Abstract
Immunoregulatory receptors are categorized as stimulatory or inhibitory based on their engagement of unique intracellular signaling networks. These proteins also display functional plasticity, which adds versatility to the control of innate immunity. Here we demonstrate that an inhibitory catfish leukocyte immune-type receptor (IpLITR) also displays stimulatory capabilities in a representative myeloid cell model. Previously, the receptor IpLITR 1.1b was shown to inhibit natural killer cell-mediated cytotoxicity. Here we expressed IpLITR 1.1b in rat basophilic leukemia-2H3 cells and monitored intracellular signaling and functional responses. Although IpLITR 1.1b did not stimulate cytokine secretion, activation of this receptor unexpectedly induced phagocytosis as well as extracellular signal-related kinase 1/2- and protein kinase B (Akt)-dependent signal transduction. This novel IpLITR 1.1b-mediated response was independent of an association with the FcRγ chain and was likely due to phosphotyrosine-dependent adaptors associating with prototypical signaling motifs within the distal region of its cytoplasmic tail. Furthermore, compared to a stimulatory IpLITR, IpLITR 1.1b displayed temporal differences in the induction of intracellular signaling, and IpLITR 1.1b-mediated phagocytosis had reduced sensitivity to EDTA and cytochalasin D. Overall, this is the first demonstration of functional plasticity for teleost LITRs, a process likely important for the fine-tuning of conserved innate defenses.
Key Words: Phagocytosis, Intracellular signaling, Phosphorylation, Immunoregulatory receptors, Kinases, Phosphatases, Leukocyte immune-type receptors, Teleost, Channel catfish, Comparative immunology
Introduction
Macrophages, granulocytes, and natural killer (NK) cells are key cellular components of innate immunity that execute antimicrobial effector functions including phagocytosis, degranulation, cellular cytotoxicity, and cytokine secretion. These cellular functions are vital for immune defense against pathogens and are in part controlled by surface receptors that initiate intracellular signaling modules that translate environmental cues into effector responses [1, 2, 3]. In mammals, many of these receptors belong to structurally distinct receptor families such as the Ig superfamily [4] or the C-type lectin superfamily [5], which share common mechanisms of intracellular signaling through stimulatory or inhibitory transduction pathways [6].
In general, inhibitory immunoregulatory receptors have long cytoplasmic tails (CYT) that contain one or more immunoreceptor tyrosine-based inhibition motifs (ITIMs) that are phosphorylated upon receptor engagement [7, 8]. Tyrosine phosphorylation can lead to the recruitment of Src homology 2 (SH2) domain-containing cytoplasmic phosphatases (SHP-1, SHP-2, and SHIP), which dephosphorylate stimulatory pathway signaling intermediates [9]. Stimulatory receptor types possess a positively charged transmembrane (TM) segment that facilitates receptor associations with the negatively charged TM of adaptor proteins possessing cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) or through a DAP10-dependent, non-ITAM motif, i.e. YxxM [10]. ITAMs are phosphorylated by Src family protein tyrosine kinases, which then interact with downstream mediators such as spleen tyrosine kinase (Syk) and/or phosphoinositide-3 kinases (PI3K) that in turn activate specific intracellular cascades responsible for controlling immune cell responses [11, 12, 13, 14, 15]. These classical paradigms of inhibition and activation are well accepted, but newly described mechanisms of cellular signaling provide evidence that the versatility of immunoregulatory receptors goes beyond the archival classifications of strictly inhibitory or stimulatory types.
Recently, several ITIM-encoding immunoregulatory receptors have been reported to also stimulate immune cell responses [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]. For example, in humans, immune receptor expressed on myeloid cells 1 (IREM-1) contains ITIMs, recruits the phosphatase SHP-1, and downregulates immune cell responses [16]. However, when its ITIM tyrosine residues were mutated, IREM-1 no longer recruited SHP-1 but associated with the p85α regulatory subunit of PI3K and induced mast cell degranulation [16]. Leukocyte mono-Ig-like receptor 3 (LMIR3) is a mouse homolog of IREM-1, which also encodes ITIMs and inhibits bone marrow-derived mast cell cytokine production when coengaged with FcεRI [17]. Interestingly, when this receptor was cross-linked alone, LMIR3 stimulated interleukin (IL)-6 production in mast cells, an activity that was dependent on its unique ability to associate with the ITAM-containing adaptor FcRγ chain [17]. Platelet endothelial cell adhesion molecule-1 (PECAM-1) is also an ITIM-encoding receptor that can both inhibit and activate immune cell responses through a series of signal transduction pathways that regulate integrin activation, apoptosis, and endothelial cell responses to fluid shear stress [18]. An ITIM-encoding member of the killer cell Ig-like receptor (KIR) family, i.e. KIR2DL4, also inhibits and activates NK cell responses [19, 20, 21, 22] and its stimulatory activities are associated with recruitment of the FcRγ chain to KIR2DL4 [23]. Members of the receptors for the Fc fragment of IgG (FcγR) present additional examples of immunoregulatory receptor functional plasticity. For example, the ITIM-containing inhibitory receptor FcγRIIB is classically described as a potent inhibitor of immune cell responses [24, 25], but this protein also stimulates phagocytosis through a C-terminal tyrosine residue found outside of the prototypical ITIMs [26]. This suggests that an ITIM-independent mechanism is responsible for FcγRIIB-mediated phagocytosis in mammals. FcγRIIB also appears to enhance the antiviral immune response of porcine pulmonary alveolar macrophages since engagement of this receptor upregulated IFN-α and TNF-α mRNA levels [27]. Overall, these findings have revealed functional plasticity among mammalian immunoregulatory receptor types, and in particular unexpected stimulatory functions for prototypical inhibitory receptors. Such plasticity likely serves as an important regulatory mechanism for the fine control of innate immune cell effector functions.
In the present study, we demonstrate that immunoregulatory receptor signaling versatility is not limited to mammals. The teleost leukocyte immune-type receptor (LITR) family, originally discovered in the channel catfish (Ictalurus punctatus), represents a large repertoire of Ig superfamily members displaying both structural and distant phylogenetic relationships with members of the mammalian FcR family and the leukocyte receptor complex [28, 29, 30]. Known IpLITRs are categorized as putative stimulatory or inhibitory receptors coexpressed by a variety of catfish myeloid and lymphoid cell types such as macrophages, NK cells, T cells, and B cells [28, 29]. Consequently, we predict that IpLITRs may participate in the regulation of several immune cell effector responses including cytotoxicity, cytokine secretion, degranulation, and phagocytosis akin to the actions of related mammalian immunoregulatory receptors. To date, IpLITR ligands have not been discovered and this has hindered our ability to understand their specific roles in teleost immunity. However, by expressing these receptors in representative mammalian myeloid and lymphoid cell types, important insights into IpLITR signaling capabilities and control of immune cell effector responses have been revealed [31, 32, 33, 34]. Although heterologous overexpression of fish immune proteins in mammalian cells has potential drawbacks, in the absence of monoclonal antibodies (mAb) and reliable transfection procedures for expression in fish cells, this approach has provided important new information regarding the immunoregulatory potential of these teleost proteins.
Previously, we demonstrated that the stimulatory IpLITR 2.6b directly associated with ITAM-encoding adaptors and induced cellular degranulation and phagocytosis [32, 33] when expressed in transfected rat basophilic leukemia (RBL)-2H3 cells, a representative myeloid cell line. Alternatively, inhibitory IpLITR types abrogated NK cell-mediated killing via SHP-dependent and SHP-independent mechanisms [31, 34], which was revealed after transfection and expression of these proteins in primary mouse NK cells. Since catfish myeloid cells also express inhibitory IpLITR types, we examined the signaling and functional potential of an inhibitory IpLITR type in RBL-2H3 cells and compared its activities to that of a previously characterized stimulatory IpLITR [33]. Here, we demonstrate that the prototypical inhibitory (i.e. ITIM-containing) IpLITR 1.1b unexpectedly stimulates the phagocytosis of opsonized microspheres and induces the activation of extracellular signal-related kinase 1/2 (ERK1/2) and protein kinase B (Akt) in transfected RBL-2H3 cells. However, when compared with a stimulatory counterpart (IpLITR 2.6b), engagement of IpLITR 1.1b failed to induce the secretion of IL-3, IL-4, IL-6, and TNF-α. In addition, IpLITR 1.1b and IpLITR 2.6b displayed temporal differences in the induction of phosphorylated ERK1/2 and Akt as well as differential susceptibilities to EDTA- and cytochalasin D (CytoD)-mediated inhibition of phagocytosis. IpLITR 1.1b-mediated phagocytosis required an intact tyrosine-containing CYT region and was not facilitated by an association with the ITAM-containing adaptor protein FcεRIγ chain in RBL-2H3 cells. This study represents the first demonstration of functional plasticity for an ITIM-containing teleost immunoregulatory receptor. At present, the precise mechanisms of IpLITR 1.1b-induced phagocytosis and induction of ERK1/2 and Akt signaling are unknown, but the events leading to this outcome are predicted to be distinct from those facilitated by a prototypical stimulatory IpLITR and its associated ITAM-containing adaptor. Characterization of this functional plasticity may reveal the conserved nature of innate signaling events among vertebrate immunoregulatory receptors. In addition, by understanding the dual functionality of certain teleost immunoregulatory receptors, we may also uncover new cellular mechanisms responsible for the control of important innate immune cell effector functions.
