Abstract
Phospholipases A2 (PLA2s) are important enzymes for metabolism of fatty acids in membrane phospholipids. Among the three major classes of PLA2s in the mammalian system, the group IV calcium-dependent cytosolic PLA2 alpha (cPLA2α) has received the most attention because it is widely expressed in nearly all mammalian cells and its active participation in cell metabolism. Besides Ca2+ binding to its C-2 domain, this enzyme can undergo a number of cell-specific post-translational modifications, including phosphorylation by protein kinases, S-nitrosylation through interaction with nitric oxide (NO), as well as interaction with other proteins and lipid molecules. Hydrolysis of phospholipids by cPLA2 yields two important lipid mediators, arachidonic acid (AA) and lysophospholipids. While AA is known to serve as a substrate for cyclooxygenases and lipoxygenases, which are enzymes for synthesis of eicosanoids and leukotrienes, lysophospholipids are known to possess detergent-like properties capable of altering micro-domains of cell membranes. An important feature of cPLA2 is its link to cell surface receptors that stimulate signaling pathways associated with activation of protein kinases and production of reactive oxygen species (ROS). In the central nervous system (CNS), cPLA2 activation has been implicated in neuronal excitation, synaptic secretion, apoptosis, cell-cell interaction, cognitive and behavioral function, oxidative-nitrosative stress and inflammatory responses that underline the pathogenesis of a number of neurodegenerative diseases. However, the types of extracellular agonists that target intracellular signaling pathways leading to cPLA2 activation among different cell types and under different physiological and pathological conditions have not been investigated in detail. In this review, special emphasis is given to metabolic events linking cPLA2 to activation in neurons, astrocytes, microglial cells, and cerebrovascular cells. Understanding the molecular mechanism(s) for regulation of this enzyme is deemed important in the development of new therapeutic targets for treatment and prevention of neurodegenerative diseases.
Keywords: Arachidonic acid, cytosolic phospholipase A2, neurons, astrocytes, microglial cells, cerebrovascular cells, ERK1/2, lipopolysaccharide, interferon gamma, ROS
Introduction
Fatty acids are essential components of phospholipids and are integral parts of cell membranes. Four decades ago, Dr. Nicolas Bazan made the novel observation that fatty acids in the brain undergo rapid release upon excitation due to electroconvulsive shock and cerebral ischemia [1, 2]. This observation, later regarded as the “Bazan effect”, has attracted extensive interest for neuroscientists to search for the underlying biochemical mechanism(s) for fatty acid release from membrane phospholipids. Subsequent studies led to evidence that fatty acids in membrane phospholipids undergo dynamic turnover through an “acylation-deacylation” mechanism mediated by phospholipases A2 (PLA2) and ATP-dependent acyl-coenzyme A: lysophospholipid acyltransferases. It is further recognized that this “acylation-deacylation” process provides an important mechanism for the release of selective fatty acids in different types of membrane phospholipids upon special demand.
Phospholipids in the central nervous system (CNS) are enriched with polyunsaturated fatty acids (PUFAs), and PLA2s are the key enzymes for hydrolysis of the PUFAs in the sn-2 position of the glycerol moiety. More than 20 isoforms of PLA2s are present in mammalian cells, and they are roughly divided into three major categories, namely, secretory (sPLA2), calcium independent (iPLA2), and calcium-dependent (cPLA2). In recent years, there are increasing interests to uncover the role of different PLA2s in regulating cell functions under physiological and pathological conditions [3–6]. Arachidonic acid (AA) and docosahexaenoic acid (DHA) are two important PUFAs offering unique physiological functions to the brain. While the release of AA has been linked to action of cPLA2, the PLA2 for release of DHA is less clear, although action of iPLA2 has been suggested [7, 8]. AA is substrate for cyclooxygenases and lipoxygenases, and serves as the precursor for synthesis of eicosanoids and prostanoids that mediate a wide variety of inflammatory responses [9]. On the other hand, DHA is effective in mediating anti-inflammatory and neuroprotective responses [8], and is the precursor for biosynthesis of neuroprotectin D (NPD1) and resolvins [9–14]. An important goal here is to provide a comprehensive review of the extracellular agonists and receptor signaling pathways regulating cPLA2 activity and/or expression in different cell types in the CNS.
The calcium-dependent group IV cytosolic PLA2 (cPLA2α) is a 97 kDa protein constitutively expressed in nearly all brain cells. Investigation on cPLA2 has gained special attention lately because not only is its requirement for calcium binding in the C2 domain, this molecule can also undergo a number of post-translational modifications through phosphorylation and S-nitrosylation. In particular, several active serine/threonine residues are susceptible to phosphorylation by protein kinases that are involved in important signaling pathways, e.g., ERK1/2 and p38 MAPK at Ser505, Mnk1 at Ser727, and CaMKII at Ser515 [15]. In human epithelial cells, S-nitrosylation by nitric oxide (NO) of an active cysteine residue can enhance cPLA2 activity by several folds [16]. cPLA2 also contains cationic domains for binding zwitterionic lipids such as phosphatidylinositol 4,5-bisphosphate (PIP2) [5], ceramide-1-phosphate (C1P), and lactosylceramide [5, 17, 18]. These properties suggest active modulation of cPLA2 through interactions with lipids and intracellular signaling molecules.
