Abstract
Renal histology results are very scarce in dengue-associated rhabdomyolysis patients developing acute kidney injury (AKI). We report a case of dengue fever-induced AKI associated to rhabdomyolysis with a renal biopsy showing acute tubular necrosis (ATN) and renal deposition of myoglobin. A 28-year-old patient who presented dengue fever (DF) complicated by severe AKI and rhabdomyolysis is described. The patient required hemodialysis for three weeks. A renal biopsy revealed ATN with positive staining for myoglobin in the renal tubuli. The patient was discharged with recovered renal function. In conclusion, this case report described a biopsy proven ATN associated to DF-induced rhabdomyolysis, in which renal deposition of myoglobin was demonstrated. We suggest that serum creatine phosphokinase should be monitored in DF patients to allow for an early diagnosis of rhabdomyolysis and the institution of renal protective measures.
Keywords: Dengue fever, Acute kidney injury, Acute tubular necrosis, Renal histology, Myoglobin, Rhabdomyolysis, Creatine phosphokinase
Abstract
Resultados de histologia renal são muito escassos em pacientes com rabdomiólise e injúria renal aguda (IRA) associada a dengue. Descrevemos caso de dengue complicado por rabdomiólise e IRA no qual a biópsia renal mostrou necrose tubular aguda (NTA) e deposição renal de mioglobina. Paciente de 28 anos que apresentou dengue complicado por IRA grave e rabdomiólise é descrito. Ele necessitou de diálise por três semanas. A biópsia renal mostrou NTA, com imunohistoquímica fortemente positiva para mioglobina nos túbulos renais. O paciente recebeu alta com recuperação da função renal. Em conclusão, descrevemos caso de dengue complicado por IRA e rabdomiólise, em que a biópsia renal mostrou NTA e deposição de mioglobina. Sugerimos que creatinofosfoquinase deve ser monitorizada em pacientes com dengue para permitir o diagnóstico precoce de rabdomiólise e a instituição de medidas protetoras para o rim.
INTRODUCTION
Dengue is currently the most important infectious viral mosquito-borne disease in the world. In recent years, there has been an explosive outbreak of this infectious disease, mainly affecting tropical countries, but also the warmer areas of developed countries, such as the southern United States. In fact, the number of cases worldwide that are annually reported to the World Health Organization (WHO) increased from approximately 900 in the 1950s to almost one million at present41.
Dengue is a multifaceted disease that can manifest as an undifferentiated fever, classical dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS)41.
Different renal injuries have been described in dengue patients, such as an increase in serum creatinine, proteinuria, glomerulonephritis, hemolytic-uremic syndrome and acute kidney injury (AKI)4,10,16,37,39. Most cases of AKI have been associated with DHF or DSS6,7,20–23,25,27,34,40, but AKI has also been described in DF13–15,30,31, albeit less commonly. Similarly, rhabdomyolysis with1,5,7,19 and without AKI9,24,28 has been described in dengue patients. Renal histology results from patients with dengue-associated AKI are scarce and to the best of our knowledge, there were no previous cases of biopsy proven ATN with renal myoglobin deposition due to rhabdomyolysis associated with DF31,37,39.
We report a case of a young patient who developed AKI and rhabdomyolysis associated with DF. A renal biopsy revealed acute tubular necrosis and positive staining for myoglobin in the renal tubuli. The literature on dengue-associated renal injury and rhabdomyolysis is reviewed and discussed.
CASE REPORT
A 28-year-old married male painter, from Sao Paulo, SP, Brazil, previously healthy, presented a 20-day history of fever, myalgia, muscle weakness, cough, nausea and vomiting, diarrhea, epigastric pain and lower limb edema. The patient history did not disclose any epidemiological clue for a specific infectious disease nor had he receive any nephrotoxic drug.