Materials and Methods
IpLITR-Expressing RBL-2H3 Cells
Transfection, selection, sorting, and stable expression of N-terminal hemagglutinin (HA) epitope-tagged pDISPLAY IpLITR 2.6b/IpFcRγ-L and pDISPLAY IpLITR 1.1b in RBL-2H3 cells was performed according to previously described protocols [31, 33]. IpLITR 2.6b/IpFcRγ-L encodes the extracellular Ig-like domains of IpLITR 2.6b (GenBank accession ABI23557) fused with the TM and ITAM-encoding CYT of IpFcRγ-L (GenBank accession AF543420) [33]. IpLITR 1.1b encodes full-length TS32.17 L1.1b (GenBank accession ABI16050) [31] that contains 4 Ig-like domains, an uncharged TM segment, and a tyrosine-containing CYT with 6 tyrosine residues [29, 31]. Two of these residues are encoded in ITIMs, one in an immunoreceptor tyrosine-based switch motif (ITSM), a TM proximal tyrosine that is required for the recruitment of C-src kinase (Csk), and 2 additional TM proximal tyrosine residues with unknown functions [28]. In the present study, a truncated version of IpLITR 1.1b (NCBI accession ABI16050) termed IpLITR 1.1b ΔCYT was also created by site-directed mutagenesis, which introduced a premature stop codon at nucleotide position 1195 within the CYT region of IpLITR 1.1b. This mutation removed the cytoplasmic tyrosine residues within the CYT, leaving only 13 TM proximal residues. We also generated IpLITR 1.2a-expressing cells; IpLITR 1.2a is an inhibitory IpLITR type (GenBank accession ABI16051) that is closely related to IpLITR 1.1b (88% amino acid identity) [31]. This receptor contains 4 Ig-like domains, an uncharged TM segment, and a tyrosine-containing CYT with 2 tyrosine residues [29, 31]. One of these residues is encoded in an ITIM and the other in an ITSM [28]. Both of these motifs correspond to the ITIM and ITSM located in the TM distal CYT region of IpLITR 1.1b. As described for the other IpLITR constructs, pDISPLAY IpLITR 1.1b ΔCYT and 1.2a were transfected and stably expressed in the RBL-2H3 cells. All constructs used in this study are schematically represented in online supplementary figure 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000356963), which also displays their CYT region sequences. In addition, an amino acid alignment of IpLITR 1.1b and IpLITR 1.2a is also provided (online suppl. fig. 2). The surface expression of receptor constructs was monitored by flow cytometry using the anti-HA mAb clone HA.C5 (Cedarlane Laboratories Ltd.) or mouse IgG3 as an isotype control (Beckman Coulter), followed by incubation with the phycoerythrin-conjugated goat anti-mouse IgG (H+L) polyclonal antibody (pAb; Beckman Coulter) diluted in antibody staining buffer [ASB; Dulbecco's phosphate-buffered saline (D-PBS)/ethylenediaminetetraacetic acid (EDTA), 0.05% NaN3, 1% bovine serum albumin (BSA)], as described by Mewes et al. [32]. Transfected RBL-2H3 cells were cultured in the presence of 400 µg/ml G418 disulfate salt solution (Sigma-Aldrich) and tested weekly for surface expression. RBL-2H3 cell cultures were grown at 37°C and 5% CO2 in culture media; MEM/EBSS (HyClone) supplemented with 2 mML-glutamine (Gibco), 100 U/ml penicillin (Gibco), 100 µg/ml streptomycin (Gibco), and 10% heat-inactivated fetal bovine serum (FBS, characterized; Hyclone). Prior to use, the culture media (MEM/EBSS/FBS) was filter sterilized using 0.22-µm filter units (Corning). The relative cell surface expression levels of all constructs used in this study are shown in figure 1.
Fig. 1.
Cell surface expression of IpLITR types stably expressed in RBL-2H3 cells (b-e). Detection of the cell surface expression of IpLITR 2.6b/IpFcRγ-L, IpLITR 1.1b, IpLITR 1.2a, and IpLITR 1.1b ΔCYT in transfected, selected, and sorted RBL-2H3 cells. Stable expressing cells were stained by incubation with an anti-HA mAb (20 μg/ml) or IgG3 (isotype control) followed by staining with 0.5 μg goat anti-mouse IgG pAb coupled to PE. Surface expression was then detected as an increase in fluorescence intensity (i.e. HA staining, FL-2; grey line) in comparison with IgG3-stained cells (black line). Shown are representative stains for each receptor construct, which demonstrated consistent surface staining throughout this study. For comparison, parental (i.e. untransfected) RBL-2H3 cells are also shown (a).
Examination of IpLITR-Induced Cytokine Production and Activation of Intracellular Signaling Using Proteome Profilers
For parallel determination of the relative levels of secreted cytokines and to analyze the phosphorylation profiles of kinases induced by IpLITR engagement, antibody array-based proteome profilers were used. Specifically, a Rat Cytokine Array Panel A Array Kit and Phospho-MAPK Array Kits (R&D Systems) were used to determine the relative amount of cytokines released, as well as to monitor the phosphorylation of signaling intermediates, respectively, by transfected RBL-2H3 cells after antibody-mediated cross-linking of the N-terminal HA epitope-tagged IpLITR 2.6b/IpFcRγ-L or IpLITR 1.1b. Cells were grown to confluence in 6-well plates (∼2.5 × 106 cells/well) and washed in D-PBS; each well was used for either cytokine secretion or generation of cell lysates for analysis of intracellular signaling. For cytokine profiling, IpLITR 2.6b/IpFcRγ-L or IpLITR 1.1b was sensitized by incubating the cells for 20 min at 4°C in ASB containing 0.625 µg/ml anti-HA mAb (or mouse IgG3 isotype control). After sensitizations, the cells were washed with ice-cold 1× D-PBS and then resuspended in culture media containing 1.25 µg/ml cross-linking antibody, i.e. goat anti-mouse IgG3 pAb (H+L; Beckman Coulter), for 24 h at 37°C. Cytokine profiling was also performed on the IpLITR-expressing RBL-2H3 cells by sensitizing them for 1 h with IgE (200 ng/ml) using the monoclonal anti-DNP IgE antibody clone SPE-7 (Sigma-Aldrich) followed by the addition of 0.1 ng/ml dinitrophenyl [DNP(23)]-human serum albumin (HSA; Biosearch Technologies Inc.) for 24 h at 37°C or by treatment with 50 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 0.5 µM calcium (Ca2+) ionophore A23187 (Sigma-Aldrich) for 24 h at 37°C. After activation, the culture media containing secreted cytokines was collected and centrifuged for 10 min at 16,000 g to remove cellular debris. The cleared media was immediately harvested for assessment of relative cytokine levels according to the manufacturer's protocol. For determination of phosphokinase levels the cells were treated as described above, except that they were incubated for 10 min at 37°C in ASB containing 1.25 µg/ml goat anti-mouse IgG3 pAb during the cross-linking step. After this incubation, the antibody solution was removed and cells were washed with ice-cold 1× D-PBS prior to cell lysis using ice-cold Lysis Buffer 6 provided by the manufacturer. Lysates were cleared of debris by centrifugation and processed according to the manufacturer's recommended protocol.
Time Course Examination of IpLITR-Induced Intracellular Signaling
Untransfected RBL-2H3 cells (∼2.5 × 106) or RBL-2H3 cells expressing the N-terminal HA epitope-tagged IpLITR 2.6b/IpFcRγ-L or IpLITR 1.1b were activated by cross-linking the receptors with the anti-HA mAb [33]. Briefly, cells were incubated for 20 min at 4°C with 0.625 µg/ml anti-HA mAb (or mouse IgG3 isotype control) diluted in ASB. The ASB was removed and cells were treated with ASB containing 1.25 µg/ml goat anti-mouse IgG3 pAb for 2, 4, 8, 16, or 32 min at 37°C. In some cases RBL-2H3 cells were sensitized for 1 h with anti-DNP IgE (200 ng/ml) at 37°C followed by the addition of 0.1 ng/ml DNP-HSA for 4 min. IpLITR-cross-linked or IgE-sensitized RBL-2H3 cells were lysed as described [31], and Western blots were performed using anti-ERK1(p44)/ERK2(p42) MAPK (L34F12) mouse mAb, anti-phospho-p44/p42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) mouse mAb, anti-Akt rabbit pAb, or anti-phospho-Akt (Ser473) (D9E) XP® rabbit mAb (all purchased from Cell Signaling Technologies) at a final dilution of 1:2,000 (v/v). Immunoreactive bands were detected using goat anti-mouse or goat anti-rabbit IgG (H+L) HRP-conjugated pAb (Bio-Rad). For each representative gel, individual lanes represent ∼0.5 × 106 total RBL-2H3 cells. All of the procedures for cellular lysis, SDS-PAGE gel separations, transfer to nitrocellulose membranes, incubation of membranes, and detection of protein bands using an enhanced chemiluminescent substrate kit (Thermo Scientific) have been previously described [31, 32, 33]. The analysis of band intensity was performed using ImageJ v1.44 software, which was downloaded from http://rsbweb.nih.gov/ij/download.html.
Co-Immunoprecipitations
Untransfected RBL-2H3 cells or those expressing IpLITR 1.1b (∼2.5 × 106) were washed with D-PBS and then treated with sodium pervanadate (Na3VO4) in D-PBS (or D-PBS alone) for 10 min at 37°C as described previously [31, 32, 33] and then lysed for 30 min with ice-cold immunoprecipitation (IP) buffer (50 mM Tris-HCL, 150 mM NaCl, 1% Triton X-100, supplemented with complete mini EDTA-free protease inhibitor tablets and PhosSTOP phosphatase inhibitor tablets; Roche). Cellular debris was removed by centrifugation for 10 min at 16,000 g. Cell lysates were transferred to a new 1.5-ml Eppendorf tube. Fifty microliters of cell lysates were mixed with equal volumes of 2× SDS-PAGE reducing buffer (Bio-Rad) and the remaining lysate was immunoprecipitated by the addition of either 2 µg anti-HA mAb clone HA.C5 or anti-FcεRI α subunit mouse mAb (Upstate Cell Signaling Solutions) for 14-16 h at 4°C on a rotary mixer. Prewashed (IP buffer) protein G agarose beads (Roche) were added to the samples and incubated for a further 2 h at 4°C. The beads were washed 3 times with IP buffer followed by the addition of 100 µl 2× SDS-PAGE reducing buffer. Samples (equivalent to ∼0.5 × 106 lysed cells) were boiled for 10 min and then separated on 8 or 10% SDS-PAGE gels and Western blotting was performed as previously described [31, 32, 33] using an HRP-conjugated goat anti-HA pAb (GenScript Corp.), the anti-FcεRIγ subunit rabbit pAb (Upstate Cell Signaling Solutions), or an anti-FcεRI α subunit rabbit pAb (Santa Cruz Biotechnology).
