Abstract
Objectives
To provide a history on nursing science within the Gynecology Oncology Group (GOG); to discuss challenges and facilitators of nursing science in the cooperative group (CG) using a current nurse-led protocol (GOG-0259) as an exemplar; and to propose recommendations aimed at advancing nursing science in the CG setting.
Data Source
GOG reports and protocol databases, online databases of indexed citations, and experiences from the development and implementation of GOG-0259.
Conclusions
Benefits of CG research include opportunities for inter-disciplinary collaboration and ability to rapidly accrue large national samples. Challenges include limited financial resources to support non-treatment trials, a cumbersome protocol approval process, and lack of experience with nursing/quality of life intervention studies. Formal structures within GOG need to be created to encourage nurse scientists to become active members; promote collaboration between experienced GOG advanced practice nurses and new nurse scientists to identify nursing research priorities; and consider innovative funding structures to support pilot intervention studies.
Implications for Nursing Practice
Understanding the CG research process is critical for nurse scientists. A multi-disciplinary team of CG leaders can help investigators navigate a complex research environment and can increase awareness of the value of nursing research.
The Gynecologic Oncology Group (GOG) was established in 1970 and received initial funding from the National Cancer Institute in 1971. It is the only NCI-sponsored Cancer Cooperative Group to focus exclusively on pelvic malignancies. Since its inception, the GOG has placed an emphasis on multi-disciplinary collaboration across the three major committees (Committees on Cancers of the Ovary; Cancers of the Cervix and Vulva; and Cancers of the Uterine Corpus) and specialty committees (Developmental Therapeutics; Experimental Medicine; Quality of Life/Health Outcomes Research; Cancer Prevention and Control). In addition, specific modality committees (Gynecologic Oncology, Medical Oncology, Nursing, Pathology, Radiation Oncology) were designed to ensure quality control and to discuss discipline-specific concerns across protocols.1
The Role of Nurses in GOG
From the earliest days of the GOG, nurses have been valued for their essential role in the design and conduct of GOG trials. The Nursing Committee was initiated as an informal committee in 1977, and was later authorized as a subcommittee of the Quality Control Committee with in the 1980's. Since 1994, the Nursing Committee has been recognized as a separate modality committee. The Nursing Committee takes on diverse roles in the GOG, including review of concepts and protocols in order to “identify, from a nursing perspective, any errors, omissions or inconsistencies that might affect patient eligibility, patient registration, safety protocol compliance and completion.” 2(p76) The committee reviews approximately 70 concepts/protocols per year. Nurses also serve on all major and specialty committees.
Other important activities of the Nursing Committee include the Nursing Manual and Educational Programs. The Nursing Manual includes procedural guideline activities important to GOG protocols, such as management of allergic and anaphylactic guidelines and intra-peritoneal chemotherapy administration. The focus of the Educational Programs is to provide continuing education to GOG nurses and to promote awareness and appreciation for nursing research and research utilization. Examples of educational programs include: “Development of Nursing Research (1990; Fran Lewis, PhD, RN), “Utilization of Nursing Research” (1992; Deborah McGuire, RN, PhD), “Quality of Life in Gynecologic Cancer Survivors” (1999; Lari Wenzel, PhD); “Update on HPV and Cervical Cancer Screening” (2004; Mary Rubin, RN, PhD), and “Symptom Experiences of Women with Recurrent Ovarian Cancer” (2011; Heidi Donovan, PhD, RN).
Nursing Science within GOG prior to 2007
The Nursing Committee does not formally sponsor concepts/protocols within the GOG. However, nurses have contributed to the GOG research enterprise in three significant ways: 1) nurse scientists as the study chair or as principal investigator (PI) of an ancillary or companion study; 2) nurse scientist as key collaborators taking lead roles in the development and dissemination of quality of life goals within a GOG-sponsored clinical trial; and 3) nurses as important members and co-authors on research teams. Table 1 provides examples of research conducted by or in collaboration with nurse scientists in the cooperative group and associated publications. These include ancillary and companion studies to phase III trials to evaluate the reliability and validity of new measures of chemotherapy-induced peripheral neuropathy3 and the measurement of vaginal stenosis4; randomized clinical trials of nursing interventions for alopecia5; quality of life outcomes for phase III trials6; and prospective studies of quality of life after treatment for germ cell ovarian cancers.7-11 In addition, GOG research nurses have been involved as co-authors on multiple GOG publications.12-21
Table 1.
