Abstract
A systematic review and meta-analyses were performed to identify the risk factors associated with carbapenem-resistant Pseudomonas aeruginosa and to identify sources and reservoirs for the pathogen. A systematic search of PubMed and Embase databases from 1 January 1987 until 27 January 2012 identified 1,662 articles, 53 of which were included in a systematic review and 38 in a random-effects meta-analysis study. The use of carbapenem, use of fluoroquinolones, use of vancomycin, use of other antibiotics, having medical devices, intensive care unit (ICU) admission, having underlying diseases, patient characteristics, and length of hospital stay were significant risk factors in multivariate analyses. The meta-analyses showed that carbapenem use (odds ratio [OR] = 7.09; 95% confidence interval [CI] = 5.43 to 9.25) and medical devices (OR = 5.11; 95% CI = 3.55 to 7.37) generated the highest pooled estimates. Cumulative meta-analyses showed that the pooled estimate of carbapenem use was stable and that the pooled estimate of the risk factor “having medical devices” increased with time. We conclude that our results highlight the importance of antibiotic stewardship and the thoughtful use of medical devices in helping prevent outbreaks of carbapenem-resistant P. aeruginosa.
INTRODUCTION
Pseudomonas aeruginosa is one of the most common nosocomial pathogens (1). P. aeruginosa can cause infections in patients with serious underlying disorders, such as a suppressed immune system or cystic fibrosis (CF), or in patients in intensive care units (ICU) (2, 3). Further, infections with P. aeruginosa in such patients lead to increased morbidity and mortality (2–4).
P. aeruginosa is intrinsically resistant to various antibiotics and is capable of acquiring additional resistance by either chromosomal mutations or horizontal gene transfer (5). The most important mechanisms are loss or alteration of outer membrane porins and increased efflux pump activity (6–8). The emergence of multidrug-resistant (MDR) P. aeruginosa is a problem of global concern, and there are currently reports of hospital outbreaks of MDR P. aeruginosa from countries around the world, including the Netherlands (9–13). These outbreaks are frequently caused by Pseudomonas aeruginosa clones with metallo-β-lactamases, such as Verona integron-encoded metallo-β-lactamase (VIM) and imipenemase (IMP). Importantly, outbreaks may be large and sustained, despite the adoption of infection control measures (12, 14).
In 2006, a summary on this subject was published by Falagas and Kopterides, who published a systematic review of the problem (15). However, there have been many more published reports regarding nosocomial (MDR) P. aeruginosa since 2006. Therefore, in the current publication, a more extensive and up-to-date systematic review was performed, focusing on carbapenem resistance and non-CF patients and including conventional and cumulative meta-analyses. The aim of the analysis was to answer the following two questions. First, what are the risk factors for the presence of carbapenem-resistant P. aeruginosa among hospitalized patients? Second, what environmental sources and/or reservoirs were identified in these outbreaks? This knowledge will be useful for worldwide health care centers that are facing the threat of MDR P. aeruginosa and will help in designing strategies to stop the emergence of spread of these MDR pathogens.
MATERIALS AND METHODS
The systematic review and meta-analyses presented in this publication include all of the items in the checklist detailed in the PRISMA guideline (16).
Study and data collection.
Eligible articles were identified by searching PubMed (Medline) and Embase databases. Additional articles were identified by hand searching the reference lists of included reviews. Searches were performed for the period from 1 January 1987 until 27 January 2012. Search terms included “Pseudomonas” as a title word, in combination with the keywords “resistant,” “multidrug resistance,” “VIM,” “IMP,” “metallo-beta-lactamase” or “MBL” and “risk factors,” “determinants,” “outbreak,” “transmission,” “nosocomial,” “health care related,” “health care associated,” “epidemiology,” or “source,” including all possible ways of writing. The authors included peer-reviewed articles relating to carbapenem-resistant P. aeruginosa that also described the risk factors associated with the presence of carbapenem-resistant P. aeruginosa using a multivariate model and in which a nosocomial infection was described. We excluded studies relating to nonhuman infections, studies that included only patients with CF, reviews, commentaries, editorials, letters, and abstracts. We also excluded studies published before 1987, the year of the U.S. approval of imipenem (17). Environmental sources and reservoirs were searched for in both included and excluded studies. A study was excluded from the meta-analyses (i) if it reported only hazard ratios, (ii) if it reported only prevalence ratios or risk ratios, (iii) when confidence intervals were missing, and (iv) if it included only patients with P. aeruginosa bacteremia.
We extracted detailed information from the included studies. We based the classification of studies regarding the different study designs on the description of the methods in a particular study, not on the study design claimed to be used by the authors (e.g., a reported retrospective cohort study can methodologically be a case-control study). We contacted the corresponding and/or first authors of 47 articles by e-mail in order to retrieve the full-text articles or to retrieve missing information.
Study quality.
To assess the quality, risk of bias, and generalizability of the included studies, a quality assessment was performed using the STROBE guidelines for included cross-sectional studies as well as the Newcastle-Ottawa quality assessment scale for included case-control and cohort studies (18, 19). The quality of the studies was not considered an exclusion criterion.
Statistical analysis.
We merged all reported risk factors with a reported odds ratio (OR) and 95% confidence interval (95% CI) into 10 different groups: group 1, carbapenem use; group 2, quinolone use; group 3, vancomycin use; group 4, other antibiotic use; group 5, medical devices; group 6, ICU admission; group 7, underlying diseases; group 8, patient characteristics; group 9, length of hospital stay; and group 10, other. We selected the 10 groups using the results of the systematic review. For each of the first nine groups, a meta-analysis was performed. That was not possible for group 10 (other), as the risk factors were too diverse. An additional meta-analysis was performed for the risk factors quinolone use, vancomycin use, and other antibiotic use together. All meta-analyses were performed using StatsDirect statistical software (Altrinchem, United Kingdom). The risk factors reported by the studies included in the analyses were diverse; therefore, a random-effects model was fitted to the data based on the method of DerSimonian and Laird (20). A P value of <0.05 was considered statistically significant, and no correction was made for multiple testing. The risk of publication bias across the studies was assessed by the Egger and Begg-Mazumdar (Kendall's tau) indicators. Both bias indicators had to show a significant result before it was concluded that publication bias was present. Additionally, two cumulative meta-analyses were performed for groups 1 (carbapenem use) and 2 (medical devices), as these two groups showed highly significant results using conventional meta-analyses. A random-effects model, based on the method of DerSimonian and Laird, was also fitted to these cumulative meta-analyses (20).
RESULTS
Description of included studies.
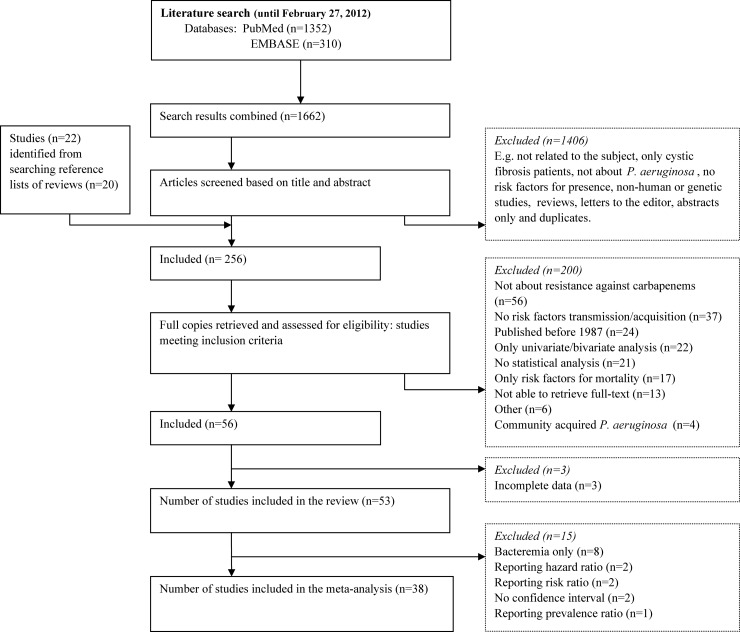
A total of 1,662 articles were identified when the search results of PubMed and Embase were combined (Fig. 1). After applying exclusion criteria as described in Materials and Methods, 256 articles were read in their entirety (full text) (Fig. 1). The corresponding and/or first authors of 47 of 256 articles were contacted by e-mail. Authors from 19 of 47 articles responded to e-mail requests. Nine full-text articles were received by mail or e-mail, and from four articles missing information was retrieved. For two articles, the requested information was not available. Fifty-three studies were finally included in the analyses after exclusion of articles that did not meet our inclusion criteria as described in Materials and Methods (Fig. 1). These studies represented 3,966 patient cases (ranging from 5 to 345 cases per publication) from 15 different countries (Tables 1 and 2). Eight of 53 of the studies included patients with bacteremia only and are shown in Table 2. All 53 studies had an observational study design and were written in English. Eight studies reported that a retrospective cohort study was performed, whereas conceptually they could be considered case-control studies. Five multicenter studies were also included. The percentage of male gender ranged from 38.6% to 84.0%. Patient age ranged from several days old (neonates) to very old, with 97 years as oldest.
