Abstract
Myocardial infarction (MI) provokes regional inflammation which facilitates the healing, whereas excessive inflammation leads to adverse cardiac remodelling. Our aim was to determine the role of macrophage migration inhibitory factor (MIF) in inflammation and cardiac remodelling following MI.
Wild type (WT) or global MIF deficient (MIFKO) mice were subjected to coronary artery occlusion. Compared to WT mice, MIFKO mice had a significantly lower incidence of post-MI cardiac rupture (27% vs. 53%) and amelioration of cardiac remodelling. These were associated with suppressed myocardial leukocyte infiltration, inflammatory mediators’ expression, and reduced activity of MMP-2, MMP-9, p38 and JNK MAPK. Infarct myocardium-derived or exogenous MIF mediated macrophage chemotaxis in vitro that was suppressed by inhibition of p38 MAPK or NF-κB. To further dissect the role of MIF derived from different cellular sources in post-MI cardiac remodelling, we generated chimeric mice with MIF deficiency either in bone marrow derived-cells (WTKO) or in somatic-cells (KOWT). Compared to WT and KOWT mice, WTKO mice had reduced rupture risk and ameliorated cardiac remodelling, associated with attenuated regional leukocyte infiltration and expression of inflammatory mediators. In contrast, KOWT mice had delayed healing and enhanced expression of M1 macrophage markers, but diminished expression of M2 markers during the early healing phase.
In conclusion, global MIF deletion protects the heart from post-infarct cardiac rupture and remodelling through suppression of leukocyte infiltration and inflammation. Leukocyte-derived MIF promotes inflammatory responses after MI, whereas cardiac-derived MIF affects early but not ultimate healing process.
Keywords: macrophage migration inhibitory factor, myocardial infarction, inflammation, healing
Introduction
Myocardial infarction (MI) is the leading cause of cardiac death worldwide and its occurrence is expected to increase with population aging [1]. A great challenge to modern cardiology is to limit cardiac damage and prevent adverse left ventricular (LV) remodelling and dysfunction after MI [2, 3]. Many studies on the role of inflammatory responses to MI have highlighted the dual nature of the contribution of inflammation to disease progression. Following myocardial ischemia, necrotic cardiomyocytes release a wide range of inflammatory molecules such as reactive oxygen species and cytokines that stimulate regional inflammatory infiltration [4]. Recruitment of leukocytes to the injured myocardium is essential for healing via phagocytosis of cellular debris, activation of matrix metalloproteinases (MMPs) to remodel extracellular matrix (ECM), and secretion of growth factors to promote granular angiogenesis and deposition of ECM proteins [5, 6]. However, either excessive or inadequate inflammation in the injured myocardium can lead to adverse outcomes such as cardiac rupture, adverse ventricular remodelling and heart failure [6, 7].
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine with a number of unique biological actions and is recognised as an important regulator of innate and acquired immunity [8, 9]. Pro-inflammatory actions of MIF have been reported in various inflammatory diseases such as sepsis, rheumatoid arthritis and atherosclerosis [9–11]. MIF is released from intracellular stores in response to various cellular stressors, such as hypoxia or bacterial proteins [12]. MIF is rapidly released from the heart, if subjected to a brief period of ischemia, and enhances glucose uptake via activation of AMP-activated protein kinase (AMPK) [13, 14], whilst it also inhibits c-Jun N-terminal kinase (JNK) [15] and attenuates oxidative stress [16], thereby reducing infarct size and preserving cardiac function. However, prolonged ischemia, which is frequently seen in clinical practice [1], provokes severe cardiac inflammatory responses. We recently reported that prolonged ischemia in mice leads to substantial myocardial damage and regional inflammation, effects which are attenuated in mice with a global MIF deletion (MIFKO) [17]. These distinct observations of the effects of MIF in different contexts highlight the complexity of pathophysiological processes following ischemic cardiac injury and warrant further investigations on the role of MIF, especially when anti-MIF therapies are to be considered for MI.
In the current study, we investigated the phenotype of MIFKO mice subjected to MI, by measuring post-MI inflammation, healing, and acute and chronic cardiac remodelling. MIF is expressed in multiple cell types including both leukocytes [18] and cardiomyocytes [19], but the relative contributions of these sources of MIF to myocardial injury are unknown. We hypothesized that MIF derived from different cellular sources would have different effects in the heart following ischemic injury. This hypothesis was investigated in chimeric mice generated by bone marrow transplant. The results confirm that MIF plays a vital role in mediating cardiac inflammatory injury after MI, and show that while leukocyte MIF enhances damage, non-leukocyte MIF enhances myocardial healing.
Methods
A detailed methodology section can be found in the online supplemental materials
Animals
All animal investigations were approved by a local animal ethics committee complying with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (7th edition). Ten-week-old male global MIFKO mice and wild type (WT) littermates with C57Bl/6 genetic background were used [20].
Induction of MI
Mice underwent coronary artery occlusion to induce MI or sham operation, as previously described [21, 22]. After surgery, mice were monitored daily for 4 weeks. Autopsy was performed for evidence of post-MI cardiac rupture or heart failure, as described previously [22]. Mice were killed at various time points following MI, infarct size was assessed and infarct and non-infarct myocardium were separated, snap frozen in liquid nitrogen and stored at −80°C for molecular assays. Further, some LVs were fixed in 10% formalin or fresh frozen for histological analyses.
Echocardiography
Echocardiography was performed prior to surgery and 1, 2 or 4 weeks post-surgery, as previously described [17, 23].
Immunofluorescence staining
Immunofluorescence was performed, as previously described [17]. Briefly, LV sections were stained with an anti CD45 antibody for leukocyte, and 4′, 6-diamidino-2-phenylindole (DAPI) for nucleus, to identify leukocytes. Visualisation of capillaries in border zones was performed using Alexa Fluor® 568 isolectin GS-IB4 conjugate. Images were acquired using an Olympus BX61 fluorescence microscope and densities of leukocytes and capillaries were analysed using Image Pro Plus software (Media Cybernetics, Inc, USA).
Quantitative real time-PCR
RNA was extracted from cardiac tissues. Gene expression of monocyte chemotactic protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), interleukin-1β (IL-1β), IL-10, MMP-9, MMP-2 and transforming growth factor β1 (TGFβ1) was assessed by quantitative real-time PCR (qPCR) using Applied Biosystems 7500 fast real-time PCR system and normalised to GAPDH, as previously described [17, 24].
Enzyme linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed, in duplicates, using a commercial mouse IL-1β (Life Research, Australia) and MIF (ElAab Science Co. Ltd. Wuhan, China) ELISA kits according to the manufactures’ instructions.
Gelatin zymography
Proteins were extracted from infarct tissues and sham-operated hearts and concentrations were determined using Bradford protein quantification assay. Gelatin zymography was performed on a 7.5% acrylamide, 0.5% gelatine SDS page, as previously described [24].
Immunoblotting
Western blotting was performed with primary antibodies to phospho- and total-p38 mitogen-activation protein kinase (p-p38 and t-p38 MAPK), p-JNK and t-JNK, as reported previously [17]. Membranes were re-probed with GAPDH antibody to verify loading consistency.
Histology
Formalin-fixed paraffin-embedded LV sections were stained for hemotoxylin and eosin to assess remaining necrotic areas and scar thickness while picosirius red was used to determine collagen deposition in the infarct region. Images were captured on using an Olympus light microscope and analysed using Image-Pro Plus 6.0 software, as described previously [21, 25].
