Abstract
Background
Echocardiographic measurements of diastolic function have not been validated against invasive pressure-volume loop (PVL) analysis in the single ventricle population. We hypothesized that echocardiographic measures of diastolic function would correlate with PVL indices of diastolic function in patients with single ventricle physiology.
Materials and Methods
Conductance-derived PVL measures of diastolic function included the isovolumic relaxation time constant (tau), maximum rate of ventricular pressure decline (peak -dP/dt), and a measure of passive diastolic stiffness, μ. Echocardiographic measures included Doppler inflow patterns of the dominant atrioventricular valve (DAVV), tissue Doppler velocities (TDI) at the lateral (ventricular free wall) component of the DAVV annulus, and TDI-derived isovolumic relaxation time (IVRT′). The correlation between PVL and echocardiographic measures was examined.
Results
Thirteen patients were enrolled at various stages of surgical palliation. Median age was 3yr (range 3mo to 19yr). Tau correlated well with Doppler E:A (r = 0.832, p = 0.005), lateral E:E′ (r = 0.747, p = 0.033), and IVRT′ (r = 0.831, p = 0.001). There was also correlation between peak -dP/dt and IVRT′ (r = 0.609, p = 0.036) while μ also correlated with IVRT′ (r = 0.884, p = 0.001).
Conclusion
This study represents the first-ever comparison of diastolic echocardiographic and PVL indices in a single ventricle population. We found that Doppler E:A, lateral E:E′, and IVRT′ correlate well with PVL measures of diastolic function. This study supports the further validation of echocardiographic measures of diastolic function vs. PVL measures of diastolic function in the single ventricle population..
Keywords: Pressure-volume loop, single ventricle, echocardiography, diastolic function
Introduction
The current management strategy for children with single ventricle physiology is staged surgical palliation, ultimately leading to total cavopulmonary connection (TCPC) circulation. This physiology requires blood to flow passively from the systemic veins directly through the pulmonary vasculature. The presence of systolic or diastolic dysfunction in the systemic ventricle of these patients often translates to higher pressures within the TCPC pathway, which increases the risk for significant post-operative morbidity, early surgical palliation failure, and death [1–4]. Moreover, there is evidence of abnormal baseline diastolic function in this population when compared to normal patients, likely due to abnormal loading conditions and morphologic adaptation of the single ventricle [5–9]. Unfortunately, assessment of diastolic function in this population is challenging and largely relies upon adapting non-invasive indices designed to evaluate heart failure in adults with normal biventricular anatomy. Therefore, the development and validation of accurate, non-invasive indices to assess diastolic function in the single ventricle population is paramount.
There has been considerable interest in the contribution of diastolic dysfunction to adult heart failure. Research in this area has produced a number of echocardiographic methods to evaluate diastolic dysfunction. To identify more sensitive measures of diastolic dysfunction, echocardiographic measurements of diastolic function have been compared with gold-standard measures derived from pressure-volume loop (PVL) analysis via conductance in the adult heart failure population [10–12]. However, such comparison has not been performed in the single ventricle population. Fortunately, the availability of safe pediatric-sized conductance catheters offers a unique opportunity to compare echocardiographic diastolic measures with those derived from PVL analysis in the single ventricle population [13].
The aim of this investigation was to compare echocardiographic measures of diastolic function in patients with a single ventricle against reference standard markers obtained by conductance-derived PVL analysis. We hypothesized that non-invasive echocardiographic measures of diastolic function would correlate with PVL indices of diastolic function in these patients.
Materials and Methods
This study represents a secondary analysis of a multi-institutional cross-sectional study - the Multi-Scale Modeling of Single Ventricle Hearts for Clinical Decision Support study by the Modeling of Congenital Hearts Alliance (MOCHA) investigators. The goal of this primary study was to enroll a heterogenous sample of patients with single ventricle physiology to develop 1) computational and engineering models of single ventricle physiology and 2) develop a software application for use in clinical decision making.
Patient population
All patients with functionally single ventricle anatomy undergoing routine diagnostic heart catheterization were recruited at the following time points: 1) prior to stage 2 procedure, 2) after stage 2 procedure (superior cavopulmonary connection - hemi-Fontan or bidirectional Glenn), or 3) after stage 3 procedure (total cavopulmonary connection – intra- or extracardiac Fontan procedure, with or without fenestration). Exclusion criteria included: 1) medical status for which participation in the study presented more than minimal risk as determined by the attending physician, 2) non-sinus rhythm, 3) interrupted inferior caval vein, 4) anomalous pulmonary venous drainage, 5) renal insufficiency, or 6) any condition which precludes magnetic resonance imaging (MRI). The protocol was approved by our institutional review board. Informed consent was obtained from the parent or legal guardian of minors or from the participants of age 18 or older.