In the CNS, cPLA2 activation has been implicated in the pathogenesis of a number of neurodegenerative diseases, including experimental autoimmune encephalomyelitis [19, 20], Alzheimer’s disease [21–26], Neimann-Pick disease [27], tumorigenesis [28], stroke [29–31], alcoholism [32, 33] as well as a number of affective disorders [34]. However, few studies have elucidated the extracellular agonists and signaling pathways leading to cPLA2 activation in these disease conditions.
Role of cPLA2 in neuronal excitation and synaptic plasticity
Neuronal stimulation by ionotropic glutamate receptor agonists, such as N-methyl-D-aspartic acid (NMDA), is known to elicit a massive and rapid influx of Ca2+ and leading to activation of Ca2+-dependent enzymes, mitochondrial dysfunction and neuronal apoptosis. Activation of the NMDA receptor is also marked by a rapid production of reactive oxygen species (ROS) which is attributed to activation of NADPH oxidase [35, 36]. Studies in our laboratory further linked NMDA-induced ROS production with activation of the MEK1/2-ERK1/2 pathway and phosphorylation of cPLA2 [35]. These findings demonstrated activation of cPLA2 together with neuronal excitation and oxidative stress. In primary cultures of cortical and hippocampal neurons, treatment with a PLA2 inhibitor methylarachidonyl-fluorophosphonate (MAFP), resulted in altered neuronal morphology, and reduced neurite outgrowth and viability [37]. Stereotaxic injection of the PLA2 inhibitor to the hippocampal CA1 area also caused a reduction of neuronal membrane fluidity which is thought to contribute to impairment in memory function [38].
In the peripheral nervous system, cPLA2 is implicated in injury of primary sensory neurons and pain behavior (tactile allodynia) [39]. The increase in cPLA2 activity is attributed to stimulation of the ionotropic P2X receptors and subsequent activation of MAPK and CaMKII [40]. In fact, phosphorylation of cPLA2 by CaMKII was also observed in vascular smooth muscle cells following stimulation by norepinephrine. Under this condition, phosphorylation of cPLA2 by CaMKII (at S515) occurred prior to the phosphorylation by ERK1/2 (at S505), and complete phosphorylation by both kinases were required for AA release [41]. There is further evidence that upon stimulation by norepinephrine, CaMKII promoted translocation of cPLA2 from cytosol to the nuclear envelope [42]. In fact, cPLA2 and CaMKII appeared to be present in close proximity in the dorsal root ganglion cells, and stimulation of the ganglion cells with ATP promoted the translocation of p-cPLA2 to the plasma membrane [40]. Since CaMKII is regarded an important protein kinase in modulation of synaptic plasticity and long-term potentiation [43], these studies presented a possible link between CaMKII and cPLA2 in modulating neuronal activities under disease conditions.
Organization of the pre- and post-synaptic structures is extremely complex. The presence of cPLA2 in the synaptic area suggests possible role for this enzyme in regulating synaptic activity. There is evidence for cPLA2 to modulate vesicle formation, for promoting neurite outgrowth and maintenance of growth cone activity. In the dorsal root ganglion, Semaphorin 3A (a class of secreted and membrane protein) can trigger a signaling pathway involving cPLA2 activation, AA release and synthesis of 12(S)-hydroxyeicosatetraenoic acid (12(S) HETE), and in turn, mediates neuronal growth cone activity [44]. In agreement with the role of cPLA2 in neuronal membrane and synaptic activity, hippocampal neurons isolated from cPLA2 knockout (KO) mice showed measurable differences in the nuclei and soma volume, and structure of the synaptic cleft as compared with neurons from wild-type animals [45]. In the hippocampus and cerebellum, the PKC-ERK1/2-cPLA2 pathway has also been implicated in long-term synaptic plasticity and regulation of AMPA receptor trafficking [46].
Albeit at lower levels as compared to stimulation by NMDA, oligomeric amyloid-beta peptide (Aβ) can also stimulate ROS production in neurons and subsequently, phosphorylate ERK1/2 and cPLA2, [35]. However, prolonged exposure of neurons to Aβ led to a decrease in NMDA response and an increase in mitochondrial dysfunction [47]. Studies by Kriem’s group demonstrated the ability for Aβ to activate cPLA2 and subsequently mitochondrial dysfunction and neuronal apoptosis [21, 48]. Besides stimulation of phospho-cPLA2, Aβ exposure also led to activation of neutral sphingomyelinase and ceramide, and together, neuronal apoptosis [49]. Mitigation of ROS production in neurons, such as that using gp91ds-tat, a specific inhibitor for the gp91phox subunit of NADPH oxidase, could protect neurons from the deleterious effects of Aβ [47]. Studies with cPLA2 deficient mice, as well as with different experimental models and pharmacological agents, have also demonstrated the critical role for cPLA2 in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and stroke [50–55]. These studies further underscore the role of cPLA2 in neuronal metabolic signaling pathways linking to Ca2+ homeostasis, protein kinases activation, and oxidative-nitrosative stress.
cPLA2 in oxidative and inflammatory signaling pathways in astrocytes
Our earlier studies with astrocytes demonstrated the ability for ATP/UTP to stimulate G-protein-coupled P2Y2 receptor and signaling pathways leading to activation of PKC-dependent and independent phosphorylation of ERK1/2 and cPLA2 [56]. Besides ATP, phorbol ester (PMA) can also increase in phospho-cPLA2, AA release and production of prostaglandin E2 (PGE2) in astrocytes [57]. This last study further demonstrated a priming effect for pro-inflammatory cytokines (such as TNFα, IL-1β and IFNγ) to enhance the production of PGE2 following short term exposure to ATP and PMA [57]. Other agents such as lipopolysaccharides (LPS) can stimulate cPLA2 and PGE2 production through an ERK1/2-dependent pathway in astrocytes [58]. In fact, a number of other stimuli, including ammonia [59], alcohol [60], ceramide [61], bradykinin [62, 63], and diethylmaleate/iodoacetate [64] have been shown stimulate cPLA2 and engage in astrocytic inflammatory and oxidative pathways.
