Abstract
Phospholemman (PLM), when phosphorylated at Ser68, inhibits cardiac Na+/Ca2+ exchanger 1 (NCX1) and relieves its inhibition on Na+-K+-ATPase. We have engineered mice in which expression of the phosphomimetic PLM S68E mutant was induced when dietary doxycycline was removed at 5 wk. At 8–10 wk, compared with noninduced or wild-type hearts, S68E expression in induced hearts was ∼35–75% that of endogenous PLM, but protein levels of sarco(endo)plasmic reticulum Ca2+-ATPase, α1- and α2-subunits of Na+-K+-ATPase, α1c-subunit of L-type Ca2+ channel, and phosphorylated ryanodine receptor were unchanged. The NCX1 protein level was increased by ∼47% but the NCX1 current was depressed by ∼34% in induced hearts. Isoproterenol had no effect on NCX1 currents but stimulated Na+-K+-ATPase currents equally in induced and noninduced myocytes. At baseline, systolic intracellular Ca2+ concentrations ([Ca2+]i), sarcoplasmic reticulum Ca2+ contents, and [Ca2+]i transient and contraction amplitudes were similar between induced and noninduced myocytes. Isoproterenol stimulation resulted in much higher systolic [Ca2+]i, sarcoplasmic reticulum Ca2+ content, and [Ca2+]i transient and contraction amplitudes in induced myocytes. Echocardiography and in vivo close-chest catheterization demonstrated similar baseline myocardial function, but isoproterenol induced a significantly higher +dP/dt in induced compared with noninduced hearts. In contrast to the 50% mortality observed in mice constitutively overexpressing the S68E mutant, induced mice had similar survival as wild-type and noninduced mice. After ischemia-reperfusion, despite similar areas at risk and left ventricular infarct sizes, induced mice had significantly higher +dP/dt and −dP/dt and lower perioperative mortality compared with noninduced mice. We propose that phosphorylated PLM may be a novel therapeutic target in ischemic heart disease.
Keywords: FXYD proteins, intracellular Ca2+ regulation, ischemic cardiomyopathy, in vivo hemodynamics
phospholemman (PLM), a 72-amino acid phosphoprotein with a single transmembrane domain (27), is highly expressed in the heart (28). PLM regulates the activities of Na+-K+-ATPase (13–15, 43) and Na+/Ca2+ exchanger 1 (NCX1) (1, 45, 48) in the heart and L-type Ca2+ channels (18, 44) in heterologous expression systems. In addition, we have preliminary data showing that the L-type Ca2+ current is significantly larger in PLM knockout (KO) compared with wild-type (WT) myocytes (J. Y. Cheung, unpublished observations). The regulation of three major cardiac sarcolemmal ion transporters in close proximity by PLM makes it extremely complex to predict the effects of PLM on intracellular Na+ and Ca2+ concentrations ([Na2+]i and [Ca2+]i, respectively) in the heart under both resting and catecholamine-stressed conditions. It requires detailed knowledge of how the kinetics of each of the ion transporters (in isolation) are affected by PLM, the stoichiometry of the interaction between PLM and the three ion transporters in the cardiac myocyte, and sophisticated mathematical modeling to integrate the effects of PLM on [Na+]i and [Ca2+]i.
Although PLM is intimately involved in the regulation of [Ca2+]i and [Na+]i and would be expected to have major impact on cardiac excitation-contraction (E-C) coupling, under resting conditions, both in vitro myocyte contraction amplitudes (37) and in vivo myocardial contractility (4, 43) are similar between WT and PLM KO mice, suggesting that PLM is functionally quiescent. Under catecholamine stress when PLM is phosphorylated at Ser68 by PKA (28, 39), inhibition on Na+-K+-ATPase by PLM is relieved (13, 43), but the activity of NCX1 is simultaneously suppressed (45). Phosphorylated (p-)PLM acts in concert to decrease risks of arrhythmias (by minimizing intracellular Ca2+ and Na+ overload via enhanced Na+-K+-ATPase activity) (14, 43) and preserve inotropy (by decreasing Ca2+ efflux via inhibition of NCX1) (42) during fight or flight situations. Therefore, PLM has been proposed to be a novel cardiac “stress” protein (9, 10).
NCX1 mediates Ca2+ efflux and influx during an action potential and regulates both [Ca2+]i and [Na+]i during E-C (5). Alterations in NCX1 expression and activity have been observed in many models of cardiac hypertrophy and heart failure (32), including human end-stage cardiomyopathy (19). Although the role of NCX1 in causing cardiac dysfunction in disease states remains controversial (22, 26, 30, 32, 41), modulation of NCX1 expression or activity has been proposed to be a potential therapeutic option in heart failure (20). Interpretation of experimental results obtained with commonly available inhibitors of NCX1, such as KB-R7943 and SEA-0400, is complicated by nonspecific effects. Mutation of Ser68 in PLM to glutamic acid (S68E mutant) results in the inhibition of NCX1 without any effect on Na+-K+-ATPase in adult cardiac myocytes (36, 42). The PLM S68E mutant may therefore act as a specific NCX1 inhibitor. The purpose of the present study was to characterize a novel transgenic (TG) mouse model in which expression of the PLM S68E mutant transgene was under the control of a cardiac-specific promoter driving the expression of a tetracycline transactivator (tTA). When doxycycline (Dox) was removed from the mouse feed, expression of the S68E transgene was induced. In addition, we used this model to test the hypotheses that 1) cardiac-specific inhibition of NCX1 by S68E enhanced the inotropic response to catecholamine stimulation and 2) cardiac-specific inhibition of NCX1 improved contractile function after ischemia-reperfusion (I/R) injury.
METHODS
Generation of inducible PLM S68E TG mice.
Founder mice (line 39, FVB background) harboring a sequence-verified S68E transgene in the TREMHC vector was engineered as previously described (33). Founder mice were crossed to cardiac tTA TG mice on the FVB background (MHC-tTA). Littermates that were heterozygous for tTA but negative for the S68E transgene were referred to as WT control mice. To prevent S68E transgene expression, Dox (300 mg/kg mouse diet, Bio-Serv) was administered to all pregnant mothers and offsprings of S68E TG groups (noninduced group). To induce S68E expression (induced group), Dox was removed from the feed at 5 wk of age, and mice were studied 3–5 wk later. In another series of experiments, the S68E transgene was constitutively overexpressed by avoiding Dox exposure in both pregnant mothers and offsprings. The MHC-tTA mouse line expresses tTA at very low levels. Expression of tTA in WT mice did not affect mouse heart weight and function up to 12 wk. Similarly, Dox alone did not affect WT mouse heart size or function (40).
Mice were housed and fed on a 12:12-h light-dark cycle at Temple University Animal Facility and were supervised by veterinary staff members. Standard care was provided to all mice used for experiments. All protocols applied to the mice in this study were approved and supervised by the Institutional Animal Care and Use Committee of Temple University.
Echocardiographic and hemodynamic analyses of cardiac function.
Transthoracic two-dimensional echocardiography was performed in anesthetized (2% inhaled isoflurane) mice with a 12-MHz probe as previously described (33, 40–43). For in vivo hemodynamic measurements, a 1.4-Fr micromanometer-tipped catheter (SPR-671, Millar Instruments) was inserted into the right carotid artery and advanced into the left ventricle (LV) of lightly anesthetized [tribromoethanol-amylene hydrate, Avertin; 2.5% (wt/vol), 8 μl/g ip] mice with spontaneous respirations and placed on a heated (37°C) pad (33, 40–43). Hemodynamic parameters, including heart rate (in beats/min), LV end-diastolic pressure, and maximal first time derivatives of the LV pressure rise (+dP/dt) and fall (−dP/dt), were recorded in the closed-chest mode both at baseline and in response to increasing doses of isoproterenol (Iso; 0.1, 0.5, 1, 5, and 10 ng) (33, 40–43).
I/R surgery in mice.