FIG 1.
Flow diagram of study selection for the systematic review on carbapenem-resistant P. aeruginosa.
TABLE 1.
Sources and characteristics of included studies (n = 45) and risk factors for transmission and acquisition of carbapenem-resistant P. aeruginosa based on multivariate analysesa
| Risk factor | No. offactors | Sourcesb | No. of cases |
Case-control studies |
|
|---|---|---|---|---|---|
| Range | No. | OR range | |||
| Carbapenem use | 19 | Harris, 2011 (33); Lautenbach, 2010 (34); Lepelletier, 2010 (35); Cezario, 2009 (36); Mueller, 2008 (37); Onguru, 2008 (27); Pena, 2007 (38); Mentzelopoulos, 2007 (39); Fortaleza, 2006 (25); Ohmagari, 2005 (40); Ozkurt, 2005 (41); 2× Zavascki, 2005 (42); Cao, 2004 (43); Harris, 2002 (26); Troillet, 1997 (44); Carmeli, 1999 (45); Lodise, Jr., 2007 (46); Montero, 2010 (47) | 5–354 | 12 | 3.6–76.0 |
| Quinolone use | 11 | van der Bij, 2011 (12); Kohlenberg, 2010 (48); Pena, 2009 (49); Yang, 2009 (50); Pena, 2007 (38); Lautenbach, 2006 (51); Zavascki, 2006 (52); Nouer, 2005 (53); Defez, 2004 (54); Lodise, Jr., 2007 (46); Montero, 2010 (47) | 15–354 | 5 | 2.5–48.4 |
| Vancomycin use | 3 | Harris, 2002 (26); Fortaleza, 2006 (25); Onguru, 2008 (27) | 75–120 | 3 | 1.8–2.9 |
| Other antibiotic use | 18 | 2× Furtado, 2010 (55); Lepelletier, 2010 (35); 2× Martinez, 2009 (56); 2× Onguru, 2008 (27); 2× Aloush, 2006 (57); Fortaleza, 2006 (25); Zavascki, 2006 (52); Nouer, 2005 (53); Ozkurt, 2005 (41); Zavascki, 2005 (42); Defez, 2004 (54); 2× Harris, 2002 (26); Richard, 1994 (58) | 15–120 | 9 | 2.2–43.7 |
| Medical devices | 21 | Nagao, 2011 (59); Park, 2011 (60); Kohlenberg, 2010 (48); 2× Cezario, 2009 (36); Cortes, 2009 (61); Fortaleza 2009 (62); Martinez, 2009 (56); Pena, 2009 (49); Mueller, 2008 (37); Onguru, 2008 (27); Pereira, 2008 (63); Zavascki, 2005 (42); 2× Defez, 2004 (54); Cao, 2004 (43); 2× Dropulic, 1995 (64); Talon, 1995 (65); Thuong, 2003 (66); Lodise, Jr., 2007 (46) | 6–204 | 13 | 2.1–64.3 |
| ICU admission | 8 | van der Bij, 2011 (12); Lepelletier, 2010 (35); Eagye, 2009 (67); Furtado, 2009 (68); Mueller, 2008 (37); Aloush, 2006 (57); Zavascki, 2006 (52); Harris, 2002 (26) | 35–120 | 5 | 1.1–13.3 |
| Underlying disease | 12 | Furtado, 2010 (55); 3× Fortaleza 2009 (62); Pena, 2007 (38); 3× Zavascki, 2006 (52); Fortaleza, 2006 (25); Ohmagari, 2005 (40); Troillet, 1997 (44); Talon, 1995 (65) | 17–260 | 6 | 1.0–25.0 |
| Patient characteristics | 19 | Park, 2011 (60); 2× Furtado, 2010 (55); Lepelletier, 2010 (35); 2× Eagye, 2009 (67); Cezario, 2009 (36); Aloush, 2006 (57); Zavascki, 2005 (42); Ohmagari, 2005 (40); 2× Defez, 2004 (54); Berthelot, 2001 (69); Carmeli, 1999 (2); 2× Mammina, 2008 (70); 3× Montero, 2010 (47) | 18–354 | 10 | 1.0–13.9 |
| Length of hospital stay | 13 | Harris, 2011 (33); Furtado, 2010 (55); Lautenbach, 2010 (34); Lepelletier, 2010 (35); Yang, 2009 (50); Pereira, 2008 (63); Onguru, 2008 (27); Ozkurt, 2005 (41); Harris, 2002 (26); Carmeli, 1999 (2); 2× Montero, 2010 (47); Arruda, 1999 (71) | 20–354 | 8 | 1.0–6.7 |
| Other | 18 | van der Bij, 2011 (12); Harris, 2011 (33); Lautenbach, 2010 (34); Furtado, 2010 (55); Montero, 2010 (47); Pena, 2009 (49); 2× Aloush, 2006 (57); Fortaleza, 2006 (25); Zavascki, 2006 (52); 2× Ozkurt, 2005 (41); 2× Defez, 2004 (54); Paramythiotou, 2004 (72); Berthelot, 2001 (69); Carmeli, 1999 (45); Dropulic, 1995 (64) | 34–354 | 10 | 1.7–13.2 |
From the initial 53 studies, those focused on only patients with bacteremia (n = 8) were excluded. OR, odds ratio.
Sources are identified by first author, year, and reference number. 2× or 3×, two or three different factors per reference.
TABLE 2.
Summary of studies (n = 8) regarding P. aeruginosa bacteremia, reporting risk factors for transmission and acquisition of carbapenem-resistant P. aeruginosa, based on multivariate analysesa
| Studyc | Country | Study design | Hospital setting | No. of cases | Quality scoreb | Risk factors |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| For what | Factor | OR estimate | 95% CI | P value | ||||||
| Joo, 2011 (73) | South Korea | cc | mix | 46 | 4 | imp | Aminoglycoside use | 3.60 | 1.39–7.31 | 0.025 |
| Urinary catheter | 3.19 | 1.39–7.31 | 0.006 | |||||||
| Carbapenem use | 2.87 | 1.26–6.56 | 0.012 | |||||||
| Fluoroquinolone use | 2.54 | 1.08–5.96 | 0.033 | |||||||
| Tumbarello, 2011 (74) | Italy | cc | mix | 106 | 6 | mr | Central venous catheter | 17.99 | 6.45–50.09 | <0.001 |
| Previous antibiotic therapy | 2.79 | 1.10–7.07 | 0.03 | |||||||
| Corticosteroid use | 2.73 | 1.06–7.00 | 0.03 | |||||||
| Yang, 2011 (75) | South Korea | cc | pea | 7 | 4 | mr | Admission to ICU | 6.82 | 1.3–35.8 | 0.023 |
| Johnson, 2009 (76) | USA | rc | mix | 113 | 7 | mr | Hospital-acquired BSI | 2.41 | 1.39–4.18 | 0.002 |
| Previous transplantation | 2.38 | 1.51–3.76 | <0.001 | |||||||
| Admission to ICU | 2.04 | 1.15–3.63 | 0.015 | |||||||
| Tam, 2007 (77) | USA | cc | mix | 18 | 4 | car | Additional wk of hospitalization | 1.25 | 1.04–1.51 | 0.019 |
| Falagas, 2006 (78) | Greece | cc | mix | 16 | 4 | mr | Carbapenem use | 9.0 | 2.4–34.3 | 0.001 |
| Kang, 2005 (79) | South Korea | rc | mix | 28 | 6 | imp | Carbapenem use | 40.96 | 8.92–188.3 | <0.001 |
| Fluoroquinolone use | 5.60 | 1.64–19.11 | 0.006 | |||||||
| Invasive procedure within previous 72 h | 4.51 | 1.56–13.04 | 0.005 | |||||||
| El Amari, 2001 (80) | Switzerland | cc | mix | 81 | 4 | mr | Previous monotherapy (including imipenem) | 2.5 | 1.3–4.8 | 0.006 |
OR, odds ratio; CI, confidence interval; cc case control; rc, retrospective cohort; mix, mixed; pea, pediatric general; imp, imipenem; mr, multiresistance, including carbapenems; car, carbapenem; BSI, bloodstream infection.
According to the Newcastle-Ottowa quality assessment scale.
Studies are reported by first author, year, and reference number.