Catheterization
All invasive hemodynamic data were collected following the patient’s primary diagnostic procedure. All patients underwent general anesthesia per the institution’s protocol. Pressure-volume loops were obtained through direct measurement using microconductance catheters (CD Leycom, Zoetermeer, Netherlands). The 4 French pigtail microconductance catheters we used in this study were designed to be placed using a retrograde approach and sit mid-ventricle avoiding the ventricular walls. We placed them antegrade across the atrioventricular valve in post-stage 1 and post-stage 2 patients to avoid the need for arterial sheath placement. A 5 French trans-septal sheath was used to aid in stable positioning of the catheter. In the post-stage 3 patients, a 4 French microconductance catheter was placed into the single ventricle using a retrograde approach. Pressure and volume data was obtained using standard equipment approved for use in human subjects (INCA® intracardiac analyzer; CD Leycom, Netherlands). Careful attention was given to place the conductance catheter in the center of the ventricle and eliminate volume segments outside of the ventricle.
Pressure-volume loop analysis was performed offline using specialized software (ConductNT® version 3.18; CD Leycom, Netherlands). Conductance volumes were calibrated using end-systolic and end-diastolic volumes obtained from magnetic resonance imaging (MRI).
Gold-standard measures of active diastolic relaxation included the isovolumic relaxation time constant (tau) by method of Weiss [14] and maximum rate of ventricular pressure decline (peak - dP/dt). To avoid the need for additional vascular access, load alteration was not performed. Therefore, passive ventricular relaxation was assessed using a previously validated measure, μ.
Where EDP = end-diastolic pressure, EDV = end-diastolic volume, and where β is a diastolic stiffness constant derived from single-beat estimation [15–19]. To adjust for size range of the study population, μ was indexed to body surface area.
Magnetic Resonance Imaging
All MRIs were performed using a 1.5 Tesla scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany), using a standardized protocol including cine imaging, phase contrast analysis, magnetic resonance angiography and respiratory-navigated 3D imaging. The cine imaging included retrospectively gated, balanced steady-state free-precession cine images in the ventricular long-axis, four-chamber, and short-axis covering the entirety of the systemic ventricle. Assessment of single ventricle volumes was performed by manual segmentation of short-axis cine images at end diastole and end systole (Argus; Siemens Medical Systems, Erlangen, Germany). End diastolic and end systolic volumes were calculated by use of the modified Simpson’s rule, and from these volumes, stroke volume and ejection fraction were calculated. Per the standard practice at our institution, all patients under the age of 10 were scanned under general anesthesia. In those patients, the MRI was performed immediately after the catheterization procedure, under the same general anesthesia. Older patients (age > 10) were scanned while awake, immediately prior to the catheterization.
Echocardiography
Transthoracic echocardiograms were performed using a Phillips iE33 system (Philips Medical Systems, Andover, MA). All studies were obtained under general anesthesia immediately prior to heart catheterization allowing for near identical hemodynamic and physiologic states as invasive measurements. All measurements were made off-line by a single blinded reviewer (SC) and averaged over three beats. Measurements were repeated 4 weeks later to assess intraobserver variability. Measurements were also repeated by a second blinded reviewer (JB) to assess interobserver variability.
Echocardiograms were performed by a single experienced sonographer following a protocol which included a complete set of standardized views to evaluate ventricular mechanics. To evaluate single ventricular diastolic function, peak early (E) and late (A) diastolic Doppler velocities across the dominant atrioventricular valve (DAVV) were analyzed. Tissue Doppler (TDI) velocities of the DAVV annulus (E′ and A′) were obtained using pulsed spectral Doppler. Lateral E′ and A′ were measured at the ventricular free wall component of the DAVV annulus. Septal E′ and A′ were measured at the septal component on the DAVV annulus. Fused E and A waves were excluded from analysis. Derived ratios (E:A, E:E′) were calculated. Isovolumic relaxation time (IVRT′) was derived using pulse wave tissue Doppler at the lateral DAVV and measured from the end of the S′ wave to the beginning of the E′ wave. Myocardial performance index (MPI) was calculated as [(isovolumic relaxation time + isovolumic contraction time)/ejection time], measured using pulsed spectral TDI at the lateral DAVV annulus.