Aggregated Aβ can also confer cytotoxic effects to astrocytes. Using specific inhibitors for cPLA2 and iPLA2, the study by Zhu et al. (2006) demonstrated ability for Aβ to incur a time-difference activation of cPLA2 and iPLA2 in alteration of mitochondrial membrane depolarization [65]. Oligomeric Aβ(1–42) increased phosphorylation of cPLA2 in astrocytes through activating the NADPH oxidase and MAPK pathways, and subsequently, this led to perturbation of mitochondrial function, impairment in ATP production, and increase in oxidative stress. Menadione, a redox-active compound capable of inducing intracellular ROS production in astrocytes, was shown to stimulate cPLA2 through p38 MAPK and ERK1/2, and subsequently, leading to an increase in actin polymerization and cytoskeletal protrusions [66]. Astrocyte plasma membranes became more molecularly ordered due to oxidative stress induced by menadione [66]. Down-regulation of cPLA2, either with a pharmacological inhibitor or by RNA interference, could abrogate the menadione-induced morphological changes in astrocytes. These studies suggest an important role for ROS in activation of astrocytes, and signaling pathways involving cPLA2 to elicit biochemical, morphological, and biophysical changes reminiscent of reactive astrocytes in pathological conditions.
cPLA2 in oxidative and inflammatory signaling pathways in microglial cells
Microglial cells are myeloid lineage cells and the principle resident immune cells in the CNS. Microglial cells exhibit multiple functions and are regarded as a major source for inflammatory responses associated with different types of brain injury. Microglial cells respond vividly to LPS which stimulates cPLA2 and PGE2 production through a signal transduction pathway involving sphingomyelinase and p38 MAPK [67]. ATP also can induce cPLA2 and PGE2 release from microglial cells and this action is attributed to the activation of P2X7 receptors [68]. However, PGE2 production due to stimulation of P2X7 receptors appeared to involve COX1 and not COX2. In another study, infection of macrophage with Candia albicans resulted in rapid activation of cPLA2, AA release, and production of eicosanoids, and the production of PGE2 was also mediated through COX1 [69]. Since COX1 is constitutive as compared to COX2 which is induced through the NF-κB pathway, these studies demonstrate that rapid response of cPLA2 to agonists can produce inflammatory events without involvement of the transcriptional processes. Chronic infusion of LPS to brains has been shown to cause inflammatory responses with increases in TNFα, iNOS and microglial activation [70]. Under this condition, the increase in cPLA2 and production of 5-LOX was attributed to the action of COX-2.
Studies from our laboratory have demonstrated the involvement of ERK1/2 in LPS-IFNγ-induced production of NO and ROS in microglial cells [71, 72]. In agreement with results from a study by Ribeiro et al. (2013), our study also indicated an increase in phospho-cPLA following LPS-IFNγ treatment (unpublished data) [73]. A study with rat primary microglial cells further showed an increase in the expression of total cPLA2 at 6–8 hours after treatment with LPS [74]. In the BV-2 microglial cells, LPS-induced cPLA2 activation was mediated by ERK1/2 and JNK but not p38 MAPK [73]. Furthermore, cPLA2 siRNA or its inhibitor, AACOCF3, attenuated LPS-induced NO and ROS production as well as iNOS and p67phox expression in microglial cells [73, 74]. Taken together, these studies demonstrated the critical role of cPLA2 in mediating inflammatory responses in microglial cells.
Superoxide anions generated by NADPH oxidase can react with NO to form peroxinitrite (ONOO-), a highly toxic radical with potent ability to damage cell membranes. Oxidation to PUFAs in membrane phospholipids can produce 4-hydroxy-2-nonenal (4-HNE), another reactive lipid peroxidation product which can form protein adducts [75] and thus is used as a good marker for assessing oxidative stress in brain tissue and brain injury [36]. In a study with the Ra2 murine microglial cells, 4-HNE was shown to upregulate cPLA2 expression as well as increased phosphorylation through a pathway involving ERK1/2 and p38 MAPK [75].
Similar to neurons and astrocytes, aggregated Aβ can also confer toxic effects on microglial cells, as demonstrated by increased production of ROS and upregulation of phospho-cPLA2 expression and cPLA2 activity [76]. Antisense cPLA2 and pyrrophenone, a cPLA2 specific inhibitor, were effective in abolishing ROS, iNOS and PGE2 production induced by Aβ.