Cell culture experiments
To further understand potential influence of MIF on post-infarct healing, we studied effects of MIF on cardiac fibroblast biology in cell culture models. First, we examined whether fibroblasts and cardiomyocytes are able to release MIF under hypoxic stimulation. Second, fibroblasts were prepared from the infarct tissue of adult MIFKO and WT mice subjected to MI for 4 days, fibroblast proliferation, collagen deposition and fibrosis-related gene expression including TGFβ, α-smooth muscle actin (α-SMA), collagen-1 and -3 were investigated.
Trans-well migration assay
Macrophages were isolated from the peritoneal cavities of WT and global MIFKO mice. Using a trans-well migration assay, cells were exposed to homogenised infarct or normal cardiac tissue, or recombinant human MIF (rMIF). Additionally, WT macrophages were pre-treated with either a p38 MAPK inhibitor, SB203580, or NF-κB inhibitor, Bay11-7082, prior to migration assay. The number of trans-well migrated macrophages was counted.
Generation of chimeric mice
A detailed description of the bone marrow transplantation (BMT) of WT and global MIFKO mice can be found in the Online Supplemental Material. Briefly, after lethal irradiation (550 rads twice), WT mice received bone marrow from MIFKO mice via a tail vein injection to create (WTKO) mice with MIF deficiency in bone marrow derived cells (or leukocytes). In parallel, irradiated MIFKO mice received WT bone marrow to create (KOWT) mice deficient in MIF in somatic cells (or the heart). After BMT, animals were allowed to recover for 4 weeks before coronary artery ligation or sham operation. Chimeric mice were monitored up to 2 weeks after surgery. Echocardiography, immunofluorescence, histology and qPCR for gene expression of MIF, M1 macrophage markers [IL-1β, interferon-γ (IFN-γ), tumor necrosis factor α (TNFα, IL-6] and M2 macrophage markers [TGFβ1, arginase 1 (Arg-1), macrophage mannose receptor 1 (MRC-1) and CD163] [26] were performed as described above. To confirm success of BMT, peripheral blood cells were collected and purified using Maxwell DNA extraction and genotyped (Online Figure 1).
To clarify the potential influence of irradiation on observed phenotypes, we reconstituted WT bone marrow to irradiated WT mice (WTWT), or MIFKO bone marrow to irradiated MIFKO mice (KOKO), then subjected mice to coronary artery occlusion. Incidence of cardiac rupture within 2 weeks was investigated.
Statistics
Results are presented as mean±SEM unless otherwise stated. Graphpad Prism software (version 5.0) was used for the statistical analysis. Survival was analysed by Kaplan-Meier analysis and compared by the log-rank, Chi-square or Fisher exact test. One- or two-way analysis of variance (ANOVA) followed by Newman-Keuls post-hoc tests were used to detect differences between groups. P<0.05 was considered statistically significant.
Results
Global MIF deficiency improved survival by reducing post-MI cardiac rupture
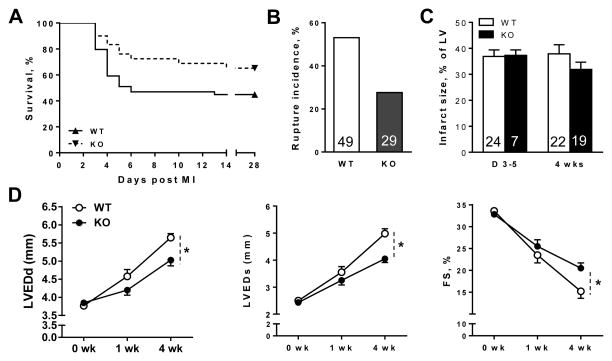
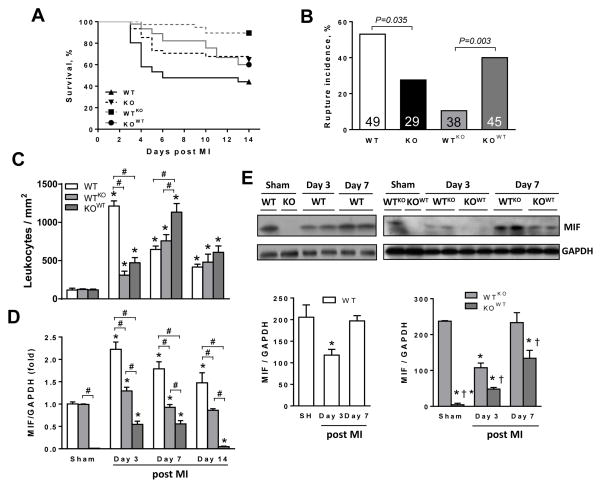
At 4 weeks after MI, survival was much better in MIFKO (66%) than WT littermates (45%, P<0.05, Figure 1A). Over the first week, significantly more WT than MIFKO mice died of cardiac rupture (P<0.05) (Figure 1B), indicating deleterious effects of MIF on cardiac rupture post MI. Typically, cardiac rupture occurred during 3–6 days following MI, consistent with our previous reports [22, 27]. One WT and two MIFKO mice developed heart failure and were killed prematurely in the second week, the remaining mice were killed at 4 weeks post MI. Infarct size was comparable between WT and MIFKO mice, when measured at autopsy in those mice dying of rupture within 3–5 days (acute phase) and in surviving mice at 4 weeks post MI (chronic phase, Figure 1C).
Figure 1. Global MIF deletion (MIFKO) reduced incidence of cardiac rupture and alleviated cardiac remodelling and dysfunction following myocardial infarction (MI).
A, Kaplan-Meier survival analysis of wild type (WT) and MIFKO mice up to 4 weeks following MI, all sham operated animals survived (not shown). n=49 for WT and 29 for MIFKO group. B, Cumulative incidence of cardiac rupture leading to mortality in WT and MIFKO mice. Numbers represent the group size. C, Quantitative analysis of infarct size at autopsy in those mice dying of rupture within 3–5 days (acute phase) and in surviving mice at 4 weeks post MI (chronic phase). Numbers in the bar represent the group size. D, Echocardiographic data showing that left ventricular end-diastolic and end-systolic dimensions (LVEDd, LVESd) were smaller and fractional shortening (FS) was greater in MIFKO than in WT mice at 4 weeks after MI, indicating less severe LV remodelling and dysfunction in MIFKO mice. *P<0.05 vs. WT, n=15–20 per time point.
Global MIF deficiency ameliorated post-MI cardiac remodelling
Echocardiography prior to MI revealed no significant differences in cardiac dimensions and function between WT and MIFKO mice. At week-1 and week-4 following MI, WT and MIFKO mice displayed gradually increasing LV dimensions and decreasing fractional shortening (FS) when compared with baseline values (Figure 1D). However, MIFKO mice had significantly smaller LV dimensions and better FS when compared to WT group at 4 weeks following MI (P<0.05, Figure 1D, Online Table 1). These results indicate that MIF contributes to adverse cardiac remodelling post MI.
