Abstract
Sensory information in the visual, auditory and somatosensory systems is organized topographically, with key sensory features ordered in space across neural sheets. Despite the existence of a spatially stereotyped map of odor identity within the olfactory bulb, it is unclear whether the higher olfactory cortex uses topography to organize information about smells. Here, we review recent work on the anatomy, microcircuitry and neuromodulation of two higher-order olfactory areas: the piriform cortex and the olfactory tubercle. The piriform is an archicortical region with an extensive local associational network that constructs representations of odor identity. The olfactory tubercle is an extension of the ventral striatum that may use reward-based learning rules to encode odor valence. We argue that in contrast to brain circuits for other sensory modalities, both the piriform and the olfactory tubercle largely discard any topography present in the bulb and instead use distributive afferent connectivity, local learning rules and input from neuromodulatory centers to build representations of behaviorally relevant properties of olfactory stimuli.
Introduction
Many mammalian sensory brain areas are organized such that physically nearby neurons respond to related stimuli [1–3]. Indeed, topographic neural maps — in which stimulus space parameters are converted into spatial relationships amongst neurons — seem to be a fundamental property of brain circuits in the visual, auditory and somatosensory systems. For instance, the visual system maps the position of objects in visual space onto the two-dimensional surface of the retina. This retinotopic map is faithfully projected via organized axonal projections to thalamic and cortical visual centers. Hierarchically organized higher-order cortical areas exploit correlations and differences between local positional features to extract information like object identity, depth and motion [4–6]. Unlike the small number of continuous sensory parameters that characterize vision, audition and touch (such as position, frequency and amplitude), olfactory parameter space is poorly defined and highly multidimensional [7]. For example, any given monomolecular odorant can be described in terms of its functional groups, molecular weight, chain length, bond substitution, resonance frequency or any number of additional chemical descriptors. Furthermore, olfactory space is inherently discrete — not only are individual odorants structurally unique but many of the molecular descriptors typically used for individual odorants (such as functional group or bond substitution) cannot be mapped continuously in any scheme for chemical space. Nevertheless, the brain somehow transforms this complex stimulus space into a neural code capable of specifying odor object identity and valence, higher-order features that are crucial for allowing animals to learn associations with the entire universe of odorants and to innately find food, avoid predators and negotiate conspecific interactions.
The surface of the olfactory bulb, the first processing center for olfactory information within the brain, organizes incoming information into a spatially stereotyped map of the olfactory world; however, it is unclear how cortical olfactory areas make use of this map, or otherwise construct higher-order representations for odor space. Here we argue that two higher-order olfactory regions dynamically construct representations of stimulus parameters using distributive afferent connectivity, local learning rules and the input from neuromodulatory centers. We review what is known about the anatomy, microcircuitry, response properties and overall function of the piriform cortex and the olfactory tubercle, and illustrate how these features position each region to differentially encode two key parameters of olfactory stimuli: odor identity and odor valence. Note that here, for reasons of clarity and brevity, we focus on the specific role of macro- and microcircuits in building representations for odorants in which encoding of stimulus-related features is achieved through the distribution of information in space. Because of this focus on spatial maps for olfaction (particularly within the cortex), we neither discuss important work that addresses the role of temporal coding in the olfactory system, nor do we review the potential role for the olfactory bulb in odor learning and odor valence encoding. These processes are well reviewed elsewhere [8–14].
Anatomy of the Olfactory System
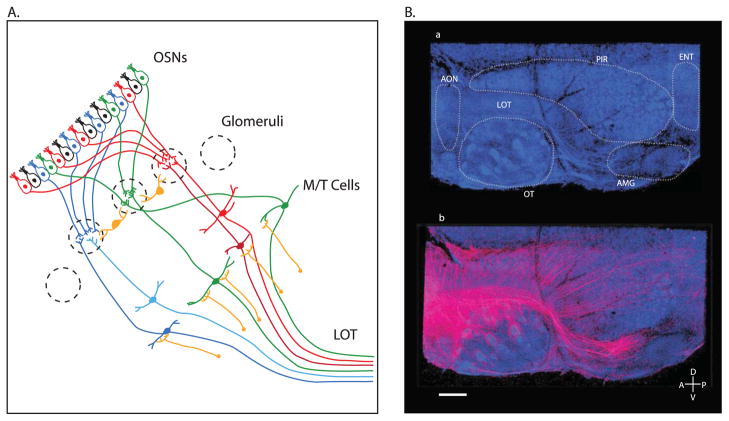
The anatomy of the mammalian olfactory system has been elaborated over the last century using a combination of anatomical tracing, genetics, imaging and electrophysiology [15–17] (Figure 1A). Specialized olfactory sensory neurons (OSNs), which detect odorants via expression of odorant receptor (OR) proteins, are distributed across the nasal epithelium. Although a typical mammalian genome encodes hundreds of potential OR genes, each OSN is thought to exclusively express one type of OR protein [18]. Every neuron expressing a given OR sends its axon to a genetically stereotyped region of neuropil on the surface of the olfactory bulb, termed a glomerulus, where it forms synapses with projection neurons whose cell bodies reside deeper in the bulb. Each OR is thought to bind odorants through interactions with specific molecular features, and on the whole ORs exhibit relatively loose tuning across odor space. Thus odors activate a specific spatiotemporal pattern of activity within glomeruli distributed across the olfactory bulb that can be taken to encode odor identity, as any given odorant activates a unique constellation of glomeruli whose spatial distribution is conserved from animal to animal. The primary projection neurons of the olfactory bulb, mitral and tufted (M/T) cells, each innervate a single bulbar glomerulus and elaborate axons that fasciculate and form the lateral olfactory tract (LOT), which courses along the ventro-lateral surface of the brain. Due to differences in intrinsic properties and intrabulbar processing, mitral and tufted cells exhibit distinct odor tuning and response properties, with tufted cells responding more quickly and more broadly to odorants [16,19–21]. The extensive axonal arbors of M/T cells can span distances of up to a centimeter (in the mouse) and innervate several areas collectively known as “olfactory cortex” [22], including the piriform cortex (PCTX), olfactory tubercle (OT), cortical amygdala (CoA), anterior olfactory nucleus (AON), tenia tecta and lateral entorhinal cortex.
Figure 1. General Anatomy of the Mammalian Olfactory System.
A) Anatomy of the peripheral olfactory system. Odorant sensory neurons (OSNs) distributed across the nasal epithelium express a single odorant receptor (OR). Every OSN expressing a particular OR sends its axons to a genetically stereotyped location on the surface of the olfactory bulb, termed a glomerulus (dashed circles). The bulb contains a number of interneuron types (yellow) including periglomerular and granule cells. Mitral and the more superficial tufted cells (M/T) send their dendrites into a single glomerulus and their axons fasciculate to form the lateral olfactory tract (LOT), which projects to olfactory cortex. As noted in the text this review focuses on feedforward afferents to the olfactory cortex; for simplicity this diagram therefore excludes the many cell types and wiring relevant to intrabulbar processing of olfactory information.
B) Axonal projection patterns in olfactory cortex from a single glomerulus. Top, flattened preparation of olfactory cortex with nuclei stained in blue. Major sub-regions of the olfactory cortex are outlined and labeled: piriform cortex (PIR), olfactory tubercle (OT), anterior olfactory nucleas (AON), cortical amygdala (AMG), lateral entorhineal cortex (ENT). Bottom, same preparation where a single glomerulus has been electroporated with TMR-dextran (pink). Each sub-region of the olfactory cortex is innervated, but projection patterns vary extensively from region to region. Scale bar 700 um; A, anterior; P, posterior; D, dorsal; V, ventral. Figure from [39].