Laboratory blood analysis at the time of the first hospital admission revealed the following: creatinine (SCr) 11.6 mg/dL, urea 277 mg/dL, sodium 124 mEq/L, potassium 6.6 mEq/L, bicarbonate 15.6 mEq/L, SGOT 1,206 IU/L, SGPT 853 IU/L, amylase 121 IU/L, total bilirubin 0.38 mg/dL, alkaline phosphatase 68 IU/L, gamma-glutamyltransferase 104 IU/L, hemoglobin 14.2 g/dL, leukocytes 10,200/mm3, platelets 292,000/mm3 and C-reactive protein (CRP) 1.95 mg/dL. The urinalysis disclosed a specific gravity of 1,027, pH 5.0, protein 3+, glucose traces, red blood cells > 1 million/mL, leukocytes > 1 million/mL and no casts. Renal replacement therapy (RRT) was initiated.
After one week, he was transferred to Hospital das Clinicas, University of Sao Paulo Medical School, Sao Paulo, Brazil. At admission, he was pale, had a blood pressure of 140/90 mmHg and oliguric (150 m/24 h), with a dialysis catheter in his right internal jugular vein and had no evidence of systemic hemorrhagic phenomena. His physical exam was otherwise unremarkable. Laboratory tests performed at admission revealed: SCr 14.8 mg/dL, serum urea 323 mg/dL, hemoglobin 11.8 g/dL, leukocytes 9,400/mm3, platelets 469,000/mm3, albumin 3.6 g/L and CRP 2.5 mg/dL. The patient presented serum creatine phosphokinase (CK) 4,063 IU/L, serum lactic dehydrogenase 657 IU/L, uric acid 13.4 mg/dL and phosphorus 9.2 mg/dL, suggesting rhabdomyolysis. The urinalysis showed protein > 1 g/L, red blood cells > 100/field, and leukocytes 20/field. A subsequent 24 h proteinuria showed 0.83 g of protein/24 h. Anti-nuclear factor, antineutrophil cytoplasmic antibodies, C3 and C4 complement fractions, rheumatoid factor and anticardiolipin antibody levels were normal. A renal ultrasound disclosed normal kidneys. Serology for HIV, leptospirosis, hepatitis C, hepatitis B and syphilis were negative. Patient tested positive for hepatitis A and cytomegalovirus immunoglobulin G. Five days after admission, a positive test for dengue was obtained (IgM, ELISA assay). As the patient had a clinical picture and serology consistent with dengue infection without hemorrhagic phenomena, low platelet count or evidence of plasma leakage, he was diagnosed with a case of dengue fever complicated by rhabdomyolysis and AKI.
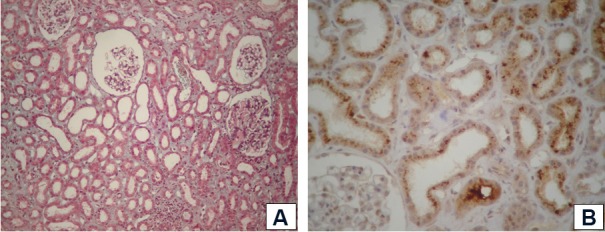
After two weeks on RRT, due to non-recovery of renal function, a percutaneous renal biopsy was performed. The biopsy included 11 glomeruli with normal volume and cellularity, dilated tubules with foci of epithelial cellular necrosis, interstitial area with focal fibrosis (approximately 10%) and normal arterioles. Immunofluorescence assays for IgA, IgG, IgM, C1q, C3 and fibrinogen were negative. The pathological diagnosis was acute tubular necrosis. Myoglobin immunohistochemistry staining was positive in the renal tubuli (Fig. 1 A and B).
Fig. 1. A. Light microscopy of renal tissue stained with Masson's trichrome (20X). Note the presence of diffuse acute tubular necrosis and preserved glomeruli. In the interstitial area, thin fibrosis lines and edema are evident. B. Immunostaining of renal tissue. Note the positive immunostaining for myoglobin in the cytoplasm of the tubular cells.