Gynecologic Oncology Group Studies with Nurse Scientists as Study Chair, Principal Investigator of Ancillary/Companion Studies, or Key Collaborator/Author on Publications Related to Nurse-Specific Study Objectives.
| GOG Trial # (Year Opened) | GOG Study Title | Nurse Scientist or Collaborator (Role on Study) | Nurse-Specific Publications or Objectives |
|---|---|---|---|
| GOG0062 (1986) | Data Collection Form For Extravasation Injury With Doxorubicin | Mary Cullen, RN (Protocol Chair) | Objective: Record clinical observations and treatment of doxorubicin (adriamycin) extravasation for the purpose of future development of standardized protocol for treatment. |
| GOG0111 (1990) | A Phase III Randomized Study Of Cyclophosphamide and Cisplatin versus Taxol and Cisplatin in Patients with Suboptimal Stage III and Stage IV Epithelial Ovarian Carcinoma (Study Chair: William McGuire) | Lois Almadrones (PI on Ancillary Study to assess neuropathy and functional status funded by Oncology Nursing Society Foundation) | Almadrones, L., McGuire, D.B., Walczak, J.R., Florio, C.M., Tian, C. (2004). Psychometric evaluation of two scales assessing functional status and peripheral neuropathy associated with chemotherapy for ovarian cancer: A Gynecologic Oncology Group study. Oncology Nursing Forum 31, 615–623. |
| GOG9102 (1993) | The Effect Of Alopecia On Cancer Patients' Body Image And The Role Of Audiovisual Information On Body Image | Susan Nolte, PhD, CRNP (Study Chair) | Nolte, S., Donnelly, J., Kelly, S., Conley, P., Cobb, R. (2006). A randomized clinical trial of a video intervention for women with chemotherapy-induced alopecia: a Gynecologic Oncology Group study. Oncology Nursing Forum, 33, 305–11. |
| GOG0147/122 (1994) | A Quality Of Life Companion Study to GOG #122 -- Whole Abdominal Radiotherapy versus Combination Doxorubicin-Cisplatin Chemotherapy in Advanced Endometrial Carcinoma (Marcus Randall, Study Chair) | Deborah Watkins Bruner, PhD, RN, FAAN (Study Chair for Companion Study) | Nursing Objective: To compare changes over time in specific parameters that impact quality of life (fatigue, changes in elimination, and neurologic impairments) Bruner, D.W., Barsevick, A., Tian, C., Randall, M., Mannel, R., Cohn, D.E., Sorosky, J., Spirtos, N.M. (2007). Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Quality of Life Research, 16, 89–100. |
| GOG9901 (1999) | Quality of Life in Ovarian Germ Cell Cancer Survivors (Stephen Williams, Study Chair) | Victoria L. Champion; RN, DNS; Anna M. Miller, RN; DNS; and Melinda Swenson, PhD, RN (Collaborator and Co-Authors on Publications with Nurse-Specific Objectives) |
Swenson, M., MacLeod, J., Williams, S., Miller, A., Champion, V. (2003). Quality of living among ovarian germ cell cancer survivors: a narrative analysis. Oncology Nursing Forum, 30. Champion, V., Williams, S.D., Miller, A., Reuille, K.M., Monahan, P., Zhao, Q. (2007). Predicting quality of life in long-term ovarian germ cell survivors: A Gynecologic Oncology Group Study. Gynecologic Oncology, 105, 687–694. Gershenson, D.M., Miller, A.M., Champion, V.L., Monahan, P.O., Zhao, Q., Cella, D., Williams, S.D. (2007). Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group study. Journal of Clinical Oncology, 25, 2792–7. Monahan, P.O., Champion, V.L., Zhao, Q., Miller, A., Gershenson, D., Williams, S.D., Cella, D. (2008). Case-control comparison of quality of life in long-term ovarian germ cell tumor survivors: a Gynecologic Oncology Group study. Journal of Psychosocial Oncology, 26, 19–42. Matei, D., Miller, A.M., Monahan, P., Gershenson, D., Zhao, Q., Cella, D., Champion, V.L., Williams, S.D. (2009). Chronic physical effects and health care utilization in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group study. Journal of Clinical Oncology, 27, 4142–9. |