Statistics
The relationship between PVL and echocardiographic measures of diastolic function was determined using a Spearman’s correlation analysis given the non-parametric distribution of data. A p-value of < 0.05 was considered significant. The analysis for differences in measures of diastolic function between stages of palliation was not possible due to the small subgroup sample sizes. Intra- and inter- observer variability was assessed by intraclass correlation coefficient using a random effects model measuring absolute agreement. Measurements were made by the original observer and a second observer 4 weeks after initial measurements were performed on all echocardiograms while blinded to the original results. An intraclass correlation coefficient of > 0.80 was deemed acceptable intra- or inter-observer variability. All statistics were performed using IBM® SPSS® Statistics software v. 20.
Results
Patient population
Between November 2010 and February 2012, 25 patients were eligible for enrollment. Six met exclusion area, six elected not to participate, and 13 patients were enrolled. Demographic, catheterization, and MRI data are presented in Table 1. Ages ranged from 3 months to 19 years (median 32 months). Nine patients were right ventricular dominant and 4 patients were left ventricular dominant. Stages of surgical palliation were as follows: post-stage 1 palliation (n = 4), post-stage 2 (n = 6), and post-stage 3 (n = 3). Heart rate was higher in patients post stage 1 (median 134bpm, range 115–153bpm) vs. stage 2 palliation (median 97bpm, range 90–117bpm) and in patients post stage 2 vs stage 3 palliation (median 62, range 56–67). The median end-diastolic pressure was 8mmHg (range 4mmHg to 12mmHg). No significant catheter-based interventions were performed. The median ejection fraction was 59% (range 32% to 71%). The MRI ejection fraction was less than 55% in four patients. All patients had tau and peak –dP/dt measured from micromanometer data. Four patients did not have μ measured secondary to either lack of MRI data or poor quality PVLs.
Table 1.
Patient Characteristics, Hemodynamic and MRI Data
| Patient | Palliation Stage | Age | Diagnosis | Heart Rate (bpm) | Systemic Sat (%) | MV O2 Sat (%) | PVRi (units x m2) | EDP (mmHg) | MRI EF (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 - Norwood | 5 months | HLHS | 153 | 71 | 56 | 2.6 | 6 | 70 |
| 2 | 3 - Fontan | 16 years | HLHS | 138 | 93 | 66 | 2.2 | 11 | 58 |
| 3 | 1 - mBTs | 3 months | TA | 108 | 72 | 42 | 3.3 | 10 | 61 |
| 4 | 3 - Fontan | 19 years | HLHS | 130 | 91 | 66 | 1.7 | 12 | 72 |
| 5 | 2 - BDG | 3 years | HLHS | 117 | 89 | 65 | 1.4 | 7 | 51 |
| 6 | 1 - mBTs | 5 months | TA | 97 | 78 | 58 | 1.9 | 7 | 71 |
| 7 | 2 - BDG | 2 years | HLHS | 67 | 86 | 69 | 0.6 | 5 | 66 |
| 8 | 2 - BDG | 7 years | HLHS | 92 | 86 | 65 | 2.7 | 8 | n/a |
| 9 | 1 – Norwood | 5 months | HLHS | 113 | 77 | 41 | 1.4 | 10 | 34 |
| 10 | 3 - Fontan | 17 years | TA | 53 | 93 | 37 | 1.1 | 12 | n/a |
| 11 | 2 - BDG | 2 years | HLHS | 97 | 72 | 55 | 2.1 | 10 | 59 |
| 12 | 2 - BDG | 2 years | DILV | 62 | 75 | 54 | 0.8 | 8 | 50 |
| 13 | 2 - BDG | 3 years | HLHS | 90 | 77 | 59 | 2.4 | 8 | 32 |
MV O2 Sat = mixed venous oxygen saturation; PVRi = pulmonary vascular resistance indexed to body surface area; EDP = end-diastolic pressure; MRI EF = magnetic resonance imaging derived ejection fraction; mBTs = modified Blalock-Taussig shunt; BDG = bidirectional Glenn, HLHS = hypoplastic left heart syndrome, TA = tricuspid atresia, DILV = double-inlet left ventricle.
Pressure-volume loop analysis
Results of PVL analysis from the total population and stratified values between stages of palliation can be seen in Table 2. Of note, end-diastolic pressure (EDP) did not correlate with invasive measures of diastolic function. A PVL in a patient with a systemic right ventricle post stage 2 palliation is presented in Figure 1.
Table 2.
