Abstract
In the past 20 years, multiple genetic mutations have been identified in patients with congenital nephrotic syndrome (CNS) and both familial and sporadic focal segmental glomerulosclerosis (FSGS). Characterization of the genetic basis of CNS and FSGS has led to the recognition of the importance of podocyte injury to the development of glomerulosclerosis. Genetic mutations induce injury due to effects on the podocyte’s structure, actin cytoskeleton, calcium signaling, and lysosomal and mitochondrial function. Transgenic animal studies have contributed to our understanding of podocyte pathobiology. Podocyte endoplasmic reticulum stress response, cell polarity, and autophagy play a role in maintenance of podocyte health. Further investigations related to the effects of genetic mutations on podocytes may identify new pathways for targeting therapeutics for nephrotic syndrome.
Keywords: Focal segmental glomerulosclerosis, Nephrotic syndrome, Steroid resistant nephrotic syndrome, Genetic mutation, Podocyte signaling
Introduction
Podocytes are highly differentiated and specialized pericyte-like cells with a complex cyto-architecture that form a major component of the glomerular filtration barrier. The podocyte consists of a cell body that extends major (primary) processes. These processes ramify and terminate in specialized structures called foot processes that wrap around the glomerular capillaries. Neighboring foot processes interdigitate and link to each other by specialized cell–cell junctions spanning distances of 40 nm, known as slit diaphragms. The podocyte foot processes with slit diaphragms act as molecular sieves that help establish the permselectivity of the glomerular filter. The three-dimensional structure of the podocyte is supported by its complex cytoskeleton. The podocyte foot processes contain a central actin bundle surrounded by a network of cortical actin fibers [1]. The extensive actin cytoskeleton allows for dynamic contraction of podocyte foot processes in response to different stimuli, such as changes in glomerular capillary hydrostatic pressure (about 60 mmHg), which is much greater than pressures typical of other capillary beds [2].
Podocyte injury and loss are thought to be the initiating factor leading to glomerulosclerosis. Why is podocyte loss so critical? The predominant view is that podocytes are terminally differentiated cells that cannot repopulate after podocyte loss. Recent studies have demonstrated a subpopulation of parietal epithelial cells that may contribute to podocyte regeneration; however, the capacity for regeneration appears to be limited [3–6]. Thus, podocyte loss beyond this regenerative capacity leads to glomerular hyperfiltration and hypertrophy of the remaining podocytes [7], which results in additional podocyte stress, injury, loss, and ultimately scar formation [7].
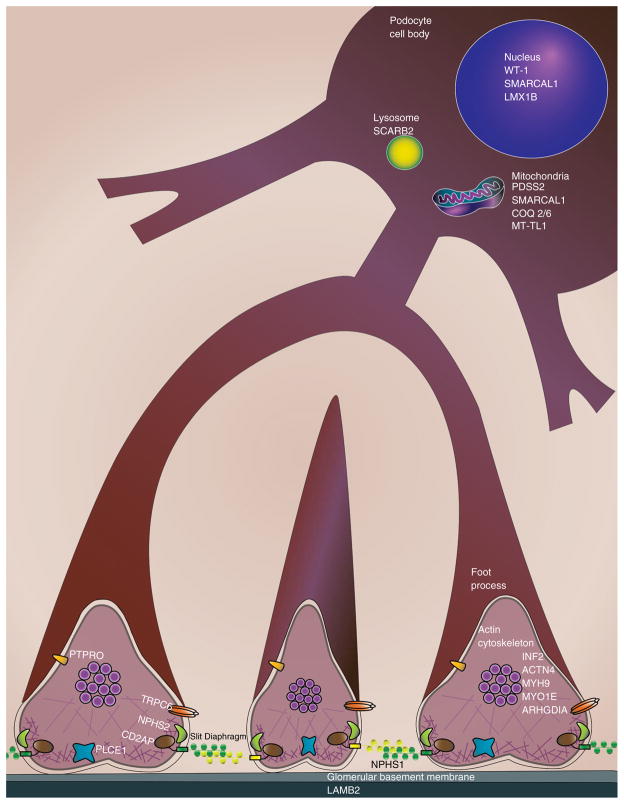
The identification of genetic mutations in familial nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) over the past few decades (Table 1) has advanced our understanding of podocyte biology. These genetic mutations affect proteins that are expressed in a variety of locations within the podocyte, including the cell membrane, nucleus, cytoskeleton, lysosomes and mitochondria (Fig. 1). Here we review some of the mechanisms by which these genetic mutations lead to podocyte injury.
Table 1.
Genetic causes of proteinuria
| Gene | Protein* | Mode of inheritance | Phenotype | Selected references |
|---|---|---|---|---|
| Slit diaphragm and cell signaling proteins | ||||
| NPHS1 | nephrin | AR | CNS, SRNS | [8, 18, 118] |
| NPHS2 | podocin | AR | CNS, SRNS | [9, 119] |
| PLCE1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase epsilon-1 | AR | DMS, SRNS | [72, 120] |
| TRPC6 | short transient receptor potential channel 6 | AD | SRNS | [66] |
| CD2AP | CD2-associated protein | AD/AR | SRNS | [35, 43, 44] |
| Cytoskeleton components | ||||
| ACTN4 | α-actinin-4 | AD | Late onset SRNS | [33] |
| INF2 | inverted formin-2 | AD | SRNS, Charcot-Marie-Tooth disease with glomerulopathy | [34, 64] |
| MYH9 | myosin-9 | AD | Macrothrombocytopenia with sensorineural deafness, Epstein syndrome, Sebastian syndrome, Fechtner syndrome | [112, 121, 122] |
| MYO1E | unconventional myosin-Ie | AR | SRNS | [53] |
| ARHGDIA | rho GDP-dissociation inhibitorα 1 | AR | SRNS; seizures, cortical blindness | [65] |
| Nuclear proteins | ||||
| WT1 | Wilms tumor protein | AD/AR | SRNS, Denys-Drash syndrome, Frasier syndrome | [99, 123] |
| LMX1B | LIM homeobox transcription factor 1-β | AD | Nail-patella syndrome/irregular GBM thickening with patchy lucent (“moa-eaten”) areas | [124, 125] |
| SMARCAL1 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1 | AR | Schimke immuno-osseous dysplasia | [126, 127] |
| GBM proteins | ||||
| LAMB2 | laminin subunit β–2 | AR | Pierson syndrome | [103, 128] |
| Mitochondrial proteins | ||||
| COQ2 | 4-hydroxybenzoate polyprenyltransferase, mitochondrial | AR | Early-onset SRNS, CoQ10 deficiency | [79] |
| COQ6 | ubiquinone biosynthesis monooxygenase COQ6 | AR | NS with sensorineural deafness | [81] |
| PDSS2 | decaprenyl-diphosphate synthase subunit 2 | AR | Leigh syndrome/CoQ10 deficiency | [80] |
| MT-TL1** | N/A | Maternal | Maternally-inherited diabetes or hearing loss presenting with FSGS/MELAS syndrome | [78, 129–131] |
| Lysosomal proteins | ||||
| SCARB2 | lysosome membrane protein 2 (LIMP II) | AR | Action myoclonus-renal failure syndrome | [82] |
| Other proteins | ||||
| APOL1 | apolipoprotein L1 | n/a | Sporadic FSGS in African-American patients | [110] |
| PTPRO | receptor-type tyrosine-protein phosphatase O (aka glomerular epithelial protein 1/GLEPP1) | AR | SRNS | [91, 132] |
Red: mutations causing non-syndromal renal disease
Blue: mutations causing syndromal renal disease
For simplicity in the text, protein products are indicated by non-italicized gene symbols
this encodes a tRNA; no protein is encoded by this gene Official full names: ACTN4 actinin, alpha 4; APOL1 apolipoprotein L, 1; CD2AP CD2-associated protein; COQ2 coenzyme Q2 4-hydroxybenzoate polyprenyltransferase; COQ6 coenzyme Q6 monooxygenase; INF2 inverted formin, FH2 and WH2 domain containing; LAMB2 laminin, beta 2 (laminin S); LIMP2 lysosome membrane protein 2; LMX1B LIM homeobox transcription factor 1 beta; MT-TL1 mitochondrially encoded tRNA leucine 1 (UUA/G); MYH9 myosin, heavy chain 9, non-muscle; MYO1E myosin IE; NPHS1 nephrosis 1, congenital, Finnish type (nephrin); NPHS2 nephrosis 2, idiopathic, steroid-resistant (podocin); PDSS2 prenyl (solanesyl) diphosphate synthase, subunit 2; PLCE1 phospholipase C, epsilon 1; PTPRO protein tyrosine phosphatase receptor type O; SCARB2 scavenger receptor class B, member 2; SMARCAL1 SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a-like 1; TRPC6 transient receptor potential cation channel, subfamily C, member 6; WT1 Wilms tumor 1 MELAS syndrome: mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes
Fig. 1.
