Abstract
Rationale
Calmodulin (CaM) mutations are associated with an autosomal-dominant syndrome of ventricular arrhythmia and sudden death that can present with divergent clinical features of catecholaminergic polymorphic ventricular tachycardia (CPVT)or long QT syndrome (LQTS).CaM binds to and inhibits RyR2 Ca release channels in the heart, but whether arrhythmogenic CaM mutants alter RyR2 function is not known.
Objective
To gain mechanistic insight into how human CaM mutations affect RyR2 Ca channels.
Methods and Results
We studied recombinant CaM mutants associated with CPVT (N54I, N98S) or LQTS (D96V, D130G, F142L). As a group, all LQTS-associated CaM mutants(LQTS-CaMs) exhibited reduced Ca affinity, whereas CPVT-associated CaM mutants(CPVT-CaMs) had either normal or modestly lower Ca affinity. In permeabilized ventricular myocytes, CPVT-CaMs at a physiological intracellular concentration (100nM) promoted significantly higher spontaneous Ca wave and spark activity, a typical cellular phenotype of CPVT. Compared to wild-type (WT) CaM, CPVT-CaMs caused greater RyR2 single channel open probability and showed enhanced binding affinity to RyR2. Even a 1:8 mixture of CPVT-CaM:WT-CaM activated Ca waves, demonstrating functional dominance. By contrast, LQTS-CaMs did not promote Ca waves and exhibited either normal regulation of RyR2 single channels (D96V) or lower RyR2 binding affinity (D130G, F142L). None of the CaM mutants altered Ca/CaM binding to CaM-kinase II.
Conclusions
A small proportion of CPVT-CaM is sufficient to evoke arrhythmogenic Ca disturbances, whereas LQTS-CaMs do not. Our findings explain the clinical presentation and autosomal dominant inheritance of CPVT-CaM mutations and suggest that RyR2-interactions are unlikely to explain arrhythmogenicity of LQTS-CaM mutations.
Keywords: Calmodulin, Catecholaminergic Polymorphic Ventricular Tachycardia, Long QT Syndrome, Calcium, RyR2 Calcium Release Channel, Sarcoplasmic Reticulum
INTRODUCTION
Calmodulin(CaM) is an essential Ca binding protein that transduces Ca signals in a wide range of biological processes including muscle contraction, inflammation, metabolism, memory and immune responses. CaM binds to larger proteins and functions as a Ca sensor for decoding Ca signals into downstream responses.1, 2 CaM contains 4 EF-hand Ca binding motifs located in two symmetrical globular N-terminal (CaM-N) and C-terminal (CaM-C) domains connected by a flexible linker.3, 4 Humans have 3CaM genes – CALM1, CALM2, CALM3 – encoding the identical amino acid sequence, which is perfectly conserved among vertebrates, and highly conserved in non-vertebrates. CaM gene deletion is lethal in yeast5 and Drosophila.6
Recent genetic studies have identified CaM missense mutations in humans with severe ventricular arrhythmia and sudden cardiac death susceptibility,7, 8 albeit with distinct clinical presentations: two mutations in CALM17 were associated with stress-induced polymorphic ventricular tachycardia reminiscent of catecholaminergic polymorphic ventricular tachycardia (CPVT-CaMs), where as three other mutations in either CALM1 or CALM28 led to recurrent cardiac arrest in infancy associated with severe QT prolongation reminiscent of a long QT syndrome (LQTS-CaMs). A recent report has demonstrated that all three LQTS-CaMs (D96V, D130G, F142L) suppress Ca-dependent inactivation of L-type Ca currents and cause action potential prolongation, which can explain the long QT phenotype of mutation carriers.9 In contrast to LQTS, the cardiac action potential, and hence the QT interval, are not altered in CPVT,10 and the mechanism underlying CPVT caused by CaM mutations is not known.
CPVT can be caused by mutations in genes involved in Ca release from sarcoplasmic reticulum (SR)during excitation-contraction coupling.11 The most common autosomal-dominant form of CPVT is associated with mutations in the RYR2 gene encoding the ryanodine receptor (RyR2) SR Ca release channel.11 Autosomal-recessive CPVT is less common and has been associated with mutations in the genes encoding RyR2 binding proteins calsequestrin (Casq2) and triadin.11 Disease-causing CPVT mutations render RyR2 Ca release channels prone to spontaneous opening, resulting in spontaneous Ca release and propagated Ca waves that trigger membrane depolarizations, premature beats and polymorphic ventricular tachycardia during exercise or emotional stress.12
RyR2 channels bind CaM in cardiac muscle,13 and this interaction reduces channel open probability.14 Previous work has suggested that some CPVT-linked RYR2 mutations can impair CaM binding to RyR2 and thereby increase RyR2 channel openings and spontaneous Ca release.15 While impaired binding to RyR2 by mutant CPVT-CaMs is possible, all three CaM genes are expressed in the human heart,8 and it is not clear how a single point mutation in one of 6 CaM alleles could influence RyR2 activity to produce CPVT, especially if binding of the mutant CaM to its target on RyR2 is reduced. Alternatively, impaired Ca binding to the C-lobe of CaM has recently been shown to promote spontaneous Ca release in HEK cells expressing RyR2 channels.16 Although disease-linked CaM mutants were not investigated in that study,16 all LQTS-CaMs disrupt C-lobe Ca binding to CaM.8 This result would predict that all LQTS-CaMs would also cause RyR2 dysregulation which may contribute to the severe arrhythmia phenotype of patients carrying LQTS-linked CaM mutations. Hence, in addition to CPVT-CaMs, we also investigated the effect of LQTS-CaMS on RyR2 channels and myocyte Ca handling and thereby gain mechanistic insight into how CaM mutations cause ventricular arrhythmias and sudden death in humans.
METHODS
Animal use
The use of animals in this study was approved by the Institutional Animal Care and Use Committees and performed in accordance with NIH guidelines.
