zadeh K: Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol 19: 2193–2203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beddhu S, Baird B, Ma X, Cheung AK, Greene T: Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol 74: 91–96, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Pelletier S, Vilayphiou N, Boutroy S, Bacchetta J, Sornay-Rendu E, Szulc P, Arkouche W, Guebre-Egziabher F, Fouque D, Chapurlat R: Bone microarchitecture is more severely affected in patients on hemodialysis than in those receiving peritoneal dialysis. Kidney Int 82: 581–588, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Kong X, Zhang L, Zhang L, Chen N, Gu Y, Yu X, Liu W, Chen J, Peng L, Yuan W, Wu H, Chen W, Fan M, He L, Ding F, Chen X, Xiong Z, Zhang J, Jia Q, Shi W, Xing C, Tang X, Hou F, Shu G, Mei C, Wang L, Xu D, Ni Z, Zuo L, Wang M, Wang H: Mineral and bone disorder in Chinese dialysis patients: A multicenter study. BMC Nephrol 13: 116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmez JA, Yan G, Bailey J, Beck GJ, Beddhu S, Cheung AK, Kaysen GA, Levey AS, Sarnak MJ, Schwab SJ, Hemodialysis (HEMO) Study Group : Cerebrovascular disease in maintenance hemodialysis patients: Results of the HEMO Study. Am J Kidney Dis 47: 131–138, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Schoppet M, Shanahan CM: Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 73: 989–991, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73: 1296–1302, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC: Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int 73: 1024–1030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallieni M, Caputo F, Filippini A, Gabella P, Giannattasio M, Stingone A, Farina M, ROCK-PD Study Investigators : Prevalence and progression of cardiovascular calcifications in peritoneal dialysis patients: A prospective study. Bone 51: 332–337, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millán JL: Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 22: 1700–1710, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Cheung BM, Ong KL, Cheung RV, Wong LY, Wat NM, Tam S, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, Lam KS: Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med 46: 523–527, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, Levy Y, Brook GJ, Aronson D: Association between elevated liver enzymes and C-reactive protein: Possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 25: 193–197, 2005 [DOI] [PubMed] [Google Scholar]
- 22.An X, Mao HP, Wei X, Chen JH, Yang X, Li ZB, Yu XQ, Li ZJ: Elevated neutrophil to lymphocyte ratio predicts overall and cardiovascular mortality in maintenance peritoneal dialysis patients. Int Urol Nephrol 44: 1521–1528, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr., Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr., Haffner SM: Liver markers and development of the metabolic syndrome: The insulin resistance atherosclerosis study. Diabetes 54: 3140–3147, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Drechsler C, Verduijn M, Pilz S, Krediet RT, Dekker FW, Wanner C, Ketteler M, Boeschoten EW, Brandenburg V, NECOSAD Study Group : Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol 6: 1752–1759, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Filipowicz R, Greene T, Wei G, Cheung AK, Raphael KL, Baird BC, Beddhu S: Associations of serum skeletal alkaline phosphatase with elevated C-reactive protein and mortality. Clin J Am Soc Nephrol 8: 26–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price CP: Multiple forms of human serum alkaline phosphatase: Detection and quantitation. Ann Clin Biochem 30: 355–372, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Reust CE, Hall L: Clinical inquiries. What is the differential diagnosis of an elevated alkaline phosphatase (AP) level in an otherwise asymptomatic patient? J Fam Pract 50: 496–497, 2001 [PubMed] [Google Scholar]