I/R surgery was performed as previously described (17, 24). Briefly, male mice (8–10 wk) were anesthetized with 2% isoflurane, and the heart was exposed through a left thoracotomy at the fifth intercostal space. A slipknot was tied around the left anterior descending (LAD) coronary artery 2–3 mm from its origin, and the heart was immediately returned to the chest cavity followed by evacuation of the pneumothorax and closure of muscle and skin layers. The slipknot was released after 30 min of ischemia to allow reperfusion. Sham-operated (sham) animals were subjected to the same surgical procedure except that the slipknot was not tied. Animals recovered from anesthesia within 5 min after the completion of surgery and received ibuprofen (10 mg/50 ml in drinking water) for 48 h as postsurgery analgesia. Experiments on survivors were performed on day 3 after surgery.
Infarct size measurements.
The myocardium was stained with 2% triphenyltetrazolium (TTC) to measure infarct size as previously described (17, 24). Briefly, 72 h after I/R, the slipknot around the LAD was retied followed by an injection of 2% Evans blue dye (0.2 ml). Hearts were excised, and the LV was sliced into five equally thick sections perpendicular to the short axis of the heart and incubated in PBS containing TTC. After 15 min at room temperature, slices were digitally photographed. The Evans blue-stained area (area not at risk), TTC-negative area (infarcted myocardium), and area at risk (AAR; including both TTC-negative and -positive areas) were measured with a computer-based image analyzer (SigmaScan Pro 5.0, SPSS Science, Chicago, IL). The AAR was expressed as a percentage of the total LV, whereas the infarcted myocardium was expressed as a percentage of the AAR.
Isolation of adult murine ventricular myocytes.
Cardiac myocytes were isolated from the LV free wall and septum of WT and KO mice according to the protocol of Zhou et al. (49) and modified by us (33, 36, 37, 40–43, 45). Myocytes were seeded onto laminin-coated coverslips and used within 2–8 h of isolation.
Myocyte shortening measurements.
Myocytes adherent to laminin-coated coverslips were bathed in 0.7 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 containing 1.8 mM extracellular Ca2+ concentration ([Ca2+]o). Myocytes were not superfused during the experiments, which usually lasted 2–5 min. Measurements of myocyte contraction (2 Hz), before and after Iso (1 μM), were performed using an edge detection algorithm (Ionoptix, Milton, MA) as previously described (33, 36, 37, 40–43). To eliminate any effects of fura-2 loading on myocyte contractility, contraction measurements were performed in myocytes not loaded with fura-2.
[Ca2+]i transient measurements.
Fura-2-loaded (0.67 μM fura-2 AM, 15 min, 37°C) myocytes were field stimulated to contract (2 Hz, 37°C) in medium 199 containing 1.8 mM [Ca2+]o. [Ca2+]i transient measurements before and after Iso (1 μM), daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as previously described (33, 36, 37, 40–43).
Electrophysiological measurements.
Na+-K+-ATPase current (Ipump) (33, 36, 42, 43) and NCX current (INaCa) (33, 36, 40–42, 45) were measured in isolated LV myocytes (30°C) with whole cell patch-clamp techniques. Fire-polished pipettes (tip diameter: 4–6 μm) with resistances of 0.8–1.4 MΩ when filled with pipette solutions were used. Sarcoplasmic reticulum (SR) Ca2+ content was estimated by integrating forward INaCa induced by caffeine (5 mM) exposure as previously described (36, 37, 47). The half-time (t1/2) of INaCa decline after caffeine-induced SR Ca2+ release (an alternative readout of NCX1 function) was determined.
Immunoblot analysis.
Mouse LV homogenates were prepared as previously described (37, 40, 41). For the detection of WT PLM or its S68E mutant (12% SDS-PAGE, reducing conditions with 5% β-mercaptoethanol), either monoclonal B8 (34) or polyclonal C2 (35) antibody was used. Heart homogenates were first treated with bacterial alkaline phosphatase to dephosphorylate PLM before being blotted with C2 antibody (33). B8 and C2 signals were referenced to calsequestrin levels. For the detection of α1- and α2-subunits of Na+-K+-ATPase (3–8% Tris acetate gradient gel, reducing conditions), sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2; 7.5% SDS-PAGE, reducing conditions), α1c-subunit of the L-type Ca2+ channel (Cav1.2; 5% SDS-PAGE, reducing conditions), NCX1 (7.5% SDS-PAGE, nonreducing conditions with 10 mM N-ethylmaleimide), cardiac ryanodine receptor 2 phosphorylated at Ser2808 (p-RyR2; 4–12% gradient gel, reducing conditions), and calsequestrin (used as a loading control), commercially available antibodies were used as previously described (33, 36, 37, 40–42, 44).
Since the S68E mutant was originally constructed from dog PLM (34), to estimate the fold protein overexpression of the mutant transgene over native PLM present in mouse hearts, B8 antibody, which detects the NH2-terminus of dog PLM but not rat (34) or mouse (42) PLM, was used. B8 signals obtained from the induced LV were standardized against endogenous PLM present in dog LV homogenates, as previously described (33).
In another series of experiments, myocytes isolated from induced and noninduced LVs were treated with either Iso (1 μM) or vehicle for 6 min, scraped into lysis buffer, and probed for unphosphorylated PLM (C2 antibody) and PLM phosphorylated at Ser68 (phospho-specific CP68 antibody) (36). Myocyte lysates were not treated with bacterial alkaline phosphatase before Western blot analysis in these experiments.
Statistics.
All results are expressed as means ± SE. For analysis of INaCa as a function of group (noninduced vs. induced) and voltage, in vivo hemodynamic parameters, [Ca2+]i, SR Ca2+ content, t1/2 of the INaCa decline after caffeine-induced SR Ca2+ release, [Ca2+]i transient and contraction amplitudes, and t1/2 of the [Ca2+]i transient decline as a function of group and Iso, two-way ANOVA was used. For analysis of echocardiographic parameters, AARs, infarct sizes, Ipump, and protein abundance, one-way ANOVA was used. A commercially available software package (JMP, version 7, SAS Institute, Cary, NC) was used. To analyze mortality after I/R, we calculated the relative risk of dying from the procedure and used Fisher's exact test for significance. In all analyses, P values of ≤0.05 were taken to be statistically significant.
RESULTS
Inducible S68E TG mice.
The 10 founders (4 males and 6 females) harboring the dog S68E transgene were all heterozygous. Founder line 39 was crossed with homozygous MHC-tTA mice in the presence of dietary Dox to generate the experimental (tTA+/−S68E+/−) and control WT (tTA+/−S68E−/−) groups. When Dox was absent from conception, S68E was constitutively overexpressed. When Dox was removed from the dietary feed at 5 wk of age, S68E expression was induced. When Dox was present throughout, S68E transgene expression was not induced.
At 8 wk of age (3 wk postinduction), the level of S68E mutant protein detected by B8 antibody in the induced LV (11.2 ± 2.2 arbitrary units) was ∼0.35 times that of endogenous PLM present in the dog LV (31.9 ± 2.5 arbitrary units; Fig. 1A). As expected, B8 signals were not detectable in the WT mouse LV, confirming our previous observation that B8 antibody recognizes dog PLM but not mouse PLM (33, 42). Data obtained with B8 antibody therefore suggested that induced S68E expression was ∼35% of endogenous PLM present in the dog LV.
Fig. 1.
Immunoblots of phospholemman (PLM) and S68E mutant in hearts. A: left ventricular (LV) homogenates were prepared from dogs, wild-type (WT; tTA+/−S68E−/−) mice, and transgenic (tTA+/−S68E+/−) mice (8 wk old) induced to overexpress the dog PLM S68E mutant [induced (Ind) S68E] at 5 wk of age and subjected to SDS-PAGE followed by Western blot analysis. B8 antibody (1:2,000), which recognizes the NH2-terminus of dog PLM (34) but not rat or mouse (33, 42) PLM, was used to detect dog PLM and the S68E mutant in WT and induced hearts (50 μg/lane). C2 antibody (1:10,000), which was raised against the COOH-terminus of rat PLM (35), was used to detect signals from dog, WT, and induced hearts (3 μg/lane). Calsequestrin (CLSQ) was used as the loading control. Heart homogenates were subjected to alkaline phosphatase treatment to dephosphorylate PLM before being probed with C2 antibody (46). B: phospho-specific PLM antibody CP68 (1:2,000) (29) was used to detect PLM phosphorylated at Ser68 in heart homogenates prepared from mice constitutively overexpressing the S68E mutant (ConS68E; 2 μg/lane), WT (20 μg/lane) mice, and noninduced (NonInd; 20 μg/lane) mice. In constitutively overexpressing mice, endogenous PLM represents <2.5% of the C2 signal (33). The absence of the CP68 signal in constitutively overexpressed samples indicates that CP68 does not detect the S68E mutant. As a control, C2 antibody (1:10,000) was used to detect unphosphorylated PLM or the S68E mutant in alkaline phosphatase-pretreated constitutively overexpressing (0.5 μg/lane), WT (5 μg/lane), and noninduced heart homogenates (5 μg/lane). C: myocytes isolated from induced and noninduced mice were exposed to vehicle or isoproterenol (Iso; 1 μM) for 6 min, after which they were processed for Western blot analysis. In this experiment, myocyte lysates were not treated with alkaline phosphatase before being probed with C2 (5 μg/lane, 1:10,000) or CP68 (20 μg/lane, 1:2,000) antibodies.