Phagocytosis Assays
Phagocytosis of antibody-opsonized microspheres by RBL-2H3 was performed as described [33]. Briefly, 10 µg/ml of anti-HA mAb or mouse IgG3 were adsorbed onto protein A- (from Staphylococcus aureus; Sigma-Aldrich) precoated 4.5-µm YG beads (Fluoresbrite™ Carboxy YG 4.5-µm microspheres; Polysciences). For each phagocytosis experiment, 1 × 105 cells in phagocytosis buffer (1:1 mixture of 1× D-PBS containing 2.0 mg/ml BSA and 1× OptiMEM reduced serum medium; Gibco) were incubated with 3 µl of antibody-opsonized 4.5-µm YG beads (1 × 108 beads/ml) for 60 min at 37°C (i.e. 3:1 beads to cells ratio). The cells were then centrifuged at 5,000 g for 1 min and the supernatant carefully aspirated. To detach non phagocytosed or cell-surface-bound beads, the cell pellet was gently agitated and the cells resuspended in ice-cold D-PBS/EDTA containing 0.05% trypsin (Hyclone) and 1 mM EDTA. After 15 min of incubation on ice, ice-cold D-PBS containing 2 mM EDTA and 0.5% BSA was added to each tube and the cells were centrifuged at 5,000 g for 1 min and then resuspended in 1% paraformaldehyde in D-PBS. Samples were analyzed by flow cytometry using FL-1 to distinguish between cells with and without internalized beads as described [33]. For some phagocytosis experiments, EDTA (2 mM; Sigma-Aldrich) was added during the incubation of cells with opsonized beads or the beads were added to the cells after they had been pretreated for 1 h with CytoD (10 µM; Sigma-Aldrich) or 0.1% DMSO (vehicle control).
Examination of IpLITR-Mediated Phagocytosis and Endocytosis by Confocal Microscopy
RBL-2H3 cells (1 × 105) were seeded into 6-well tissue culture plates (Costar) containing a sterile microscope glass coverslip (Fisher Scientific) and allowed to grow for 2 days at 37°C (∼70% confluence over the coverslip). After incubation, the growth media was removed and coverslips were washed with ASB prior to staining. For GM1 ganglioside membrane staining, coverslips (cell side down) were placed on a parafilm strip containing ASB containing cholera toxin B subunit-FITC (1 µg/ml; Sigma-Aldrich) for 30 min at 4°C in the dark. Cells were then washed with ASB prior to incubation with opsonized beads or IpLITR staining as described below.
For visualization of the internalized beads, GM1-stained cells were incubated with anti-HA mAb-opsonized 2.0 µm Polybead® polystyrene microspheres (Polysciences) for 30 min at 37°C. Coverslips were then washed with 1× D-PBS and cells were fixed by placing the coverslips (cell side down) on a parafilm strip containing Fixation Buffer (BioLegend) for 20 min at room temperature in the dark. The cells were again washed with ASB and the coverslip placed (cell side down) on a glass slide containing mounting media containing DAPI [kindly provided by Geraldine Barron (Cross Cancer Institute Microscopy Facility, University of Alberta, Edmonton, Alta., Canada)]. Cells were viewed with a laser scanning confocal microscope (LSCM), i.e. Zeiss LSM 710, objective 40× 1.3 oil plan-Apochromat (Cross Cancer Institute Microscopy Facility). All images were collected using Zen 2009 or 2011 software.
To visualize IpLITR surface staining and the localization of these receptors after antibody-mediated cross-linking, RBL-2H3 cells expressing the specified IpLITRs were incubated on coverslips as described above. Coverslips were placed on a parafilm strip containing ASB with 1.25 µg/ml anti-HA mAb or 1.25 µg/ml IgG3 isotype control antibody. After 30 min on ice, cells were washed with ice-cold ASB and then placed on a parafilm strip containing 2.5 µg/ml cross-linking/staining antibody goat anti-mouse IgG Cy5-conjugated pAb (Invitrogen). Cross-linking/staining of the IpLITRs was performed at 37°C for 10, 20, or 30 min, after which the cells were immediately washed in ice-cold ASB and then fixed and mounted for confocal microscopy as described above. For some experiments, cells were pretreated for 30 min with hypertonic sucrose (0.2 M) prior to performing the cross-linking and staining experiments. To examine colocalization with the GM1 ganglioside, IpLITR staining was also performed on RBL-2H3 cells after their membranes were prestained with cholera toxin B subunit-FITC.
Statistics
Experimental groups were analyzed using Student's t test (2 tails), and p ≤ 0.05 was considered statistically significant.
Results
Cytokine Production by IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-Expressing Cells
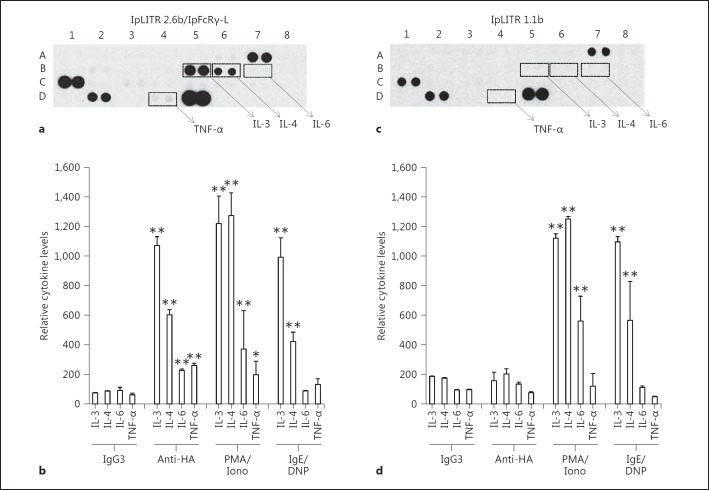
Using the Rat Cytokine Array Panel, we observed that neither IpLITR 2.6b/IpFcRγ-L- nor IpLITR 1.1b-expressing RBL-2H3 cells produced IL-3, IL-4, IL-6, or TNF-α when treated with the isotype control antibody IgG3 (fig. 2). The complete set of RBL-2H3 cytokine array data and profiler array coordinates/legend are provided in the online supplementary material. However, PMA and A23187-stimulated IpLITR 2.6b/IpFcRγ-L-expressing cells produced significantly higher levels of IL-3, IL-4, IL-6, and TNF-α compared to the untreated control. Also, when activated via the FcεRI, a significant induction of IL-3 and IL-4 was detected in the supernatants of IpLITR 2.6b/IpFcRγ-L-expressing cells (fig. 2a, b). Cells expressing IpLITR 1.1b were responsive to PMA and A23187 (fig. 2c, d), and following activation via the FcεRI these cells also produced IL-3 and IL-4 (fig. 2c, d) indicating that IpLITR-transfected RBL-2H3 cells are responsive to cytokine-inducing stimuli. When cross-linked using anti-HA mAb, cells expressing IpLITR 2.6b/IpFcRγ-L produced cytokine levels comparable to PMA/A23187 or IgE treatments (fig. 2a, b). However, IpLITR 1.1b-activated cells did not significantly increase cytokine levels compared to treatment with the IgG3 isotype antibody (fig. 2c, d). Duplicate spots of the select cytokines of interest are indicated on the profilers (fig. 2a, c). The spots located at positions A7, C1, D2, and D5 represent the cytokines sICAM-1, IL-13, CXCL7, and VEGF, respectively. These cytokines were constitutively produced by cultured RBL-2H3 cells under all conditions tested and were also detected in the supernatants of nontransfected parental cells (data not shown).
Fig. 2.
Parallel examination of the relative cytokine levels produced by IpLITR-activated RBL-2H3 cells. RBL-2H3 cells (2.5 × 106) expressing the N-terminal HA epitope-tagged IpLITR 2.6b/IpFcRγ-L (a, b) or IpLITR 1.1b (c, d) were cross-linked by treatment with 0.625 µg/ml mouse IgG3 (isotype control) or 0.625 µg/ml anti-HA mAb followed by 1.25 µg/ml anti-mouse IgG3 pAb (H+L) for 24 h at 37°C. Cell supernatants were then examined for the presence of various cytokines using the antibody capture chemiluminescent-based Rat Cytokine Array Panel Array Kit (R&D Systems). Transfected cells were also stimulated for 24 h at 37°C with 50 ng/ml PMA/0.5 µM Ca2+ ionophore A23187 (i.e. PMA/Iono) or after triggering them via their endogenous FcεRI with 200 ng/ml anti-DNP-IgE and 0.1 ng/ml DNP-HSA (i.e. IgE/DNP). Representative proteome profiler array results for anti-HA-activated IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b are shown (a, c); the calculated densitometry results of the relative cytokine levels are also displayed (b, d). The duplicate spots used for calculating the relative cytokine levels are indicated by the boxes, and these values were normalized for cross-array comparisons using the manufacturer's negative and positive controls standards. Densitometry of spot intensity was performed using ImageJ v1.44 software. The complete cytokine profiler array coordinates, representative array blots for IgG3-, PMA/Iono-, and IgE/DNP-treated cells, and the calculated densitometry results for cytokine levels following all experimental treatments are provided in the online supplementary material. Each bar represents the mean relative cytokine level ± SEM of 3 independent cytokine arrays. * p ≤ 0.01 and ** p ≤ 0.001 when comparing the relative cytokine levels for each experimental treatment to the cytokine levels of IgG3-treated cells.
Phosphorylation Status of Select Signaling Kinases following Engagement of IpLITR 2.6b/IpFcRγ-L or IpLITR 1.1b
A phospho-signaling profile of IpLITR-activated RBL-2H3 cells was obtained using the Phospho-MAPK Array Kit (R&D Systems). As shown in figure 3a, b, c, activation of IpLITR 2.6b/IpFcRγ-L by anti-HA cross-linking for 10 min at 37°C increased the phosphorylation of several signaling molecules, including ERK1/2, GSK-3α/β, GSK-3β, RSK1, CREB, JNK (pan), MEK6, MSK2, p38δ, and Akt2 (the complete Phospho-MAPK Array data and profiler array coordinates/legend are provided in the online suppl. material). This effect was not observed when the cells were treated with the IgG3 isotype control antibody. Conversely, when IpLITR 1.1b-expressing cells were activated by anti-HA cross-linking, increases in the phosphorylation of target signaling molecules were not detected and no differences between anti-HA and IgG3-treated cells were observed (fig. 3d-f). Figure 3 shows representative proteome profiler array results for both IgG3 (fig. 3a, d) and anti-HA (fig. 3b, e) cross-linked IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b-expressing RBL-2H3 cells, respectively. Also indicated are the duplicate spots used to calculate the relative phosphorylation levels of the specific signaling molecules. Figure 3c and f shows the calculated densitometry results obtained from the duplicate spots.