Genetic mutations associated with nephrotic syndrome induce injury due to effects on the podocyte’s structure, actin cytoskeleton, calcium signaling, and lysosomal and mitochondrial function
Mutations in genes encoding slit diaphragm components
Some of the earliest identified genetic defects leading to nephrotic syndrome were those in genes encoding the slit diaphragm protein nephrin (NPHS1) and podocin (NPHS2), an integral membrane protein that associates with NPHS1 [8, 9]. The slit diaphragm protein NPHS1 is a transmembrane protein of the immunoglobulin family of cell-adhesion molecules. The large extracellular portion of NPHS1 has eight immunoglobulin G-like domains and a single fibronectin type-3 motif. It forms homo- and heterodimers with proteins such as NEPH1, 2, and 3 that are expressed on adjacent podocyte foot processes to generate the zipper-like multi-protein complexes of the slit diaphragm.
In addition to forming a key structural barrier to loss of protein in the urine, a complex of NPHS1 and NEPH1 mediates “outside–in” cell signaling to regulate the podocyte actin cytoskeleton [10]. Once thought to be fairly static, the foot processes are perhaps better viewed as dynamic structures that are able to remodel due to active regulation of the actin cytoskeleton. The cytoplasmic tail of NPHS1 is characterized by multiple SH2 domains which allow Src tyrosine kinases Fyn and Yes to bind and phosphorylate NPHS1. Adapter proteins NCK1/2 are recruited to these phosphorylated NPHS1 domains, leading to actin polymerization [11–13]. Podocyte-specific deletion of NCK1/2 in mice leads to FSGS lesions, suggesting that dysregulation of NPHS1 signaling induces podocyte injury [12].
Phosphorylated NPHS1 also binds to the p85 subunit of phosphatidylinositide 3-kinase (PI3K), leading to activation of AKT signaling [13, 14]. Classically, PI3K/AKT is an anti-apoptotic and cell survival pathway, but the PI3K/AKT pathways also regulate the podocyte actin cytoskeleton via effects on cofilin (CFL1) [14]. CFL1 is an enzyme that allows for actin filament severing, facilitating actin elongation and remodeling [13]. Loss of CFL1 in cultured podocytes leads to the accumulation of poly-merized actin and impaired migration [13, 15], and in mice, it results in an inability for podocytes to regain their structure after injury [13].
Hence, NPHS1 plays critical roles in maintaining podocyte health via its effects on cell–cell adhesion, cell survival, cell signaling, and regulation of the actin cytoskeleton. Homozygous NPHS1 loss-of-function mutations result in the severe phenotype of congenital nephrotic syndrome (CNS). More than 140 different NPHS1 mutations have been identified, such as nonsense, missense, frameshift insertion/deletion, and splice-site mutations, including the classic Finmajor and Finminor mutations that are responsible for 94 % of the CNS cases in the Finnish population [16]. The Finmajor mutation is a 2-bp deletion (c.121delCT; p.L41fs) in the second exon of NPHS1 that leads to truncation of the NPHS1 polypeptide chain from 1,241 to 90 amino acids [8, 16]. Similarly, the less common Finminor mutation is a nonsense mutation which results in a truncated NPHS1 1,109-amino acid protein that lacks the 82 C-terminal amino acids that interact with NPHS2. NPHS1 missense mutant proteins are retained in the endoplasmic reticulum (ER), likely causing a null allele phenotype [17]. Recently, some less severe missense NPHS1 mutations have been identified in children and adults with FSGS [18].
NPHS2 mutations induce injury in part via effects on the NPHS1 and the actin cytoskeleton. NPHS2 is a member of the stomatin family and localizes to lipid rafts where it forms homo-oligomers [19]. Lipid rafts are microdomains in the plasma membrane that are enriched with sphingolipids and cholesterol. The lipid composition is less fluid and more rigid, and facilitates the concentration of signaling receptors to these micro-domains. NPHS2 binds cell–cell junction proteins and serves as a scaffold anchoring the actin cytoskeleton to cell–cell contacts [20]. NPHS2 also recruits NPHS1 and other signaling proteins, such as TRPC6, to lipid rafts, potentially forming a mechanosensory signaling platform to regulate the podocyte actin cytoskeleton [21–24].
More than 100 pathogenic NPHS2 mutations have been reported that involve nonsense and frameshift mutations in exons. Recessive NPHS2 mutations are the most common mutations identified in Central European patients with early-onset steroid-resistant nephrotic syndrome (SRNS) [9, 25, 26]; in contrast, NPHS2 mutations are relatively rare in African American children [27]. Complete loss of function may alter glomerular development and cause CNS [28, 29]. Mutations in the C-terminus, such as R138Q (common in European populations), cause retention of NPHS2 within the ER and away from the plasma membrane [30]. Mis-localization of NPHS2 can also result in mis-localization of its binding partners NPHS1, CD2AP, and TRPC6 [30–32]. Other NPHS2 mutations do not affect NPHS2 localization but induce podocyte apoptosis [30].
Mutations in genes encoding proteins involved in the podocyte actin cytoskeleton
Following the discovery of the role of NPHS1 and NPHS2, mutations in actin cytoskeleton-associated genes (CD2AP, ACTN4, MYO1E, INF2, ARHGDIA) were identified in patients with nephrotic syndrome [33–35]. How do defects in actin cytoskeleton regulation lead to podocyte injury? One possibility is they may impair the ability of podocyte foot processes to respond to the dynamic changes in the pressure and shape of the capillary walls. In vivo fluorescent imaging of podocytes suggests that podocytes are motile and migrate in the presence of injury [6]. Altered podocyte motility and decreased adhesion could induce detachment from the glomerular basement membrane (GBM) and eventually podocyte loss [36].
CD2AP is an 80-kDa cytoplasmic adaptor protein originally identified as a ligand interacting with the T-cell-adhesion protein CD2 [37]. In podocytes, CD2AP serves as a linker that anchors NPHS1 and NPHS2 to the actin cytoskeleton [19, 38]. In addition, CD2AP binds other regulators of the actin cytoskeleton. Cell motility requires the formation of projections of the actin cytoskeleton, known as lamellipodia. CD2AP recruits actin capping proteins to cortactin in the cortical actin cytoskeleton, promoting lamellipodia formation [39]. CD2AP also binds synaptopodin (SYNPO), an alpha-actin binding protein that promotes the formation of unbranched actin filaments and is required for actin remodeling [40, 41]. In addition to its effects on the actin cytoskeleton, CD2AP deletion induces podocyte injury and apoptosis through the upregulation of transforming growth factor beta [42]. CD2AP−/− mice develop early onset, severe nephrotic syndrome, while CD2AP+/− (heterozygous) mice develop FSGS-like lesions at 9 months [43, 44]. CD2AP mutations may be rare in humans; to date, only a few heterozygous CD2AP mutations linked to FSGS [35, 44, 45] and one case of homozygous CD2AP mutations in infantile form of nephrotic syndrome have been reported [46].