Using C2 antibody, which detects the COOH-termini of both dog and mouse PLM, the signals for endogenous dog PLM, mouse PLM (WT), and S68E mutant (induced) were 0.30 ± 0.05, 0.25 ± 0.06, and 0.50 ± 0.03 arbitrary units, respectively (Fig. 1A). Assuming similar expression of PLM between dog and WT mouse hearts, the lower C2 signal indicated that the single amino acid change from Thr69 to Ser69 in mouse PLM decreased the efficiency of C2 detection by ∼17%. This is similar to the ∼75% detection efficiency of C2 for mouse PLM we have previously reported (33). After correction for 75% detection efficiency of mouse PLM by C2, S68E expression (ΔC2 signal) in the induced LV was ∼75% that of endogenous PLM in the WT LV. Both B8 and C2 signals therefore indicated that 3 wk after induction, S68E expression was 0.35- to 0.75-fold that of endogenous PLM in 8-wk-old FVB mice. This is in sharp contrast to the 38.3- to 41.1-fold expression present in 4-wk-old mice when S68E was constitutively overexpressed (33).
To determine the fraction of endogenous PLM in induced myocytes that was phosphorylated, we used CP68 antibody, which is specific for PLM phosphorylated at Ser68 (29, 36, 46). In constitutively overexpressed S68E hearts in which endogenous PLM constitutes <2.5% of the C2 signal (33), there was no detectable CP68 signal, although the corresponding C2 signal was quite robust (Fig. 1B). In contrast, both CP68 and C2 signals were readily detectable in WT and noninduced hearts (Fig. 1B). This observation indicates that CP68 antibody does not detect the S68E mutant.
Under basal conditions, CP68 signal intensities were similar between noninduced and induced myocytes (0.35 ± 0.11 and 0.39 ± 0.09 arbitrary units, respectively, P < 0.90; Fig. 1C), suggesting similar phosphorylation levels at Ser68. Iso significantly (Iso effect, P < 0.005) increased CP68 signals in noninduced and induced myocytes to similar extents (0.94 ± 0.18 and 0.87 ± 0.14 arbitrary units, respectively, group × Iso effect, P < 0.70; Fig. 1C). Assuming that Iso resulted in 100% phosphorylation of Ser68, the percentage of p-Ser68 in PLM in unstimulated mouse myocytes ranged from 37% to 45%. This compares favorably with the 33% (16) to 46% (34) Ser68 phosphorylation previously reported for PLM in unstimulated adult rat ventricular myocytes. At baseline, C2 signals were significantly (P < 0.02) higher in induced myocytes (1.23 ± 0.13 arbitrary units) compared with noninduced myocytes (0.85 ± 0.11 arbitrary units), reflecting S68E expression in induced myocytes at ∼34% of endogenous PLM. Iso treatment decreased C2 signals in induced (0.99 ± 0.10 arbitrary units) and noninduced (0.71 ± 0.09 arbitrary units) groups. Collectively, these results suggested that moderate expression of the S68E mutant in induced myocytes did not affect basal or Iso-dependent phosphorylation of endogenous PLM.
Unlike constitutively overexpressed mice, which suffered 50% mortality at 5 wk of age, neither noninduced nor induced mice had any mortality at 15 wk of age (Fig. 2). Finally, the severe bradycardia (<250 beats/min) observed in 11of 12 constitutively overexpressed mice at 4 wk (33) was not observed in 10 of 10 induced mice at 15 wk of age (Fig. 3).
Fig. 2.
Induced overexpression of the S68E mutant does not result in mortality. Survival curves of WT mice (n = 20), mice constitutively overexpressing the S68E mutant (n = 15), mice induced at 5 wk to express the S68E mutant (n = 10), and transgenic S68E mice kept on a doxycycline diet to prevent induction of the S68E mutant (noninduced; n = 15) are shown.
Fig. 3.
Induced expression of the S68E mutant does not result in arrhythmias. Echocardiography was performed in 8-wk-old noninduced mice (top) and mice induced to express the S68E mutant (middle). For comparison, echocardiography of 4-wk-old mice constitutively overexpressing the S68E transgene (bottom) is also shown. ECG tracings demonstrated normal sinus rhythm in both induced and noninduced mice but severe bradycardia and multifocal ventricular tachycardia in the mouse constitutively overexpressing the S68E mutant.
Effects of induced S68E expression on in vivo cardiac function.
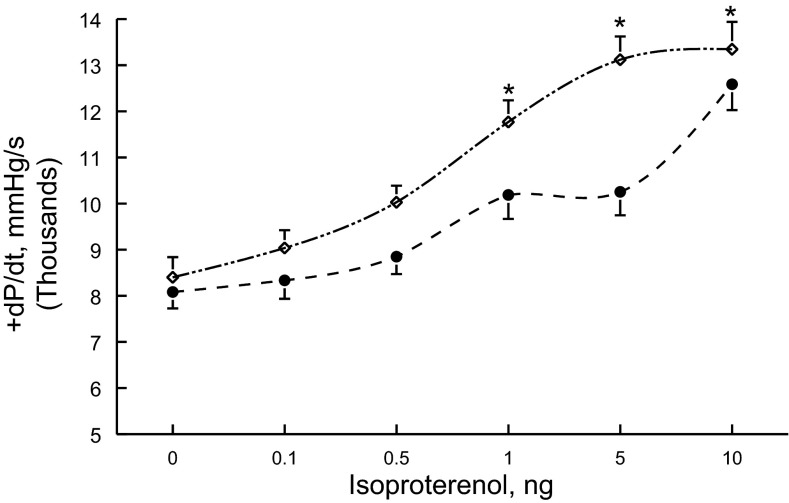
Compared with WT or noninduced hearts, induced hearts did not exhibit LV hypertrophy (Table 1). Ejection fractions were similar among WT, noninduced, and induced hearts (Table 1). In vivo hemodynamic measurements corroborated echocardiographic findings in that resting +dP/dt was similar among the three groups of animals (Table 1). However, in response to β-adrenergic stimulation, induced hearts had significantly (P < 0.006) higher +dP/dt compared with noninduced hearts (Fig. 4 and Table 1).
Table 1.
Echocardiographic and hemodynamic parameters of induced S68E mice
| WT Mice | Noninduced Mice | Induced Mice | WT Sham-Operated Mice | Noninduced I/R Mice | Induced I/R Mice | |
|---|---|---|---|---|---|---|
| LV mass, mg | 77.0 ± 3.0 (n = 6) | 76.2 ± 5.5 (n = 6) | 77.1 ± 2.1 (n = 4) | |||
| Heart rate, beats/min | 452 ± 33 | 462 ± 19 | 426 ± 20 | |||
| LVIDd, mm | 3.82 ± 0.06 | 3.91 ± 0.10 | 3.84 ± 0.06 | |||
| LVIDs, mm | 2.16 ± 0.05 | 2.30 ± 0.10 | 2.14 ± 0.06 | |||
| Ejection fraction, % | 75.2 ± 1.2 | 72.5 ± 1.6 | 72.1 ± 2.2 | |||
| Fractional shortening, % | 43.4 ± 1.1 | 41.1 ± 1.3 | 44.3 ± 2.1 | |||
| +dP/dt, mmHg/s | 8,272 ± 239 (n = 10) | 8,083 ± 357 (n = 6) | 8,403 ± 436 (n = 8) | 7,461 ± 380 (n = 7) | 7,556 ± 463 (n = 7) | 7,154 ± 341 (n = 11) |
| Maximum +dP/dt, mmHg/s | 12,792 ± 622 | 12,587 ± 559 | 13,348 ± 592* | 12,987 ± 313 | 10,949 ± 541 | 12,587 ± 546† |
Values are means ± SE; n, number of mice.