Not all studies provided detailed information regarding the microbiological methods used. However, 23 of the 53 studies did describe the method used for the identification of P. aeruginosa, of which 10 studies used the Vitek system. Only 19 of the 53 studies described isolate genotyping, with 16 studies using pulsed-field gel electrophoresis (PFGE), 1 using multiple-locus variable-number tandem repeat analysis (MLVA), 1 using restriction fragment length polymorphism (RFLP), and 1 using repetitive-element-based PCR. The median number of cases, as included in the multivariate analyses in these 19 studies, was 30 (ranging from 6 to 204 cases). The median number of genetically identical clusters identified was 2 (ranging from 1 to 8). The median size of the clusters described in these genotyping studies was 4 (ranging from 2 to 47). Seven of the 53 studies also identified the presence of blaVIM and blaIMP genes (using PCR amplification). The average number of cases in these seven studies was 32 (ranging from 5 to 47 cases).
The statistically significant risk factors calculated from the multivariate analyses, specifically the presence of carbapenem-resistant P. aeruginosa (Table 1), were extracted and merged into 10 different classes. The definitions of the risk factors from the different studies were not uniform.
When considering all statistically significant risk factors from the multivariate analyses of 45 studies (n) that had not included “only bacteremic patients,” it was observed that the presence of medical devices was the most reported risk factor (n = 21) (Table 1). The risk factors extracted from the eight studies including only patients with bacteremia are shown in Table 2. Eight of the 53 studies not only identified risk factors but also identified protective factors for presence of a carbapenem-resistant P. aeruginosa, including quinolone use, exclusive feeding by formula, and duration of antibiotic treatment (Table 3).
TABLE 3.
Summary of studies reporting protective factors for transmission and acquisition of carbapenem-resistant P. aeruginosa, based on multivariate analyses
| Studya | Country | Risk factor resultsb |
||||
|---|---|---|---|---|---|---|
| Risk factor | Risk estimate | 95% CI | P value | |||
| van der Bij, 2011 (12) | Netherlands | Cystic fibrosis as an underlying disease | OR | 0.10 | 0.1–0.6 | NR |
| Fortaleza, 2009 (62) | Brazil | Quinolone use | OR | 0.13 | 0.03–0.47 | 0.002 |
| Martinez, 2009 (56) | Spain | Quinolone use | OR | 0.27 | 0.1–0.7 | NR |
| Martinez, 2009 (56) | Spain | Antipseudomonal cephalosporin use | OR | 0.27 | 0.08–0.9 | NR |
| Mammina, 2008 (70) | Italy | Exclusive feeding by formula | HR | 0.18 | 0.05–0.61 | 0.006 |
| Mammina, 2008 (70) | Italy | Length of stay of >2 weeks | HR | 0.10 | 0.00–0.11 | 0.011 |
| Lodise, Jr., 2007 (46) | USA | Risk factor 1 + 2 + 3c | PR | 0.60 | 0.4–0.9 | 0.02 |
| Aloush, 2006 (57) | Israel | Having a malignant disease | OR | 0.20 | 0.05–0.9 | 0.03 |
| Berthelot, 2001 (69) | France | Duration of antibiotic treatment | OR | 0.78 | 0.69–0.87 | NR |
| Arruda, 1999 (71) | Brazil | Number of antimicrobial drugs | OR | 0.33 | NR | 0.006 |
Studies are reported by first author, year, and reference number.
OR, odds ratio; HR, hazard ratio; PR, prevalence ratio; CI, confidence interval; NR, not reported.
Combination of risk factors: 1, prior receipt of mechanical ventilation for 11 days or more; 2, prior carbapenem exposure for 3 days or more; 3, prior fluoroquinolone exposure of 3 days or more.
Possible sources and reservoirs.
Several environmental sources and reservoirs were identified (Table 4). In some outbreaks, a single source could be identified (e.g., a damaged bronchoscope or a contaminated automated urine collection machine), and the outbreak stopped after removing, repairing, or cleaning this source. However, often a reservoir was identified that was possibly not actually the main source of infection but rather a consequence of the presence of a colonized or infected patient that had led to contamination of the environment (e.g., via sinks or mattresses).
TABLE 4.
Environmental sources and reservoirs identified when searching 1,662 + 22 studies for carbapenem-resistant P. aeruginosa
| Environmental source/reservoir | Reference(s)a |
|---|---|
| Automated urine analyzer | Hallin, 2012 (81); Nagao, 2011* (59) |
| Urine vol-measuring device | Sekiguchi, 2007 (82) |
| Air-conditioning system | Pinna, 2009 (83) |
| Sinks | Kouda, 2011 (84); Babu, 2011 (85); Crivaro, 2009 (86); Hota, 2009 (87); Mayank, 2009 (88); Crespo, 2004 (89); Boutiba-Ben Boubaker, 2003 (90); Bertrand, 2000 (91); Bert, 1998 (92); Griffith, 1989 (93) |
| Scopes | Boutiba-Ben Boubaker, 2003 (90). Bronchoscope, DiazGranados, 2009 (94); Sorin, 2001 (95); Panzig, 1999 (96). ERCP scope, Fraser, 2004 (97). Endoscope, Pitten, 2001 (98) |
| Water tap | Mentzelopoulos, 2007* (39); Bukholm, 2002 (99) |
| Trap water | Leung, 2008 (100) |
| Tap water | Mayank, 2009 (88); Pitten, 2001 (98); Bert, 1998 (92) |
| Sanitation related contamination | Kouda, 2011 (84); Panzig, 1999 (96); Verweij, 1997 (101) |
| Contaminated patient room | Kouda, 2011 (84); Cezario, 2009* (36); Mayank, 2009 (88); Boutiba-Ben Boubaker, 2003 (90); Landman, 2002 (102) |
| Positive cultures from nurses | Crivaro, 2009 (86); Mayank, 2009 (88); Vilar-Compte, 2003 (103); Bertrand, 2000 (91); Zheng, 1990 (104) |
| Bed pan sterilizer | Verweij, 1997 (101) |
| Milk bank pasteurizer | Gras-Le Guen, 2003 (105) |
| Bottle warmer | Gras-Le Guen, 2003 (105) |
| Stethoscope | Crespo, 2004 (89) |
| Mechanical ventilation related | Cezario, 2009* (36); Kikuchi, 2007 (106); Landman, 2002 (102) |
| Suction apparatus | Babu, 2011 (85); Mentzelopoulos, 2007* (39); Bertrand, 2000 (91) |
| Ice packs | Bertrand, 2000 (91) |
| Mops | Babu, 2011 (85) |
| O2 bottles, O2 tubing | Mayank, 2009 (88) |
| Contaminated cystoscopy room | Pena, 2003 (107) |
| Contaminated urodynamic lab | Climo, 1997 (108) |
References are reported by first author, year, and reference number. Studies followed by an asterisk were included in the systematic review.
Study quality.
For all included studies (n = 53), a quality assessment was performed. Validation of case-control studies (n = 38) according to the Newcastle-Ottawa quality assessment scale resulted in all studies scoring between 4 and 6 stars of a possible 10 (19). However, the validation of cohort studies (n = 12) according to the Newcastle-Ottawa quality assessment scale resulted in scores between 6 and 7 stars of 13 (19). The most important reasons for not awarding a star were (i) the use of hospital controls, (ii) the use of medical records, (iii) no information about follow-up of patients, and (iv) different matching criteria between studies. Validation of the two cross-sectional studies and the single study with an observational study design, all according to STROBE guidelines, resulted in scores of 15, 17, and 18 points of a total of 22, respectively (18). The main reasons not to award points in these analyses were due to the limited description of the statistical analysis in the methods and results sections of the articles.
Nine meta-analyses.
Thirty-eight of 53 studies were included in the 9 conventional meta-analyses, reporting 106 risk factors and 5 protective factors. Eight studies were excluded because only risk factors for P. aeruginosa bacteremia were reported. Five studies were excluded because they reported hazard ratios (n = 2), risk ratios (n = 2), and a prevalence ratio (n = 1). Two studies were excluded because of missing confidence intervals. Thus, nine different meta-analyses were performed, plus an additional meta-analysis combining three risk factors (quinolone use, vancomycin use, and other antibiotic use). The results of the nine meta-analyses are shown in Table 5, and their forest plots are shown in Fig. 2. When combining the risk factors quinolone use, vancomycin use, and other antibiotic use and performing an additional meta-analysis, the pooled odds ratio was 3.07 (95% CI = 2.27 to 4.15). Publication bias indicators showed significant results for the risk factors carbapenem use, medical devices, patient characteristics, and length of hospital stay (Table 5). For the additional meta-analysis, publication bias indicators showed no significant results. Carbapenem use (OR = 7.09, 95% CI = 5.43 to 9.25) and medical devices (OR = 5.11, 95% CI = 3.55 to 7.37) resulted in the highest pooled ORs in the meta-analyses. Therefore, cumulative meta-analyses were performed for these two risk factors. Results are shown in a forest plot (Fig. 2a and b). For carbapenem use, all years showed statistically significant results. For the risk factor medical devices, the result was not significant when the estimate was updated the second time. When the estimate was updated for the third time, results became significant once more.