Measurement of Ca binding to CaM
To study the consequences of the human CaM disease-associated mutations on Ca binding, we bacterially expressed and purified recombinant wild-type (WT) and mutant CaMs. Ca binding affinities for WT, N54I, and N98S CaM proteins were determined as described.8 Briefly, macroscopic binding constants for the pairs of Ca binding sites in CaM-N and CaM-C were measured by monitoring the intrinsic tyrosine and phenylalanine fluorescence of the protein over the course of a Ca titration.17 The data were analyzed by plotting the normalized fluorescence signal vs free [Ca] and fitting to the model independent two site Adair function. The dissociation constants (Kd) for each domain are reported as the average value for the pair of sites by taking the square root of K2 from the Adair equation.
Ca wave experiments in ventricular myocytes
Single ventricular myocytes from 12 to 16 weeks-old mice were isolated as described.18 Myocytes were permeabilized with saponin (40µg/mL) for 60 seconds and placed in internal solution composed (in mM) of K-aspartate 100, KCl 15, KH2PO4 5, MgCl2 0.75, Dextran (40,000) 4 %, HEPES 10, MgATP 5, Phosphocreatine di-Na 10, Creatine phosphokinase 10 U/ml, Glutathione (reduced) 10, and Fluo 4 0.025. Free [Ca] was 0.120 nM. To allow equilibration of CaM binding to cellular targets, all Ca wave measurements were taken after 30 min incubation with either WT or mutant CaM alone, or with a mixture of WT and mutant CaMs. Free [CaM] was kept at the physiological concentration of 100 nM.19 Ca waves in myocytes were imaged with an confocal microscope in line-scan mode. Ca wave analysis was carried out as described.20 Given the variability between different experimental days, the Ca wave frequency and Ca amplitudes data were normalized to the mean of WT-CaM group obtained on the same day.
CaM binding to RyR2
To resolve CaM binding to RyR2, we used a FRET-based competition binding assay that detects acceptor-labeled CaM (A-CaM) binding in the proximity of donor-labeled FKBP (D-FKBP) pre-targeted to RyR2.21 Briefly, SR vesicles from porcine ventricular myocardium decorated with D-FKBP were used to prepare the FRET samples consisting of 3mg/ml SR, WT or mutant CaM, 100 nM A-CaM, 20 mM K-PIPES (pH 7.0), 150 mM KCl, 5 mM GSH, 1 mM EGTA, 0.1 mg/ml BSA, 1 µg/ml Aprotinin/Leupeptin, and sufficient CaCl2 to yield the indicated free [Ca2+]. After a 2.5 hr incubation at 25°C, the samples were transferred to a 384-well plate, and fluorescence spectra read in a fluorescence plate reader. FRET was calculated based on the fractional decrease of donor fluorescence (FD) in the presence of acceptor (FDA), according to FRET = 1 – FDA/FD.
Single channel recording of RyR2 activity
RyR2 was isolated from sheep hearts and incorporated into artificial lipid bilayers as described.22 RyR2 gating was measured at −40 mV in cytoplasmic solutions containing 250 mM Cs, 2 mM ATP, 10 µM KN93 and Ca of either 0.1 or 1 µM. Luminal solutions contained 250 mM Cs, 0.1 mM Ca, pH 7.4. A local perfusion technique that allows rapid fluid exchange (<1s)23 was used to apply vehicle solutions to RyR2 or vehicle plus 100 nM WT-CaM or CaM mutants. Single-channel recordings were analyzed for open probability using a 50% threshold method.
CaM-Kinase activation
WT and mutant CaMs binding affinity for CaMKII was measured using a plasmid encoding for FRET-based Camui as previously described.24
A more detailed description of the experimental methods can be found in the online data supplement.
RESULTS
Divergent effects of CaM mutations on C-domain Ca binding affinity
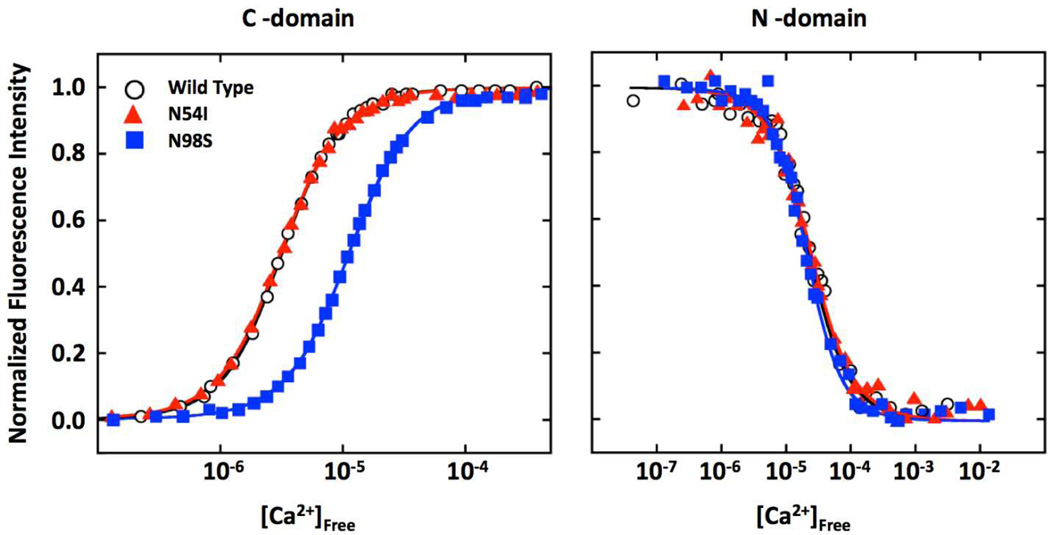
CaM has two high-affinity Ca binding sites (EF-hand III and IV) in the C-domain, and two slightly lower affinity Ca binding sites (EF-hand I and II) in the N-domain.25 The N54I mutation, which is located 2 residues before the first Ca coordinating residue of EF-hand II, had no significant effect on CaM-C or CaM-N Ca binding affinity compared to WT-CaM (Figure 1A). In contrast, the N98S mutation, which is in EF-hand III of CaM-C, decreased Ca affinity in CaM-C but had no effect on CaM-N (Figure 1). Our results are in agreement with a previous report that investigated only the C-domain Ca binding properties of these two mutants.7 Table 1 compares the Ca binding affinity of CPVT-CaMs with those reported for the three LQTS-CaMs. Note that all LQTS mutations affect CaM-C Ca affinity to a greater extent than the CPVT mutations, which either have no or only modest effects on Ca binding. These data suggest that lower Ca binding affinity is not the critical mechanism of CPVT caused by CaM mutants. Conversely, there is good correlation between impaired Ca binding and the LQTS phenotype.