WT, wild type; LVIDd, left ventricular (LV) internal dimension at end diastole; LVIDs, LV internal dimension at end-systole; +dP/dt, first time derivative of the LV pressure rise; maximum +dP/dt, +dP/dt after 10 ng isoproterenol (Iso) injection; I/R, 30 min of ischemia followed by 3 days of reperfusion.
P < 0.006, induced vs. noninduced mice;
P < 0.003, induced I/R vs. noninduced I/R or WT sham-operated vs. noninduced I/R mice. There were no differences (P < 0.84) in maximum +dP/dt between WT sham-operated and induced I/R hearts.
Fig. 4.
Induced expression of the S68E transgene enhances the in vivo contractility response to Iso. In vivo catheterization was performed in anesthetized mice (see methods), and the maximal first time derivatives of the left venticular (LV) pressure rise (+dP/dt) and fall (−dP/dt) and heart rate were continuously monitored, both at baseline and with increasing doses of Iso. The average maximal +dP/dt achieved with each dose of Iso in six noninduced mice (●) and eight induced mice (◇) is shown. Composite results are shown in Table 1. Two-way ANOVA indicated significant group (P < 0.0065) and Iso (P < 0.0001) effects. *P < 0.0065, noninduced vs. induced mice.
Effects of induced S68E expression on the expression of selected proteins involved in E-C coupling.
At 8 wk of age, there were no differences in protein levels of α1- and α2-subunits of Na+-K+-ATPase, SERCA2, Cav1.2, and p-RyR2 among WT, noninduced, and induced hearts (Fig. 5 and Table 2). Compared with WT or noninduced hearts, NCX1 levels were significantly (P < 0.025) elevated in induced hearts (Fig. 5 and Table 2).
Fig. 5.
Induced expression of the S68E transgene enhances LV expression of the Na+/Ca2+ exchanger (NCX). LV homogenates were prepared from 8-wk-old WT, noninduced, and induced mice and subjected to SDS-PAGE followed by Western blot analysis (see methods). Protein loading was 50 μg/lane for all proteins. Primary antibodies were used to detect the cardiac ryanodine receptor phosphorylated at Ser2808 (p-RyR; 1:1,000), α1c-subunit of the L-type Ca2+ channel (Cav1.2; 1:200), NCX1 (1:1,000), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2; 1:5,000), α1- (1:1,000) and α2- (1:2,000) subunits of Na+-K+-ATPase, and CLSQ (1:3,000). Signal band intensities of each protein were normalized against the corresponding CLSQ loading control signal and are shown as composite data in Table 2.
Table 2.
Effects of induced S68E expression on levels of selected proteins
| WT Mice | Noninduced Mice | Induced Mice | |
|---|---|---|---|
| p-RyR2 | 2.26 ± 0.37 (n = 5) | 2.47 ± 0.41 (n = 6) | 2.08 ± 0.40 (n = 8) |
| Cav1.2 | 0.51 ± 0.04 | 0.49 ± 0.02 | 0.55 ± 0.02 |
| NCX1 | 0.67 ± 0.04 | 0.59 ± 0.02 | 0.87 ± 0.09* |
| SERCA2 | 0.96 ± 0.13 | 0.93 ± 0.04 | 1.11 ± 0.05 |
| α1-subunit of Na+-K+-ATPase | 2.13 ± 0.09 | 1.98 ± 0.07 | 2.09 ± 0.08 |
| α2-subunit of Na+-K+-ATPase | 1.60 ± 0.03 | 1.62 ± 0.08 | 1.77 ± 0.09 |
Values (in arbitrary units) are means ± SE of ratios of protein band intensities normalized to respective calsequestrin loading controls; n, number of hearts.
p-RyR2, cardiac ryanodine receptor 2 phosphorylated at Ser2808; Cav1.2, α1c-subunit of the L-type Ca2+ channel; NCX1, cardiac Na+/Ca2+ exchanger 1; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase.
P < 0.025, induced vs. noninduced or WT mice.
Effects of induced S68E expression on INaCa and Ipump.
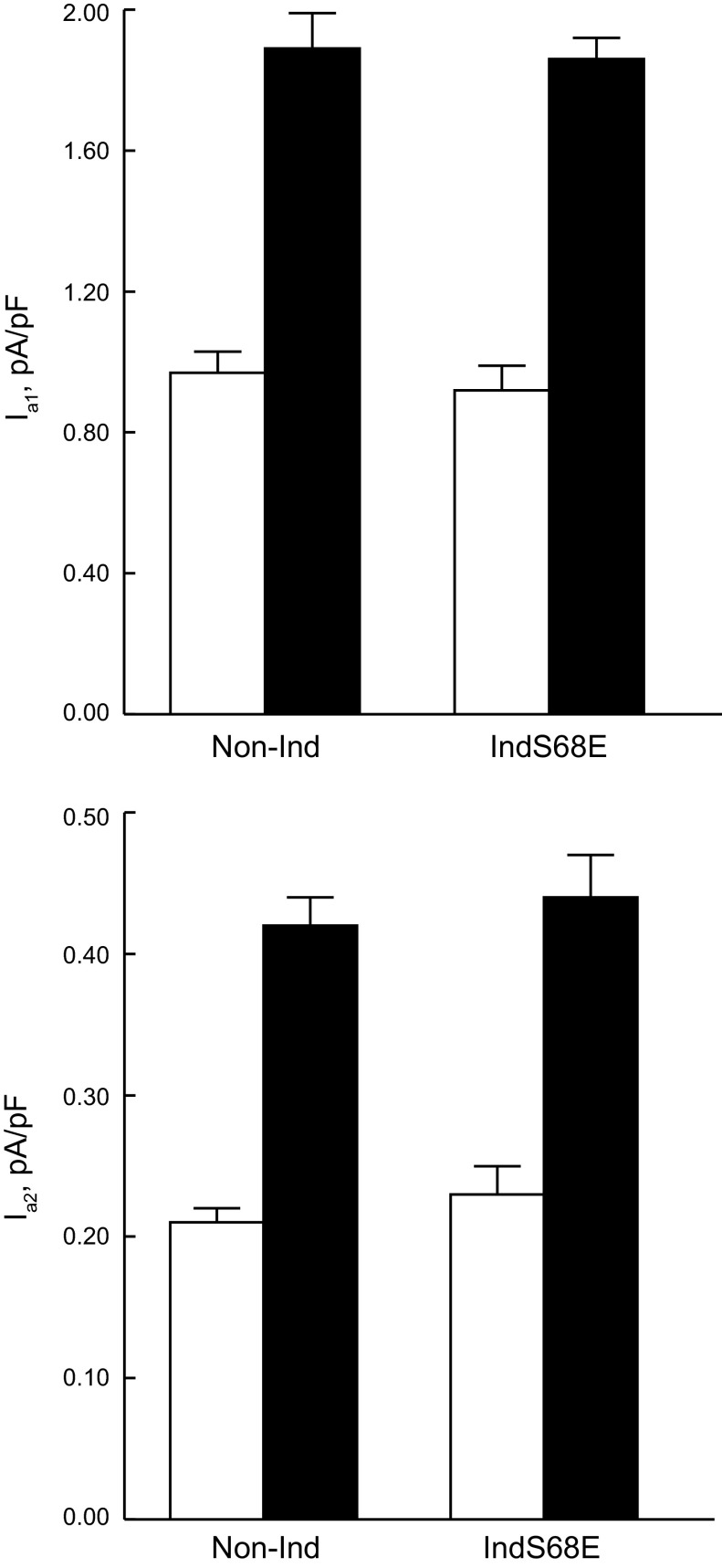
Despite elevated NCX1 protein levels (Fig. 5 and Table 2), induced expression of the S68E mutant significantly (P < 0.0001, group × voltage interaction effects) inhibited INaCa compared with noninduced myocytes (Fig. 6A). Iso had no effects on INaCa in both induced and noninduced myocytes (Fig. 6B). At baseline, Na+-K+-ATPase currents due to α1-subunits (Iα1) and α2-subunits (Iα2) were not different between induced and noninduced myocytes (Fig. 7). After Iso stimulation, both Iα1 and Iα2 doubled in induced and noninduced myocytes (Fig. 7).