TABLE 5.
Conventional meta-analyses of the different risk factors for acquisition and transmission of carbapenem-resistant P. aeruginosaa
| Risk factor | No. of factors | Pooled OR (random effects) | 95% CI | Range of OR in individual studies | Risk of publication bias |
|||
|---|---|---|---|---|---|---|---|---|
| Egger | P value | Kendall's tau | P value | |||||
| Carbapenem use | 16 | 7.09 | 5.43–9.25 | 3.6–76.0 | 1.39 | 0.02 | 0.47 | 0.01 |
| Medical devices | 19 | 5.11 | 3.55–7.37 | 2.1–64.3 | 2.30 | <0.001 | 0.49 | 0.003 |
| Other antibiotic use | 19 | 3.56 | 2.52–5.03 | 0.3–43.7 | 1.49 | 0.06 | 0.38 | 0.02 |
| ICU admission | 8 | 3.02 | 1.62–5.61 | 1.1–13.3 | 2.96 | 0.002 | 0.07 | 0.90 |
| Quinolone use | 11 | 2.73 | 1.27–5.87 | 0.1–48.4 | 0.89 | 0.56 | 0.45 | 0.06 |
| Underlying disease | 13 | 2.44 | 1.23–4.84 | 0.1–25.0 | 1.34 | 0.06 | −0.05 | 0.77 |
| Vancomycin use | 3 | 2.10 | 1.42–3.09 | 1.8–2.9 | NC | NC | NC | NC |
| Patient characteristics | 13 | 1.46 | 1.22–1.75 | 1.0–13.9 | 2.02 | <0.001 | 0.56 | 0.007 |
| Length of hospital stay | 9 | 1.06 | 1.02–1.09 | 1.0–6.7 | 3.05 | 0.0003 | 0.56 | 0.04 |
OR, odds ratio; CI, confidence interval; NC, not calculated because there were too few strata.
FIG 2.
(a) Forest plots of conventional and cumulative meta-analyses of the risk factor carbapenem use in a random-effects model, shown on a logarithmic scale. Plots: 1, conventional meta-analysis including the source given as first author and year of publication, number of case patients (in parentheses), odds ratio, and 95% confidence interval; 2, cumulative meta-analysis including number of case patients, odds ratio, and 95% confidence interval. (b) Forest plots of conventional and cumulative meta-analyses of the risk factor medical devices using a random-effects model, shown on a logarithmic scale. Plots: 1, conventional meta-analysis including source and number of case patients as indicated for panel a, odds ratio, and 95% confidence interval; 2, cumulative meta-analysis including number of case patients, odds ratio, and 95% confidence interval. (c) Forest plots of individual and pooled odds ratios for seven different risk factors of transmission and acquisition of carbapenem-resistant P. aeruginosa, using a random-effects model, shown on a logarithmic scale.
Even when excluding cohort and cross-sectional studies (n = 8) from the meta-analyses, our estimated results changed only slightly. The mean change was +0.2, ranging from −0.1 (risk factor length of hospital stay) to +1.31 (risk factor underlying diseases). All previous significant result calculations remained significant after removal of these eight studies.
DISCUSSION
Summary of evidence.
This systematic review identified the nine most significant and most reported risk factors for the presence of carbapenem-resistant P. aeruginosa and summarized the sources and reservoirs of these bacteria within the hospital environment. The nine risk factors were, in order of statistical significance, (i) carbapenem use, (ii) medical devices, (iii) other antibiotic use, (iv) ICU admission, (v) quinolone use, (vi) underlying diseases, (vii) vancomycin use, (viii) patient characteristics, and (ix) length of hospital stay. The risk factor carbapenem use showed the strongest pooled odds ratio in the meta-analysis (Table 5). However, the most frequently reported risk factor was medical devices, which showed the second strongest pooled odds ratio (Table 5). The cumulative meta-analyses (Fig. 2a and b) of these two risk factors showed that the estimate of the risk factor carbapenem use was stable for studies published after 2005. Before 2005, only a few studies published were included, and therefore the estimate fluctuated per publication. However, after 2005, the worldwide use of carbapenem increased, mainly due to the appearance of endemic and epidemic multiresistant microorganisms, especially bacteria expressing extended-spectrum beta-lactamases in ICUs (where most of the studies included in this publication were performed) (21–24). The estimate of the risk factor medical devices decreased between 1995 and 2008 and increased from 2008 to 2011. We hypothesize that the estimate increased after 2008 due to an increase in the number of medical device days during this time period. The decrease in estimate from 1995 to 2008 can be explained by the relatively few studies included in the first part of the cumulative meta-analysis.
We also looked at whether studies identified environmental sources and/or reservoirs, not only in included studies but also in those excluded. Only 31 outbreaks reported environmental sources or reservoirs (Table 4). This implies that in most epidemics a source or reservoir is not identified, not reported, or not searched for. If carbapenem-resistant P. aeruginosa was identified in the innate environment, it was often unclear or not proven that the presumed reservoir was indeed the primary source of infection. In fact, sinks are most frequently reported and thought to be the main reservoir of carbapenem-resistant P. aeruginosa in hospitals (Table 4).
It was remarkable that in three of the studies included in the analyses, vancomycin use was identified as a risk factor for acquiring carbapenem-resistant P. aeruginosa (25–27). All three articles hypothesize that this may have been due to antibiotic selection pressure, with the reduction or elimination of competing Gram-positive bacteria post-antibiotic treatment having facilitated the colonization of the skin or gastrointestinal tract of patients with Gram-negative bacteria, including P. aeruginosa.
Limitations and strengths.
The limitations of this study are mostly related to the heterogeneity of the studies included in the analyses. From our investigations, it was obvious that every reported outbreak generally involved different target populations, microbial sources, microbiological methods, active surveillance to find cases, and methods for identifying whether there was transmission or endogenous selection.
A limitation of the meta-analyses was the diverse models used by the different studies when performing multivariate regression analysis. Also, in almost all cases, the models used were not described. This problem is already known to be a major limitation of studies utilizing meta-analyses, as “confounders” can seriously alter the combined estimate. We know that the confounders that are adjusted for are different, whereas in meta-analysis we require them to be the same. However, from a clinical point of view they have to be different, because every situation (selection or transmission), outbreak, or level of endemicity is different. Even if we knew every specific model used, it would not solve the problem of heterogeneity. For all of these reasons, we used a random-effects model.
The statistical results may also have been influenced by publication bias, and the Egger and Kendall's tau publication bias indicators showed significant results for several risk factors (Table 5). However, the authors tried to include as many studies as possible, despite differences in language or size of the outbreak. Nevertheless, a full-text article was not available for 20 studies, data were incomplete for 2 studies, and there may also be unpublished studies that we could not access. However, this number of studies is small relative to the number of studies included after title/abstract selection (n = 256), so its influence on our results is likely to be limited.
We excluded studies including only patients with CF. These patients are chronically infected with P. aeruginosa, with strains acquired mostly in the community, and are a different patient population from the population of our interest (28).
Previously, a review by Falagas and Kopterides (2006) also identified risk factors associated with P. aeruginosa infection (15). Several of the current risk factors observed (Table 1) match the risk factors observed by Falagas and Kopterides. However, in contrast to the review by Falagas and Kopterides, the current study focuses on carbapenem resistance and includes only studies that analyzed data using a multivariate model. Also, almost one-half of the studies included in this publication were published after 2006. Finally, we also included all studies that indicated a source or reservoir of their P. aeruginosa outbreak, and we conducted conventional and cumulative meta-analyses, results that are not available in the review by Falagas and Kopterides.
Conclusions and implications.
This systematic review shows that the risk factors for P. aeruginosa infection and transmission are diverse. However, the use of carbapenem antibiotics was the most significant risk estimate from this meta-analysis, which highlights the importance of antibiotic stewardship in controlling P. aeruginosa outbreaks. During an outbreak involving one or more (clonal) strains, the use of these antibiotics could be a risk factor for acquisition of that clonal strain(s), by making the patient more vulnerable to colonization or infection. Importantly, antibiotic use is a risk factor that can be influenced in order to reduce the chance of outbreaks occurring. Another risk factor is the use of medical devices and reduction of device days. The use of medical devices and the number of device days are also the most frequently reported risk factors resulting from our meta-analyses. The increased use of medical devices, and for longer periods of time, means that patients are becoming more vulnerable to acquiring MDR P. aeruginosa (29). On the other hand, other important risk factors for outbreaks involving MDR P. aeruginosa such as patient characteristics, underlying diseases, or ICU admission cannot be easily influenced.