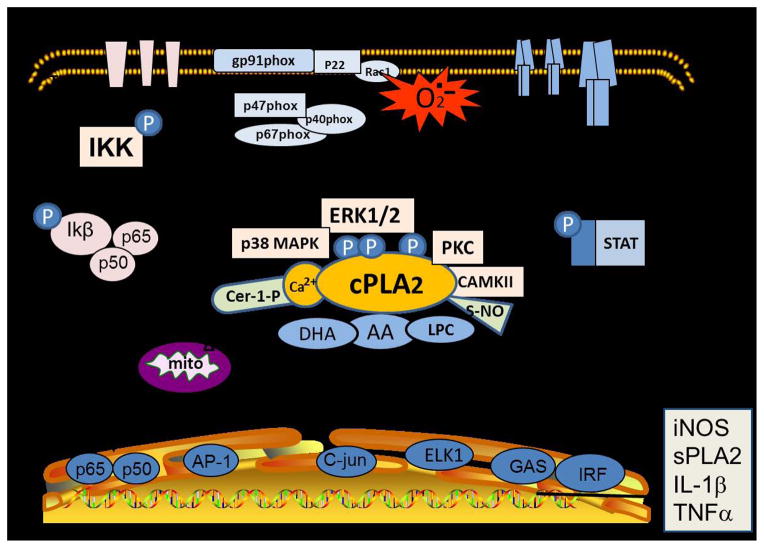
IFNγ, or type II interferon, is a cytokine critical for innate and adaptive immunity against viral and bacterial infections, and in autoinflammatory and autoimmune diseases. Although IFNγ is produced predominantly by natural killer T cells and lymphocytes, microglial cells are capable of responding to this cytokine, which is known to stimulate the canonical JAK-STAT pathway for producing transcription factors such as interferon-gamma-activated sites (GAS) and IFN regulatory factors (IRF). In microglial cells, activation of GAS is necessary for induction of the iNOS gene by IFNγ and LPS [77]. In our study with immortalized microglial cells (BV-2 and HAPI), IFNγ not only can activate the canonical JAK-STAT pathway but also induce a non-canonical pathway involving Raf-Ras and MEK1/2, which in turn, lead to activation of ERK1/2 [72] as well as cPLA2 (unpublished data). Indeed, IFNγ-induced stimulation of p-ERK1/2 has become a key signaling pathway for activation of a number of cytoplasmic proteins, including NADPH oxidase subunits for ROS production, filopodia formation, and IKKα for the NF-κB pathway in these microglial cells (Fig 1).
Fig. 1. cPLA2 in oxidative and inflammatory signaling pathways in microglial cells.
A graphical illustration depicting the role of cPLA2 in oxidative and inflammatory signaling pathways in microglial cells. This figure demonstrates the multiple signaling pathways leading to phosphorylation of ERK1/2 and cPLA2 and the multiple links for ERK1/2 to other metabolic processes including phosphorylation of NADPH oxidase subunits leading to production of ROS and development of filopodia.
Spinal microglial cells are activated during spinal cord injury and have been implicated in the pathogenesis of neuropathic pain [78]. Spinal microglial cells are susceptible to stimulation by LPS which induces the increase in COX-1 and COX-2 and production of PGE2 and NO through the p38 MAPK pathway [79]. Interestingly, a recent study indicated a role for lysophosphatidic acid (LPA) for microglial stimulation upon spinal cord injury and neuropathic pain [80]. In addition, this study further demonstrated that NMDA and neurokinin 1 receptors, cPLA2, iPLA2 and microglial activation, as well as LPA1 and LPA3 receptors, were all involved in nerve injury-induced LPA production and neuropathic pain [81]. Obviously, more studies are needed to investigate the underlying mechanisms linking spinal microglial cells and neurons in neuropathic pain.
cPLA2 and signaling pathways in cerebrovascular cells
The brain’s microvascular structure is a multicellular complex comprised of endothelial cells, astrocytes and pericytes, and together, these cells are responsible for many biological processes such as regulation of the blood-brain barrier (BBB), angiogenesis, transmigration of monocytes, and atherogenesis. Previous studies have shown that activation of the PKC-ERK1/2-cPLA2 pathway is important in endothelial functions [82]. Similar to astrocytes, cPLA2 phosphorylation/activation in cerebral endothelial cells is regulated by oxidative signaling through NADPH oxidase and ERK1/2 [83]. Vascular endothelial growth factor (VEGF) is important in endothelial angiogenesis and is responsible for cell growth, migration, and tubulogenesis in endothelial cells. VEGF receptors are coupled to the downstream signaling pathway involving activation of Src-PLD1-PKCγ, and in turn, stimulation of cPLA2 [84]. The important role of cPLA2 in mediating VEGF-induced DNA synthesis, migration, and tube formation in human retinal microvascular endothelial cells was further supported by depleting cPLA2 with siRNA [84].
Gram negative pathogens, such as E. coli, can invade brain microvascular endothelial cells and cross the BBB, and this type of infection can be a major cause of neonatal meningitis [82]. PLA2 and their metabolites have been implicated in mediating communication between endothelial cells and pericytes through signaling pathways involving PKCα and MAPK/ERK [85, 86]. In a study with human brain microvascular endothelial cells, cPLA2, AA release and synthesis of cysteinyl leukotrienes (LTs) was induced upon an invasion of Group B Streptococcus, a pathogen important in the development of neonatal meningitis [87]. Cronobacter sakazakii, another opportunistic pathogen that targets endothelial cells, is involved in neonatal sepsis and meningitis through activation of the PI3K/Akt and cPLA2 signaling pathway leading to disruption of actin microfilaments [88]. The important role of cPLA2 in C. sakazakii-induced Akt signaling and actin rearrangement can be demonstrated by treating cells with the cPLA2 inhibitor AACOCF3. Results of these studies well demonstrated the important role of cPLA2 in mediating damaging effects induced by pro-inflammatory pathogens.