Alpha-4-actinin (ACTN4) is a 100-kDa actin-binding protein that belongs to the spectrin gene superfamily. ACTN4 forms cross-links between actin filaments and binds adhesion molecules alpha-1-integrin and vinculin. Missense mutations in ACTN4are associated with incompletely penetrant and late-onset autosomal dominant (AD) FSGS [33]. Mutations in ACTN4 are relatively rare, accounting for only approximately 4 % of familial FSGS [47]. The identified mutations result in non-conservative amino acid substitutions affecting the ACTN4 binding domain. Mutant ACTN4 exhibits increased binding to filamentous actin in vitro compared with wild-type protein, and the mutant protein formed aggregates within the podocyte, impairing podocyte migration in vitro [48, 49].
In addition to effects on the actin cytoskeleton, mutant ACTN4 may have other deleterious effects on the podocyte. Transgenic “knock–in” mice that express K255E mutant ACTN4 develop FSGS lesions and demonstrate activation of the ER stress response [50, 51]. The ER is a network of membrane-enclosed tubes (cisternae). Proteins are synthesized on ribosomes attached to the ER, and the ER is enriched in chaperones that help the nascent proteins fold. These chaperones, such as GRP78/BIP, have dual roles, as they also regulate the cell’s response to stress (reviewed in [52]). In the absence of stress, members of the unfolded protein response (UPR) signaling cascade [including inositol-requiring kinase 1 (IRE1a), PRKR-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6)] are bound and inhibited by GRP78/BIP [52]. Accumulation of unfolded proteins in the ER sequesters GRP78/BIP and releases this inhibition. Mis-folded proteins are also targeted for degradation by the ubiquitin–proteasome system. Back-up (or “choking”) of the ubiquitin–proteosome system with mis-folded proteins further activates the UPR. Early on, activation of UPR elements leads to the global suppression of mRNA transcription and cell cycle arrest. This is likely to be an adaptive response to enable the cell to recover. However, continued UPR activation leads to p38 MAPK phosphorylation and increased expression of C/EBP homologous protein (CHOP) and BIM [52]. These proteins are pro-apoptotic and can induce cell death. The K255E mutant ACTN4 causes “choking” of the ubiquitin–protesome system and activation of the UPR signaling pathways [51]. Thus, mutant ACTN4 may also induce podocyte injury via ER stress.
Two mutations (A159P and Y695X) in MYO1E, the gene encoding non-muscle class I myosin, myosin 1E, have been associated with childhood-onset autosomal-recessive FSGS [53]. MYO1E is a member of the actin-dependent motor proteins. Myosins are bound to actin and generate force by hydrolysis of ATP to ADP, leading to a conformational change that stimulates movement of the actin filaments. Like other myosins, the N-terminus of MYO1E has an actin-binding domain and ATPase [54]. In addition to binding actin, MYO1E localizes to the slit diaphragm via interactions with ZO1, a cell–cell junction protein that can form a complex with slit diaphragm components [54]. MYO1E is required for the organization of podocyte actin filaments along cell–cell contacts. In one study, cultured podocytes expressing mutant A159P MYO1E failed to organize actin filaments at cell–cell junctions [54], and in another study, knockdown of MYO1E led to impaired podocyte adhesion and podocyte detachment in vitro [55]. In sum, MYO1E mutations impact both the assembly of the actin cytoskeleton and cell–cell adhesion, likely leading to podocyte injury and loss.
INF2 encodes inverted formin 2 (INF2), a member of the diaphanous formin subfamily of actin-regulating proteins (mDias). mDias are effectors for RHOA signaling. RHOA, CDC42, and RAC belong to the RHO family of GTPases that regulate the actin cytoskeleton and modulate cell shape, motility, adhesion, polarity, cell cycle, and transcription. A delicate balance of RHOA, RAC, and CDC42 signaling is required in podocytes, and excess RHOA activation induces podocyte injury and FSGS lesions in mice [56, 57]. When RHOA is bound by GTP, and it can bind and active mDias to stimulate actin polymerization. The mDias have formin homology domains that are the sites of actin nucleation and polymerization. They also have two regulatory domains: the diaphanous inhibitory domain (DID) and the diaphanous autoregulatory domain (DAD). In the absence of RHOA–GTP binding, the DID/DAD domains interact to inhibit actin polymerization. INF2 is homologous to mDias, and its DID domain can inhibit mDias and actin polymerization [58]. Thus, INF2 acts to fine-tune RHOA signaling. Loss of function disrupts the cortical actin network in cultured podocytes [58].
Most of the described mutations in INF2 are heterozygous missense variants clustered in exons 2–4, which code for the N-terminal DID of the protein [59–61]. INF2 mutations lead to loss of its inhibitory function and tip the balance towards mDia activation [58]. INF2 mutations account for up to 9–17 % of familial cases of AD FSGS but are rarely associated with the sporadic cases of FSGS [34, 59, 62, 63]. INF2 mutations have been also identified in individuals with FSGS and Charcot–Marie–Tooth disease [64].
Mutations in ARHGDIA have recently been identified in an infant with CNS and in two siblings with early onset SRNS [65]. ARHGDIA regulates GDP/GTP binding to RHO GTPases. It can act as a regulatory switch by determining the proportion of RHO GTPases bound to GDP (inactive) versus GTP (active). In cultured podocytes, wild-type ARHGDIA binds RHOA, RAC, and CDC42 and inhibits cell migration [65]. Expression of the mutant ARHGDIA leads to increased RAC1 and CDC42 activity in vitro [65]. Taken together, data on the mutations in INF2 and ARHGDIA indicate the need for tight regulation of the actin cytoskeleton to maintain podocyte health.
Mutations associated with calcium signaling in podocytes
The identification of calcium transporter TRPC6 mutations as a cause of familial FSGS brought to the forefront the concept that calcium signaling contributes to the maintenance of podocyte health [66]. Analyses suggested that an activating TRPC6 mutation led to the AD inheritance pattern [66–68]. Congruent with these findings, podocyte overexpression of TRPC6 was found to induce FSGS in mice [69]. However, the mechanisms by which excess calcium entry into podocytes results in injury remain unclear. One possibility is that podocyte TRPC6-mediated calcium influx participates in mechanosensation. In vitro studies support this hypothesis, as NPHS2 binds TPRC6 and can block stretch-induced calcium influx into TRPC6 channels [70]. Increased calcium influx into the podocyte activates RHOA, leading to perturbations of the actin cytoskeleton [71]. It can also lead to downregulation of NPHS1 and loss of podocytes, either through apoptosis or detachment [71]. Interestingly, these studies reveal a possible mechanism by which NPHS2 loss-of-function mutations may lead to podocyte injury via excess calcium influx.