This systematic review also shows that it is difficult to identify the actual source of P. aeruginosa outbreaks. Therefore, basic infection prevention measures remain very important. For example, contact isolation of patients and strict compliance with hand hygiene measures remain the major steps necessary to stop further transmission of outbreak isolates. This is important whether or not an assumed or proven exogenous source is responsible for the outbreak.
We believe that it is important that prospective studies relating to outbreaks of carbapenem-resistant P. aeruginosa report on sources and reservoirs of infection and that analysis of any data be performed using a multivariate statistical model. This information is extremely valuable with respect to planning future research and control measures for antibiotic-resistant P. aeruginosa. It is also very important for authors to genetically type strains associated with infection in order to identify clonal clusters of isolates. These data allow the infectious disease specialist to determine whether infection and spread are related to selection (risk factor carbapenem/antibiotic use) or transmission (e.g., risk factor medical devices). The systematic review and meta-analysis published here show the nine most important risk factors for the presence of carbapenem-resistant P. aeruginosa bacterial isolates among hospitalized patients. The identification of these risk factors is useful in controlling future outbreaks of by these organisms. In this case, risk factors such as antibiotic use and high numbers of device days have to be reduced or eliminated in order to help prevent the appearance and spread of carbapenem-resistant P. aeruginosa. In this study, carbapenem use was identified with the highest pooled odds ratio. Therefore, use of this class of antibiotics especially should be reduced.
Finally, it is important to decrease the use of antibiotics, especially the use of carbapenems, in order to help prevent resistant P. aeruginosa outbreaks. In addition, it is highly recommended that an infectious disease consultant with a broad view on the prevalence of MDR bacteria and knowledge of the most recent guidelines for antibiotic use in the hospital concerned be consulted. Indeed, studies have shown that consultation with an infectious disease consultant significantly increases the correct administration of microbiologically correct antibiotic therapy (30–32).
ACKNOWLEDGMENTS
We thank the infection prevention team of the Erasmus Medical Centre Rotterdam and John P. Hays for their information and comments. This systematic review would not have been possible without their cooperation.
The authors' contributions are as follows: M.C.V. conceived of the design, helped interpret the data, and contributed to the writing of the manuscript. A.F.V. performed the literature search, data collection, data analysis, data interpretation, and writing of the manuscript. J.A.S. contributed to the writing of the manuscript. E.M.E.H.L. oversaw statistical analyses and contributed to the writing of the manuscript. All authors have read and approved the final version of the manuscript.
Footnotes
Published ahead of print 18 February 2014
REFERENCES
- 1.Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, Leblebicioglu H, Fisher D, Alvarez-Moreno C, Khader IA, Del Rocio Gonzalez Martinez M, Cuellar LE, Navoa-Ng JA, Abouqal R, Guanche Garcell H, Mitrev Z, Pirez Garcia MC, Hamdi A, Duenas L, Cancel E, Gurskis V, Rasslan O, Ahmed A, Kanj SS, Ugalde OC, Mapp T, Raka L, Yuet Meng C, Thu le TA, Ghazal TAS, Gikas A, Narvaez LP, Mejia N, Hadjieva N, Gamar Elanbya MO, Guzman Siritt ME, Jayatilleke K. 2012. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am. J. Infect. Control 40:396–407. 10.1016/j.ajic.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 2.Carmeli Y, Troillet N, Karchmer AW, Samore MH. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127–1132. 10.1001/archinte.159.10.1127 [DOI] [PubMed] [Google Scholar]
- 3.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GKS, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock REW, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. 10.1038/35023079 [DOI] [PubMed] [Google Scholar]
- 4.Suarez C, Pena C, Gavalda L, Tubau F, Manzur A, Dominguez MA, Pujol M, Gudiol F, Ariza J. 2010. Influence of carbapenem resistance on mortality and the dynamics of mortality in Pseudomonas aeruginosa bloodstream infection. Int. J. Infect. Dis. 14(Suppl 3):e73–e78. 10.1016/j.ijid.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 5.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. 2011. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19:419–426. 10.1016/j.tim.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933–951 table of contents. 10.1128/CMR.14.4.933-951.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby GA, Munoz-Price LS. 2005. The new beta-lactamases. N. Engl. J. Med. 352:380–391. 10.1056/NEJMra041359 [DOI] [PubMed] [Google Scholar]
- 8.Walsh TR. 2008. Clinically significant carbapenemases: an update. Curr. Opin. Infect. Dis. 21:367–371. 10.1097/QCO.0b013e328303670b [DOI] [PubMed] [Google Scholar]
- 9.Camargo CH, Bruder-Nascimento A, Mondelli AL, Montelli AC, Sadatsune T. 2011. Detection of SPM and IMP metallo-beta-lactamases in clinical specimens of Pseudomonas aeruginosa from a Brazilian public tertiary hospital. Braz. J. Infect. Dis. 15:478–481. 10.1016/S1413-8670(11)70231-8 [DOI] [PubMed] [Google Scholar]
- 10.Lolans K, Queenan AM, Bush K, Sahud A, Quinn JP. 2005. First nosocomial outbreak of Pseudomonas aeruginosa producing an integron-borne metallo-beta-lactamase (VIM-2) in the United States. Antimicrob. Agents Chemother. 49:3538–3540. 10.1128/AAC.49.8.3538-3540.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Bij AK, Van der Zwan D, Peirano G, Severin JA, Pitout JD, Van Westreenen M, Goessens WH. 2012. Metallo-beta-lactamase-producing Pseudomonas aeruginosa in the Netherlands: the nationwide emergence of a single sequence type. Clin. Microbiol. Infect. 18:E369–E372. 10.1111/j.1469-0691.2012.03969.x [DOI] [PubMed] [Google Scholar]
- 12.Van der Bij AK, Van Mansfeld R, Peirano G, Goessens WH, Severin JA, Pitout JD, Willems R, Van Westreenen M. 2011. First outbreak of VIM-2 metallo-beta-lactamase-producing Pseudomonas aeruginosa in The Netherlands: microbiology, epidemiology and clinical outcomes. Int. J. Antimicrob. Agents 37:513–518. 10.1016/j.ijantimicag.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Mingcheng L, Dong X, Li F. 2011. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa carrying blaVIM-2 in burn wards, China. Braz. J. Infect. Dis. 15:505–506. 10.1016/S1413-8670(11)70241-0 [DOI] [PubMed] [Google Scholar]
- 14.Suarez C, Pena C, Arch Dominguez OMA, Tubau F, Juan C, Gavalda L, Sora M, Oliver A, Pujol M, Ariza J. 2011. A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect. Dis. 11:272. 10.1186/1471-2334-11-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falagas ME, Kopterides P. 2006. Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J. Hosp. Infect. 64:7–15. 10.1016/j.jhin.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 62:e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA. 2007. Comparative review of the carbapenems. Drugs 67:1027–1052. 10.2165/00003495-200767070-00006 [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. 2008. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61:344–349. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. 2011. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute, Ottawa, Canada [Google Scholar]
- 20.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin. Trials 7:177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21.Kwint HM, van der Linden PD, Roukens MM, Natsch S. 2012. Intensification of antibiotic use within acute care hospitals in the Netherlands. J. Antimicrob. Chemother. 67:2283–2288. 10.1093/jac/dks190 [DOI] [PubMed] [Google Scholar]
