Abstract
We previously reported non-aqueous silicone elastomer gels (SEGs) for sustained vaginal administration of the CCR5-targeted entry inhibitor maraviroc. Here, we describe chemically modified SEGs (h-SEGs) in which the hydrophobic cyclomethicone component was partially replaced with relatively hydrophilic silanol-terminated polydimethylsiloxanes (st-PDMS). Maraviroc and emtricitabine (a nucleoside reverse transcriptase inhibitor), both currently under evaluation as topical microbicides to counter sexual transmission of human immunodeficiency virus type 1 (HIV-1), were used as model antiretroviral (ARV) drugs. Gel viscosity and in vitro ARV release were significantly influenced by st-PDMS molecular weight and concentration in the h-SEGs. Unexpectedly, gels prepared with lower molecular weight grades of st-PDMS showed higher viscosities. h-SEGs provided enhanced release over 24 h compared with aqueous hydroxyethylcellulose (HEC) gels, did not modify the pH of simulated vaginal fluid (SVF), and were shown to less cytotoxic than standard hydroxyethylcellulose (HEC) vaginal gel. ARV solubility increased as st-PDMS molecular weight decreased (i.e. as percentage hydroxyl content increased), helping to explain the in vitro release trends. Dye ingression and SVF dilution studies confirmed the increased hydrophilicity of the h-SEGs. h-SEGs have potential for use in vaginal drug delivery, particularly for ARV-based HIV-1 microbicides.
Keywords: Silicone elastomer gel, HIV microbicide, Maraviroc, Emtricitabine, FTC, Sustained release, HIV/AIDS, Rheology, Viscosity, Formulation, Drug Delivery Systems
1. INTRODUCTION
Biomedical strategies for HIV prevention have focused primarily on three different strategies: vaccines, vaginal microbicides and oral pre-exposure prophylaxis (PrEP). Despite determined efforts over almost thirty years, a HIV vaccine remains as elusive as ever. The two most recent efficacy trials of HIV vaccine candidates (the STEP and HVTN 505 studies) were stopped early due to initial results indicating that they were ineffective in preventing HIV infections.1,2 As a relatively recent HIV prevention method, PrEP holds out greater promise of success, but will require strict adherence to prescribed daily regimens in order to be effective. In July 2012 the U.S. Food and Drug Administration approved the combination medication tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC) for use as PrEP among sexually active adults at risk of HIV infection.3–5
The development of vaginally administered products containing antiretroviral (ARV) microbicides remains a major goal in HIV prevention.6–9 To date, emphasis has been placed on microbicide-loaded aqueous gels, with formulations based predominantly on the well-established poly(acrylic acid) (Carbopol®) and hydroxyethylcellulose (HEC) polymers that are widely used gelling agents in other vaginal products.10–14 A 1% w/w tenofovir / 2.7% w/w aqueous HEC gel is currently the most advanced candidate, with Phase 1 and 2 safety and acceptability studies (HPTN 050 and HPTN 059, respectively) successfully completed.15–17 In 2010, the Phase IIb CAPRISA 004 trial results showed a 39% reduction (compared to placebo) in the incidence of new HIV-1 infections in sexually active women using the tenofovir gel.17 A 54% reduction in incidence was found for the sub-group of women who were most adherent to the two-dose, coitally dependent BAT24 dosing protocol, underscoring the critical importance of adherence for microbicide effectiveness.17 Pericoital dosing schedules, such as the one used in the CAPRISA 004 trial, can be problematic from the perspective of user adherence with timing and frequency of dosing contributing to variability in the level of protection achieved.17,27 Less coitally-dependent or coitally-independent strategies are expected to improve microbicide efficacy. While long-acting ARV-releasing vaginal ring devices are being developed to specifically address these adherence issues,18–25 they are generally only suitable for delivery of drugs with very specific physicochemical characteristics.26 A vaginal gel applied once daily and capable of maintaining protective local ARV concentrations over a 24 h period may be a satisfactory approach to the adherence problem. To this end, a once-daily 1% tenofovir aqueous HEC gel was evaluated as part of the VOICE Phase 2B safety and acceptability study (Microbicide Trials Network MTN-003).27 However, this daily dosing schedule was not shown to reduce rates of HIV acquisition.27 A recent vaginal challenge study in macaques dosed with a HEC-based gel containing maraviroc, a CCR5-targeted HIV-1 entry inhibitor, found that the protection half-life was only 4 h.28 Therefore, unless there is a strong ligand binding or a tissue depot effect (as observed with UC781, a non-nucleoside reverse transcriptase inhibitor microbicide candidate),29,30 a single application of a water-based gel may not be capable of sustained vaginal retention and maintenance of protective ARV concentrations throughout a full 24 h period. The concept of enhancing mucosal retention of vaginal gels in order to sustain local or systemic drug levels is well established using mucoadhesive gel systems.31–36 However, given the high water content of most commercial vaginal gels and their propensity for dilution in vaginal fluid, it is not surprising that retention is generally limited to a few hours.36–40
Non-aqueous silicone gels are already widely and safely used as personal lubricants for vaginal and rectal application.41,42 Recently, we demonstrated that vaginal administration of non-aqueous silicone elastomer gels (SEGs) in rhesus macaques provided higher and more sustained maraviroc concentrations in the vaginal fluid and vaginal tissue compartments compared with HEC-based gels, presumably due to their enhanced mucosal retention due to lack of water miscibility40 However, as with silicone elastomer vaginal rings, SEGs may be less useful for formulation of relatively hydrophilic drugs owing to their limited solubility in the gel matrix and giving rise to potentially non-optimal release and pharmacokinetics. SEGs typically comprise an elastomeric component (a lightly crosslinked polydimethylsiloxane, PDMS) and a non-aqueous low molecular weight dilutant (e.g. cyclomethicone) (Fig. 1). An 80/20 SEG formulation (silicone elastomer to cyclomethicone ratio) containing 33 mg/mL w/w maraviroc was previously tested in rhesus macaques.40 We hypothesized that the substitution of the cyclomethicone component with a linear, low molecular weight, silanol-terminated PDMS (st-PDMS) would enhance the relative hydrophilicity of the SEGs (although preferably not to the extent of making them aqueous miscible), thereby improving the solubility and release of incorporated hydrophilic ARVs. Here, we demonstrate the potential of these hydrophilically-modified SEGs, hereafter termed h-SEGs, for sustained release of the model ARV microbicide compounds maraviroc and emtricitabine. Both compounds used as part of multi-drug regimens for treating HIV-1-infected individuals, are being tested for their potential as orally delivered pre-exposure prophylaxis to prevent infection,3,4,43 and may be useful as topically administered vaginal or rectal microbicides.16,17,25
Figure 1.
