ABSTRACT
Despite the recent progress in the development of new antiviral agents, hepatitis C virus (HCV) infection remains a major global health problem, and there is a need for a preventive vaccine. We previously reported that adenoviral vectors expressing HCV nonstructural proteins elicit protective T cell responses in chimpanzees and were immunogenic in healthy volunteers. Furthermore, recombinant HCV E1E2 protein formulated with adjuvant MF59 induced protective antibody responses in chimpanzees and was immunogenic in humans. To develop an HCV vaccine capable of inducing both T cell and antibody responses, we constructed adenoviral vectors expressing full-length and truncated E1E2 envelope glycoproteins from HCV genotype 1b. Heterologous prime-boost immunization regimens with adenovirus and recombinant E1E2 glycoprotein (genotype 1a) plus MF59 were evaluated in mice and guinea pigs. Adenovirus prime and protein boost induced broad HCV-specific CD8+ and CD4+ T cell responses and functional Th1-type IgG responses. Immune sera neutralized luciferase reporter pseudoparticles expressing HCV envelope glycoproteins (HCVpp) and a diverse panel of recombinant cell culture-derived HCV (HCVcc) strains and limited cell-to-cell HCV transmission. This study demonstrated that combining adenovirus vector with protein antigen can induce strong antibody and T cell responses that surpass immune responses achieved by either vaccine alone.
IMPORTANCE HCV infection is a major health problem. Despite the availability of new directly acting antiviral agents for treating chronic infection, an affordable preventive vaccine provides the best long-term goal for controlling the global epidemic. This report describes a new anti-HCV vaccine targeting the envelope viral proteins based on adenovirus vector and protein in adjuvant. Rodents primed with the adenovirus vaccine and boosted with the adjuvanted protein developed cross-neutralizing antibodies and potent T cell responses that surpassed immune responses achieved with either vaccine component alone. If combined with the adenovirus vaccine targeting the HCV NS antigens now under clinical testing, this new vaccine might lead to a stronger and broader immune response and to a more effective vaccine to prevent HCV infection. Importantly, the described approach represents a valuable strategy for other infectious diseases in which both T and B cell responses are essential for protection.
INTRODUCTION
Hepatitis C virus (HCV) infection is a major global health problem affecting an estimated 170 million people worldwide, occurring among persons of all ages, genders, races, and regions of the world. Chronically infected subjects are at risk of developing progressive liver disease, including cirrhosis, and primary hepatocellular carcinoma (1). Although the recent introduction of directly acting antiviral drugs (DAAs) has improved therapy for chronic HCV and interferon (IFN)-free regimens are on the horizon (2), treatment success may be limited by a range of factors, including awareness of infection status, access to and cost of therapy, relative efficacy of different regimens for specific HCV genotypes, adverse effects, comorbidities (e.g., cirrhosis or HIV coinfection), and host factors. For these reasons, the development of a safe, effective, and affordable preventive vaccine against HCV is the optimal long-term goal to control the global epidemic (3).
Approximately 20% of infected individuals clear the virus spontaneously, and resolution is associated with HLA type and with potent, multispecific, and long-lasting T cell responses (4). T cell depletion experiments with chimpanzees confirmed the essential role of cellular immunity in controlling HCV infection (5). Moreover, antibodies targeting the HCV envelope glycoproteins have been shown to neutralize infection in vitro (6, 7) and to protect against virus challenge in the human liver-Alb-uPA/SCID murine model (8–10). A recent report demonstrated that spontaneous clearance of HCV is associated with the early appearance of a broadly neutralizing antibody response (11). Recombinant E1E2 glycoproteins have been shown to induce cross-neutralizing antibody responses against heterologous HCV genotypes in rodents, chimpanzees, and humans (12–15). Ideally, any future HCV vaccine should elicit potent antibody and cellular responses. Recent reports showing that HCV can infect cells by the classical route involving extracellular virus particles or via direct cell-to-cell contact spread (16–18) highlight a new pathway for HCV to evade the humoral antibody response. Given the high genetic diversity observed for HCV (19), any successful vaccine will need to induce immune responses that recognize diverse HCV genotypes and inhibit cell-to-cell viral transmission.
Viral vectors engineered to express foreign antigens are an effective tool to induce T cell immunity against pathogens (20–22). Adenovirus (Ad) is one of the most potent vectors for eliciting CD8 T cell and antibody responses in humans (22, 23). Ad vectors based on human serotype 6 (Ad6) and chimpanzee Ad 3 (ChAd3) expressing the HCV nonstructural proteins elicit potent, durable, and protective T cell responses in chimpanzees and were recently tested in phase I trials (24, 25). In this study, we evaluated the immunogenicity of human Ad6 vectors expressing either full-length E1E2 or a truncated version of HCV E2, alone and in a prime-boost vaccination with recombinant glycoproteins in rodents. This combined regimen induced potent T cell responses and high-titer functional Th1-type IgG responses in mice and guinea pigs. Importantly, immune sera were capable of neutralizing diverse HCV strains and, in a few cases, of limiting HCV cell-to-cell transmission.
MATERIALS AND METHODS
Plasmid construction.
To generate an Ad6 vector encoding E1E2 and p7 proteins, cDNA E1(F78)E2p7 sequence from HCV isolate T212, genotype 1b (GT1b) (GenBank accession number AB049099 [26]), was cloned under the control of the human cytomegalovirus (CMV) promoter and bovine growth hormone (BGH) polyadenylation signal between two Ad6 flanking regions. Human CMV tetracycline-regulated promoter (TetO2) was amplified from pcDNA5/FRT/TO-TOPO plasmid (Invitrogen) and inserted by homologous recombination upstream E1F78E2p7 sequence, creating pNEB(tetO)/E1F78E2p7 plasmid. To generate the Ad6/F78E2662 vector, a human codon-optimized sequence coding for tissue plasminogen activator (tPA) leader sequence, F78, and E2662 (cDNA of E2 protein truncated at 662 amino acids [aa]) from HCV strain T212 was cloned under the control of the human CMV promoter and BGH polyadenylation signal between two Ad6 flanking regions, creating pNEB/F78E2662 plasmid. In both constructs the natural HVR1 sequence at the N terminus of E2 was replaced by an artificial mimotope, F78, which was previously shown to elicit cross-reactive antibodies to diverse HVR1 variants following immunization of mice and rabbits (27).