Fig. 6.
Induced expression of the S68E mutant inhibits NCX current (INaCa). A: 3 wk after the induction of S68E expression by removing doxycycline in the diet, myocytes were isolated from the LV, and INaCa (Na+ concentration in the pipette: 12.25 mM, Ca2+ concentration in the pipette: 205 nM, extracellular Na+ concentration: 141.2 mM, and Ca2+ concentration: 5 mM) was measured at 30°C (33, 36, 40–42, 45). The reversal potential of INaCa in all myocytes examined was −60 mV, close to the theoretical reversal potential of −73 mV under prevailing ionic conditions. INaCa was significantly (P < 0.0001, group × voltage interaction effects) lower in myocytes induced to express the S68E mutant (open squares; n = 11) compared with noninduced myocytes (open circles; n = 13). Error bars are not shown if they fell within the boundaries of the symbol. B: in another series of experiments, INaCa was measured in induced (squares; n = 5) and noninduced (circles; n = 4) myocytes in the absence (open symbols) and presence (filled symbols) of 1 μM Iso. For clarity of presentation, lines are only drawn through open symbols.
Fig. 7.
Induced S68E expression had no effects on currents due to α1-subunts (Iα1) and α2-subunits (Iα2) of Na+-K+-ATPase. Currents due to α1- and α2-subunits of Na+-K+-ATPase (80 mM Na+ concentration in the pipette and 18 mM extracellular K+ concentration, 30°C) were separated by their differential sensitivities to dihydroouabain (43). Top and bottom: Iα1 and Iα2, respectively, in induced (n = 8) and noninduced (n = 8) myocytes both at baseline (open bars) and after stimulation with 1 μM Iso (solid bars).
Effects of induced S68E expression on [Ca2+]i transients, SR Ca2+ contents, and myocyte contraction.
At baseline, there were no differences in systolic and diastolic [Ca2+]i, t1/2 of the [Ca2+]i transient decline (estimate of SR Ca2+ uptake rate) (47), and [Ca2+]i transient and contraction amplitudes between noninduced and induced myocytes (Fig. 8 and Table 3). After stimulation with Iso (1 μM), diastolic [Ca2+]i was similar (P < 0.95), but maximal systolic [Ca2+]i (P < 0.04) and [Ca2+]i transient (P < 0.05) and contraction (P < 0.03) amplitudes were higher in induced compared with noninduced myocytes (Fig. 8 and Table 3). The significant group × Iso interaction effects for maximal systolic [Ca2+]i and [Ca2+]i transient and contraction amplitudes support the notion that Iso exerted larger effects on induced compared with noninduced myocytes. The observation that t1/2 of the [Ca2+]i transient decline was similar between induced and noninduced myocytes (P < 0.59) is consistent with the finding that SERCA2 proteins levels were not different (Fig. 5 and Table 2). The time courses of systolic [Ca2+]i and the contraction amplitude increase in response to Iso stimulation were equally rapid between noninduced and induced myocytes (Fig. 9). SR Ca2+ contents were similar at baseline but were significantly higher (P < 0.002) in induced myocytes in the presence of Iso (Fig. 10 and Table 3). In addition, t1/2 of the INaCa decline after caffeine-induced SR Ca2+ release (an alternative measure of NCX1 activity) was significantly (P < 0.04) longer in induced compared with noninduced myocytes (Fig. 10 and Table 3).
Fig. 8.
Intracellular Ca2+ concentration ([Ca2+]i) transients and cell shortening in induced and noninduced myocytes. A and B: representative traces of [Ca2+]i transients (A) and contraction (B) in noninduced (left) and induced (right) myocytes paced at 2 Hz, 1.8 mM extracellular Ca2+ concentration ([Ca2+]o), and 37°C. Iso, where indicated, was at 1 μM. Composite results are shown in Table 3.
Table 3.
Effects of induced S68E expression on [Ca2+]i transients, SR Ca2+ content, and myocyte contractility
| Noninduced Mice | Induced Mice | |
|---|---|---|
| Systolic [Ca2+]i, nM | ||
| Without Iso | 230 ± 22 (n = 11) | 267 ± 42 (n = 10) |
| With Iso | 557 ± 61 | 853 ± 95* |
| Diastolic [Ca2+]i, nM | ||
| Without Iso | 121 ± 13 | 130 ± 13 |
| With Iso | 126 ± 10 | 137 ± 12 |
| [Ca2+]i transient amplitude, % | ||
| Without Iso | 19.8 ± 2.5 | 20.7 ± 4.2 |
| With Iso | 58.1 ± 4.0 | 73.4 ± 3.4* |
| t1/2 of the [Ca2+]i transient decline, ms | ||
| Without Iso | 121.5 ± 12.4 | 135.9 ± 13.5 |
| With Iso | 64.6 ± 4.6 | 68.4 ± 3.5 |
| Contraction amplitude, % | ||
| Without Iso | 4.5 ± 0.7 (n = 9) | 4.4 ± 0.3 (n = 10) |
| With Iso | 14.6 ± 0.5 | 17.4 ± 0.9† |
| SR Ca2+ content, fM/fF | ||
| Without Iso | 6.6 ± 0.3 (n = 5) | 7.4 ± 0.4 (n = 7) |
| With Iso | 21.6 ± 0.7 (n = 8) | 27.4 ± 0.8‡ (n = 8) |
| t1/2 of the INaCa decline, ms | ||
| Without Iso | 386 ± 59 (n = 4) | 482 ± 22§ (n = 6) |
| With Iso | 452 ± 29 (n = 8) | 726 ± 123§ (n = 8) |
Values are means ± SE; n, number of myocytes. Both intracellular Ca2+ concentration ([Ca2+]i) transients (3 induced mice and 3 noninduced mice) and contraction (4 induced mice and 3 noninduced mice) were measured in myocytes incubated in 1.8 mM extracellular Ca2+ concentration ([Ca2+]o) and stimulated to contract at 2 Hz and 37°C. Iso, when present, was at 1 μM. The [Ca2+]i transient amplitude is given as the percent increase in the fura-2 signal. Contraction amplitude is given as the percent resting cell length. Sarcoplasmic reticulum (SR) Ca2+ content and the half-time (t1/2) of the Na+/Ca2+ exchanger current (INaCa) decline after a caffeine pulse were measured at 1.8 mM [Ca2+]o and 30°C (4 induced mice and 3 noninduced mice).
P < 0.05,
P < 0.03, and
P < 0.0015, group (induced vs. noninduced) × Iso interaction effects;
P < 0.04, group effect.
Fig. 9.
Time course of contraction and systolic [Ca2+]i changes in response to Iso. Top: myocyte contraction amplitudes (% of resting cell length) from induced (●; n = 10) and noninduced (○; n = 9) myocytes paced at 2 Hz, 1.8 mM [Ca2+]o, and 37°C are shown. Iso (1 μM) was added at the times indicated. Bottom: systolic [Ca2+]i from fura-2-loaded noninduced (○; n = 10) and induced (●; n = 11) myocytes paced at 2 Hz, 1.8 mM [Ca2+]o, and 37°C are shown. After 2 min of pacing, steady-state [Ca2+]i values were obtained (time 0) followed by the addition of Iso (1 μM), and measurements continued for 6.5 min.
Fig. 10.
Induced S68E expression increases sarcoplasmic reticulum (SR)-releasable Ca2+ content in response to Iso. Noninduced (A and C) and induced S68E (B and D) myocytes were incubated at 1.8 mM [Ca2+]o and 30°C and voltage clamped at −90 mV. To ensure steady-state SR Ca2+ load, 12 conditioning pulses (from −90 to 0 mV, 300 ms, 1 Hz) were delivered before caffeine (5 mM, 200 ms after the 12th conditioning pulse) was puffed on the myocyte for 2.4 s, both in the absence (A and B) and presence (C and D) of Iso (1 μM). A large transient inward current caused by caffeine-induced SR Ca2+ release was observed. This current represents Na+ entry accompanying Ca2+ extrusion by NCX1, and the half-time (t1/2) of current decline is a functional readout of Na+/Ca2+ exchange activity. In addition, the time integral of this current provides an estimate of SR-releasable Ca2+ (36, 37). To convert the INaCa time integral (in coulombs) to moles, the charge was divided by Faraday's constant of 96,487 coulombs/equivalent, based on 3 Na+ being exchanged for each 1 Ca2+. SR Ca2+ content was normalized to cell size (in fmol/fF). Results are shown in Table 3.