- 22.Liew YX, Krishnan P, Yeo CL, Tan TY, Lee SY, Lim WP, Lee W, Hsu LY. 2011. Surveillance of broad-spectrum antibiotic prescription in Singaporean hospitals: a 5-year longitudinal study. PLoS One 6:e28751. 10.1371/journal.pone.0028751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. 2010. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit. Care 14:R113. 10.1186/cc9062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pluss-Suard C, Pannatier A, Kronenberg A, Muhlemann K, Zanetti G. 2011. Hospital antibiotic consumption in Switzerland: comparison of a multicultural country with Europe. J. Hosp. Infect. 79:166–171. 10.1016/j.jhin.2011.05.028 [DOI] [PubMed] [Google Scholar]
- 25.Fortaleza CM, Freire MP, Filho Dde C, de Carvalho Ramos M. 2006. Risk factors for recovery of imipenem- or ceftazidime-resistant Pseudomonas aeruginosa among patients admitted to a teaching hospital in Brazil. Infect. Control Hosp. Epidemiol. 27:901–906. 10.1086/507288 [DOI] [PubMed] [Google Scholar]
- 26.Harris AD, Smith D, Johnson JA, Bradham DD, Roghmann MC. 2002. Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin. Infect. Dis. 34:340–345. 10.1086/338237 [DOI] [PubMed] [Google Scholar]
- 27.Onguru P, Erbay A, Bodur H, Baran G, Akinci E, Balaban N, Cevik MA. 2008. Imipenem-resistant Pseudomonas aeruginosa: risk factors for nosocomial infections. J. Korean Med. Sci. 23:982–987. 10.3346/jkms.2008.23.6.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugha RE, Davies JC. 2011. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and new treatments. Br. J. Hosp. Med. (Lond.) 72:614–619 [DOI] [PubMed] [Google Scholar]
- 29.von Eiff C, Jansen B, Kohnen W, Becker K. 2005. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs 65:179–214. 10.2165/00003495-200565020-00003 [DOI] [PubMed] [Google Scholar]
- 30.Beovic B, Kreft S, Seme K, Cizman M. 2009. The impact of total control of antibiotic prescribing by infectious disease specialist on antibiotic consumption and cost. J. Chemother. 21:46–51. 10.1179/joc.2009.21.1.46 [DOI] [PubMed] [Google Scholar]
- 31.Kerremans JJ, Verbrugh HA, Vos MC. 2012. Frequency of microbiologically correct antibiotic therapy increased by infectious disease consultations and microbiological results. J. Clin. Microbiol. 50:2066–2068. 10.1128/JCM.06051-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo E, Rezai K, Evans AT, Madariaga MG, Phillips M, Brobbey W, Schwartz DN, Wang Y, Weinstein RA, Trenholme GM. 2004. Why don't they listen? Adherence to recommendations of infectious disease consultations. Clin. Infect. Dis. 38:1212–1218. 10.1086/383315 [DOI] [PubMed] [Google Scholar]
- 33.Harris AD, Johnson JK, Thom KA, Morgan DJ, McGregor JC, Ajao AO, Moore AC, Comer AC, Furuno JP. 2011. Risk factors for development of intestinal colonization with imipenem-resistant Pseudomonas aeruginosa in the intensive care unit setting. Infect. Control Hosp. Epidemiol. 32:719–722. 10.1086/660763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, Kim M. 2010. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 31:47–53. 10.1086/649021 [DOI] [PubMed] [Google Scholar]
- 35.Lepelletier D, Cady A, Caroff N, Marraillac J, Reynaud A, Lucet JC, Corvec S. 2010. Imipenem-resistant Pseudomonas aeruginosa gastrointestinal carriage among hospitalized patients: risk factors and resistance mechanisms. Diagn. Microbiol. Infect. Dis. 66:1–6. 10.1016/j.diagmicrobio.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 36.Cezario RC, Duarte De Morais L, Ferreira JC, Costa-Pinto RM, da Costa Darini AL, Gontijo-Filho PP. 2009. Nosocomial outbreak by imipenem-resistant metallo-beta-lactamase-producing Pseudomonas aeruginosa in an adult intensive care unit in a Brazilian teaching hospital. Enferm. Infecc. Microbiol. Clin. 27:269–274. 10.1016/j.eimc.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 37.Mueller MR, Hayden MK, Fridkin SK, Warren DK, Phillips L, Lolans K, Quinn JP. 2008. Nosocomial acquisition of Pseudomonas aeruginosa resistant to both ciprofloxacin and imipenem: a risk factor and laboratory analysis. Eur. J. Clin. Microbiol. Infect. Dis. 27:565–570. 10.1007/s10096-008-0475-9 [DOI] [PubMed] [Google Scholar]
- 38.Pena C, Guzman A, Suarez C, Dominguez MA, Tubau F, Pujol M, Gudiol F, Ariza J. 2007. Effects of carbapenem exposure on the risk for digestive tract carriage of intensive care unit-endemic carbapenem-resistant Pseudomonas aeruginosa strains in critically ill patients. Antimicrob. Agents Chemother. 51:1967–1971. 10.1128/AAC.01483-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentzelopoulos SD, Pratikaki M, Platsouka E, Kraniotaki H, Zervakis D, Koutsoukou A, Nanas S, Paniara O, Roussos C, Giamarellos-Bourboulis E, Routsi C, Zakynthinos SG. 2007. Prolonged use of carbapenems and colistin predisposes to ventilator-associated pneumonia by pandrug-resistant Pseudomonas aeruginosa. Intensive Care Med. 33:1524–1532. 10.1007/s00134-007-0683-2 [DOI] [PubMed] [Google Scholar]
- 40.Ohmagari N, Hanna H, Graviss L, Hackett B, Perego C, Gonzalez V, Dvorak T, Hogan H, Hachem R, Rolston K, Raad I. 2005. Risk factors for infections with multidrug-resistant Pseudomonas aeruginosa in patients with cancer. Cancer 104:205–212. 10.1002/cncr.21115 [DOI] [PubMed] [Google Scholar]
- 41.Ozkurt Z, Ertek M, Erol S, Altoparlak U, Akcay MN. 2005. The risk factors for acquisition of imipenem-resistant Pseudomonas aeruginosa in the burn unit. Burns 31:870–873. 10.1016/j.burns.2005.04.015 [DOI] [PubMed] [Google Scholar]
- 42.Zavascki AP, Cruz RP, Goldani LZ. 2005. Risk factors for imipenem-resistant Pseudomonas aeruginosa: a comparative analysis of two case-control studies in hospitalized patients. J. Hosp. Infect. 59:96–101. 10.1016/j.jhin.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 43.Cao B, Wang H, Sun H, Zhu Y, Chen M. 2004. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. J. Hosp. Infect. 57:112–118. 10.1016/j.jhin.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 44.Troillet N, Samore MH, Carmeli Y. 1997. Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin. Infect. Dis. 25:1094–1098. 10.1086/516092 [DOI] [PubMed] [Google Scholar]
- 45.Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. 1999. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 43:1379–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodise TP, Jr, Miller C, Patel N, Graves J, McNutt LA. 2007. Identification of patients with Pseudomonas aeruginosa respiratory tract infections at greatest risk of infection with carbapenem-resistant isolates. Infect. Control Hosp. Epidemiol. 28:959–965. 10.1086/518972 [DOI] [PubMed] [Google Scholar]
- 47.Montero M, Sala M, Riu M, Belvis F, Salvado M, Grau S, Horcajada JP, Alvarez-Lerma F, Terradas R, Orozco-Levi M, Castells X, Knobel H. 2010. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 29:335–339. 10.1007/s10096-009-0850-1 [DOI] [PubMed] [Google Scholar]
- 48.Kohlenberg A, Weitzel-Kage D, van der Linden P, Sohr D, Vogeler S, Kola A, Halle E, Ruden H, Weist K. 2010. Outbreak of carbapenem-resistant Pseudomonas aeruginosa infection in a surgical intensive care unit. J. Hosp. Infect. 74:350–357. 10.1016/j.jhin.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 49.Pena C, Suarez C, Tubau F, Dominguez A, Sora M, Pujol M, Gudiol F, Ariza J. 2009. Carbapenem-resistant Pseudomonas aeruginosa: factors influencing multidrug-resistant acquisition in non-critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 28:519–522. 10.1007/s10096-008-0645-9 [DOI] [PubMed] [Google Scholar]
- 50.Yang K, Zhuo H, Guglielmo BJ, Wiener-Kronish J. 2009. Multidrug-resistant Pseudomonas aeruginosa ventilator-associated pneumonia: the role of endotracheal aspirate surveillance cultures. Ann. Pharmacother. 43:28–35. 10.1345/aph.1L210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. 2006. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 27:893–900. 10.1086/507274 [DOI] [PubMed] [Google Scholar]
