Abstract
The fine structures of mouse embryonic stem cells (mESCs) grown as colonies and differentiated in three-dimensional (3D) culture as embryoid bodies (EBs) were analyzed by transmission electron microscopy. Undifferentiated mESCs expressed markers that proved their pluripotency. Differentiated EBs expressed different differentiation marker proteins from the three germ layers. The ultrastructure of mESCs revealed the presence of microvilli on the cell surfaces, large and deep infolded nuclei, low cytoplasm-to-nuclear ratios, frequent lipid droplets, nonprominent Golgi apparatus, and smooth endoplasmic reticulum. In addition, we found prominent juvenile mitochondria and free ribosomes-rich cytoplasm in mESCs. Ultrastructure of the differentiated mESCs as EBs showed different cell arrangements, which indicate the different stages of EB development and differentiation. The morphologies of BALB/c and 129 W9.5 EBs were very similar at day 4, whereas C57BL/6 EBs were distinct from the others at day 4. This finding suggested that differentiation of EBs from different cell lines occurs in the same pattern but not at the same rate. Conversely, the ultrastructure results of BALB/c and 129 W9.5 ESCs revealed differentiating features, such as the dilated profile of a rough endoplasmic reticulum. In addition, we found low expression levels of undifferentiated markers on the outer cells of BALB/c and 129 W9.5 mESC colonies, which suggests a faster differentiation potential.
Introduction
Stem cells are unspecialized cells that have the ability to self-renew and differentiate into various types of cells or tissues in vivo and in vitro (Friel et al., 2005; Kruse and Völcker, 1997; Lakshmipathy and Verfaillie, 2005; Lo et al., 2003; Schlafke and Enders, 1963; Wobus, 2001). Thus, stem cells represent an important tool for conducting biomedical research (Horwitz, 2003; Kruse et al., 2006; Tielens et al., 2006). Pluripotent stem cells can produce cells from all three germ layers (ectoderm, mesoderm, and endoderm) but not from extraembryonic membranes (Kruse et al., 2006; Lakshmipathy and Verfaillie, 2005; Lo et al., 2003; Sanders et al., 2006; Wobus, 2001). In 1981, Martin was able to isolate the mouse embryonic stem cells (mESCs) from preimplanted blastocysts (Bongso and Richards, 2004; Friel et al., 2005; Martin, 1981). The mESCs can be stably maintained in an undifferentiated state in vitro using a feeder layer and the leukemia inhibitory factor (LIF) (Smith et al., 1988; Williams et al., 1988). Doetschman et al. in 1985 for the first time presented an in vitro model of mouse embryogenesis based on differentiating mESCs (Doetschman et al., 1985). mESCs can be grown in the absence of feeder cells and LIF to direct their differentiation into a three-dimensional (3D) spheroids called embryoid bodies (EBs). EBs will subsequently differentiate into cells representing the three germ layers, resulting in various committed cell types, including cardiomyocytes (Maltseva et al., 1993; Wobus et al., 1991), skeletal muscle cells (Miller-Hance et al., 1993), endothelial cells (Vittet et al., 1996), neuronal cells (Fraichard et al., 1995), adipocytes (Dani et al., 1997), and hematopoietic precursors (Schmitt et al., 1991).
The fine structure of mESC colonies was analyzed by scanning and transmission electron microscopy (TEM). They had Golgi complexes, spherical to oval mitochondria, lysosomes, typical centrioles, microfilaments and microtubules, and large nuclei containing reticulated nucleoli (Baharvand and Matthaei, 2003). In addition, the fine structure of human (h) ESC colonies was analyzed by TEM. Three morphological types of cells were identified on the basis of their fine structure: (1) Undifferentiated cells resembling inner cell mass (ICM) cells of blastocysts; (2) protein-synthesizing cells at the onset of cellular differentiation; and (3) compact masses of secretory cells resembling unicellular goblet cells of the intestine (Sathananthan et al., 2001). Newly established hESCs were studied by spontaneous differentiation into cardiomyocytes and neurons. Differentiated cardiomyocytes were processed for TEM, which revealed mononuclear cells, with parallel arrays of myofibrillar bundles oriented in an irregular manner in some cells, whereas more mature sarcomeric organization was apparent in others (Baharvand et al., 2004). A previous ultrastructural study of mESCs has shown that there is a clear increase in the cytoplasmic volume when ESCs are differentiated as EBs; in addition, there is an increase in protein synthesis (Sampath et al., 2008). In addition, many other investigations have studied ultrastructural morphology of EBs, which differentiated into various committed cell types, including cardiomyocytes (Taha et al., 2012), endothelial cells (Festag et al., 2007), hepatocytes (Kuai et al., 2014), skeletal muscle cells (Kawagoe et al., 2011), pancreatic exocrine enzyme-producing cells (Shirasawa et al., 2011), and renal cells (Kramer et al., 2006).