Figure 1. Ca binding affinity of CPVT-CaMs.
Shown are Ca titration curves for WT and CPVT-CaMs (N54I, N98S) for the CaM-C and CaM-N domain. Data were used to derive dissociation constants (Kd, in µM) for the each domain. C-domain: 3.3±0.2 (WT), 3.1±0.2 (N54I), 11±1 (N98S). N-domain: 23±3 (WT), 23 ±3 (N54I), 22±2 (N98S). Values are averages of three experiments and error was determined by analysis of the curve fits.
Table 1.
Fold reduction in Ca binding affinity of LQTS- and CPVT-CaMs. Kd values were obtained from Figure 1 and reference8 and compared to control Kd values for WT-CaM.
| CaM mutation | CaM–C domain Kd (Fold-reduction) |
CaM–N domain Kd (Fold-reduction) |
Clinical arrhythmia syndrome |
|---|---|---|---|
| D130G | 53.6 | No change | LQTS |
| D96V | 13.6 | No change | LQTS |
| F142L | 5.4 | No change | LQTS |
| N98S | 3.3 | No change | CPVT |
| N54I | No change | No change | CPVT |
Only CPVT CaM mutants promote Ca waves
We next examined the functional consequences of CPVT-CaMs and LQTS-CaMs on cellular Ca handling in saponin-permeabilized murine ventricular cardio myocytes. Experiments were carried out at physiological free [CaM] of 100 nM19 and diastolic free [Ca] of 120 nM. Under those conditions, murine myocytes exhibit spontaneous Ca release in the form of regular propagated Ca waves (Figure 2A). In this bioassay, CPVT mutations in RyR2 or Casq2 that promote higher RyR2 channel open probability cause greater frequency and lower amplitude of spontaneous Ca wave.20 Furthermore, drugs such as flecainide that inhibit RyR2 channels and reduce Ca wave frequency in this bioassay20 have been shown to prevent CPVT in mice and humans.26–28 LQTS-CaMs either had no effect or evoked slightly lower Ca wave frequency (Figure 2). In contrast, both CPVT-CaMs caused significantly greater Ca wave frequency, similar to the higher Ca wave frequency observed in WT-CaM-treated myocytes lacking Casq2 (Casq2 KO, Figure 2), an established mouse model of genetic CPVT.18 Moreover, flecainide treatment effectively suppressed Ca waves (Online Figure I), suggesting that flecainide may be useful for treating CPVT associated with CaM mutations.
Figure 2. Only CPVT-CaMs promote spontaneous Ca waves in permeabilized myocytes.
(A) Representative line-scans (red arrow) from permeabilized mouse ventricular myocytes after 30 min incubation with either WT or mutant CaMs (100nM). CPVT-CaMs promoted higher Ca wave frequency (B) and lower Ca wave amplitude (C). After permeabilization, myocytes were incubated in internal solution composed of 120 nM free [Ca] (calculated using Max Chelator), 100 µM EGTA, and 25 µM Fluo 4. Bars represent mean + SE of values normalized by WT values on each experimental day. WT (white, n=45), D96V (light grey, n=33), D130G (grey, n=15), F142L (dark grey, n=20), N54I (red, n=35), N98S (blue, n=35). Casq2KO: myocytes isolated from Casq2 null mice and incubated with WT-CaM (violet, n=21). *P < 0.05, **P < 0.01 vs WT CaM.
One plausible explanation for their differential effect on Ca waves is that CPVT and LQTS CaMs bind to RyR2 with different affinity. Hence, we used a FRET-based assay to specifically measure CaM bound to RyR2 in SR vesicles (as opposed to total CaM bound to SR).21, 29 RyR2-specific CaM binding was measured by FRET between fluorescent FKBP12.6 and fluorescent CaM (F-CaM). Figure 3A illustrates how WT and CaM mutants compete with 100 nM F-CaM and reduce FRET, at either 30 nM or 30 µM [Ca]. Strikingly, both CPVT-CaMs displaced F-CaM more effectively than did WT-CaM (p < 0.05, n=4) at low [Ca] relevant to diastolic [Ca] in the heart. At very high [Ca] (30 µM), which should saturate CaM, CPVT-CaMs were no better than WT-CaM at displacing F-CaM (Figure 3). Interestingly, 2 of 3 LQTS-CaMs (D130G and F142L) exhibited much lower RyR2 affinity than WT-CaM in nM Ca (Figure 3). On the other hand, the D96V LQTS-CaM mutant had similar RyR2 affinity as the CPVT-CaMs (Figure 3), indicating that differential RyR2 binding cannot be the sole explanation for the divergent action of mutant CaMs on Ca waves. Rather, CaM mutants may modulate RyR2 channel activity differentially.
Figure 3. CPVT-CaMs bind with higher affinity to RyR2 Ca release channels in nanomolar Ca conditions.
(A) In cardiac SR vesicles stripped of native CaM, RyR2 was pre-labeled with AF488-FKBP (donor), then incubated with 100 nM AF568-CaM (acceptor) and a series of concentrations of WT and mutant (Mut) CaMs (D96V light grey, D130G grey, F142L dark grey, N54I red, N98S blue). AF568-CaM binding to RyR2 was determined from the decrease in AF488-FKBP fluorescence due to FRET. Data are mean ± SE, n=3–4, for each concentration. (B) Mean ratio of mutant and WT IC50 of CaM binding to RyR2. *P < 0.05 vs WT-CaM, n=3–4 experiments per group.