Global MIF deficiency had moderate influence on healing
We next investigated the effect of global MIF deletion on post-infarct healing. The fractional size of remaining necrotic area in the infarct region was reduced between 7 to 14 days to a similar extent in both WT (13% to 3%) and MIFKO mice (17% to 2%). No significant difference was observed between genotypes at either time point following MI (Online Figure 2A and 2B). Between 7 and 14 days post-MI, collagen content in the infarct region was increased in both WT (24% to 59%) and MIFKO mice (19% to 48%), but collagen content was significantly lower in MIFKO mice at 14 days (P<0.05 vs. WT, Online Figure 2C and 2D). Capillary densities in the infarct border zone were comparable between WT and MIFKO mice at 7 and 14 days following MI (Online Figure 2E and 2F). Expression of TGFβ1 mRNA was similarly increased (~6.5-fold) at day-1 and day-7 post-MI in MIFKO and WT mice, but 30% higher in WT versus MIFKO mice at day-3 when the expression reached its maximum (P<0.05, Online Figure 2G). Infarct wall thickness at 7 and 14 days following MI was lower versus sham values in both WT and MIFKO mice, and the thickness was significantly greater in MIFKO than WT mice at day-14 post-MI (P<0.05, Online Figure 2H).
We further studied potential influence of MIF on cardiac fibroblast biology by in vitro cell culture experiments. First, 6 h hypoxia exposure induced substantial release of MIF from cardiomyocytes with a similar trend in fibroblasts (Online Figure 3A). Second, we dissected and cultured fibroblasts for 48 h from the infarct tissue of both global MIFKO and WT mice at day-4 after MI, and found that cell proliferation was 28% in MIFKO and 47.2% in WT group (P<0.05) compared with their respective baseline levels (Online Figure 3B). Collagen deposition by cardiac fibroblasts was 3% lower in MIFKO than in WT mice (7% vs. 10%, P=NS) over their respective baseline levels (Online Figure 3C). There was no difference in mRNA levels of TGFβ and α-SMA between MIFKO and WT mice. Collagen-1 expression was significantly higher in MIFKO than that in WT fibroblasts while there was a trend to lower collagen-3 expression in MIFKO fibroblasts (Online Figure 3D). These data suggest that global MIF deficiency may not impact on early healing post MI albeit MIF deficiency decreased proliferation of cardiac fibroblasts post MI.
Global MIF deficiency attenuates acute inflammatory responses post MI
As regional inflammation following MI leads to tissue damage contributing to subsequent cardiac remodelling, we next investigated the effect of global MIF deletion on temporal changes in leukocyte infiltration and expression of pro-inflammatory mediators post-MI.
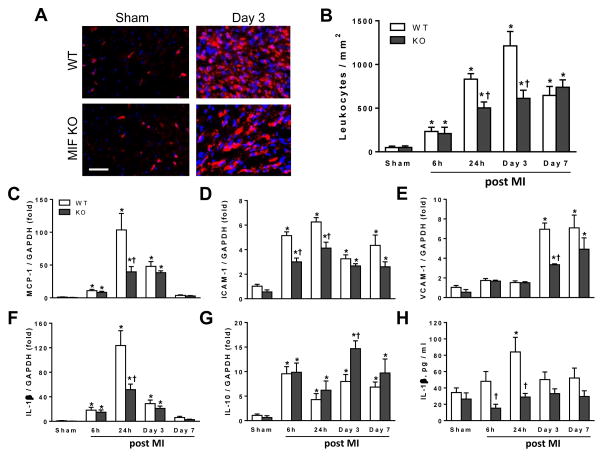
Immunohistochemistry for CD45, a marker of leukocytes, revealed increased leukocyte density in the infarct region including the border zone in both genotypes from 6 h to 7 days following MI (Figure 2B). In WT mice, leukocyte density peaked at day-3 and declined by day-7. Compared to WT mice, MIFKO mice had significantly lower leukocyte density in the infarct myocardium at days 1–3 (both P<0.05) (Figure 2A and 2B). In addition, expression of MCP-1, ICAM-1, VCAM-1 and IL-1β mRNA in the infarct tissue of both WT and MIFKO mice was significantly increased over sham values at multiple time points (Figure 2C–F), with a significantly lower expression level in MIFKO mice at 24 h and day-3 (P<0.05, Figure 2C–F). Expression of the anti-inflammatory cytokine, IL-10, was also increased in both groups as early as 6 h post-MI and it was markedly higher in MIFKO than WT heart at day-3 (Figure 2G, P<0.05). To assess whether global MIF deletion also influences systemic inflammatory responses, we measured plasma concentrations of IL-1β by ELISA. Plasma IL-1β was significantly elevated above baseline in WT mice at 24 h, and MIFKO mice had significantly lower plasma IL-1β levels at 6 and 24 h following MI (Figure 2H, P<0.05).
Figure 2. Temporal changes in leukocyte infiltration and gene expression of cytokines, chemokine and adhesion molecules in global MIF deficient (MIFKO) and wild type (WT) mice following myocardial infarction (MI).
A, Representative images of CD45 positive immunofluroescent staining for leukocytes in the infarct region at 3 days after MI. The purple colour indicates overlap of CD45 positive staining (red) with DAPI (blue) staining for nuclei. Bar=100 μm. B, Quantification of leukocytes (CD45 positive cells) in the infarct and border regions from different time points after MI. C–G, Changes in mRNA level of monocyte chemoattractant protein-1 (MCP-1), intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), interlukin-1β (IL-1β) and IL-10 in the infarct tissue of WT and MIFKO mice at different time points after MI. H, Temporal change of IL-1β plasma level in WT and MIFKO mice with either sham-operated or MI. *P<0.05 vs. respective sham, †P<0.05 vs. WT at the same time point. n=4 per sham group, n=7–8 per MI group.
Global MIF deficiency reduces MMP activity following MI
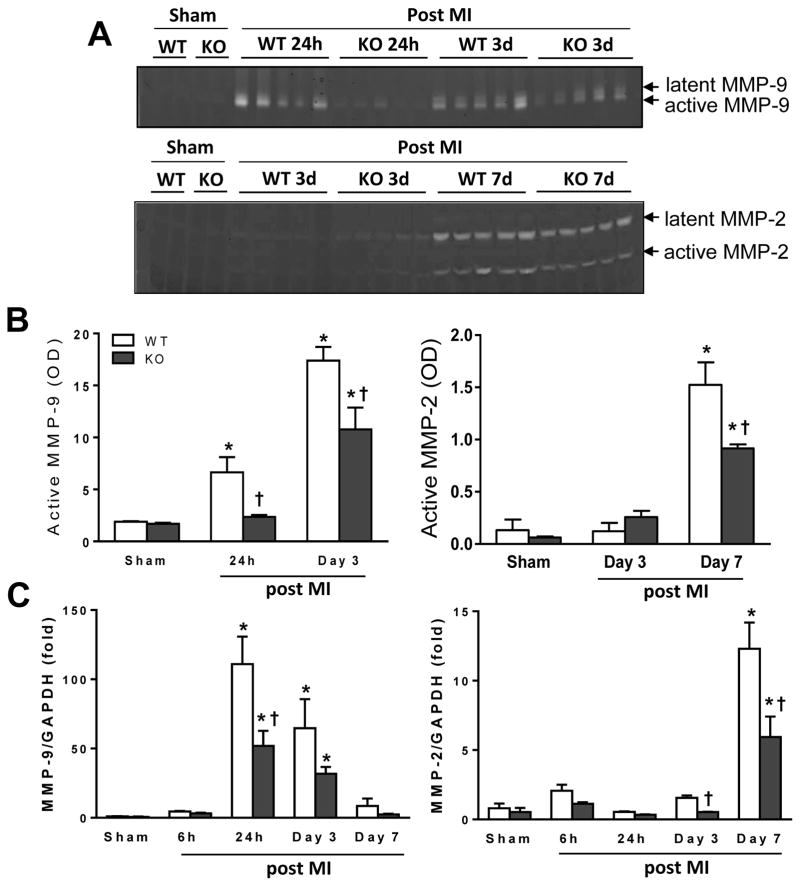
As excessive activation of MMPs has been implicated in adverse cardiac remodelling [28], we also measured mRNA expression and activity of MMP-9 and MMP-2 by qPCR and gelatine zymography. Both expression and activity of MMP-9 and MMP-2 were at very low levels in sham WT or MIFKO hearts (Figure 3A–C). Following MI, MMP-9 expression and activity increased from 24 h to day-3 in WT hearts with a blunted response in MIFKO mice (Figure 3A–C). Abundance, mRNA level and activity of MMP-2 were significantly increased until day-7 post-MI but with attenuated MMP-2 expression and activity in MIFKO hearts (P<0.05, Figure 3A–C).
