Abstract
Study Objectives:
Sleep loss is suspected to induce endothelial dysfunction, a key factor in cardiovascular risk. We examined whether sympathetic activity is involved in the endothelial dysfunction caused by total sleep deprivation (TSD).
Design:
Two groups: TSD (24-h wakefulness), using slowly rotating wheels, and wheel control (WC).
Participants:
Seven-month-old male Wistar rats.
Interventions:
Pharmacological sympathectomy (reserpine, 5 mg/kg, intraperitoneal), nitric oxide synthase (NOS) inhibition (NG-nitro-L-arginine, 20 mg/kg, intraperitoneally 30 min before experiment) and cyclooxygenase (COX) inhibition (indomethacin, 5 mg/kg, intraperitoneally 30 min before experiment).
Measurements and Results:
In protocol 1, changes in heart rate (HR) and blood pressure were continuously recorded in the sympathectomized and non-sympathectomized rats. Blood pressure and HR increased during TSD in non-sympathectomized rats. In protocol 2, changes in skin blood flow (vasodilation) were assessed in the sympathectomized and non-sympathectomized rats using laser-Doppler flowmetry coupled with iontophoretic delivery of acetylcholine (ACh), sodium nitroprusside (SNP), and anodal and cathodal currents. ACh- and cathodal current-induced vasodilations were significantly attenuated after TSD in non-sympathectomized and sympathectomized rats (51% and 60%, respectively). In protocol 3, ACh-induced vasodilation was attenuated after NOS and COX inhibition (66% and 49%, respectively). Cathodal current-induced vasodilation decreased by 40% after COX inhibition. In TSD compared to WC a decrease in ACh-induced vasodilation was still observed after COX inhibition. No changes in SNP- and anodal current-induced vasodilation were detected.
Conclusion:
These results demonstrate that total sleep deprivation induces a reduction in endothelial-dependent vasodilation. This endothelial dysfunction is independent of blood pressure and sympathetic activity but associated with nitric oxide synthase and cyclooxygenase pathway alterations.
Citation:
Sauvet F; Florence G; Van Beers P; Drogou C; Lagrume C; Chaumes C; Ciret S; Leftheriotis G; Chennaoui M. Total sleep deprivation alters endothelial function in rats: a nonsympathetic mechanism. SLEEP 2014;37(3):465-473.
Keywords: Acetylcholine, arterial pressure, current-induced vasodilation, endothelial dysfunction, iontophoresis, skin blood flow, sodium nitroprusside, sympathectomy, vascular reactivity
INTRODUCTION
Sleep loss resulting from increased shift work and various other challenges imposed by modern society1 may represent a serious threat to health. Decreased sleep time is notably associated with the development of cardiovascular diseases including stroke, coronary events, sudden cardiac death, and hypertension.2–4 In a recent study, we showed that 29 h of continuous wakefulness is sufficient stress to trigger a decrease in endothelial-dependent cutaneous vasodilation5 in healthy humans. Other authors have confirmed this endothelial dysfunction in healthy subjects after acute sleep restriction.6–9 Endothelial dysfunction is already known to be a key factor in the physiopathology of cardiovascular disease.10 The effect of total sleep deprivation (TSD) on the endothelial function of rodents has never before been published to the best of our knowledge.
Among the possible mechanisms linking sleep disorders to cardiovascular diseases are activation of the sympathetic nervous system suspected of inducing an increase in blood pressure11 and an endothelial dysfunction6 that may trigger the cardiovascular events more frequently observed in chronic short sleepers (< 6 h)12 or after an acute lack of sleep.13,14 Indeed, many studies have observed that chronic sleep disorders or acute sleep restriction are associated with activation of the sympathetic nervous system, increased blood pressure, and baroreflex sensitivity changes in both humans6,11,15 and rodents.16 However, the effects of acute TSD on sympathovagal balance are less clear. Although some studies showed increased sympathetic activity and heart rate (HR),11 others reported no significant changes in resting HR, increased arterial pressure, and a reduction in muscle sympathetic nerve activity.17–19 Moreover, we observed that endothelial dysfunction appears before the increase in sympathetic activity and blood pressure5 during 40 h of wakefulness (i.e., TSD) in healthy humans. In other words, the available data still do not provide a clear picture regarding the role of sympathetic activation in the development of endothelial dysfunction during sleep loss.
Because the skin is readily accessible in humans and rodents, it provides an appropriate site to assess peripheral microvascular endothelial function with noninvasive methods.20,21 In particular, vascular response to ACh administration, a substance that induces cutaneous vasodilation via endothelium-dependent releases of a variety of vasoactive substances such as nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factors (EDHF), is correlated with cardiac and large-vessel endothelial function.22–24 Currently, low ACh-induced cutaneous vasodilation is used as a surrogate marker of cardiovascular risk.25
The purposes of this study were (1) to characterize the effect of TSD (24 h of wakefulness) on blood pressure and HR in sympathectomized and non-sympathectomized rats; (2) to assess the effect of TSD and sympathectomy on cutaneous endothelial-dependent vasodilation; and (3) to evaluate the effects of TSD on the NO and PGI2 metabolic pathways implicated in endothelial-dependent vasodilation.
METHODS
Animals
Experiments were performed with male Wistar rats (250-350 g, 7 to 9 w old, Janvier laboratory, Le Genest-St-Isle, France). Procedures for the maintenance and use of the animals were carried out in accordance with French regulations and approved by the institutional ethics committee for animal experimentation.