HCV T212 expression cassettes were inserted by homologous recombination into the Ad6 backbone on plasmid pAd6/NSmut (28). PmeI and PacI fragments, respectively, from pNEB/E1F78E2p7 and pNEB/F78E2662 containing expression cassette and Ad6 E1 flanking regions were recombined to SnaBI-linearized pAd6/NSmut in BJ5183 cells, creating plasmids pAd6/E1F78E2p7 and pAd6/F78E2662 containing the Ad6 genome with deletions of E1 and E3 genes and with introduced transgenes.
Rescue, amplification, and purification of viruses.
The plasmids pAd6/E1F78E2p7 and pAd6/F78E2662 were cut with PacI to release the adenovirus inverted terminal repeats (ITRs) and transfected into producer cells. The Ad6/E1F78E2p7 (called Ad6E1E2p7 hereafter) vector containing TetO2 promoter was rescued in Flp-In T-REx-293 cells (Invitrogen), cultured in complete Dulbecco modified Eagle medium (DMEM) with 100 μg/ml of Zeocin (Invitrogen) and 15 μg/ml of blasticidin (Invitrogen). Ad6/F78E2662 (called Ad6E2662 hereafter) vector containing human CMV promoter was rescued in PER.C6 cells (Crucell), cultured in complete DMEM with 10 mM MgCl2. Cells together with culture medium were lysed by freeze-thawing 5 to 10 days posttransfection. Vectors were amplified by serial passage on PER.C6 or Flp-In T-REx-293 cells. Large-scale amplification was performed on 3 × 108 cells for each virus. Viruses were purified by ultracentrifugation on a cesium chloride gradient and dialyzed against A195 buffer (29). Viral particles were quantified by quantitative PCR of the CMV promoter region with specific primers (CMV-for, CATCTACGTATTAGTCATCGCTATTACCA; CMV-rev, GACTTGGAAATCCCCGTGAGT) and probe (CMV-6-carboxyfluorescein [FAM]-6-carboxytetramethylrhodamine [TAMRA], ACATCAATGGGCGTGGATAGCGGTT).
E2 expression analysis.
HeLa cells (8 × 105) plated in 2.5-cm culture dish in complete DMEM were infected with Ad6E1E2p7 or Ad6E2662 at different multiplicities of infection (MOI). Forty-eight hours postinfection (hpi), cells were lysed in buffer containing TEN (10 mM Tris, 1 mM EDTA, 100 mM NaCl) plus 1% Triton X-100. Proteins were separated by 10% SDS-PAGE and blotted onto a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% milk in phosphate-buffered saline (PBS)–0.05% Tween 20 and probed with anti-E2 monoclonal antibody (MAb) clone BDI167 (Biodesign) diluted 1:200. After extensive washing, membranes were incubated with goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) and developed with enhanced chemiluminescence (ECL). For immunocytochemical staining, infected HeLa cells monolayers were washed with PBS, frozen at −70°C for 15 min, and fixed with 4% paraformaldehyde. Cells were probed with anti-E2 MAb (AP33; gift from A. Patel, MRC, Glasgow, Scotland) diluted 1:2,000 in PBS–5% fetal bovine serum (FBS)–0.1% sodium azide–1% Tween 80. Secondary HRP-conjugated anti-mouse antibody (Santa Cruz) diluted 1:2,000 in PBS–5% FBS–1% Tween 80 was used. The reaction was developed with the NovaRED substrate kit (Vector Laboratories).
Animals and vaccinations.
Animal experiments were approved by local animal ethics committees and were performed in accordance with national and international laws and policies. Dose ranges and schedules are detailed in Table 1. Groups of five female 6-week-old C57BL/6 (H-2b) or BALB/c (H-2d) mice (Charles River, Como, Italy) or groups of six outbred female Hartley guinea pigs (Harlan) were vaccinated intramuscularly with Ad6 vectors bilaterally into the quadriceps muscles (50 μl per side). Recombinant protein (HCV genotype 1a E1E2p7, aa 192 to 809; Novartis [15]) was formulated by being mixed 1:1 with MF59 adjuvant immediately before immunization. Blood samples were collected from the retro-orbital sinus and by cardiac puncture for mice and guinea pigs, respectively, and mouse splenocytes were isolated according to standard techniques. All in vivo procedures were performed under deep anesthesia.
TABLE 1.
Vaccination regimensa
| Species | Group (code) | Vaccine 1 |
Vaccine 2 |
||||
|---|---|---|---|---|---|---|---|
| Component(s) (genotype) | Dose | Schedule (wk) | Component(s) (genotype) | Dose | Schedule (wk) | ||
| C57BL/6 mice | A (AA) | Ad6E1E2p7 (1b) | 109 vp | 0, 3 | |||
| B (AtAt) | Ad6E2662 (1b) | 109 vp | 0, 3 | ||||
| BALB/c mice | C (AA) | Ad6E1E2p7 (1b) | 109 vp | 0, 3 | |||
| D (PPP) | E1E2 protein (1a), MF59 adjuvant | 5 µg | 0, 3, 6 | ||||
| E (PPA) | E1E2 protein (1a), MF59 adjuvant | 5 µg | 0, 3 | Ad6E1E2p7 (1b) | 109 vp | 6 | |
| F (AAPP) | Ad6E1E2p7(1b) | 109 vp | 0, 3 | E1E2 protein (1a), MF59 adjuvant | 5 µg | 6, 9 | |
| Guinea pig | G (PPP) | E1E2 protein (1a), MF59 adjuvant | 10 µg | 0, 4, 12 | |||
| H (AAPP) | Ad6E1E2p7 (1b) | 1010 vp | 0, 4 | E1E2 protein (1a), MF59 adjuvant | 10 µg | 12, 20 | |
| I (AtAtPP) | Ad6E2662 (1b) | 1010 vp | 0, 4 | E1E2 protein (1a), MF59 adjuvant | 10 µg | 12, 20 | |
Regimens included one vaccine component or a combination of two vaccine components administered to different animal species. Vaccine component identity, HCV genotype, dosage, and timing (week from first vaccination) of each different injection are specified. vp, viral particles; genotype, vaccine antigen HCV genotype; P, protein; A, Ad6E1E2p7; At, Ad6E2662 truncation
IFN-γ ELISpot assays, intracellular staining, and antigenic peptides.