Three weeks after admission, RRT was interrupted and patient was discharged with SCr 2.4 mg/dL, normal urine output and urinalysis with pH 5.5, specific gravity 1015, blood +, protein 0.3 g/dL, 12 WBCs and 3 RBCs. After three weeks, he returned to the outpatient facility with no complains and an unremarkable physical examination. Laboratory tests performed at that time revealed a SCr of 0.98 mg/dL.
DISCUSSION
To the best of our knowledge, the current case is the first biopsy proven description of rhabdomyolysis and AKI associated with DF in which ATN and myoglobin staining in the renal tubuli was demonstrated.
There are only four previously reported cases of dengue-induced rhabdomyolysis and AKI (Table 1), but renal biopsy was not performed on those patients1,7,15,19. All of them presented elevated CK levels, three studies reported myalgia, two reported muscle weaknesses and three studies presented dark urine, positive urinary myoglobin and oliguria. Renal replacement therapy was performed in two cases.
Table 1. Comparison of the current case with the cases of dengue with rhabdomyolysis and AKI previously described.
| Author, year, country | Gender, age | Type of dengue | Myalgia | Muscle weakness | Dark urine | UMyo | Oliguria | CK (IU/L) | Cr (mg/dL) | Renal biopsy | RRT | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gunasekera et al, 2000, Ceylon (ref 14) | Female, 28 y | NR | yes | yes | + | + | yes | >5,000 | 8.8 | no | PD, HF | recovery |
| Davis & Bourke, 2004, East Timor (ref 7) | Male, 33 y | DHF | NR | NR | NR | NR | NR | 17,548 | NR | no | NR | death |
| Karakus et al, 2007, Suriname (ref 19) | Male, 66 y | DSS | yes | NR | + | + | yes | 156,900 | 3.6 | no | NR | death |
| Acharya et al, 2010, India (ref 1) | Male, 40 y | NR | yes | yes | + | + | yes | 29,000 | 2.6 | no | NR | NR |
| Current case, 2013, Brazil | Male, 28 y | DF | yes | ND | yes | 4,063 | 14.8 | ATN | HD | recovery |
UMyo: urinary myoglobin; CK: serum creatine phosphokinase; Cr: serum creatinine; RRT: renal replacement therapy; NR: not reported; PD: peritoneal dialysis; HF: hemofiltration; DHF: dengue hemorrhagic fever; DSS: dengue shock syndrome; DF: dengue fever; ND: not done; ATN: acute tubular necrosis; HD: hemodialysis.
There are also case descriptions of dengue-associated rhabdomyolysis with myalgia, dark urine and highly elevated CK levels without AKI development5,7,9,24. In addition, AKI was not reported in series of patients with dengue-induced quadriparesis or myositis and high CK levels28,33. Although rhabdomyolysis is a well-known risk factor for AKI, the presence of other simultaneous injury factors, such as hypovolemia or dehydration, acidosis and aciduria, must be present to cause clinically relevant myoglobinuria-induced renal injury.
AKI has also been reported in DHF, DSS and DF without rhabdomyolysis. Hemodynamic instability, hemolysis, glomerular injury and direct action of viral particles on renal tissue have been considered as possible injury mechanisms6,10,25,30,32,34,37. In fact, the current patient presented proteinuria but the renal biopsy did not disclose glomerular injury.
Myositis and rhabdomyolysis have been considered as rare complications of dengue infection but few authors have systematically assessed their occurrences. MALHEIROS et al. 26 performed muscle biopsies in 15 patients with DF and myalgia during an epidemic outbreak in Brazil. Only three patients presented mild increased CK levels. They found mononuclear infiltrates in 12 biopsies, lipid accumulations in 11 and rare foci of myonecrosis in three (one case had an elevated CK level). Seven out of 16 patients with serologically proven dengue infections in India presented muscle weakness and increased CK levels18. A muscle biopsy was performed in one patient, revealing myositis18. SAID et al. prospectively assessed CK levels and the incidence of myositis in 101 patients with serologically proven dengue in Saudi Arabia35. In that study, 91% of the patients had elevated CK, 63.4% complained of myalgia and 2.9% had muscle weakness. MISRA et al. evaluated 39 patients with positive dengue serology during a dengue outbreak in Northern India29. Transient muscle dysfunction with CK levels of approximately 1,000 U/L was observed in 11 (73.3%) patients with DF, in 18 (81.8%) with DHF and in the two patients with DSS. This collection of data suggests that muscle injury is not uncommon in dengue and has probably been underreported.