PVL Analysis Comparisons between Stages of Palliation
| Measure | All Patients | Post stage 1 | Post stage 2 | Post stage 3 |
|---|---|---|---|---|
| Tau (ms) | 25 (12–34) | 22 (12–25) | 25 (20–28) | 31 (29–34) |
| n = 13 | n = 4 | n = 6 | n = 3 | |
| Peak -dP/dt (mmHg/sec) | 1021 (901–3433) | 1144 (1009–3433) | 1015 (901–1236) | 957 (908–1057) |
| n = 13 | n = 4 | n = 6 | n = 3 | |
| μ (mmHg*m2/mL) | 0.55 (0.31–1.16) | 0.44 (0.38–0.55) | 0.59 (0.31–0.84) | 0.93 (0.69–1.16) |
| n = 9 | n = 3 | n = 4 | n = 2 |
Values are reported as median (range). tau = the isovolumic relaxation time constant, peak -dP/dt = maximum rate of ventricular pressure decline, μ = β(end-diastolic pressure/end-diastolic volume).
Figure 1.
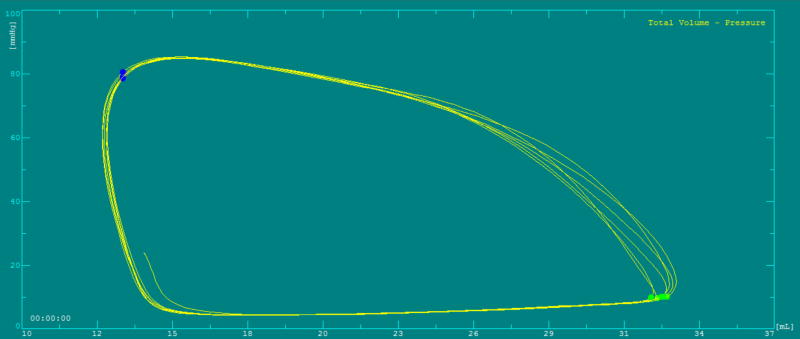
Pressure-volume loop in a single right ventricle post stage 2 palliation.
Echocardiographic analysis
Four patients did not have complete Doppler interrogation secondary to E and A wave fusion. Two of these patients were post-stage 1, one was at post-stage 2, and one at post-stage 3 palliation. All patients had IVRT′ and MPI measured. No patients had greater than mild DAVV regurgitation.
Results of echocardiographic analysis from the total population and stratified values between stages of palliation can be seen in Table 3. Intra-observer variability for all echocardiographic measures had an intraclass correlation coefficient of r > 0.85. Inter-observer variability for all echocardiographic measures had an intraclass correlation coefficient of r > 0.85.
Table 3.
Echocardiographic Analysis Comparisons between Stages of Palliation
| Measure | All Patients | Post stage 1 | Post stage 2 | Post stage 3 |
|---|---|---|---|---|
| Doppler E (cm/s) | 66.5 (41.0–114.7) | 55.5 (55.3–55.6) | 66.5 (41.0–81.6) | 104.4 (94.1–114.7) |
| n = 9 | n = 2 | n = 5 | n = 2 | |
| Doppler A (cm/s) | 70.3 (39.9–85.5) | 65.8 (61.8–69.7) | 70.3 (39.9–85.5) | 78.3 (77.4–79.1) |
| n = 9 | n = 2 | n = 5 | n = 2 | |
| Doppler E:A | 0.95 (0.80–1.45) | 0.85 (0.80–0.89) | 0.95 (0.85–1.13) | 1.33 (1.22–1.45) |
| n = 9 | n = 2 | n = 5 | n = 2 | |
| TDI Lateral E′ (cm/s) | 5.8 (3.9–8.1) | 6.2 (5.3–7.1) | 6.5 (4.8–8.1) | 5.1(3.9–6.3) |
| n = 9 | n = 2 | n = 5 | n = 2 | |
| Lateral E:E′ | 11.5 (7.8–24.1) | 9.2 (7.8–10.4) | 10.9 (9.3–17.0) | 21.2 (18.2–24.1) |
| n = 9 | n = 2 | n = 5 | n = 2 | |
| IVRT′ (s) | 0.08 (0.05–0.11) | 0.06 (0.05–0.07) | 0.08 (0.07–0.09) | 0.10 (0.09–0.11) |
| n = 13 | n = 4 | n = 6 | n = 3 | |
| MPI | 0.54 (0.31–0.76) | 0.40 (0.31–0.57) | 0.55 (0.35–0.69) | 0.67 (0.49–0.76) |
| n = 13 | n = 4 | n = 6 | n = 3 |
Values are reported as median (range). TDI = tissue Doppler imaging, IVRT′ = isovolumic relaxation time, MPI = myocardial performance index.