Induced S68E expression protected hearts from contractile dysfunction after I/R.
The significantly higher [Ca2+]i transient and contraction amplitudes in induced compared with noninduced myocytes stimulated with Iso (Fig. 8; Table 3) suggested that induced expression of modest levels of S68E may improve contractile dysfunction in disease states where circulating catecholamine levels are elevated. We tested this hypothesis by subjecting 8-wk-old mice to 30 min of ischemia followed by reperfusion. Three days after I/R, both AARs and infarct sizes were similar between noninduced and induced hearts (Fig. 11). Compared with WT sham hearts, noninduced I/R hearts had significant decreases in +dP/dt (P < 0.0003; Fig. 12) and −dP/dt (P < 0.0001; data not shown). Induced expression of S68E improved both +dP/dt (P < 0.003; Fig. 12) and −dP/dt (P < 0.0006) after I/R compared with noninduced I/R hearts. Indeed, there were no significant differences in maximal +dP/dt (P < 0.84; Table 1) or −dP/dt (P < 0.06) between WT sham and induced I/R hearts. Induced S68E expression ameliorated myocardial dysfunction after I/R.
Fig. 11.
Infarct sizes were similar in noninduced and induced S68E hearts subjected to ischemia/reperfusion (I/R). Hearts were subjected to 30 min of ischemia followed by reperfusion for 3 days (see methods). Top: area at risk (AAR) [2,3,5-triphenyltetrazolium (TTC) positive and TTC negative], infarct area (TTC negative), and area not at risk (Evans blue dye stained) were determined for both noninduced and induced hearts. Bottom: summary for AAR and infarct size for noninduced I/R (n = 6) and induced I/R (n = 7) hearts. There were no differences in AAR (P < 0.90) and infarct size (P < 0.79) between noninduced and induced hearts.
Fig. 12.
Cardiac performance after I/R was better in induced compared with noninduced hearts. Hearts were subjected to sham operation (sham) or 30 min of ischemia followed by 3 days of reperfusion. In vivo catheterization was performed in anesthetized mice. Both +dP/dt and −dP/dt were continuously measured, both at baseline and at increasing doses of Iso. There were 7 WT sham mice (□), 7 noninduced I/R mice (●), and 11 induced I/R mice (◇), respectively. Error bars are not shown if they fell within the boundaries of the symbol. Composite results are shown in Table 1. Two-way ANOVA indicated P < 0.0003 for WT sham vs. noninduced I/R mice and P < 0.003 for induced I/R vs. noninduced I/R mice. There were no differences (P < 0.84) in +dP/dt between WT sham and induced I/R mice. *P < 0.003, noninduced I/R vs. induced I/R or WT sham mice.
Induced S68E expression reduced perioperative mortality after I/R.
Perioperative mortality (death within 24 h of surgery) was 0 of 8 (0%) in WT sham mice, 3 of 6 (50.0%) in WT I/R mice, 10 of 18 (55.6%) in noninduced I/R mice, and 6 of 26 (23.1%) in induced I/R mice. All except two deaths occurred within 2 h after I/R. Fisher's exact test indicated that perioperative mortality was significantly (P ≤ 0.05) lower in induced I/R mice compared with noninduced I/R mice.
DISCUSSION
PLM colocalizes and coimmunoprecipates with Na+-K+-ATPase (9, 46), NCX (1, 48), and the L-type Ca2+ channel in adult cardiac myocytes (44). Under catecholamine stimulation, PLM phosphorylated at Ser68 (39) performs the dual function of reducing risks of arrhythmogenesis (by disinhibition of Na+-K+-ATPase) (14, 43) and preserving inotropy (by inhibition of NCX1)(42). Manipulating PLM expression or its activity is thus a tempting and rational therapeutic target for a myriad of pathological stressful conditions such as ischemia, volume overload, and pressure overload in which circulating catecholamine levels are expected to be elevated. Our initial attempt to increase p-PLM activity involved directly injection of recombinant adeno-associated virus serotype 9 (rAAV9) expressing the phosphomimetic S68E transgene into the LV of PLM KO mice (42). Despite the fact only that ∼40% of LV myocytes expressed the S68E mutant, cardiac contractility in the presence of Iso was significantly enhanced compared with control PLM KO mice injected with rAAV9 expressing green fluorescent protein. Although these results provide proof of principle, the invasive transgene delivery method renders further evaluation of any beneficial effects of S68E expression on pathological conditions such as transverse aortic constriction, myocardial infarction, and I/R injury technically challenging since multiple survival surgeries are necessary. Our next attempt was to engineer a mouse expressing the S68E transgene. Constitutive overexpression of the S68E transgene is plagued by cardiac hypertrophy, severe bradyarrhythmias, and multifocal ventricular tachycardia (Fig. 3) as well as heart failure and early mortality (∼50% within 5 wk of age; Fig. 2) compared with WT mice (33). This is most likely due to the exuberant overexpression of the S68E transgene (∼41 times that of endogenous PLM) (33), which gives rise to “molecular torture” in which high levels of protein expression cause a nonspecific and toxic phenotype (12). In the present series of experiments, we used the tet-off system to induce expression of the S68E transgene.
Results using both B8 and C2 antibodies suggested that at 3 wk postinduction, S68E expression was ∼35–75% that of endogenous PLM in WT FVB mice (Fig. 1). This implies that in induced mice, both WT PLM and the S68E mutant were expressed in the heart. The interpretation that not all of endogenous WT PLM was replaced by the S68E mutant in induced mice is supported by two observations. First, in response to Iso, the phospho-specific CP68 signal increased with corresponding decreases in the C2 signal in both induced and noninduced myocytes (Fig. 1), indicating that endogenous PLM was phosphorylated at Ser68 by PKA. Second, Ipump was increased by ∼100% in both noninduced and induced myocytes treated with Iso (Fig. 7). If endogenous WT PLM was entirely replaced with the S68E mutant in induced myocytes, one would not expect any β-agonist-induced increase in pump currents since the S68E mutant had no effect on pump currents, both in the absence and presence of β-agonists (33, 42). That the S68E mutant was functional was attested by the observation that INaCa (Fig. 6) but not Ipump (Fig. 7) was inhibited in induced compared with noninduced myocytes. To our knowledge, this is one of the few studies demonstrating that the induced transgene did not overwhelm the endogenous WT gene, both in terms of expression level and function.
The first major finding is that unlike mice constitutively overexpressing the S68E mutant (33), mice in which cardiac-specific expression of the S68E mutant was induced exhibited normal survival (Fig. 2) and baseline cardiac function (Table 1). This observation suggested that modest expression of the S68E mutant did not engender life-threatening arrhythmias since survival was 100% at 17–18 wk after induction (Fig. 2). In addition, with the exception of NCX1, levels of proteins involved in E-C coupling, such as SERCA2, p-RyR2, the L-type Ca2+ channel, and α1- and α2-subunits of Na+-K+-ATPase, were similar to those measured in WT or noninduced hearts (Fig. 5 and Table 2). The lack of drastic changes in expression of these proteins likely accounted for the normal basal systolic and diastolic [Ca2+]i, SR Ca2+ content, and [Ca2+]i transient and contraction amplitudes in induced compared with noninduced myocytes (Figs. 8 and 10 and Table 3).