On arrival the rats were housed five per cage in a constant environment (temperature: 22 ± 2°C; relative hygrometry: 50 ± 15%) and a 12/12 light/dark cycle (300 Lux) with lights on from 07:00 to 19:00. Animals had access to standard rodent pellets and water ad libitum. All animals were given at least 1 w before the experiment to acclimatize to the environment. The rats were then randomly distributed into two groups: TSD (total sleep deprivation) or WC (wheel control). Each rat's weight was recording daily in both groups (TSD and WC).
Total Sleep Deprivation
Rats were singly housed throughout the TSD experiments in activity wheels (Lafayette Instruments, Lafayette, IN, USA) customized as previously described and validated.26 Briefly, these are large motorized stainless-steel activity wheels that allow free access to food and water. In the 5 days preceding the TSD experiment the rats were habituated to the activity wheel with a 30-min session at 09:00 and 15:00 every day. TSD (09:00-09:00) was produced by the rotation of the activity wheel, programmed on a schedule of 3 sec “on” at a speed of 3 m.min-1 and 12 sec “off”. Similar protocols have previously been shown to result in wakefulness for more than 93% of a 24-h period.26 In our experimental condition, we observed that the animals had the following sleep pattern: 97.1 ± 2.5% of wakefulness, 2.8 ± 2.1% of non-rapid eye movement (NREM) sleep and 0.1 ± 0.1% of rapid eye movement (REM) sleep.
To control the nonspecific effects of the activity wheel (i.e., locomotor activity and restraint) a WC group was used. These rats were housed in the wheel moving for 36 min in every 3-h period for a 24-h period (09:00-09:00). The speed of the wheel was the same as that of the TSD group. In this group the rats experienced the same amount of locomotor activity as in TSD (≈ 800 m/24 h) but they had the opportunity to enter and maintain deep sleep (56.7 ± 4.5% of wakefulness, 37.0 ± 3.1% of NREM sleep and 6.3 ± 2.1% of REM sleep). We confirmed that no differences in endothelial function parameters were observed between the WC and control cage (CC) groups (see supplemental material for CC condition values). In the CC condition, rats were housed in the rodent room during all the experiments.
Telemetry Surgical Implantation
Telemetry transmitters, for the recording of arterial blood pressure, electrocardiogram (ECG) and body temperature (Tco), were chronically implanted in rats (C50-PXT, Data Sciences Int., St Paul, MN, USA). We used a surgical procedure that guarantees high-quality recordings in rats, even during physical activity.16 Briefly, the rats were anesthetized with isoflurane [5% (induction) / 1-2%, oxygen (maintenance)] and buprenorphine (0.05 mg/kg, subcutaneously, 20 min before surgery). The transmitter catheter was inserted into the abdominal aorta, just proximal to the iliac bifurcation. The two electrodes for ECG recording were fixed to the dorsal surface of the xiphoid process and the anterior mediastinum, close to the right atrium. The transmitter body was introduced into the abdominal cavity and attached to the abdominal wall with a 3-0 proline suture. Subsequently, the rats were injected with a prophylactic antibiotic (benzathine benzylpenicilline, Extencilline®, 60,000 UI, intramuscularly) and received postoperative analgesic treatment for 3 days (carprofen, 4 mg/kg subcutaneously per day).27 After surgery, the rats were singly housed and allowed to recover for 14 days.
Endothelial Function Assessment
Two days before the experiment, a depilatory lotion was used to remove the rats' hair from the skull and along the back to avoid any skin irritation at the time of the study. For the experiment, the animals were tranquilized (phenobarbital sodium, Nesdonal, 40 mg/kg intraperitoneally) and placed in the prone position in a warm (30 ± 0.5°C) incubator to maintain a stable rectal temperature throughout the experiment (ML295/R Home-othermic Controller, ADInstruments Ltd, Oxford, UK).21,28,29 Skin blood flows (SkBf) were assessed using laser-Doppler flowmeters (PF 5001 Master Periflux, Perimed, Sweden) and heat-controlled (33°C) laser-Doppler probes (PF 481-1, Perimed) coupled with iontophoresis devices. After gentle cleaning with an alcoholic solution, the probes were positioned on the hairless skin of the back. Iontophoresis is an established method in which an applied electric field is used to facilitate the cutaneous penetration of vasoactive drugs.20 Combined with laser-Doppler measurements, iontophoresis allows the assessment of a drug's effect on SkBf. All drugs for iontophoresis were dissolved in deionized water and placed in the 1.2-cm2 sponge of the iontophoretic drug-delivery electrode, which also contained the laser-Doppler probe at its center. A battery-driven power supply provided constant anodal or cathodal currents for drug delivery. All drugs were delivered using a 30 sec continuous current (100 μA, 0.4 mC).
Each rat was implanted with four iontophoresis/laser-Doppler probes for delivery of an acetylcholine chloride solution (ACh, 5.5 mM, Sigma-Aldrich CO, St. Louis, MO, USA.), a sodium nitroprusside solution (SNP, 67 mM, SERB laboratory, Paris, France), and the carrier alone (deionized water, in the other two probes) in order to control the effect of anodal and cathodal current-induced vasodilation.28 ACh delivered with an anodal current induces endothelium-dependent vasodilation (Figures 1 and 2). The nitric oxide (NO) donor SNP delivered with a cathodal current induces an endothelium-independent vasodilation. Although the current applications are not painful, it is generally admitted that iontophoretic current-induced vasodilation relies on an axon reflex due to excitation of cutaneous nociceptors by the current.30 Moreover, among the various observations regarding current-induced vasodilation, it has been reported that cathodal current induces a vasodilation dependent on cyclooxygenase-1 (COX-1) activation both in rats28 and in humans.30
Figure 1.