Chemical structures of silanol-terminated polydimethylsiloxane (st-PDMS), cyclomethicone, ST Elastomer 10, the nucleoside reverse transcriptase inhibitor emtricitabine and the HIV-1 entry inhibitor maraviroc.
2. MATERIALS AND METHODS
2.1. Materials
ST-Elastomer 10 and cyclomethicone, used for preparation of the SEGs and h-SEGs, were kindly donated by Dow Corning® (Midland, USA). Various st-PDMS compounds (DMS S12, viscosity 16–32 cST, MW 400–700 Da; DMS S14, viscosity 35–45 cST, MW 700–1500 Da; DMS S15, viscosity 45–85 cST, MW 2000–3500 Da; DMS S21, viscosity 90–120 cST, MW 4200 Da; DMS S27, viscosity 700–800 cST, MW 18,000 Da; DMS S35, viscosity 5,000 cST, MW 49,000 Da; DMS S51, viscosity 90,000–150,000 cST, MW 139,000 Da) were supplied by Fluorochem (Hadfield, Derbyshire, UK). Various grades of HEC (Natrosol® 250 HX, Natrosol® 250 M-Pharm and Natrosol® 250 HXX) were obtained from Aqualon Hercules (Wilmington, USA), sodium chloride from Sigma Aldrich® (St. Louis, MO, USA), potassium chloride and potassium dihydrogen orthophosphate from AnalaR® VWR (West Chester, Pennsylvania, USA) and disodium hydrogen orthophosphate from Fisher Scientific (Loughborough, Leicestershire, UK). Maraviroc and emtricitabine were supplied by ViiV Healthcare (Brentford, Middlesex, UK) and CONRAD (Arlington, Virginia, USA), respectively. All other materials and solvents were supplied by Sigma Aldrich® and were used as received. Commercial vaginal gels Gynofit® (Tentan AG), Replens® (Anglian Pharma), Metrogel® (Galderma), Gygel® (Marlborough Pharmaceuticals), Balance Activ® (Kullgren Pharma) and RepHresh® (Anglian Pharma) were obtained from AAH Pharmaceuticals (Belfast, UK).
2.2. Preparation of HEC and silicone elastomer gels
SEGs and h-SEGs were prepared by mixing the required quantities of ST-Elastomer 10, cyclomethicone and st-PDMS in a SpeedMixer™ (DAC 150 FVZ-K, Synergy Devices Ltd., UK, 1 min, 3000 rpm). Aqueous HEC gels were prepared according to the method described previously.40 Briefly, HEC was added to phosphate buffered saline with mixing (SpeedMixer™, 3 min, 3000 rpm), hydrated overnight and adjusted to pH 7.
2.3. Rheological assessment of gels
Continuous flow rheological assessment of the gels was performed using a TA Instruments AR 1500 Rheometer (T.A. Instruments, Surrey, England) fitted with a 40 mm diameter stainless steel parallel plate assembly. Each gel sample was applied to the rheometer base plate, the upper plate lowered to a gap depth of 1000 µm, and excess gel removed before initiating the test. Experiments were conducted at 37 ± 0.1° in continuous ramp mode with the shear stress increased from 0 to 200 Pa over 10 min (40 sampling points) while shear rate measurements were recorded. Gel viscosities and power law indices (n) were determined by applying the Power Law to the linear portion of the resulting log-log plot of viscosity against shear rate.44 Four replicates were assessed for each formulation.
2.4. In vitro release of maraviroc and emtricitabine from silicone gels
SEG and h-SEG placebo gel compositions having a viscosity of 50 Pa.S were selected from the viscosity-composition plots. Active gels containing 5% w/w micronized maraviroc or emtricitabine were then prepared and assessed for in vitro release over 24 h using both simulated vaginal fluid (SVF)45 and isopropyl alcohol (IPA) / water (1:1 ratio) solutions as release media. Gel samples (1.0 g) were syringed into sealed plastic flasks containing 20 mL of release medium and the flasks placed in a shaking orbital incubator (Analab, Infors AGCH-4103, Bottmingen, Switzerland, 37°C, 60 rpm). Four replicates were assessed for each gel formulation. The release medium was sampled with volume replacement (1 mL) at 1, 2, 4, 6, 8 and 24 h. Maraviroc and emtricitabine concentrations were quantified by HPLC using a Waters Alliance e2695 HPLC (Waters, Dublin, Ireland) installed with Empower data handling software and connected to a Waters UV (2489) detector. A Phenomenex® Luna 5µ C18 (2) 100A 150 × 4.6 mm column was used for both compounds. For maraviroc, the mobile phase for the gradient flow method comprised 0.1% v/v trifluoroacetic acid (TFA) solution (A) and acetonitrile (B) at a flow rate of 1 mL/min; 70% A 0–4.0 min, 20% A 4.0–4.5 min, 70% A 4.5–7.0 min. An isocratic flow method involving 80% water and 20% methanol was used to quantify emtricitabine. Maraviroc and emtricitabine were detected at 210 nm and 301 nm, respectively with retention times of 3.0 min and 4.0 min, respectively (based on 10 µL sample injections).
2.5. Solubility determination of maraviroc and emtricitabine in cyclomethicone and st-PDMS
The solubilities of maraviroc and emtricitabine in cyclomethicone and the various low molecular weight st-PDMSs (DMS S12, DMS S14, DMS S15, DMS S21) were determined by a shake flask method. Briefly, each drug was added to 5 mL of each solvent in sealed glass vials (n = 4) to form saturated suspensions. The vials were placed in a shaking orbital incubator (37 °C, 60 rpm) for 5 days, then removed and equilibrated at room temperature (18 ± 1 °C, 24 h). The suspensions were filtered (Millipore™ Millex-GS 0.22 µm syringe filters, Cork, Ireland), the filtrates (1 mL) added to methanol (5 mL), and the solution then analysed for maraviroc and emtricitabine content using the previously described HPLC methods.
2.6. Aqueous dye ingression studies
The relative hydrophilic character of the gels was assessed by ingression of aqueous methylene blue solution (0.01% w/v). Each silicone gel sample (3 g) was syringed into a glass vial and allowed to settle under gravity overnight. Methylene blue solution (5 mL) was pipetted on top of the gel sample, the glass vial sealed and placed in a shaking orbital incubator (37 °C, 60 rpm), and the extent of dye ingression observed at 0, 6, 24 and 48 h.
2.7. In vitro gel dilution
In vivo dilution of the gels was modelled rheologically. Test samples of the SEG and h-SEGs (5 g) were placed into plastic containers containing SVF (5 mL) (n=4). The containers were sealed and placed in an orbital shaking incubator (37 °C, 60 rpm). At various time points (0, 6, 24 and 48 h), excess SVF was removed, the remaining gel sample speed-mixed (3000 rpm, 1 min), and rheological flow analysis performed to determine gel viscosity.