| GOG8003 (2003) | Vaginal length, elasticity, lubrication and sexual function in women with stage Ib2-cervice carcinoma | Deborah Watkins Bruner, PhD, RN, FAAN (Study Chair) | Bruner, D.W., Nolte, S., Shahin, M.S., Huang, H. Sobel, E. Ballup, D. Cella, D. (2006). Measurement of vaginal stenosis after cancer therapy: development and reliability of the vaginal sound: A Gynecologic Oncology Group study. International Journal of Gynecologic Caner, 16, 1749–55. |
| GOG0259 (2010) | Nurse-delivered WRITE Symptoms vs. Self-directed WRITE Symptoms vs. Care as Usual for Optimal Symptom Management for Women with Recurrent Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Heidi S. Donovan, PhD, RN (Study Chair and NIH/NINR PI) | Objective: Compare the efficacy of nurse-delivered WRITE Symptoms and self-directed WRITE Symptoms vs. usual care in improving target symptom severity, symptom-related distress, and symptom consequences in patients with recurrent or persistent ovarian, fallopian tube, or primary peritoneal cancer. |
| GOG0244 (2012) | The Lymphedema and Gynecologic Cancer (LEG) Study: Incidence, Risk Factors and Impact in Newly Diagnosed Patients (Richard Barakat, Study Chair) | Jane Armer, PhD, RN, FAAN (Consortium PI) | Objective: To prospectively estimate the incidence of lymphedema over time in patients undergoing surgery and lymphadenectomy for uterine cancer, cervical cancer, and vulvar cancer. |
Development of Infra-structure to Support Nursing Science within GOG
Despite a very active nursing committee and the involvement of several nurse scientists in evaluating quality of life outcomes within GOG clinical trials, prior to 2007 there were no nurse-scientists (i.e. research as their primary professional role) who were active members of GOG, nor was there specific infrastructure to support nurse scientists within the GOG.
In 2005 an Oncology Nursing Society (ONS) multi-site research working group proposed a plan for increasing the capacity of nurses to conduct multi-site research, including research within the cooperative groups.22, 23. A workshop for nurse investigators new to cooperative group research was subsequently developed with the goals of fostering nurse-led interdisciplinary research within cooperative groups and facilitating the development of research concepts by interdisciplinary teams.
GOG leadership committed to the goals of this initiative, selecting an interdisciplinary team of experienced cooperative group investigators to not only attend the workshop, but to continue providing mentorship to a new nurse investigator following the workshop. Experienced investigators included the Chair of the GOG Nursing Committee, the Chair of the Quality of Life Committee, a senior statistician, and a physician investigator. The team attended a three-day workshop in October of 2006 with teams from four other NCI cooperative groups. The new nurse investigators were trained on the structure and process of cooperative groups by senior nurses and other interdisciplinary members of the groups and each interdisciplinary team, led by the new nurse investigator, worked to develop research concepts for submission to their respective CG.
GOG-0259 Experience
At the time of the workshop, Dr. Donovan had just completed a National Institute of Nursing Research (NIH/NINR)-sponsored pilot study (NR009275) establishing the feasibility and acceptability of WRITE Symptoms, a web-based symptom management intervention for women with recurrent ovarian cancer.24 At the workshop, she presented a new proposal for a 3-arm randomized clinical trial (RCT) to compare the efficacy of nurse-delivered WRITE Symptoms to computer-mediated (self-directed) WRITE Symptoms to care-as-usual. The interdisciplinary team then worked to refine the proposal to be feasible for a multi-site cooperative group trial and to plan the process of obtaining concept and protocol approval through the GOG.