Only CPVT-CaMs increase RyR2 single-channel open probability (Po)
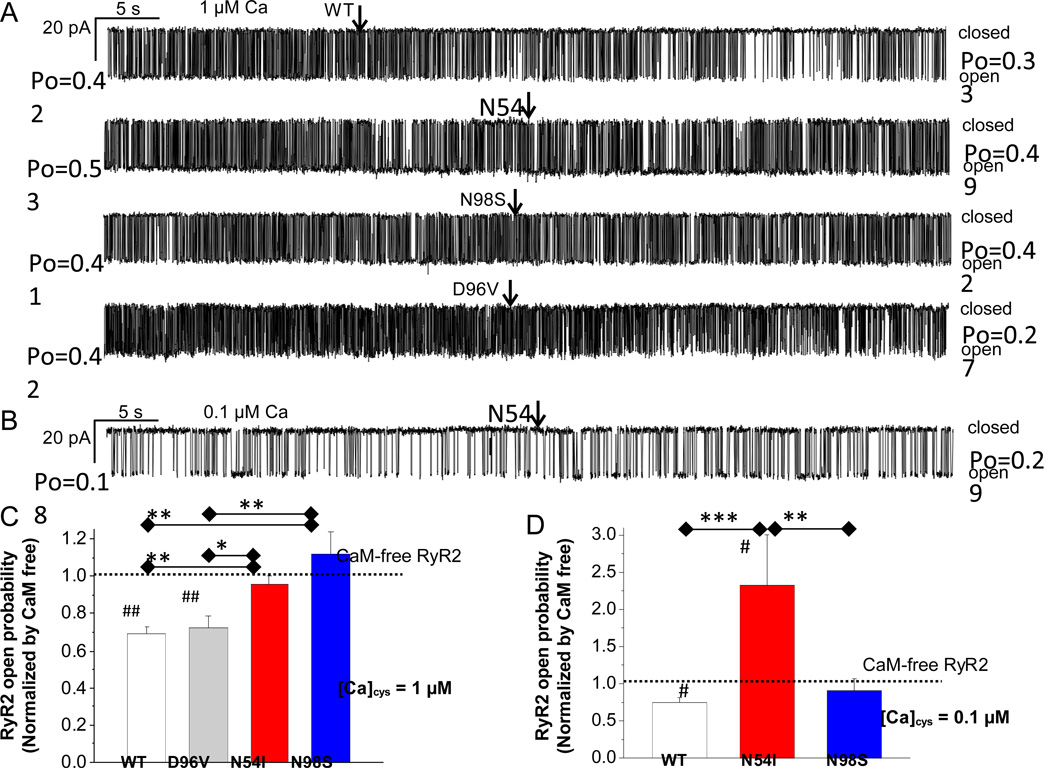
To test this hypothesis directly, we next applied 100 nM of WT-CaM, CPVT-CaM (N54S, N98S) or LQTS-CaM(D96V) to single RyR2 channels incorporated into lipid bilayers. Experiments were carried out at cytosolic [Ca] of 1 µM to activate RyR2 channels. All solutions contained KN93 (10 µM) to prevent CaMKII activation. As illustrated in Figure 4A, adding WT-CaM to CaM-free RyR2 channels significantly reduced their activity (i.e., lowered Po), which is consistent with the inhibitory action of CaM on RyR2 channels.30 In contrast, single channel Po was unchanged after application of either CPVT-CaM (N54I, N98S), resulting in a significantly higher RyR2 single-channel Po compared to WT-CaM (Figure 4A,C). On the other hand, the effect of LQTS-CaM D96V on single RyR2 channels was comparable to that of WT-CaM (Figure 4A,C), a result that is consistent with the lack of Ca wave activation by D96V (Figure 2). We next performed experiments at cytosolic [Ca] of 0.1 µM to mimic diastolic conditions. Applying CPVT-CaM N54I to RyR2 channels devoid of endogenous CaM significantly increased RyR2 channel activity (Figure 4B,D),whereas N98S-CaM had no significant effects on RyR2 channel activity(Figure 4D). As a result, N54I-CaM significantly increased RyR2 channel Po compared to either WT-CaM or N98S-CaM (Figure 4D). These results indicate that the N98S CPVT mutant fails to inhibit RyR2 channels, whereas N54I not only lacks inhibitory action, but also directly activates RyR2 channels at diastolic [Ca].
Figure 4. Abnormal regulation of single RyR2 channels by CPVT-associated CaM mutants.
(A) Continuous records of RyR2 gating in the absence of CaM. Cytoplasmic bath contained 1 µM [Ca]. Arrows indicate addition of 100nM of WT-CaM, CPVT-CaMs (N54I, N98S) or LQTS-CaM (D96V). Solution changes were achieved within <5 s using local perfusion of the RyR2 (see methods). Average open probability (Po) values before and after adding CaM are given at the left and right of each trace, respectively. (B) Continuous records of a single RyR2 beginning in the absence of CaM at 0.1 µM [Ca]cyt. Addition of 100 nM N54I CaM (arrow) increased RyR2 channel activity. Average effect of WT and mutant CaMs on RyR2 Po, at 1 µM (C) and 0.1 µM cytosolic [Ca] (D). Po values were obtained from 1–2 min of continuous recording under each condition. Before averaging, each Po value was normalized to the Po of the same RyR2 channel obtained before adding CaM. *P < 0.05, **P < 0.01, **P < 0.001 by Mann-Whitney Test, #P < 0.05, ##P < 0.01 by Wilcoxon Signed-Rank Test versus CaM-free RyR2. n=10–14 records per group.
We next measured the effect of CPVT-CaMs on Ca sparks, which are Ca release events by clusters of RyR2 channels in myocytes.31 To prevent wave propagation, Ca sparks were measured at 50 nM free [Ca] with strong buffering by 0.5 mM EGTA. SR Ca content was assessed by rapid caffeine application because it strongly influences SR Ca leak,32 Ca spark frequency, and amplitude.33 Adding 100 nM WT-CaM to myocytes pre-depleted of endogenous CaM evoked lower Ca spark frequency (Figure 5A,B) and lower SR Ca leak, which also resulted in greater SR Ca content (Figure 5C,D). The rise in SR Ca content by itself would tend to promote Ca sparks, so the lower Ca spark frequency underestimates the RyR2 effect of CaM. Compared to WT-CaM, both CPVT-CaMs evoked significantly greater Ca spark frequency (Figure 5A,B), and this effect was enhanced by cyclic AMP (Online Figure II),which was used to model adrenergic stress. Despite our conditions, which robustly suppress Ca waves in WT myocytes, Ca waves were observed occasionally with CPVT-CaMs (especially N98S), but not in either CaM-free or WT-CaM exposure. This agrees with the promotion of Ca waves by N54I and N98S illustrated in Figure 2 (at higher [Ca] and lower [EGTA]). CPVT-CaMs also reduced spark amplitude, increased spark duration and spark width (Online Table I). These data are consistent with the single-channel results (Figure 4), and indicate that CPVT-CaMs promote rather than inhibit RyR2 channel activity, resulting in an increased SR Ca leak and reduced SR Ca content.