Sets of 15-mer peptides overlapping by 11 amino acids, dissolved in 100% dimethyl sulfoxide (DMSO) and arranged in pools covering all the E1E2p7 genotype T212 transgene (3 pools: E1, E2, and p7) or E1E2 genotype H77 transgene (2 pools: E1 and E2), were used as stimulants at a final concentration of 2 μg/ml for each single peptide. Standard 16-h enzyme-linked immunosorbent spot (ELISpot) assays were done as previously described (30), with the following minor modifications: MSIP S4510 plates (Millipore) were used, and gamma interferon (IFN-γ) antibody pairs were purchased from U-CyTech. DMSO and concanavalin A (ConA) were used, respectively, as negative and positive controls. Plates were read by an automated reader (A.EL.VIS) and data expressed as IFN-γ spot-forming cells (SFC) per million splenocytes. A response was defined as positive when all of the following applied: IFN-γ production was present in ConA-stimulated wells, >40 specific spots/million splenocytes were generated against at least one pool, and the number of spots in antigen wells was more than three times higher than in control wells (containing DMSO). IFN-γ intracellular cytokine staining (ICS) and fluorescence-activated cell sorter (FACS) analysis were performed as previously described (25). Stained cells were acquired on a FACS Canto II and analyzed using DIVA software (BD Biosciences). At least 30,000 CD8+ CD3+ gated events were acquired for each sample. Data are expressed as the percentage of antigen-specific IFN-γ-secreting CD4+ or CD8+ cells over total CD8+ or CD4+ cells. Representative FACS plots and gating strategy are shown in Fig. S1 in the supplemental material.
ELISA.
To measure antibody reactivity for immunizing antigens, whole-cell extracts of pNebF78E2662- or pNebE1F78E2p7-transfected human embryonic kidney (HEK293) cells obtained by lysing cells with TEN–1% Triton X-100–protease inhibitors (Complete protease inhibitor cocktail; Boehringer Mannheim) were used as a source of HCV glycoproteins in a Galanthus nivalis lectin (GNA; Sigma) capture enzyme-linked immunosorbent assay (ELISA) as previously described (30). Alternatively, a standard ELISA with direct coating (100 ng/well in PBS) of recombinant HCV E1E2 protein was used. Immune sera were tested in duplicate from 1:100 to 1:72,900. Plates were developed either with anti-mouse or anti-guinea pig total IgG alkaline phosphatase (AP) conjugate (Sigma) and p-nitrophenyl phosphate substrate (SigmaFast; Sigma) or with horseradish peroxidase (HRP)-conjugated anti-guinea pig IgG1 (Acris Antibodies) or anti-guinea pig IgG2 (LifeSpan BioSciences) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma). Plates were read (Envision; PerkinElmer) at 405 and 620 nm (AP) or 450 and 620 nm (HRP). The endpoint titer was defined as the highest serum dilution that resulted in an absorbance value (optical density [OD]) 5 times the background value (preimmune sera at 1:100 dilution).
HCVcc and HCVpp genesis and neutralization.
Plasmids encoding chimeric JFH cell culture-derived HCV (HCVcc) strains were used to generate RNA as previously described (31). Briefly, RNA was electroporated into Huh-7.5 cells, and 72 to 96 h later, extracellular virus was harvested and frozen at −80°C. HCVcc infectivity of the extracellular virus was quantified by inoculating Huh-7.5 cells for 48 h, and NS5A viral antigen-expressing cells were enumerated. Luciferase reporter pseudoparticles expressing HCV envelope glycoproteins (HCVpp) or a no-envelope control were generated as previously reported (32). Virus-specific neutralization was performed by incubating comparable stocks of infectious virus (1,000 focus-forming units/ml of approximately 500,000 RLU/ml) with preimmune or immune sera for 1 h and infecting Huh-7.5 cells for 48 h. Data are presented as the dilution of sera able to neutralize 50% of HCVpp infectivity (ID50) or the percent neutralization of virus infection at a final serum dilution of 1/500 compared to preimmune sera. All infections were performed in triplicate wells, and neutralization values are the means of two independent assessments.
Assay of cell-to-cell transmission.
HCV H77/JFH-infected Huh-7.5 cells were labeled with fluorescent cell tracker 5-chloromethylfluorescein diacetate (CMFDA) and cocultured with an equal number of naive Huh-7.5 cells at 2.5 × 104 cells/cm2, as previously reported (18). Extracellular virus was neutralized by treating the cultures with control purified polyclonal human anti-HCV Ig (100 μg/ml); preimmune or postimmune sera (1/100) were added to the cultures, and the cultures were incubated for 24 h. Culture supernatants were assessed for the level of infectious virus by infecting Huh-7.5 cells. Cells were collected and fixed, and the frequency of cells expressing HCV NS5A was quantified by flow cytometry. Newly infected target cells are identified as NS5A+ CMFDA−, and cell contact-dependent virus transmission occurs when >99% of extracellular virus is neutralized.
Statistical analysis.
Statistical analysis and graphs were made using Prism software (GraphPad). As populations were normally distributed, an unpaired, 2-tailed t test (paired or unpaired as appropriate) was used to determine if there were significant differences. Statistically significant differences are shown as follows: *, P = 0.01 to 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001. For in vitro assays, statistical analyses were performed using a nonparametric one-way analysis of variance (ANOVA) (Kruskal-Wallis test) or Student's t test in Prism 4.0, where necessary corrections for multiple comparisons were made, while Bonferroni's multiple-comparison test was used to assess the degree of difference from the wild-type positive control.
RESULTS
Construction of recombinant Ad6 encoding E2662 or E1E2p7 of HCV.
Replication-defective Ad6 vectors encoding HCV genotype 1b, strain T212, truncated E2 (Ad6E2662) and E1E2p7 (Ad6E1E2p7) were constructed (Fig. 1A). The tissue plasminogen activator (tPA) signal sequence was introduced to facilitate E2662 secretion. Ad6E2662 was successfully rescued in the PER.C6 cell line, but several attempts to rescue Ad6E1E2p7 failed. We previously reported that overexpression of some transgenes is associated with impaired adenovirus growth and a selective disadvantage (33). We therefore generated an inducible promoter system in which two copies of the tetracycline operator 2 were inserted in tandem downstream of the TATA box in the human CMV promoter (TetO2 [34, 35]). This modification enabled the amplification and recovery of Ad6E1E2p7 in a cell line stably expressing the tetracycline repressor protein (Flp-I T-REx-293 cells). E2 expression was confirmed by Western blotting (Fig. 1B) or immunohistochemical staining (Fig. 1C). We noted higher levels of E2 expression by the Ad6E2662 vector, which may reflect the codon optimization of the E2 sequence in this construct.