Fig. 3.
Parallel examination of the relative amounts of MAPK and other serine/threonine kinase phosphorylation levels induced by IpLITR-activated RBL-2H3 cells. RBL-2H3 cells (2.5 × 106) expressing the N-terminal HA epitope-tagged IpLITR 2.6b/IpFcRγ-L (a) or IpLITR 1.1b (b) were cross-linked by treatment with 0.625 µg/ml mouse IgG3 (isotype control) or 0.625 µg/ml anti-HA mAb followed by 1.25 µg/ml anti-mouse IgG3 pAb (H+L) for 10 min at 37°C. Cell lysates were then examined for the presence of phosphorylated proteins using the antibody capture chemiluminescent-based Human Phospho-MAPK Array Kit (R&D Systems). Representative proteome profiler array results for IgG3 isotype and anti-HA-activated IpLITR 2.6b/IpFcRγ-L (a, b) and IpLITR 1.1b (d, e); the calculated densitometry results of relative phospho-MAPK levels are also displayed (c, f). The duplicate spots used for calculating the relative levels of protein phosphorylation are indicated by the boxes, and these values were normalized for cross-array comparisons using the manufacturer's negative and positive control standards. Densitometry of spot intensity was performed using ImageJ v1.44 software. Each bar represents the mean spot intensity of the 2 indicated spots, and the data is representative of 2 independent experiments which gave similar profiler results. The complete phospho-MAPK array coordinates and the calculated densitometry results are provided in the online supplementary material.
Temporal Activation of ERK1/2 and Akt Activation in RBL-2H3 Cells by IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b Engagement
IpLITR-expressing RBL-2H3 cells were cross-linked with anti-HA mAb and a time course induction of ERK1/2 and Akt phosphorylation was measured by Western blot using phospho-specific primary antibodies. IpLITR 2.6b/IpFcRγ-L cross-linking with anti-HA mAb induced ERK1/2 phosphorylation at 2 min, which peaked at 4 min and then gradually declined after 8, 16, and 32 min (fig. 4a, top). This analysis correlated with the data obtained using the Phospho-MAPK Array Kit, demonstrating a relative increase in ERK1/2 phosphorylation after 10 min (fig. 3a-c). Also, 3 immunoreactive phospho-ERK proteins were detected at ∼42, ∼44, and ∼45 kDa. The ∼45-kDa protein is probably an ERK-like protein or nonspecific immunoreactivity. When RBL-2H3 cells expressing the inhibitory IpLITR 1.1b were cross-linked with anti-HA mAb, a different temporal pattern of ERK1/2 phosphorylation was observed: although IpLITR 1.1b-triggered cells showed no levels of phosphorylated ERK1/2 in their lysates after 8, 16, and 32 min, an early and transient phosphorylation was observed after 2 min, which rapidly diminished by 4 min (fig. 4c, top). This time course for ERK1/2 activation was also in agreement with the data obtained using the Phospho-MAPK Array Kit in that no phosphorylation of ERK1/2 was detected in IpLITR 1.1b-activated RBL-2H3 cells after 10 min (fig. 3d-f). Importantly, parental (i.e. nontransfected) RBL-2H3 cells did not demonstrate any levels of phosphorylated ERK1/2 after they were cross-linked with anti-HA mAb over the same time course (fig. 4i, j). Although IpLITR 1.1b-expressing cells demonstrated differential temporal ERK1/2 activation compared to IpLITR 2.6b/IpFcRγ-L-expressing cells, when activated via the FcεRI both cell types induced a similar level of ERK1/2 phosphorylation as previously reported [33] (data not shown).
Fig. 4.
Examination of ERK1/2 and Akt activation in IpLITR-activated RBL-2H3 cells. RBL-2H3 cells (2.5 × 106) expressing the N-terminal HA epitope-tagged IpLITR 2.6b/IpFcRγ-L (a, b) or IpLITR 1.1b (c, d) were cross-linked by treatment with 0.625 µg/ml anti-HA mAb followed by 1.25 µg/ml anti-mouse IgG3 pAb (H+L) for 0, 2, 4, 8, 16, and 32 min at 37°C. Cell lysates were then blotted with either the anti-phospho-p44/p42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) mouse mAb (a, c; top) or anti-p44/p42 MAPK (Erk1/2) (L34F12) mouse mAb (Endo; a, c; bottom) followed by a goat anti-mouse IgG (H+L) HRP-conjugated pAb. Using conditions identical to those described above, cross-linked IpLITR 2.6b/IpFcRγ-L (e, f) or IpLITR 1.1b (g, h) were then blotted with either anti-phospho-Akt (Ser473) (D9E) XP rabbit mAb (e, g; top) or anti-Akt rabbit pAb (Endo; e, g; bottom) followed by a goat anti-mouse IgG (H+L) HRP-conjugated pAb. i-l Parental (i.e. nontransfected) RBL-2H3 cells were also cross-linked as described using anti-HA mAb and their cell lysates were probed for phospho- and endo-ERK1/2 (i, j) or phospho- and endo-Akt (k, l) levels. Band intensity values were obtained by densitometry using ImageJ v1.44 software. Changes in phospho-ERK1/2 levels are reported as fold induction values relative to the untreated RBL-2H3 cells (i.e. 0 min), set to 1.0 as calculated below. Fold induction values of phospho-ERK1 expression in each lane were calculated using the following equation: [(ERK1 densitometry value for the time point/ERK1 value for the time point) × 100]. This value was then divided by the calculated relative fold induction value of ERK1 expression obtained for 0 min (i.e. untreated cells). Grey and white bars represent the relative fold induction values of phospho-ERK1 and phospho-ERK2, respectively. Phospho-Akt band intensity levels were corrected for endogenous Akt levels using the following equation: [(phospho-Akt densitometry value for the time point/endo-Akt value for the time point) × 100]. This corrected value was then converted into a percent change in phospho-Akt values (% ΔpAkt) as follows: [1 - (0 min corrected phospho-Akt value/corrected phospho-Akt value for the time point) × 100]. b, d, f, h, j, l % ΔpAkt values are displayed, including the % ΔpAkt values for cells triggering via their FcεRI using DNP-HSA (i.e. IgE). Results are representative of 2 independent experiments that gave similar results.
IpLITR 2.6b/IpFcRγ-L cross-linking with anti-HA mAb also resulted in a rapid and sustained phosphorylation of Akt (fig. 4e, top). Phosphorylated Akt levels were maintained for up to 32 min following receptor cross-linking, and the IpLITR 2.6b-activated Akt levels were similar to those induced by FcεRI. For IpLITR 1.1b, there was also a rapid increase in the phosphorylated levels of Akt following receptor cross-linking (fig. 4g, top). However, unlike the sustained levels of Akt activation observed for IpLITR 2.6b/IpFcRγ-L-activated cells, the relative amount of phosphorylated Akt diminished after 4, 8, 16, and 32 min, respectively. Engagement of the endogenous FcεRI in IpLITR 1.1b-expressing RBL-2H3 cells also increased phosphorylated Akt levels (fig. 4g, h), and this was also observed with the IpLITR 2.6b/IpFcRγ-L-expressing RBL-2H3 cells. In these experiments IgE served as a positive control for Akt activation. When parental RBL-2H3 cells were cross-linked with anti-HA, no sustained or consistent patterns of phosphorylated Akt levels were observed between 0 and 32 min that matched those of the IpLITR 2.6b/IpFcRγ-L- or IpLITR 1.1b-triggered RBL-2H3 cells (fig. 4k, l).
IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-Induced Phagocytosis
In stable transfected RBL-2H3 cells, the relative IpLITR 1.1b surface staining was higher than that observed for IpLITR 2.6b/IpFcRγ-L (fig. 1), and when untransfected RBL-2H3 cells were stained with the anti-HA mAb their fluorescence intensity was no different from IgG3 isotype antibody staining levels (fig. 1). Without a surface-expressed IpLITR, untransfected RBL-2H3 cells demonstrated <10% phagocytosis after incubation with either IgG3- or anti-HA mAb-coated beads (fig. 5a). Cells expressing IpLITR 2.6b/IpFcRγ-L also demonstrated <10% phagocytosis of IgG3-coated beads but this response increased to ∼35% when anti-HA-opsonized beads were used (fig. 5a). IpLITR 1.1b-expressing cells also phagocytosed low levels of the IgG3 beads (i.e. 7.4%), but surprisingly these cells increased their phagocytic response to ∼50% when incubated with the anti-HA mAb-coated beads (fig. 5a). In each of the experiments performed, IpLITR 1.1b-expressing RBL-2H3 cells had a ∼15–20% increased ability to phagocytose anti-HA mAb beads over IpLITR 2.6b/IpFcRγ-L-expressing cells, which may be in part due to their increased surface expression.
Fig. 5.
IpLITR-mediated phagocytosis of antibody-coated microspheres. Transfected cells were incubated with IgG3 mouse antibody- or anti-HA mAb-coated 4.5-µm YG microspheres and, using a flow cytometry-based phagocytosis assay [33], the % phagocytosis of untransfected RBL-2H3 cells, IpLITR 2.6b/IpFcRγ-L-expressing cells, and IpLITR 1.1b-expressing cells was determined (a). Each bar represents the mean % phagocytosis value ± SEM of 6 independent experiments. * p ≤ 0.001 when comparing the % phagocytosis of anti-HA beads vs. IgG3 beads for each group. Following phagocytosis, cells were also examined by confocal microscopy (b-e). DIC (b) and FITC (c) images of IpLITR 1.1b-expressing cells after phagocytosis of 2-µm anti-HA-opsonized microspheres. Cells are also stained with DAPI (nuclei) and FITC-cholera toxin B subunit as a marker for the GM1 ganglioside. The boxes highlight the similar locations of internalized microspheres for the DIC and FITC images. d, e Representative IpLITR 1.1b-expressing RBL-2H3 cells internalizing 4.5-µm anti-HA-coated microspheres are also shown. Consecutive images from top to bottom are serial confocal Z-stack images of the same cell, and the locations of phagocytosed beads are indicated by circles or arrows. These fluorescence confocal images are also stained with DAPI and the FITC-cholera toxin B subunit.