Figure 5. CPVT-CaMs promote Ca sparks and lower SR Ca content.
(A) Representative line-scan images of Ca sparks in permeabilized rat ventricular myocytes in CaM-free, WT-CaM, and CPVT-CaMs (N54I, N98S). Line plots of spark events (red arrow) are indicated below their images. (B) Average Ca spark frequency. Bars represent mean + SE. CaM-free (black, n=5), WT (white, n=9), N54I (red, n=8), N98S (blue, n=10).(C) Line-scan (top) and line plot (bottom) examples of SR Ca2+ content evaluated by 10 mM caffeine-evoked Ca transient in WT-CaM. (D) Average SR Ca content. CaM-free (black, n=5), WT (white, n=6), N54I (red, n=6), N98S (blue, n=7). [Ca]i = 50 nM, EGTA = 0.5 mM, [WT] or [mutant CaMs] = 100 nM. **P < 0.01, ***P < 0.001 vs. WT-CaM.
CPVT-CaMs exhibit dominant activating effects on Ca waves
Given that only 1 out of 6 CaM alleles in patients has a mutation, another important question is whether CPVT-CaMs can exert a dominant effect on RyR2 function in the presence of excess WT-CaM. To address this question, we performed mixing studies that tested the effects of various ratios of CPVT-CaMs and WT-CaM on Ca wave frequency (Figure 6). We found that even in the presence of 8-fold excess of WT-CaM (87.5%), CPVT-CaMs promoted significantly higher Ca wave frequencies (Figure 6B). These results are consistent with the higher affinity of CPVT-CaMs for RyR2 at low Ca (Figure 3), which suggests that the fraction of RyR2 bound to N54I or N98S (vs. WT-CaM) would be higher than their relative expression level.
Figure 6. CPVT-CaMs exhibit a dominant effect on Ca waves.
(A) Representative line-scans (red arrow) from permeabilized mouse myocytes after 30 min incubation with either WT-CaM or CPVT-CaMs mixed with WT-CaM (0%, 50%, 75%, and 87.5% (total free [CaM] = 100nM) for 30 min. (B) Ca wave frequency and (C) amplitude for each group. Bars represent mean + SE of values normalized by WT values on each experimental day. WT (white, n=39); N54I (red, n=21–37 each), N98S (blue, n=27–37 each)*P < 0.05, **P < 0.01. vs. WT-CaM.
CaMKII does not contribute to RyR2 activation by CPVT-CaMs
An important CaM target in cardiac muscle is Ca/CaM-dependent protein kinase II (CaMKII). CaMKII activation has been implicated in a number of heart diseases associated with increased SR Ca leak and Ca triggered arrhythmias including CPVT.34 RyR2 phosphorylation by CaMKII increases RyR2 Po and the frequency of spontaneous Ca waves.35, 36 Hence, we tested whether CaMKII activation by disease-associated CaM mutants contributes to their effect on Ca waves. Blocking CaMKII activation by KN93 significantly reduced Ca wave frequency in myocytes incubated with WT-CaM or LQTS-CaM D96V but had no effect on Ca wave frequency in myocytes exposed to the other CaM mutants (Figure 7 A,B). The results raise the possibility that (other than D96V) CaM mutants might impede CaMKII activation by Ca/CaM. To test this idea more directly, we measured the [CaM]-dependence of CaMKII activation using Camui, a FRET-based reporter of CaMKII activation state.24 Camui FRET is reduced when Ca/CaM binds to and opens up the CaMKII structure, a critical step in activation. None of the CaM mutants significantly altered the apparent CaMKII affinity at 200 µM [Ca] (Figure 7C,D). However, WT and D96V had the lowest Kd values, which would be consistent with their having some CaMKII-dependent effect on Ca waves (Figure 7A). Taken together, these results essentially exclude CaMKII activation as a mechanism responsible for activation of RyR2 and arrhythmogenic Ca waves generated by CPVT-CaMs.
Figure 7. CaMKII activation is not responsible for the effect of CPVT-CaMs on Ca waves.
(A), (B) Effect of CaMKII inhibition with KN93 on Ca wave frequency and amplitude. Permeabilized myocytes were incubated with 100nM of CaM mutants in presence or absence of KN93 (1µM, 30 min pre-incubation). Bars represent mean + SE. WT (white, n=40), D96V (light grey, n=20), D130G (grey, n=15), F142L (dark grey, n=20), N54I (red, n=29), N98S (blue, n=12), *P < 0.05 vs + KN93. With the exception of WT-CaM and LQTS-CaM D96V, CaMKII activation by CaM did not contribute to the effect of CaM mutants on Ca waves. (C) CaM-dependent activation of CaMKII (WT-Camui) measured in HEK 293 cell lysate at 37°C with saturating Ca (200 µM). CaM±1mM EGTA (black, n=13 each), N54I (red, n=9), N98S (blue, n=10), D96V (light grey, n=11), F142L (grey, n=9) and D130G (dark grey, n=10). Decreases in FRET were normalized for each mutant (maximal FRET change was similar to WT for all mutants, except F142L and D96V where it was smaller). (D) [CaM] for half-maximal change in FRET (K0.5) obtained from panel C for WT and mutant CaM.