Challenges and Strategies
Proposal Approval
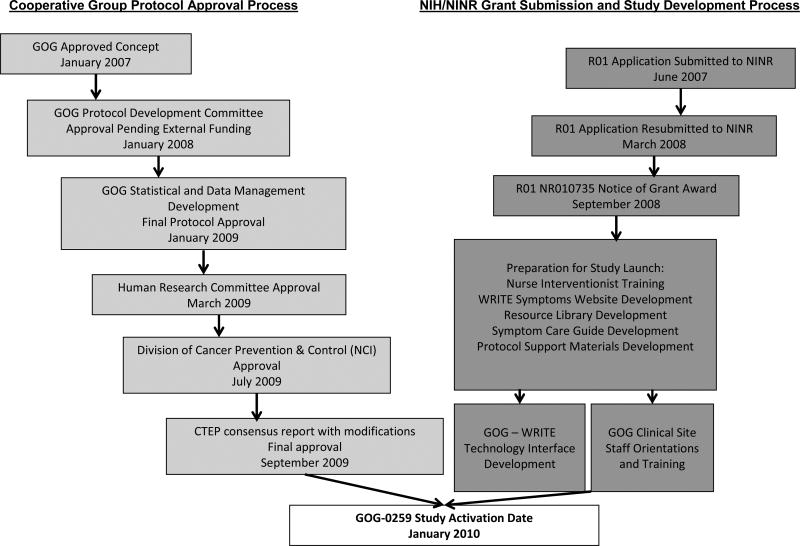
The team faced multiple challenges during the proposal development process. The complexity of simultaneously shepherding the proposal through the GOG approval process and obtaining external funding was the primary barrier. Based on the current funding structure of CGs, the team was required to seek external funding to support the development, coordination, and oversight of intervention delivery and collection of online patient-reported outcomes. Figure 1 provides a description of the key steps in the 36-month path to protocol approval within GOG, successful funding through NIH/NINR, and subsequent study activation.
Figure 1. GOG-0259 Protocol Development and Activation Timeline.
Several factors were essential to our success. With the guidance and support of GOG's leadership, we initially sought concept approval through GOG (Figure 1). A letter of support documenting concept approval was included on the initial R01 grant proposal. Following a competitive, but not fundable, score on the initial R01, the team then sought and obtained GOG protocol approval “pending external funding.” A stronger letter of support documenting a commitment on the part of GOG to activate the proposal as a group-wide study if funding was obtained was included in the NINR resubmission. This proposal was ultimately funded.
The other essential component to our success in obtaining GOG protocol approval was the ongoing support of the multi-disciplinary mentoring team who acted as champions of the proposal as it moved through the necessary committees in the approval process (Quality of Life; Ovarian; Protocol Development). These champions were able to answer questions about the proposal and to speak to the importance of the study in the context of the larger mission of GOG. At the same time, the chair of the nursing committee worked to ensure that GOG-0259 was recognized and supported as “nursing research”. Although the nursing committee does not sponsor research protocols, it was important that the nurses within the GOG feel ownership over the protocol. To promote this, updates on the status of the protocol were given to the Nursing Committee at each semi-annual meeting and two continuing education programs related to symptom assessment and management at the meetings were provided. The nurse scientist was also appointed to the Quality of Life committee and later to the Ancillary Data Committee in order to better understand and contribute to the research mission of the GOG.
Study Activation
Challenges to activating the study included: 1) cultural differences between decision-making processes common to externally-funded research studies conducted in academic settings and that of the cooperative group research environment; and 2) differences in the infra-structure necessary to support psycho-educational intervention research compared to treatment trials.
Principal investigators of investigator-initiated trials have a great deal of autonomy in decision-making regarding how to implement a funded study. This is often not the case in cooperative group trials, where there is a highly structured set of policies and procedures that guide the development, activation, implementation, and dissemination of group protocols. Even with training and mentorship, a new nurse scientist can be overwhelmed, confused, and frustrated by this complex process. Although many of the policies are explicit, there is also an implicit culture within the group that may take a great deal of time to understand.
In addition, nurse scientists interested in conducting intervention research outside the typical domain of disease treatment trials face the challenge of integrating study procedures within a system that is not set up to support this type of trial. Many of the GOG forms that are typically used to track disease status, adverse events, and other clinical factors were not consistent with the proposed study variables. This issue posed a significant challenge to the GOG Statistical and Data Center to modify existing forms and led to some confusion on the part of site staff on how to accurately report patient status for GOG-0259. A wide-range of challenges in connecting the two web-based systems necessary to ensure timely, secure transfer of data at key points in the study (patient consent, baseline questionnaire completion, and patient registration and randomization) were encountered. This was critical so that all members of the research team (University of Pittsburgh study staff; GOG site staff; and GOG data and statistical core staff) had accurate and up-to-date information to ensure seamless participant coordination.