Afferent Input
The dramatic crystalline array of glomeruli tiling the surface of the olfactory bulb (Fig. 1) raises the possibility that sensory information is organized into discrete glomerular channels and further suggests that the glomerular array itself might be organized topographically — the surface of an “unrolled” olfactory bulb might organize olfactory information (i.e. inputs to single glomeruli) into a two-dimensional map that represents features of olfactory space. The unusual anatomy of the olfactory bulb therefore potentially reconciles the idea that olfactory space is discrete (via molecular feature encoding within discrete glomeruli) with the notion that continuous sensory maps are often built in two-dimensional sheets, raising the possibility that the bulb contains a topographically-organized map for smell not so different from topographic maps for other sensory modalities. Of course, maps are useful only insofar as they are read: thus more than 50 years before molecular biologists revealed the underlying functional organization of the bulb, researchers injected dyes, enzymes and radioactive tracers to ask whether there was a topographical relationship between spatial domains within the olfactory bulb and target regions in the olfactory cortex [23–31]. In principle the results from such experiments could fall anywhere between one of two extremes, from point-to-point topography (where nearby glomeruli project to nearby areas in olfactory cortex, reminiscent of retinal projections to the lateral geniculate nucleus of the thalamus), to all-to-all topography (where all glomeruli project to an equal extent to all regions of olfactory cortex, abandoning any spatial order apparent in the olfactory bulb). These early labeling studies revealed a dense interconnectivity between the bulb and the olfactory cortex more suggestive of all-to-all than point-to-point topography, but with intriguing hints of underlying organization. For example, the PCTX was shown to receive input from predominantly mitral cells, while the OT was shown to get most of its projections from tufted cells; clear gradients of axons were also revealed, with the anterior PCTX receiving more afferents than the posterior PCTX, and the lateral OT receiving denser innervation from the bulb than the medial OT [23–31]. However, labeling of smaller bulb regions and filling of single mitral cells (which only partially highlighted their cortical extent) revealed patches of axonal branches that focally innervated multiple loci within the PCTX [32,33]. For many years this and other similar results served as a sort of Rorschach test, with some researchers arguing that these focal patches reveal all-to-all patterns of projection between the bulb and olfactory cortex, and others concluding that those same patches demonstrate underlying point-to-point topography. Consistent with notions of a topographic mapping of the bulb onto the cortex, later experiments in which small amounts of dye were introduced into the bulb under the guidance of a fluorescence microscope revealed that projections to the AON pars externa are topographically organized (with a matched dorsal-to-ventral pattern of projections), raising the possibility that other regions of the olfactory cortex also receive spatially ordered patterns of projections from the bulb [34,35].
The discovery that the spatial position of any given glomerulus (as defined by OR expression) is hardwired into the bulb and invariant from animal to animal revealed that odor identity can be encoded through spatial patterns of glomerular activity within the bulb [18,36–38]. While local circuits within the olfactory bulb likely modify these patterns via lateral interactions between glomeruli [14], the stereotyped nature of OSN axon projections into the bulb imposes a structured input to the population of M/T cells that is unique for any given olfactory stimulus. The fundamental unit of computation within the olfactory bulb is therefore the glomerulus: glomeruli are genetically-specified channels that uniquely subsample olfactory space within a stereotyped bulbar map of odor identity [18]. Thus, independent of any notion of topography, characterizing how information from a single glomerulus is distributed to the rest of the brain is crucial for understanding the function of the olfactory system. Recent advances in multiphoton microscopy, genetics and viral tracing technologies have provided direct experimental access to this question, and in doing so shed new light on the question of topography in the olfactory cortex. Electroporation of tetramethylrhodamine (TMR)-dextran into single genetically identified glomeruli revealed that projections from the bulb, regardless of spatial location or identity, are dispersed across the entire surface of the PCTX [39] (Figure 1B). This result was consistent with others obtained by introducing anterograde viral tracers into “sister” mitral cells that innervate the same glomerulus [40]. In this case, each mitral cell was found to target a unique set of subdomains within the PCTX, with the summed axonal arbors of those sister mitral cells that innervate a given glomerulus effectively tiling the entire surface of the PCTX. While these anterograde tracing experiments reveal that each glomerulus distributes its projections across the expanse of the PCTX, retrograde trans-synaptic viral tracing from single PCTX cells also demonstrates that single PCTX primary neurons receive inputs from glomeruli distributed across the bulb [41]. Thus, at least with respect to the PCTX, single glomerulus and single neuron tracing strongly suggests an all-to-all distribution of sensory information from the bulb to the cortex — every region of the bulb projects to every region of the PCTX. It is important to note that the all-to-all nature of projections from the bulb to the PCTX breaks down a certain spatial scale because every glomerulus does not project to every neuron; rather it appears that PCTX neurons sample from local axons that contain information from spatially dispersed glomeruli within the bulb. In contrast, anterograde tracing via dye electroporation revealed a starkly different pattern of projections from individual glomeruli to the CoA, in which individual genetically identified glomeruli were found to project to focal and spatially stereotyped regions of the CoA. Axonal projections from the OB to the CoA and to the OT were also found to exhibit a crude set of topographical relationships: dorsal glomeruli tended to project to ventral regions of the CoA and OT, while ventral bulb regions projected to corresponding dorsal regions in the these areas ([39], preliminary data). This topography is not strict: individual glomeruli were identified that break this general rule, further supporting the notion that the relevant unit of computation in this system is the glomerulus itself. Retrograde tracing also revealed features of topographic order in the olfactory system; primary neurons in the AON revealed topographically ordered patterns of projection from the bulb that preserve dorsal-ventral relationships (consistent with previous dye tracing results [35]), and infection of neurons within the CoA revealed a slightly higher overall density of innervation from the dorsal bulb [41].
Taken together these results suggest that at the anatomic level the genetically stereotyped glomerular map of odor identity within the olfactory bulb is dramatically rewritten through projections to the cortex: projections to the PCTX are dispersive and effectively destroy any notion of spatial order originating within the olfactory bulb, while in contrast the CoA receives topographically organized patterns of projection, suggesting that it may make use of information that is spatially organized across the olfactory bulb. The OT seems to sit between these two extremes: although Sosulski et al. [39] did not analyze the pattern of projections from single glomeruli to the OT comprehensively, there seems to be little order to the bulbar projections other than the overall dorsal-ventral axis mapping mentioned above, and the preferential innervation of the “crest” regions of the OT (see below). Taken together, these observations suggest that the OT receives a “hybrid” transformation of the glomerular map, although much work remains to be done to quantify afferent patterns of projection to the OT.