PVL vs echocardiographic correlations
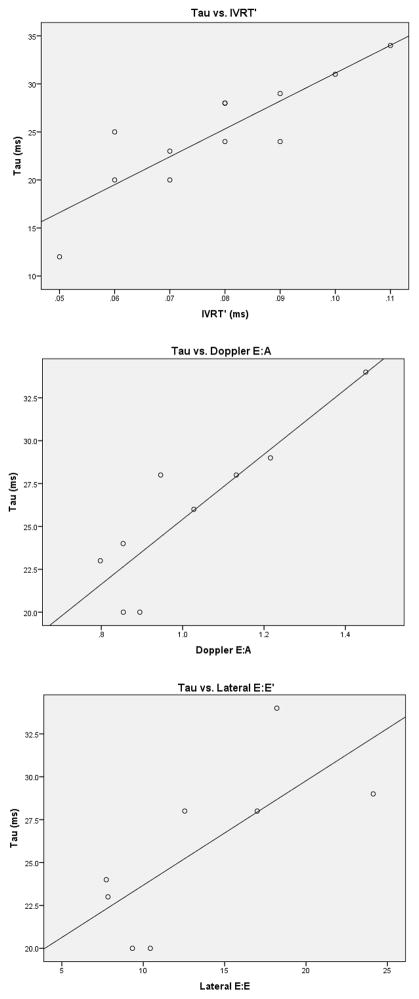
Comprehensive results can be viewed in Table 4. Of note, Doppler E:A and lateral E:E′ showed strong correlation with tau. IVRT′ exhibited a strong correlation with tau and μ; it had a modest correlation with peak -dP/dt. MPI had a modest correlation with EDP and μ. Septal TDI Doppler velocities and associated ratios did not show correlation to the invasive indices, and were not included in the table. Of note, when corrected for heart rate by dividing by the square root of the RR interval, IVRT′ continued to correlate with invasive measures tau (r = 0.79, p < 0.01) and μ (r = 0.65, p < 0.01), though less strongly. Scatterplots of tau vs. IVRT′, Doppler E:A, and Lateral E:E′ can be seen in Figure 2.
Table 4.
PVL and Echocardiographic Correlations
| Measure | EDP | Tau | Peak -dP/dt | μ |
|---|---|---|---|---|
|
| ||||
| Doppler E | r = 0.14 | r = 0.67 | r = −0.31 | r = 0.52 |
| NS | NS | NS | NS | |
| n = 9 | n = 9 | n = 9 | n = 8 | |
|
| ||||
| Doppler E:A | r = 0.15 | r = 0.83 | r = 0.48 | r = 0.60 |
| NS | p < 0.01 | NS | NS | |
| n = 9 | n = 9 | n = 9 | n = 8 | |
|
| ||||
| TDI Lateral E′ | r = −0.13 | r = −0.52 | r = −0.70 | r = 0.34 |
| NS | NS | NS | NS | |
| n = 9 | n = 9 | n = 9 | n = 8 | |
|
| ||||
| Lateral E:E′ | r = 0.21 | r = 0.75 | r = 0.48 | r = 0.50 |
| NS | p = 0.03 | NS | NS | |
| n = 9 | n = 9 | n = 9 | n = 8 | |
|
| ||||
| IVRT′ | r = 0.21 | r = 0.83 | r = 0.61 | r = 0.88 |
| NS | p < 0.01 | p = 0.04 | p < 0.01 | |
| n = 13 | n = 13 | n = 13 | n = 13 | |
|
| ||||
| MPI | r = 0.58 | r = 0.48 | r = 0.40 | r = 0.76 |
| p = 0.05 | NS | NS | p = 0.03 | |
| n = 13 | n = 13 | n = 13 | n = 13 | |
Results given as Spearman’s correlation r-values with their associated p-values. NS = non-significant p-value. EDP = ventricular end diastolic pressure, tau = the isovolumic relaxation time constant, peak -dP/dt = maximum rate of ventricular pressure decline, TDI = tissue Doppler imaging, IVRT′ = isovolumic relaxation time, MPI = myocardial performance index.
Figure 2.
Correlation scatterplots of tau vs. IVRT′, Doppler E:A, and lateral E:E′. IVRT′ = isovolumic relaxation time.