In this study, we identified several differences between cultured mESCs and their differentiated derivatives as EBs. By examining the ultrastructures of both mESCs and EBs, we looked for consistencies between the three different mESC lines (BALB/c, 129 W9.5, and C57BL/6). Furthermore, by studying the ultrastructure of the EBs, we clarified the types of early changes that occurred in the microstructure following differentiation. Finally, this study demonstrated that these changes are cell line dependent.
Materials and Methods
ESC culture
Previously established mESCs were a gift from Professor Klaus Matthaei (The John Curtin School of Medical Research, Australian National University, Canberra, Australia). We used the mESCs derived from three different mouse strains, BALB/c, 129 W9.5, and C57BL/6, and propagated cells between passages 9 and 20. The mESCs were cultured in T25 tissue culture flasks coated with 0.1% bovine gelatin with a feeder layer of mitomycin-C (1 mg/100 mL of mitomycin C; Sigma, cat. no. M4287) treated mouse embryonic fibroblasts (MEFs). The MEFs were grown in medium composed of Dulbecco's Modified Eagle Medium (DMEM; Gibco, cat. no. 41966052) supplemented with 10% fetal bovine serum (FBS; Gibco, cat. no. 26140087), 1% pen/strep (10,000 units of penicillin and 10,000 μg of streptomycin/mL; Gibco, cat. no. 15140122), and 1% nonessential amino acids (NEAA; X100; Gibco, cat. no. 11140035). The mESCs were cultured in medium composed of knockout D-MEM (Gibco, cat. no. 10829018) supplemented with 15% FBS-ES qualified (Gibco, cat. no. 16141079), 1% pen/strep (10,000 units of penicillin and 10,000 μg of streptomycin/mL; Gibco, cat. no. 15140122), 1% NEAA (X100; Gibco, cat. no. 11140035), 1% l-glutamine 200 mM (100×; Gibco, cat. no. 25030024), 1000 Units/mL of LIF (Millipore, cat. no. ESG1107), and 0.1 mM of 2-mercaptoethanol (Sigma, cat. no. M7522). ESC medium was changed every day, including fresh LIF. Experimental conditions were the same throughout the study for all three cell lines. No changes were made regarding cell culture conditions or EB differentiation.
EB formation
Once the flask became confluent (80–90%), mESCs were trypsinized to ensure an equal amount of the cells. The sizes of mESC colonies were very carefully analyzed under a light microscope, and only at the right confluency were they used for EB formation. This was true for each cell line. The trypsinized mESCs were placed in adherent cell culture flasks for 1 h in the incubator to separate the mESCs from the MEF feeder layer. Then the mESCs were cultured into nonadherent culture dishes, in which EBs were formed. The EB medium was composed of knockout DMEM supplemented with 15% FBS-ES qualified, 1% pen/strep, 1% NEAA, 1% l-glutamine 200 mM, and 0.1 mM of 2-mercaptoethanol. EBs were grown in this medium for up to 11 days. Histological analysis and TEM were performed.