Local Circuit Anatomy and Response Properties
Piriform Cortex
Anatomy
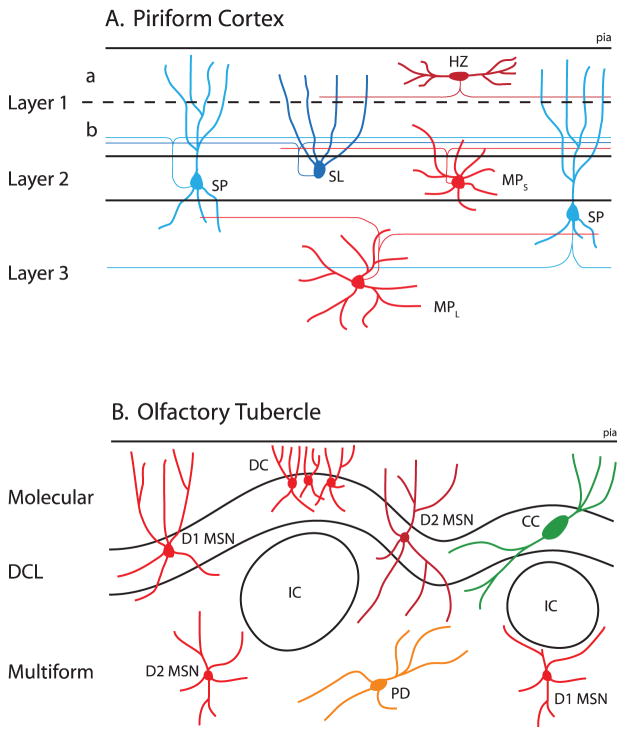
The PCTX, the largest and best-studied subregion of the olfactory cortex, is a trilaminar archicortical structure heavily innervated by the olfactory bulb [42–45] (Figure 2A). Layer 1a contains primarily afferent axons from the bulb, while layer 1b contains associational axons from neurons located throughout the PCTX; the dendrites of the principal cells of the PCTX span both sub-layers. Layer 1 also includes GABAergic horizontal (HZ) and neurogliaform (NG) interneurons [46–50], whose superficially localized axons are poised to provide dendritic feedforward inhibition to other neurons of the PCTX. Layer 2 contains the principal neurons of the PCTX, the glutamatergic semilunar (SL) and spiny pyramidal (SP) cells. Both SL and SP cells extend apical dendrites up to the pial surface where they receive synaptic input from the LOT and other cells of the PCTX, innervate downstream regions like the entorhinal and prefrontal cortices and elaborate extensive associational collaterals in layers 1b through 3 [51–53]. SL cells are located in the more superficial layer 2a and do not have basal dendrites, while SP cells are densely packed in layer 2b and have basal dendrites extending into Layer 3. Layer 2 also contains several GABAergic interneurons, including bitufted, and small and large multipolar cells [46–50]. Layer 3 is predominantly neuropilar, containing relatively few somata, including deep pyramidal cells and a number of interneuron types. As mentioned above, the PCTX has been traditionally divided into anterior and posterior portions, and the ratio of afferent to associational fibers in layer 1 decreases as one moves more posterior [22,54]. Thus, the primary neurons of the PCTX are anatomically poised to respond to activity in the bulb conveyed by the LOT in a manner that is strongly modulated by local feedforward and feedback excitation and inhibition.
Figure 2. Microcircuits of the Piriform Cortex and Olfactory Tubercle.
A) Major cell types and anatomy of the Piriform Cortex. Excitatory neurons are colored in blue, inhibitory neurons in red. Axons from the LOT are restricted to layer 1a, where they synapse onto spiny pyramidal cells (SP), semilunar cells (SL) and interneurons such as horizontal cells (HZ). Interneurons present in layer 1a provide feedforward inhibition to SL/SP cells. Collaterals of SP and SL axons ramify extensively across layers 1b through 3. These collaterals excite other SP cells as well as small and large multipolar neurons (MPS, MPL). Multiform neurons provide strong feedback inhibition that balances excitation and keeps odor representations sparse. Dendrites are represented by thick lines, axons as thin lines.
B) Major cell types and anatomy of the Olfactory Tubercle. Axons from the LOT are restricted primarily to the superficial molecular layer of the OT. There, they synapse onto the dendrites of D1R- and D2R-type medium spiny neurons (MSNs, D1 colored in light red, D2 in dark red). The somata of these cells are located in the dense cell layer (DCL), which undulates across the extent of the OT. Also present are various interneuron types, such as crescent cells (CC, green). Below the DCL in the multiform layer are tight clusters of granule cells, the Islands Of Cajella (IC). The ICs displace the DCL to form crests that approach the pial surface containing dwarf cells (DC). Intermingled within the multiform layer are other MSNs and regions of ventral pallidum and displaced pallidal cells (PD, orange).
Microcircuitry
Several studies using in vitro approaches have characterized microcircuits in the PCTX [46,50,53,55–57]. As expected from anatomy, both SL and SP neurons are directly excited by LOT stimulation, but unitary excitatory post-synaptic currents (EPSCs) in SL cells are 3–4 times larger on average, suggesting a greater sensitivity of SL neurons to bulbar activity [53,56]. A recent study using glutamate uncaging to activate different numbers of random glomeruli in the bulb showed that SL/SP neurons typically only fire action potentials when 3 or more glomeruli are co-active [58] (but see [59]). HG and NG interneurons within layer 1 are also directly activated by LOT fibers; paired recordings between HZ/NG and SL/SP cells show that they indeed mediate feedforward dendritic inhibition onto the principal cells of the PCTX and thus temper bulbar excitation [46,50]. Interestingly, the responses of HZ/NG cells to LOT stimulation attenuate over frequencies and time-scales similar to rodent breathing rates. This has lead to the idea that layer 1 interneurons might filter out spurious, weak or asynchronous LOT activity in the dendrites of SL and SP neurons [46]. Furthermore, SL/SP neurons have low spontaneous firing rates, again demonstrating that the relatively high spontaneous firing rates observed in mitral and tufted cells in the bulb are filtered before transmission to primary neurons in the PCTX [59–62]. Finally, while unitary EPSCs evoked by single-fiber LOT stimulation are equivalent in amplitude between layer 1 interneurons and SP cells, LOT-evoked compound EPSCs are ~6 times larger in layer 1 interneurons, presumably due to greater convergence of LOT axons onto the interneurons [46]. Combined with the distributed nature of afferents in the piriform, this leads to the prediction that layer 1 interneurons should be more broadly tuned to odors than SL/SP cells.
The prominent associational connectivity of the PCTX likely plays a critical role in shaping PCTX network activity. While unitary associational EPSCs in SL and SP cells are equal in amplitude, compound associational EPSCs are much larger in SP cells, suggesting that SP neurons receive more associational inputs [53,54]. These data, taken together with the LOT stimulation experiments discussed above, demonstrate an important distinction between SL and SP cells: the activity of SL cells is relatively more sensitive to bulbar input, whereas SP cell activity is primarily driven by local feedforward excitation. Recent work using optogenetics and whole-cell recordings demonstrated that feedforward excitation in the PCTX is spatially widespread — cells up to 2 mm away are reliably excited by presynaptic activation [54,57]. While individual associational connections are sparse and weak, with connection probabilities less than 1% and unitary EPSCs of 25–35 pA, the number of such synapses is high — estimated to be an order of magnitude greater than the number of afferent inputs [57,58]. These factors combine to make associational inputs onto SP cells very strong in aggregate. Indeed, recent work found examples of neurons that were excited by activating large numbers of glomeruli even when direct inputs from individual glomeruli were negligible [58]. This result indicates a critical role for the associational network of the PCTX in activating neurons within an odor-evoked ensemble of responsive cells.
The extensive recurrent network in the PCTX implies an important role for inhibition in preventing runaway excitation; indeed, the PCTX is prone to seizures in both rodents and humans, demonstrating the importance of the excitatory/inhibitory balance in this brain area [63,64]. This has been explored in experiments in which researchers record post-synaptic currents in SL/SP cells while repeatedly stimulating the LOT; under these conditions feedback inhibitory post-synaptic currents (IPSCs) increase in amplitude [46,50]. This observed increase in feedback inhibition is likely due to a progressive recruitment of SL/SP cells during the stimulus train, which in turn evokes increased feedback inhibition mediated primarily by fast-spiking multipolar cells located in layers 2 and 3. These synaptic dynamics, along with those mentioned above for layer 1 interneurons, suggest that activity in the PCTX may shift over time from an input-dominated mode to an association-dominated mode, underscoring the importance of deep layer interneurons in controlling overall activity. The spatial spread of feedback inhibition is essentially equivalent to the spatial distribution of feedforward excitation, although the resulting IPSCs are stronger than the EPSCs [57]. As a consequence, any group of “starter” neurons triggered by an odor stimulus will activate a particular ensemble of PCTX neurons via feedforward excitation, which will in turn activate even more neurons. The scale of this feedforward excitation is such that the recruited ensemble is large (spanning most of the piriform) but the larger amplitude of inhibition relative to excitation keeps this representation relatively sparse and prevents epileptic activity.