Discussion
To our knowledge, this study represents the first comparison of echocardiographic and conductance derived PVL measures of diastolic function in patients with single ventricle physiology. Of note, Doppler E:A and lateral E:E′ correlated well with tau suggesting they may be useful in evaluating the early, active relaxation phase of diastole. This is similar to findings in adults suffering from heart failure with a normal ejection fraction [10]. We found no correlation of lateral E′ and tau, dissimilar to what is seen in adults. This may be secondary to our limited, heterogeneous sample, or perhaps the fact that most of our patients had normal systolic function – a condition that decreases the correlation of tau and E′ [20]. IVRT′ correlated well with most measures of diastolic function, which suggests it also may be useful in the evaluation of overall diastolic function in single ventricle patients. MPI is a measure of combined systolic and diastolic performance. It correlated well with μ and may be useful in characterizing ventricular chamber compliance. Septal TDI measures did not correlate well with invasive measures. This is likely secondary to the variable effects the hypoplastic ventricle has on the myocardial mechanics of the septal wall [21].
The importance of diastolic function in single ventricle physiology is evidenced by the fact that EDP is used prior to staged surgical palliation in single ventricle patients for risk stratification [22, 23]. While ventricular EDP is an established marker of diastolic function, it has limitations including load-sensitivity and response to changes in systolic function. Furthermore, there is evidence that elevation of EDP occurs in the later stage of diastolic dysfunction, making it an insensitive marker of early diastolic dysfunction [24]. Menon et al. examined the correlation of echocardiographic measures of diastolic function to EDP in patients with single ventricle physiology and found a modest correlation of lateral E:E′ with EDP [20]. In our study, EDP did not correlate with lateral E:E′ or measures of diastolic function obtained using PVL analysis. This could be due to the fact that no patients in our cohort displayed abnormal EDP and our limited patient numbers. The PVL derived diastolic indices we used reflect early diastole, while EDP reflects the properties of late diastole. Therefore, it is difficult to know whether the lack of correlation is due to the characteristics of our patient population, or an insensitivity of EDP to identify early phase diastolic abnormalities. This is supported by multiple studies that have had mixed results when investigating the correlation of early diastolic echocardiographic measures to EDP [20, 25, 26].
Tau is a marker of active ventricular relaxation in diastole and has been shown to be prolonged in adults with heart failure and a normal ejection fraction [27]. It has also been shown to correlate with short-term post-surgical outcomes after stage 3 palliation [28]. While it was not possible to assess for statistically significant differences in tau between stages of palliation, we did observe that tau was higher in patients after stage 3 versus stage 2 palliation. This agrees with the findings of Penny et al. showing prolonged tau immediately following Fontan procedure [29]. One might presume that diastolic function worsens with each cardiopulmonary bypass run, which could explain the differences we found in tau between stages of surgical palliation.
Doppler and TDI measurements based on the E and A wave or E′ and A′ wave could not be measured in 31% of our patients due to fused E and A waves – a common finding in patients with high heart rates [30]. However, when attainable, their ratios correlated quite well with invasive measures of diastolic function. This suggests these measures of diastolic function may not be feasible or reliable in children with a single ventricle. The time-based measures of diastolic function, IVRT′ and MPI, could be measured in all patients. Therefore, they represent a reasonable alternative when Doppler and TDI measures cannot be used due to E and A wave fusion.
Stratification of echocardiographic measures between surgical stages of palliation yielded another observation. There was an increase in IVRT′ between stages of palliation. Like tau, the prolongation of IVRT′ in our population could be secondary to the decrease in heart rate between the groups. All patients post-stage 3 palliation demonstrated lateral E:E′ ratios that were elevated. This is consistent with the baseline evidence of diastolic dysfunction in single ventricle patients as described by others [5–9]. Analysis of intra- and inter-observer variability revealed excellent reproducibility for these indices of diastolic function, similar to what has been reported by others [31].
It is important to note the influence of heart rate on tau. In healthy adult subjects, tau has an upper limit of normal value of 48msec and an inverse relationship with heart rate [10]. However, it is unlikely that applying adult normal values in children with single ventricle physiology is appropriate, and, there is a lack of established normal data for tau in children. One small study demonstrated similar values of tau in children with single ventricle physiology post-stage 2 palliation compared to control subjects. At resting heart rates, all values for both groups were below 30 msec [32]. It is possible that children have a lower threshold for an abnormal tau. This would be consistent with our findings in that post stage 3 palliation, our patients had a tau value of 34 ms or less while all of their lateral E:E′ ratios would be considered abnormal (>15).
Heart rate’s influence on the relationship between tau and IVRT′ is also important to consider. Both have an inverse relationship to heart rate which could account for a portion of the strong correlation we found between tau and IVRT′. Schmitz et al. examined this issue by indexing IVRT′ to heart rate and found that the corrected IVRT′ had a constant value from infancy to adolescence [33]. However, simply indexing IVRT′ and tau to heart rate is not appropriate because tau does not have a linear correlation to heart rate in this population [32]. Unfortunately, our sample size was not large enough to perform a multivariable analysis to correct for heart rate. Studies further establishing heart rate’s on diastolic indices (both invasive and non-invasive) are needed in children, especially those with single ventricle physiology.