Mechanisms involved in iontophoretic-induced vasodilation. SNP, sodium nitroprusside; NO, nitric oxide; COX-1, cyclooxygenase-1; PGI2, prostacyclin (prostaglandin I2); Indo, indomethacin, an inhibitor of cyclooxygenases; L-NNA, NG-Nitro-L-arginine, an inhibitor of nitric oxide synthase.
Figure 2.
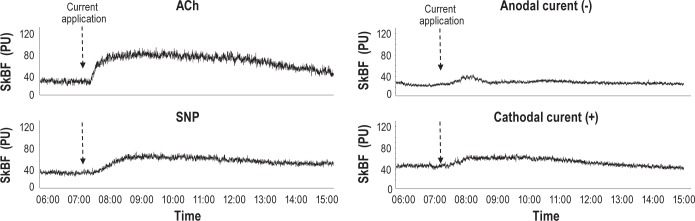
Examples of changes in skin blood flow (SkBf) after iontophoretic application (0.1 mA, 30 sec) of acetylcholine (ACh), anodal current (-), sodium nitroprusside (SNP) and cathodal current (+).
SkBF signals were digitized (500 Hz sampling frequency) using a computerized acquisition system (PowerLab, ADIinstruments Ltd). Data collection started with a 10-min control period before the current stimulations began and was continued for 20 min. SkBf is expressed in arbitrary perfusion units (PU). Recordings started only if the SkBf control values were between 20 and 100 PU.
Systolic and diastolic arterial pressure (SAP and DAP) was noninvasively assessed with a pulse transducer and a pressure cuff system placed on the tail (ML125/R NIBP system, ADInstruments Ltd). They were determined before iontophoresis and at the end of the experiment.
Sympathectomy
In some experiments sympathectomy was induced by injection of reserpine (5 mg/kg intraperitoneally per day) on 2 consecutive days: 24 h and 1 h before the experiment.31 The reserpine was dissolved in 2% ascorbic acid in a 0.9% saline solution (solvent).31 Similarly, the non-sympathectomized rats received an equivalent volume of solvent (intraperitoneally). Such a chronic sympathectomy is reported not to alter the blood pressure and HR baseline levels but to markedly enhance blood pressure variability.31,32 At the end of the experiment, the efficacy of the sympathectomy was checked by the attenuated response to an injection of a sympathomimetic agent (tyramine, 250 μg/kg intravenously).33 With tyramine, mean arterial pressure (MAP) increased by 13 ± 2 mmHg in the sympathectomized rats versus 57 ± 4 mmHg in non-sympathectomized rats (P < 0.01).33 All chemicals were obtained from Sigma-Aldrich CO, St. Louis, MO, USA.
Protocols
Three protocols were performed with different sets of animals.
In protocol 1, we assessed the effect of sleep deprivation on subsequent recovery of the daily rhythm of blood pressure, HR and Tco. Fourteen days after surgery, data from the sympathectomized and non-sympathectomized rats (n = 6 per condition) were continuously recorded the day before (baseline), during TSD and WC, and the following day (Dataquest ART Acquisition program, Data Science Int., St. Paul, MN, USA). The ASCI files were sent to Labchart (ADInstruments Ltd) for post-acquisition analysis.
In protocol 2, we examined the effect of TSD on vascular reactivity (ACh-, SNP-, anodal current- and cathodal current-induced vasodilation) in sympathectomized and non-sympathectomized rats (n = 8 per group).
In protocol 3, a pharmacological approach was used to identify the involvement of the underlying signaling pathways implicated in cutaneous vascular reactivity. Vascular reactivity was evaluated in rats (n = 6) previously treated (30 min before the experiment) with a saline solution (Control), a nonspecific COX inhibitor (INDO, indomethacin, 5 mg/kg intraperitoneally, Sigma-Aldrich CO, St. Louis, MO, USA.), a nonspecific nitric oxide synthase (NOS) inhibitor (L-NNA, NG-Nitro-L-arginine, 20 mg/kg intraperitoneally, Sigma-Aldrich), and a combination of INDO + L-NNA.28,29
Data Analysis
In protocol 1, arterial pressure, ECG, and Tco signals were analyzed offline using Labchart software (ADInstruments Ltd). Average R-R interval (RR, ms) and HR (bpm), SAP (mmHg), DAP (mmHg), MAP (mmHg), and Tco were quantified and expressed as the mean value of successive 3-h periods.
In protocols 2 and 3, SkBf signals were averaged every 10 sec to reduce the effects of instantaneous variability due to vasomotion. Baseline values were calculated as the mean of the 2-min control period before applying the current. SNP-, ACh-, cathodal- and anodal-induced vasodilation responses were reported as peak changes (increase in SkBf from baseline, as a % of baseline) in response to the iontophoretic delivery. In order to evaluate the effect of ACh and SNP without any influence of the current on the vascular structure, the vasodilations observed with anodal or cathodal currents were subtracted from ACh and SNP values, respectively.
Statistics
In protocol 1, differences between TSD and WC animals were evaluated using a two-way analysis of variance: “time of day” (repeated factor, 12 per day, i.e., 230 degrees of freedom) and “experimental situation” (non-repeated factor, three levels: TSD and WC, i.e., 10 degrees of freedom). If appropriate, we compared the values measured at the same time of day using a post hoc Bonferroni test. We had previously confirmed that the distribution of values followed a normal law.