In each of these cases, open communication and support from the GOG mentors was essential to navigating the different systems and creating compromise. They were able to advise the nurse scientist regarding when it was important to adapt to the GOG system and to assist in brokering for modifications to existing GOG policy when necessary. Additional experts, including leadership in information technology at GOG and at the University of Pittsburgh, were also brought in when necessary to ensure success in linking the two systems.
Post-Activation Study Coordination
Coordinating the study activities necessary to successfully conduct a multi-site RCT of a web-based psycho-educational intervention to improve symptom management for women with recurrent ovarian cancer presented a wide range of challenges to the team. The study teams had to coordinate recruitment, retention, intervention delivery, and collection of clinical and patient-reported outcomes for approximately 500 women with recurrent ovarian cancer from 68 GOG sites across the country. From the onset, it was decided that intervention delivery would be carried out by a team of nurse interventionists at the University of Pittsburgh to ensure intervention fidelity (accurate and consistent delivery according to the theory-driven protocol). In addition, all patient-reported outcomes (PRO) were captured and therefore coordinated through the WRITE Symptoms web-based-system. See Table 2 for GOG-0259 study design, sample, and division of coordination activities between UPSN and GOG staff.
Table 2. GOG-259 (The WRITE Symptoms Study).
| Study Design: 3-arm Randomized Clinical Trial |
|
| Eligibility: |
|
| Study Sample: 497 women from 68 clinical sites across 27 states |
| Coordination of Study Activities: | |
|---|---|
| University of Pittsburgh School of Nursing (UPSN) | Gynecologic Oncology Group Clinical Sites and Statistical & Data Core |
|
|
Because a majority of study activities were coordinated through the University of Pittsburgh, multiple activities were implemented to ensure that GOG study staff were knowledgeable about the study and able to carry out screening, informed consent, orientation to the study website, and collection of clinical data. These activities included GOG site staff training sessions at bi-annual GOG meetings, training webinars for all institutions at the time of site IRB approval, and a full-time project director at UPSN dedicated to communicating with and supporting GOG staff.
Although these activities were very helpful, some difficulties remained. It was very challenging for the nurse scientist to ensure consistent implementation of recruitment procedures across all of the clinical sites. It was not feasible to meet with GOG site research staff in person and not all staff at each institution attended a training session or webinar. Research procedures and staff roles are highly variable across sites and we found that participants were enrolled in the study with a wide range of understanding about the expectations for participating in the study. In future studies, nurse investigators might consider a more limited access study (e.g. 10 highly committed institutions), with more frequent and explicit collaboration on intervention delivery and collection of PROs between the GOG recruiting sites and the academic research staff. This would require intensive training and monitoring of site staff and a formal commitment to study accrual from selected sites in order to ensure consistent study operations and successful recruitment.
Benefits of Cooperative Group Research
Despite the challenges that may be faced by nurse researchers engaged in Cooperative Group Research, there is a wide range of potential benefits. First among these is the ability to rapidly accrue a national sample. The NCI cooperative group mechanism provides financial resources and a well-established infra-structure to support recruitment, data collection, data analysis, and dissemination efforts. In GOG-0259 we were able to surpass our accrual goal of 480 women in 34 months, which would have been impossible without the support of the Gynecologic Oncology Group leadership and member institutions. Furthermore, the cooperative group setting is an ideal setting for the nurse scientist to collaborate and learn from interdisciplinary clinical and research experts from across the country. The bi-annual meetings provide an excellent forum for keeping up-to-date on the latest advances in the field, for discussing ideas and collaborations for future research, and for getting feedback on proposed studies. In addition, the many cooperative group policies and procedures that can feel burdensome and rigid are there to ensure expert interdisciplinary input into proposals and to provide a structure for ensuring clear, accurate protocol development, implementation, and dissemination.