Immunocytochemical analysis of mESCs
The mESCs were seeded on 0.1% gelatin-coated chamber slides. At day 4 of culture, the cells were fixed with 4% paraformaldhyde (PFA) at room temperature for 30 min, permeabilized with 0.1% Triton X-100 (Sigma, cat. no. T8787) for 10–15 min at room temperature, and blocked with 3% bovine serum albumin (BSA; Sigma, cat. no. A3311) at room temperature for 30–60 min. The slides were incubated overnight at 4°C with primary antibodies used as markers associated with the state of pluripotency of the stem cells, specifically rabbit polyclonal anti-SOX2 (sex determining region Y-box 2) (3 μg/mL; Abcam, cat. no. ab15830), rabbit polyclonal anti-Oct4 (1–5 μg/mL; Abcam, cat. no. ab19857), and mouse monoclonal anti-SSEA-1 (stage-specific embryonic antigen-1) (1/100; Abcam, cat. no. ab16285). The slides were incubated for 30–60 min at room temperature with diluted secondary antibodies, specifically goat polyclonal anti-rabbit immunoglobulin G (IgG)–heavy and light chains (H&L) (fluorescein isothiocyanate, FITC) at 1/1000 dilution (Abcam, cat. no. ab6717) and goat polyclonal anti-mouse IgG–H&L (FITC) at 1/1000 dilution (Abcam, cat. no. ab6785). For nuclei staining, we dispensed one drop of Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs, cat. no. H-100) onto each histological section.
Immunohistotochemical analysis of EBs
Immunostaining of EBs was performed at 4, 7, and 11 days following mESC differentiation. After washing the cells with phosphate-buffered saline (PBS), the EBs were fixed in 4% PFA at room temperature for 30 min. The EBs were then dehydrated in increasing concentrations of ethyl alcohol (10%, 30%, 70%, and 95%; BDH, cat. no. 101077Y) for 10 min each and in two changes of 100% alcohol for 5 min each. The clearing was accomplished with xylene (Riedel-de Haen, cat. no. 33817) 2X for 10 min each, followed by infiltration by adding melted paraffin wax to the cleared sections (Sigma-Aldrich, cat. no. 32,720-4). The section widths were obtained at 5 μm using a rotary microtome (Sakura, SRM 200 CW). A peroxidase detection system (Leica, RE7110-K) was used for immunostaining as per company recommendations. The primary antibodies used for identifying the markers associated with the state of pluripotency of the stem cells included anti-SOX2 at 1/1500 dilution and rabbit polyclonal anti-Oct4 at 1/250 dilution. For identifying the differentiation markers, we used purified mouse anti-vimentin, expressed in mesenchymal cells, with 1/2000 dilution (BD, cat. no. 550513) and a mouse monoclonal antibody to neuron-specific βIII tubulin, with 1/500 dilution (Abcam, cat. no. ab14545).
Electron microscopy
After trypsinizing, the mESCs from the flasks and EBs were collected in tubes. The samples were washed with PBS and the pellets were resuspended directly in 2.5% glutaraldehyde fixative (Electron Microscopy Sciences, cat. no. 16500) in 0.1 M phosphate buffer (pH 7.2) and kept at 4°C for 4 h. First, the cells were washed in 0.1 M phosphate buffer (pH 7.2) 3× for 30 min each. After washing, the cells were transferred to 1% osmium tetroxide (OsO4) in 0.1 M phosphate buffer (pH 7.2) for 2 h. The fixed cells were dehydrated in ascending grades of ethanol (10%, 30%, 50%, and 70%) for 15 min each, followed by 90% ethanol 2× for 15 min, ending with 100% ethanol 2× for 15 min each. The cells were then resuspended in acetone 2× for 15 min each. The resulting cells in suspension were aliquoted into BEEM® embedding capsules and infiltrated with an acetone:resin mixture (2:1) for 1 h followed by an acetone:resin mixture (1:2) for 1 h. After each infiltration step, the BEEM® capsules were centrifuged at 2500 rpm for 5 min and embedded in a pure resin mixture for 2 h. Polymerization of the resin was accomplished in an oven at 70°C for 12 h. After trimming the blocks using an ultramicrotome (Leica, UCT, Australia), the semithin sections (0.5 μm thickness) were prepared and stained with 1% Toluidine Blue. Ultrathin sections (70-nm thickness) were prepared and mounted on copper grids. Ultrathin sections were first contrasted with uranyl acetate (saturated ethanol solution) for 30 min, rinsed with double-distilled water, contrasted with Reynold's lead citrate for 5 min, and finally rinsed with distilled water. The contrasted ultrathin sections were examined and photographed under a TEM (Jeol 1010, Jeol, Tokyo, Japan).