The second important finding is that despite similar [Ca2+]i dynamics and myocyte contractility at baseline, under catecholamine stimulation, systolic [Ca2+]i, SR Ca2+ content, and [Ca2+]i transient and contraction amplitudes were significantly higher in induced compared with noninduced myocytes (Figs. 8–10 and Table 3). In addition, the enhanced myocyte performance translated to improved myocardial contractility in the presence of Iso (Fig. 4). The enhanced contractility observed only with catecholamine stimulation but not under basal conditions appears to be a unique property of the induced S68E mouse. For comparison, phospholamban ablation resulted in the expected increase in contractility under basal conditions, but the response to β-agonists was attenuated (11). Another comparison is that overexpression of rat SERCA2 resulted in increased contractility at baseline but no difference in +dP/dt at higher Iso doses (21). The ability to maintain normal baseline contractility but increased cardiac performance “on demand” during stress (above and beyond the catecholaminergic response) suggests modest S68E expression as an intriguing therapeutic option for heart failure.
The significantly higher steady-state systolic [Ca2+]i in Iso-treated induced myocytes implies that inhibition of forward Na+/Ca2+ exchange by S68E resulted in elevated SR Ca2+ contents. Indeed, SR Ca2+ content was significantly higher in induced myocytes in the presence of Iso (Fig. 10 and Table 3). The absence of changes in Cav1.2 and p-RyR2 levels (Fig. 5) suggests, but does not prove, that neither the trigger (L-type Ca2+ current) nor the “gain” (RyR2) for SR Ca2+ release was affected by modest S68E expression. In addition, neither SERCA2 (Fig. 5 and Table 2) nor SR Ca2+ uptake activity (t1/2 of the [Ca2+]i transient decline; Fig. 8 and Table 3) was different between induced and noninduced myocytes, indicating that the enhanced SR Ca2+ content was not a result of increased SR Ca2+ uptake activity. Therefore, the increased [Ca2+]i transient amplitudes and elevated SR Ca2+ contents in induced myocytes exposed to Iso were most likely due to an inhibition of forward Na+/Ca2+ exchange by S68E.
In rat and mouse cardiac ventricular myocytes in which cAMP does not activate the cAMP-dependent and Ni2+-sensitive Cl− current (23), Iso or forskolin treatment does not alter the amplitude of INaCa (25, 45). Indeed, under patch-clamp conditions in which [Ca2+]i, [Na+]i, [Ca2+]o, extracellular Na+ concentration, and membrane potential were tightly controlled, Iso had no effect on INaCa in both induced and noninduced myocytes (Fig. 6B), confirming that Iso does not affect the intrinsic activity of NCX in rodent myocytes. The functional consequence of Na+/Ca2+ exchange inhibition by S68E, however, can be inferred from caffeine-induced SR Ca2+ release experiments in which the released and unbuffered Ca2+ (47) is extruded by forward Na+/Ca2+ exchange. t1/2 of the INaCa decline is an estimate of how fast NCX extrudes the released SR Ca2+. As a group, induced myocytes had significantly longer t1/2 of the INaCa decline than noninduced myocytes (Fig. 10 and Table 3).
The most dramatic and clinically relevant finding is that modest expression of S68E almost completely ameliorated cardiac contractile dysfunction after I/R (Fig. 12 and Table 1). In rat hearts subjected to acute ischemia, phosphorylation of PLM is increased (15). Likewise, in a rabbit model of volume overload heart failure, PLM phosphorylation at Ser68 is dramatically increased (7). Phosphorylation of PLM at Ser68 relieves its inhibition of Na+-K+-ATPase while simultaneously inhibiting NCX. The results of the present study indicate that enhancing PLM's ability to inhibit NCX, without compromising its regulation of Na+-K+-ATPase, may be a rational approach to improve myocardial function after ischemia. In this context, it is relevant to note that in a canine pacing-induced tachycardic heart failure model (22), partial inhibition of NCX normalized [Ca2+]i transient amplitudes concomitant with an increase in SR Ca2+ content. These observations support the hypothesis that partial inhibition of NCX by a specific agent is a potentially effective therapeutic strategy for improving contractility in heart failure.
In heart failure and acute coronary ischemia, circulating catecholamine levels are elevated and PLM is hyperphosphorylated. β-Adrenergic blockers, which are widely used in heart failure and acute cardiac ischemia, will be expected to decrease the phosphorylation of PLM at Ser68. How, then, do β-adrenergic blockers exert their beneficial effects if hyperphosphorylated PLM is protective? The answer probably lies in the specificity of p-PLM (mimicked by the S68E mutant) effects on NCX and Na+-K+-ATPase, which does not faithfully capture the hyperadrenergic state in which a myriad of cellular functions are altered.
A totally unexpected outcome is that compared with noninduced mice, the odds ratio of dying at 24 h after I/R was significantly (P ≤ 0.05) lower in induced mice. We speculate that this is due to a reduction in sudden death associated with I/R injury by modest levels of S68E expression, which left regulation of Na+-K+-ATPase activity by endogenous PLM intact (Fig. 7). The hypothesis that p-PLM minimizes the risk of arrhythmogenesis was first advanced by Despa et al. (14). The cellular mechanisms by which induced S68E expression enhanced cardiac performance and reduced the risk of sudden death after ischemic injury remain to be delineated with further studies. One potential mechanism by which S68E confers protection in I/R injury or increases the inotropic response to Iso is that modest induced expression of S68E leads to an upregulation of NCX1 (Fig. 5 and Table 2) in an attempt to maintain INaCa at baseline. Thus, there is a greater reserve for NCX1 inhibition (and the ensuing inotropy) by WT PLM.
In adult cardiac myocytes isolated from rodents, Iso increases Ipump both by decreasing its Km for Na+ (13) and increasing Vmax (4, 15, 43). In the present study in which Ipump was measured under Vmax conditions, Iso increased pump currents due to both α1- and α2-subunits of Na+-K+-ATPase, in apparent contradiction of our previous report (43) showing that Iso increases Iα1 but not Iα2 measured in cardiac myocytes under similar Vmax conditions. Since Iα1 is the predominant pump current, Iα2 was derived from subtraction of two large numbers, which by its very nature is imprecise. The methodological limitation precludes our ability to consistently detect small but significant changes in Iα2 in response to Iso. In contrast, using “SWAP” mice in which the ouabain affinities of the α-subunits are reversed, Bossuyt et al. (8) demonstrated that PLM regulates the activities of both α1- and α2-subunits of Na+-K+-ATPase. In addition, in heterologous expression systems, Bibert et al. (6) showed that p-PLM enhances the activities of both α1- and α2-subunits of Na+-K+-ATPase. The weight of evidence favors the concept that PLM modulates both α1- and α2-subunits of Na+-K+-ATPase. Finally, we wish to note that our previous study (43) was performed in mice of the C57BL/6 background, whereas the inducible S68E mice in the present study were of the FVB background. It has recently been appreciated that genetic background differences have a significant impact, not only on cardiac responses to pathological stimuli such as transverse aortic constriction (3), in vivo acute hypoxia (2), and myocardial infarction (38) but also on baseline myocardial function, responses to β-adrenergic antagonists, and intracellular Ca2+ regulation (2, 31).
In summary, induced expression of S68E mutant resulted in 0.3- to 0.7-fold expression of the transgene compared with endogenous phospholemman, with no cardiac phenotype at baseline. Despite increased protein levels of Na+/Ca2+ exchanger, Na+/Ca2+ exchange activity was inhibited in myocytes in which expression of S68E mutant was induced. Under catecholamine stimulation, induced S68E myocytes exhibited higher systolic [Ca2+]i, [Ca2+]i transient and contraction amplitudes, and SR Ca2+ content compared with non-induced myocytes. In the presence of isoproterenol, induced S68E hearts had higher +dP/dt compared with non-induced hearts. After ischemia/reperfusion injury, induced S68E mice had lower mortality compared with non-induced mice. In addition, despite similar areas at risk and infarct sizes, induced S68E hearts contracted better than non-induced hearts when stimulated with isoproterenol. We conclude that targeting serine68 of phospholemman may be a useful therapeutic strategy to enhance cardiac performance under stress, especially for ischemic injury.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1-HL-58672 and RO1-HL-74854 (to J. Y. Cheung); RO1-HL-56205, RO1-HL-61690, RO1-HL-85503, PO1-HL-75443, and PO1-HL-91799 (to W. J. Koch); and PO1-HL-91799 (Project 2; to A. M. Feldman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W., W.J.K., A.M.F., and J.Y.C. conception and design of research; J.W., J.S., E.G., X.-Q.Z., T.G., and J.Y.C. performed experiments; J.W., J.S., E.G., X.-Q.Z., T.G., D.Y., and J.Y.C. analyzed data; J.W., J.S., E.G., X.-Q.Z., D.Y., W.J.K., A.M.F., and J.Y.C. interpreted results of experiments; J.W., W.J.K., A.M.F., and J.Y.C. edited and revised manuscript; J.W., J.S., E.G., X.-Q.Z., T.G., D.Y., W.J.K., A.M.F., and J.Y.C. approved final version of manuscript; X.-Q.Z. and J.Y.C. prepared figures; J.Y.C. drafted manuscript.