2.8. Effect of gels on the pH of SVF
Three silicone gel formulations (80/20 SEG, DMS S12 h-SEG and DMS S52 h-SEG), representing the unmodified and the most and least hydrophilic modified silicone gels, respectively, were tested for their potential to modify the pH of SVF (pH = 4.2). SVF medium is specifically formulated to model the pH, osmolarity and minimal buffer capacity observed with native vaginal fluid.45 Gel samples (5.00 g) were placed in individual glass vials and SVF added (5.0 mL). The pH of the SVF phase was measured at 0, 1, 2, 4, 8 and 24 h.
2.9. MTT assay of gel cytotoxicity
Dulbecco’s Modified Eagle Medium (DMEM) supplemented with penicillin and streptomycin (5 mL) was added to polypropylene sample bottles containing either no gel (control), HEC, SEG or DMS S12 h-SEG gels and incubated at 37°C for 5 days without agitation. TZM-bl cells were plated at a concentration of 2×104 cells/well in a 96 well format and allowed to form an adherent monolayer overnight. The following day, the media were removed from the gels and supplemented with 10% foetal calf serum and 1% L-glutamine. 200 µL of media was added to each well of cells. Controls included fresh DMEM, 5 day old DMEM and 100 µg/mL nonoxynol-9 as a measure of complete cytotoxicity. The plate was incubated for 24 h at 37 °C. The media were then removed from the cells and replaced with 200 µL/well of fresh DMEM containing 0.5 mg/mL MTT. The plate was incubated for 2 h then MTT removed and cells lysed with 98% isopropanol and 2% 1N HCl. After thorough resuspension, absorbance was read at 570 nm with background at 630 nm.
2.9. Statistical analysis
Statistical analysis was performed using GraphPad Prism software. Data were analyzed using either one or two-way ANOVA, depending on the number of variables to be compared. Significance was noted when p < 0.05.
3. RESULTS
3.1. Rheological assessment of gels
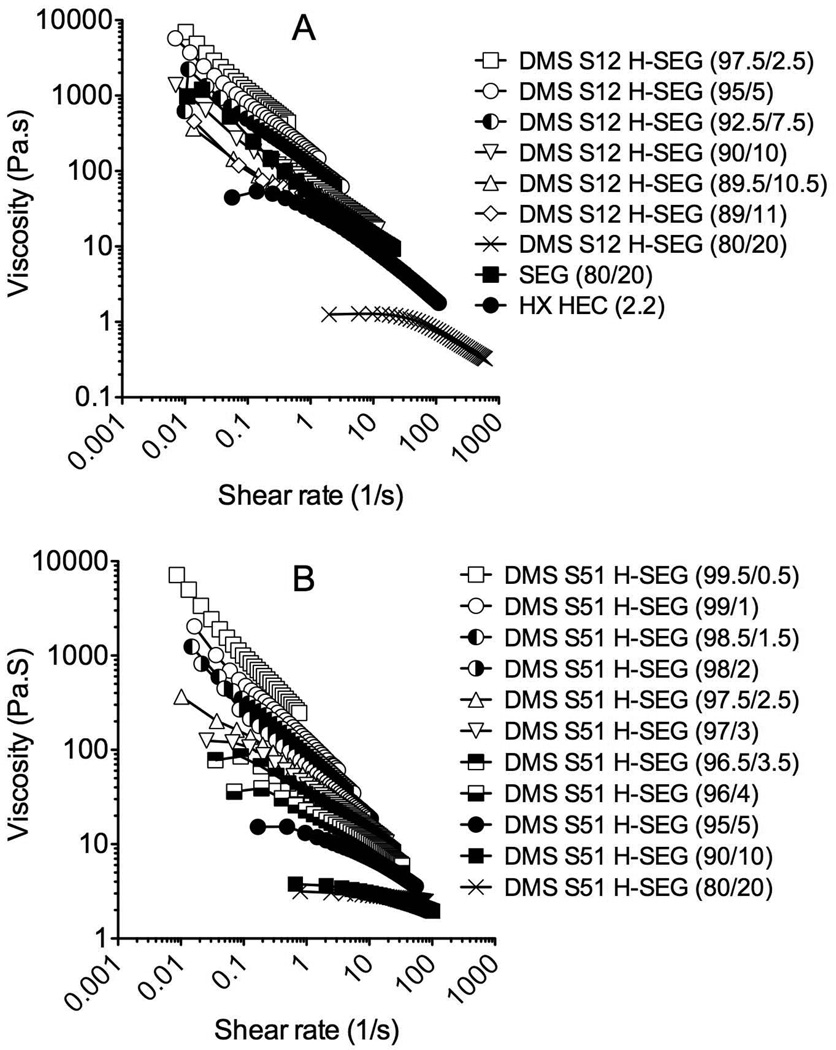
All the h-SEGs displayed shear-thinning (i.e. pseudoplastic) behaviour, whereby viscosity decreased with increasing shear rate. Representative viscosity vs. shear rate rheograms for DMS S12 and DMS S51 h-SEGs with various concentrations of the DMS component are presented in Figure 2. h-SEGs prepared with the other st-PDMS components showed similar rheological properties. Comparative rheograms for the 80/20 SEG and 2.2% w/w HEC gel (250 HX grade) are also presented in Figure 2A. Apparent viscosity values for the 80/20 SEG and each of the h-SEGs and HEC gels were determined from the gradient of the linear portions of the log-log plots of shear rate vs. viscosity (Power Law equation) and are plotted as a function of the percentage ST-Elastomer 10 and HEC concentrations in Figures 3A and 3B, respectively. The apparent viscosities of the SEGs and h-SEGs decreased significantly as the ST-Elastomer 10 component decreased (Fig. 3A), demonstrating the diluent nature of the cyclomethicone / DMS components. All the gels had power law index values of less than one, indicative of strongly shear-thinning behavior (data not presented).
Figure 2.
Representative flow rheograms for h-SEGs prepared from (A) DMS S12 and (B) DMS S51. Data for SEG 80/20 and HEC 250 HX control gels are also presented in A.
Figure 3.
Viscosities (Pa.S) of (A) placebo SEGs and h-SEGs having different ST-Elastomer 10 / cyclomethicone and ST-Elastomer 10 / st-PDMS ratios, and (B) placebo HEC gels having different HEC concentrations. The viscosities of five commercially available vaginal gels are presented in C.