Limitations
Although strong correlations were identified, our patients had normal EDPs and no more than mild atrioventicular valve regurgitation, which may affect generalizability to patients with elevated EDPs and greather than mild atrioventricular valve regurgitation. Also, had we used load-alteration during our measurements, the correlation of load-dependent and load-independent measures may not have been as strong. Our small sample size did not allow for a meaningful statistical analysis to assess for differences in measures of diastolic function between stages of palliation, ventricular morphology, or systolic function. The small sample size also limits the strengths of our conclusions – future studies are needed to determine the usefulness of echocardiographic measures of diastolic function in the single ventricle population. Missing data secondary to E and A wave fusion and missing μ data points secondary to technical issues decreases the strength of our conclusions regarding these measures.
Two patients had inadequate PVL tracing for analysis due to contact with the ventricular wall. Both of these patients were infants with a systemic right ventricle whose small ventricular size and abnormal ventricular morphology did not permit adequate antegrade catheter placement. The measure of passive ventricular relaxation, μ, was calculated from single-beat methods. While this method has been validated in both animals and humans with biventricular circulation, it has not yet been validated in the single ventricle population.
Conclusion
To our knowledge, this study represents the first-ever comparison of diastolic echocardiographic indices to invasive conductance-derived PVL indices in a single ventricle population. We demonstrated that IVRT′ correlates well with most invasive measures of diastolic function. When obtainable, Doppler E:A and lateral E:E′ correlated well with invasive measures of active relaxation, and MPI correlated well with invasive measures of stiffness. Our findings support the further validation of echocardiographic measures of diastolic function vs. PVL measures of diastolic function in the single ventricle population.
Acknowledgments
This study was funded by a grant obtained through the Leducq Foundation.
Footnotes
Author Disclosures
The authors have no financial disclosures to disclose.
References
- 1.Williams IA, Sleeper LA, Colan SD, Lu M, Stephenson EA, Newburger JW. Functional state following the Fontan procedure. Cardiol Young. 2009;19:320–30. doi: 10.1017/S1047951109990382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Border WL, Syed AU, Michelfelder EC, Khoury P, Uzark KC, Manning PB. Impaired systemic ventricular relaxation affects postoperative shortterm outcome in Fontan patients. J Thorac Cardiovasc Surg. 2003;126:1760–4. doi: 10.1016/j.jtcvs.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Mair DD, Hagler DJ, Puga FJ, Schaff HV, Danielson GK. Fontan operation in 176 patients with tricuspid atresia. Results and a proposed new index for patient selection. Circulation. 1990;82:164–9. [PubMed] [Google Scholar]
- 4.Garofalo CA, Cabreriza SE, Quinn TA, Weinberg AD, Printz BF, Hsu DT. Ventricular diastolic stiffness predicts perioperative morbidity and duration of pleural effusions after the Fontan operation. Circulation. 2006;114:I56–61. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 5.Reich O, Vorísková M, Ruth C, Krejcír M, Marek J, Skovránek J. Long-term ventricular performance after intra-atrial correction of transposition: left ventricular filling is the major limitation. Heart. 1997;78:376–81. doi: 10.1136/hrt.78.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel M, Derrick G, White PA, Cullen S, Aichner H, Deanfield J. Systemic ventricular function in patients with transposition of the great arteries after atrial repair: a tissue Doppler and conductance catheter study. J Am Coll Cardiol. 2004;43:100–6. doi: 10.1016/j.jacc.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Ho PK, Lai CT, Wong SJ, Cheung YF. Three-dimensional mechanical dyssynchrony and myocardial deformation of the left ventricle in patients with tricuspid atresia after Fontan procedure. J Am Soc Echocardiogr. 2012;25:393–400. doi: 10.1016/j.echo.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Bassareo PP, Tumbarello R, Piras A, Mercuro G. Evaluation of regional myocardial function by Doppler tissue imaging in univentricular heart after successful Fontan repair. Echocardiography. 2010;27:702–8. doi: 10.1111/j.1540-8175.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 9.Vitarelli A, Conde Y, Cimino E, D’Angeli I, D’Orazio S, Ventriglia F. Quantitative assessment of systolic and diastolic ventricular function with tissue Doppler imaging after Fontan type of operation. Int J Cardiol. 