The pathogenesis of dengue-associated muscle injury is unclear. Different mechanisms have been hypothesized, such as direct viral invasion of and immune-mediated injury to the muscle fibers. Striated skeletal muscles from mice inoculated with dengue type 2 viruses exhibited myofibril destruction, sarcoplasm involution, mitochondrial changes and aggregates of electron-dense material and cytoplasmic glycogen particles32. The dengue virus has demonstrated high efficiency in infecting and replicating in human primary muscle satellite cells38 and muscle cells were demonstrated to be highly susceptible to in vitro infection by the dengue 2 virus. The infected cells exhibited increased expression of inflammatory genes and the IP-10 protein and elevated intracellular calcium36. Dengue virus infection has also been associated with increased production of inflammatory cytokines which may cause muscle injury11,12.
In conclusion, DF may cause rhabdomyolysis-associated ATN. Dengue virus-associated muscle injury is probably under-recognized and underreported. CK levels should be monitored in dengue patients to allow for an early diagnosis of rhabdomyolysis and the institution of renal protective measures.
CONSENT
Written informed consent was obtained from the patient for the publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Series Editor of this journal.
REFERENCES
- 1.Acharya S, Shukla S, Mahajan SN, Diwan SK. Acute dengue myositis with rhabdomyolysis and acute renal failure. Ann Indian Acad Neurol. 2010;13:221–222. doi: 10.4103/0972-2327.70882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto DF, Takiya CM, Paes MV, Farias-Filho J, Pinhão AT, Alves AM, et al. Histopathological aspects of dengue-2 virus infected mice tissues and complementary virus isolation. J Submicrosc Cytol Pathol. 2004;36:121–130. [PubMed] [Google Scholar]
- 3.Basílio-de-Oliveira CA, Aguiar GR, Baldanza MS, Barth OM, Eyer-Silva WA, Paes MV. Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz J Infect Dis. 2005;9:341–347. doi: 10.1590/s1413-86702005000400012. [DOI] [PubMed] [Google Scholar]
- 4.Basu G, Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, et al. Acute kidney injury in tropical acute febrile illness in a tertiary care centre - RIFLE criteria validation. Nephrol Dial Transplant. 2011;26:524–531. doi: 10.1093/ndt/gfq477. [DOI] [PubMed] [Google Scholar]
- 5.Beauvais P, Quinet B, Richardet JM. Dengue. A propos of 2 cases. Arch Fr Pediatr. 1993;50:905–907. [PubMed] [Google Scholar]
- 6.Chacko B, John GT, Jacob CK, Vijayakumar TS. Dengue shock syndrome in a renal transplant recipient. Transplantation. 2004;77:634–635. doi: 10.1097/01.tp.0000114608.44000.d7. [DOI] [PubMed] [Google Scholar]
- 7.Davis JS, Bourke P. Rhabdomyolysis associated with dengue virus infection. Clin Infect Dis. 2004;38:e109–111. doi: 10.1086/392510. [DOI] [PubMed] [Google Scholar]
- 8.Araújo JM de, Schatzmayr HG, Filippis AM de, Santos FB Dos, Cardoso MA, Britto C, et al. A retrospective survey of dengue virus infection in fatal cases from an epidemic in Brazil. J Virol Methods. 2009;155:34–38. doi: 10.1016/j.jviromet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Finsterer J, Kongchan K. Severe, persisting, steroid-responsive dengue myositis. J Clin Virol. 2006;35:426–428. doi: 10.1016/j.jcv.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Futrakul P, Poshyachinda V, Mitrakul C, Kun-Anake C, Boonpucknavig V, Boompucknavig S, et al. Renal involvement and reticulo-endothelial-system clearance in dengue hemorrhagic fever. J Med Assoc Thai. 1973;56:33–39. [PubMed] [Google Scholar]