Statistical analysis
The morphometric parameters of EBs area were measured by ImageJ 1.47V software and compared by means of the Kruskal–Wallis test or Mann–Whitney U-test.
Results
Expression of pluripotent markers in mESCs
To detect the state of pluripotency, immunocytochemistry was performed on mESC colonies using undifferentiated markers. All three mESC lines were positive for Oct4, Sox2, and SSEA-1 (Fig. S1A–C). (Supplementary Data are available at www.liebertpub.com/cell/.) However, the outer cells of 129 W9.5 and BALB/c ESC colonies showed low expression levels of these markers of undifferentiation. Additionally, this was not observed in C57BL/6 mESC colonies (Fig. S1).
Derivation of the three germ layers in EBs of the three cell lines
The mESCs were allowed to differentiate spontaneously into EBs in medium without LIF for 11 days. At different time points, the EBs were collected and used for histological studies to identify cells from the three germ layers. Sections of the EBs were obtained at different time points of differentiation and stained with Hematoxylin & Eosin (H&E) and Toluidine Blue. The histological studies showed similar structures between EBs of C57BL/6 from day 4 (Figs. S2C, D) and day 7 (Fig. S3A). We found significant differences in the size of the EBs at day 7, which were larger than EBs at day 4 (p<0.05), and between EBs at day 7 and EBs at day 11 (p<0.05) (Tables S1 and S2). At EB day 11, a few cysts were formed and many layers of the peripheral cells were observed (Fig. S3B). EBs from 129 W9.5 cells harvested at days 7 and 11 (Fig. S3C, D) were larger and contained larger cysts than EBs at day 4. The difference was statistically significant (p<0.05) (Figs. S2E, F, Tables S1 and S2). However, no significant difference was seen across the three cell lines when examined on the individual days (Tables S1 and S2). Histological analysis of 129 W9.5 EBs showed the presence of cell debris in the middle layer of all EBs starting with EBs at day 4 (Fig. S2E, F) and in C57BL/6 EBs starting from day 7 (Fig. S3A).
Day-4 EBs differentiated from the three mESC lines still stained positive for the pluripotency marker Oct4. However, day-4 EBs showed a lower expression level of Sox2 protein (Fig. S4). We also observed that the outer layer of EB cells stained strongly positive for markers of the undifferentiated state compared with the inner EB cell layer (Fig. S4).
All EBs from the three mESC lines stained positive for vimentin and βIII tubulin, a marker of mesodermal and neuroectoderm differentiation, respectively (Fig. S5). Thus, the EBs exhibited pluripotency, as evidenced by their germ-layer differentiation potential. In general, EBs at day 4 from the 129 W9.5, BALB/c, and C57BL/6 mouse lines exhibited low expression of βIII tubulin. The protein expression level of βIII tubulin in C57BL/6 EBs increased gradually and reached its highest level at EB day 11 (Fig. S5G). On the other hand, during the 129 W9.5 EB differentiation process, we found that the protein expression of βIII tubulin had reached its maximum level at day 7 (Fig. S5K); thereafter, the protein expression level decreased by day 11 (Fig. S5M). The protein expression pattern of vimentin was similar to βIII tubulin, with no changes among the three cell lines. However, the peripheral cells at day 7 of 129 W9.5 EBs showed very clear expression of vimentin protein; at the other time points, vimentin was uniformly distributed over the entire EBs (Fig. S5L).