Figure 3. Global MIF deletion (MIFKO) attenuated activity and gene expression of matrix metallanoprotienase-9 (MMP-9) and MMP-2 following myocardial infarction (MI).
A, Representative images of gelatine zymography demonstrating temporal changes of MMP-9 and MMP-2 activities in WT and MIFKO mice following MI. B, Quantitative analyses of active MMP-9 and MMP-2 by zymography in WT and MIFKO mice from sham-operated and infarcted tissues. n=3 for each sham group, n=5 for each MI group. C, temporal changes in mRNA levels of MMP-9 and MMP-2 from WT and MIFKO mice following MI. n=4 per sham group, n=7–8 per MI group. *P<0.05 vs. sham, †P<0.05 vs. WT at the same time point.
Global MIF deficiency attenuates MI induced phosphorylation of p38 MAPK and JNK
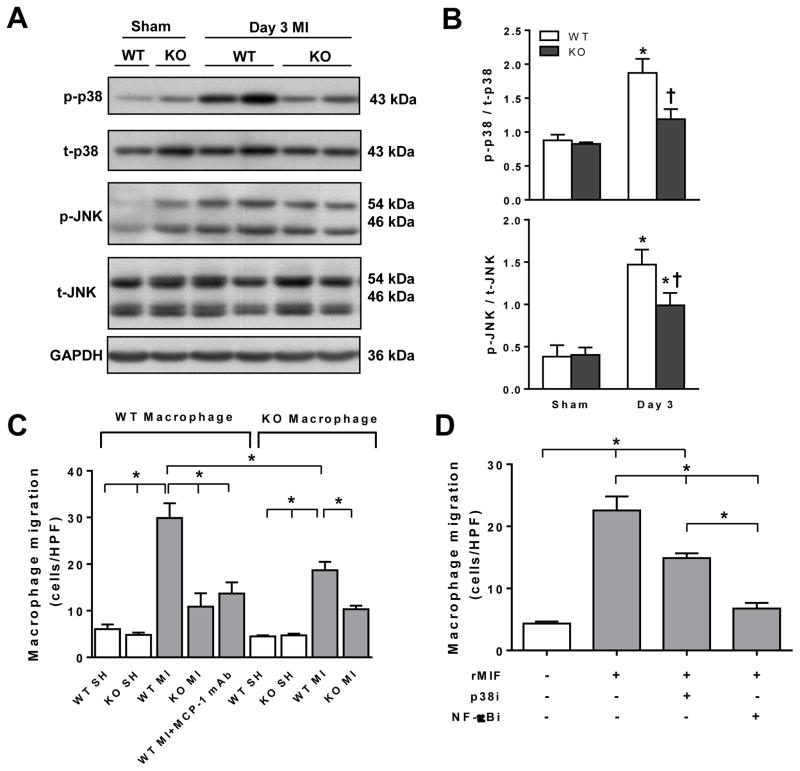
To elucidate how MIF regulates inflammatory signalling following MI, we performed immunoblotting for p38 and JNK MAPK, both are important in inflammatory signalling post MI [29–31]. There was no difference between sham-operated WT and MIFKO groups in levels of phosphorylated p38 or JNK (Figure 4A and 4B). At day-3 following MI, phosphorylation of both p38 MAPK and JNK was significantly elevated in both genotypes with such increment significantly attenuated in MIFKO infarct hearts (P<0.05, Figure 4A and B).
Figure 4. Phosphorylation of pro-inflammatory signalling kinases was reduced in global MIF deficient (MIFKO) mice and MIF promoted macrophage infiltration in the infarct myocardium.
A, Representative immunoblotting images for phospho- and total-p38 mitogen-activation protein kinase (p/t-p38) and c-Jun N-terminal kinase (p/t-JNK) in hearts of sham-operated (SH) or infarct wild type (WT) and MIFKO mice. B, Quantitative analysis of p/t-p38 MAPK and p/t-JNK in WT and MIFKO mice. *P<0.05 vs. sham, †P<0.05 vs. WT at day 3, n=3 per sham group, n=5 per MI group). C, Macrophage chemotaxis in response to sham or homogenised infarct tissue from WT or MIFKO mice at day 3. Cells/HPF, cells/high power field. *P<0.05 vs. other conditions. MCP-1 Ab, monocyte chemoattrant protein-1 neutralizing monoclonal antibody (eBioscience, 5 μg/ml). D, Chemotaxis by quantitative migration analysis of WT macrophage or pre-treated WT macrophages with inhibitors for p38 MAPK (p38i, SB203580, 10 μM) or NF-κB inhibitor (NF-κBi, Bay 11-7082, 2 μM) for 1 hr in response to recombinant MIF (rMIF, 40 ng/ml) in trans-well experiments. *P<0.05. Experiments were performed in triplicates.
MIF mediates macrophage migration to the infarct myocardium
To seek direct evidence for MIF promoting inflammatory infiltration, we tested, using an ex vivo trans-well migration assay [18], migration of peritoneal macrophages prepared from WT or global MIFKO mice in response to homogenised infarct myocardium. Macrophages from either genotype displayed a similar and minimal migration to homogenised sham-operated cardiac tissue of WT or MIFKO mice (Figure 4C). WT macrophages showed a 4-fold increase in migration in response to homogenised WT infarct tissue, but significantly attenuated in response to MIFKO infarct tissue (Figure 4C). Moreover, addition of anti-MCP-1 monoclonal antibody to the WT infarct tissue homogenate significantly inhibited migration (Figure 4C). When MIFKO macrophages were exposed to WT infarct tissue, migration was also lower than WT macrophages, and cell migration was further reduced when MIFKO macrophages were exposed to MIFKO infarct tissue (Figure 4C). To confirm the chemoattractant property of MIF, rMIF was added to the lower chamber and the top chamber loaded with WT macrophages. We observed a dramatic macrophage migration (Figure 4D) under these conditions i.e. in the absence of other chemotracttants such as would be found in the infarct tissue. To further illustrate mechanisms involved in MIF-mediated macrophage migration, we investigated effects of specific inhibitors on the macrophage migration evoked by rMIF. Compared with control media, rMIF induced significant migration of WT macrophages, which was partially or largely inhibited by pre-treatment of WT macrophages with the p38 MAPK inhibitor, SB203580, or the NF-κB inhibitor, Bay11-7082 (Figure 4D).