Phospholipase C epsilon 1 (PLCE1) mutations were initially described in children who develop early onset nephrotic syndrome [72]. In this study, children with truncating mutations had characteristic histologic lesions of diffuse mesangial sclerosis (DMS), whereas those with missense mutations had FSGS [72]. PLCE1 is a member of the phosphoinositide-specific phospholipase C (PLC) family. PLCs catalyze the hydrolysis of membrane phospholipids to generate the second messengers inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses through the cytoplasm to the ER, where it triggers release of the ER’s calcium storage pool. DAG meanwhile remains in the phospholipid bilayer, where it activates protein kinase C (PKC) and the RAS/RAF/MEK signaling pathways [73–75]. DAG also activates TRPC6, and in podocytes this leads to increased calcium influx and the production of reactive oxidative species by NOX2 [24]. A renal phenotype has not been found in PLCE1 knockout mice [72]. Enhanced podocyte PLC signaling in transgenic mice, however, results in podocyte injury and proteinuria [76]. PLCE1 likely also affects podocyte differentiation. PLCE1 is expressed in the S-shaped body and capillary loop glomeruli. Children with PLCE1 mutations have immature capillary loop glomeruli and a decreased expression of proteins that are characteristic of terminally differentiated podocytes such as NPHS1 and NPHS2 [72].
Recent findings support the possibility of cross-talk between the actin cytoskeleton regulators, slit diaphragm proteins, and podocyte calcium signaling. PLCE1 has a guanine nucleotide exchange factor domain on the N-terminus which allows PLCE1 to be stimulated by small GTPases that regulate the actin cytoskeleton, such as RAS and RHO [73]. PLCE1 forms a complex with Ras GTPase-activating-like protein IQGAP1 in podocytes [72]. IQGAP1 can form a complex with podocyte slit diaphragm proteins, including NPHS1 and NPHS2 [77], and is also regulated by binding to members of the RHO GTPase family. It can shift the balance between cell adhesion and migration, as it interacts with cell–cell adhesion molecules and the actin cytoskeleton. Silencing of IQGAP1 in podocytes leads to the depolymerization of F-actin and inhibits migration [77]. Thus, PLCE1 mutations likely have multiple mechanisms of inducing podocyte injury, including effects on calcium signaling, the actin cytoskeleton, and podocyte differentiation.
Mutations in genes encoding mitochondrial proteins
The identification of mutations in mitochondrial genes led to the recognition of the importance of mitochondria to podocyte health. This includes a discovery of an A3243G mutation in the MT-TL1 gene encoding leucine tRNA that causes a respiratory chain defect and induces FSGS [78]. Several genetic defects in the synthesis of mitochondrial coenzyme Q10 (CoQ10) have been described that result in podocytopathies: mutations in COQ2 gene (which encodes para-hydroxybenzoate-polyprenyl-transferase) were identified in some patients with early-onset nephrotic syndrome with or without neuromuscular symptoms [79]. Mutations in PDSS2, a gene coding the subunit 2 of the enzyme decaprenyl diphosphate synthase, were identified in some patients with Leigh syndrome with nephrotic-range proteinuria [80]. Mutations have also been identified in the COQ6 gene, which encodes CoQ10 biosynthesis monooxygenase 6, in families with early-onset SRNS and sensorineural deafness [81].
How do mitochondria play a role in maintenance of podocyte health? CoQ10 is a component of the electron transport chain that is required for the synthesis of ATP. The finding of podocytopathy with CoQ10 deficiency suggests that podocytes may have a relatively high energy requirement to maintain podocyte health. In addition to energy production, the mitochondrial electron transport chain is the source of reactive oxygen species (ROS). CoQ10 acts to scavenge oxygen free radicals and limits the oxidation of DNA, RNA, and proteins by ROS. Genetic defects in CoQ10 synthesis are therefore likely to induce mitochondrial dysfunction and excessive generation of ROS, resulting in podocyte injury and apoptosis. Congruent with this concept, knockdown of COQ6 in podocyte cell lines and in zebrafish embryos caused apoptosis that was partially reversed by CoQ10 treatment [81].
Mutations in genes encoding lysosomal proteins
Homozygous truncating mutations of SCARB2, the gene that encodes lysosomal integral membrane protein LIMP-II, a β-glucocerebrosidase receptor, have been associated with action myoclonus–renal failure syndrome [82]. This is an autosomal recessive (AR) syndrome that presents in adolescents and young adults as collapsing FSGS and progressive myoclonic epilepsy [82]. The neurologic phenotype is similar to that seen with lysosomal storage diseases. Defects in autophagy, a cellular process of degradation of cell components that allows for recycling of cellular material, are associated with lysosomal storage diseases [83]. The components to be broken down are first engulfed in autophagosomes that then fuse with lysosomes. In lysosomal storage diseases, the auto-phagosomes are unable to fuse with the lysosomes, resulting in the accumulation of unfolded proteins, mitochondrial dysfunction, and cell death [83]. A role for autophagy in the maintenance of podocyte health is supported by genetic studies in mice. In one study, deletion of ATG5 (a major component of the autophagy machinery) in podocytes was found to increase susceptibility to glomerular disease [84]. Similarly, in another study, disruption of podocyte mammalian target of rapamycin (mTOR) signaling, a regulator of autophagy, resulted in disturbed autophagic flux and induced glomerulosclerosis in mice [85]. Thus, the dysregulation of autophagy can be considered as a potential mechanism for SCARB2-mediated podocyte injury.
Mutations affecting cell polarity
During embryogenesis, podocytes evolve from columnar epithelial cells of the S-shaped body into mature arborated cells with a complex polarity. Namely, mature podocytes have basal domains that attach to the GBM, apical domains that face the urinary space, and junctional domains of cell–cell contact at the slit diaphragm. These domains express distinct sets of membrane proteins, as is characteristic of polarized cells. The membrane on the apical side of foot processes contains negatively charged proteins, such as podocalyxin, podoplanin, podoendin, and protein tyrosine phosphatase receptor type O (PTPRO, also known as glomerular epithelial protein or GLEPP-1), which form a glycocalyx [86]. Podocalyxin is linked to the actin cytoskeleton and is necessary for normal foot process structure in mice [87]. Integrins are expressed in the basal domains and slit diaphragm proteins at the junctional domains. Consequently, polarized expression of proteins in podocytes may support proper cell–matrix and cell–cell adhesion.
Studies in mice have identified a role for the apico-basal polarity proteins partioning defective (PARD3/PAR6) and atypical protein kinase C (aPKC λ/ι) in establishing podocyte structure during nephrogenesis and in the development of glomerulosclerosis [88–90]. Mutations of the apical protein, PTPRO, have been identified in children with AR SRNS [91]. Another apical polarity protein, Crumbs (CRB2B), is required for proper podocyte structure and NPHS1 localization to the slit diaphragm in zebrafish [92]. Mutations in the human crumbs homolog CRB2, identified by exome sequencing in patients with FSGS, have recently been reported [93]. Actin dynamics may also affect podocyte polarity, as the deletion of podocyte RHO GTPase CDC42 in mice led to congenital nephrotic syndrome with decreased expression of NPHS1, Pard3, and aPKC [94]. Together, these data indicate that defects in cell polarity may induce podocyte injury and loss.
Genetic mutations in transcription factors
Mutations in WT-1, a nuclear transcription factor, are associated with both syndromic and sporadic SRNS. WT-1 is required for renal development, but its function in the mature podocyte remains incompletely understood. WT-1 likely affects podocyte differentiation, as NPHS1 and podocalyxin genes are downregulated in mice with decreased levels of WT-1 [95]. WT-1 defects also induce podocyte apoptosis and loss [96].