REFERENCES
- 1.Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genomics 42A: 103–113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292: H2119–H2130, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterisation of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na/K ATPase activity. Am J Physiol Heart Circ Physiol 294: H613–H621, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bibert S, Roy S, Schaer D, Horisberger JD, Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J Biol Chem 283: 476–486, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bossuyt J, Ai X, Moorman JR, Pogwizd SM, Bers DM. Expression and phosphorylation of the Na-pump regulatory subunit phospholemman in heart failure. Circ Res 97: 558–565, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bossuyt J, Despa S, Han F, Hou Z, Robia SL, Lingrel JB, Bers DM. Isoform-specificity of the Na/K-ATPase association and regulation by phospholemman. J Biol Chem 284: 26749–26757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung JY, Zhang XQ, Song J, Gao E, Rabinowitz JE, Chan TO, Wang J. Phospholemman: a novel cardiac stress protein. Clin Transl Sci 3: 189–196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung JY, Zhang XQ, Song J, Gao E, Chan TO, Rabinowitz JE, Koch WJ, Feldman AM, Wang J. Coordinated regulation of cardiac Na+/Ca2+ exchanger and Na+-K+-ATPase by pPhospholemman (FXYD1). Adv Exp Med Biol 961: 175–190, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Chu G, Ferguson DG, Edes I, Kiss E, Sato Y, Kranias EG. Phospholamban ablation and compensatory responses in the mammalian heart. Ann NY Acad Sci 853: 49–62, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: a user's guide for genetically manipulating the mouse in cardiac research. Circ Res 111: 761–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the β-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Despa S, Tucker A, Bers D. PLM-mediated activation of Na/K-ATPase limits [Na]i and inotropic state during β-adrenergic stimulation in mouse ventricular myocytes. Circulation 117: 1849–1855, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller W, Eaton P, Bell JR, Shattock MJ. Ischemia-induced phosphorylation of phospholemman directly activates rat cardiac Na/K-ATPase. FASEB J 18: 197–199, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Fuller W, Howie J, McLatchie L, Weber R, Hastie CJ, Burness K, Pavlovic D, Shattock MJ. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine69 is a novel substrate for protein kinase C. Am J Physiol Cell Physiol 296: C1346–C1355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107: 1445–1453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo K, Wang X, Gao G, Huang C, Elmslie KS, Peterson BZ. Amino acid substitutions in the FXYD motif enhance phospholemman-induced modulation of cardiac L-type calcium channels. Am J Physiol Cell Physiol 299: C1203–C1211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenfuss G. Alteration of calcium-regulatory proteins in heart failure. Cardiovasc Res 37: 279–289, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss G, Schillinger W. Is modulation of sodium-calcium exchange a therapeutic option in heart failure? Circ Res 95: 225–227, 2004 [DOI] [PubMed] [Google Scholar]
- 21.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, Dillmann WH. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest 100: 380–389, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hobai IA, Maack C, O'Rourke B. Partial inhibition of sodium/calcium exchange restores cellular calcium handling in canine heart failure. Circ Res 95: 292–299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levesque PC, Hume JR. ATPo but not cAMPi activates a chloride conductance in mouse ventricular myocytes. Cardiovasc Res 29: 336–343, 1995 [PubMed] [Google Scholar]
- 24.Li X, Mikhalkova D, Gao E, Zhang J, Myers V, Zincarelli C, Lei Y, Song J, Koch WJ, Peppel K, Cheung JY, Feldman AM, Chan TO. Myocardial injury after ischemia-reperfusion in mice deficient in Akt2 is associated with increased cardiac macrophage density. Am J Physiol Heart Circ Physiol 301: H1932–H1940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X, Jo H, Sakakibara Y, Tambara K, Kim B, Komeda M, Matsuoka S. β-Adrenergic stimulation does not activate Na+/Ca2+ exchange current in guinea pig, mouse and rat ventricular myocytes. Am J Physiol Cell Physiol 290: C601–C608, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ozdemir S, Bito V, Holemans P, Vinet L, Mercadier JJ, Varro A, Sipido KR. Pharmacological inhibition of Na/Ca exchange results in increased cellular Ca2+ load attributable to the predominance of forward mode block. Circ Res 102: 1398–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem 266: 11126–11130, 1991 [PubMed] [Google Scholar]
- 28.Presti CF, Jones LR, Lindemann JP. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J Biol Chem 260: 3860–3867, 1985 [PubMed] [Google Scholar]
- 29.Rembold CM, Ripley ML, Meeks MK, Geddis LM, Kutchai HC, Marassi FM, Cheung JY, Moorman JR. Serine68 phospholemman phosphorylation during forskolin-induced swine carotid artery relaxation. J Vasc Res 42: 483–491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos KP, Jordan MC, Fishbein MC, Ritter MR, Friedlander M, Chang HC, Rahgozar P, Han T, Garcia AJ, Maclellan WR, Ross RS, Philipson KD. Hypertrophy and heart failure in mice overexpressing the cardiac sodium-calcium exchanger. J Cardiac Failure 13: 318–329, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah AP, Siedlecka U, Gandhi A, Navaratnarajah M, Al-Saud SA, Yacoub MH, Terracciano CM. Genetic background affects function and intracellular calcium regulation of mouse hearts. Cardiovasc Res 87: 683–693, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Sipido KR, Volders PGA, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res 53: 782–805, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Song J, Gao E, Wang J, Zhang XQ, Chan TO, Koch WJ, Shang X, Joseph JI, Peterson BZ, Feldman AM, Cheung JY. Constitutive overexpression of phospholemman S68E mutant results in arrhythmias, early mortality and heart failure: potenial involvement of Na+/Ca2+ exchanger. Am J Physiol Heart Circ Physiol 302: H770–H781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H2342–H2354, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Song J, Zhang XQ, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alter contractility and [Ca2+]i transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H576–H583, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman: Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 295: H1615–H1625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]i homeostasis in phospholemman-deficient murine myocytes: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 291: H2199–H2209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 84: 273–282, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Waalas SI, Czernik AJ, Olstad OK, Sletten K, Walaas O. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochem J 304: 635–640, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents and action potential duration. Am J Physiol Heart Circ Physiol 297: H590–H601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Gao E, Chan TO, Zhang XQ, Song J, Shang X, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger does not aggravate myocardial dysfunction induced by transverse aortic constriction. J Cardiac Failure 19: 60–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Gao E, Rabinowitz J, Song J, Zhang XQ, Koch WJ, Tucker AL, Chan TO, Feldman AM, Cheung JY. Regulation of in vivo cardiac contractility by phospholemman: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 300: H859–H868, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Gao E, Song J, Zhang XQ, Li J, Koch WJ, Tucker AL, Philipson KD, Chan TO, Feldman AM, Cheung JY. Phospholemman and β-adrenergic stimulation in the heart. Am J Physiol Heart Circ Physiol 298: H807–H815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Gao G, Guo K, Yarotskyy V, Huang C, Elmslie KS, Peterson BZ. Phospholemman modulates the gating of cardiac L-type calcium channels. Biophys J 98: 1149–1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XQ, Moorman JR, Ahlers BA, Carl LL, Lake DE, Song J, Mounsey JP, Tucker AL, Chan YM, Rothblum LI, Stahl RC, Carey DJ, Cheung JY. Phospholemman overexpression inhibits Na+-K+-ATPase in adult rat cardiac myocytes: relevance to decreased Na+ pump activity in post-infarction myocytes. J Appl Physiol 100: 212–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. J Appl Physiol 87: 2143–2150, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI, Cheung JY. Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 284: H225–H233, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000 [DOI] [PubMed] [Google Scholar]