Bone marrow derived-cell MIF deficiency (WTKO) lowers risk of cardiac rupture and ameliorates cardiac remodelling post-MI
As MIF is derived from both the myocardium and immune cells [8, 14], we next investigated the respective roles of MIF derived from different cellular sources on post-MI inflammation and cardiac remodelling. Chimeric mice were created with MIF deficiency either in somatic-cells (KOWT) or in bone marrow derived-cells (WTKO) (confirmation of genotype, Online Figure 1). Compared with WT counterparts (serving as the baseline control), KOWT mice had only a slight reduction in the incidence of cardiac rupture. In contrast, WTKO mice had a significantly reduced occurrence of rupture events which was even lower than that in MIFKO mice (Figure 5A and 5B). Interestingly, while the time window for rupture was slightly prolonged in WTKO mice it was significantly extended in KOWT compared to WT mice (P<0.05, Figure 5A) [22, 27]. Further, echocardiography revealed that relative to leukocyte-MIF deficient or WT mice, KOWT mice displayed greater LV end-diastolic dimension and lower FS when studied at day-7 and day-14 post-MI (Table 1).
Figure 5. Survival, incidence of cardiac rupture, cardiac expression of MIF and leukocytes infiltration in wild type (WT), global MIF deficient (KO) or chimeric mice with either bone marrow derived cell-MIF deficiency (WTKO) or somatic cell-MIF deficiency (KOWT) following myocardial infarction (MI).
A, Kaplan-Meier survival analyses for WT, KO, WTKO and KOWT mice after MI. B, Cumulative incidence of cardiac rupture leading to mortality in WT, KO, WTKO and KOWT mice. Numbers represent group size. C, Time course of CD45 positive leukocyte densities in the infarct and border zones of WT, WTKO and KOWT mice following MI. D, Temporal change in MIF gene expression by qPCR in WT mice and both chimeric models following MI. *P<0.05 vs. sham, #P<0.05. n=3 per sham group, n=5–8 per MI group. E, Temporal change in MIF protein expression in WT mice and both chimeric models following MI. *P<0.05 vs. sham, †P<0.05 vs. WTKO at each time point. n=3 per sham group, n=5 per MI group.
Table 1.
Echocardiographic parameters of wild type and chimeric mice (WTKO and KOWT) following myocardial infarction
| Day-0 | Day-7 | Day-14s | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| WT n=25 | WTKO n=21 | KOWT n=26 | WT n=13 | WTKO n=15 | KOWT n=18 | WT n=13 | WTKO n=15 | KOWT n=11 | |
|
|
|
|
|||||||
| HR (bpm) | 598±13 | 604±11 | 582±13 | 600±10 | 588±17 | 593±34 | 590±15 | 610±10 | 613±11 |
| LVEDd (mm) | 3.77±0.06 | 3.65±0.04 | 3.50±0.07 | 4.58±0.17* | 4.45±0.16* | 4.89±0.28* | 5.03±0.22* | 4.65±0.31* | 5.34±0.31*†‡ |
| LVESd (mm) | 2.50±0.05 | 2.35±0.05 | 2.28±0.07 | 3.45±0.14* | 3.28±0.18* | 3.86±0.31*†‡ | 4.21±0.22* | 3.82±0.14* | 4.40±0.34*‡ |
| PWd (mm) | 0.80±0.01 | 0.83±0.03 | 0.82±0.02 | 0.80±0.03 | 0.84±0.02 | 0.79±0.05 | 0.76±0.03 | 0.77±0.04 | 0.88±0.03 |
| PWs (mm) | 1.24±0.03 | 1.28±0.03 | 1.29±0.03 | 1.03±0.04* | 1.13±0.04* | 1.02±0.08* | 0.98±0.05* | 1.11±0.04* | 0.94±0.06* |
| FS (%) | 33.6±1.4 | 35.6±1.2 | 34.8±1.6 | 24.6±1.7* | 26.3±1.8* | 21.1±1.7*†‡ | 16.3±1.6* | 18.0±1.1* | 13.6±0.9*†‡ |
Values are mean ± SEM,
P<0.05 vs. baseline,
P<0.05 vs. WT at the same time point.
P<0.05 vs. WTKO at the same time point.
WT, wild type; WTKO, bone marrow cell/leukocyte-MIF deficient mice; KOWT, somatic cell/heart-MIF deficient mice; HR, heart rate; LVEDd, left ventricular end-diastolic diameter; LVESd, LV end-systolic diameter; PWd, LV posterior wall thickness at diastole; PWs, LV posterior wall thickness at systole; FS, fractional shortening.
To clarify the potential effect of irradiation on observed phenotypes in chimeric mice, we reconstituted WT bone marrow to irradiated WT mice (WTWT), or global MIFKO bone marrow to irradiated MIFKO mice (KOKO), and then subjected these mice to MI. We observed that rate and time-window of cardiac rupture as well as infarct size (data not shown) were comparable between KO and KOKO, or between WT and WTWT mice (Online Figure 4), indicating that irradiation had minimal influence on the phenotypes observed.
Somatic-cell MIF deficiency (KOWT) delays wound healing following MI
We further examined the impact of cardiac- or leukocyte-MIF deficiency on the healing process by determining the size of residual necrotic area, collagen deposition, infarct wall thickness and capillary densities. Residual necrotic area was markedly reduced in WT, KOWT and WTKO mice during 7 to 14 days post-MI. However, KOWT mice had much greater necrotic area than WT or WTKO mice at day-7, although no difference remained by day-14 after MI (Table 2). While infarct wall thickness was reduced by 40–50% in all 3 groups from 7 to 14 days, a thicker infarct wall was observed in KOWT mice at 14 days post-MI (Table 2). In contrast, compared to sham-operated hearts of all 3 genotypes (data not shown), there was a marked increase in collagen density in the infarct region in WT mice which was significantly abrogated in both chimeric groups at day-7 post-MI (Table 2). At day-14, while collagen density was further increased in all 3 groups it remained lower in both chimeric groups with a more significant reduction in KOWT than WTKO mice (P<0.05, Table 2). Capillary densities in sham-operated hearts were comparable among the 3 groups, but increased from 7 to 14 days post-MI in WT and WTKO mice, whereas in KOWT mice significantly less increase in capillary density was observed (Table 2). These data suggest a delayed infarct healing in those mice with MIF deficiency in somatic cells but still expressing MIF in bone marrow-derived cells. However by examining these healing-related parameters at 4 weeks after MI, we found there was no difference in collagen content (69±4% vs. 68±5%) or wall thickness (293±22 vs. 290±16 μm) between WTKO and KOWT group. Clearance of necrotic tissues was also complete in both groups. These indicated that although somatic cell/cardiac MIF deficiency delayed the healing process acutely, chronic healing was not impaired.
Table 2.