In protocol 2 and 3, differences between TSD and WC and between the sympathectomized and non-sympathectomized rats were evaluated using a Kruskal-Wallis one-way analysis of variance on ranks followed by a between-groups pairwise Mann-Whitney rank sum test where appropriate. All statistical analyses were made using Statistica (StatSoft, Maisons-Alfort, France). Data are reported as the mean ± standard error of the mean (SEM).
RESULTS
Protocol 1: Effect of TSD on Arterial Pressure, HR, and Tco
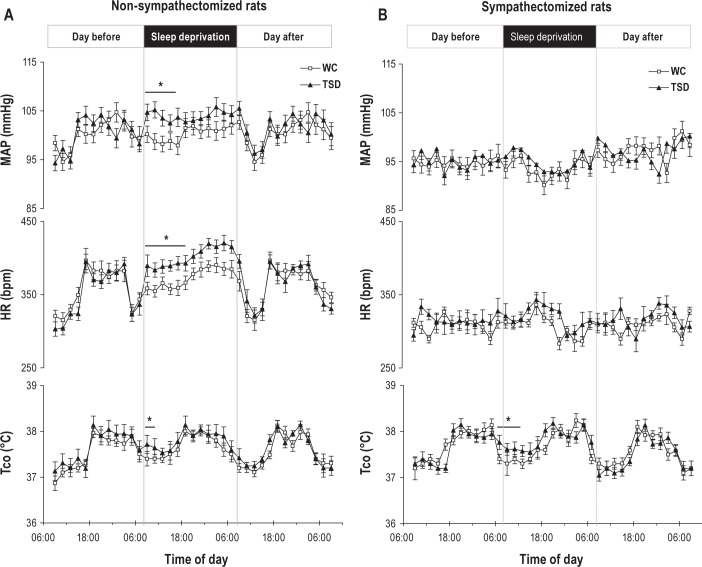
In non-sympathectomized TSD rats, we observed an increase in MAP (Figure 3) and SAP between 09:00 and 18:00 compared to WC (interaction between the “time of day” and the “experimental situation”, F(10, 230) = 97.2, P < 0.01 for MAP and F(10, 230) = 99.7, P < 0.01 for SAP). For HR, a significant interaction between the “time of day” and the “experimental situation” was detected (F(10, 230) = 102.7, P < 0.01). HR in non-sympathectomized TSD rats was higher compared to WC between 09:00 and 18:00. No significant increase in TSD was noticed during the recovery day. Tco in non-sympathectomized TSD rats was greater than in WC rats between 09:00 and 12:00 (interaction between the “time of day” and the “experimental situation”, F(10, 230) = 57.2, P < 0.05).
Figure 3.
Effects of 24 h of wakefulness on the circadian rhythm of mean arterial pressure (MAP), heart rate (HR), and body temperature (Tco) in nonsympathectomized (A) and sympathectomized (B) rats. Groups are wheel control (WC) and total sleep deprivation (TSD). Values are mean ± standard error of the mean. n = 6. *Differences between TSD and WC (P < 0.05).
In sympathectomized rats, no significant difference in SAP, DAP, MAP, and HR between the groups was evidenced whatever the day. Tco was higher between 09:00 and 13:00 in the TSD group compared to WC (interaction between the “time of day” and the “experimental situation”, F(10, 230) = 53.5, P < 0.05).
Protocol 2: TSD Effect on Cutaneous Vasodilation
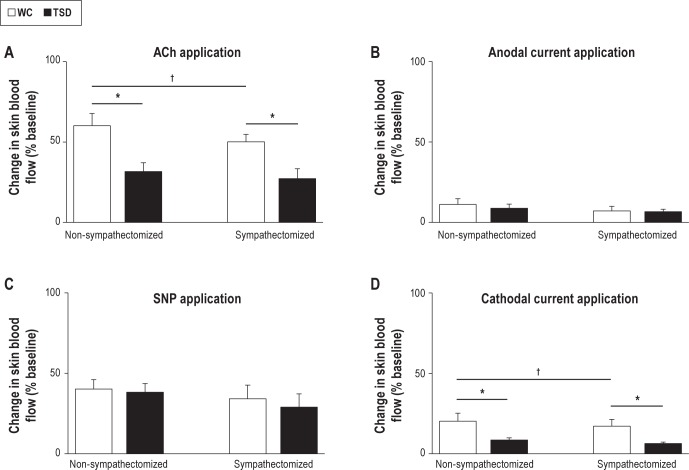
The iontophoretic delivery of ACh increased SkBF from baseline in non-sympathectomized (64.3 ± 8.2%) and in sympathectomized rats (52.7 ± 5.6%, P = 0.12; Figure 4). In TSD compared to WC, we observed a reduced ACh-induced vasodilation in both non-sympathectomized (31.7 ± 5.5%, P < 0.05) and sympathectomized rats (28.3 ± 6.1%, P < 005). Sympathectomy induced a decrease in ACh-induced vasodilation in WC rats (60.2 ± 7.6% versus 50.2 ± 4.6%, P < 0.05).
Figure 4.
Maximal relative increases in skin blood flow in non-sympathectomized and sympathectomized rats (A) After acetylcholine (ACh) application. (B) After anodal current application. (C) After sodium nitroprusside (SNP) application. (D) After cathodal current application. Groups are wheel control (WC) and total sleep deprivation (TSD). n = 8. Values are mean ± standard error of the mean. *Difference versus WC (P < 0.05); †Difference versus nonsympathectomized rats (P < 0.05).