Targeting cPLA2 and upstream signaling pathways to mitigate neurodegenerative processes
Increased oxidative stress and chronic inflammation are common factors associated with many age-related neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and stroke. In a cerebral ischemia model induced by middle cerebral artery occlusion (MCAO) in mice, a transient and rapid increase in total and phosphorylated-cPLA2 expression was observed in the ischemic hemisphere immediately after MCAO and returned to control levels by 2 hours after reperfusion [55]. The early increase in cPLA2 expression was coupled to the increase in COX-2, oxidative stress, and upregulation of phospho-p38 and pERK1/2 MAPK [55]. In another study using the MCAO model in rats, increases in expressions of phospho-p38 MAPK were observed from 6 hours and phospho-cPLA2 three days after ischemia/reperfusion [89]. Under this condition, administration of the p38 MAPK inhibitor SB203580 could suppress phospho-cPLA2 activation and attenuate BBB extravasation and subsequent induction of edema due to ischemia/reperfusion. AACOCF3, the cPLA2 inhibitor, is also effective in reducing ischemia-induced infarction [90]. Recent studies also suggest effective application of AACOCF3 to ameliorate injuries in animal models of other neurodegenerative diseases, including experimental autoimmune encephalomyelitis [74].
There is increasing evidence supporting the use of botanical antioxidants to suppress oxidative and inflammatory pathways in different cell types in the CNS. A recent comprehensive review outlined the mechanisms of actions of a number of flavonoids found in cocoa, tea, berries and citrus, to mitigate multiple age-associated events including impaired cognition and neuro-inflammation [91]. As an example, the leaf extract of Centella asiatica, used as an alternative medicine for memory improvement in the Indian Ayurvedic system, was shown to inhibit cPLA2 and sPLA2 activities in primary cortical neurons [92]. Another study with Ginkgo biloba extract (EGb761) showed the contribution of cPLA2 and ERK1/2 to the signaling pathway to protect spinal cord neurons from glutamate excitotoxicity [93]. Although our studies did not include analysis of cPLA2, we demonstrated the ability of EGCG from green tea [47] and honokiol, a polyphenol from Magnolia bark, to inhibit NMDA-induced neuronal excitation and ROS production from primary cortical neurons [72]. Study with Buddleja officinalis extract, a traditional Korean herbal medicine used to treat strokes, headaches, vascular diseases and neurological disorders, was shown to exert anti-inflammatory effects (LPS-induced iNOS) by suppressing the ERK1/2 and NF-κB pathways [94]. Wogonin, an active component from the root of the Chinese herbal plant, Huang qin, was shown to attenuate endotoxin/IFNγ-induced PGE2 and NO production in microglial cells through the Src-ERK1/2-NF-κB pathway, albeit the exact involvement of cPLA2 remains to be further demonstrated [95].
Conclusions and perspectives
Taken together, these studies suggest involvement of cPLA2 in oxidative and inflammatory signaling pathways and provide evidence for its link to multiple cell specific receptors and agonists in the CNS. These characteristic properties of cPLA2 underscore its role in regulating cell metabolism under different physiological and pathological conditions. Studies here also highlight an important link between cPLA2 and MAPKs, especially ERK1/2 and p38 MAPK in different cell types. Recognizing this metabolic link is important because these kinases are involved in phosphorylation of many other cellular proteins, including phosphorylation of NADPH oxidase subunits required for ROS production [96]. In both neurons and microglial cells, signaling pathways leading to ERK1/2, ROS, and cPLA2 activation have been shown to explain neuronal excitotoxicity as well as glial oxidative and inflammatory responses [35, 71]. Our studies with microglial cells further place ERK1/2 and cPLA2 in a cross-talk mechanism between NF-κB and JAK-STAT pathways induced by LPS and IFNγ, respectively (Fig. 1). Since other types of PLA2 are present in these cells, future studies should be directed to understanding the relationship between cPLA2 and other PLA2s, in particular, iPLA2 and sPLA2, in mediating inflammatory responses.
Acknowledgments
This work was supported in part by NIH Grants 2P01 AG08357 from NIA and P50AT006273 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). The help of Ms. Deborah Ratliff to proof-read and edit the manuscript is much appreciated.