DISCUSSION
The major finding reported here is that unlike WT-CaM, CPVT-CaMs exert an activating effect on RyR2 Ca release channels and evoke higher frequencies of spontaneous Ca waves in murine ventricular myocytes. The cellular Ca handling defect induced by CPVT-CaMs is similar to that reported for CPVT-linked mutations in RYR2 andCASQ2.18, 37 CPVT-CaMs bind to RyR2 with higher affinity than WT-CaM, which likely contributes to their dominant action in the heart. At the same time, CPVT-CaMs have near normal Ca binding affinities and may function normally as Ca sensors, which could explain why two mutations in the ubiquitously expressed CALM1 gene are only associated with a specific CPVT phenotype. In contrast, LQTS-CaMs lack direct effects on SR Ca release, which can be explained either by their reduced RyR2 binding affinity (D130G, F142L), or normal inhibitory regulation of RyR2 channels (D96V). Collectively, our results provide new mechanistic insight into the divergent human arrhythmia phenotypes caused by CaM mutations.
Studying mouse models expressing human CPVT-linked mutations, investigators have consistently found higher rates of spontaneous Ca release, delayed after depolarizations and triggered beats in cardiac muscle.12 Furthermore, drugs that suppress the spontaneous SR Ca release in single cells are also effective in preventing exercise or stress-induced CPVT in mice and humans.28, 38 Hence, it is generally accepted that the dysfunctional and premature spontaneous Ca release from the SR is the culprit of the CPVT observed in vivo. Investigators have proposed several different mechanisms to explain the propensity of spontaneous SR Ca release associated with CPVT mutations: loss of regulation of RyR2 mutant channels by luminal Ca resulting in store-overload induced Ca release,39 defective inter-domain interaction of RyR2 mutant channels,40 reduced FKBP12.6 binding to RyR2,41 loss of luminal Ca sensing and Ca release refractoriness due to loss of Casq2 and/or triadin,18, 42, 43 or impaired CaM binding to PKA-phosphorylated RyR2-mutant channels.15 Our results suggest a new mechanism that is responsible for CPVT caused by CALM1 mutations, a mechanism that is distinct from those reported previously for CPVT mutations in other genes: CPVT-CaMs bind with greater affinity to RyR2 channels, but at the same time fail to inhibit or even activate RyR2 channels, rendering them hyperactive, which generates arrhythmogenic Ca waves and leads to SR Ca store depletion. However, the CPVT-CaMs produced this Ca wave activation by distinct RyR2 single-channel phenotypes. The N-domain mutation N54I appears to directly activate RyR2 channels in anagonist-like action similar to caffeine. In contrast, N98S located in the C-domain does not activate RyR2 channels by itself, but fails to inhibit RyR2 channels, especially at higher cytosolic [Ca]. These different single channel mechanisms of action could explain why the N54I mutant had more potent activating effects on Ca waves and Ca sparks at low [Ca] than N98S (Figures 2 and 5).
Both CPVT-CaMs exhibit dominant-activating effects on Ca waves when mixed with WT-CaM (Figure 6), which is consistent with an autosomal-dominant inheritance pattern in humans. What are possible reasons for this functional dominance? One possibility is based on the fact that the tetrameric RyR2 Ca release channel complex has 4 CaM binding sites.44 Binding of only a single mutant CaM may be sufficient to disrupt the CaM-dependent regulation of the RyR2 channel complex, analogous to the dominant effects of point mutations in K-channel monomers that function as tetrameric complexes in the cell membrane. Thus, single amino-acid changes in CaM may substantially alter the gating of a large RyR macromolecular complex. For example, WT-CaM has an activating effect on skeletal RyR1 channels at nanomolar [Ca], but an inhibitory effect at high micromolar [Ca],45 suggesting that the CaM binding domain (CBD) in both RyR1 and RyR2 can transduce large changes in gating that can be either stimulatory or inhibitory. It is possible that the CPVT-CaMs exert an activating effect on RyR2 gating at diastolic [Ca] (analogous to effects of WT CaM on RyR1) despite having only slightly altered Ca affinity (Table 1).46 In their initial report of CaM mutations associated with a clinical CPVT phenotype, Nyegaard et al. assessed the interaction of the two CPVT-CaMs with a RyR2-derived peptide encompassing the putative CaM binding domain by monitoring the RyR2 peptide’s Trp3586 fluorescence-emission spectra.7 While results for N54I were not different from WT-CAM, the interaction of N98S-CaM with the RyR2 peptide was impaired, selectively at 100 nM [Ca] (but not at 1–200 µM [Ca]).CaM binding to native, full-length RyR2 channels or RyR2 function was not assessed in that report. Based on their results, Nyegaard and colleagues concluded that the loss-of-function with regard to the RyR2 interaction may lead to inappropriate RYR2 leakiness, similar to CPVT caused by RYR2 mutations that reduce CaM binding to RyR2.15 In contrast, our studies using native RyR2 channels suggest a mechanism of action that is quite different from that proposed for RYR2 mutations: CaM mutations that reduce binding affinity to RyR2 (i.e., LQTS CaM D130G and F141L, Figure 3) do not cause CPVT. Rather, we found that CaM mutations resulting in CPVT (CPVT CaM N54I and N98S) bind to native RyR2 with higher affinity than WT-CaM(Figure 3) and that this interaction changes the CaM effect on RyR2 from an inhibitory to a stimulatory effect, especially for N54I. Given that there are 3 genes encoding identical CaM proteins which are all expressed in the human heart,8 it is likely that both mechanisms (increased RyR2 binding and stimulatory action) contribute to the dominant effect of the CALM1 mutations observed in CPVT patients. Even a minority of RyR2 channels with mutant CaM bound could be enough to nucleate Ca-induced Ca-release as spontaneous Ca waves and trigger CPVT in vivo.