The type of podocyte injury induced by WT-1 likely depends upon the location of the mutations. Mutations in exons 8 and 9, which code for zinc finger domains 2 and 3, are associated with Denys–Drash syndrome. Denys–Drash is characterized by the triad of congenital or infantile SRNS with diffuse mesangial sclerosis, XY pseudohermaphroditism (male-to-female sex reversal), and a high prevalence of Wilms tumors. Such mutations may lead to a truncation of WT-1 [97]. Truncated WT-1 may act as a dominant negative suppressor of wild-type WT-1, explaining the early onset and developmental phenotype [97].
In contrast, Frasier syndrome [98] is caused by the mutations in the donor splice site at intron 9 of the WT1 gene [99] and is characterized by FSGS, XY pseudohermaphroditism, and high risk of gonadoblastoma. The donor splice site mutations lead to a change in the balance of two splice variants (+KTS and −KTS). The balance of +KTS/−KTS is usually 2:1. Mutations at intron 9 changes the balance with increased −KTS versus +KTS variants [99]. The two KTS variants have distinct roles in the podocyte, with the −KTS variant tending to bind DNA and the +KTS variant being more prone to bind RNA than DNA [100]. Thus, the −KTS variant cannot compensate for loss of the +KTS variant. The +KTS variant binds alpha-actinin1 mRNA; thus, dysregulation of the actin cytoskeleton may be the mechanism by which these WT-1 mutations induce podocyte injury [100].
LMX1B mutations are associated with the rare AD disorder Nail–Patella syndrome that is characterized by glomerulo-sclerosis and hereditary onychoosteodysplasia. LMX1B is a LIM homeodomain transcription factor. Mutations in this transcription factor typically occur in either its protein-binding LIM domain or its DNA binding domain. Loss of LMX1B leads to defective podocyte differentiation and GBM formation in mice [101]. Studies with podocyte-specific LMX1B knockout mice suggest that LMX1B also regulates podocyte motility, possibly via effects on the transcription of actin cytoskeleton-associated proteins [102].
Mutations in the GBM components
Podocyte injury can be the result of defects in other parts of the glomerular filtration barrier, such as in components of the GBM. LAMB2 mutations were first described in patients with CNS characterized by DMS, in combination with complex ocular abnormalities and severe neuro-developmental deficits, known as Pierson syndrome [103]. LAMB2 encodes laminin β2, an important glycoprotein component of the GBM, which binds α3β1 integrin, thereby linking podocytes to the GBM [104, 105]. Laminin binding to the GBM may also induce modulation of the actin cytoskeleton, as α3β1 integrin is coupled to the actin cytoskeleton through focal adhesion complexes. The original studies of LAMB2−/− mice suggested that podocyte injury occurs subsequent to GBM abnormalities, possibly due to excessive endocytosis of the filtered albumin [106].
The full Pierson syndrome phenotype is present when truncating mutations in LAMB2 occur, whereas patients with missense mutations, such as R246Q and C321R, have nephrotic syndrome with significantly milder extra-renal defects [107]. Transgenic mice expressing R246Q mutant LAMB2 have impaired laminin secretion [108]. The retention of mis-folded LAMB2 has been found to induce podocyte ER stress (detected by the production of the unfolded response protein CHOP) and autophagy activation [109]. Thus, increased podocyte ER stress is an alternative mechanism by which this genetic defect may induce podocyte injury.
Genetic variants associated with FSGS
Genetic variants in APOL1 were identified initially in a genome-wide association study (GWAS) examining the association of single nucleotide polymorphisms (SNPs) with the development of hypertensive end-stage kidney disease in African Americans [110]. The initial analysis of the GWAS identified an association of this disease with SNPs in MYH9, which encodes a non-muscle myosin IIA heavy chain [111]. Missense mutations of MYH9 have been found to be associated with AD giant-platelet syndromes, which may include sensorineuronal deafness, cataracts, and FSGS, consistent with a role for MYH9 in podocyte health [112].
However, mutations in MYH9 were not identified in the GWAS study, and further analysis revealed stronger linkage to two SNPs in the APOL1 gene, termed G1 (S342G and I384M substitutions) and G2 (deletion of two amino acid residues, N388 and Y389) [110, 113]. These variants likely provide a selective advantage in Africa, where homozygous or compound heterozygous carriers of the APOL1 G1 and G2 alleles have an improved capability to lyse the parasite Trypanosoma brucei rhodensiense, the cause of human African sleeping sickness [110]. APOL1 risk alleles were subsequently identified in patients with FSGS.
The physiologic functions of APOL1 are not fully understood beyond its anti-trypanosomal effect. APOL1 is widely expressed in different tissues, including podocytes, and also circulates as a component of high-density lipoprotein. There is some evidence that APOL1 overexpression promotes autophagic cell death [114], but it is not clear whether circulating or podocyte-specific APOL1 is responsible for glomerular disease. However, glomerular staining for APOL1 was found to be decreased in cases of FSGS and human immunodeficiency virus-nephropathy [115]. It was also reported that transplanted kidneys with two APOL1 risk alleles experience higher rates of early failure than kidneys with other genotypes [116]. These data suggest that the APOL1 expressed in the kidney may play some role in the development of glomerular disorders.
Conclusions and implications for future therapeutics
One of the most exciting prospects of the contributions of genetics to our improved understanding of the mechanisms that maintain podocyte health is the potential for new therapeutic options and personalized medicine. Therapeutics targeting regulation of the actin cytoskeleton, calcium signaling, ER stress, and autophagy are potential areas for investigation opened up by this knowledge. Furthermore, the increasingly less expensive potential to perform next-generation sequencing is likely to revolutionize our approach to the care of patients with FSGS and suggests the possibility for developing personalized treatment for specific genetic mutations [117]. However, some barriers remain to translating our understanding of genetics and podocyte health into optimization of patient care and clinical outcomes. We are only now starting to have large-scale studies of ethnically diverse populations of children with nephrotic syndrome and FSGS to provide us with a detailed understanding of genotype–phenotype–environmental correlations, including response to therapy, risk for end-stage kidney disease, and recurrence after transplant.
Acknowledgments
O.A. is a trainee in an NIH T32-supported pediatric nephrology training program. K.R. is supported by NIH NIDDK K08-DK091507. We would like to acknowledge Hillary Guzik for her assistance with the Figure.