Odor Response Properties
The receptive fields of individual neurons in the PCTX have been determined at the single cell level with in vivo extracellular and intracellular recordings, and at the population level with immediate early gene staining and in vivo two-photon calcium imaging [51,52,59,62,65–75]. Electrophysiological studies have indicated that individual neurons can be excited by multiple odors, often in a respiration-modulated manner, and that cells responsive to any one odor are distributed across the anterior and posterior PCTX, consistent with the anatomy summarized above. In general odors activate around 10% of recorded SL/SP cells [51,62,71]. However, one study using a 25-odor set suggested that multiple sub-classes of SL/SP cells might exist [62]. This study identified at least two types of cells: a larger class that was broadly tuned to structurally dissimilar odors, and a smaller class that was narrowly tuned. It would be interesting if these classes reflected SP and SL cells, respectively, but it is as likely that these data simply reflect the peculiarities of associational connections in the PCTX. One consistent finding is that interneurons in layer 1 are much more broadly tuned than the principal neurons in layers 2/3 [51,52,62]; this result is consistent with the microcircuit structure described above, and suggests an important role for global feedforward inhibition in this brain area. To explore the organization of odor-driven responses at the ensemble level, in vivo two-photon calcium imaging has been used to look at odor responses distributed across wide stretches of the PCTX [72]. These experiments confirm that odor responses are spatially distributed across the PCTX, and provide direct evidence that odor-evoked responses form overlapping ensembles, consistent with previous work using c-fos staining [76]. In addition, these experiments demonstrated that odor responses “add” sublinearly and thus odor mixtures tend to activate similar numbers of neurons as their components, and that the density of ensemble responses is only weakly dependent on odor concentration.
These results suggest that the PCTX generates odor representations such that any given odor or odor mixture (at a wide range of concentrations) recruits a unique, spatially dispersed and approximately equally sized ensemble of primary neurons. Receptive fields within the PCTX are not organized topographically, and individual neurons respond to multiple structurally distinct odors. The extensive excitatory and inhibitory associational network present in the PCTX plays a key role in normalizing responses to any given olfactory stimulus and in building dispersive ensembles that are well-suited to encode odor object identity.
Neuromodulation
The PCTX is innervated by a number of neuromodulatory centers, including the noradrenergic locus coeruleus and the cholinergic nucleus of the horizontal limb of the diagonal band [77,78], which alter activity and plasticity within the PCTX. Acetylcholine (ACh) has been shown to affect a wide variety of cellular processes in the PCTX, including increasing the intrinsic excitability of principal cells and interneurons and altering both inhibitory and excitatory synaptic transmission, via the activation of pre- and post-synaptic metabotropic ACh receptors [79–82]. In particular, ACh has been shown to suppress associative fiber responses while leaving afferent fiber responses relatively unchanged. However, long-term potentiation of the associational pathways is enhanced in the presence of ACh, likely due to a suppression of local inhibition [81–83]. These findings suggest that ACh might allow for plasticity in the associational fibers, but prevents indiscriminate changes across the entire network. This hypothesis is supported by behavioral experiments in which animals were first habituated to a mixture of odors and then components of these mixtures were tested for cross-habituation [65]. When metabotropic ACh signaling was blocked with scopolamine, cross-habituation between mixtures and their components increased, suggesting that the animal failed to “learn” the mixture as a unique odor object. Increased cholinergic tone is associated with increases in attention and arousal, and so this may serve as a way for the animal to selectively enhance its ability to discriminate important olfactory features of the environment [84–86]. Taken together, these results have led to theoretical models wherein ACh allows for the formation of unique neuronal ensembles representing odor objects via rapid synaptic plasticity of the associational network within the PCTX [83,87]. These intriguing models are ripe for more detailed investigation.
Olfactory Tubercle
Anatomy
The OT lies ventral/medial and slightly anterior to the PCTX, and is apparent on the surface of the brain as a slight bulge separated from the PCTX by the LOT. Historically, the OT has been described as a laminar structure, which led early investigators to assume that it was cortical in nature [88,89]. Later histological and anatomical studies lent support to the idea that the OT is the ventral-most extension of the striatum, the input structure of the basal ganglia [88]. Indeed, the OT is physically contiguous with the nucleus accumbens (NucAcc) and shares common patterns of inputs and outputs [90–92]. Like the NucAcc, the OT receives inputs from the prefrontal cortex, hippocampus and amygdala. The OT projects to the ventral striatum and pallidum, thalamus, hypothalamus, and various brainstem nuclei that control feeding, drinking and locomotor behavior [88]. Notably, the OT is heavily and bidirectionally connected with the ventral tegmental area (VTA), a dopaminergic center that also targets the NucAcc core and shell [93]. In addition, the OT is a bona fide olfactory area, receiving direct input from the bulb, and extensive inputs from the other parts of olfactory cortex, including the PCTX and CoA [30,31,94]. Interestingly, the OT does not send associational axons to the other higher-order olfactory areas, a situation similar to the dorsal sensorimotor striatum. As a whole, these features strongly suggest that the OT should be considered olfactory/limbic striatum.
The OT is traditionally divided into a molecular layer, a so-called dense cell layer (DCL) and a sparser multiform layer [88] (Figure 2B). As its name suggests, the molecular layer predominantly consists of the axons of the LOT and other olfactory cortical regions, and the dendrites of cells residing deeper in the OT. Unlike layer 2/3 in the PCTX, the DCL is not a regular lamina — instead it undulates across the extent of the whole OT. The principal cell type within the DCL is the medium spiny neuron (MSN). These GABAergic neurons are the principal cells of the OT, and as is true in the rest of the striatum these neurons express D1- and D2-type dopamine receptors [95,96]. The dendrites of OT MSNs extend to the pial surface and their axons project to the deeper multiform layer, forming collateral bridges to the rest of the ventral striatum, as well as projecting to the ventral pallidum and several other brain areas. Finally, the multiform layer, which is loosely intermingled with the ventral pallidum, contains axon bundles from a wide variety of brain areas and sparse cellular somata (likely representing interneurons) [88].
One of the most conspicuous anatomical features of the OT is the presence of the Islands of Calleja (IC) — large regions of tightly packed granular cells that lie just above the DCL and extend dorsally into the NucAcc [97–99]. These islands, which generally number between 10 and 20, are heterogeneously distributed from animal to animal [100]. Interestingly, the ICs seem to form topographically organized and reciprocal connections with the NucAcc, amygdala and PCTX. The ICs develop after other cell types in the OT, presumably displacing the DCL outward and contributing to its rippled structure [100,101]. The undulating DCL forms caps of cells called “crests” close to the pial surface overlaying the ICs, and within these crests reside a sub-type of MSNs with smaller cell bodies termed dwarf cells [88]. The functional significance of ICs and crests of dwarf cells, which are unique to this otherwise striatum-like structure, is unknown.
Microcircuitry
In stark contrast to the PCTX, the microcircuitry of the OT remains essentially uncharacterized. Little is known about the properties of direct inputs from the bulb, associational connections within the OT, or the extensive connections from other olfactory cortical areas. In vivo recordings of field potentials within the OT elicited while stimulating the LOT demonstrated paired-pulse facilitation, although this finding also holds true for every other higher-order olfactory region examined [102]. Field responses in the DCL can be evoked by stimulating the molecular layer or the multiform layer, and these responses are differentially sensitive to cholinergic agonists and antagonists [103]. However, the significance and site of action of these drugs remains uncertain. Finally, voltage-sensitive dye imaging in an ex vivo guinea pig preparation revealed a biphasic response in the OT to LOT stimulation [104]. This response is strongest in the lateral OT, and severing input into the PCTX selectively reduces the second phase of the response. Taken together, these results confirm that the OT functionally responds to LOT stimulation and to associational inputs from the PCTX, as predicted by anatomy.