FIG 1.

Construction and characterization of recombinant adenovirus vectors. (A) Schematic diagram of expression cassettes inserted into the Ad6 backbone. The N-terminal methionine residue is indicated as Met; tetracycline operator binding sites (TetO2) within the CMV promoter (CMV) are indicated as T. (B) Western blot detection of E2 protein expression in HeLa cells 48 h after infection with Ad6E1E2p7 or Ad6E2662 at different MOI. A lysate of noninfected cells represents the negative control (cells). β-Actin immunodetection is shown for normalization of lysate protein content. (C) Detection of E2 protein by immunohistochemical staining of HeLa cells infected with Ad6E1E2p7 (1) or Ad6E2662 (2) or left uninfected (3).
Ad6E2662 and Ad6E1E2p7 alone or in a prime-boost regimen with E1E2 adjuvanted protein induce humoral and cellular responses in mice.
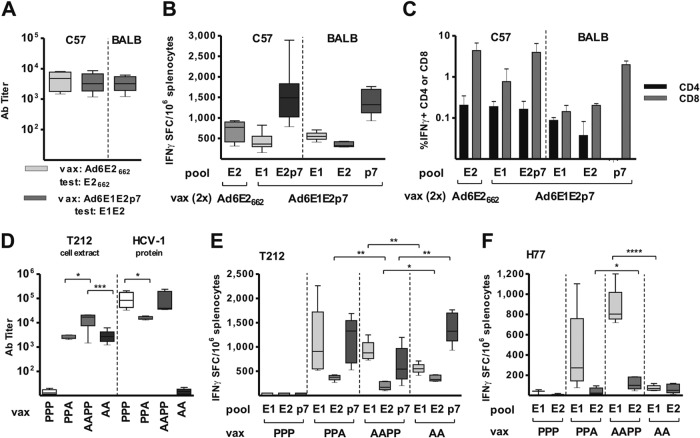
For the initial assessment of adenovirus immunogenicity, two doses of Ad6E2662 orAd6E1E2p7 were given to C57BL/6 mice. The two vectors induced HCV-specific antibody responses at comparable levels (Fig. 2A). Ad6E1E2p7 immunogenicity was confirmed in a second strain of mice, BALB/c, which showed similar antibody titers (Fig. 2A). ELISpot analysis of splenocytes isolated from animals of the two strains demonstrated the induction of antigen-specific IFN-γ-secreting T cells upon immunization with both adenovirus vectors (Fig. 2B). Notably, Ad6E1E2p7 elicited a broad T cell response directed against all three encoded HCV proteins, comprising CD4 and CD8 subsets (Fig. 2C) with a general skew toward CD8 T cells, a common feature of adenoviral vector-based vaccines.
FIG 2.
Humoral and cellular immune responses in C57 and BALB/c mouse strains receiving Ad6 or Ad6-protein vaccinations. Sera and splenocytes were collected 2 weeks after the last vaccination (regimens are detailed in Table 1 and schematically reproduced at graph bottom). (A and D) Antibody endpoint titers measured by ELISA using as the antigen E1E2p7 or E2662 prepared from extracts of HEK293 cells transfected with plasmids expressing the same sequence as encoded by adenovirus and captured on an ELISA plate with GNA lectin. Titer was defined as the highest serum dilution that resulted in an absorbance value (OD) 5 times the value of preimmune sera. (D) Antibody titers in vaccine-responding animals (i.e., groups PPP on T212 and AA on HCV-1 excluded) measured on homogeneous ELISA antigens were compared by unpaired t test; only statistically significant differences are shown. (B, E, and F) The number of antigen-specific IFN-γ-secreting cells was determined by ELISpot assay on splenocytes. ELISpot data corrected for background are expressed as IFN-γ spot-forming cells (SFC) per million splenocytes. Peptide pools of T212 (B, C, and E) or H77 (F) origin were used. (E and F) T cell responses on each peptide pool from responding animals only (i.e., PPP group excluded) were compared by unpaired t test; only statistically significant differences are shown. Ab titer and ELISpot data are shown as box (median and interquartile range) and whisker (minimum to maximum value) plots. A, Ad6E1E2p7; P, E1E2 protein vaccinations (D to F). (C) IFN-γ intracellular staining and FACS analysis. Data are expressed as percentage of IFN-γ-secreting CD4 or CD8 cells in response to T212 peptides after subtraction of DMSO background, presented as group means ± standard deviations.
To explore the potential synergy of the different vaccine platforms, we evaluated mixed vaccination regimens combining Ad6E1E2p7 and MF59-adjuvanted recombinant E1E2 (GT1a; strain HCV-1) in BALB/c mice (Table 1, groups E and F) compared to those involving Ad or protein alone (Table 1, groups C and D). HCV genotype 1b and 1a antigens were used to demonstrate B and T cell responses against HCV-1 and T212 strains. All vaccination regimens induced antibody responses to the homologous HCV glycoproteins (T212 GT1b for Ad and HCV-1 GT1a for protein). However, only Ad-protein combination regimens induced antibodies able to recognize both strains (HCV-1 and T212 [Fig. 2D]), and Ad prime followed by two protein boosts (but not one [data not shown]) induced the strongest humoral response to both HCV strains.
Mice vaccinated with protein alone failed to mount a detectable T cell response to homologous or heterologous antigens, confirming the superiority of the gene-based vaccine platform for inducing cellular immune responses (Fig. 2E and F). T cells specific for the T212 (GT1b) antigens raised in mice receiving Ad alone were poorly reactive to heterologous HCV H77 (GT1a) E1E2-derived peptide pools. However, combined Ad6 and protein immunization induced the strongest and most cross-reactive antigen-specific T cells, with responses that were highest for antigens derived from each genotype depending on which component (Ad for GT1b or protein for GT1a) was injected last (Fig. 2E and F). Interestingly, T cell responses to E1 of both HCV genotypes were boosted by protein vaccination over levels already induced by two Ad injections (Fig. 2E and F), suggesting that while protein is unable to prime a T cell response after repeated administration, it can improve T cell responses in animals receiving mixed vaccination regimens. Flow cytometry confirmed that the cellular responses included both CD4 and CD8 T cells (data not shown). Collectively, these data show that combining Ad and protein platforms induces stronger and broader B and T cell responses than either vaccine alone.