Abbreviations
- Aβ
amyloid beta peptides
- AA
arachidonic acid
- AACOCF3
arachidonyl trifluoromethyl ketone
- BEL
bromoenol lactone
- COX
cyclooxygenase
- CNS
central nervous system
- CAMK-II
calcium/calmodulin-dependent protein kinase-II
- cPLA2α
cytosolic phospholipase A2 alpha
- DHA
docosahexaenoic acid
- ERK1/2
extracellular signal regulated kinases 1/2
- IFNγ
interferon gamma
- LOX
lipoxygenase
- LPS
lipopolysaccharides
- MAFP
methylarachidonyl-fluorophosphonate
- MAPK
mitogen activated protein kinases
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- PGE2
prostaglandin E2
- PKC
protein kinase C
- PMA
phorbol myristoyl acetate
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bazan NG, Jr, Rakowski H. Increased levels of brain free fatty acids after electroconvulsive shock. Life Sci. 1970;9(9):501–7. doi: 10.1016/0024-3205(70)90205-5. [DOI] [PubMed] [Google Scholar]
- 2.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 3.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103(1):1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun GY, et al. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010;12(2):133–48. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M, et al. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog Lipid Res. 2011;50(2):152–92. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Sun GY, et al. Integrating cytosolic phospholipase A(2) with oxidative/nitrosative signaling pathways in neurons: a novel therapeutic strategy for AD. Mol Neurobiol. 2012;46(1):85–95. doi: 10.1007/s12035-012-8261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport SI. Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):79–85. doi: 10.1016/j.plefa.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheon Y, et al. Disturbed brain phospholipid and docosahexaenoic acid metabolism in calcium-independent phospholipase A(2)-VIA (iPLA(2)beta)-knockout mice. Biochim Biophys Acta. 2012;1821(9):1278–86. doi: 10.1016/j.bbalip.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):101–8. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Niemoller TD, Bazan NG. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediat. 2010;91(3–4):85–9. doi: 10.1016/j.prostaglandins.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eady TN, et al. Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One. 2012;7(10):e46151. doi: 10.1371/journal.pone.0046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol Neurobiol. 2010;41(2–3):367–74. doi: 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- 13.Orr SK, et al. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127(3):378–93. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mas E, et al. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58(10):1476–84. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 15.Linkous A, Yazlovitskaya E. Cytosolic phospholipase A2 as a mediator of disease pathogenesis. Cell Microbiol. 2010;12(10):1369–77. doi: 10.1111/j.1462-5822.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, et al. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide synthase and cyclooxygenase-2. J Biol Chem. 2008;283(6):3077–87. doi: 10.1074/jbc.M705709200. [DOI] [PubMed] [Google Scholar]
- 17.Ward KE, et al. The molecular basis of ceramide-1-phosphate recognition by C2 domains. J Lipid Res. 2013;54(3):636–48. doi: 10.1194/jlr.M031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura H, et al. Lactosylceramide interacts with and activates cytosolic phospholipase A2alpha. J Biol Chem. 2013;288(32):23264–72. doi: 10.1074/jbc.M113.491431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41(3):323–35. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 20.Kalyvas A, et al. Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain. 2009;132(Pt 5):1221–35. doi: 10.1093/brain/awp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriem B, et al. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. FASEB J. 2005;19(1):85–7. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Mejia RO, Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):784–90. doi: 10.1016/j.bbalip.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Mejia RO, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11(11):1311–8. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florent-Bechard S, et al. The essential role of lipids in Alzheimer’s disease. Biochimie. 2009;91(6):804–9. doi: 10.1016/j.biochi.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Gentile MT, et al. Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer’s disease pathogenesis. Mol Neurobiol. 2012;45(3):596–604. doi: 10.1007/s12035-012-8279-4. [DOI] [PubMed] [Google Scholar]
- 26.Schaeffer EL, Forlenza OV, Gattaz WF. Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berl) 2009;202(1–3):37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H, et al. Arachidonic acid metabolism via cytosolic phospholipase A2 alpha induces cytotoxicity in niemann-pick disease type C cells. J Cell Physiol. 2012;227(7):2847–55. doi: 10.1002/jcp.23025. [DOI] [PubMed] [Google Scholar]
- 28.Linkous AG, Yazlovitskaya EM, Hallahan DE. Cytosolic phospholipase A2 and lysophospholipids in tumor angiogenesis. J Natl Cancer Inst. 2010;102(18):1398–412. doi: 10.1093/jnci/djq290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saluja I, et al. Activation of cPLA2, PKC, and ERKs in the rat cerebral cortex during ischemia/reperfusion. Neurochem Res. 1999;24(5):669–77. doi: 10.1023/a:1021004525979. [DOI] [PubMed] [Google Scholar]
- 30.Stephenson D, et al. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27(2):110–28. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Bonventre JV, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390(6660):622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 32.Moon KH, et al. Phospholipase A2, Oxidative Stress, and Neurodegeneration in Binge Ethanol-Treated Organotypic Slice Cultures of Developing Rat Brain. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tajuddin NF, et al. Effect of repetitive daily ethanol intoxication on adult rat brain: significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation. Alcohol. 2013;47(1):39–45. doi: 10.1016/j.alcohol.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao JS, et al. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord. 2012;136(1–2):63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Shelat PB, et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106(1):45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 36.Brennan AM, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12(7):857–63. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forlenza OV, et al. Inhibition of phospholipase A2 reduces neurite outgrowth and neuronal viability. Prostaglandins Leukot Essent Fatty Acids. 2007;76(1):47–55. doi: 10.1016/j.plefa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Forlenza OV, Schaeffer EL, Gattaz WF. The role of phospholipase A2 in neuronal homeostasis and memory formation: implications for the pathogenesis of Alzheimer’s disease. J Neural Transm. 2007;114(2):231–8. doi: 10.1007/s00702-006-0597-0. [DOI] [PubMed] [Google Scholar]