- 11.Gagnon SJ, Mori M, Kurane I, Green S, Vaughn DW, Kalayanarooj S, et al. Cytokine gene expression and protein production in peripheral blood mononuclear cells of children with acute dengue virus infections. J Med Virol. 2002;67:41–46. doi: 10.1002/jmv.2190. [DOI] [PubMed] [Google Scholar]
- 12.Gandini M, Reis SR, Torrentes-Carvalho A, Azeredo EL, Freire M da S, Galler R, et al. Dengue-2 and yellow fever 17DD viruses infect human dendritic cells, resulting in an induction of activation markers, cytokines and chemokines and secretion of different TNF-α and IFN-α profiles. Mem Inst Oswaldo Cruz. 2011;106:594–605. doi: 10.1590/s0074-02762011000500012. [DOI] [PubMed] [Google Scholar]
- 13.George R, Liam CK, Chua CT, Lam SK, Pang T, Geethan R, et al. Unusual clinical manifestations of dengue virus infection. Southeast Asian J Trop Med Public Health. 1988;19:585–590. [PubMed] [Google Scholar]
- 14.Gunasekera HH, Adikaram AV, Herath CA, Samarasinghe HH. Myoglobinuric acute renal failure following dengue viral infection. Ceylon Med J. 2000;45:181. [PubMed] [Google Scholar]
- 15.Hommel D, Talarmin A, Reynes JM, Hulin A. Acute renal failure associated with dengue fever in French Guiana. Nephron. 1999;83:183. doi: 10.1159/000045506. [DOI] [PubMed] [Google Scholar]
- 16.Hutspardol S, Prommalikit O, Upiya N, Chataroopwijit J, Khemakanok K, Assadamongkol K. Heavy proteinuria following dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2011;42:579–582. [PubMed] [Google Scholar]
- 17.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 18.Kalita J, Misra UK, Mahadevan A, Shankar SK. Acute pure motor quadriplegia: is it dengue myositis? Electromyogr Clin Neurophysiol. 2005;45:357–631. [PubMed] [Google Scholar]
- 19.Karakus A, Banga N, Voorn GP, Meinders AJ. Dengue shock syndrome and rhabdomyolysis. Neth J Med. 2007;65:78–81. [PubMed] [Google Scholar]
- 20.Kuo MC, Lu PL, Chang JM, Lin MY, Tsai JJ, Chen YH, et al. Impact of renal failure on the outcome of dengue viral infection. Clin J Am Soc Nephrol. 2008;3:1350–1356. doi: 10.2215/CJN.00020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laoprasopwattana K, Pruekprasert P, Dissaneewate P, Geater A, Vachvanichsanong P. Outcome of dengue hemorrhagic fever-caused acute kidney injury in Thai children. J Pediatr. 2010;157:303–309. doi: 10.1016/j.jpeds.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Lee IK, Liu JW, Yang KD. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;79:149–153. [PubMed] [Google Scholar]
- 23.Lee IK, Liu JW, Yang KD. Clinical characteristics, risk factors, and outcomes in adults experiencing dengue hemorrhagic fever complicated with acute renal failure. Am J Trop Med Hyg. 2009;80:651–655. [PubMed] [Google Scholar]
- 24.Lim M, Goh HK. Rhabdomyolysis following dengue virus infection. Singapore Med J. 2005;46:645–646. [PubMed] [Google Scholar]
- 25.Lima EQ, Gorayeb FS, Zanon JR, Nogueira ML, Ramalho HJ, Burdmann EA. Dengue haemorrhagic fever-induced acute kidney injury without hypotension, haemolysis or rhabdomyolysis. Nephrol Dial Transplant. 2007;22:3322–3326. doi: 10.1093/ndt/gfm431. [DOI] [PubMed] [Google Scholar]