The combination of Ad6E2662 or Ad6E1E2p7 prime and E1E2 protein boost induces a balanced Th1-Th2 cellular response and cross-neutralizing antibodies in guinea pigs.
Immune sera from vaccinated mice displayed modest HCV-neutralizing activity (data not shown), consistent with previous reports showing limited immunogenicity in mice compared to that in guinea pigs (15). We therefore evaluated our optimal immunization regimen (Ad prime and protein boost) in guinea pigs, reintroducing Ad6E2662 in comparison to Ad6E1E2p7 to ascertain any potential additional advantages of E1 and p7 coexpression. Vaccination with protein alone elicited antibody responses to the homologous antigen that showed minimal reactivity with HCV T212 (Fig. 3A). Animals vaccinated with either of the Ad6 vectors mounted a modest antibody response capable of recognizing homologous T212 antigens only. As noted for mice, vaccination with Ad6E1E2p7 prime-protein boost generated the highest HCV-1 antibody titers, comparable to those elicited by the protein vaccine alone, and T212 titers significantly higher than in animals receiving Ad6E1E2p7 only. The prime-boost regimen involving Ad6E2662 was less effective, with lower responses for both HCV antigens (Fig. 3A).
FIG 3.

Quantification and characterization of humoral responses induced by Ad6 and protein vaccines in guinea pigs. Vaccination regimens are described in Table 1 and schematically indicated at the bottom of each graph (P, protein; A, Ad6E1E2p7; At, Ad6E2662 truncation). Sera were collected 2 weeks after the second Ad6 or final protein immunization. Data are shown as box (median and interquartile range) and whisker (minimum to maximum value) plots. (A) Antibody endpoint titers measured by ELISA using antigen E1E2p7 or E2662 (T212 strain) prepared from extracts of HEK293 cells transfected with plasmids expressing the same sequence encoded by adenovirus and captured on an ELISA plate with GNA lectin, or E1E2 recombinant protein (strain HCV-1) used to directly coat the plate. Titer was defined as the highest serum dilution that resulted in an absorbance value (OD) 5 times the value of preimmune sera. Antibody titers in vaccine-responding animals (i.e., groups AA and AtAt, HCV-1 E2 protein excluded) measured on homogeneous ELISA antigens are compared either within groups after Ad and protein immunization strains (paired t test [solid line]) or among groups at the end of vaccination (unpaired t test [dashed line]), with only statistically significant differences shown. (B) Ratios of IgG2a to IgG1 titers, calculated to characterize the Th profile (ratios of >1 indicate Th1 response, and ratios of <1 indicate Th2 response).
Due to the lack of reagents to directly assess T cell responses in guinea pigs, we measured differential induction of antigen-specific IgG1 and IG2a as an indirect marker of Th1- or Th2-biased T cell responses. As expected, adenoviruses induced higher levels of IgG2a, indicative of a Th1-skewed phenotype. Vaccination with E1E2 protein plus MF59 induced mostly IgG1, suggesting a dominant Th2 response. Interestingly, heterologous prime-boost immunization induced similar levels of the two Ig isotypes, suggesting a more balanced Th1-Th2 response (Fig. 3B).
To assess the functionality of the polyclonal antibody responses, we evaluated the ability of the immune sera to neutralize the infectivity of HCVpp expressing heterologous GT1a H77 strain glycoproteins (HCVpp-H77) for Huh-7.5 hepatoma cells. HCVpp expressing HCV-1 or T212 strain glycoproteins was noninfectious, and we were unable to measure the efficacy of the autologous antibody response. In contrast to the ELISA data, all three immunization regimens induced comparable neutralization titers against HCVpp-H77 (Fig. 4A). Importantly, all three regimens induced antibody responses that were able to neutralize chimeric cell culture-derived HCV (HCVcc) strains expressing a panel of structural proteins derived from different HCV genotypes (Fig. 4B). The prime-boost regimen did not induce higher titers of neutralizing antibody than did protein immunization alone, but in the latter, three doses of E1E2 protein plus MF59 were needed (data not shown) and there was a skewed Th2 T cell response.
FIG 4.

Quantification and characterization of neutralizing antibody responses induced by Ad6 and protein vaccines in guinea pigs. Sera were collected 2 weeks after final immunization with the vaccination regimens detailed in Table 1 and schematically depicted at the bottom of each graph (P, protein; A, Ad6E1E2p7; At, Ad6E2662 truncation. (A) Neutralization endpoint titer (ID50) for HCVpp-H77, with median value and interquartile range presented. (B) Percent neutralization of a panel of chimeric HCV JFH strains expressing structural proteins from diverse genotypes (Gt). Sera were tested at a final 1/500 dilution, and the mean neutralization values are presented, where the dotted line represents the assay cutoff. (C) Schematic diagram of single-cycle HCV transmission assay (18). (D) HCV H77/JFH cell-free or cell-to-cell de novo infection events in untreated (control) anti-HCV Ig (100 μg/ml), guinea pig preimmune sera (black bars) or postimmune sera (gray and white bars) at a final 1/100 dilution. Each bar represents the mean + standard deviation of newly infected targets cells per 10% producer cells. The amount of extracellular virus at the end of the 24-h incubation period was determined by endpoint titration, and the conditions under which >99% of cell-free virus was eliminated are indicated by the horizontal line. Inhibition of cell-cell transmission was defined as reduced infection of naive cells in the absence of cell-free virus, and significant inhibition is indicated with an asterisk. Statistical analyses were performed using a nonparametric one-way ANOVA (Kruskal-Wallis test) or Student's t test in Prism 4.0, where necessary corrections for multiple comparisons were made, and Bonferroni's multiple-comparison test was used to assess the degree of difference from the wild-type positive control.
Given the ability of the various immune sera to neutralize HCVcc, we evaluated their ability to limit cell-to-cell virus transmission using a recently reported assay (Fig. 4C) (18). All of the immune sera neutralized extracellular virus by >95% (data not shown), consistent with the data presented in Fig. 4B. Importantly, sera from three vaccinated animals (Fig. 4D, highlighted with an asterisk) reduced de novo transmission events beyond that observed with control anti-HCV Ig, representing pooled Ig from over 600 chronically infected donors, consistent with a partial inhibition of cell-to-cell transmission.