Histological analysis of post-infarct healing in hearts of WT and chimeric mice (WTKO, KOWT).
| Day-7 MI
|
Day-14 MI
|
|||||
|---|---|---|---|---|---|---|
| WT (n=7–8) | WTKO (n=6–8) | KOWT (n=7) | WT (n=7–8) | WTKO (n=7) | KOWT (n=7–8) | |
|
|
|
|||||
| Infarct Size (%) | 29.6±3.2 | 29.3±3.4 | 31.5±3.7 | 34.6±2.4 | 34.6±3.3 | 34.5±2.6 |
| Residual necrotic area (%infarct area) | 17.8±5.7 | 18.9±3.6 | 30.4±2.2†‡ | 2.6±0.8* | 4.3±1.6* | 4.9±0.9* |
| Infarct wall thickness (μm) | 600±46 | 492±66 | 559±78 | 316±31* | 245±13*† | 344±40*‡ |
| Collagen deposition (% of infarct area) | 24.0±8.0 | 3.8±0.5† | 3.7±0.9† | 59.0±2.0* | 43.7±1.8*† | 38.3±1.4*†‡ |
| Capillary density (vessels No./mm2) | 1689±237 | 1906±188 | 1766±175 | 1881±123 | 2301±201*† | 1812±112‡ |
Values are mean ± SEM,
P<0.05 vs. respective values at day-7 MI,
P<0.05 vs. WT at same time point,
P<0.05 vs. WTKO at the same time point.
WT, Wild Type; WTKO, bone marrow cell/leukocyte-MIF deficient mice; KOWT, somatic cell/heart-MIF deficient mice; MI, myocardial infarction.
MIF expressed in the heart by different cells differentially influences leukocyte infiltration
Immunohistochemical staining revealed a temporal change of infiltrating leukocytes in the infarct myocardium including the border zone in chimeric mice following MI. Although similarly lower versus WT values at day-3 following MI, leukocyte densities in both chimeric groups were markedly increased at day-3 from respective sham values (Figure 5C). Compared to the peak level at day-3 in WT mice, both KOWT and WTKO mice had a lower leukocyte density at the same time point, but peaked at day-7 then declined by day-14 post-MI, corresponding with the extended time window for rupture that was observed (Figure 5A and 5C). Interestingly, KOWT mice displayed increased leukocyte infiltration at day-7 post MI compared to WTKO and WT mice. There were no differences in leukocyte density in the infarct region at day-14 among the 3 groups (Figure 5C).
To explore the linkage between MIF expression and inflammatory cell infiltration, we studied MIF gene and protein expression in the infarct myocardium at similar time points following MI. There was no difference in MIF gene expression between sham-operated WT and WTKO mice, whereas MIF expression in KOWT mice was undetectable (Figure 5D). Following MI, MIF mRNA levels in WT mice were considerably increased and sustained up to 14 days with the peak at day-3, consistent with the temporal pattern of leukocyte infiltration (Figure 5C and D). In WTKO mice, although leukocyte infiltration steadily increased after MI with a peak at day-7 with maintained elevation till day-14 (all P<0.05 vs. sham), MIF mRNA levels did not change during the 2-week study period (Figure 5C and D). In KOWT mice, leukocyte density was significantly increased post MI with a peak at day-7 and then a reduction by day-14 with corresponding upregulation of MIF expression at day-3 and day-7 and significant decrease at day-14 (Figure 5C and D). Notably, although the absolute MIF mRNA level in KOWT mice with MI was significantly lower than other two genotypes, the increment at day-3 and day-7 relative to KOWT sham level (undetectable) was remarkable (Figure 5D). Among the 3 groups, elevation of MIF mRNA level was the highest in WT. Intriguingly, when adding up MIF mRNA values from WTKO and KOWT groups, it was very close to the WT values at day-3 and day-7 post-MI (Figure 5D), indicating that leukocytes are also an important cellular source of regional MIF. Meanwhile Western blotting revealed a different pattern in MIF protein expression. At day-3 post-MI, MIF content in the infarct myocardium of WT and WTKO mice was significantly reduced by approximately 50% compared to their respective sham values, but was restored by day-7 (Figure 5E). While there was a stepwise and drastic increase in MIF protein expression in KOWT mice, in keeping with leukocyte infiltration following MI, the absolute levels were lower relative to WT or WTKO mice (Figure 5E).
Somatic-cell MIF deficiency (KOWT) promoted M1, but attenuated M2 macrophage marker genes post-MI
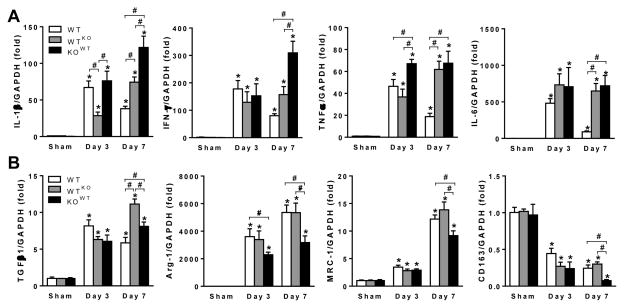
M1 macrophages are considered pro-inflammatory post-MI whereas M2 macrophages facilitate the healing process [32]. We investigated the impact of MIF derived from different cellular sources on macrophage polarisation following MI. Studies using qPCR revealed that cardiac expression of M1 macrophage markers, IL-1β, IFN-γ, TNFα and IL-6 was markedly increased at 3 and 7 days post-MI in all 3 groups. Whilst expression had declined at day-7 from day-3 levels in WT mice, expression of M1 marker genes was either increased or maintained at day-7 in both chimeric groups (Figure 6A). These changes are consistent with peak leukocyte infiltration at day-7 in both chimeric genotypes, particularly in KOWT mice (Figure 5C). For M2 macrophage markers, TGFβ1, Arg-1 and MRC-1 were all increased at both days 3 and 7 after MI whilst CD163 expression was decreased in all 3 groups. Notably, cardiac expression of all M2 macrophage markers in KOWT mice was significantly lower than that of WT or WTKO mice at day 7 (Figure 6B), suggesting that poor post-infarct healing is partly attributable to impaired M2 activation as a consequence of cardiac MIF deficiency (Table 2).
Figure 6. Expression pattern of markers for M1 and M2 macrophages in wild type (WT) and chimeric mice with either bone marrow derived cell-MIF deficiency (WTKO) or somatic cell-MIF deficiency (KOWT) following myocardial infarction (MI).
A, mRNA expression of M1 macrophage markers, interleukin 1β (IL-1β), interferon-γ (IFN-γ), tumor necrosis factor α (TNFα) and IL-6 in WT, WTKO and KOWT mice. B, mRNA expression of M2 macrophage markers, transforming growth factor β1 (TGFβ1), arginase 1 (Arg-1), macrophage mannose receptor 1 (MRC-1) and CD163 in WT, WTKO and KOWT mice. Data expressed as fold change from WT sham expression. n=4 per sham group, n=6 per MI group. *P<0.05 vs. sham, #P<0.05.
Discussion
Using global MIFKO mice or chimeric mice with deficiency of MIF either in cardiac and non-leukocyte tissues, or in bone marrow derived cells, we examined the role of MIF in inflammation, wound healing and cardiac remodelling following MI. First, we observed that global MIF deletion protected against post-MI cardiac rupture and adverse cardiac remodelling. This effect of MIF deficiency was associated with suppressed cardiac inflammatory responses, evidenced by attenuation of leukocyte infiltration, expression of pro-inflammatory molecules and activity of MMP-9 and MMP-2. Second, we observed that MIF is a potent facilitator of the recruitment of macrophages to the site of injury, via mechanisms which depend upon the activation of p38 MAPK and NF-κB. Third, deficiency of MIF in leukocytes lowered risk of post-infarct cardiac rupture, reduced extent of post-infarct LV dilatation and dysfunction, and attenuated inflammatory responses, phenotypes similar to that seen in global MIFKO mice. In contrast, KOWT mice with deficiency of MIF in the heart (and other non-leukocyte tissues) but expressing MIF in leukocytes, had a higher incidence of cardiac rupture and delayed healing associated with delayed leukocyte infiltration and enhanced M1 but impaired M2 macrophage marker expression. These results suggest distinct roles of cardiac and leukocyte MIF in regulating post-MI inflammation, healing and cardiac remodelling.