Recommendations
Based on our experiences, the following recommendations are aimed at advancing nursing science within the CGs. First, more nurse scientists are needed as active members of CGs. Nurse scientists should pro-actively foster relationships with their CG colleagues at their local CG member institutions and ask to be placed as a team member on the CG roster. Nurse scientists should also be encouraged to attend and network at the semi-annual CG meetings as a way to stay current on the developing science in the field. CG leadership and member institutions should consider funding new nurse scientists to attend the meetings in order to promote interdisciplinary collaborations in concept development. Experienced CG nurses should also be encouraged to identify, encourage, and support nurse scientists at their local academic institutions to become familiar with the CG process.
Once nurse scientists are drawn into the cooperative group setting, activities should focus on mentoring and support to become successful and productive scientists who are fully integrated within the group. Nurse scientist should actively seek out positions on CG committees, as this is one of the best ways to learn about CG processes while also providing service to the group. In addition, CGs with fewer numbers of active nurse scientists should look to other CGs with more established nursing research programs to identify models for successfully supporting nursing research.27-34 The Children's Oncology Group has a long history of success based on a scholar team model in which nurse scientists novel to the CG were paired with experienced APNs (see Kelly et al article in this issue). Not only did the nurse scientists become well-integrated into the group, but several APN team members were motivated to pursue doctoral education. Perhaps more importantly, the teams came together to identify nursing research priorities, develop a conceptual model to guide nursing research, and develop a plan for addressing the priority research areas.27,28,30,31
Finally, nurse scientists and cooperative groups must work to identify novel funding sources and develop infra-structure to facilitate nursing intervention research in the CGs. Given the constraints of funding for nursing intervention research within the CGs, many of these studies will continue to be investigator-initiated trials funded outside of the CG. Existing CG procedures may need to be modified to recognize the potential for and facilitate coordination of data collection/management, intervention delivery, and dissemination efforts outside of the CG. Nurse scientist should also explore novel funding sources for CG research including partnerships with industry, the Biomarker, Imaging, & Quality of Life Studies Funding Program (BIQSFP),35-36 and foundation funding. Professional organizations committed to nursing research, such as the Oncology Nursing Foundation, could consider specific Requests for Applications for nursing research conducted within CG settings.
Conclusion
There is a lack of familiarity with nursing intervention studies in most CGs and often little experience about the CG process on the part of nurse scientists. These factors lead to a wide range of challenges at every stage of protocol development and implementation and misunderstandings can easily occur. However, the potential benefits of CG research far outweigh the challenges. With the support of GOG leaders, this team was able to successfully conduct a large RCT of a web-based nursing intervention within the CG. Lessons learned from this trial can be used to create models within GOG to attract and mentor more nurse scientists in CG group and to create procedures to facilitate the complex process of proposal development, activation, implementation, and dissemination for nursing intervention research.
Acknowledgments
The authors would like to thank the following groups of individuals: Mark Brady, PhD, and Steven Plaxe, MD, for their time and expertise in mentoring Dr. Donovan in the GOG process; the WRITE study team at the University of Pittsburgh, in particular Judy Knapp, PhD, LCSW, and Howard Stein, MS, who were essential in coordinating study activities between the University of Pittsburgh and GOG institutions; and the GOG nursing committee and all of the clinical site research nurses and research assistants, in particular HeeSun Kim-Suh, RN, Heather Stipanovich, and Nancy Fusco, RN, for their commitment to recruiting participants to GOG-0259.