DISCUSSION
The need for a prophylactic HCV vaccine must be considered in the context of a range of preventive strategies, as well as screening and provision of safe blood products in all settings. Although such interventions have reduced HCV transmission, the ability of a vaccine to protect against and, potentially, to treat persistent infection remains an attractive goal. This is analogous to the case with hepatitis B virus infection, for which high-risk groups are readily identifiable and the availability of an effective vaccine has led to its extended use in many countries.
It is well established that strong and broad cellular responses are needed for successful HCV clearance, and recent findings suggest that antibodies targeting envelope proteins may play a major role in the early stages of infection (4, 7, 10, 11). In chimpanzees, antibody responses to E2 were inversely associated with HCV viral loads, and a meta-analysis of candidate HCV vaccine efficacy in this animal model demonstrated that immunogens including structural proteins were more significantly associated with protective immune responses than vaccines based solely on nonstructural proteins (36, 37).
Several preclinical studies targeting a range of infectious diseases have demonstrated that the combined use of adenovirus prime and protein boost immunization regimens induces high antibody titers together with potent T cell responses (reviewed in reference 38). In this study, we combined an adenovirus vector known to induce potent T cell responses with an adjuvanted recombinant HCV E1E2 protein previously reported to induce neutralizing antibody responses to evaluate the potential to induce HCV-specific T and B cell responses.
Adenoviruses are excellent delivery systems for gene-based vaccines, as they drive high and long-lasting antigen expression leading to the induction of strong immune responses that can be further enhanced by boosting with other vaccine platforms (23). Since adenovirus immunogenicity can be hampered by anti-Ad preexisting immunity (23), we constructed vectors based on human serotype 6 (Ad6), which is less prevalent than commonly used serotype 5 and can escape anti-Ad5 immunity (28, 39). Two Ad6 vectors encoding different forms of the envelope glycoproteins (Ad6E2662 andAd6E1E2p7) were constructed. We could rescue the latter only after introducing TetO sites into the HCMV promoter and growth of the virus in the Flp-In T-REx-293 cells. This observation demonstrates the value of inducible systems to regulate vaccine transgene expression (33, 40), enabling the large-scale production of vectors encoding any antigen of interest. Both Ad6 vectors induced T cell responses in the experimental animals, and as expected, a broader response targeting all encoded antigens (E1, E2, and p7) was induced by Ad6E1E2p7, suggesting that this vector is a superior candidate vaccine (41, 42). However, only modest levels of HCV-specific antibodies were raised.
To improve antibody responses, we combined Ad6 with MF59-adjuvanted E1E2 protein, which, as we previously reported, was able to induce neutralizing antibodies in guinea pigs and chimpanzees (14, 15). Recent reports from clinical trials demonstrate that antibodies induced in humans vaccinated with HCV E1E2/MF59 were able to neutralize homologous and heterologous HCV strains (13, 43, 44). However, in-depth analyses of T cell responses were not reported for these studies. We performed prime-boost immunizations with different vaccine combinations and found that priming with Ad and boosting with HCV E1E2 protein constituted an optimal approach to induce cellular and humoral immune responses. Similarly, Desjardins and coworkers reported that priming with Ad encoding HCV E1E2 and boosting with DNA generating recombinant retrovirus-like particles (retroVLPs) induced higher cellular responses than priming with retroVLPs and boosting with Ad (45). These data confirm that the order of prime-boost administration is relevant and that Ad encoding HCV envelope proteins can prime immune responses. In our study, combining Ad6E1E2p7 and heterologous E1E2/MF59 induced antibodies that recognized proteins of both genotypes 1b (T212) and 1a (HCV-1) as well as cross-reactive T cell responses. However, we could not ascertain whether the increased heterologous response was due to the combined adenovirus vector and protein vaccination or to the use of heterologous antigens (HCV-1 for the recombinant protein and T212 for the adenovirus-expressed antigen). Only follow-up vaccination studies with adenovirus vectors expressing antigens homologous to the protein will clarify the impact of combination regimens versus the use of heterologous antigens.
Previous reports showed that recombinant HCV E1E2 immunization of guinea pigs elicited higher-titer cross-neutralizing antibody responses than in mice (15). We confirmed these findings, showing that Ad6E1E2p7 and E1E2/MF59 induced cross-neutralizing antibodies in guinea pigs while maintaining a balanced Th1-Th2 response. Compared to a regimen based on protein alone, the heterologous prime-boost did not increase neutralizing antibody titers but required a lower number of protein doses.
Importantly, we demonstrate that recombinant antigen can induce polyclonal responses that partially limit cell-to-cell HCV transmission. This is in contrast to our previous report showing that polyclonal antibody responses from chronically infected subjects show minimal inhibition of cell-to-cell transmission (16, 17), highlighting potential differences in antibody responses raised during natural infection compared to experimental vaccination. Of note, we recently reported that a nanobody targeting the HCV E2 CD81 binding site limits HCV cell-to-cell transmission, potentially due to reduced size of the nanobody compared to a complete IgG molecule (34). However, our current data showing that polyclonal anti-E1E2 sera from immunized guinea pigs reduce cell-to-cell viral transmission suggest that factors in addition to the molecular weight or size of the immunoglobulin are important, including the structure or binding sites of the antibodies. Regardless, the ability of these antibodies to limit cell-to-cell transmission may allow for combination therapy with newly developed DAAs to limit viral escape from treatment postinfection in addition to working as a prophylactic vaccine.
Several approaches using E1E2 as vaccine antigen delivered in various forms have been reported to date, including purified recombinant proteins, viral vectors, virus-like particles, and plasmid DNA (46). In general, previous reports demonstrated that HCV glycoproteins can induce cellular and humoral responses. Some studies (47, 48) showed that a booster immunization with recombinant protein enhanced humoral immune responses; however, the neutralizing properties of the immune sera were not studied. Recently, two reports described cross-neutralizing antibody responses in rodents vaccinated with viral vectors and recombinant envelope proteins. Lin and coworkers (49) reported optimal induction of HCV-specific CD4+ and CD8+ T cell responses and cross-neutralizing antibodies against heterologous genotype 2a HCV in mice primed with E1E2 and boosted with alphavirus particles encoding HCV E1E2. In another study, mice immunized with measles virus vector expressing HCV core and E1E2 and boosted with soluble E2 developed neutralizing antibodies against homologous and heterologous HCV strains, but T cell responses were not studied (50). An additional study by Garrone and coworkers (51) reported a weaker induction of immune responses by a measles virus vector encoding HCV E1 compared to adenovirus vector encoding E1E2. In that study, priming with adenovirus expressing E1E2 and boosting with VLPs pseudotyped with HCV envelope proteins induced broadly neutralizing antibodies in macaques. However, vaccination with VLPs alone required multiple injections (more than five) to induce specific antibody responses, and neutralization titers were modest. Furthermore, T-cell responses were not demonstrated with this prime-boost strategy. E1E2 protein in adjuvant used in our study might outperform VLPs in boosting responses after adenovirus priming.