We also performed confocal microscopy to observe IpLITR-mediated phagocytosis of opsonized beads. This was done in conjunction with FITC-conjugated cholera toxin B subunit staining, a marker for lipid rafts (fig. 5b-e). When IpLITR 1.1b-expressing cells were incubated with anti-HA-opsonized 2-µm microspheres (merging of DIC, DAPI, and FITC), we observed multiple beads internalized by each cell (fig. 5b). Interestingly, in figure 5c, in which only the cholera toxin B subunit FITC stain is shown, the majority of the internalized beads are surrounded by intense FITC staining indicative of lipid raft compartment-containing membranes surrounding the beads. We also imaged the phagocytosis of 4.5-µm beads (the same size used in the flow cytometric assays) to show that the RBL-2H3 cells expressing IpLITR 1.1b also effectively internalized larger beads. Transverse sectioning (Z-stack analysis) allowed us to visualize the interface between the cell membrane and the surface of these associated beads. For the cells imaged in figure 5d and e [read from top to bottom, representing consecutive transverse sections (Z-stack) of the same cell], the FITC staining surrounding the bead gradually changes from a bright green ring around the bead (color only in online version) to a more diffuse pattern at the point where the edge of the bead and the cell membrane are in contact (areas highlighted by circles). Although all images shown in figure 5b, c, d, e are for IpLITR 1.1b-expressing cells, similar images were captured for IpLITR 2.6b/IpFcRγ-L-expressing RBL-2H3 cells (data not shown).
IpLITR Internalization and Colocalization into GM1-Containing Membrane Compartments Are Induced by Receptor Cross-Linking
Confocal microscopy was also used to examine the cellular location of IpLITRs expressed in RBL-2H3 cells. As shown in figure 6, neither IpLITR 2.6b/IpFcRγ-L- nor IpLITR 1.1b-expressing cells were stained when incubated with isotype control IgG3 followed by the goat-anti-mouse IgG Cy5-labeled pAb for 30 min at 4°C. However, using the anti-HA mAb, we observed distinct circular/ring-like cell surface staining patterns for both IpLITR types (fig. 6a, b) after 30 min at 4°C. We then performed staining experiments at 37°C for 10-30 min after cross-linking with the goat-anti-mouse IgG Cy5-labeled pAb. Both IpLITR 2.6b/IpFcRγ-L (fig. 6a) and IpLITR 1.1b (fig. 6b) displayed a punctate or patchy surface-staining pattern after 10 min at 37°C, and after 20 and 30 min the majority of the cross-linked receptors appeared to have been internalized. Transverse sections through the cells (not shown) confirmed that the Cy5 label was inside the cells, which can also be observed in the DIC/DAPI/Cy5 overlays shown in figure 6. When the IpLITR-expressing RBL-2H3 cells were pretreated for 30 min with hypertonic sucrose (0.2 M, inhibitor of clathrin-dependent endocytosis) [35] prior to cross-linking, both receptors remained on the cell surface even after 30 min at 37°C (fig. 6). Figure 7 is a representative set of Cy5/cholera toxin B subunit-FITC costaining images for IpLITR 1.1b before and after receptor cross-linking. At 4°C the surface stainings of IpLITR 1.1b (Cy5) and GM1 (FITC) are present but do not appear to colocalize at the cell surface (fig. 7a). When the cells were incubated at 37°C for 10 min, the punctate surface-staining pattern of IpLITR 1.1b was observed and, when overlaid with GM1, staining patterns of receptor with GM1 colocalization were detected as indicated by the arrows (fig. 7b). After 30 min at 37°C, aggregates of IpLITR 1.1b staining were observed inside the cells, and in some cases they were colocalized with GM1-containing subcellular compartments (fig. 7c). Similar staining and colocalization effects were observed when IpLITR 2.6b/IpFcRγ-L was examined (data not shown).
Fig. 6.
Internalization of IpLITRs following antibody-mediated cross-linking. RBL-2H3 cells expressing IpLITR 2.6b/IpFcRγ-L (a) or IpLITR 1.1b (b) were stained with anti-HA mAb (or IgG3 isotype control antibody) and then incubated with the goat anti-mouse IgG Cy5 pAb for 30 min at 4°C or for 10, 20, and 30 min at 37°C prior to examination by confocal microscopy. Each image shows Cy5 staining (top) or DIC with a DAPI/Cy5 merge (bottom). For the +sucrose treatments, cells were preincubated with a 0.2 M hypertonic sucrose solution prior to staining. The staining patterns that we observed after each treatment are shown, and these images are representative of the ∼20 cells that were imaged per treatment group.
Fig. 7.
Cross-linking of IpLITRs induces their association with lipid raft compartments. RBL-2H3 cells expressing IpLITR 1.1b were prestained with FITC-cholera toxin B subunit prior to staining with anti-HA mAb and the goat anti-mouse IgG Cy5 pAb. a From left to right: Cy5, FITC, Cy5/FITC, and DIC/Cy5/FITC/DAPI merged images for IpLITR 1.1b cells incubated at 4°C for 30 min following receptor cross-linking. b, c These images are the same as above but these cells were incubated for 10 and 30 min at 37°C after receptor cross-linking, respectively. The arrows indicate the location of staining predicted to correspond to the location of IpLITR 1.1b-Cy5 colocalizing with the GM1 ganglioside-FITC lipid raft compartments. The staining patterns that we observed after each treatment are shown, and they are representative of the ∼10 cells that were imaged per treatment group.
IpLITR 1.1b-Mediated Phagocytosis Is Not due to an Association with the RBL-2H3 FcεRIγ Chain but Is Dependent on Its CYT Region
To examine whether IpLITR 1.1b-mediated phagocytosis could be due to its association with the ITAM-bearing FcεRIγ chain endogenously expressed in RBL-2H3 cells, co-immunoprecipitation experiments were performed. IP of the FcεRI α subunit in association with the FcεRIγ chain was done in both untransfected and IpLITR 1.1b-expressing RBL-2H3 cells (fig. 8a). This association was not affected by stimulation of the cells with Na3VO4. When we immunoprecipitated IpLITR 1.1b with anti-HA mAb, a 70-kDa protein corresponding to the expected size of IpLITR 1.1b was detected which was not present in untransfected cells (fig. 8b, bottom). When we probed the anti-HA mAb immunoprecipitates for an associated FcεRIγ chain, unlike what was observed for the FcεRI α subunit, no associating adaptor protein was detected in unstimulated or Na3VO4-stimulated cells (fig. 8b, bottom) even though the FcεRIγ chain was present in the cell lysates (fig. 8b, top).
Fig. 8.
IpLITR 1.1b does not associate with the FcεRIγ chain and requires an intact CYT region for phagocytosis. Untransfected RBL-2H3 cells and those expressing IpLITR 1.1b (∼2.5 × 106) were treated with (+) or without (-) Na3VO4 and then lysed and immunoprecipiated with either 2 µg anti-FcεRI α subunit mouse mAb (a) or anti-HA mAb (b). Immunoprecipitated samples were then blotted with the anti-FcεRIγ rabbit pAb or an anti-FcεRI α rabbit pAb as indicated. Cellular lysates were also blotted with an HRP-conjugated anti-HA antibody (b). As described earlier, the % phagocytosis of untransfected RBL-2H3 cells, IpLITR 1.1b-expressing cells, IpLITR 1.1b ΔCYT-expressing cells, and IpLITR 1.2a-expressing cells was determined (c). Each bar represents the mean % phagocytosis value ± SEM of 4 independent experiments. * p ≤ 0.001 when comparing the % phagocytosis of anti-HA beads vs. IgG3 beads for each group.
A CYT-deficient mutant of IpLITR 1.1b was generated lacking all intracellular tyrosines, and a representative flow cytometric staining profile of IpLITR 1.1b ΔCYT expression is shown (fig. 1). Phagocytosis experiments were performed with untransfected RBL-2H3 cells, IpLITR 1.1b-expressing cells, and IpLITR 1.1b ΔCYT mutant-expressing cells (fig. 8c). As expected, RBL-2H3 cells demonstrated <10% phagocytosis of IgG3- and anti-HA-coated 4.5-µm beads, whereas IpLITR 1.1b-expressing cells demonstrated 11.2% phagocytosis of IgG3 beads and 59.5% phagocytosis of anti-HA beads, which is consistent with the results observed in figure 5a. When the IpLITR 1.1b ΔCYT was tested, we observed an 8.9% phagocytosis of IgG3 beads and only 14.3% phagocytosis in the presence of anti-HA-opsonized beads. This represented a ∼90% reduction in phagocytic capacity compared to the intact wild-type IpLITR 1.1b (fig. 8c). We then tested IpLITR 1.2a to see if a related inhibitory IpLITR type that only encoded 2 of the 6 tyrosines found within IpLITR 1.1b also promoted phagocytosis. RBL-2H3 cells expressing this receptor demonstrated phagocytosis levels of 11.5 and 57.3% when treated with IgG3 beads and anti-HA beads, respectively (fig. 8c), indicating that the ability of prototypical inhibitory IpLITRs to induce phagocytosis is not limited to IpLITR 1.1b. This also suggests that the phagocytic activities of these two receptors may be mediated by the TM distal ITIM and/or ITSM that are shared between these two receptors (online suppl. fig. 1, 2). IpLITR 1.2a surface expression is also similar to that of IpLITR 1.1b (fig. 1).