Small changes in the st-PDMS concentration within the h-SEG formulations caused very significant decreases in gel viscosity. Surprisingly, this concentration-dependent decrease in viscosity was more marked with the higher molecular weight st-PDMS systems. For example, the addition of 5% w/w DMS S51 produced a very significant decrease in viscosity (measured at a shear rate of 1 s−1) from 277 Pa.S (100% ST-Elastomer 10) to 19.5 Pa.S (95% ST-Elastomer 10). Similar dramatic decreases in viscosity were observed with the DMS S27 and DMS S35 h-SEGs (Fig. 3A). By comparison, the DMS S12 h-SEG, prepared with the lowest molecular weight DMS component (and therefore having the greatest percentage of hydroxyl groups), showed a less dramatic decrease in viscosity with increasing DMS S12 concentration; at 5% and 10% w/w DMS S12 contents, the gel viscosity had declined to 178 and 69 Pa.S, respectively. This unexpected result effectively permits a relatively hydrophilic h-SEG formulation to be prepared while maintaining gel viscosity within a range deemed appropriate for vaginal application (see following section for details).
HEC gel viscosity was significantly influenced by both the HEC grade (molecular weight) and concentration (Fig. 3B). Gels prepared with the HX and HXX grades produced very similar viscosity profiles, ranging from 9 Pa.S at 1% w/w to 618 Pa.S at 5% w/w. The M-Pharm grade material produced lower viscosity gels (14 to 176 Pa.S). By comparison, the viscosities of five commercially available aqueous vaginal gels (Metrogel®, Gygel®, Replens®, Gynofit® and RepHresh®) were also measured (Fig. 3C), with viscosity values ranging from 33 Pa.S (Gynofit®) to 166 Pa.S (RepHresh®). The 80/20 SEG (Fig. 3A) and the 2.2% w/w HEC HX gel (Fig. 3B) had viscosity values close to 50 Pa.S, which falls within the range measured for the commercial products. h-SEG compositions with similar viscosity values are presented in Table 1. All viscosity values quoted were obtained at a shear rate of 1 s−1. Viscosity-matched gel formulations were next used for maraviroc and emtricitabine release testing in vitro.
Table 1.
Composition of h-SEGs that produced a viscosity equivalent to the 80/20 conventional SEG and 2.2% w/w HEC (HX) gel (~50 Pa.S) used for in vitro release testing.
| Formulation | Composition | Viscosity (Pa.S) |
|---|---|---|
| SEG | 80/20 | 48.49 ± 4.87 |
| DMS S12 h-SEG | 89/11 | 50.90 ± 1.20 |
| DMS S21 h-SEG | 95/5 | 52.13 ± 3.33 |
| DMS S27 h-SEG | 97.4/2.6 | 47.98 ± 1.98 |
| DMS S35 h-SEG | 97.7/2.3 | 52.97 ± 2.90 |
| DMS S51 h-SEG | 97.5/2.5 | 51.73 ± 0.89 |
| HEC (HX) | 2.2 | 43.57 ± 1.03 |
3.2. In vitro release of maraviroc and emtricitabine from gels
In vitro maraviroc release profiles into SVF and IPA/water are presented in Figures 4A and 4B for the 80/20 SEG, the DMS S12, DMS S21, DMS S27, DMS S35 and DMS S51 h-SEGs and the 2.2% w/w HEC gel, each loaded with 5% w/w maraviroc. For both media, release was greatest for the HEC gel, with 51 mg released into IPA/water and 55 mg released into SVF after 24 h (equivalent to ~100% release). By comparison, the maximum cumulative release from a silicone gel was 18 mg (36%) (SVF) and 21 mg (42%) (IPA/water) for DMS S12 h-SEG (the silicone gel formulation containing the greatest percentage of silanol groups), reflecting the fact that the h-SEGs are not aqueous miscible. Significantly more maraviroc was released into both media for the DMS S12 and DMS S21 h-SEGs than for the 80/20 SEG. In SVF media, the DMS S12 and DMS S21 h-SEGs released 18 mg (36%) and 16 mg (31%) respectively after 24 h, compared to only 3 mg (7%) released from the 80/20 SEG. After 24 h in IPA/water, the DMS S12 and DMS S21 h-SEGs released 21 mg (42%) and 16 mg (31%), respectively, while 12 mg (24%) was released from the 80/20 SEG. The release of maraviroc from the DMS S27, DMS S35 and DMS S51 h-SEGs into both media was comparable to the 80/20 SEG after 24 h.
Figure 4.
In vitro mean cumulative release versus time plots for maraviroc and emtricitabine from 80/20 SEG, DMS S12, DMS S21, DMS S27, DMS S35 and DMS S51 h-SEGs and the 2.2% w/w HEC gel into SVF (A) and IPA/ water (1:1) (B) over 24 h (n=4)
In vitro release profiles for emtricitabine (5% w/w loading) are presented in Figures 4C and 4D for the various SEG, h-SEG and HEC gels into SVF and IPA/water, respectively. Once again, emtricitabine release was greatest for the HEC gel in both media, with 43 mg (86%) released into SVF and 45 mg (90%) into IPA/water, respectively. Emtricitabine release after 24 h from the DMS S12 (3.9 mg, 7.8%), DMS S21 (3.2 mg, 6.3%), DMS S27 (3.2 mg, 6.4%) and DMS S35 h-SEGs (3.3 mg, 6.6%) was significantly greater than from the 80/20 SEG (2.4 mg, 5.6%) in SVF. The release of emtricitabine from the DMS S51 h-SEG (2.2 mg, 4.5%) after 24 h was comparable to the 80/20 SEG in SVF. After 24 h in IPA/water, only the release of emtricitabine from the DMS S12 h-SEG (14 mg, 28%) was significantly greater than from the 80/20 SEG (8.8 mg, 18%). For the 80/20 SEG and DMS S21, S27, S35 and S51 h-SEGs, no significant differences in release into IPA/ water were observed (Figure 4D).
3.3. Solubility of maraviroc and emtricitabine in st-PDMS and cyclomethicone
No numerical value for the solubility of maraviroc and emtricitabine in cyclomethicone could be determined as concentrations were below the limit of detections for both HPLC methods (maraviroc − 5.0 µg/mL, emtricitabine − 1.1 µg/mL). The solubilities of maraviroc and emtricitabine in the various st-PDMS materials were measurable, and increased in proportion to the weight percentage of hydroxyl groups (Fig. 5). It was also noted that the solubility of maraviroc in the st-PDMS was up to 100-fold greater than emtricitabine when the percentage hydroxyl group values were comparable. For example, the solubility of maraviroc in DMS S12 was 60 mg/mL whereas the emtricitabine solubility was only 0.46 mg/mL.
Figure 5.
Solubility of maraviroc and emtricitabine in st-DMS S12 (6% hydroxyl groups), DMS S14 (3.5% hydroxyl groups), DMS S15 (1.05% hydroxyl groups) and DMS S21 (0.85% hydroxyl groups). Solubility was also assessed in cyclomethicone (0% hydroxyl groups), although values were below the limit of HPLC quantification.