The most comprehensive in vitro electrophysiological survey of OT neurons was performed by Chiang and Strowbridge [105], in a study in which they recorded from neurons distributed across the DCL and multiform layers. They examined the intrinsic properties of these cells and found that they were divided into three broad classes: regular-spiking, intermittently-spiking and bursting cells. Regular spiking cells were spiny and likely correspond to MSNs. Their intermittently spiking and bursting cells seemed to include several morphological cell classes, and are probably a combination of distinct types. There are several poorly characterized interneuron types in the OT, most of which probably represent variants on the major neurons of the striatum including crescent cells (possibly cholinergic interneurons), and spine-poor and spindle cells (possibly GABAergic interneurons) [88]. Electrophysiological characterization of IC granule cells reveals that they are coupled via gap junctions and that this coupling is modulated by dopamine; however, the relationship of the ICs and the rest of the OT remains a mystery [106]. Future in vitro slice experiments targeting genetically identified subtypes of cells will be crucial in understanding the organization and function of microcircuits within the OT.
Odor Response Properties
Recent in vivo studies have used extracellular recordings to investigate odor responses in all three layers of the OT in anesthetized rats [70,107,108]. The majority of units in the OT are spontaneously active at around 5 Hz and modulated by breath rate. These spontaneous firing rates are consistent with MSNs in the rest of the striatum, but higher than those observed in the PCTX. Odor exposure increases the firing rate of a subset of units in the OT by several Hz, and these odor-responsive cells exhibit similar tuning properties and firing latencies to those described for primary neurons within the PCTX. Neurons in the OT were also found to respond to mixtures as well as to monomolecular odorants. Like the recordings performed to date in the PCTX, conclusions from this work in the OT are constrained by relatively small odor stimulus sets and low number of recorded units. Furthermore, the heterogeneous anatomy of the OT makes targeted recording of specific layers and cell types particularly challenging. Given these limitations, perhaps it is not surprising that odor response properties in the OT and the PCTX are superficially similar. However, the OT might encode information about olfactory stimuli in properties that extracellular recordings might miss, such as cell-type identity, large-scale topography, layer specificity or responses to neuromodulation. Finally, we note that a subset of units in the OT responds to auditory as well as olfactory stimuli, presumably through inputs from the hippocampus [108]. Thus, like other parts of the striatum, the OT may integrate olfactory input and other kinds of sensory stimuli with the motivational state of the animal via inputs from the rest of the basal forebrain.
Neuromodulation
Like the adjacent NucAcc, the OT is densely interconnected with the VTA, a dopaminergic center whose activity is tightly associated with reward and reward-based learning [93]. Indeed, lesions of the OT disrupt attention and social behaviors, and rats self-administer cocaine into the tubercle even more readily than into the NucAcc or ventral pallidum [109–111]. These results suggest that dopaminergic modulation of activity in the OT is reinforcing and likely crucial to its proper function [112–115].
Microcircuit Models of the Piriform Cortex and the Olfactory Tubercle
The differences in bulbar input, axonal projection patterns, microcircuitry, and cell types in the PCTX and the OT suggest that while they both receive extensive olfactory input, they likely encode different aspects of odor stimuli and perform distinct types of computations.
Based on anatomical similarity to the hippocampus (in terms of recurrent feedforward and feedback connectivity, both within the PCTX itself and between the PCTX and other olfactory cortical regions), and increasing experimental evidence, the PCTX has been hypothesized to be an associational memory circuit, binding molecular features of olfactory input into holistic odor representations [42,45,116] (Figure 3). Current data suggest that projections from any given glomerulus in the olfactory bulb tile the surface of the piriform cortex, offering any particular PCTX neuron the opportunity to sample afferents from a subset of bulbar axons without respect to glomerular location within the bulb. This distributive olfactory input from the bulb excites SP and especially SL cells, which seed activation of spatially distributed ensembles of SP cells via the extensive associational network present in the PCTX. Feedback inhibition driven by layer 2/3 interneurons keeps these odor representations relatively sparse (10 percent of cells or less), thereby increasing discriminability and preventing runaway excitation. Because each ensemble of recruited neurons is specific for a given odorant, the PCTX is well-suited to dynamically and synthetically represent the identity of the almost unlimited number of unique olfactory stimuli (both monomolecular odorants and complex odor objects) an organism might encounter in a lifetime. Information from the PCTX is projected to both to other regions of the olfactory system (e.g., bulb, OT, AON) and to regions of the brain involved in behavioral decision making and cognition (e.g. entorhinal cortex, orbitofrontal cortex) which may use these ensembles as a means to facilitate odor discrimination and behavioral coupling. Recent work using optogenetics supports the notion that PCTX ensembles can be dynamically linked to adaptive behaviors, as essentially random light-activated ensembles of SL/SP neurons can be associated with appetitive and aversive stimuli, and these associations can be used to entrain behaviors [117].
Figure 3. Odor Representations and Learning in the Piriform Cortex.
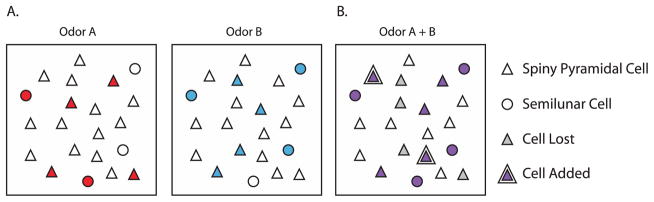
A) Odor representations in the Piriform Cortex. Distributed input from the olfactory bulb activates unique, overlapping and sparse patterns of neuronal activation across the extent of the piriform cortex, ideal for encoding odor identity. Semilunar cells are especially strongly activated by afferent input from the bulb (circles), while spiny pyramidal cells (triangles) are excited primarily by local network activity. Ensembles for two distinct odors (A and B) are shown, active cells are colored.
B) Odor mixtures are dynamically learned and stabilized by Acetylcholine. When presented with a mixture of odors (A + B), activity at new excitatory and inhibitory synapses drive some previously silent neurons to fire (double triangles) and some previously excited cells to fall silent (grey centers). The presence of acetylcholine in the piriform allows for rapid synaptic plasticity, which stabilizes the representation of A+B as a unique odor object. Note that the semilunar cells remain activated, while spiny pyramidal cells are added and lost in the new representation.
Current evidence also suggests an important role for neuromodulation in stabilizing patterns of activity and in entraining particular ensembles of neurons within the PCTX to respond to a particular odorant. When ACh levels are high, cellular and network properties of the PCTX change such that exposure to an odor can induce long-term plasticity at synapses of the associational network, thus linking the presence of an odor in the environment with the activation of a complete neuronal ensemble; this crystallization plays a key role in olfactory pattern completion, as subsequent partial or weak olfactory inputs are then sufficient to recruit a consistent subpopulation of neurons in the PCTX. Indeed, when a single component of an odorant mixture is removed, responses in the PCTX remain more correlated than in the olfactory bulb [65]. When one component is swapped for another, however, responses in both M/T cells and the PCTX became significantly decorrelated. It will be interesting to test if blocking cholinergic signaling — which also has broad effects on attention and salience — interferes in general with olfactory learning paradigms.
In contrast to the PCTX, there is no well-formed model for what the OT might encode or what computations it might execute. In general, the striatum is thought to facilitate action selection, integrating sensory information and motivational state of the animal to activate appropriate motor programs while suppressing unwanted behaviors [118–121]. At a microcircuit level, MSNs have been theorized to implement this function via local collaterals, with mutually inhibitory MSN ensembles competing for dominance using a “winner-take-all” mechanism [122–124]. However, MSN collateral synapses are relatively weak and sparse compared to the feedforward excitation present in the associational network of the PCTX, and circuit-scale MSN dynamics in vivo have been difficult to determine [125–127]. Nevertheless, these models are useful as a framework for how the OT might process olfactory information.