Differential Effects of EDTA and CytoD on IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-Mediated Phagocytosis
The addition of EDTA (2 mM) reduced the phagocytosis of anti-HA beads from 36.7 to 23.2% by IpLITR 2.6b/IpFcRγ-L-expressing RBL-2H3 cells (fig. 9a). This represents a 52% inhibition of the phagocytic response using the formula described in the figure legend. Conversely, when EDTA (2 mM) was added to the IpLITR 1.1b-expressing cells, the phagocytosis of anti-HA beads surprisingly increased from 44.5% (no EDTA) to 52.5% (fig. 9b), representing a 27.5% increase in their phagocytic response. Pretreatment with CytoD (10 µM) produced a significant reduction in the phagocytosis of anti-HA beads from 47.3 to 19.8% by IpLITR 2.6b/IpFcRγ-L-expressing RBL-2H3 cells (fig. 9a). This translated into a 72.1% inhibition of their phagocytic response. In comparison, when pretreated with Cyto D (10 µM), IpLITR 1.1b-expressing cells demonstrated only a 37.9% overall inhibition of their phagocytic response (fig. 9d).
Fig. 9.
Effects of EDTA and CytoD on IpLITR 2.6b/IpFcRγ-L- and IpLITR1.1b-mediated phagocytosis of 4.5-µm microspheres. RBL-2H3 cells expressing IpLITR 2.6b/IpFcRγ-L (a, c) or IpLITR 1.1b (b, d) were incubated with IgG3 mouse antibody-coated (white bars) or anti-HA mAb-coated (grey bars) 4.5-µm microspheres for 1 h at 37°C. Incubations were performed in the absence (control) or presence of 2 mM EDTA (a, b) or using cells pretreated for 1 h at 37°C with 10 µM CytoD or the DMSO vehicle control (c, d) prior to analysis by flow cytometry. Each bar represents the mean ± SEM of 3 independent phagocytosis experiments. a, b** p ≤ 0.001 when comparing the % phagocytosis of anti-HA beads by IpLITR 2.6b/IpFcRγ-L-expressing cells with and without 2 mM EDTA; * p ≤ 0.05 when comparing the % phagocytosis of anti-HA beads by IpLITR 1.1b-expressing cells with and without 2 mM EDTA. c, d** p ≤ 0.001 when comparing the % phagocytosis of anti-HA beads by IpLITR-expressing cells pretreated with DMSO or with 10 µM CytoD. Percent inhibition for each treatment were calculated using the following formula: [1 - [EDTA or CytoD values for; (%Phagocytosis HA beads - %Phagocytosis G3 beads)]/[control or DMSO values for; (%Phagocytosis HA beads - %Phagocytosis G3 beads)]] × 100.
Discussion
Receptors within the IpLITR family are structurally related to Ig superfamily receptors in vertebrates that are known to participate in the regulation of innate immunity [28, 29]. IpLITRs also exhibit distant phylogenetic relationships with mammalian FcRs and those encoded within the leukocyte receptor complex [28, 30]. However, we have yet to identify all IpLITR members, to fully explore their expression patterns on fish immune cell subtypes, and to identify the endogenous ligand(s) that engage IpLITRs. Consequently, our studies have relied on transfection and expression of epitope-tagged IpLITRs in mammalian immune cell lines. This approach has allowed us to examine the biochemistry of IpLITR-mediated signal transduction and to determine how IpLITRs control innate immune cell effector functions [33, 34]. As expected, stimulatory IpLITR types associate with ITAM-containing adaptor molecules [32] and activate kinase-dependent intracellular signaling events that promote degranulation and phagocytosis [33]. Conversely, inhibitory IpLITR types recruit the phosphatases SHP-1 and SHP-2 [31] and effectively abrogate NK cell killing responses using both SHP-dependent and SHP-independent mechanisms [34]. To date, functional studies of specific IpLITRs as stimulatory or inhibitory immunoregulatory receptor types have been in agreement with the presence of cytoplasmic ITAMs or ITIMs, respectively [31, 32, 33, 34]. Here we compared the cellular functions mediated by a previously characterized classical stimulatory IpLITR and its associated ITAM-containing adaptor (i.e. IpLITR 2.6b/IpFcRγ-L) and, for the first time, an inhibitory ITIM-containing IpLITR type (IpLITR 1.1b) expressed in the myeloid cell line RBL-2H3. Overall, this study represents the first demonstration of functional plasticity for an ITIM-containing teleost immunoregulatory receptor.
IpLITR 2.6b/IpFcRγ-L- but not IpLITR 1.1b-activated RBL-2H3 cells produced IL-3, IL-4, IL-6, and TNF-α at levels significantly higher than the isotype antibody (IgG3) control. IpLITR 1.1b-expresssing cells were capable of producing cytokines if stimulated via endogenous FcεRI triggering or the addition of PMA/A23187. Untransfected RBL-2H3 cells also generated cytokines if stimulated via FcεRI triggering or the addition of PMA/A23187 (data not shown), indicating that these cells can be activated in an IpLITR-independent manner. As expected, several activated intracellular kinases were detected in the lysates of RBL-2H3 following activation for 10 min by IpLITR 2.6b/IpFcRγ-L cross-linking. By comparison, no relative change in phosphorylated kinases for IpLITR 1.1b-induced RBL-2H3 cells was observed at 10 min poststimulation. This suggests either that IpLITR 1.1b does not target the 26 kinases represented on the array or that there are temporal differences in signaling events initiated by IpLITR 1.1b when compared to IpLITR 2.6b/IpFcRγ-L. To address the potential for temporal differences in signaling dynamics, we examined a time course (up to 32 min) for the activation of 2 classical immunoregulatory receptor signaling targets: ERK1/2 and Akt. We observed that IpLITR 1.1b induced a rapid but transient phosphorylation of ERK1/2 that peaked early after receptor cross-linking (2 min) and no activated ERK1/2 was detected after 4 min. This transient activation of ERK1/2 by IpLITR 1.1b explains the absence of activated ERK1/2 detected 10 min after cross-linking using the signaling array. In comparison, ERK1/2 phosphorylation induced by IpLITR 2.6b/IpFcRγ-L was sustained for up to 32 min after cross-linking, which reached maximum levels at 4 min. Unlike ERK1/2, activated Akt was detected in unstimulated RBL-2H3 cells but these levels greatly increased when cells were activated via IpLITR 2.6b/IpFcRγ-L. IpLITR 1.1b-expressing cells also displayed a similar induction of phosphorylated Akt following cross-linking. However, IpLITR 2.6b/IpFcRγ-L-mediated increases in Akt activation were sustained for up to 32 min, whereas IpLITR 1.1b-stimulated Akt phosphorylation steadily diminished at 4 min after cross-linking. Importantly, when nontransfected RBL-2H3 cells were cross-linked with anti-HA mAb they did not demonstrate any ERK1/2 phosphorylation for up to 32 min poststimulation and their patterns of Akt phosphorylation were distinct from that observed in the IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-expressing cells, indicating that the results observed following IpLITR engagements were receptor-specific signaling events.
Temporal differences in the patterns of ERK1/2 and Akt activation also suggest that the dynamics of the signaling events induced by IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b are unique to each receptor type. Previously we hypothesized that the ITAM-dependent signaling by IpLITR 2.6b likely occurs through classical stimulatory kinase-dependent cascades, which are shared among other ITAM encoding immunoregulatory receptor types [33]. However, unexpectedly, activation of ERK1/2 and Akt in RBL-2H3 cells by IpLITR 1.1b may reflect functional plasticity in ITIM-encoding immunregulatory receptor-mediated responses. IpLITR 1.1b has ITIMs in its CYT region, recruits phosphatases (including SHP-1/2), and was originally demonstrated to function as an inhibitory receptor when expressed in mouse primary NK cells [34]. The stimulatory signaling by IpLITR 1.1b in RBL-2H3 cells may result from differential recruitment of signaling mediators in specific immune cell types, thereby facilitating a context-dependent plasticity of receptor-mediated inhibitory or stimulatory control of cellular processes, which will be discussed below.
We also monitored IpLITR staining patterns in RBL-2H3 cells before and after receptor cross-linking. Confocal imaging demonstrated ring-like surface-staining patterns for both IpLITR types on resting cells and this staining pattern became more punctate after 10 min of receptor cross-linking. When cells were activated for 20 or 30 min, both IpLITR types were internalized. Receptor internalization was blocked by treatment with hypertonic sucrose, suggesting that clathrin-dependent endocytosis was involved as hypertonic treatments eliminate the formation of coated pits by interfering with the clathrin polymerization process [35]. Since both IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b encode endocytic motifs (i.e. YxxΦ) [36] in their CYT regions (online suppl. fig. 1), it is not surprising that they were both endocytosed after antibody-mediated cross-linking, which likely results in receptor degradation and/or recycling. IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b also colocalized with the GM1 ganglioside, which is known to associate with lipid rafts [37]. These sphingolipid-enriched domains are thought to function as membrane platforms that facilitate receptor aggregation and association with signaling effector molecules such as Src family protein tyrosine kinases [38]. Lipid rafts are also involved in the activation of lymphocytes and participate in the control of effector cell functions involved in innate immunity, including phagocytosis [39, 40]. Recently, we demonstrated that GM1 is a marker of lipid rafts in teleost immune cells and that during phagocytosis GM1 concentrates in the phagosomal membranes of goldfish macrophages [41]. In the present study, both IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b aggregated into raft-like compartments during receptor engagement and these IpLITR-containing aggregates were concentrated in phagosomal compartments surrounding internalized microspheres. IpLITR surface staining patterns, aggregation, and internalization after cross-linking were relatively similar for both IpLITR 2.6b/IpFcRγ-L and IpLITR 1.1b, unlike what was observed for cytokine production and intracellular signaling.