- 52.Zavascki AP, Barth AL, Gaspareto PB, Goncalves AL, Moro AL, Fernandes JF, Goldani LZ. 2006. Risk factors for nosocomial infections due to Pseudomonas aeruginosa producing metallo-beta-lactamase in two tertiary-care teaching hospitals. J. Antimicrob. Chemother. 58:882–885. 10.1093/jac/dkl327 [DOI] [PubMed] [Google Scholar]
- 53.Nouer SA, Nucci M, de-Oliveira MP, Pellegrino FL, Moreira BM. 2005. Risk factors for acquisition of multidrug-resistant Pseudomonas aeruginosa producing SPM metallo-beta-lactamase. Antimicrob. Agents Chemother. 49:3663–3667. 10.1128/AAC.49.9.3663-3667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Defez C, Fabbro-Peray P, Bouziges N, Gouby A, Mahamat A, Daures JP, Sotto A. 2004. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 57:209–216. 10.1016/j.jhin.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 55.Furtado GH, Gales AC, Perdiz LB, Santos AE, Wey SB, Medeiros EA. 2010. Risk factors for hospital-acquired pneumonia caused by imipenem-resistant Pseudomonas aeruginosa in an intensive care unit. Anaesth. Intensive Care 38:994–1001 [DOI] [PubMed] [Google Scholar]
- 56.Martinez JA, Delgado E, Marti S, Marco F, Vila J, Mensa J, Torres A, Codina C, Trilla A, Soriano A, Alquezar A, Castro P, Nicolas JM. 2009. Influence of antipseudomonal agents on Pseudomonas aeruginosa colonization and acquisition of resistance in critically ill medical patients. Intensive Care Med. 35:439–447. 10.1007/s00134-008-1326-y [DOI] [PubMed] [Google Scholar]
- 57.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. 2006. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob. Agents Chemother. 50:43–48. 10.1128/AAC.50.1.43-48.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richard P, Le Floch R, Chamoux C, Pannier M, Espaze E, Richet H. 1994. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J. Infect. Dis. 170:377–383. 10.1093/infdis/170.2.377 [DOI] [PubMed] [Google Scholar]
- 59.Nagao M, Iinuma Y, Igawa J, Saito T, Yamashita K, Kondo T, Matsushima A, Takakura S, Takaori-Kondo A, Ichiyama S. 2011. Control of an outbreak of carbapenem-resistant Pseudomonas aeruginosa in a haemato-oncology unit. J. Hosp. Infect. 79:49–53. 10.1016/j.jhin.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 60.Park YS, Lee H, Chin BS, Han SH, Hong SG, Hong SK, Kim HY, Uh Y, Shin HB, Choo EJ, Song W, Jeong SH, Lee K, Kim JM. 2011. Acquisition of extensive drug-resistant Pseudomonas aeruginosa among hospitalized patients: risk factors and resistance mechanisms to carbapenems. J. Hosp. Infect. 79:54–58. 10.1016/j.jhin.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 61.Cortes JA, Cuervo SI, Urdaneta AM, Potdevin G, Arroyo P, Bermudez D, Correa A, Villegas MV. 2009. Identifying and controlling a multiresistant Pseudomonas aeruginosa outbreak in a Latin-American cancer centre and its associated risk factors. Braz. J. Infect. Dis. 13:99–103 [DOI] [PubMed] [Google Scholar]
- 62.Fortaleza CM, Figueiredo LC, Beraldo CC, Melo EC, Pola PM, Aragao VD. 2009. Risk factors of oropharyngeal carriage of Pseudomonas aeruginosa among patients from a medical-surgical intensive care unit. Braz. J. Infect. Dis. 13:173–176. 10.1590/S1413-86702009000300004 [DOI] [PubMed] [Google Scholar]
- 63.Pereira GH, Levin AS, Oliveira HB, Moretti ML. 2008. Controlling the clonal spread of Pseudomonas aeruginosa infection. Infect. Control Hosp. Epidemiol. 29:549–552. 10.1086/588163 [DOI] [PubMed] [Google Scholar]
- 64.Dropulic LK, Leslie JM, Eldred LJ, Zenilman J, Sears CL. 1995. Clinical manifestations and risk factors of Pseudomonas aeruginosa infection in patients with AIDS. J. Infect. Dis. 171:930–937. 10.1093/infdis/171.4.930 [DOI] [PubMed] [Google Scholar]
- 65.Talon D, Capellier G, Boillot A, Michel-Briand Y. 1995. Use of pulsed-field gel electrophoresis as an epidemiologic tool during an outbreak of Pseudomonas aeruginosa lung infections in an intensive care unit. Intensive Care Med. 21:996–1002. 10.1007/BF01700661 [DOI] [PubMed] [Google Scholar]
- 66.Thuong M, Arvaniti K, Ruimy R, de la Salmoniere P, Scanvic-Hameg A, Lucet JC, Regnier B. 2003. Epidemiology of Pseudomonas aeruginosa and risk factors for carriage acquisition in an intensive care unit. J. Hosp. Infect. 53:274–282. 10.1053/jhin.2002.1370 [DOI] [PubMed] [Google Scholar]
- 67.Eagye KJ, Kuti JL, Nicolau DP. 2009. Risk factors and outcomes associated with isolation of meropenem high-level-resistant Pseudomonas aeruginosa. Infect. Control Hosp. Epidemiol. 30:746–752. 10.1086/603527 [DOI] [PubMed] [Google Scholar]
- 68.Furtado GH, Bergamasco MD, Menezes FG, Marques D, Silva A, Perdiz LB, Wey SB, Medeiros EA. 2009. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: risk factors and mortality. J. Crit. Care 24:625.e9-14. 10.1016/j.jcrc.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 69.Berthelot P, Grattard F, Mahul P, Pain P, Jospe R, Venet C, Carricajo A, Aubert G, Ros A, Dumont A, Lucht F, Zeni F, Auboyer C, Bertrand JC, Pozzetto B. 2001. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med. 27:503–512. 10.1007/s001340100870 [DOI] [PubMed] [Google Scholar]
- 70.Mammina C, Di Carlo P, Cipolla D, Casuccio A, Tantillo M, Plano MR, Mazzola A, Corsello G. 2008. Nosocomial colonization due to imipenem-resistant Pseudomonas aeruginosa epidemiologically linked to breast milk feeding in a neonatal intensive care unit. Acta Pharmacol. Sin. 29:1486–1492. 10.1111/j.1745-7254.2008.00892.x [DOI] [PubMed] [Google Scholar]
- 71.Arruda EA, Marinho IS, Boulos M, Sinto SI, Caiaffa HH, Mendes CM, Oplustil CP, Sader H, Levy CE, Levin AS. 1999. Nosocomial infections caused by multiresistant Pseudomonas aeruginosa. Infect. Control Hosp. Epidemiol. 20:620–623. 10.1086/501683 [DOI] [PubMed] [Google Scholar]
- 72.Paramythiotou E, Lucet JC, Timsit JF, Vanjak D, Paugam-Burtz C, Trouillet JL, Belloc S, Kassis N, Karabinis A, Andremont A. 2004. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 38:670–677. 10.1086/381550 [DOI] [PubMed] [Google Scholar]
- 73.Joo EJ, Kang CI, Ha YE, Kang SJ, Park SY, Chung DR, Peck KR, Lee NY, Song JH. 2011. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia: clinical impact of antimicrobial resistance on outcome. Microb. Drug Resist. 17:305–312. 10.1089/mdr.2010.0170 [DOI] [PubMed] [Google Scholar]
- 74.Tumbarello M, Repetto E, Trecarichi EM, Bernardini C, De Pascale G, Parisini A, Rossi M, Molinari MP, Spanu T, Viscoli C, Cauda R, Bassetti M. 2011. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol. Infect. 139:1740–1749. 10.1017/S0950268810003055 [DOI] [PubMed] [Google Scholar]
- 75.Yang MA, Lee J, Choi EH, Lee HJ. 2011. Pseudomonas aeruginosa bacteremia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J. Korean Med. Sci. 26:612–618. 10.3346/jkms.2011.26.5.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson LE, D'Agata EM, Paterson DL, Clarke L, Qureshi ZA, Potoski BA, Peleg AY. 2009. Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transpl. Infect. Dis. 11:227–234. 10.1111/j.1399-3062.2009.00380.x [DOI] [PubMed] [Google Scholar]
- 77.Tam VH, Chang KT, LaRocco MT, Schilling AN, McCauley SK, Poole K, Garey KW. 2007. Prevalence, mechanisms, and risk factors of carbapenem resistance in bloodstream isolates of Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 58:309–314. 10.1016/j.diagmicrobio.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 78.Falagas ME, Koletsi PK, Kopterides P, Michalopoulos A. 2006. Risk factors for isolation of strains susceptible only to polymyxin among patients with Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 50:2541–2543. 10.1128/AAC.00224-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Risk factors for antimicrobial resistance and influence of resistance on mortality in patients with bloodstream infection caused by Pseudomonas aeruginosa. Microb. Drug Resist. 11:68–74. 10.1089/mdr.2005.11.68 [DOI] [PubMed] [Google Scholar]