3.4. Aqueous dye ingression analysis of SEGs and h-SEGs
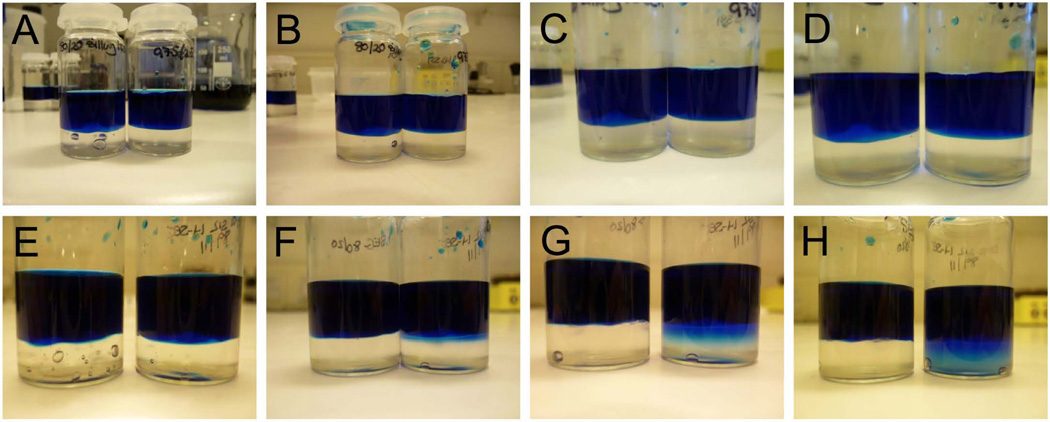
Ingression of methylene blue dye into the 80/20 SEG and the DMS S51 h-SEG (97.5/2.5) was not observed over 48 h (Fig. 6), reflecting the highly hydrophobic character of these gels. DMS S51 is the most hydrophobic of the st-PDMS materials, having the lowest weight percentage of silanol groups. By contrast, ingression of methylene blue dye was very apparent for the relatively hydrophilic DMS S12 h-SEG (89/11) at 6 h (Fig. 6F); by 48 h, the dye had diffused throughout the entire gel sample (Fig. 6H). However, the gel layer and dye solution remained as two immiscible phases.
Figure 6.
Ingression of aqueous methylene blue solution (blue top layer) into silicone elastomer gels (bottom layer). Bottom layer in photographs A–D comprise either SEG (80/20) (left vial in each photo) or DMS S51 h-SEG (97.5/2.5) (right vial in each photo) after 0 h (A), 6 h (B), 24 h (C) and 48 h (D). Photographs E–H show ingression into SEG (80/20) (left vial) and DMS S12 h-SEG (89/11) (right vial) at the same time points. The main point to note here is that the aqueous methylene blue solution only ingresses into the relatively hydrophilic DMS S12 gel.
3.5. In vitro gel dilution
The viscosities of the 80/20 SEG, DMS S12 h-SEG and DMS S51 h-SEG gels decreased by a relatively small 10 – 20% after 48 h placement in SVF. Initial viscosities were 43, 53 and 49 Pa.S, respectively, and final viscosities were 39, 43 and 42 Pa.S, respectively (Fig. 7). The latter three values were not significantly different from each other. By contrast, the initial viscosity of the 2.2% HEC (HX grade) gel was 61 Pa.S, but at 6 h it was too low to be measured using the rheological method due to its complete dilution in SVF.
Figure 7.
Viscosity of placebo SEG, DMS S12 h-SEG and DMS S51 h-SEG expressed as a percentage of initial viscosity following dilution with SVF at 0, 6, 24 and 48 h. A 2.2% w/w HEC gel was also tested, but the viscosity had declined to non-measurable values by 6 h.
3.6. Influence of gels on pH of simulated vaginal fluid
The pH of SVF was not significantly modified by the addition of SEG, DMS S12 h-SEG or DMS S52 h-SEG, with pH values maintained within the range 4.1 – 4.3 over a 24 h period (Fig. 8) and no significant differences observed between the gel formulations.
Figure 8.
Influence of silicone gel formulation on pH of simulated vaginal fluid.
3.7. Gel cytotoxicity
Results are presented as raw optical density (OD) values then as a percentage of ODs obtained for the 5 day DMEM control. The results showed minimal difference between the 5-day media control and the fresh media control. The viability recorded for cells exposed to HEC gel plus media was 10.9% ± 6.6%. Cells exposed to media incubated with hSEG gel demonstrated viability of 24.6% ± 5.7%. Cells exposed to media incubated with SEG gel demonstrated viability of 108% ± 14%. Percentage viability values above 100% are considered to be an artefact of the analysis method and should be considered completely viable.
4. DISCUSSION
Aqueous gels based on polyacrylic acid (Carbopol®) and HEC have been studied extensively as vaginal delivery systems for HIV-1 microbicides.10,11,13–15,17,28,46–48 In fact, a HEC-based gel formulation has been developed as a ‘universal placebo’ for use in clinical trials of vaginal HIV microbicide gels.49 The greatest advantages associated with use of aqueous HEC-based vaginal gels include good safety data, ease of manufacture and low cost. Some surveys have reported that women tend to favour use of gels over other vaginal products due to their convenience and ease of insertion.49–52 Gels are also versatile and easily manipulated to achieve optimum performance and efficacy.
Gel viscosity, which is largely influenced by gel composition, is one of the key determinants in the efficacy of vaginally administered pharmaceutical gels.53–56 For example, viscosity affects vaginal distribution and retention; lower viscosity gels tend to distribute more rapidly but are also more likely to be poorly retained.55–57 After application, aqueous gels are subjected to both high shear rates (related to the user’s physical activity) and the diluting effects of vaginal fluid.36,55 For these reasons, aqueous-based HIV microbicide gels are generally intended for administration immediately prior to sexual intercourse, so as to maximize local drug levels and increase efficacy.17,58 A major challenge remains for the development of vaginal gel formulations that can provide rapid and complete coverage of the tissue surfaces while also being retained for up to 24 h to permit once-daily application.59
Few studies have reported the effect of vaginal gel viscosity on retention and/or clinical efficacy. For HIV microbicide application, it is essential that the gel be distributed rapidly and widely throughout the vaginal cavity so as to form a protective physical barrier against transmission of virus and to deliver effective quantities of the constituent ARV(s) to the underlying tissue. A higher gel viscosity with poor spreadability characteristics has been linked to an increased risk of HIV-1 infection in hu-SCID mice.60 The universal placebo gel commonly used in microbicide trials contains 2.7% w/w HEC (HX grade) and has a viscosity of 68 Pa.S.49 A 2.2% w/w HEC gel used for vaginal delivery of maraviroc to rhesus macaques was determined to have a viscosity of 44 Pa.S (at a shear rate of 1 s−1).28 The 80/20 SEG gel evaluated in this study (48 Pa.S) has also been tested in rhesus macaques.40 To facilitate comparisons of in vitro release of model ARV microbicides across SEG, h-SEG and HEC gel formulations, it was important that gels had similar viscosity values, since viscosity differences were also likely to influence release. Based on rheological testing of the 2.2% w/w HEC gel and the 80/20 SEG, we selected h-SEG gel compositions with viscosities of ~50 Pa.S to use for in in vitro release testing (Table 1), a value within the range of the five commercial vaginal gel products we also tested (Fig. 3).