Supported by: NIH/NINR R01NR010735; NIH/GOG-0259
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thigpen T, Blessing J. Gynecologic Oncology Group. The Gynecologic Oncology Group: Report of 35 Years of Excellence in Clinical Research. 2006. The Gynecologic Oncology Group scientific process: A unique approach to cooperative clinical trials. P. DiSaia, Chair. [Google Scholar]
- 2.Creasman W, Nolte S. Gynecologic Oncology Group. The Gynecologic Oncology Group: Report of 35 Years of Excellence in Clinical Research. 2006. Modality and Quality Control Committees. P. DiSaia, Chair. [Google Scholar]
- 3.Almadrones L, McGuire DB, Walczak JR, Florio CM, Tian C. Psychometric evaluation of two scales assessing functional status and peripheral neuropathy associated with chemotherapy for ovarian cancer: A Gynecologic Oncology Group study. Oncol Nurs Forum. 2004;31(3):615–623. doi: 10.1188/04.ONF.615-623. [DOI] [PubMed] [Google Scholar]
- 4.Bruner DW, Nolte S, Shahin MS, et al. Measurement of vaginal stenosis after cancer therapy: development and reliability of the vaginal sound: A Gynecologic Oncology Group study. Int J Gynecol Cancer. 2006;16(5):1749–55. doi: 10.1111/j.1525-1438.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 5.Nolte S, Donnelly J, Kelly S, Conley P, Cobb R. A randomized clinical trial of a video intervention for women with chemotherapy-induced alopecia: a Gynecologic Oncology Group study. Oncol Nurs Forum. 2006;33(2):305–11. doi: 10.1188/06.ONF.305-311. [DOI] [PubMed] [Google Scholar]
- 6.Bruner DW, Barsevick A, Tian C, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Qual Life Res. 2007;16(1):89–100. doi: 10.1007/s11136-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 7.Swenson M, MacLeod J, Williams S, Miller A, Champion V. Quality of living among ovarian germ cell cancer survivors: a narrative analysis. Oncol Nurs For. 2003;30(3):E48–E54. doi: 10.1188/03.ONF.E48-E54. doi: 0.1188/03.ONF. [DOI] [PubMed] [Google Scholar]
- 8.Champion V, Williams SD, Miller A, Reuille KM, Monahan P, Zhao Q. Predicting quality of life in long-term ovarian germ cell survivors: A Gynecologic Oncology Group Study. Gynecol Oncol. 2007;105(3):687–694. doi: 10.1016/j.ygyno.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershenson DM, Miller AM, Champion VL, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group study. J Clin Oncol. 2007;25(19):2792–7. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- 10.Monahan PO, Champion VL, Zhao Q, et al. Case-control comparison of quality of life in long-term ovarian germ cell tumor survivors: a Gynecologic Oncology Group study. J Psychosoc Oncol. 2008;26(3):19–42. doi: 10.1080/07347330802115715. [DOI] [PubMed] [Google Scholar]
- 11.Matei D, Miller AM, Monahan P. Chronic physical effects and health care utilization in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27(25):4142–9. doi: 10.1200/JCO.2008.20.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markman M, Rowinsky E, Hakes T, et al. Phase I Trial of Intraperitoneal Taxol: A Gynecologic Oncology Group Study. J Clin Oncol. 1992;10(9):1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- 13.O'Reilly S, Fleming GF, Baker S, et al. A phase I trial and pharmacologic trial of sequences of paclitaxel and topotecan in previously treated ovarian epithelial malignancies: A Gynecologic Oncology Group Study. J Clin Oncol. 1997;15(1):177–186. doi: 10.1200/JCO.1997.15.1.177. [DOI] [PubMed] [Google Scholar]
- 14.Rose P, Rodriguez M, Walker J, Greer B, Fusco N, McGuire W. Phase I study of liposomal doxorubicin (Doxil) and prolonged etoposide as second-line therapy in recurrent or persistant ovarian, tubal and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2002;85(1):136–9. doi: 10.1006/gyno.2002.6584. [DOI] [PubMed] [Google Scholar]
- 15.Moore DH, Donnelly J, McGuire W, et al. A limited access trial using amifostine for protection against cisplatin and 3-hour paclitaxel-induced neurotoxicity: a phase II study of the Gynecologic Oncology Group. Study J Clin Oncol. 2003;21(22):4207–13. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 16.Rose PG, Greer BE, Horowitz IR, Markman M, Fusco N. Paclitaxel, carboplatin and liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a phase I study of the Gynecologic Oncology Group. Gynecol Oncol. 2007;104(1):114–119. doi: 10.1016/j.ygyno.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Markman M, Bell J, Fusco N. Sequential prolonged oral topotecan and prolonged oral etoposide as second line therapy in ovarian or peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;102(2):236–9. doi: 10.1016/j.ygyno.