As expected, IpLITR 2.6b/IpFcRγ-L-expressing cells engulfed anti-HA mAb-coated beads significantly more than beads opsonized with IgG3 but, surprisingly, IpLITR 1.1b-expressing cells also displayed a robust phagocytic response. Differential effects on the phagocytic responses induced by these two IpLITR types were observed after treatments with EDTA and CytoD. IpLITR 2.6b/IpFcRγ-L-mediated phagocytosis of anti-HA mAb-opsonized beads was significantly reduced in the presence of EDTA (2 mM), suggesting the involvement of extracellular Ca2+. This effect is consistent with previous reports of EDTA inhibition of phagocytosis in fibroblasts [42] and murine peritoneal macrophages [43]. Phagocytosis induced by IpLITR 1.1b was refractory to EDTA-induced inhibition and instead showed a slight enhancement of bead internalization in EDTA-treated cells. One possible explanation for this observation is that IpLITR 1.1b employs a Ca2+-independent signaling mechanism to control phagocytosis. IgG-mediated phagocytosis in human neutrophils can also be Ca2+ independent [44], and in fact protein kinase C translocation to the plasma membrane is known to be Ca2+ independent in RBL-2H3 cells [45]. Alternatively, human monocytes utilize a Ca2+-independent phagocytic phospholipase/phospholipase A2 to carry out IgG-mediated phagocytosis [46]. Thus, IpLITR 1.1b may potentially recruit protein kinase C or phagocytic phospholipase/phospholipase A2 to control phagocytosis in RBL-2H3 cells, which remains to be determined.
Treatment with CytoD, an inhibitor of actin polymerization, significantly inhibited both IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-mediated phagocytosis. However, the effect of CytoD was more potent for IpLITR 2.6b/IpFcRγ-L-expressing cells. The role of actin polymerization during phagocytosis is well studied [42, 47, 48]; however, the cellular mechanisms responsible for the differential susceptibility to CytoD observed for IpLITR 2.6b/IpFcRγ-L- and IpLITR 1.1b-induced phagocytosis are unknown. Regardless, these results again suggest that IpLITR 1.1b is capable of promoting immune cell effector responses by controlling signaling events that are likely unique when compared to those induced by the ITAM-encoding IpLITR 2.6b/IpFcRγ-L.
To further characterize the unexpected ability of IpLITR 1.1b to activate phagocytosis, we determined whether or not IpLITR 1.1b associated with the RBL-2H3 FcεRIγ chain. No association between IpLITR 1.1b and the ITAM-containing adaptor was observed in our co-immunoprecipitation experiments although the FcεRIγ chain did directly interact with the endogenous FcεRI, demonstrating that we could observe these immune receptor-adaptor interactions in RBL-2H3 cells. Examination of whether or not IpLITR 1.1b could associate with the FcεRIγ chain was prompted by a recently identified but unconventional stimulatory capability of murine LMIR3 [17]. Under certain conditions, this prototypical inhibitory protein was shown to associate with the FcεRIγ chain and induce cellular activation [17]. This was an unexpected finding since LMIR3 does not encode any charged TM residues that are predicted to facilitate adaptor recruitment [17]. We also reasoned that a teleost immunoregulatory receptor could associate with an endogenous mammalian ITAM-containing adaptor protein based on previous work from our lab and others. For example, fish encode a full complement of ITAM-containing adaptor proteins [49] and, in the case of the FcεRIγ chain, the human, mouse, and catfish proteins are highly similar in their TM segments, with similar positioning of their aspartic acid residues and other residues identified as important for an interaction between these adaptors and immunoregulatory receptors [10, 32]. Consequently, if cross-species adaptor-immunoregulatory receptor interactions can occur in a conventional manner as shown by Feng et al. [10], then perhaps the stimulatory properties of IpLITR 1.1b could also occur by cross-species adaptor-receptor interactions; although this would be more akin to the unconventional mechanism displayed by LMIR3 [17]. It is also worth noting that RBL-2H3 cells do not express the ITAM-containing adaptors DAP12 and CD3ζ although they have been reported to express DAP10 [50, 51, 52]. Whether or not DAP10 can directly associate with IpLITR 1.1b in RBL-2H3 cells has yet to be determined, but our results suggest that the FcεRIγ chain is not involved. More likely, the novel stimulatory functions of IpLITR 1.1b observed here are due to signals transmitted by its CYT region, which correspond with the stimulatory mechanisms of other ITIM-bearing immunoregulatory receptors like IREM-1 [16], PECAM-1 [18], and FcγRIIB [26].
To further reinforce that IpLITR 1.1b-induced phagocytosis was independent of associations with RBL-2H3 adaptors (i.e. the FcεRIγ chain and DAP10), we deleted the CYT region of IpLITR 1.1b and reassessed its phagocytic ability. Without the CYT region, IpLITR 1.1b was still expressed on the cell surface but it did not stimulate phagocytosis, indicating that the signal to initiate phagocytosis is independent of an associated ITAM-bearing adaptor and is more likely due to events promoted by the CYT region. We then tested the phagocytic activity of the closely related inhibitory receptor IpLITR 1.2a (88% identical to IpLITR 1.1b) since this receptor completely lacks the TM proximal CYT region of IpLITR 1.1b that encodes Y433, Y453, and Y463. In addition, the corresponding position of Y499 in IpLITR 1.1b is a serine residue (S429) in IpLITR 1.2a. Therefore, IpLITR 1.2a effectively provided us with a naturally mutated version of IpLITR 1.1b as it is missing 4 of the 6 tyrosine residues thought to facilitate these unique CYT-derived phagocytic signaling events. In transfected RBL-2H3 cells, IpLITR 1.2a induced phagocytosis of anti-HA-coated beads at levels comparable to IpLITR 1.1b (∼55%). This allowed us to hypothesize that it is either IpLITR 1.1b Y477 and/or Y503 that is responsible for IpLITR 1.1b-mediated phagocytosis as discussed below. These residues, and the full CYT region sequences of IpLITR 1.1b and IpLITR 1.2a are shown in online supplementary figure 1 and an amino acid alignment of IpLITR 1.1b and IpLITR 1.2a is shown in online supplementary figure 2.
We predict that the IpLITR 1.1b-induced signaling events observed in this study likely involve Y477 and/or Y503, which are contained within an ITIM and an ITSM-like region, respectively. Switch motifs bind SHP-1 and SHP-2, resulting in cellular inhibition [53], and can also activate cellular signaling by selectively recruiting adaptor proteins such as SH2 domain protein 1A (SH2D1A) and Ewing's sarcoma-activated transcript 2 (EAT-2) [54]. Interestingly, ITSMs also facilitate PI3K-dependent signaling [55, 56] and can trigger both ERK1/2 and Akt phosphorylation [reviewed in [57]]. ITSM induction of ERK1/2 activation has also been shown to involve recruitment of the cellular phosphatase SHP-2 [58]. SHP-2 recruitment to ITIMs and/or ITSMs causes its specific phosphorylation at a C-terminal tyrosine that serves as a binding site for the SH2-containing adaptor molecule growth factor receptor-bound 2 (Grb2) [59] and this can then promote the activation of the Ras-MEK1/2-ERK1/2 pathway via interactions with members of the Dab/Dos family of scaffolding proteins, known as the Grb2-associated binders (Gab) [60, 61, 62]. During IpLITR 1.1b-mediated phagocytosis, we predict that Gab2 recruitment to the activated IpLITR 1.1b would likely rely on an indirect mode of interaction. For example, SHP-2 binding of the phosphorylated IpLITR 1.1b ITIM (Y477) and/or the ITSM (Y503) could promote SHP-2-Gab2 interactions or formation of SHP-2-Grb2-Gab2 ternary complexes [60, 61, 62]. The demonstration of SHP-2 involvement as a scaffold for Gab2 and/or Grb2 binding suggests that this cellular phosphatase can also play important roles as an intracellular adaptor capable of organizing receptor-mediated cellular activation [63, 64, 65], independent of classical inhibitory roles in immune cell function. For IpLITR 1.1b, this provides an explanation for context-dependent functional outcomes contrasting with our previously reported SHP-dependent inhibition of NK cell killing [34]. We have previously shown that SHP-2 is recruited to IpLITR 1.1b [31] and, taken together, this suggests that IpLITR 1.1b-mediated activities may be in part achieved by ITIM/ITSM-dependent recruitment of SHP-2-mediated signaling, which requires Y477 and or Y503. Further studies including site-directed mutagenesis of these residues and co-immunoprecipitation experiments are required to decipher the unique mechanisms behind IpLITR-mediated phagocytosis. These studies are ongoing in our laboratory.
In conclusion, recruitment of intracellular signaling adaptors allows surface receptors to selectively integrate with specific downstream effectors that can differentially control cellular responses. Understanding how IpLITR 1.1b-induced signaling mediates the activation of immune cell phagocytosis will help to elucidate the importance of immunoregulatory receptor plasticity in teleosts. Furthermore, whether functional plasticity is conserved among the ITIM/ITSM-containing vertebrate immunoregulatory proteins is unknown. However, it seems likely that new mechanisms of stimulatory responses induced by previously defined inhibitory innate immune receptor types will continue to be uncovered. From these mechanisms we can decipher the precise roles that various immunoregulatory receptor types play in the fine-tuning of essential innate defense responses like phagocytosis.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
This work was supported by grants from: the Natural Sciences and Engineering Council of Canada (NSERC) awarded to J.L.S. and J.P.C.; an NSERC CGS-M and an Alberta Innovates Technology Futures Graduate Student Scholarship (GSS) awarded to H.D.C.; graduate teaching assistantships awarded by the Department of Biological Sciences to H.D.C., D.M.E.L., and M.A.Z.; an NSERC USRA awarded to A.O.; an NSERC CGS-D awarded to B.C.S.M., and an NSERC PGS-D, Alberta Innovates Health Solutions Graduate Studentship (GSS), and an Honorary Izaak Walter Killam Memorial Scholarship awarded to J.G.P.