References
- 1.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in vertebrate glomerular podocytes. Cell Tissue Res. 2007;329:541–557. doi: 10.1007/s00441-007-0440-4. [DOI] [PubMed] [Google Scholar]
- 2.Neal CR, Muston PR, Njegovan D, Verrill R, Harper SJ, Deen WM, Bates DO. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am J Renal Physiol. 2007;293:F1787–F1798. doi: 10.1152/ajprenal.00157.2007. [DOI] [PubMed] [Google Scholar]
- 3.Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol. 2013;183:542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Renal Physiol. 2013;304:F1375–F1389. doi: 10.1152/ajprenal.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackl MJ, Burford JL, Villanueva K, Lam L, Susztak K, Schermer B, Benzing T, Peti-Peterdi J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19:1661–1666. doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23:1351–1363. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 9.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 10.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 13.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285:22676–22688. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 15.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, Bollig F, Staggs L, Roberts IS, Park JK, Haller H, Schiffer M. Cofilin-1 inactivation leads to proteinuria—studies in zebrafish, mice and humans. PLoS One. 2010;5:e12626. doi: 10.1371/journal.pone.0012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltcheva O, Martin P, Lenkkeri U, Tryggvason K. Mutation spectrum in the nephrin gene (NPHS1) in congenital nephrotic syndrome. Hum Mutat. 2001;17:368–373. doi: 10.1002/humu.1111. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Done SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, Tryggvason K. Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet. 2001;10:2637–2644. doi: 10.1093/hmg/10.23.2637. [DOI] [PubMed] [Google Scholar]
- 18.Santin S, Garcia-Maset R, Ruiz P, Gimenez I, Zamora I, Pena A, Madrid A, Camacho JA, Fraga G, Sanchez-Moreno A, Cobo MA, Bernis C, Ortiz A, de Pablos AL, Pintos G, Justa ML, Hidalgo-Barquero E, Fernandez-Llama P, Ballarin J, Ars E, Torra R. Nephrin mutations cause childhood- and adult-onset focal segmental glomerulosclerosis. Kidney Int. 2009;76:1268–1276. doi: 10.1038/ki.2009.381. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T. Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol. 2007;18:2525–2533. doi: 10.1681/ASN.2006101084. [DOI] [PubMed] [Google Scholar]
- 21.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276:41543–41546. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- 23.Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet. 2003;12:3397–3405. doi: 10.1093/hmg/ddg360. [DOI] [PubMed] [Google Scholar]
- 24.Kim EY, Anderson M, Wilson C, Hagmann H, Benzing T, Dryer SE. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol. 2013;305:C960–C971. doi: 10.1152/ajpcell.00191.2013. [DOI] [PubMed] [Google Scholar]
- 25.Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A. Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2002;13:388–393. doi: 10.1681/ASN.V132388. [DOI] [PubMed] [Google Scholar]
- 26.Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2) Pediatrics. 2007;119:e907–e919. doi: 10.1542/peds.2006-2164. [DOI] [PubMed] [Google Scholar]
- 27.Chernin G, Heeringa SF, Gbadegesin R, Liu J, Hinkes BG, Vlangos CN, Vega-Warner V, Hildebrandt F. Low prevalence of NPHS2 mutations in African American children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2008;23:1455–1460. doi: 10.1007/s00467-008-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, Niaudet P, Antignac C. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 29.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2003;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Q, Zhang H, Ding J, Liu S, Miao J, Xing Y, Yu Z, Guan N. R168H and V165X mutant podocin might induce different degrees of podocyte injury via different molecular mechanisms. Genes Cells. 2009;14:1079–1090. doi: 10.1111/j.1365-2443.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 31.Nishibori Y, Liu L, Hosoyamada M, Endou H, Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw D, Ruotsalainen V, Tryggvason K, Khoshnoodi J, Yan K. Disease-causing missense mutations in NPHS2 gene alter normal nephrin trafficking to the plasma membrane. Kidney Int. 2004;66:1755–1765. doi: 10.1111/j.1523-1755.2004.00898.x. [DOI] [PubMed] [Google Scholar]
- 32.Philippe A, Weber S, Esquivel EL, Houbron C, Hamard G, Ratelade J, Kriz W, Schaefer F, Gubler MC, Antignac C. A missense mutation in podocin leads to early and severe renal disease in mice. Kidney Int. 2008;73:1038–1047. doi: 10.1038/ki.2008.27. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 34.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42:72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gigante M, Pontrelli P, Montemurno E, Roca L, Aucella F, Penza R, Caridi G, Ranieri E, Ghiggeri GM, Gesualdo L. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) Nephrol Dial Transplant. 2009;24:1858–1864. doi: 10.1093/ndt/gfn712. [DOI] [PubMed] [Google Scholar]
- 36.Kemeny E, Mihatsch MJ, Durmuller U, Gudat F. Podocytes loose their adhesive phenotype in focal segmental glomerulosclerosis. Clin Nephrol. 1995;43:71–83. [PubMed] [Google Scholar]
- 37.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 38.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J, Bruck S, Cemerski S, Zhang L, Butler B, Dani A, Cooper JA, Shaw AS. CD2AP links cortactin and capping protein at the cell periphery to facilitate formation of lamellipodia. Mol Cell Biol. 2013;33:38–47. doi: 10.1128/MCB.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber TB, Kwoh C, Wu H, Asanuma K, Godel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest. 2006;116:1337–1345. doi: 10.1172/JCI27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiffer M, Mundel P, Shaw AS, Bottinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 43.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 44.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 45.Lowik M, Levtchenko E, Westra D, Groenen P, Steenbergen E, Weening J, Lilien M, Monnens L, van den Heuvel L. Bigenic heterozygosity and the development of steroid-resistant focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2008;23:3146–3151. doi: 10.1093/ndt/gfn208. [DOI] [PubMed] [Google Scholar]
- 46.Lowik MM, Groenen PJ, Pronk I, Lilien MR, Goldschmeding R, Dijkman HB, Levtchenko EN, Monnens LA, van den Heuvel LP. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int. 2007;72:1198–1203. doi: 10.1038/sj.ki.5002469. [DOI] [PubMed] [Google Scholar]
- 47.Weins A, Kenlan P, Herbert S, Le TC, Villegas I, Kaplan BS, Appel GB, Pollak MR. Mutational and biological analysis of alpha-actinin-4 in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2005;16:3694–3701. doi: 10.1681/ASN.2005070706. [DOI] [PubMed] [Google Scholar]
- 48.Michaud JL, Chaisson KM, Parks RJ, Kennedy CR. FSGS-associated alpha-actinin-4 (K256E) impairs cytoskeletal dynamics in podocytes. Kidney Int. 2006;70:1054–1061. doi: 10.1038/sj.ki.5001665. [DOI] [PubMed] [Google Scholar]
- 49.Yao J, Le TC, Kos CH, Henderson JM, Allen PG, Denker BM, Pollak MR. Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2004;2:e167. doi: 10.1371/journal.pbio.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michaud JL, Lemieux LI, Dube M, Vanderhyden BC, Robertson SJ, Kennedy CR. Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol. 2003;14:1200–1211. doi: 10.1097/01.asn.0000059864.88610.5e. [DOI] [PubMed] [Google Scholar]
- 51.Cybulsky AV, Takano T, Papillon J, Bijian K, Guillemette J, Kennedy CR. Glomerular epithelial cell injury associated with mutant alpha-actinin-4. Am J Renal Physiol. 2009;297:F987–F995. doi: 10.1152/ajprenal.00055.2009. [DOI] [PubMed] [Google Scholar]