No significant difference in anodal current-induced vasodilation was observed between sympathectomized (10.8 ± 4.2%, in WC) and non-sympathectomized rats (6.6 ± 3.8%, in WC) or between TSD and WC.
Regarding the SNP-induced vasodilation, no differences were observed between non-sympathectomized (40.2 ± 5.1% for WC) and sympathectomized rats (38.7 ± 4.3% for WC) or between TSD and WC groups.
The cathodal current-induced dilation was less in TSD (8.6 ± 1.3%) compared to WC (20.3 ± 5.0%, P < 0.05). We showed no significant changes in WC and TSD in non-sympathectomized compared with sympathectomized rats.
No significant differences were observed in the basal SkBf (57.4 ± 12.1 PU) or MAP (88 ± 5 mmHg) between TSD and WC or between sympathectomized and non-sympathectomized rats. As expected, the effect of tyramine on MAP was higher in non-sympathectomized compared to sympathectomized rats (54 ± 4 mmHg versus 13 ± 1 mmHg in WC, P < 0.05 and 53 ± 3 mmHg versus 14 ± 3 mmHg for TSD, P < 0.05).
Protocol 3: TSD Effect on Endothelial-Dependent Vasodilation Metabolic Pathways
The inhibition type (sympathectomized and non-sympathectomized) and groups (WC and TSD) did not significantly change the basal SkBf (mean value 55.3 ± 10.5 PU) or the MAP (mean 85 ± 5 mmHg), except the L-NNA treatment that induced an increase in MAP (mean value 30 ± 8%) showing the efficiency of the NOS inhibition.
The ACh-induced vasodilation (70.7 ± 7.6%) was almost abolished after NO synthase inhibition (7.7 ± 2.5%, P < 0.001) and was reduced after COX inhibition (40.8 ± 7.7%, P < 0.01; Figure 5). In TSD after COX inhibition compared to WC, we observed that the decrease in ACh-induced vasodilation persisted (15.1 ± 5.3% in TSD, P < 0.05), whereas no significant changes were observed after NOS inhibition and COX + NOS inhibition.
Figure 5.
Maximal relative increases in skin blood flow in rats treated with saline solution (Control) or indomethacin [Cyclooxygenase (COX) inhibition] or L-NNA [Nitric oxide synthase (NOS) inhibition] or with a combination of indomethacin and L-NNA [COX + NOS inhibition]. (A) After acetylcholine (ACh) application. (B) After anodal current application. (C) After sodium nitroprusside (SNP) application. (D) After cathodal current application. Groups are wheel control (WC) and total sleep deprivation (TSD). n = 8. Values are mean ± standard error of the mean. *Difference versus WC (P < 0.05); †Difference versus control (i.e., rats treated with a saline solution) (P < 0.05).
No significant differences in anodal current- (10.9 ± 4.5%) and SNP- (33.0 ± 7.5%) induced vasodilation were observed after COX, NOS, or COX + NOS inhibition or between TSD and WC.
The cathodal current-induced vasodilation (43.3 ± 7.5%) decreased after COX inhibition (6.5 ± 4.8%, P < 0.01), whereas no difference was observed after NOS inhibition. Moreover, the cathodal current-induced vasodilation was also lower in TSD when compared to WC after NOS inhibition (32.5 ± 6.9%, P < 0.05), whereas no significant differences were detected after COX or COX + NOS inhibition.
DISCUSSION
In this study we observed that 24 h of continuous wakefulness (i.e., TSD) induced a transient increase in blood pressure and HR in rats. These changes were related to an increase in sympathetic activity that disappeared the day after TSD. We also showed for the first time that TSD is sufficient stress to trigger a decrease in endothelium-dependent vasodilation in rodents. Moreover, our results demonstrate that this endothelial dysfunction is a sympathetic-independent mechanism because it is still obvious after pharmacological sympathectomy. Finally, we showed that the endothelial dysfunction induced by TSD is related to an alteration of both the COX-1 and NOS pathways.
Sleep Deprivation Alters Endothelial Function
In this study on rats we demonstrated for the first time that 24 h of wakefulness alter ACh-induced vasodilation, a sign of endothelial dysfunction.23 This result confirms previous studies showing endothelial dysfunction after acute sleep deprivation5,9,34 or acute sleep restriction6–8 in healthy humans. Indeed, we showed a significant effect of sleep deprivation on endothelium-dependent vasodilation in TSD rats compared to WC groups. Moreover, as previously described in humans34 we showed no significant effect of TSD on endothelium-independent vasodilation as assessed with SNP delivery. These results support the view that acute TSD in rats is sufficient stress to trigger an alteration of the endothelial-dependent vasodilation that is a sign of endothelial dysfunction.
A Sympathetic Independent Mechanism
According to previously published studies of rats and humans, 24 h of wakefulness induce a transient increase in blood pressure and HR that did not persist the day following TSD.16,35 These changes were not observed in sympathectomized rats. On the contrary, it has been reported that 24 h of paradoxical sleep deprivation do not induce significant increases in HR and MAP (96 h are necessary).36 So, a decrease in total sleep time produces an increase in sympathetic activity during the wakefulness period, leading to a transient rise in blood pressure and HR. Sympathetic activity enhancement has been involved in the decrease of endothelial-dependent vasodilation37 and suspected to link sleep deprivation and endothelial dysfunction.6 However, these results show for the first time that endothelial dysfunction induced by TSD persists in sympathectomized rats. Thus, we may conclude that the initial endothelial dysfunction observed after 24 h of wakefulness is a sympathetic independent mechanism. This is an important result for understanding the kinetics of the vascular alterations observed after sleep loss.