- 39.Tsuda M, Hasegawa S, Inoue K. P2X receptors-mediated cytosolic phospholipase A2 activation in primary afferent sensory neurons contributes to neuropathic pain. J Neurochem. 2007;103(4):1408–16. doi: 10.1111/j.1471-4159.2007.04861.x. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa S, et al. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Mol Pain. 2009;5:22. doi: 10.1186/1744-8069-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res. 2008;49(4):724–37. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Fatima S, et al. CaM kinase IIalpha mediates norepinephrine-induced translocation of cytosolic phospholipase A2 to the nuclear envelope. J Cell Sci. 2003;116(Pt 2):353–65. doi: 10.1242/jcs.00242. [DOI] [PubMed] [Google Scholar]
- 43.Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia. 2012;53(Suppl 1):45–52. doi: 10.1111/j.1528-1167.2012.03474.x. [DOI] [PubMed] [Google Scholar]
- 44.Sanford SD, et al. Group IVA phospholipase A(2) is necessary for growth cone repulsion and collapse. J Neurochem. 2012;120(6):974–84. doi: 10.1111/j.1471-4159.2012.07651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu BX, et al. cPLA2alpha knockout mice exhibit abnormalities in the architecture and synapses of cortical neurons. Brain Res. 2013;1497:101–5. doi: 10.1016/j.brainres.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Antunes G, De Schutter E. A stochastic signaling network mediates the probabilistic induction of cerebellar long-term depression. J Neurosci. 2012;32(27):9288–300. doi: 10.1523/JNEUROSCI.5976-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, et al. Prolonged exposure of cortical neurons to oligomeric amyloid-beta impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro. 2011;3(1):e00050. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaplate-Armand C, et al. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23(1):178–89. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Sagy-Bross C, Hadad N, Levy R. Cytosolic phospholipase Aalpha upregulation mediates apoptotic neuronal death induced by aggregated amyloid-beta peptide. Neurochem Int. 2013;63(6):541–550. doi: 10.1016/j.neuint.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Desbene C, et al. Critical role of cPLA2 in Abeta oligomer-induced neurodegeneration and memory deficit. Neurobiol Aging. 2012;33(6):1123 e17–29. doi: 10.1016/j.neurobiolaging.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Chalimoniuk M, et al. Involvement of multiple protein kinases in cPLA2 phosphorylation, arachidonic acid release, and cell death in in vivo and in vitro models of 1-methyl-4-phenylpyridinium-induced parkinsonism--the possible key role of PKG. J Neurochem. 2009;110(1):307–17. doi: 10.1111/j.1471-4159.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 52.Last V, Williams A, Werling D. Inhibition of cytosolic Phospholipase A2 prevents prion peptide-induced neuronal damage and co-localisation with Beta III Tubulin. BMC Neurosci. 2012;13:106. doi: 10.1186/1471-2202-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundaram JR, et al. Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci. 2012;32(3):1020–34. doi: 10.1523/JNEUROSCI.5177-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang XX, et al. Neuroprotection of interleukin-6 against NMDA-induced neurotoxicity is mediated by JAK/STAT3, MAPK/ERK, and PI3K/AKT signaling pathways. Cell Mol Neurobiol. 2013;33(2):241–51. doi: 10.1007/s10571-012-9891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kishimoto K, et al. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J Neuroinflammation. 2010;7:42. doi: 10.1186/1742-2094-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, et al. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. J Neurochem. 2002;83(2):259–70. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- 57.Xu J, et al. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):437–48. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Xiang Y, et al. Inhibition of sPLA2-IIA Prevents LPS-Induced Neuroinflammation by Suppressing ERK1/2-cPLA2alpha Pathway in Mice Cerebral Cortex. PLoS One. 2013;8(10):e77909. doi: 10.1371/journal.pone.0077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24(1):103–17. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- 60.Floreani NA, et al. Alcohol-induced interactive phosphorylation of Src and toll-like receptor regulates the secretion of inflammatory mediators by human astrocytes. J Neuroimmune Pharmacol. 2010;5(4):533–45. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad VV, Nithipatikom K, Harder DR. Ceramide elevates 12-hydroxyeicosatetraenoic acid levels and upregulates 12-lipoxygenase in rat primary hippocampal cell cultures containing predominantly astrocytes. Neurochem Int. 2008;53(6–8):220–9. doi: 10.1016/j.neuint.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh HL, et al. BK-induced COX-2 expression via PKC-delta-dependent activation of p42/p44 MAPK and NF-kappaB in astrocytes. Cell Signal. 2007;19(2):330–40. doi: 10.1016/j.cellsig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh HL, et al. BK-induced cytosolic phospholipase A2 expression via sequential PKC-delta, p42/p44 MAPK, and NF-kappaB activation in rat brain astrocytes. J Cell Physiol. 2006;206(1):246–54. doi: 10.1002/jcp.20457. [DOI] [PubMed] [Google Scholar]
- 64.Liao SL, et al. Diethylmaleate and iodoacetate in combination caused profound cell death in astrocytes. J Neurochem. 2013;127(2):271–82. doi: 10.1111/jnc.12291. [DOI] [PubMed] [Google Scholar]
- 65.Zhu D, et al. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26(43):11111–9. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu D, et al. NAD(P)H oxidase-mediated reactive oxygen species production alters astrocyte membrane molecular order via phospholipase A2. Biochem J. 2009;421(2):201–10. doi: 10.1042/BJ20090356. [DOI] [PubMed] [Google Scholar]