- 52.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 53.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. New Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bi J, Chase SE, Pellenz CD, Kurihara H, Fanning AS, Krendel M. Myosin 1e is a component of the glomerular slit diaphragm complex that regulates actin reorganization during cell-cell contact formation in podocytes. Am J Renal Physiol. 2013;305:F532–F544. doi: 10.1152/ajprenal.00223.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao J, Wang D, Mataleena P, He B, Niu D, Katayama K, Xu X, Ojala JR, Wang W, Shu Q, Du L, Liu A, Pikkarainen T, Patrakka J, Tryggvason K. Myo1e impairment results in actin reorganization, podocyte dysfunction, and proteinuria in zebrafish and cultured podocytes. PLoS One. 2013;8:e72750. doi: 10.1371/journal.pone.0072750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 2013;84:920–930. doi: 10.1038/ki.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H, Schlondorff J, Higgs HN, Pollak MR. Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J Am Soc Nephrol. 2013;24:917–929. doi: 10.1681/ASN.2012080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyer O, Benoit G, Gribouval O, Nevo F, Tete MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, Grunfeld JP, Legendre C, Joly D, Rieu P, Mohsin N, Hannedouche T, Moal V, Gubler MC, Broutin I, Mollet G, Antignac C. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:239–245. doi: 10.1681/ASN.2010050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun H, Schlondorff JS, Brown EJ, Higgs HN, Pollak MR. Rho activation of mDia formins is modulated by an interaction with inverted formin 2 (INF2) Proc Natl Acad Sci USA. 2011;108:2933–2938. doi: 10.1073/pnas.1017010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chhabra ES, Higgs HN. INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem. 2006;281:26754–26767. doi: 10.1074/jbc.M604666200. [DOI] [PubMed] [Google Scholar]
- 62.Gbadegesin RA, Lavin PJ, Hall G, Bartkowiak B, Homstad A, Jiang R, Wu G, Byrd A, Lynn K, Wolfish N, Ottati C, Stevens P, Howell D, Conlon P, Winn MP. Inverted formin 2 mutations with variable expression in patients with sporadic and hereditary focal and segmental glomerulosclerosis. Kidney Int. 2012;81:94–99. doi: 10.1038/ki.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barua M, Brown EJ, Charoonratana VT, Genovese G, Sun H, Pollak MR. Mutations in the INF2 gene account for a significant proportion of familial but not sporadic focal and segmental glomerulosclerosis. Kidney Int. 2012;83:316–322. doi: 10.1038/ki.2012.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Cong EH, Arrondel C, Tete MJ, Montjean R, Richard L, Karras A, Pouteil-Noble C, Balafrej L, Bonnardeaux A, Canaud G, Charasse C, Dantal J, Deschenes G, Deteix P, Dubourg O, Petiot P, Pouthier D, Leguern E, Guiochon-Mantel A, Broutin I, Gubler MC, Saunier S, Ronco P, Vallat JM, Alonso MA, Antignac C, Mollet G. INF2 mutations in Charcot–Marie–Tooth disease with glomerulop-athy. New Engl J Med. 2011;365:2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- 65.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 67.Gigante M, Caridi G, Montemurno E, Soccio M, D’Apolito M, Cerullo G, Aucella F, Schirinzi A, Emma F, Massella L, Messina G, De Palo T, Ranieri E, Ghiggeri GM, Gesualdo L. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol. 2011;6:1626–1634. doi: 10.2215/CJN.07830910. [DOI] [PubMed] [Google Scholar]
- 68.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson M, Kim EY, Hagmann H, Benzing T, Dryer SE. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am J Physiol Cell Physiol. 2013;305:C276–C289. doi: 10.1152/ajpcell.00095.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang L, Ding J, Tsai H, Li L, Feng Q, Miao J, Fan Q. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp Biol Med. 2011;236:184–193. doi: 10.1258/ebm.2010.010237. [DOI] [PubMed] [Google Scholar]
- 72.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Muller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O’Toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nurnberg P, Hildebrandt F. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 73.Wing MR, Bourdon DM, Harden TK. PLC-epsilon: a shared effector protein in Ras-, Rho-, and G alpha beta gamma-mediated signaling. Mol Interv. 2003;3:273–280. doi: 10.1124/mi.3.5.273. [DOI] [PubMed] [Google Scholar]
- 74.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C (epsilon): a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaib H, Hoskins BE, Ashraf S, Goyal M, Wiggins RC, Hildebrandt F. Identification of BRAF as a new interactor of PLCepsilon1, the protein mutated in nephrotic syndrome type 3. Am J Renal Physiol. 2008;294:F93–F99. doi: 10.1152/ajprenal.00345.2007. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Fields TA, Pazmino K, Dai Q, Burchette JL, Howell DN, Coffman TM, Spurney RF. Activation of Galpha q-coupled signaling pathways in glomerular podocytes promotes renal injury. J Am Soc Nephrol. 2005;16:3611–3622. doi: 10.1681/ASN.2005020167. [DOI] [PubMed] [Google Scholar]
- 77.Rigothier C, Auguste P, Welsh GI, Lepreux S, Deminiere C, Mathieson PW, Saleem MA, Ripoche J, Combe C. IQGAP1 interacts with components of the slit diaphragm complex in podocytes and is involved in podocyte migration and permeability in vitro. PLoS One. 2012;7:e37695. doi: 10.1371/journal.pone.0037695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowik MM, Hol FA, Steenbergen EJ, Wetzels JF, van den Heuvel LP. Mitochondrial tRNALeu (UUR) mutation in a patient with steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2005;20:336–341. doi: 10.1093/ndt/gfh546. [DOI] [PubMed] [Google Scholar]
- 79.Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 80.Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V, Killen PD, Raphael Y, Ashraf S, Ovunc B, Schoeb DS, McLaughlin HM, Airik R, Vlangos CN, Gbadegesin R, Hinkes B, Saisawat P, Trevisson E, Doimo M, Casarin A, Pertegato V, Giorgi G, Prokisch H, Rotig A, Nurnberg G, Becker C, Wang S, Ozaltin F, Topaloglu R, Bakkaloglu A, Bakkaloglu SA, Muller D, Beissert A, Mir S, Berdeli A, Varpizen S, Zenker M, Matejas V, Santos-Ocana C, Navas P, Kusakabe T, Kispert A, Akman S, Soliman NA, Krick S, Mundel P, Reiser J, Nurnberg P, Clarke CF, Wiggins RC, Faul C, Hildebrandt F. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berkovic SF, Dibbens LM, Oshlack A, Silver JD, Katerelos M, Vears DF, Lullmann-Rauch R, Blanz J, Zhang KW, Stankovich J, Kalnins RM, Dowling JP, Andermann E, Andermann F, Faldini E, D’Hooge R, Vadlamudi L, Macdonell RA, Hodgson BL, Bayly MA, Savige J, Mulley JC, Smyth GK, Power DA, Saftig P, Bahlo M. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:673–684. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- 84.Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cina DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, Jurisicova A, Quaggin SE. MTOR regulates autophagic flux in the glomerulus. Autophagy. 2012;8:696–698. doi: 10.4161/auto.19386. [DOI] [PubMed] [Google Scholar]
- 86.Simons M, Hartleben B, Huber TB. Podocyte polarity signalling. Curr Opin Nephrol Hypertens. 2009;18:324–330. doi: 10.1097/MNH.0b013e32832e316d. [DOI] [PubMed] [Google Scholar]
- 87.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, Walz G, Haller H, Schiffer M. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol. 2009;20:798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, Matsusaka T, Ichikawa I, Noda T, Ohno S. An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PLoS One. 2009;4:e4194. doi: 10.1371/journal.pone.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem. 2008;283:23033–23038. doi: 10.1074/jbc.M803143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozaltin F, Ibsirlioglu T, Taskiran EZ, Baydar DE, Kaymaz F, Buyukcelik M, Kilic BD, Balat A, Iatropoulos P, Asan E, Akarsu NA, Schaefer F, Yilmaz E, Bakkaloglu A. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am J Hum Genet. 2011;89:139–147. doi: 10.1016/j.ajhg.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol. 2009;334:1–9. doi: 10.1016/j.ydbio.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 93.Majumdar AE, Lwaki AS, Lovric S, Hildebrandt F. Genetic loss of function mutations in zebrafish and human CRB2, a regulator of epithelial polarity, are associated with podocyte foot process effacement and focal segmental glomerulosclerosis. American Society of Nephrology abstract. 2012 Available at: http://www.asn-online.org/education/kidneyweek/archives/