Although a number of experimental studies, including ours, have investigated the influence of MIF in ischemic heart injury [13–17], no previous study has defined the role of MIF in post-infarct healing and cardiac remodelling. Coronary artery occlusion in mice results in severe ischemic injury, strong inflammatory responses and cardiac remodelling, which mimic the clinical scenario of MI in humans. Using this model, we demonstrated that global MIF deletion protected the heart from acute wall rupture during the first week post MI. Further, echocardiography showed that by 4 weeks after MI, global MIFKO mice had smaller LV dimensions and preserved contractile function, confirming a detrimental role of MIF in post-infarct pathophysiology.
The inflammatory response following MI is critical for the removal of cellular debris and for fibrotic healing [6, 33]. However, excessive inflammatory responses and subsequent ECM degradation due to activation of MMPs, particularly MMP-9 and MMP-2, contribute to acute LV rupture and chronic chamber dilatation [34–36]. When compared to WT counterparts, global MIFKO mice had a lower risk of cardiac rupture and less severe LV dilatation and dysfunction. This was associated with reduced myocardial leukocyte infiltration, and suppressed expression of chemokines, pro-inflammatory cytokines and adhesion molecules, whereas expression of the anti-inflammatory cytokine, IL-10, was upregulated in MIFKO mice. These contributions of MIF to post-MI inflammation are similar to that we previously reported in the setting of prolonged cardiac ischemia-reperfusion [17]. MIF has been shown to upregulate MMP-9 expression in osteoblasts to promote bone resorption [37] and in macrophages to destabilize atherosclerotic plaques [38]. Our current study revealed that global MIF deletion attenuated expression and activity of MMP-9 and MMP-2 in the heart following MI. In keeping with these findings, our recent study demonstrated that inhibitory interventions using an anti-MIF monoclonal antibody or a small molecule inhibitor of MIF ex vivo attenuated the expression of MIF, MMP-9 and IL-6 by cultured peripheral blood mononuclear cells from human MI patients [39].
Macrophages from WT mice displayed significantly decreased chemotaxis in response to MIFKO infarct tissue, suggesting the importance of MIF located in the infarct myocardium in the recruitment of inflammatory cells. Similarly, MIFKO macrophages exhibited reduced capability of migration, indicating that both myocardial and leukocyte sources of MIF contribute to regional infiltration of immune cells following MI. This finding is congruent with recent reports showing that MIF increases leukocyte–endothelial interactions in human endothelial cells via promoting expression of adhesion molecules [40], and that MIF possesses chemokine-like function acting as a major regulator of inflammatory cell recruitment and atherogenesis [18]. It is also consistent with two recent reports showing that MIF facilitates chemokine- or inflammation-induced migration of macrophages and neutrophils [41, 42].
p38 and JNK are major MAPK family members and play a vital role in myocardial injury through promoting cardiomyocyte apoptosis and inflammation [31, 43, 44]. The MAPK pathways and the NF-κB signal transduction cascade, are activated by cellular stress signals, such as inflammatory cytokines. MIF has been reported to activate p38 MAPK in rheumatoid arthritis [44] and colitis [45] or JNK in T lymphocytes and fibroblasts [46], and has been shown to enhance NF-κB activation in endothelial cells [40]. We therefore investigated the potential involvement of p38 MAPK and JNK as well as NF-κB signalling in MIF-mediated inflammatory action. We observed that global MIFKO hearts had reduced phosphorylation of both p38 MAPK and JNK. Interestingly, in the in vitro trans-well migration assays, migration of WT macrophages in response to exogenous MIF was abrogated by pre-treatment of macrophages with inhibitors of p38 MAPK or NF-κB, suggesting that these intracellular pathways are essential for MIF-mediated macrophage migration.
Previous studies reported protection of MIF against myocardial injury following a brief ischemia episode [13–16]. In the present study using MI model, as well as in our previous study using a prolonged ischemia/reperfusion model [17], we demonstrated a detrimental effect of MIF in MI via promotion of inflammatory responses and subsequent cardiac remodelling. As MIF is expressed in a variety of cell types including inflammatory cells [18] and cardiomyocytes [19], it is important to know whether MIF derived from different sources has different actions in these phenomena. We created chimeric mice with either somatic-cell (KOWT) or bone-marrow derived-cell (WTKO) MIF deficiency, to elucidate the contribution of MIF from these pools following MI. Compared to WT control mice, WTKO mice were largely protected from post-MI cardiac rupture; in contrast, KOWT mice had no protection against rupture, which was accompanied by more severe LV dilatation and dysfunction. Further, distinct temporal changes in leukocyte infiltration and MIF gene expression after MI were also observed. Notably, the temporal change in MIF gene expression in the infarct myocardium of WT and KOWT mice corresponded with the changes in leukocyte infiltration. WT mice had the highest leukocyte density and MIF gene expression in the infarct myocardium among the 3 groups. As leukocytes in WTKO mice do not express MIF, such infiltration did not affect MIF mRNA levels post MI. In contrast, leukocytes in KOWT mice express MIF thereby contributing to elevated MIF mRNA levels. Thus, infiltrated inflammatory cells are a predominant cellular source of MIF in the infarct myocardium of WT and KOWT mice at the time points studied. While in WTKO hearts, cardiac cells are the only cellular source of MIF. Interestingly, different from MIF at mRNA level, MIF protein content in both WT and WTKO groups was decreased at day-3, relative to respective sham values, and restored at day-7 post MI. The reason for such dynamic changes may be due to release of cardiac MIF, in a large quantity, upon severe ischemic insult [47] albeit MIF gene transcription had already been upregulated. By day-7, upregulated MIF gene expression from various cells restored tissue MIF content, a similar phenomenon as previously reported [48]. Whereas, in KOWT hearts, infiltrated leukocytes are the only source of MIF post MI, a steady increase in MIF protein level was associated with gradually increased leukocyte infiltration. Thus, deletion of MIF from immune cells (WTKO) diminished MIF expression in the infarct myocardium, consistent with reduced inflammatory infiltration, cardiac rupture risk and attenuated adverse remodelling. Whereas, deficiency of MIF in somatic-cells or in the heart (KOWT) resulted in delayed inflammatory response and extended the time window for rupture occurrence.