- 26.Malheiros SM, Oliveira AS, Schmidt B, Lima JG, Gabbai AA. Dengue. Muscle biopsy findings in 15 patients. Arq Neuropsiquiatr. 1993;51:159–164. doi: 10.1590/s0004-282x1993000200001. [DOI] [PubMed] [Google Scholar]
- 27.Méndez A, González G. Dengue hemorrágico en niños: diez años de experiencia clínica. Biomedica(Bogotá) 2003;23:180–193. [PubMed] [Google Scholar]
- 28.Mishra UK, Kalita J. Spectrum of neurological manifestations of dengue in India. Dengue Bull. 2006;30:107–113. [Google Scholar]
- 29.Misra UK, Kalita J, Maurya PK, Kumar P, Shankar SK, Mahadevan A. Dengue-associated transient muscle dysfunction: clinical, electromyography and histopathological changes. Infection. 2012;40:125–130. doi: 10.1007/s15010-011-0203-8. [DOI] [PubMed] [Google Scholar]
- 30.Mohsin N, Mohamed E, Gaber M, Obaidani I, Budruddin M, Al Busaidy S. Acute tubular necrosis associated with non-hemorrhagic dengue fever: a case report. Ren Fail. 2009;31:736–739. doi: 10.3109/08860220903003404. [DOI] [PubMed] [Google Scholar]
- 31.Nair VR, Unnikrishnan D, Satish B, Sahadulla MI. Acute renal failure in dengue fever in the absence of bleeding manifestations or shock. Infect Dis Clin Pract. 2005;13:142–143. [Google Scholar]
- 32.Nath P, Agrawal DK, Mehrotra RM. Ultrastructural changes in skeletal muscles in dengue virus-infected mice. J Pathol. 1982;136:301–305. doi: 10.1002/path.1711360405. [DOI] [PubMed] [Google Scholar]
- 33.Paliwal VK, Garg RK, Juyal R, Husain N, Verma R, Sharma PK, et al. Acute dengue virus myositis: a report of seven patients of varying clinical severity including two cases with severe fulminant myositis. J Neurol Sci. 2011;300:14–18. doi: 10.1016/j.jns.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Radakovic-Fijan S, Graninger W, Muller C, Honigsmann H, Tanew A. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430–433. doi: 10.1067/mjd.2002.111904. [DOI] [PubMed] [Google Scholar]
- 35.Said SM, Elsaeed KM, Alyan Z. Benign acute myositis in association with acute dengue viruses' infections. Egypt J Neurol Psychiat Neurosurg. 2008;45:193–200. [Google Scholar]
- 36.Salgado DM, Eltit JM, Mansfield K, Panqueba C, Castro D, Vega MR, et al. Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J. 2010;29:238–242. doi: 10.1097/INF.0b013e3181bc3c5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhaya BK, Sharma A, Khaira A, Dinda AK, Agarwal SK, Tiwari SC. Transient IgA nephropathy with acute kidney injury in a patient with dengue fever. Saudi J Kidney Dis Transpl. 2010;21:521–5. [PubMed] [Google Scholar]
- 38.Warke RV, Becerra A, Zawadzka A Schmidt DJ, Martin KJ, Giaya K, et al. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J Gen Virol. 2008;89:1605–1615. doi: 10.1099/vir.0.2008/000968-0. [DOI] [PubMed] [Google Scholar]
- 39.Wiersinga WJ, Scheepstra CG, Kasanardjo JS, Vries PJ de, Zaaijer H, Geerlings SE. Dengue fever-induced hemolytic uremic syndrome. Clin Infect Dis. 2006;43:800–801. doi: 10.1086/507111. [DOI] [PubMed] [Google Scholar]
- 40.Wiwanitkit V. Acute renal failure in the fatal cases of dengue hemorrhagic fever, a summary in Thai death cases. Ren Fail. 2005;27:647. doi: 10.1080/08860220500200916. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) >Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: WHO; 2009. [Google Scholar]