- 80.El Amari EB, Chamot E, Auckenthaler R, Pechere JC, Van Delden C. 2001. Influence of previous exposure to antibiotic therapy on the susceptibility pattern of Pseudomonas aeruginosa bacteremic isolates. Clin. Infect. Dis. 33:1859–1864. 10.1086/324346 [DOI] [PubMed] [Google Scholar]
- 81.Hallin M, Deplano A, Roisin S, Boyart V, De Ryck R, Nonhoff C, Byl B, Glupczynski Y, Denis O. 2012. Pseudo-outbreak of extremely drug-resistant Pseudomonas aeruginosa urinary tract infections due to contamination of an automated urine analyzer. J. Clin. Microbiol. 50:580–582. 10.1128/JCM.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sekiguchi J, Teruya K, Horii K, Kuroda E, Konosaki H, Mizuguchi Y, Araake M, Kawana A, Yoshikura H, Kuratsuji T, Miyazaki H, Kirikae T. 2007. Molecular epidemiology of outbreaks and containment of drug-resistant Pseudomonas aeruginosa in a Tokyo hospital. J. Infect. Chemother. 13:418–422. 10.1007/s10156-007-0560-5 [DOI] [PubMed] [Google Scholar]
- 83.Pinna A, Usai D, Sechi LA, Zanetti S, Jesudasan NC, Thomas PA, Kaliamurthy J. 2009. An outbreak of post-cataract surgery endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology 116:2321–2326. e2321-2324. 10.1016/j.ophtha.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 84.Kouda S, Fujiue Y, Watanabe Y, Ohara M, Kayama S, Kato F, Hisatsune J, Tsuruda K, Matsubara A, Doi M, Kuwabara M, Sugai M. 2011. Sporadic isolations of a multi-drug resistant Pseudomonas aeruginosa clone during a 14-month epidemic in a general hospital in Hiroshima. Infection 39:247-253. 10.1007/s15010-011-0111-y [DOI] [PubMed] [Google Scholar]
- 85.Babu KV, Kumara A, Vijayanath V. 2011. Study of imipenem resistant metallo-beta-lactamase positive Pseudomonas aeruginosa from different hospital environmental sources. J. Pure Appl. Microbiol. 5:195–203 [Google Scholar]
- 86.Crivaro V, Di Popolo A, Caprio A, Lambiase A, Di Resta M, Borriello T, Scarcella A, Triassi M, Zarrilli R. 2009. Pseudomonas aeruginosa in a neonatal intensive care unit: molecular epidemiology and infection control measures. BMC Infect. Dis. 9:70. 10.1186/1471-2334-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, Gardam MA. 2009. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect. Control Hosp. Epidemiol. 30:25–33. 10.1086/592700 [DOI] [PubMed] [Google Scholar]
- 88.Mayank D, Anshuman M, Singh RK, Afzal A, Baronia AK, Prasad KN. 2009. Nosocomial cross-transmission of Pseudomonas aeruginosa between patients in a tertiary intensive care unit. Indian J. Pathol. Microbiol. 52:509–513. 10.4103/0377-4929.56143 [DOI] [PubMed] [Google Scholar]
- 89.Crespo MP, Woodford N, Sinclair A, Kaufmann ME, Turton J, Glover J, Velez JD, Castaneda CR, Recalde M, Livermore DM. 2004. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-8, a novel metallo-beta-lactamase, in a tertiary care center in Cali, Colombia. J. Clin. Microbiol. 42:5094–5101. 10.1128/JCM.42.11.5094-5101.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boutiba-Ben Boubaker I, Boukadida J, Triki O, Hannachi N, Ben Redjeb S. 2003. Outbreak of nosocomial urinary tract infections due to a multidrug resistant Pseudomonas aeruginosa. Pathol. Biol. (Paris) 51:147–150 (In French.). 10.1016/S0369-8114(03)00040-3 [DOI] [PubMed] [Google Scholar]
- 91.Bertrand X, Bailly P, Blasco G, Balvay P, Boillot A, Talon D. 2000. Large outbreak in a surgical intensive care unit of colonization or infection with Pseudomonas aeruginosa that overexpressed an active efflux pump. Clin. Infect. Dis. 31:E9–E14. 10.1086/318117 [DOI] [PubMed] [Google Scholar]
- 92.Bert F, Maubec E, Bruneau B, Berry P, Lambert-Zechovsky N. 1998. Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J. Hosp. Infect. 39:53–62. 10.1016/S0195-6701(98)90243-2 [DOI] [PubMed] [Google Scholar]
- 93.Griffith SJ, Nathan C, Selander RK, Chamberlin W, Gordon S, Kabins S, Weinstein RA. 1989. The epidemiology of Pseudomonas aeruginosa in oncology patients in a general hospital. J. Infect. Dis. 160:1030–1036. 10.1093/infdis/160.6.1030 [DOI] [PubMed] [Google Scholar]
- 94.DiazGranados CA, Jones MY, Kongphet-Tran T, White N, Shapiro M, Wang YF, Ray SM, Blumberg HM. 2009. Outbreak of Pseudomonas aeruginosa infection associated with contamination of a flexible bronchoscope. Infect. Control Hosp. Epidemiol. 30:550–555. 10.1086/597235 [DOI] [PubMed] [Google Scholar]
- 95.Sorin M, Segal-Maurer S, Mariano N, Urban C, Combest A, Rahal JJ. 2001. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect. Control Hosp. Epidemiol. 22:409–413. 10.1086/501925 [DOI] [PubMed] [Google Scholar]
- 96.Panzig B, Schroder G, Pitten FA, Grundling M. 1999. A large outbreak of multiresistant Pseudomonas aeruginosa strains in north-eastern Germany. J. Antimicrob. Chemother. 43:415–418. 10.1093/jac/43.3.415 [DOI] [PubMed] [Google Scholar]
- 97.Fraser TG, Reiner S, Malczynski M, Yarnold PR, Warren J, Noskin GA. 2004. Multidrug-resistant Pseudomonas aeruginosa cholangitis after endoscopic retrograde cholangiopancreatography: failure of routine endoscope cultures to prevent an outbreak. Infect. Control Hosp. Epidemiol. 25:856–859. 10.1086/502309 [DOI] [PubMed] [Google Scholar]
- 98.Pitten FA, Panzig B, Schroder G, Tietze K, Kramer A. 2001. Transmission of a multiresistant Pseudomonas aeruginosa strain at a German university hospital. J. Hosp. Infect. 47:125–130. 10.1053/jhin.2000.0880 [DOI] [PubMed] [Google Scholar]
- 99.Bukholm G, Tannaes T, Kjelsberg AB, Smith-Erichsen N. 2002. An outbreak of multidrug-resistant Pseudomonas aeruginosa associated with increased risk of patient death in an intensive care unit. Infect. Control Hosp. Epidemiol. 23:441–446. 10.1086/502082 [DOI] [PubMed] [Google Scholar]
- 100.Leung CH, Wang NY, Liu CP, Weng LC, Hsieh FC, Lee CM. 2008. Antimicrobial therapy and control of multidrug-resistant Pseudomonas aeruginosa bacteremia in a teaching hospital in Taiwan. J. Microbiol. Immunol. Infect. 41:491–498 [PubMed] [Google Scholar]
- 101.Verweij PE, Bijl D, Melchers WJ, De Pauw BE, Meis JF, Hoogkamp-Korstanje JA, Voss A. 1997. Pseudo-outbreak of multiresistant Pseudomonas aeruginosa in a hematology unit. Infect. Control Hosp. Epidemiol. 18:128–131. 10.1086/647567 [DOI] [PubMed] [Google Scholar]
- 102.Landman D, Quale JM, Mayorga D, Adedeji A, Vangala K, Ravishankar J, Flores C, Brooks S. 2002. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned. Arch. Intern. Med. 162:1515–1520. 10.1001/archinte.162.13.1515 [DOI] [PubMed] [Google Scholar]
- 103.Vilar-Compte D, Jacquemin B, Diaz-Gonzalez A, Velasquez C, Volkow P. 2003. Pseudomonas aeruginosa outbreak, in the area of surgical wound ambulatory care, in postmastectomy patients. Salud Publica Mex. 45:371–378 (In Spanish.) 10.1590/S0036-36342003000500007 [DOI] [PubMed] [Google Scholar]
- 104.Zheng YN, Xu ZY, Weng XH. 1990. Experimental study and case control study of nosocomial infection caused by Pseudomonas aeruginosa. Zhonghua Nei Ke Za Zhi 29:217–220, 253 (In Chinese.) [PubMed] [Google Scholar]
- 105.Gras-Le Guen C, Lepelletier D, Debillon T, Gournay V, Espaze E, Roze JC. 2003. Contamination of a milk bank pasteuriser causing a Pseudomonas aeruginosa outbreak in a neonatal intensive care unit. Arch. Dis. Child Fetal Neonatal Ed. 88:F434–F435. 10.1136/fn.88.5.F434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kikuchi T, Nagashima G, Taguchi K, Kuraishi H, Nemoto H, Yamanaka M, Kawano R, Ugajin K, Tazawa S, Marumo K. 2007. Contaminated oral intubation equipment associated with an outbreak of carbapenem-resistant Pseudomonas in an intensive care unit. J. Hosp. Infect. 65:54–57. 10.1016/j.jhin.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 107.Pena C, Dominguez MA, Pujol M, Verdaguer R, Gudiol F, Ariza J. 2003. An outbreak of carbapenem-resistant Pseudomonas aeruginosa in a urology ward. Clin. Microbiol. Infect. 9:938–943. 10.1046/j.1469-0691.2003.00686.x [DOI] [PubMed] [Google Scholar]
- 108.Climo MW, Pastor A, Wong ES. 1997. An outbreak of Pseudomonas aeruginosa related to contaminated urodynamic equipment. Infect. Control Hosp. Epidemiol. 18:509–510. 10.1086/647657 [DOI] [PubMed] [Google Scholar]