Based on structural homology with the NucAcc, one hypothesis is that the OT maps molecular features of any given olfactory stimulus onto a valence (such as pleasant or aversive), facilitating the execution of appropriate motivated behaviors [128]. Indeed, neurons in the ventral striatum have been shown to acquire sensory responses to odors predictive of value after various learning tasks, and in other studies respond to innately aversive and attractive stimuli [129,130]. How valence might be encoded within the OT is not yet clear, but it may be constructed through the topographic distribution of differentially connected MSNs in space, or perhaps through biases in the balance of D1 and D2 MSNs that are recruited in response to any given stimulus [131–133]. The OT receives crudely topographic inputs along the dorso-ventral axis from the OB; genetic studies have suggested that dorsal regions of the olfactory bulb are enriched in glomeruli that specify innate behaviors, suggesting that the ventromedial OT may be particularly important in this regard [134]. The OT may also use reward-based learning algorithms to update this encoding of valence over time, integrating olfactory information, dopamine from the VTA and motivational state from the rest of the basal forebrain. Such mechanisms could then promote appropriate odor-based action selection via its projections to the ventral pallidum, hypothalamus and brainstem nuclei [110]. This model predicts that rewarding or aversive stimuli would have distinct representations in the OT, and that the representation of neutral odors would shift accordingly as they are paired with appetitive or aversive stimuli. Further characterization of odor responses with carefully selected olfactory stimuli and reward-based learning paradigms will be crucial in determining if the OT is involved in the representation of odor valence.
Conclusions/Future Directions
In conclusion, while the OT and the PCTX both receive olfactory input from the bulb, they differ significantly in terms of the nature of this input, their anatomy, cell types, microcircuitry and neuromodulation. However in both cases these brain areas likely construct representations for olfactory stimuli using local, circuit-specific learning algorithms. Neuromodulation likely plays a critical role in both circuits, gating and shaping ongoing neural activity. Interestingly, the PCTX seems to largely discard any spatial organization present in the olfactory bulb, perhaps because this information is not useful for the computations it executes. Instead, the evenly distributed inputs from all areas of the olfactory bulb suggest that the PCTX comprehensively samples olfactory space. This makes intuitive sense based on its proposed function: odor identity is unlikely to be specific to any given molecular feature and instead is a holistic aspect of odor stimuli. Although the jury is still out, the OT also appears to receive relatively distributed inputs from the bulb, which would enable comprehensive odor space sampling, facilitating the assignment of valence to arbitrary odors.
Olfactory stimuli are complex and perhaps it is no surprise that the neural map of the olfactory bulb is demultiplexed by parallel, specialized higher-order brain areas. Indeed, the discrete and multidimensional nature of olfactory space suggests that a single topographic map is likely insufficient to extract all relevant features of any given odor. Some regions of olfactory cortex may use aspects of the genetically defined glomerular organization present in the olfactory bulb, and others may discard it, instead building representations that are based on the life history of the animal. Understanding olfaction will require dissection of microcircuitry and untangling the complex relationships between the sub-regions of the olfactory system. Relative comparisons between different higher-order olfactory areas through a combination of genetic, electrophysiological, imaging and behavior approaches will help us understand how the brain can make sense of such a complex, but fundamental sensory modality.
Highlights.
The glomerular organization in the mammalian olfactory bulb is differentially transformed in higher-order olfactory areas
The piriform cortex acts as an associative memory circuit, binding molecular features into holistic odor representations
Neuromodulation of plasticity in the of the piriform, enabling synaptic plasticity and flexible odor object encoding.
The olfactory tubercle is a striatal circuit that may encode odor valence using mesolimbic reward-based learnings
Acknowledgments
We thank Ofer Mazor, James Jeane, Ian Davison, Venkatesh Murthy, and members of the Datta lab for helpful comments. A.G. is supported by a fellowship from the Nancy Lurie Marks foundation. S.R.D. is supported by fellowships from the Burroughs Wellcome Fund, the Searle Scholars Program, the McKnight Foundation and by grants DP2OD007109 (Office of the Director) and RO11DC011558 (NIDCD) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murthy VN. Olfactory maps in the brain. Annu Rev Neurosci. 2011;34:233–258. doi: 10.1146/annurev-neuro-061010-113738. [DOI] [PubMed] [Google Scholar]
- 2.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Layton OW, Mingolla E, Yazdanbakhsh A. Dynamic coding of border-ownership in visual cortex. J Vis. 2012;12:8. doi: 10.1167/12.13.8. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez F, Perez R. Neural mechanisms underlying stereoscopic vision. Prog Neurobiol. 1998;55:191–224. doi: 10.1016/s0301-0082(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 6.Clifford CW, Ibbotson MR. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog Neurobiol. 2002;68:409–437. doi: 10.1016/s0301-0082(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 7.Amoore JE. Stereochemical and vibrational theories of odour. Nature. 1971;233:270–271. doi: 10.1038/233270a0. [DOI] [PubMed] [Google Scholar]
- 8.Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich RW, Stopfer M. Recent dynamics in olfactory population coding. Current opinion in neurobiology. 2001;11:468–474. doi: 10.1016/s0959-4388(00)00236-1. [DOI] [PubMed] [Google Scholar]
- 10.Gire DH, Restrepo D, Sejnowski TJ, Greer C, De Carlos JA, Lopez-Mascaraque L. Temporal processing in the olfactory system: can we see a smell? Neuron. 2013;78:416–432. doi: 10.1016/j.neuron.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nature reviews Neuroscience. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- 12.Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101:2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: modified olfactory bulb output response patterns to learned attractive odors. J Neurosci. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RI, Mainen ZF. Early events in olfactory processing. Annual review of neuroscience. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 15.Kandel ER. Principles of neural science. 5. New York: McGraw-Hill Medical; 2013. [Google Scholar]
- 16.Sheperd GM, Chen WR, Greer CA. In: Olfactory Bulb. 5. Shepherd GM, editor. Oxford; New York: Oxford University Press; 2004. [Google Scholar]
- 17.Ramón y Cajal S. Histologie du systeme nerveux de l’homme et des vertebres. Paris: Maloine; 1911. [Google Scholar]
- 18.Axel R. The molecular logic of smell. Sci Am. 1995;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- 19.Mori K, Shepherd GM. Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Seminars in cell biology. 1994;5:65–74. doi: 10.1006/scel.1994.1009. [DOI] [PubMed] [Google Scholar]
- 20.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Seminars in cell & developmental biology. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. Journal of neurophysiology. 1989;61:1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- 22.Neville KRaHLB. In: Olfactory Cortex. 5. Shepherd GM, editor. Oxford; New York: Oxford University Press; 2004. [Google Scholar]
- 23.Allison AC. The structure of the olfactory bulb and its relationship to the olfactory pathways in the rabbit and the rat. J Comp Neurol. 1953;98:309–353. doi: 10.1002/cne.900980206. [DOI] [PubMed] [Google Scholar]
- 24.Broadwell RD. Olfactory relationships of the telencephalon and diencephalon in the rabbit. I. An autoradiographic study of the efferent connections of the main and accessory olfactory bulbs. J Comp Neurol. 1975;163:329–345. doi: 10.1002/cne.901630306. [DOI] [PubMed] [Google Scholar]
- 25.Haberly LB, Price JL. The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res. 1977;129:152–157. doi: 10.1016/0006-8993(77)90978-7. [DOI] [PubMed] [Google Scholar]
- 26.Heimer L. Synaptic distribution of centripetal and centrifugal nerve fibres in the olfactory system of the rat. An experimental anatomical study. J Anat. 1968;103:413–432. [PMC free article] [PubMed] [Google Scholar]
- 27.Land LJ, Eager RP, Shepherd GM. Olfactory nerve projections to the olfactory bulb in rabbit: demonstration by means of a simplified ammoniacal silver degeneration method. Brain Res. 1970;23:250–254. doi: 10.1016/0006-8993(70)90044-2. [DOI] [PubMed] [Google Scholar]
- 28.Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- 29.Scalia F, Halpern M, Knapp H, Riss W. The efferent connexions of the olfactory bulb in the frog: a study of degenerating unmyelinated fibres. J Anat. 1968;103:245–262. [PMC free article] [PubMed] [Google Scholar]