Ultrastructural analysis of undifferentiated ESCs
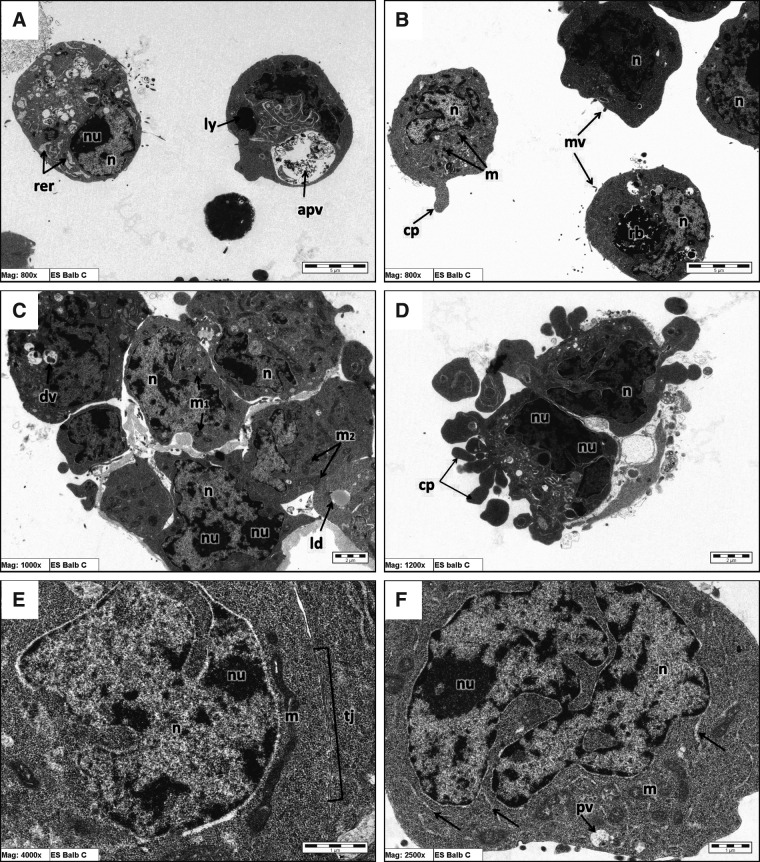
TEM experiments showed that the undifferentiated mESCs were separated as a result of a trypsin disaggregating effect. The mESCs from all three lines revealed clear polygonal shapes. We observed processes extending from BALB/c and 129 W9.5 ESCs, although not in C57BL/6 ESCs (Figs. 1B, D and 3C, below). Cell surfaces displayed microvilli of varying lengths and frequencies; C57BL/6 ESCs had numerous microvilli. In contrast, microvilli occurred rarely on BALB/c and 129 W9.5 ES cells (Figs. 1B, 2A, and 3A).
FIG. 1.
TEM of BALB/c ESCs. (A) n, nucleus; nu, nucleolus; rer, dilated rER; ly, lysosomes; apv, autophagocytic vacuole. (B) mv, microvilli; rb, residual bodies; m, juvenile mitochondria; cp, cell process. (C) m1, poorly developed mitochondria; m2, well-developed mitochondria; ld, lipid droplets; dv, digestive vacuole. (E) m, reticular form of mitochondria. (F) m, large poorly developed mitochondria, stalks of rER (arrows).
FIG. 3.
TEM of 129 W9.5 ES cells. (A) m, elongated mitochondria. (B) m, large poorly developed mitochondria, reticular form of mitochondria (*). (C) m, juvenile mitochondria lipid droplets (arrowheads). (E) (arrows) Stalks of RER; g, Golgi apparatus. (E) m, small poorly developed mitochondria.
FIG. 2.
TEM of C57BL/6 ES cells. (B) pv, phagocytic vacuole; (arrowheads) stalks of RER. (C) m, large poorly developed mitochondria. (D) (*) Elongated mitochondria; m, juvenile mitochondria; (arrowheads) lipid droplets. (E) dj, desmosomal-like junction; pv, pinocytotic vacuole. (F) m, small poorly developed mitochondria.
Lysosomes were frequently seen in C57BL/6 ESCs as rounded electron-dense cytoplasmic structures, and these were rarely seen in the other two cell lines (Figs. 1A, 2A, and 3D). We observed phagocytic vacuoles as rounded, membrane-bounded cytoplasmic structures containing particulate material of moderate electron density structures (Figs 1F, 2B, 2D, 2E, and 3D). In contrast, the digestive vacuoles were rounded, electron-lucent structures containing dense irregular bodies (Figs. 1C and 2B). These electron-dense bodies were likely formed after the fusion of the lysosomes with the phagocytic vacuoles. Phagolysosomes and digestive vacuoles occurred more frequently in the C57BL/6 ESCs than in the 129 W9.5 and BALB/c ESCs. Residual bodies containing highly electron-dense structures were also observed, although they were not prominent in the BALB/c ESCs and rarely seen in the C57BL/6 and 129 W9.5 ESCs (Figs. 1B, 2A, 2D, 3B, 3D, and 3F). Occasional autophagic vacuoles, round in shape and containing membranous structures, were observed in the three ESC lines (Figs. 1A, 2B, and 2F).