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Rose PG, DeGeest K, McMeekin DS, Fusco N. A phase I study of gemcitabine followed by cisplatin concurrent with whole pelvic radiation therapy in locally advanced cervical cancer: A Gynecologic Oncology Group Study. Gynecol Oncol. 2007;107:274–9. doi: 10.1016/j.ygyno.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Greene MH, Piedmonte M, Alberts D, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: design and baseline characteristics: A Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2008;17(3):594–604. doi: 10.1158/1055-9965.EPI-07-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia A, Blessing J, Nolte S, Mannel R. A phase II evaluation of weekly docetaxel (NSC #628503) in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;111(1):22–6. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Rose PG, Sill MW, McMeekin DS, et al. A phase I study of concurrent weekly topotecan and cisplatin chemotherapy with whole pelvic radiation therapy in locally advanced cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:158–62. doi: 10.1016/j.ygyno.2011.12.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oncology Nursing Society. ONS role in multi-site research. Pittsburgh, PA: Author; 2004. Panel report. [Google Scholar]
- 23.O'Mara A, Bauer-Wu S, Berry D, Lillington L. A Needs Assessment of Oncology Nurses' Perceptions of National Cancer Institute-Supported Clinical Trial Networks. Oncol Nur Forum. 2007;34:E23–E27. doi: 10.1188/07.ONF.E23-E27. [DOI] [PubMed] [Google Scholar]
- 24.Donovan HS, Ward SE, Sereika S, et al. Web-Based Symptom Management for Women with Recurrent Ovarian Cancer: A Pilot Randomized Controlled Trial of the WRITE Symptoms Intervention. J Pain Symptom Manage. 2013 doi: 10.1016/j.jpainsymman.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–6. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 26.Donovan HS, Ward S, Song MK, Heidrich SM, Gunnarsdottir S, Phillips C. An update on the Representational Approach to patient education. J Nurs Scholarsh. 2007;39(3):259–65. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinds PS, Baggott C, DeSwarte-Wallace J, et al. Online exclusive: Functional integration of nursing research into a Pediatric Oncology Cooperative Group: Finding common ground. Oncol Nurs Forum. 2003;30:E121–E126. doi: 10.1188/03.onf.e121-e126. [DOI] [PubMed] [Google Scholar]
- 28.Landier W, Leonard M, Ruccione KS. Children's Oncology Group's 2013 blueprint for research: Nursing discipline. Pediatric Blood Cancer. 2012;60:1031–1036. doi: 10.1002/pbc.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawks R. Complementary and Alternative Medicine Research Initiatives in the Children's Oncology Group and the Role of the Pediatric Oncology Nurse. J Pediatr OncolNurs. 2006;23:261–264. doi: 10.1177/1043454206291358. [DOI] [PubMed] [Google Scholar]
- 30.Ruccione K, Patterson K. Pediatric oncology nursing in cooperative group clinical trials comes of age. Semin Oncol Nurs. 2000;16:253–260. doi: 10.1053/sonu.2000.16576. [DOI] [PubMed] [Google Scholar]
- 31.Ruccione KS, Hinds PS, DeSwarte-Wallace J, et al. Creating a novel structure for nursing research in a cooperative clinical trials group: The Children's Oncology Group Experience. Semin Oncol Nurs. 2005;21:79–88. doi: 10.1016/j.soncn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Hinds PS, DeSwarte-Wallace J. Positioning nursing research to contribute to the scientific mission of the Pediatric Oncology Cooperative Group. Semin Oncol Nurs. 2000;16:251–252. doi: 10.1053/sonu.2000.16575. [DOI] [PubMed] [Google Scholar]
- 33.Hinds PS, DeSwarte J. Pediatric oncology nursing research in the Children's Oncology Group: The second generation. Semin Oncol Nurs. 2005;21:77–78. doi: 10.1016/j.soncn.2004.12.001. 2005. [DOI] [PubMed] [Google Scholar]
- 34.Smith EL, Skosey C, Armer J, Berg D, Cirrincione C, Henggeler M. The cancer and leukemia group B oncology nursing committee (1983–2006): A history of passion, commitment, challenge, and accomplishment. Clin Cancer Res. 2006;12(11, Pt. 2):3638s–3641s. doi: 10.1158/1078-0432.CCR-06-9013. [DOI] [PubMed] [Google Scholar]
- 35.National Institutes of Health (May, 2013) Biomarker, Imaging, & Quality of Life Studies Funding Program (BIQSFP) [Accessed 10 2013]; http://biqsfp.cancer.gov/May2013.
- 36.IOM (Institute of Medicine) Multi-Center Phase III Clinical Trials and NCI Cooperative Groups: Workshop Summary. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]