2005;102:61–9. doi: 10.1016/j.ijcard.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K. Utility of Doppler Echocardiography and Tissue Doppler Imaging in the Estimation of Diastolic Function in Heart Failure with Normal Ejection Fraction: A Comparative Doppler-Conductance Catheterization Study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 11.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 12.Rudko R, Przewlocki T, Pasowicz M, Biernacka B, Kablak-Ziembicka A, Tracz W. IVRT′/IVRT index is a useful tool for detection of elevated left ventricular filling pressure in patients with preserved ejection fraction. Echocardiography. 2008;25:473–81. doi: 10.1111/j.1540-8175.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanter, Joshua P, Hellenbrand William E. Recent advances in non-interventional pediatric cardiac catheterization. Current Opinion in Cardiology. 2005;20:75–79. doi: 10.1097/01.hco.0000154067.99851.4a. [DOI] [PubMed] [Google Scholar]
- 14.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976;58:751–60. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–12. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 16.ten Brinke EA, Burkhoff D, Klautz RJ, Tschöpe C, Schalij MJ, Bax JJ. Single-beat estimation of the left ventricular end-diastolic pressure-volume relationship in patients with heart failure. Heart. 2010;96:213–9. doi: 10.1136/hrt.2009.176248. [DOI] [PubMed] [Google Scholar]
- 17.Klotz S, Dickstein ML, Burkhoff D. A computational method of prediction of the end-diastolic pressure-volume relationship by single beat. Nat Protoc. 2007;2:2152–8. doi: 10.1038/nprot.2007.270. [DOI] [PubMed] [Google Scholar]
- 18.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–12. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ferrazzi P, Senni M, Iascone MR, Merlo M, Triggiani M, Lorusso R. Implantation of an elastic ring at equator of the left ventricle influences cardiac mechanics in experimental acute ventricular dysfunction. J Am Coll Cardiol. 2007;50:1791–8. doi: 10.1016/j.jacc.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 21.Fogel MA, Weinberg PM, Gupta KB, Rychik J, Hubbard A, Hoffman EA. Mechanics of the single left ventricle: a study in ventricular-ventricular interaction II. Circulation. 1998;98:330–8. doi: 10.1161/01.cir.98.4.330. [DOI] [PubMed] [Google Scholar]
- 22.Menon SC, Gray R, Tani LY. Evaluation of ventricular filling pressures and ventricular function by Doppler echocardiography in patients with functional single ventricle: correlation with simultaneous cardiac catheterization. J Am Soc Echocardiogr. 2011;24:1220–5. doi: 10.1016/j.echo.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol. 1993;22:318–25. doi: 10.1016/0735-1097(93)90850-z. [DOI] [PubMed] [Google Scholar]
- 24.Ohno M, Cheng CP, Little WC. Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation. 1994;89:2241–50. doi: 10.1161/01.cir.89.5.2241. [DOI] [PubMed] [Google Scholar]
- 25.Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116:2702–8. doi: 10.1161/CIRCULATIONAHA.107.698985. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao SH, Chiou KR, Lin KL, Lin SK, Huang WC, Kuo FY. Left atrial distensibility and E/e′ for estimating left ventricular filling pressure in patients with stable angina. -A comparative echocardiography and catheterization study- Circ J. 2011;75:1942–50. doi: 10.1253/circj.cj-11-0033. [DOI] [PubMed] [Google Scholar]
- 27.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 28.Border WL, Syed AU, Michelfelder EC, Khoury P, Uzark KC, Manning PB. Impaired systemic ventricular relaxation affects postoperative short-term outcome in Fontan patients. J Thorac Cardiovasc Surg. 2003;126:1760–4. doi: 10.1016/j.jtcvs.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Penny DJ, Lincoln C, Shore DF, Xiao HB, Rigby ML, Redington AN. The early response of the systemic ventricle during transition to the Fontan circulation: an acute hypertrophic cardiomyopathy? Cardiol Young. 1992;2:78–84. [Google Scholar]
- 30.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–854.e6. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erenberg FG, Banerjee A. Systolic and diastolic properties of univentricular hearts in children: insights from physiologic indices that reflect calcium cycling. Pediatr Res. 2003;54:885–91. doi: 10.1203/01.PDR.0000090930.17613.D8. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz L, Schneider MB, Lange PE. Isovolumic relaxation time corrected for heart rate has a constant value from infancy to adolescence. J Am Soc Echocardiogr. 2003;16:221–2. doi: 10.1067/mje.2003.17. [DOI] [PubMed] [Google Scholar]