Further development of the Ad-based vaccine described in this study will likely require the use of nonhuman Ad, such as chimpanzee adenoviruses that were recently shown to be safe, highly immunogenic in humans, and insensitive to human Ad preexisting immunity (25). Moreover, the combination of the new vaccine described in this report with the clinically validated ChAd3-based NS vaccine (22) may induce stronger and broader humoral and cell-mediated immunity against both structural and nonstructural HCV proteins and therefore counteract HCV genetic diversity. The requirement to induce potent and broadly reactive T cell and antibody responses is not unique to HCV vaccine development. Therefore, immunization regimens combining vectored vaccines with protein antigen in adjuvant represent a valuable vaccine platform for infectious diseases in which both T and B cells are crucial for protection.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by the European Union (FP6, Hepacivac, grant no. LSH-2005-1.2.4-2). A. M. Chmielewska was partly supported by Polish National Science Centre grant no. UMO-2012/04/A/NZ1/00056.
Footnotes
Published ahead of print 5 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03574-13.
REFERENCES
- 1.Lavanchy D. 2011. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17:107–115. 10.1111/j.1469-0691.2010.03432.x [DOI] [PubMed] [Google Scholar]
- 2.Lange CM, Zeuzem S. 2013. Perspectives and challenges of interferon-free therapy for chronic hepatitis C. J. Hepatol. 58:583–592. 10.1016/j.jhep.2012.10.019 [DOI] [PubMed] [Google Scholar]
- 3.Shi C, Ploss A. 2013. Hepatitis C virus vaccines in the era of new direct-acting antivirals. Expert Rev. Gastroenterol. Hepatol. 7:171–185. 10.1586/egh.12.72 [DOI] [PubMed] [Google Scholar]
- 4.Neumann-Haefelin C, Thimme R. 2013. Adaptive immune responses in hepatitis C virus infection. Curr. Top. Microbiol. Immunol. 369:243–262. 10.1007/978-3-642-27340-7_10 [DOI] [PubMed] [Google Scholar]
- 5.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645–1655. 10.1084/jem.20030239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. U. S. A. 104:6025–6030. 10.1073/pnas.0607026104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc. Natl. Acad. Sci. U. S. A. 101:10149–10154. 10.1073/pnas.0403519101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat. Med. 14:25–27. 10.1038/nm1698 [DOI] [PubMed] [Google Scholar]
- 9.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, Alter H, Purcell RH, Leroux-Roels G. 2011. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 53:755–762. 10.1002/hep.24171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball JL, Tarr WA, McKeating JA. 26 February 2014. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res. 10.1016/j.antiviral.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. 15 January 2014. Clearance of hepatitis C infection is associated with early appearance of broad neutralizing antibody responses. Hepatology 10.1002/hep.27013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, B. Moss B, Cummins LB, Houghton M, Muchmore E. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 91:1294–1298. 10.1073/pnas.91.4.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamataki Z, Coates S, Abrignani S, Houghton M, McKeating JA. 2011. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J. Infect. Dis. 204:811–813. 10.1093/infdis/jir399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier JC, Gottwein JM, Houghton M, Russell RS, Emerson SU, Bukh J, Purcell RH. 2011. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis. 204:1186–1190. 10.1093/infdis/jir511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamataki Z, Coates S, Evans MJ, Wininger M, Crawford K, Dong C, Fong YL, Chien D, Abrignani S, Balfe P, Rice CM, McKeating JA, Houghton M. 2007. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine 25:7773–7784. 10.1016/j.vaccine.2007.08.053 [DOI] [PubMed] [Google Scholar]
- 16.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. 2011. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J. Virol. 85:596–605. 10.1128/JVI.01592-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P, McKeating JA. 2008. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology 47:17–24. 10.1002/hep.21959 [DOI] [PubMed] [Google Scholar]
- 18.Meredith LW, Harris HJ, Wilson GK, Fletcher NF, Balfe P, McKeating JA. 2013. Early infection events highlight the limited transmissibility of hepatitis C virus in vitro. J. Hepatol. 58:1074–1080. 10.1016/j.jhep.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 19.Simmonds P. 2004. Genetic diversity and evolution of hepatitis C virus—15 years on. J. Gen. Virol. 85:3173–3188. 10.1099/vir.0.80401-0 [DOI] [PubMed] [Google Scholar]
- 20.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305–6313. 10.1128/JVI.77.11.6305-6313.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barefoot B, Thornburg NJ, Barouch DH, Yu JS, Sample C, Johnston RE, Liao HX, Kepler TB, Haynes BF, Ramsburg E. 2008. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine 26:6108–6118. 10.1016/j.vaccine.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draper SJ, Heeney JL. 2010. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 8:62–73. 10.1038/nrmicro2240 [DOI] [PubMed] [Google Scholar]
- 23.Tatsis N, Ertl HC. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616–629. 10.1016/j.ymthe.2004.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4:115ra111. 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O'Hara GA, Ewer KJ, Anagnostou N, Bliss C, Hill AV, Traboni C, Klenerman P, Cortese R, Nicosia A. 2012. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4:115ra112. 10.1126/scitranslmed.3002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Iwata K, Matsumoto M, Matsumoto H, Nakao K, Hatahara T, Ohta Y, Kanai K, Maruo H, Baba K, Hijikata M, Mishiro S. 2001. Hepatitis C virus (HCV) genotype 1b sequences from fifteen patients with hepatocellular carcinoma: the ‘progression score' revisited. Hepatol. Res. 20:161–171. 10.1016/S1386-6346(00)00141-8 [DOI] [PubMed] [Google Scholar]
- 27.Roccasecca R, Folgori A, Ercole BB, Puntoriero G, Lahm A, Zucchelli S, Tafi R, Pezzanera M, Galfre G, Tramontano A, Mondelli MU, Pessi A, Nicosia A, Cortese R, Meola A. 2001. Induction of cross-reactive humoral immune response by immunization with mimotopes of the hypervariable region 1 of the hepatitis C virus. Int. Rev. Immunol. 20:289–300. 10.3109/08830180109043040 [DOI] [PubMed] [Google Scholar]