However, we cannot exclude that a persistent increase in sympathetic activity would favor endothelial dysfunction during repeated TSD or prolonged sleep restriction. Indeed, Amir et al.34 and Wehrens et al.38 showed that endothelial dysfunction after a night shift was greater in subjects with a longer history of night-shift duty and was associated with an increase in sympathetic activity. Moreover, Sgoifo et al.16 demonstrated that sleep deprivation changes baroreflex sensitivity. In their study, animals were exposed to an acute restraining stress and sleep deprivation induced a blunted parasympathetic antagonism following sympathetic activation, together with an increased susceptibility to cardiac arrhythmia. So, when TSD is associated with stress, we cannot exclude that a large increase in sympathetic activity could be related to endothelial dysfunction.
Metabolic Pathways Implicated
Our results confirm previous findings28,30 that concluded that ACh-induced vasodilation is almost abolished after NOS inhibition and is reduced after COX inhibition in rat skin, thus demonstrating the major role of NO in the in vivo endothelium-dependent vasodilation and the significant participation of prostaglandins.28,30 Moreover, although current-induced vasodilation (CIV) exists at both anodal and cathodal electrodes, we corroborate previous findings in rats28 that CIV amplitude is almost three times greater at the cathode (35%) than the anode (10%) for the same charge. Our results also confirm that indomethacin (COX inhibitor) delivery decreases cathodal CIV by almost 80%, proving the major contribution of the COX-1 pathway in cathodal CIV, as previously reported in humans30 and rats.28
In this study, we found that after COX inhibition (i.e., with only the NO pathway available) there was less ACh-induced vasodilation in TSD rats compared to WC. Our results suggest for the first time that in rodents 24 h of wakefulness alter the NO pathway that is the most important endothelium-dependent vasodilation pathway. However, because we also observed that TSD decreased cathodal-induced vasodilation, we conclude that acute TSD also decreases the COX-1 dependent vasodilation pathway. Our results demonstrate that acute TSD alters cutaneous endothelial function, and in particular both the NOS and COX-1 pathways. However, the evidence suggests that there is considerable ‘‘cross talk’’ between NO and prostaglandins biosynthetic pathways.39 The effect of PGI2 is closely related to NO effects because PGI2 potentiates NO release, and in turn NO potentiates the effect of PGI2 on vascular smooth cells via a cyclic AMP turnover pathway.40
Many authors have observed that sleep deprivation in rodents changed COX-1 and NOS bioavailability in several brain regions and showed a decrease in cortical neural NOS activity during partial sleep deprivation and subsequent 24-h recovery.41,42 Moreover, 5 days of TSD inhibited neuronal NOS activity in the cardiovascular afferent neurons (i.e., nodose neurons) of adult rats.43 Because NO may play an inhibitory role in the regulation of sympathetic tone in the cardiovascular afferent neurons, these authors related the decrease in NO production to the increased sympathetic activity observed during sleep disorders. Furthermore, systemic injection of an NOS inhibitor suppressed sleep rebound induced by sleep deprivation in rats, in particular NREM sleep rebound.44 Others showed that paradoxical sleep deprivation and TSD (72 h) are associated with oxidative stress in the cortex and hippocampus and the protective effect of an antioxidant treatment on behavior and memory.45,46 It is well established that the NO production may play a role as a signaling molecule in the homeostatic regulation of sleep, and that TSD alters NOS activity.44,47 In addition, sleep deprivation also inhibits COX activity in the cardiovascular afferents of adult rats.43
Sleep deprivation adversely affects several systems that could change vascular response. In particular, many studies have demonstrated that TSD is associated with low-grade systemic inflammation with increased levels of tumor necrosis factor (TNF-α)48, interleukin-649 and C-reactive protein49 that are suspected to induce endothelial dysfunction and impair NO availability by a COX-2 dependent pathway finally leading to increased production of oxidative stress.50 The vascular reactivity of skin to ACh is significantly reduced during low-grade chronic inflammation.51 Furthermore, an increase in endothelin-1 (the main endothelial vasoconstrictive hormone) plasma levels has been observed after 96 h of partial sleep deprivation in rats52 and 27 h of wakefulness in humans.53 Endothelin-1 is secreted in particular during sympathetic activation or inflammation.54
Limitations
Although our results are consistent with previous findings in humans, they were obtained in tranquilized rats. Sodium pentobarbital was used because it does not alter the COX and NOS portions of ACh vasodilation in rodents,55 although it modifies the EDHF pathway that also contributes to ACh-dependent vasodilation.29,55 However, the physiological role of EDHF is more important in older rodents in which the age-related decrease in NO availability is in part compensated with an EDHF-dependent backup.21 Previous findings in humans showed that TSD induces a greater increase in SAP and MAP in the elderly than in young adults,56 so it would be pertinent to study the specific effect of TSD on the EDHF pathway.
The pharmacological sympathectomy induced by reserpine appears to be incomplete after 24 h of TSD or WC because there remained a small increase in MAP (< 15 mmHg versus > 50 mmHg in non-sympathectomized rats) that could be explained by the half-life of the reserpine-induced sympathectomy (about 20-24 h).31–33 Longer subchronic sympathectomy methods would need to be used for sleep deprivation/restriction protocols of longer than 24 h.