Hydrophobic silicone gels may overcome the poor vaginal retention observed with conventional aqueous gels by forming a non-dissolvable physical barrier layer at the vaginal mucosa. In a recent study, we found better in vitro retention and improved pharmacokinetics in rhesus macaques following a single dose vaginal application of maraviroc in a SEG 80/20 compared with a HEC-based gel.40 We hypothesized that partly or fully substituting the hydrophobic cyclomethicone component of a SEG with a relatively hydrophilic, low molecular weight, st-PDMS (to form an h-SEG) might increase the release of the incorporated ARV while maintaining the site retention characteristics. The rationale was that the solubility of the ARV in the gel system would be improved without compromising the other advantageous properties of SEGs. As predicted, increased solubility of maraviroc and emtricitabine in the st-PDMS materials was observed with increasing weight percentage of silanol groups (Fig. 5), leading to increased release rates (Fig. 4). This observation is in accordance with the Higuchi model which describes diffusion-controlled release polymer matrices containing dispersed drugs.61,62 In fact, the general trend in in vitro release as a function of DMS type in the h-SEGs (Fig. 4) mirrors the solubility data for maraviroc and emtricitabine in the DMS compounds (Fig. 5).
In vitro release of maraviroc and emtricitabine was greatest for the 2.2% w/w HEC gels, irrespective of the release medium used (Fig. 4). In fact, 100% drug release was achieved, consistent with a rapid breakdown in gel structure and complete dissolution of the drug. While such rapid drug release might be advantageous in providing high levels in vivo, it actually mitigates against a coitally independent microbicide gel product, since the drug release is unlikely to be sustained in vivo over more than a few hours at best. This supposition is consistent with results from a previous macaque study where the degree of protection afforded by a maraviroc HEC gel steadily declined from 86% to 0% as the interval between vaginal gel application and subsequent vaginal challenge was extended from 30 min to 12 h.28 A subsequent pharmacokinetic study testing the same maraviroc gel formulations provided additional insights into how the measured in vivo maraviroc concentrations (vaginal fluid, vaginal tissue, and plasma) correlated with the extent of protection.63 In that study, we noted that vaginal maraviroc concentrations typically declined by an order of magnitude over a 24 h period, presumably due in part to dilution and loss of the aqueous gel.
Release of maraviroc from the DMS S12 h-SEG into SVF was four times greater than for emtricitabine after 24 h (Fig. 4), despite emtricitabine having a much greater aqueous solubility (100 mg/mL vs. 1 mg/mL for maraviroc). This discrepancy is directly attributable to the solubility difference of the two ARVs in DMS S12 PDMS; maraviroc was 100-fold more soluble (Fig. 6), reaffirming the importance of drug solubility in the polymeric matrix when optimizing its release from a delivery system. However, drug solubility was not the only factor contributing to the enhanced release of maraviroc and emtricitabine from the DMS S12 h-SEG formulation. The increased hydrophilicity of the DMS S12 h-SEG (relative to the SEG 80/20 and the other DMS gels) facilitated the ingress of the aqueous release media into the gel matrix (Fig. 6), similar to that observed with the HEC gels. The ingress of fluid increases the surface area available for drug release and disrupts the gel matrix, although not to the extent that the rheological performance were greatly compromised (Fig. 7). For example, the final viscosity of the DMS S12 h-SEG was not significantly different from those of the 80/20 SEG and DMS S51 h-SEG, indicating that rheological structure is maintained. By comparison, the rheological properties of aqueous HEC gels were compromised shortly after vaginal administration due to dilution by vaginal fluids.32,55,64 At 0 h, the viscosities of the 2.2% w/w HEC and DMS S12 h-SEG were similar (~50 Pa.S), but by the 6 h time-point the HEC gel had been diluted to such an extent that its viscosity was too low to be measured using the rheological technique. This outcome has major implications for in vivo retention. The advantage of the DMS S12 h-SEG is that ARV release from this system can be enhanced while maintaining rheological structure. Taken together, these two properties may provide optimized drug coverage and retention within the vagina.
We used an established MMT cytotoxicity assay to measure and compare the toxicity of the new gel formulations with the widely used HEC-based vaginal placebo gel. Surprisingly, the HEC gel displayed the greatest toxicity. The h-SEG also displayed some cytotoxicity, though less than that seen with the HEC gel, while the SEG was free from negative effects. Unlike the SEG and the h-SEG gels, the HEC gel dissolved in the DMEM, helping explain why this well-established and safe gel formulation showed an apparently high measure of cytotoxicity. The lack of aqueous solubility of the SEG and h-SEG gels contributed to their low cytotoxicity measurements. Using a slug mucosal irritation model, we have previously shown that the SEG and hSEG gels do not cause mucosal irritation.40 Compared to the HEC gel, the SEG and hSEG gels performed well. Considered in conjunction with their lack of toxicity in the slug mucosal irritation model, these results suggest that they are relatively non-toxic. However, the apparent toxicity of the HEC gel suggests that this assay may not be the most suitable for assessing formulation toxicity and further testing would be required to definitively establish formulation safety.
CONCLUSIONS
The study describes a novel, non-aqueous, semi-solid drug delivery system for the sustained release of small molecule ARVs after vaginal delivery. In particular, the hydrophilically modified silicone elastomer gels offer certain advantages over both aqueous HEC-based gels and standard silicone elastomer gels. They have considerable potential for development as a vaginally administered, once-daily, coitally independent microbicide product for prevention of HIV-1 transmission. In vivo studies, both in relevant animal models and women, are required to assess the safety, drug pharmacokinetics and efficacy of these gels.
ACKNOWLEDGEMENTS
The work was funded through the European Union Seventh Framework Programme (CHAARM project, Health-F3-2009-242135) and National Institutes of Health (grant number U19 AI076982). We acknowledge supply of maraviroc and emtricitabine by ViiV Healthcare and CONRAD, respectively.
ABBREVIATIONS
- ARV
antiretroviral
- FTC
emtricitabine
- HEC
hydroxyethylcellulose
- HIV
human immunodeficiency virus
- IPA
isopropyl alcohol
- h-SEG
hydrophilically modified silicone elastomer gel
- PDMS
polydimethylsiloxane
- PrEP
pre-exposure prophylaxis
- MVC
maraviroc
- SEG
silicone elastomer gel
- st-PDMS
silanol-terminated polydimethylsiloxane
- SVF
simulated vaginal fluid
- TFA
trifluoroacetic acid
Footnotes
Conflicts of Interest: No conflicts of interest are declared for any of the authors.