CaM binds to multiple other targets in the heart, hence, we cannot exclude that other mechanisms contribute to the autosomal-dominant inheritance of CPVT caused by CALM1 mutations. One important target is CaMKII, which after activation by Ca/CaM can phosphorylate RyR2 channels, produce spontaneous Ca waves, deplete SR Ca stores and triggered arrhythmia.36 However, based on our findings that CaM mutants did not differentially regulate CaMKII (Figure 7) and CaMKII inhibition by KN93 did not prevent the effects of CPVT-CaMs on Ca waves (Figure 7) and RyR2 single channels (Figure 4), altered CaMKII regulation is probably not a culprit in CPVT caused by CaM mutations. Another important CaM target involved in arrhythmogenesis is the L-type Ca channel CaV1.2. Apo-CaM pre-bound the C-terminus of Cav1.2 importantly regulates Ca-dependent channel inactivation.47 CaM mutations in EF-hand motifs that prevent Ca-binding to CaM severely disrupt Ca-dependent inactivation of Cav1.2 channels,48 an effect that has been recently reported for the three LQTS-CaMs (D96V, D130G, N142L).9 Accordingly, impaired Ca-dependent inactivation and the ensuing excess L-type Ca current during the phase III of the action potential has been proposed as the underlying mechanism responsible for the QT prolongation caused by LQTS-CaMs,9 all of which are located in a EF-hand and significantly impair Ca binding to CaM (Table 1). In contrast, CPVT-CaMs exhibit only modest or no impairment of Ca binding (Table 1), a likely explanation as to why these mutations do not cause QT prolongation. Other potentially arrhythmogenic CaM targets in the heart include the cardiac Na channels, K channels, and calcineurin.2, 36 It may be worthwhile to explore how mutant CaMs interact with and regulate those targets as well.
An important result of our studies is that LQTS-CaMs had no effect on SR Ca release (Figure 2), which is consistent with the lack of a clinical CPVT phenotype in this group of mutation carriers.8 For the LQTS-CaM D96V, which has normal RyR2 binding affinity (Figure 3), we show that it is capable of physiological inhibition of single RyR2 channels akin to WT-CaM (Figure 4). For the other two LQTS-CaMs (D130G and F142L), the absence of Ca waves modulation may be explained by their lower binding affinity to RyR2 compared to WT-CaM (Figure 3).Because all LQTS-CaMs also drastically impair C-lobe Ca binding (Table 1), our results appear at odds with a recent report suggesting that impaired Ca binding to the C-lobe of CaM promotes spontaneous Ca release.16 A possible explanation for the differences is that those experiments were conducted in HEK cells over expressing mutant CaMs and LQTS-CaM mutations were not studied,16 whereas our experiments were carried out at physiological free [CaM] of 100 nM in native ventricular myocytes. Although we did not study the effect of CaM mutants on arrhythmias at the cellular or in vivo level, we nevertheless find that the divergent actions of the mutant CaMs on Ca binding, Ca wave modulation, and RyR2 regulation were consistent with the distinct human arrhythmia phenotypes. Thus, the in vitro assays described in our report could be useful for evaluating the pathogenicity of other human CaM mutations that undoubtedly will be discovered in the future.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Calmodulin (CaM) is an essential Ca binding protein that functions as a Ca sensor for decoding Ca signals into downstream responses.
The ryanodine receptor Ca release channel (RyR2) is a major CaM binding site in heart muscle and CaM binding inhibits the opening of RyR2.
Recent genetic studies have identified CaM missense mutations in humans with different ventricular arrhythmia and sudden cardiac death syndromes: Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) and congenital long QT syndrome (LQTS).
What New Information Does This Article Contribute?
CaM mutations that produce a CPVT phenotype in humans (CPVT-CaMs) bind to RyR2 with higher affinity than wild-type CaM and activate rather than inhibit RyR2 channels.
Small amounts of CPVT-CaMs in presence of physiological concentrations of wild-type (WT) CaM are sufficient to produce spontaneous Ca release in myocytes, which likely represents the mechanism underlying autosomal dominant inheritance of CPVT-CaM mutations.
LQTS-associated CaM mutations (LQTS-CaMs) have either lower RyR2 binding affinity or regulate RyR2 normally, suggesting that RyR2 interactions are unlikely to explain arrhythmogenicity of LQTS-CaM mutations.
CaM missense mutations are associated with arrhythmia and sudden cardiac death susceptibility in humans, but how mutant CaMs cause arrhythmia susceptibility is not known. Since RyR2 channels are regulated by CaM, and abnormal RyR2 channel activity can trigger ventricular arrhythmias, we hypothesized that arrhythmogenic CaM mutants alter RyR2 function. We find that unlike WT-CaM, CPVT-CaMs activate RyR2 and promote spontaneous Ca waves in myocytes. The cellular Ca handling defect induced by CPVT-CaMs is similar to that reported for CPVT-linked mutations in RYR2 and CASQ2 genes. CPVT-CaMs bind to RyR2 with higher affinity than WT-CaM, which likely contributes to their dominant action in the heart. At the same time, CPVT-CaMs have near normal Ca binding affinities and may function normally as Ca sensors, which could explain why mutations in the ubiquitously expressed CaM are only associated with a specific CPVT phenotype. In contrast, LQTS-CaMs lack direct effects on Ca release, either due to their reduced RyR2 binding affinity or normal inhibitory regulation of RyR2 channels. Collectively, our results provide new mechanistic insight into the divergent human arrhythmia phenotypes caused by CaM mutations. Furthermore, the in vitro assays described here could be useful for evaluating the pathogenicity of other human CaM mutations that undoubtedly will be discovered in the future.
ACKNOWLEDGEMENTS
DRL acknowledges technical assistance with experiments from Paul Johnson.
SOURCES OF FUNDING
This work was supported in part by the US National Institutes of Health (NIH) grants HL88635 & HL71670 (to BCK), HL083374 (ALG), R37-HL30077 (to DMB), R01-HL92097 (DMB & RLC), T32 AR007612 (FRN), by an American Heart Association (AHA) Innovative Research Grant (to BCK), AHA Scientist Development Grant (to HSH), AHA Postdoctoral Fellowship (to CNJ, LP), and an NH&MRC grant to DRL, BCK.