- 67.Akundi RS, et al. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia. 2005;51(3):199–208. doi: 10.1002/glia.20198. [DOI] [PubMed] [Google Scholar]
- 68.Anrather J, et al. Purinergic signaling induces cyclooxygenase-1-dependent prostanoid synthesis in microglia: roles in the outcome of excitotoxic brain injury. PLoS One. 2011;6(10):e25916. doi: 10.1371/journal.pone.0025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suram S, et al. Cytosolic phospholipase A(2)alpha and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS One. 2013;8(7):e69002. doi: 10.1371/journal.pone.0069002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kellom M, et al. Dose-dependent changes in neuroinflammatory and arachidonic acid cascade markers with synaptic marker loss in rat lipopolysaccharide infusion model of neuroinflammation. BMC Neurosci. 2012;13:50. doi: 10.1186/1471-2202-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Sheng W, et al. Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia. J Neuroinflammation. 2011;8:121. doi: 10.1186/1742-2094-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chuang DY, et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J Neuroinflammation. 2013;10:15. doi: 10.1186/1742-2094-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribeiro R, et al. Involvement of ERK1/2, cPLA2 and NF-kappaB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat. 2013;100–101:1–14. doi: 10.1016/j.prostaglandins.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Vana AC, et al. Arachidonyl trifluoromethyl ketone ameliorates experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter. Exp Neurol. 2011;231(1):45–55. doi: 10.1016/j.expneurol.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 75.Shibata N, et al. 4-Hydroxy-2-nonenal upregulates and phosphorylates cytosolic phospholipase A(2) in cultured Ra2 microglial cells via MAPK pathways. Neuropathology. 2011;31(2):122–8. doi: 10.1111/j.1440-1789.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 76.Szaingurten-Solodkin I, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1-42 in microglia. Glia. 2009;57(16):1727–40. doi: 10.1002/glia.20886. [DOI] [PubMed] [Google Scholar]
- 77.Gao J, et al. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272(2):1226–30. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 78.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsui T, et al. Release of prostaglandin E(2) and nitric oxide from spinal microglia is dependent on activation of p38 mitogen-activated protein kinase. Anesth Analg. 2010;111(2):554–60. doi: 10.1213/ANE.0b013e3181e3a2a2. [DOI] [PubMed] [Google Scholar]
- 80.Ma L, Nagai J, Ueda H. Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J Neurochem. 2010;115(3):643–53. doi: 10.1111/j.1471-4159.2010.06955.x. [DOI] [PubMed] [Google Scholar]
- 81.Ma L, et al. An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Mol Pain. 2013;9(1):29. doi: 10.1186/1744-8069-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alberghina M. Phospholipase A(2): new lessons from endothelial cells. Microvasc Res. 2010;80(2):280–5. doi: 10.1016/j.mvr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Askarova S, et al. Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience. 2011;199:375–85. doi: 10.1016/j.neuroscience.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q, et al. Activation of cytosolic phospholipase A2 downstream of the Src-phospholipase D1 (PLD1)-protein kinase C gamma (PKCgamma) signaling axis is required for hypoxia-induced pathological retinal angiogenesis. J Biol Chem. 2011;286(25):22489–98. doi: 10.1074/jbc.M110.217786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anfuso CD, et al. Endothelial cell-pericyte cocultures induce PLA2 protein expression through activation of PKCalpha and the MAPK/ERK cascade. J Lipid Res. 2007;48(4):782–93. doi: 10.1194/jlr.M600489-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Salmeri M, et al. Involvement of PKCalpha-MAPK/ERK-phospholipase A(2) pathway in the Escherichia coli invasion of brain microvascular endothelial cells. Neurosci Lett. 2012;511(1):33–7. doi: 10.1016/j.neulet.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 87.Maruvada R, et al. Host cytosolic phospholipase A(2)alpha contributes to group B Streptococcus penetration of the blood-brain barrier. Infect Immun. 2011;79(10):4088–93. doi: 10.1128/IAI.05506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Q, et al. PI3K-dependent host cell actin rearrangements are required for Cronobacter sakazakii invasion of human brain microvascular endothelial cells. Med Microbiol Immunol. 2010;199(4):333–40. doi: 10.1007/s00430-010-0168-8. [DOI] [PubMed] [Google Scholar]
- 89.Nito C, et al. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28(10):1686–96. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J, et al. Inhibition of cytosolic phospholipase A(2) alpha protects against focal ischemic brain damage in mice. Brain Res. 2012;1471:129–37. doi: 10.1016/j.brainres.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 91.Spencer JP, et al. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 92.Defillipo PP, et al. Inhibition of cPLA2 and sPLA2 activities in primary cultures of rat cortical neurons by Centella asiatica water extract. Nat Prod Commun. 2012;7(7):841–3. [PubMed] [Google Scholar]
- 93.Zhao Z, et al. Inhibition of cPLA2 activation by Ginkgo biloba extract protects spinal cord neurons from glutamate excitotoxicity and oxidative stress-induced cell death. J Neurochem. 2011;116(6):1057–65. doi: 10.1111/j.1471-4159.2010.07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh WJ, et al. Inhibition of lipopolysaccharide-induced proinflammatory responses by Buddleja officinalis extract in BV-2 microglial cells via negative regulation of NF-kB and ERK1/2 signaling. Molecules. 2013;18(8):9195–206. doi: 10.3390/molecules18089195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh CH, et al. Wogonin attenuates endotoxin-induced prostaglandin E2 and nitric oxide production via Src-ERK1/2-NFkappaB pathway in BV-2 microglial cells. Environ Toxicol. 2013 doi: 10.1002/tox.21847. [DOI] [PubMed] [Google Scholar]
- 96.Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277(1):22–9. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]