CONTRIBUTIONS FROM AUTHORS
CJF, CFMcC, DJM and AE completed the laboratory experiments. RKM, JPM, RJS and ADW designed the studies and supervised the work. All authors contributed to drafting of the manuscript.
REFERENCES
- 1.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, Casapia M, Santiago S, Gilbert P, Corey L. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant Adenovirus HIV vaccine. J Infect Dis. 2012;206(2):258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH news statement. NIH discontinues immunizations in HIV vaccine study. 2013 Available from: http://www.niaid.nih.gov/news/newsreleases/2013/Pages/HVTN505 April2013.aspx.
- 3.García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong M, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JH, Jaffe HS, Martinez AI, Burns DN, Glidden DV. iPrEx Study Team, Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New Eng J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration press release. FDA approves first medication to reduce HIV risk. 2012 Available from: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm311821.htm.
- 6.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quifiones-Mateu ME, Vanham G. HIV microbicides: where are we now? Curr HIV Res. 2012;10:1–2. doi: 10.2174/157016212799304724. [DOI] [PubMed] [Google Scholar]
- McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis. 2010;23:26–31. doi: 10.1097/QCO.0b013e328334fe70. [DOI] [PubMed] [Google Scholar]
- Morris GC, Lacey CJN. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr Opin Infect Dis. 2010;23:57–63. doi: 10.1097/QCO.0b013e328334de6d. [DOI] [PubMed] [Google Scholar]
- Malcolm RK, Forbes CJ, Geer L, Veazey RS, Goldman L, Klasse PJ, Moore JP. Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J Antimicrob Chemother. 2013;68:678–683. doi: 10.1093/jac/dks422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Cosgrove Sweeney YT, Balkus JE, Rohan LC, Moncla BJ, Parniak MA, Hillier SL. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608–1615. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim SSA, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, Kapina M, Maslankowski L, Coletti A, Profy A, Moench TR, Piwowar-Manning E, Mâsse B, Hillier SL, Soto-Torres L. On behalf of the HPTN 035 study team, Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25:957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AM, Coplan P, van der Wijgert JH, Kapiga SH, von Mollendorf C, Geubbels E, Vyankandondera J, Rees HV, Masenga G, Kiwelu I, Moyes J, Smythe SC. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS. 2009;23:1531–1538. doi: 10.1097/QAD.0b013e32832c413d. [DOI] [PubMed] [Google Scholar]
- 14.Nel AM, Coplan P, Smythe SC, McCord K, Mitchnick M, Kaptur PE, Romano J. Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res Hum Retroviruses. 2010;26:1181–1190. doi: 10.1089/aid.2009.0227. [DOI] [PubMed] [Google Scholar]
- 15.Carballo-Diéguez A, Balan IC, Morrow K, Rosen R, Mantell JE, Gai F, Hoffman S, Maslankowski L, El-Sadr W, Mayer K. Acceptability of tenofovir gel as a vaginal microbicides by US male participants in a Phase I clinical trial (HPTN 050) AIDS Care. 2007;19:1026–1031. doi: 10.1080/09540120701294237. [DOI] [PubMed] [Google Scholar]
- 16.HPTN 059 press statement. HPTN 059: A multi-center clinical trial evaluating the safety and acceptability of the candidate microbicide tenofovir topical gel. 2008 Available from: http://www.mtnstopshiv.org/news/studies/hptn059/backgrounder. [Google Scholar]
- 17.Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. On behalf of the CAPRISA 004 trial group, Effectiveness and safety of tenofovir gel an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta KM, Pearce SM, Poursaid AE, Aliyar HA, Tresco PA, Mitchnick MA, Kiser PF. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008;97:4228–4239. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 19.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 20.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. Advances in microbicide vaginal rings. Antiviral Res. 2010;88S:S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Nel A, Smythe S, Young K, Malcolm K, McCoy C, Rosenberg Z, Romano J. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 22.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325:82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Fetherston SM, Geer L, Veazey RS, Goldman L, Murphy DJ, Ketas TJ, Klasse PJ, Blois S, La Colla P, Moore JP, Malcolm RK. Partial protection against multiple RT-SHIV162P3 vaginal challenge of rhesus macaques by a silicone elastomer vaginal ring releasing the NNRTI MC1220. J Antimicrob Chemother. 2013;68:394–403. doi: 10.1093/jac/dks415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer R, Mawson P, Derby N, Rodriguez A, Kizima L, Menon R, Goldman D, Kenney J, Aravantinou M, Seidor S, Gettie A, Blanchard J, Piatak M, Lifson JD, Fernández-Romero JA, Robbiani M, Zydowsky TM. An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci Trans Med. 2012;4:150ra123. doi: 10.1126/scitranslmed.3003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau CP, Srinivasan P, Smith JM, Baum MM. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob Agents Chemother. 2012;56:5952–5960. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malcolm K, Woolfson D, Russell J, Tallon P, McAuley L, Craig D. Influence of silicone elastomer solubility and diffusivity on the in vitro release of drugs from intravaginal rings. J Control Release. 2003;90:217–225. doi: 10.1016/s0168-3659(03)00178-0. [DOI] [PubMed] [Google Scholar]
- 27.MTN press statement. MTN statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. [01/02/12] Available from: http://www.mtnstopshiv.org/node/3909.