- 30.Scott JW, McBride RL, Schneider SP. The organization of projections from the olfactory bulb to the piriform cortex and olfactory tubercle in the rat. The Journal of comparative neurology. 1980;194:519–534. doi: 10.1002/cne.901940304. [DOI] [PubMed] [Google Scholar]
- 31.White LE. Olfactory bulb projections of the rat. The Anatomical Record. 1965;152:465–479. [Google Scholar]
- 32.Buonviso N, Revial MF, Jourdan F. The Projections of Mitral Cells from Small Local Regions of the Olfactory Bulb: An Anterograde Tracing Study Using PHA-L (Phaseolus vulgaris Leucoagglutinin) Eur J Neurosci. 1991;3:493–500. doi: 10.1111/j.1460-9568.1991.tb00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Ojima H, Mori K, Kishi K. The trajectory of mitral cell axons in the rabbit olfactory cortex revealed by intracellular HRP injection. The Journal of comparative neurology. 1984;230:77–87. doi: 10.1002/cne.902300107. [DOI] [PubMed] [Google Scholar]
- 34.Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain research bulletin. 1978;3:59–72. doi: 10.1016/0361-9230(78)90062-x. [DOI] [PubMed] [Google Scholar]
- 35.Yan Z, Tan J, Qin C, Lu Y, Ding C, Luo M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58:613–624. doi: 10.1016/j.neuron.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 37.Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 38.Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 39.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. This paper labeled identified olfactory bulb glomeruli by dye electroporation and characterized axonal arborization patterns of mitral and tufted cells across multiple areas of olfactory cortex, including the piriform cortex and cortical amygdala. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. This paper used an anterograde viral tracing strategy to infect individual mitral and tufted cells and characterized the projection patterns of cells innervating the same glomerulus in the anterior olfactory nucleus and piriform cortex. [DOI] [PubMed] [Google Scholar]
- 41.Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. The authors infected neurons across the olfactory cortex with a trans-synaptic retrograde virus to identify the distribution of pre-synaptic mitral and tufted cells across the olfactory bulb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haberly LB, Bower JM. Olfactory cortex: model circuit for study of associative memory? TRENDS in Neurosciences. 1989;12:258–264. doi: 10.1016/0166-2236(89)90025-8. [DOI] [PubMed] [Google Scholar]
- 43.Isaacson JS. Odor representations in mammalian cortical circuits. Current opinion in neurobiology. 2010;20:328–331. doi: 10.1016/j.conb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: plasticity and perception. Seminars in cell & developmental biology. 2006;17:462–470. doi: 10.1016/j.semcdb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokes CCA, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki N, Bekkers JM. INHIBITORY INTERNEURONS IN THE PIRIFORM CORTEX - Suzuki - 2007 - Clinical and Experimental Pharmacology and Physiology - Wiley Online Library. Clinical and experimental pharmacology & physiology. 2007;34:1064–1069. doi: 10.1111/j.1440-1681.2007.04723.x. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki N, Bekkers JM. Distinctive Classes of GABAergic Interneurons Provide Layer-Specific Phasic Inhibition in the Anterior Piriform Cortex. Cerebral Cortex. 2010;20:2971–2984. doi: 10.1093/cercor/bhq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. The Journal of comparative neurology. 2010;518:1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki N, Bekkers JM. Microcircuits mediating feedforward and feedback synaptic inhibition in the piriform cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:919–931. doi: 10.1523/JNEUROSCI.4112-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poo C, Isaacson JS. A major role for intracortical circuits in the strength and tuning of odor-evoked excitation in olfactory cortex. Neuron. 2011;72:41–48. doi: 10.1016/j.neuron.2011.08.015. The authors used in vivo whole cell recordings to characterize the odor coding properties of neurons in the piriform cortex. In particular, they found a crucial role for associational connections in determining the odor tuning of neurons in the piriform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki N, Bekkers JM. Two layers of synaptic processing by principal neurons in piriform cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2156–2166. doi: 10.1523/JNEUROSCI.5430-10.2011. This paper carefully distinguished the differences between semilunar and spiny pyramidal cells in the piriform, characterizing their differential afferent and associational inputs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagiwara A, Pal SK, Sato TF, Wienisch M, Murthy VN. Optophysiological analysis of associational circuits in the olfactory cortex. Frontiers in neural circuits. 2012;6:18. doi: 10.3389/fncir.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekkers JM, Suzuki N. Neurons and circuits for odor processing in the piriform cortex. TRENDS in Neurosciences. 2013;36:429–438. doi: 10.1016/j.tins.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 57.Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. This paper exploited optogenetics and paired whole-cell recordings to study the spatial extent of feedforward and feedback inhibition onto the principal cells of the piriform cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davison IG, Ehlers MD. Neural Circuit Mechanisms for Pattern Detection and Feature Combination in Olfactory Cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. By combining glutamate uncaging in the olfactory bulb with in vivo extra- and intracellular recordings the authors examined the functional integration properties of the principal cells of the of piriform cortex. Activation of multiple glomeruli was required to evoke action potentials in the piriform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nature neuroscience. 2013;16:949–957. doi: 10.1038/nn.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output. Neuron. 2012;75:320–329. doi: 10.1016/j.neuron.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Gire DH, Whitesell JD, Doucette W, Restrepo D. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nature neuroscience. 2013;16:991–993. doi: 10.1038/nn.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhan C, Luo M. Diverse patterns of odor representation by neurons in the anterior piriform cortex of awake mice. Journal of Neuroscience. 2010;30:16662–16672. doi: 10.1523/JNEUROSCI.4400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gale K. Subcortical Structures and Pathways Involved in Convulsive Seizure Generation. Journal of Clinical Neurophysiology. 1992;9:264–277. doi: 10.1097/00004691-199204010-00007. [DOI] [PubMed] [Google Scholar]
- 64.LÖSCHER W, EBERT U. THE ROLE OF THE PIRIFORM CORTEX IN KINDLING. Progress in neurobiology. 1996;50:427–481. doi: 10.1016/s0301-0082(96)00036-6. [DOI] [PubMed] [Google Scholar]
- 65.Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nature neuroscience. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. Journal of neurophysiology. 2006;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. European Journal of Neuroscience. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- 69.Miura K, Mainen ZF, Uchida N. Odor representations in olfactory cortex: distributed rate coding and decorrelated population activity. Neuron. 2012;74:1087–1098. doi: 10.1016/j.neuron.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Payton CA, Wilson DA, Wesson DW. Parallel odor processing by two anatomically distinct olfactory bulb target structures. PloS one. 2012;7:e34926. doi: 10.1371/journal.pone.0034926. This study (and others from the same group) used in vivo extracellular recordings to identify odor response properties of neurons in the olfactory tubercle and piriform cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rennaker RL, Chen C-FF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stettler DD, Axel R. Representations of Odor in the Piriform Cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. Using in vivo two photon calcium imaging, the authors studied topography, odor tuning, concentration dependence, and responses to mixtures and their components in populations of neurons across large regions of the piriform cortex. [DOI] [PubMed] [Google Scholar]
- 73.Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. Journal of neurophysiology. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- 74.Wilson DA. Odor specificity of habituation in the rat anterior piriform cortex. Journal of neurophysiology. 2000;83:139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]
- 75.Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. Journal of neurophysiology. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- 76.Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. The Journal of comparative neurology. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- 77.Linster C, Wyble BP, Hasselmo ME. Electrical Stimulation of the Horizontal Limb of the Diagonal Band of Broca Modulates Population EPSPs in Piriform Cortex. [DOI] [PubMed] [Google Scholar]