CONCLUSION
These original results demonstrate that 24 h of wakefulness in rats induce a decrease in endothelial-dependent vasodilation that is not related to changes in blood pressure or sympathetic activation. Ours findings also suggest that this endothelial dysfunction is associated with a decrease in the activity of both the NOS and COX-1 pathways.
DISCLOSURE STATEMENT
This was not an industry supported study. The General Directorate for Armament (DGA, Ministry of Defense, contracts 08Ca704 and 10Ca704) provided financial support for this research project. Pascal Van Beers participates in research activities supported by Physip SA, Paris, France. Fabien Sauvet, Pascal Van Beers and Mounir Chennaoui received free use of material provided by Philips SA, Surennes, France. The material presented in this manuscript has no relationship with any of these potential conflicts. The other authors have indicated no financial conflicts of interest. This work was performed at the Armed Forces Biomedical Research Institute (IRBA), Brétigny sur Orge, France.
ACKNOWLEDGMENTS
The authors thank Mr Francis Boutet, Thierry Leloup, Sergent Renaud Duquenoy, and Adjudant-chef Xavier Bitaud for their technical assistance and Wanda Lipski for English language editing.
ABBREVIATIONS
- ACh
acetylcholine chloride
- CC
control cage group
- CIV
current induced vasodilation
- COX
cyclooxygenase
- DAP
diastolic arterial pressure
- EDHF
endothelium-derived hyperpolarizing factors
- HR
heart rate
- INDO
indomethacin, an inhibitor of cyclooxygenase 1 & 2
- L-NNA
NG-Nitro-L-arginine, an inhibitor of nitric oxide synthase
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- PU
perfusion units
- SAP
systolic arterial pressure
- SkBF
skin blood flow
- SNP
sodium nitroprusside
- Tco
body temperature
- TSD
total sleep deprivation (i.e., 24-h of continuous wakefulness)
- WC
24-h wheel control group
SUPPLEMENTAL MATERIAL
Blood pressure, heart rate, and body temperature values in non-sympathectomized and sympathectomized rats in the control cage experimental condition
Effect of sympathectomy on endothelial function in the control cage experimental condition
Effect of nitric oxide and cyclooxygenase on endothelial function inhibition, in the control cage experimental condition
REFERENCES
- 1.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 2.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 3.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev. 2010;6:54–61. doi: 10.2174/157340310790231635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamazaki Y, Morikawa Y, Nakamura K, et al. The effects of sleep duration on the incidence of cardiovascular events among middle-aged male workers in Japan. Scand J Work Environ Health. 2011;37:411–7. doi: 10.5271/sjweh.3168. [DOI] [PubMed] [Google Scholar]
- 5.Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 6.Dettoni JL, Consolim-Colombo FM, Drager LF, et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol. 2012;113:232–6. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- 7.Takase B, Akima T, Uehata A, Ohsuzu F, Kurita A. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol. 2004;27:223–7. doi: 10.1002/clc.4960270411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekine T, Daimon M, Hasegawa R, et al. The impact of sleep deprivation on the coronary circulation. Int J Cardiol. 2010;144:266–7. doi: 10.1016/j.ijcard.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Kim W, Park HH, Park CS, Cho EK, Kang WY, Lee ES. Impaired endothelial function in medical personnel working sequential night shifts. Int J Cardiol. 2011;151:377–8. doi: 10.1016/j.ijcard.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 10.Khazaei M, Moien-Afshari F, Laher I. Vascular endothelial function in health and diseases. Pathophysiology. 2008;15:49–67. doi: 10.1016/j.pathophys.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12:63–8. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Tanaka H. Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men. Occup Environ Med. 2002;59:447–51. doi: 10.1136/oem.59.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tofler GH, Stone PH, Maclure M, et al. Analysis of possible triggers of acute myocardial infarction (the MILIS study) Am J Cardiol. 1990;66:22–7. doi: 10.1016/0002-9149(90)90729-k. [DOI] [PubMed] [Google Scholar]
- 15.Muenter NK, Watenpaugh DE, Wasmund WL, Wasmund SL, Maxwell SA, Smith ML. Effect of sleep restriction on orthostatic cardiovascular control in humans. J Appl Physiol. 2000;88:966–72. doi: 10.1152/jappl.2000.88.3.966. [DOI] [PubMed] [Google Scholar]
- 16.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31:197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26:986–9. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 19.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302:H1991–7. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tesselaar E, Sjoberg F. Transdermal iontophoresis as an in-vivo technique for studying microvascular physiology. Microvasc Res. 2011;81:88–96. doi: 10.1016/j.mvr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Gaubert ML, Sigaudo-Roussel D, Tartas M, Berrut G, Saumet JL, Fromy B. Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. J Physiol. 2007;585:617–26. doi: 10.1113/jphysiol.2007.143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debbabi H, Bonnin P, Ducluzeau PH, Leftheriotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens. 2009;23:541–6. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–73. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008;115:295–300. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 25.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–2. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 26.Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008;17:376–84. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zegre Cannon C, Kissling GE, Goulding DR, King-Herbert AP, Blankenship-Paris T. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim (NY) 2011;40:85–93. doi: 10.1038/laban0311-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gohin S, Sigaudo-Roussel D, Conjard-Duplany A, Dubourg L, Saumet JL, Fromy B. What can current stimulation tell us about the vascular function of endogenous prostacyclin in healthy rat skin in vivo? J Invest Dermatol. 2011;131:237–44. doi: 10.1038/jid.2010.267. [DOI] [PubMed] [Google Scholar]