References
- 1.Steevels TAM, Meyaard L. Immune inhibitory receptors: essential regulators of phagocyte function. Eur J Immunol. 2011;41:575–587. doi: 10.1002/eji.201041179. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 4.Barclay AN. Membrane proteins with immunoglobulin-like domains - a master superfamily of interaction molecules. Semin Immunol. 2003;14:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 5.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 6.Barrow AD, Trowsdale J. The extended leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev. 2008;224:98–123. doi: 10.1111/j.1600-065X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 8.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;90:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 9.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Call ME, Wucherpfennig KW. The assembly of diverse immune receptors is focused on a polar membrane-embedded interaction site. PLoS Biol. 2006;4:e142. doi: 10.1371/journal.pbio.0040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 12.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;377:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 13.Bezbradica JS, Medzhitov R. Role of ITAM signaling module in signal integration. Curr Opin Immunol. 2012;24:58–66. doi: 10.1016/j.coi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunol Rev. 2009;232:59–71. doi: 10.1111/j.1600-065X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Àlvarez-Errico D, Sayós J, López-Botet N. The IREM-1 (CD300f) inhibitory receptor associates with the p85 α subunit of phosphoinositide 3-kinase. J Immunol. 2007;178:808–816. doi: 10.4049/jimmunol.178.2.808. [DOI] [PubMed] [Google Scholar]
- 17.Izawa K, Kitaura J, Yamanishi Y, Matsuoka T, Kaitani A, Sugiuchi M, Takahashi M, Maehara A, Enomoto Y, Oki T, Takai T, Kitamura T. An activating and inhibitory signal from an inhibitory receptor LMIR3/CLM-1: LMIR3 augments lipopolysaccharide response through association with FcRγ in mast cells. J Immunol. 2009;183:925–936. doi: 10.4049/jimmunol.0900552. [DOI] [PubMed] [Google Scholar]
- 18.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 19.Faure M, Long EO. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential. J Immunol. 2002;168:6208–6214. doi: 10.4049/jimmunol.168.12.6208. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD185d) in resting NK cells. J Immunol. 2001;167:1877–1881. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 21.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168:5047–5057. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi-Maki A, Yusa S, Catina TL, Campbell KS. KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. J Immunol. 2003;171:3415–3425. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor gamma protein. J Immunol. 2005;174:3859–3863. doi: 10.4049/jimmunol.174.7.3859. [DOI] [PubMed] [Google Scholar]
- 24.Koncz G, Pecht I, Gergely J, Sármay G. Fcgamma receptor-mediated inhibition of human B cell activation: the role of SHP-2 phosphatase. Eur J Immunol. 1999;29:1980–1989. doi: 10.1002/(SICI)1521-4141(199906)29:06<1980::AID-IMMU1980>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Takai T. Role of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 26.Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhou Y, Yang Q, Mu C, Duan E, Chen J, Yang M, Xia P, Cui B. Ligation of Fc gamma receptor IIB enhances levels of antiviral cytokine in response to PRRSV infection in vitro. Vet Microbiol. 2012;160:473–480. doi: 10.1016/j.vetmic.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Stafford JL, Bengtén E, Du Pasquier L, McIntosh RD, Quiniou SM, Clem LW, Miller NW, Wilson M. A novel family of diversified immunoregulatory receptors in teleosts is homologous to both mammalian Fc receptors and molecules encoded within the leukocyte receptor complex. Immunogenetics. 2006;58:758–773. doi: 10.1007/s00251-006-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stafford JL, Bengtén E, Du Pasquier L, Miller NW, Wilson M. Channel catfish leukocyte immune-type receptors contain a putative MHC class I binding site. Immunogenetics. 2007;59:77–91. doi: 10.1007/s00251-006-0169-3. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery BC, Cortes HD, Mewes-Ares J, Verheijen K, Stafford JL. Teleost IgSF immunoregulatory receptors. Dev Comp Immunol. 2011;35:1223–1237. doi: 10.1016/j.dci.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery BC, Mewes J, Davidson C, Burshtyn DN, Stafford JL. Cell surface expression of channel catfish leukocyte immune-type receptors (IpLITRs) and recruitment of both Src homology 2 domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-2. Dev Comp Immunol. 2009;33:570–582. doi: 10.1016/j.dci.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Mewes J, Verheijen K, Montgomery BC, Stafford JL. Stimulatory catfish leukocyte immune-type receptors (IpLITRs) demonstrate a unique ability to associate with adaptor signaling proteins and participate in the formation of homo- and heterodimers. Mol Immunol. 2009;47:318–331. doi: 10.1016/j.molimm.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Cortes HD, Montgomery BC, Verheijen K, García-García E, Stafford JL. Examination of the stimulatory signaling potential of a channel catfish leukocyte immune-type receptor and associated adaptor. Dev Comp Immunol. 2012;36:62–73. doi: 10.1016/j.dci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery BC, Cortes HD, Burshtyn DN, Stafford JL. Channel catfish leukocyte immune-type receptor mediated inhibition of cellular cytotoxicity is facilitated by SHP-1-dependent and -independent mechanisms. Dev Comp Immunol. 2012;37:151–163. doi: 10.1016/j.dci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clatherin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozik P, Francis RW, Seaman MNJ, Robinson MS. A screen for endocytic motifs. Traffic. 2010;11:843–855. doi: 10.1111/j.1600-0854.2010.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 39.Baumruker T, Prieschl EE. Sphingolipids and the regulation of the immune response. Semin Immunol. 2002;14:57–63. doi: 10.1006/smim.2001.0342. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizaki F, Nakayama H, Iwahara C, Takamori K, Ogawa H, Iwabuchi K. Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochim Biophys Acta. 2008;1780:383–392. doi: 10.1016/j.bbagen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Garcia E, Grayfer L, Stafford JL, Belosevic M. Evidence for the presence of functional lipid rafts in immune cells of ectothermic organisms. Dev Comp Immunol. 2012;37:257–269. doi: 10.1016/j.dci.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Gronski MA, Kinchen JM, Juncadella IJ, Franc NC, Ravichandran KS. An essential role for calcium flux in phagocytes for apoptotic cell engulfment and the α-inflammatory response. Cell Death Differ. 2009;10:1323–1331. doi: 10.1038/cdd.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hishikawa T, Cheung JY, Yelamarty RV, Knutson DW. Calcium transients during Fc receptor-mediated and nonspecific phagocytosis by murine peritoneal macrophages. J Cell Biol. 1991;115:59–66. doi: 10.1083/jcb.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosales C, Brown EJ. Two mechanisms for IgG Fc-receptor-mediated phagocytosis by human neutrophils. J Immunol. 1991;146:3937–3944. [PubMed] [Google Scholar]
- 45.Altrichter J, Guse AH, Resch K, Brock J, Daëron M, Hückel C. Phosphatidylinositol hydrolysis and an increase in Ca2+ concentration in the signal-transduction process triggered by murine FcγRIII are not required for protein kinase C translocation. Eur J Biochem. 1994;228:587–595. doi: 10.1111/j.1432-1033.1995.0587m.x. [DOI] [PubMed] [Google Scholar]
- 46.Lennartz MR, Lefkowith JB, Bromley FA, Brown EJ. Immunoglobulin G-mediated phagocytosis activates a calcium-independent, phosphatidylethanolamine-specific phospholipase. J Leukoc Biol. 1993;54:389–398. doi: 10.1002/jlb.54.5.389. [DOI] [PubMed] [Google Scholar]
- 47.Groves E, Dart AE, Covarelli V, Caron E. Molecular mechanisms of phagocytic uptake in mammalian cells. Cell Mol Life Sci. 2008;65:1957–1976. doi: 10.1007/s00018-008-7578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113:2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- 49.Yoder JA, Orcutt TM, Traver D, Litman GW. Structural characteristics of zebrafish orthologs of adaptor molecules that associate with transmembrane immune receptors. Gene. 2007;401:154–164. doi: 10.1016/j.gene.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomasello E, Cant Charles, Bühring H-J, Vély F, André P, Seiffert M, Ullrich A, Vivier E. Association of signal-regulatory proteins β with KARAP/DAP12. Eur J Immunol. 2000;30:2147–2156. doi: 10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Anfossi N, Lucas M, Diefenbach A, Bühring H-J, Raulet D, Tomasello E, Vivier E. Contrasting roles of DAP10 and KARAP/DAP12 signaling adaptors in activation of the RBL-2H3 leukemic mast cell line. Eur J Immunol. 2003;33:3514–3522. doi: 10.1002/eji.200324573. [DOI] [PubMed] [Google Scholar]
- 52.Schleinitz N, Chiche L, Guia S, Bouvier G, Vernier J, Morice A, Houssaint E, Harlé JR, Kaplanski G, Montero-Julian FA, Vély F. Pattern of DAP12 expression in leukocytes from both healthy and systemic lupus erythematosus patients. PLoS One. 2009;4:e6264. doi: 10.1371/journal.pone.0006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 54.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 55.Mikhalap SV, Shlapatska LM, Berdova AG, Law CL, Clark EA, Sidorenko SP. CDw150 associates with src-homology 2-containing inositol phosphatase and modulates CD96-mediated apoptosis. J Immunol. 1999;15:5719–5727. [PubMed] [Google Scholar]
- 56.Aoukaty A, Tan R. Association of the X-linked lymphoproliferative disease gene product SAP/SH2D1A with 2B4, a natural killer cell-activating molecule, is dependent on phosphoinositide 3-kinase. J Biol Chem. 2002;277:13331–13337. doi: 10.1074/jbc.M112029200. [DOI] [PubMed] [Google Scholar]
- 57.Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- 58.Li C, Iosef C, Jia CY, Han VK, Li SS. Dual functional roles for the X-linked lymphoproliferative syndrome gene product SAP/SH2D1A in signaling through the signaling lymphocyte activation molecule (SLAM) family of immune receptors. J Biol Chem. 1993;278:3852–3859. doi: 10.1074/jbc.M206649200. [DOI] [PubMed] [Google Scholar]
- 59.Brummer T, Schmitz-Peiffer C, Daly RJ. Docking proteins. FEBS J. 2010;277:4356–4369. doi: 10.1111/j.1742-4658.2010.07865.x. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Nishimura R, Kashishian A, Batzer AG, Kim WJ, Cooper JA, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;12:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc Natl Acad Sci USA. 1991;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belov AA, Mohammadi N. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci Signal. 2012;5:pe49. doi: 10.1126/scisignal.2003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 64.Qu C-K. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim Biophys Acta. 2002;1592:297–301. doi: 10.1016/s0167-4889(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Jenkins B, Shin JL, Rohrschneider LR. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptors tyrosine kinase. Mol Cell Biol. 2001;21:3047–3056. doi: 10.1128/MCB.21.9.3047-3056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data