- 94.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol. 2012;23:1149–1154. doi: 10.1681/ASN.2011121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11:651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 96.Loeb DM. WT1 influences apoptosis through transcriptional regulation of Bcl-2 family members. Cell Cycle. 2006;5:1249–1253. doi: 10.4161/cc.5.12.2807. [DOI] [PubMed] [Google Scholar]
- 97.Patek CE, Little MH, Fleming S, Miles C, Charlieu JP, Clarke AR, Miyagawa K, Christie S, Doig J, Harrison DJ, Porteous DJ, Brookes AJ, Hooper ML, Hastie ND. A zinc finger truncation of murine WT1 results in the characteristic urogenital abnormalities of Denys–Drash syndrome. Proc Natl Acad Sci USA. 1999;96:2931–2936. doi: 10.1073/pnas.96.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frasier SD, Bashore RA, Mosier HD. Gonadoblastoma associated with pure gonadal dysgenesis in monozygous twins. J Pediatr. 1964;64:740–745. doi: 10.1016/s0022-3476(64)80622-3. [DOI] [PubMed] [Google Scholar]
- 99.Barbaux S, Niaudet P, Gubler MC, Grunfeld JP, Jaubert F, Kuttenn F, Fekete CN, Souleyreau-Therville N, Thibaud E, Fellous M, McElreavey K. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet. 1997;17:467–470. doi: 10.1038/ng1297-467. [DOI] [PubMed] [Google Scholar]
- 100.Morrison AA, Venables JP, Dellaire G, Ladomery MR. The Wilms tumour suppressor protein WT1 (+KTS isoform) binds alpha-actinin 1 mRNA via its zinc-finger domain. Biochem Cell Biol. 2006;84:789–798. doi: 10.1139/o06-065. [DOI] [PubMed] [Google Scholar]
- 101.Miner JH, Morello R, Andrews KL, Li C, Antignac C, Shaw AS, Lee B. Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest. 2002;109:1065–1072. doi: 10.1172/JCI13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burghardt T, Kastner J, Suleiman H, Rivera-Milla E, Stepanova N, Lottaz C, Kubitza M, Boger CA, Schmidt S, Gorski M, de Vries U, Schmidt H, Hertting I, Kopp J, Rascle A, Moser M, Heid IM, Warth R, Spang R, Wegener J, Mierke CT, Englert C, Witzgall R. LMX1B is essential for the maintenance of differentiated podocytes in adult kidneys. J Am Soc Nephrol. 2013;24:1830–1848. doi: 10.1681/ASN.2012080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 104.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nurnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Broking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nurnberg P, Zenker M, Hildebrandt F. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 2006;70:1008–1012. doi: 10.1038/sj.ki.5001679. [DOI] [PubMed] [Google Scholar]
- 108.Chen YM, Kikkawa Y, Miner JH. A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J Am Soc Nephrol. 2011;22:849–858. doi: 10.1681/ASN.2010060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen YM, Zhou Y, Go G, Marmerstein JT, Kikkawa Y, Miner JH. Laminin beta2 Gene Missense Mutation Produces Endoplasmic Reticulum Stress in Podocytes. J Am Soc Nephrol. 2013;24:1223–1233. doi: 10.1681/ASN.2012121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seri M, Pecci A, Di Bari F, Cusano R, Savino M, Panza E, Nigro A, Noris P, Gangarossa S, Rocca B, Gresele P, Bizzaro N, Malatesta P, Koivisto PA, Longo I, Musso R, Pecoraro C, Iolascon A, Magrini U, Rodriguez Soriano J, Renieri A, Ghiggeri GM, Ravazzolo R, Balduini CL, Savoia A. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine. 2003;82:203–215. doi: 10.1097/01.md.0000076006.64510.5c. [DOI] [PubMed] [Google Scholar]
- 113.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;1:1025–1030. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCarthy HJ, Bierzynska A, Wherlock M, Ognjanovic M, Kerecuk L, Hegde S, Feather S, Gilbert RD, Krischock L, Jones C, Sinha MD, Webb NJ, Christian M, Williams MM, Marks S, Koziell A, Welsh GI, Saleem MA. Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8:637–648. doi: 10.2215/CJN.07200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999;64:51–61. doi: 10.1086/302182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kerti A, Csohany R, Szabo A, Arkossy O, Sallay P, Moriniere V, Vega-Warner V, Nyiro G, Lakatos O, Szabo T, Lipska BS, Schaefer F, Antignac C, Reusz G, Tulassay T, Tory K. NPHS2 p.V290M mutation in late-onset steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2012;28:751–757. doi: 10.1007/s00467-012-2379-2. [DOI] [PubMed] [Google Scholar]
- 120.Boyer O, Benoit G, Gribouval O, Nevo F, Pawtowski A, Bilge I, Bircan Z, Deschenes G, Guay-Woodford LM, Hall M, Macher MA, Soulami K, Stefanidis CJ, Weiss R, Loirat C, Gubler MC, Antignac C. Mutational analysis of the PLCE1 gene in steroid resistant nephrotic syndrome. J Med Genet. 2010;47:445–452. doi: 10.1136/jmg.2009.076166. [DOI] [PubMed] [Google Scholar]
- 121.Ghiggeri GM, Caridi G, Magrini U, Sessa A, Savoia A, Seri M, Pecci A, Romagnoli R, Gangarossa S, Noris P, Sartore S, Necchi V, Ravazzolo R, Balduini CL. Genetics, clinical and pathological features of glomerulonephritis associated with mutations of nonmuscle myosin IIA (Fechtner syndrome) Am J Kidney Dis. 2003;41:95–104. doi: 10.1053/ajkd.2003.50028. [DOI] [PubMed] [Google Scholar]
- 122.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L, Fine RN, Silverman BL, Haber DA, Housman D. Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys–Drash syndrome. Cell. 1991;67:437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- 124.Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet. 1998;19:47–50. doi: 10.1038/ng0598-47. [DOI] [PubMed] [Google Scholar]
- 125.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- 126.Zivicnjak M, Franke D, Zenker M, Hoyer J, Lucke T, Pape L, Ehrich JH. SMARCAL1 mutations: a cause of prepubertal idiopathic steroid-resistant nephrotic syndrome. Pediatr Res. 2009;65:564–568. doi: 10.1203/PDR.0b013e3181998a74. [DOI] [PubMed] [Google Scholar]
- 127.Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, Andre JL, Bogdanovic R, Burguet A, Cockfield S, Cordeiro I, Frund S, Illies F, Joseph M, Kaitila I, Lama G, Loirat C, McLeod DR, Milford DV, Petty EM, Rodrigo F, Saraiva JM, Schmidt B, Smith GC, Spranger J, Stein A, Thiele H, Tizard J, Weksberg R, Lupski JR, Stockton DW. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet. 2002;30:215–220. doi: 10.1038/ng821. [DOI] [PubMed] [Google Scholar]
- 128.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seidowsky A, Hoffmann M, Glowacki F, Dhaenens CM, Devaux JP, de Sainte L, Foy C, Provot F, Gheerbrant JD, Hummel A, Hazzan M, Dracon M, Dieux-Coeslier A, Copin MC, Noel C, Buob D. Renal involvement in MELAS syndrome - a series of 5 cases and review of the literature. Clin Nephrol. 2012;80:456–463. doi: 10.5414/CN107063. [DOI] [PubMed] [Google Scholar]
- 130.Dinour D, Mini S, Polak-Charcon S, Lotan D, Holtzman EJ. Progressive nephropathy associated with mitochondrial tRNA gene mutation. Clin Nephrol. 2004;62:149–154. doi: 10.5414/cnp62149. [DOI] [PubMed] [Google Scholar]
- 131.Kurogouchi F, Oguchi T, Mawatari E, Yamaura S, Hora K, Takei M, Sekijima Y, Ikeda S, Kiyosawa K. A case of mitochondrial cytopathy with a typical point mutation for MELAS, presenting with severe focal-segmental glomerulosclerosis as main clinical manifestation. Am J Nephrol. 1998;18:551–556. doi: 10.1159/000013406. [DOI] [PubMed] [Google Scholar]
- 132.Wharram BL, Goyal M, Gillespie PJ, Wiggins JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC, Wiggins RC. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. The J Clin Invest. 2000;106:1281–1290. doi: 10.1172/JCI7236. [DOI] [PMC free article] [PubMed] [Google Scholar]