- 29.Garry A, Fromy B, Blondeau N, et al. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Rep. 2007;8:354–9. doi: 10.1038/sj.embor.7400916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Current-induced vasodilation during water iontophoresis (5 min, 0.10 mA) is delayed from current onset and involves aspirin sensitive mechanisms. J Vasc Res. 2002;39:59–71. doi: 10.1159/000048994. [DOI] [PubMed] [Google Scholar]
- 31.Raasch W, Dominiak P, Ziegler A, Dendorfer A. Reduction of vascular noradrenaline sensitivity by AT1 antagonists depends on functional sympathetic innervation. Hypertension. 2004;44:346–51. doi: 10.1161/01.HYP.0000138406.13413.0e. [DOI] [PubMed] [Google Scholar]
- 32.Varma DR, Chemtob S. Endothelium- and beta-2 adrenoceptor-independent relaxation of rat aorta by tyramine and certain other phenylethylamines. J Pharmacol Exp Ther. 1993;265:1096–104. [PubMed] [Google Scholar]
- 33.Huang F, Villafana S, Hong E. Role of central and sympathetic nervous systems in pressor effect of L-NAME. J Cardiovasc Pharmacol. 2003;41:68–72. doi: 10.1097/00005344-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Amir O, Alroy S, Schliamser JE, et al. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93:947–9. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–11. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 36.Perry JC, Bergamaschi CT, Campos RR, et al. Sympathetic and angiotensinergic responses mediated by paradoxical sleep loss in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:146–52. doi: 10.1177/1470320310391504. [DOI] [PubMed] [Google Scholar]
- 37.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–8. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 38.Wehrens SM, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2012;38:171–81. doi: 10.5271/sjweh.3197. [DOI] [PubMed] [Google Scholar]
- 39.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–52. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 40.Marcelin-Jimenez G, Escalante B. Functional and cellular interactions between nitric oxide and prostacyclin. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:349–59. doi: 10.1016/s1532-0456(01)00210-1. [DOI] [PubMed] [Google Scholar]
- 41.Clement P, Sarda N, Cespuglio R, Gharib A. Changes occurring in cortical NO release and brain NO-synthases during a paradoxical sleep deprivation and subsequent recovery in the rat. J Neurochem. 2004;90:848–56. doi: 10.1111/j.1471-4159.2004.02529.x. [DOI] [PubMed] [Google Scholar]
- 42.Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells Tissues Organs. 2003;173:242–54. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- 43.Chang HM, Wu UI, Lin TB, et al. Total sleep deprivation inhibits the neuronal nitric oxide synthase and cytochrome oxidase reactivities in the nodose ganglion of adult rats. J Anat. 2006;209:239–50. doi: 10.1111/j.1469-7580.2006.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro AC, Gilligan JG, Kapas L. Systemic injection of a nitric oxide synthase inhibitor suppresses sleep responses to sleep deprivation in rats. Am J Physiol. 2000;278:R1048–56. doi: 10.1152/ajpregu.2000.278.4.R1048. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Garg R. A role of nitric oxide mechanism involved in the protective effects of venlafaxine in sleep deprivation. Behav Brain Res. 2008;194:169–73. doi: 10.1016/j.bbr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Khadrawy YA, Nour NA, Aboul Ezz HS. Effect of oxidative stress induced by paradoxical sleep deprivation on the activities of Na+, K+-ATPase and acetylcholinesterase in the cortex and hippocampus of rat. Transl Res. 2011;157:100–7. doi: 10.1016/j.trsl.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigne S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 2012;16:265–79. doi: 10.1016/j.smrv.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Chennaoui M, Sauvet F, Drogou C, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56:318–24. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 50.Taddei S, Caraccio N, Virdis A, et al. Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2006;91:5076–82. doi: 10.1210/jc.2006-1075. [DOI] [PubMed] [Google Scholar]
- 51.Egan CG, Lockhart JC, Ferrell WR. Pathophysiology of vascular dysfunction in a rat model of chronic joint inflammation. J Physiol. 2004;557:635–43. doi: 10.1113/jphysiol.2004.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palma BD, Gabriel A, Jr., Bignotto M, Tufik S. Paradoxical sleep deprivation increases plasma endothelin levels. Braz J Med Biol Res. 2002;35:75–9. doi: 10.1590/s0100-879x2002000100011. [DOI] [PubMed] [Google Scholar]
- 53.Sauvet F, Bourrilhon C, Besnard Y, et al. Effects of 29-h total sleep deprivation on local cold tolerance in humans. Eur J Appl Physiol. 2012;112:3239–50. doi: 10.1007/s00421-011-2297-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Park Y, Wu J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–30. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Wit C, Esser N, Lehr HA, Bolz SS, Pohl U. Pentobarbital-sensitive EDHF comediates ACh-induced arteriolar dilation in the hamster microcirculation. Am J Physiol. 1999;276:H1527–34. doi: 10.1152/ajpheart.1999.276.5.H1527. [DOI] [PubMed] [Google Scholar]
- 56.Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep. 2011;34:335–9. doi: 10.1093/sleep/34.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood pressure, heart rate, and body temperature values in non-sympathectomized and sympathectomized rats in the control cage experimental condition
Effect of sympathectomy on endothelial function in the control cage experimental condition
Effect of nitric oxide and cyclooxygenase on endothelial function inhibition, in the control cage experimental condition