- 78.Luskin MB, Price JL. The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques. The Journal of comparative neurology. 1982;209:249–263. doi: 10.1002/cne.902090304. [DOI] [PubMed] [Google Scholar]
- 79.Barkai E, Bergman RE, Horwitz G, Hasselmo ME. Modulation of associative memory function in a biophysical simulation of rat piriform cortex. Journal of neurophysiology. 1994;72:659–677. doi: 10.1152/jn.1994.72.2.659. [DOI] [PubMed] [Google Scholar]
- 80.Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. Journal of neurophysiology. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- 81.Patil MM, Hasselmo ME. Modulation of inhibitory synaptic potentials in the piriform cortex. Journal of neurophysiology. 1999;81:2103–2118. doi: 10.1152/jn.1999.81.5.2103. [DOI] [PubMed] [Google Scholar]
- 82.Patil MM, Linster C, Lubenov E, Hasselmo ME. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. Journal of neurophysiology. 1998;80:2467–2474. doi: 10.1152/jn.1998.80.5.2467. [DOI] [PubMed] [Google Scholar]
- 83.Linster C, Hasselmo ME. Neuromodulation and the functional dynamics of piriform cortex. Chemical senses. 2001;26:585–594. doi: 10.1093/chemse/26.5.585. [DOI] [PubMed] [Google Scholar]
- 84.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progress in brain research. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- 86.Chapuis J, Wilson DA. Cholinergic modulation of olfactory pattern separation. Neuroscience letters. 2013;545:50–53. doi: 10.1016/j.neulet.2013.04.015. This paper tests the effects of blocking acetylcholine signaling in the piriform on discriminability between odors. Enhancement of cholinergic signaling increased performance on an odor discrimination task, while blocking acetylcholine receptors had the opposite effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. Journal of neurophysiology. 1992;67:1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- 88.Millhouse OE, Heimer L. Cell configurations in the olfactory tubercle of the rat. The Journal of comparative neurology. 1984;228:571–597. doi: 10.1002/cne.902280409. [DOI] [PubMed] [Google Scholar]
- 89.Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neuroscience and biobehavioral reviews. 2011;35:655–668. doi: 10.1016/j.neubiorev.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heimer L, Wilson RD. The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. Golgi Centennial Symposium Proceedings; Raven, New York. 1975. [Google Scholar]
- 91.Heimer L, Zaborszky L, Zahm DS, Alheid GF. The ventral striatopallidothalamic projection: I. The striatopallidal link originating in the striatal parts of the olfactory tubercle. The Journal of comparative neurology. 1987;255:571–591. doi: 10.1002/cne.902550409. [DOI] [PubMed] [Google Scholar]
- 92.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. The Journal of comparative neurology. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- 93.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain research reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. This anatomy paper traces connections between the dopaminergic ventral tegmental area and the ventral striatum, including the olfactory tubercle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. The Journal of comparative neurology. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 95.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault J-A. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: Sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAS in distinct neuronal populations of the dorsal and ventral striatum. The Journal of comparative neurology. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 97.Fallon JH. The islands of Calleja complex of rat basal forebrain II: connections of medium and large sized cells. Brain research bulletin. 1983;10:775–793. doi: 10.1016/0361-9230(83)90210-1. [DOI] [PubMed] [Google Scholar]
- 98.Fallon JH, Loughlin SE, Ribak CE. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. The Journal of comparative neurology. 1983;218:91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- 99.Fallon JH, Riley JN, Sipe JC, Moore RY. The islands of Calleja: organization and connections. The Journal of comparative neurology. 1978;181:375–395. doi: 10.1002/cne.901810209. [DOI] [PubMed] [Google Scholar]
- 100.Adjei S, Houck AL, Ma K, Wesson DW. Age-dependent alterations in the number, volume, and localization of islands of Calleja within the olfactory tubercle. Neurobiology of aging. 2013 doi: 10.1016/j.neurobiolaging.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 101.Hsieh Y-C, Puche AC. Development of the Islands of Calleja. Brain Research. 2013;1490:52–60. doi: 10.1016/j.brainres.2012.10.051. [DOI] [PubMed] [Google Scholar]
- 102.McNamara AM. Characterization of the Synaptic Properties of Olfactory Bulb Projections. Chemical senses. 2004;29:225–233. doi: 10.1093/chemse/bjh027. [DOI] [PubMed] [Google Scholar]
- 103.Owen GS, Halliwell JV. Electrophysiological characterization of laminar synaptic inputs to the olfactory tubercle of the rat studied in vitro: modulation of glutamatergic transmission by cholinergic agents is pathway-specific. European Journal of Neuroscience. 2001;13:1767–1780. doi: 10.1046/j.0953-816x.2001.01556.x. [DOI] [PubMed] [Google Scholar]
- 104.Carriero G, Uva L, Gnatkovsky V, de Curtis M. Distribution of the olfactory fiber input into the olfactory tubercle of the in vitro isolated guinea pig brain. Journal of neurophysiology. 2009;101:1613–1619. doi: 10.1152/jn.90792.2008. [DOI] [PubMed] [Google Scholar]
- 105.Chiang E, Strowbridge BW. Diversity of neural signals mediated by multiple, burst-firing mechanisms in rat olfactory tubercle neurons. Journal of neurophysiology. 2007;98:2716–2728. doi: 10.1152/jn.00807.2007. This is the most comprehensive whole-cell recording study of cells in the olfactory tubercle. The authors recorded from neurons across all layers and characterized their intrinsic firing properties. [DOI] [PubMed] [Google Scholar]
- 106.Halliwell JV, Horne AL. Evidence for enhancement of gap junctional coupling between rat island of Calleja granule cells in vitro by the activation of dopamine D3 receptors. The Journal of physiology. 1998;506 (Pt 1):175–194. doi: 10.1111/j.1469-7793.1998.175bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rampin O, Bellier C, Maurin Y. Electrophysiological responses of rat olfactory tubercle neurons to biologically relevant odours. European Journal of Neuroscience. 2011;35:97–105. doi: 10.1111/j.1460-9568.2011.07940.x. [DOI] [PubMed] [Google Scholar]
- 108.Wesson DW, Wilson DA. Smelling sounds: olfactory-auditory sensory convergence in the olfactory tubercle. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3013–3021. doi: 10.1523/JNEUROSCI.6003-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hagamen TC, Greeley HP, Hagamen WD, Reeves AG. Behavioral asymmetries following olfactory tubercle lesions in cats. Brain, behavior and evolution. 1977;14:241–250. doi: 10.1159/000125664. [DOI] [PubMed] [Google Scholar]
- 110.Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. Journal of comparative and physiological psychology. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 112.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 114.Lerner TN, Kreitzer AC. Neuromodulatory control of striatal plasticity and behavior. Current opinion in neurobiology. 2011;21:322–327. doi: 10.1016/j.conb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicola SM, Hopf FW, Hjelmstad GO. Contrast enhancement: a physiological effect of striatal dopamine? Cell and tissue research. 2004;318:93–106. doi: 10.1007/s00441-004-0929-z. [DOI] [PubMed] [Google Scholar]
- 116.Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neuroscience and biobehavioral reviews. 2003;27:307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 117.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behavioural brain research. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 120.Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 121.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 122.Ponzi A, Wickens J. Cell assemblies in large sparse inhibitory networks of biologically realistic spiking neurons. Advances in Neural Information …. 2008 [Google Scholar]
- 123.Ponzi A, Wickens J. Sequentially Switching Cell Assemblies in Random Inhibitory Networks of Spiking Neurons in the Striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:5894–5911. doi: 10.1523/JNEUROSCI.5540-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ponzi A, Wickens J. Input dependent cell assembly dynamics in a model of the striatal medium spiny neuron network. Frontiers in systems neuroscience. 2012;6:6. doi: 10.3389/fnsys.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taverna S, Ilijic E, Surmeier DJ. Recurrent Collateral Connections of Striatal Medium Spiny Neurons Are Disrupted in Models of Parkinson's Disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. TRENDS in Neurosciences. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 127.Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. Journal of neurophysiology. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- 128.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature neuroscience. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 130.Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- 131.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]