Macrophage polarisation results in dual actions of macrophages in inflammation and healing. Classically activated macrophages (M1) are recruited quickly into the damaged myocardium mediating further production of an array of pro-inflammatory mediators and cellular infiltrates [49, 50]. In contrast, alternatively activated macrophages (M2) accumulate later in the injured site, acting to terminate inflammation and promote synthesis of growth factors for angiogenesis and fibrotic healing [51, 52]. Compared to WTKO counterparts, KOWT mice displayed poor wound healing evidenced by increased necrotic area, greater infarct wall thickness, and decreased collagen deposition or capillary density at day-7 and day-14 post-MI, implying an essential role of cardiac MIF in post-infarct healing. Moreover, KOWT mice but not WTKO mice exhibited diminished activation of M2 macrophages indicated by down-regulated expression of M2 macrophage markers. These findings were in keeping with greater leukocyte infiltration, higher risk of rupture and adverse cardiac remodelling and dysfunction in KOWT versus WTKO mice. However, although global MIFKO mice had moderately delayed healing indicated by a lower collagen deposition and thicker infarct wall versus WT mice at day-14, there was no difference in all healing parameters studied at 7 days and in necrosis absorption at 14 days between MIFKO and WT mice. Further, collagen deposition and gene expression of TGFβ, α-SMA by cultured cardiac fibroblasts isolated from the infarct tissue at day-4 post MI were similar between MIFKO and WT mice though collagen-1 mRNA level were higher in MIFKO fibroblasts. These observations suggest that MIF had a modest influence on the early healing process. It is worthwhile to point out that incidence of cardiac rupture follows the order of WT > KOWT > global MIFKO ≥ WTKO mice. WTKO mice showed a trend to a lower rupture risk compared to global MIF deficiency, possibly due to a loss of protective endogenous cardiac MIF in the latter. Collectively, results from chimeric studies indicate that intrinsic cardiac MIF facilitates healing while inflammatory cell-derived MIF is detrimental to the heart following MI. These different effects may also partly explain the discrepancy between the findings of studies on the effects of MIF on ischemia-related events depending on the timing of observation, as cardiac healing effects and inflammatory effects, while coupled, are not simultaneous.
In conclusion, global MIF deletion attenuates MI-induced inflammatory responses thereby protecting the heart from cardiac rupture and adverse remodelling. Leukocyte-derived MIF promotes inflammatory infiltration, whereas cardiac-derived MIF affects healing although such influence on early healing process is minimal. The distinction between the contribution of cardiac, compared to leukocyte, MIF, may be important in considering anti-MIF therapeutic interventions, which would be likely to target both leukocyte and non-leukocyte MIF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants (ID 472628, ID 1004235) from the National Health and Medical Research Council (NHMRC) of Australia and the Victorian Government’s Operational Infrastructure Support Program. D.W. was a recipient of Australian National Heart Foundation PhD Scholarship (PB 10M 5481). A.M.D. and X.J.D. were NHMRC research fellows.
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474–81. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 7.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 11.Morand EF, Bucala R, Leech M. Macrophage migration inhibitory factor: an emerging therapeutic target in rheumatoid arthritis. Arthritis Rheum. 2003;48:291–9. doi: 10.1002/art.10728. [DOI] [PubMed] [Google Scholar]
- 12.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–4. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 13.Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–92. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–82. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 15.Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest. 2009;119:3807–16. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga K, Kenessey A, Powell SR, Sison CP, Miller EJ, Ojamaa K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid Redox Signal. 2011;14:1191–202. doi: 10.1089/ars.2010.3163. [DOI] [PubMed] [Google Scholar]
- 17.Gao XM, Liu Y, White D, Su Y, Drew BG, Bruce CR, et al. Deletion of macrophage migration inhibitory factor protects the heart from severe ischemia-reperfusion injury: a predominant role of anti-inflammation. J Mol Cell Cardiol. 2011;50:991–9. doi: 10.1016/j.yjmcc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Nishihira J, Shimpo M, Mizue Y, Ueno S, Mano H, et al. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res. 2001;52:438–45. doi: 10.1016/s0008-6363(01)00408-4. [DOI] [PubMed] [Google Scholar]
- 20.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, et al. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci U S A. 2003;100:9354–9. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao XM, Dilley RJ, Samuel CS, Percy E, Fullerton MJ, Dart AM, et al. Lower risk of postinfarct rupture in mouse heart overexpressing beta 2-adrenergic receptors: importance of collagen content. J Cardiovasc Pharmacol. 2002;40:632–40. doi: 10.1097/00005344-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–77. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res. 2000;45:330–8. doi: 10.1016/s0008-6363(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 24.Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007;43:535–44. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, et al. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens. 2006;24:1663–70. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res. 2013;112:675–88. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao XM, Ming Z, Su Y, Fang L, Kiriazis H, Xu Q, et al. Infarct size and post-infarct inflammation determine the risk of cardiac rupture in mice. Int J Cardiol. 2010;143:20–8. doi: 10.1016/j.ijcard.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, et al. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 29.Chen LL, Zhu TB, Yin H, Huang J, Wang LS, Cao KJ, et al. Inhibition of MAPK signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through reduction of inflammation. Mol Biol Rep. 2010;37:3067–72. doi: 10.1007/s11033-009-9879-6. [DOI] [PubMed] [Google Scholar]
- 30.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–9. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. The Journal of biological chemistry. 1996;271:24313–6. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 33.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–42. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 37.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, et al. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. The Journal of biological chemistry. 2002;277:7865–74. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 38.Kong YZ, Yu X, Tang JJ, Ouyang X, Huang XR, Fingerle-Rowson G, et al. Macrophage migration inhibitory factor induces MMP-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis. 2005;178:207–15. doi: 10.1016/j.atherosclerosis.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 39.White DA, Fang L, Chan W, Morand EF, Kiriazis H, Duffy SJ, et al. Pro-Inflammatory Action of MIF in Acute Myocardial Infarction via Activation of Peripheral Blood Mononuclear Cells. PLoS One. 2013;8:e76206. doi: 10.1371/journal.pone.0076206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Q, McKeown SJ, Santos L, Santiago FS, Khachigian LM, Morand EF, et al. Macrophage migration inhibitory factor increases leukocyte-endothelial interactions in human endothelial cells via promotion of expression of adhesion molecules. J Immunol. 2010;185:1238–47. doi: 10.4049/jimmunol.0904104. [DOI] [PubMed] [Google Scholar]
- 41.Fan H, Hall P, Santos LL, Gregory JL, Fingerle-Rowson G, Bucala R, et al. Macrophage migration inhibitory factor and CD74 regulate macrophage chemotactic responses via MAPK and Rho GTPase. J Immunol. 2011;186:4915–24. doi: 10.4049/jimmunol.1003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos LL, Fan H, Hall P, Ngo D, Mackay CR, Fingerle-Rowson G, et al. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum. 2011;63:960–70. doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–91. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 44.Santos LL, Lacey D, Yang Y, Leech M, Morand EF. Activation of synovial cell p38 MAP kinase by macrophage migration inhibitory factor. J Rheumatol. 2004;31:1038–43. [PubMed] [Google Scholar]
- 45.Ishiguro Y, Ohkawara T, Sakuraba H, Yamagata K, Hiraga H, Yamaguchi S, et al. Macrophage migration inhibitory factor has a proinflammatory activity via the p38 pathway in glucocorticoid-resistant ulcerative colitis. Clin Immunol. 2006;120:335–41. doi: 10.1016/j.clim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Lue H, Dewor M, Leng L, Bucala R, Bernhagen J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal. 2011;23:135–44. doi: 10.1016/j.cellsig.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan W, White DA, Wang XY, Bai RF, Liu Y, Yu HY, et al. Macrophage migration inhibitory factor for the early prediction of infarct size. J Am Heart Assoc. 2013;2:e000226. doi: 10.1161/JAHA.113.000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu CM, Lai KW, Chen YX, Huang XR, Lan HY. Expression of macrophage migration inhibitory factor in acute ischemic myocardial injury. J Histochem Cytochem. 2003;51:625–31. doi: 10.1177/002215540305100508. [DOI] [PubMed] [Google Scholar]
- 49.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 50.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, et al. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–46. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–97. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.