- 28.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motakis D, Parniak MA. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:1851–1856. doi: 10.1128/AAC.46.6.1851-1856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Cruz OJ, Uckun FM. Dawn of non-nucleoside inhibitor-based anti-HIV microbicides. J Antimicrob Chemother. 2006;57:411–423. doi: 10.1093/jac/dki464. [DOI] [PubMed] [Google Scholar]
- 31.Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Andrews GP, Donnelly L, Jones DS, Curran RM, Morrow RJ, Woolfson AD, Malcolm RK. Characterization of the rheological, mucoadhesive, and drug release properties of highly structured gel platforms for intravaginal drug delivery. Biomacromolecules. 2009;10:2427–2435. doi: 10.1021/bm9003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenta C, Kast CE, Harich I, Bernkop-Schnürch A. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for progesterone. J Control Release. 2001;77:323–332. doi: 10.1016/s0168-3659(01)00520-x. [DOI] [PubMed] [Google Scholar]
- 34.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–1712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Vermani K, Garg S, Zaneveld LJD. Assemblies for in vitro measurement of bioadhesive strength and retention characteristics in simulated vaginal environment. Drug Del Ind Pharm. 2002;28:1133–1146. doi: 10.1081/ddc-120014580. [DOI] [PubMed] [Google Scholar]
- 36.Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Brown J, Hooper G, Kenyon CJ, Haines S, Burt J, Humphries JM, Newman SP, Davis SS, Sparrow RA, Wilding IR. Spreading and retention of vaginal formulations in post-menopausal women as assessed by gamma scintigraphy. Pharm Res. 1997;14:1073–1078. doi: 10.1023/a:1012113714552. [DOI] [PubMed] [Google Scholar]
- 38.Chatterton BE, Penglis S, Kovacs JC, Presnell B, Hunt B. Retention and distribution of two 99mTc-DTPA labelled vaginal dosage forms. Int J Pharm. 2004;271:137–143. doi: 10.1016/j.ijpharm.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Witter FR, Barditch-Crovo P, Rocco L, Trapnell CB. Duration of vaginal retention and potential duration of antiviral activity for five nonoxynol-9 containing intravaginal contraceptives. Int J Gynecol Obstet. 1999;65:165–170. doi: 10.1016/s0020-7292(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 40.Forbes CJ, Lowry D, Greer L, Veazey RS, Shattock RJ, Klasse PJ, Mitchnick M, Goldman L, Doyle LA, Muldoon BCO, Woolfson AD, Moore JP, Malcolm RK. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release. 2011;156:161–169. doi: 10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, Wang L, Uranker K, Kunjara RP, Na Ayudhya K, Pryke K, Pickett J, LeBlanc MA, Rohan RC. Is Wetter Better? An Evaluation of Over-the-Counter Personal Lubricants for Safety and Anti-HIV-1 Activity. PLoS ONE. 2012;7(11):e48328. doi: 10.1371/journal.pone.0048328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Schnaare RL, Dezzutti C, Anton PA, Rohan LC. Rectal microbicides:clinically relevant approach to the design of rectal specific placebo formulations. Aids Res Therapy. 2011;8:12. doi: 10.1186/1742-6405-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutu G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, Barin B, Rooney JF, Mark D, Chetty P, Fast P, Priddy FH. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PloS ONE. 2012;7:e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fresno MJC, Ramírez AD, Jiménez MM. Systematic study of the flow behaviours and mechanical properties of Carbopol® Ultrex™ 10 hydroalcoholic gels. Eur J Pharm Biopharm. 2002;54:329–335. doi: 10.1016/s0939-6411(02)00080-2. [DOI] [PubMed] [Google Scholar]
- 45.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 46.Mahalingam A, Simmons AP, Ugaonkar SR, Watson KM, Dezzutti CS, Rohan LC, Buckheit RW, Kiser PF. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with dual mechanism of action against HIV-1. Antimicrob Agents Chemother. 2011;55:1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haaland RE, Evans-Strickfaden T, Holder A, Pau CP, McNicholl JM, Chaikummao S, Chonwattana W, Hart CE. UC781 microbicide gels retains anti-HIV activity in cervicovaginal lavage fluids collected following twice-daily vaginal application. Antimicrob Agents Chemother. 2012;56:3592–3596. doi: 10.1128/AAC.00452-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiser PF, Mahalingam A, Fabian J, Smith E, Damian FR, Peters JJ, Katz DF, Elgendy H, Clark MR, Friend DR. Design of tenofovir-UC781 combination microbicide vaginal gels. J Pharm Sci. 2012;101:1852–1864. doi: 10.1002/jps.23089. [DOI] [PubMed] [Google Scholar]
- 49.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 50.Hardy E, Jiménez AL, de Padua KS, Zaneveld LJD. Women’s preferences for vaginal antimicrobial contraceptives III: Choice of a formulation, applicator, and packaging. Contraception. 1998;58:245–249. doi: 10.1016/s0010-7824(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 51.Coggins C, Elias CJ, Atisook R, Bassett MT, Ettiègne-Traoré V, Ghys PD, Jenkins-Woelk L, Thongkrajai E, VanDevanter NL. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS. 1998;12:1389–1391. doi: 10.1097/00002030-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 52.Rosen RK, Morrow KM, Carballo-Diéguez A, Mantell JE, Hoffman S, Gai F, Maslankowski L, El-Sadr WM, Mayer KH. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: A mixed-methods study. J Womens Health. 2008;17:383–392. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 53.Owen DH, Peters JJ, Lavine ML, Katz DF. Effect of temperature and pH on contraceptive gel viscosity. Contraception. 2003;67:57–64. doi: 10.1016/s0010-7824(02)00430-4. [DOI] [PubMed] [Google Scholar]
- 54.Katz DF, Henderson MH, Owen DH, Plenys AM, Walmer DK. What is needed to advance vaginal formulation technology? In: Rencher WF, editor. Vaginal microbicide formulations workshop. Philadelphia: Lippincott-Raven Publishers Philadelphia; 1998. pp. 90–96. [Google Scholar]
- 55.Owen DH, Peters JJ, Katz D. Rheological properties of contraceptive gels. Contraception. 2000;62:321–326. doi: 10.1016/s0010-7824(00)00184-0. [DOI] [PubMed] [Google Scholar]
- 56.das Neves J, da Silva MV, Gonçalves MP, Amaral MH, Bahia MH. Rheological properties of vaginal hydrophilic polymer gels. Curr Drug Deliv. 2009;6:83–92. doi: 10.2174/156720109787048294. [DOI] [PubMed] [Google Scholar]
- 57.Yu T, Malcolm K, Woolfson D, Jones DS, Andrews GP. Vaginal gel drug delivery systems: understanding rheological characteristics and performance. Expert Opin Drug Deliv. 2011;8:1309–1322. doi: 10.1517/17425247.2011.600119. [DOI] [PubMed] [Google Scholar]
- 58.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12:272–283. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baloglu E, Sengyigit ZA, Karavana SY, Bernkop-Schürch A. Strategies to prolong the intravaginal residence time of drug delivery systems. J Pharm Pharmaceut Sci. 2009;12:312–336. doi: 10.18433/j3hp41. [DOI] [PubMed] [Google Scholar]
- 60.Di Fabio S, Van Roey J, Giannini G, van den Mooter G, Spada M, Binelli A, Pirillo MF, Germinario E, Belardelli F, de Bethune MP, Vella S. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS. 2003;17:1597–1604. doi: 10.1097/00002030-200307250-00003. [DOI] [PubMed] [Google Scholar]
- 61.Higuchi T. Mechanisms of sustained action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 62.Siepmann J, Peppas NA. Higuchi equation: Derivation, applications, use and misuse. Int J Pharm. 2011;418:6–12. doi: 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 63.Malcolm RK, Veazey RS, Greer L, Lowry D, Fetherston SM, Murphy DJ, Boyd P, Major I, Shattock RJ, Klasse PJ, Doyle LA, Rasmussen LL, Goldman L, Ketas TJ, Moore JP. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56:2251–2258. doi: 10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai BE, Xie YQ, Lavine ML, Szeri AJ, Owen DH, Katz DF. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J Pharm Sci. 2008;97:1030–1038. doi: 10.1002/jps.21132. [DOI] [PubMed] [Google Scholar]