Nonstandard Abbreviations and Acronyms
- CaM
Calmodulin
- Casq2
calsequestrin, cardiac isoform
- CaMKII
Ca/CaM kinase II
- CPVT
Catecholaminergic Polymorphic Ventricular Tachycardia
- FRET
Fluorescence resonance energy transfer
- LQTS
Long QT Syndrome
- Po
Single channel open probability
- RyR2
Ryanodine receptor Ca release channel
- SR
Sarcoplasmic Reticulum
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 2.Saucerman JJ, Bers DM. Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes. J Mol Cell Cardiol. 2012;52:312–316. doi: 10.1016/j.yjmcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu YS, Sack JS, Greenhough TJ, Bugg CE, Means AR, Cook WJ. Three-dimensional structure of calmodulin. Nature. 1985;315:37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- 4.Barbato G, Ikura M, Kay LE, Pastor RW, Bax A. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 1992;31:5269–5278. doi: 10.1021/bi00138a005. [DOI] [PubMed] [Google Scholar]
- 5.Davis TN, Urdea MS, Masiarz FR, Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986;47:423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Sullivan KMC, Beckingham K. Drosophila Calmodulin Mutants With Specific Defects in the Musculature or in the Nervous System. Genetics. 2003;165:1255–1268. doi: 10.1093/genetics/165.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyegaard M, Overgaard MT, Sondergaard MT, Vranas M, Behr ER, Hildebrandt LL, Lund J, Hedley PL, Camm AJ, Wettrell G, Fosdal I, Christiansen M, Borglum AD. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotti L, Johnson CN, Graf E, De Ferrari GM, Cuneo BF, Ovadia M, Papagiannis J, Feldkamp MD, Rathi SG, Kunic JD, Pedrazzini M, Wieland T, Lichtner P, Beckmann BM, Clark T, Shaffer C, Benson DW, Kaab S, Meitinger T, Strom TM, Chazin WJ, Schwartz PJ, George AL., Jr Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limpitikul W, Joshi-Mukherjee R, Dick IE, George AL, Yue DT. Abstract 17783: Calmodulin Mutants Associated With Long QT Syndrome Suppress Inactivation of Cardiac L-type Ca2+ Currents and Prolong Action Potentials in Guinea-Pig Ventricular Myocytes. Circulation. 2013;128:A17783. [Google Scholar]
- 10.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 11.Chopra N, Knollmann BC. Genetics of sudden cardiac death syndromes. Curr Opin Cardiol. 2011;26:196–203. doi: 10.1097/HCO.0b013e3283459893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe H, Knollmann BC. Mechanism underlying catecholaminergic polymorphic ventricular tachycardia and approaches to therapy. J Electrocardiol. 2011 doi: 10.1016/j.jelectrocard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Meissner G. Mechanism of calmodulin inhibition of cardiac sarcoplasmic reticulum Ca2+ release channel (ryanodine receptor) Biophys J. 2004;86:797–804. doi: 10.1016/S0006-3495(04)74155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ikemoto N, Matsuzaki M. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–666. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian X, Tang Y, Liu Y, Wang R, Chen SR. Calmodulin modulates the termination threshold for cardiac ryanodine receptor-mediated Ca2+ release. Biochem J. 2013;455:367–375. doi: 10.1042/BJ20130805. [DOI] [PubMed] [Google Scholar]
- 17.VanScyoc WS, Sorensen BR, Rusinova E, Laws WR, Ross JB, Shea MA. Calcium binding to calmodulin mutants monitored by domain-specific intrinsic phenylalanine and tyrosine fluorescence. Biophys J. 2002;83:2767–2780. doi: 10.1016/S0006-3495(02)75286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2007;41:353–364. doi: 10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galimberti ES, Knollmann BC. Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca2+ waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol. 2011;51:760–768. doi: 10.1016/j.yjmcc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo T, Fruen BR, Nitu FR, Nguyen TD, Yang Y, Cornea RL, Bers DM. FRET detection of calmodulin binding to the cardiac RyR2 calcium release channel. Biophys J. 2011;101:2170–2177. doi: 10.1016/j.bpj.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Imtiaz MS, Beard NA, Dulhunty AF, Thorne R, vanHelden DF, Laver DR. ss-Adrenergic stimulation increases RyR2 activity via intracellular Ca2+ and Mg2+ regulation. PLoS One. 2013;8:e58334. doi: 10.1371/journal.pone.0058334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill ER, Sakowska MM, Laver DR. Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophysical Journal. 2003;84:1674–1689. doi: 10.1016/S0006-3495(03)74976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circ Res. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seamon KB. Calcium- and magnesium-dependent conformational states of calmodulin as determined by nuclear magnetic resonance. Biochemistry. 1980;19:207–215. doi: 10.1021/bi00542a031. [DOI] [PubMed] [Google Scholar]
- 26.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, Willems AR, van der Veen MJ, Watanabe H, Laborderie J, Haissaguerre M, Knollmann BC, Wilde AA. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H, Knollmann BC. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornea RL, Nitu F, Gruber S, Kohler K, Satzer M, Thomas DD, Fruen BR. FRET-based mapping of calmodulin bound to the RyR1 Ca2+ release channel. Proc Natl Acad Sci U S A. 2009;106:6128–6133. doi: 10.1073/pnas.0813010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JS, Rousseau E, Meissner G. Calmodulin modulation of single sarcoplasmic reticulum Ca2+-release channels from cardiac and skeletal muscle. Circ Res. 1989;64:352–359. doi: 10.1161/01.res.64.2.352. [DOI] [PubMed] [Google Scholar]
- 31.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 32.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 33.Zima AV, Bovo E, Bers DM, Blatter LA. Ca(2)+ spark-dependent and -independent sarcoplasmic reticulum Ca(2)+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. J Mol Cell Cardiol. 2010;50:214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 36.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu N, Priori SG. Disruption of calcium homeostasis and arrhythmogenesis induced by mutations in the cardiac ryanodine receptor and calsequestrin. Cardiovasc Res. 2008;77:293–301. doi: 10.1093/cvr/cvm004. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of Disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3:43–52. doi: 10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]
- 41.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 42.Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal Interactions of Calsequestrin With the Ryanodine Receptor Calcium Release Channel Complex Linked to Exercise-Induced Sudden Cardiac Death. Circ Res. 2006 doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 43.Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD, Franzini-Armstrong C, Knollmann BC. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca(2+) release channel (ryanodine receptor) J Biol Chem. 2003;278:23480–23486. doi: 10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 45.Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Membr Biol. 2002;185:1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 46.Fruen BR, Balog EM, Schafer J, Nitu FR, Thomas DD, Cornea RL. Direct detection of calmodulin tuning by ryanodine receptor channel targets using a Ca2+-sensitive acrylodan-labeled calmodulin. Biochemistry. 2005;44:278–284. doi: 10.1021/bi048246u. [DOI] [PubMed] [Google Scholar]
- 47.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 48.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.