- 28.Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A, Fu TM, Bett A, Colloca S, Cortese R, Nicosia A, Folgori A. 2006. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J. Virol. 80:1688–1699. 10.1128/JVI.80.4.1688-1699.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S, Chen M, Zhu DM, Shiver JW, Volkin DB. 2004. Development of stable liquid formulations for adenovirus-based vaccines. J. Pharm. Sci. 93:2458–2475. 10.1002/jps.20157 [DOI] [PubMed] [Google Scholar]
- 30.Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D, Simon AJ, Laufer R, La Monica N, Cortese R, Nicosia A. 2000. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J. Virol. 74:11598–11607. 10.1128/JVI.74.24.11598-11607.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottwein JM, Bukh J. 2008. Cutting the gordian knot—development and biological relevance of hepatitis C virus cell culture systems. Adv.Virus Res. 71:51–133. 10.1016/S0065-3527(08)00002-X [DOI] [PubMed] [Google Scholar]
- 32.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. U. S. A. 100:7271–7276. 10.1073/pnas.0832180100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cottingham MG, Carroll F, Morris SJ, Turner AV, Vaughan AM, Kapulu MC, Colloca S, Siani L, Gilbert SC, Hill AV. 2012. Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors. Biotechnol. Bioeng. 109:719–728. 10.1002/bit.24342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillen W, Berens C. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345–369. 10.1146/annurev.mi.48.100194.002021 [DOI] [PubMed] [Google Scholar]
- 35.Hillen W, Gatz C, Altschmied L, Schollmeier K, Meier I. 1983. Control of expression of the Tn10-encoded tetracycline resistance genes. Equilibrium and kinetic investigation of the regulatory reactions. J. Mol. Biol. 169:707–721 [DOI] [PubMed] [Google Scholar]
- 36.Dahari H, Feinstone SM, Major ME. 2010. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 139:965–974. 10.1053/j.gastro.2010.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youn JW, Park SH, Lavillette D, Cosset FL, Yang SH, Lee CG, Jin HT, Kim CM, Shata MT, Lee DH, Pfahler W, Prince AM, Sung YC. 2005. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology 42:1429–1436. 10.1002/hep.20934 [DOI] [PubMed] [Google Scholar]
- 38.de Cassan SC, Draper SJ. 2013. Recent advances in antibody-inducing poxviral and adenoviral vectored vaccine delivery platforms for difficult disease targets. Expert Rev. Vaccines 12:365–378. 10.1586/erv.13.11 [DOI] [PubMed] [Google Scholar]
- 39.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957. 10.1016/j.vaccine.2009.10.145 [DOI] [PubMed] [Google Scholar]
- 40.Matthews DA, Cummings D, Evelegh C, Graham FL, Prevec L. 1999. Development and use of a 293 cell line expressing lac repressor for the rescue of recombinant adenoviruses expressing high levels of rabies virus glycoprotein. J. Gen. Virol. 80(Part 2):345–353 [DOI] [PubMed] [Google Scholar]
- 41.Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR, Perrone MP, Girelli G, Del Porto P, Piccolella E, Mondelli MU, Amoroso P, Cortese R, Nicosia A, Vitelli A, Folgori A. 2004. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 53:1673–1681. 10.1136/gut.2003.037788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. 2005. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology 42:104–112. 10.1002/hep.20749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray R, Meyer K, Banerjee A, Basu A, Coates S, Abrignani S, Houghton M, Frey SE, Belshe RB. 2010. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J. Infect. Dis. 202:862–866. 10.1086/655902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P, Hill H, Wolff MC, Schultze V, Han JH, Scharschmidt B, Belshe RB. 2010. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 28:6367–6373. 10.1016/j.vaccine.2010.06.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desjardins D, Huret C, Dalba C, Kreppel F, Kochanek S, Cosset FL, Tangy F, Klatzmann D, Bellier B. 2009. Recombinant retrovirus-like particle forming DNA vaccines in prime-boost immunization and their use for hepatitis C virus vaccine development. J. Gene Med. 11:313–325. 10.1002/jgm.1307 [DOI] [PubMed] [Google Scholar]
- 46.Swadling L, Klenerman P, Barnes E. 2013. Ever closer to a prophylactic vaccine for HCV. Expert Opin. Biol. Ther. 13:1109–1124. 10.1517/14712598.2013.791277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YP, Kang HN, Babiuk LA, Liu Q. 2006. Elicitation of strong immune responses by a DNA vaccine expressing a secreted form of hepatitis C virus envelope protein E2 in murine and porcine animal models. World J. Gastroenterol. 12:7126–7135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song MK, Lee SW, Suh YS, Lee KJ, Sung YC. 2000. Enhancement of immunoglobulin G2a and cytotoxic T-lymphocyte responses by a booster immunization with recombinant hepatitis C virus E2 protein in E2 DNA-primed mice. J. Virol. 74:2920–2925. 10.1128/JVI.74.6.2920-2925.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Y, Kwon T, Polo J, Zhu YF, Coates S, Crawford K, Dong C, Wininger M, Hall J, Selby M, Coit D, Medina-Selby A, McCoin C, Ng P, Drane D, Chien D, Han J, Vajdy M, Houghton M. 2008. Induction of broad CD4+ and CD8+ T-cell responses and cross-neutralizing antibodies against hepatitis C virus by vaccination with Th1-adjuvanted polypeptides followed by defective alphaviral particles expressing envelope glycoproteins gpE1 and gpE2 and nonstructural proteins 3, 4, and 5. J. Virol. 82:7492–7503. 10.1128/JVI.02743-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes-del Valle J, de la Fuente C, Turner MA, Springfeld C, Apte-Sengupta S, Frenzke ME, Forest A, Whidby J, Marcotrigiano J, Rice CM, Cattaneo R. 2012. Broadly neutralizing immune responses against hepatitis C virus induced by vectored measles viruses and a recombinant envelope protein booster. J. Virol. 86:11558–11566. 10.1128/JVI.01776-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Du Chene I, LeGrand R, Mangeot I, Lavillette D, Bellier B, Cosset FL, Tangy F, Klatzmann D, Dalba C. 2011. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci. Transl. Med. 3:94ra71. 10.1126/scitranslmed.3002330 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.