The nuclei of the three cell lines were large and deeply infolded. The nuclei possessed heterogeneous structures containing electron-dense and electron-lucent areas (Figs. 1D, 2B, and 3B). In the dense areas, we observed heterochromatin formed irregularly as clumps at the nuclear periphery. The electron-lucent nuclear material (or euchromatin) occupied most of the nuclear space. The nuclei displayed low-density chromatin and contained one to two reticular-shaped nucleoli (Figs. 1C–D, 2B, and 3A–B). The cytoplasm-to-nucleus ratio was visibly low in all cell lines (Figs. 1F, 2C, and 3B). In both the C57BL/6 and BALB/c ESCs, features of cell division were evident (Figs. 1D and 2E).
Even after we disaggregated the cells with trypsin, some junctions remained visible between the ESC colonies. Gap junctions between the cells were evident in the BALB/c and 129 W9.5 ESCs (Fig. 1E), but not in the C57BL/6 ESCs. Instead, desmosomal-like junctions occurred between the C57BL/6 ESCs (Figs. 2E and 3E). Lipid droplets, cytoplasmic rounded structures of moderate electron-dense areas within the cytoplasm, were frequently observed in the C57BL/6 ESCs, although they were observed with low frequency in the BALB/c and 129 W9.5 ESCs (Figs. 1C, 2B, 2D, and 3C). The Golgi apparatus and smooth endoplasmic reticulum (sER) were unnoticeable in the cytoplasm of any of the ESC lines (Fig. 3E).
The ESC mitochondria were of varied shapes and sizes. The large well-developed form of mitochondria with remarkable cristae was observed infrequently in all the ESC lines and was mostly observed in BALB/c ESCs (Fig. 1C). Poorly developed mitochondria, which were smaller than the well-developed mitochondria in size with less distinct cristae, were occasionally observed in the ESC populations (Figs. 1C, 1F, 2C, 3B). In addition, there were small poorly developed mitochondrial forms with obviously thin cristae in all ESC lines, with a high number of this form observed in C57BL/6 ESCs (Fig. 2F). Juvenile mitochondria of the smallest size were also observed in all ESC lines (Figs. 1B, 2D, and 3C). Noticeably elongated mitochondria were seen in all mESC lines (Figs. 1E, 2D, and 3A). Reticular-shaped mitochondria, with interdigitating cristae in a network configuration were typically observed in 129 W9.5 ESCs (Fig. 3B).
Long stalks of ribosome-studded rough endoplasmic reticulum (rER) were observed in all mESC lines. This form of rER was mainly seen in C57BL/6 ESCs (Figs. 2F, 2B, and 3E). Dilated rER were observed most often in the BALB/c and 129 W9.5 ESC lines (Figs. 1A and 3C). The cytoplasm of these cells from the three cell lines was rich in free ribosomes and polysomes.
Ultrastructural analysis of BALB/c EBs
TEM examinations revealed that the cell distribution in the EBs of the three cells lines was different from the cell arrangement seen in the undifferentiated ESCs. The BALB/c EBs were composed of three cell types arranged in a configuration so that the terminal cells were localized along the EB edges with peripheral cells surrounding the middle cells. A basement-like membrane surrounded the entire EB, segregating between the terminal and the peripheral cells (Fig. 4C). The BALB/c EB cells were rounded in shape, whereas the terminal cells took on a flattened contour (Fig. 4B). On the apical surface of the terminal cells, we observed numerous microvilli of varying lengths (Fig. 4B). Microvilli were absent on the peripheral and middle cells.
FIG. 4.
TEM of cells in BALB/c EBs. (A) m, poorly developed mitochondria; microvilli (arrows); (arrowheads) tight junction. (B) m, poorly developed mitochondria; (arrows) microvilli; (arrowheads) tight junction. (C) m, well-developed mitochondria; (arrow) basement-like membrane. (D) (arrowheads) Desmosomal-like junction (E) (arrowhead) Tight junction; m, well-developed mitochondria; gj, gap junction. (G) m, well-developed mitochondria; (*) lipid droplets. (I) (Arrowheads) Desmosomal-like junction; m, well-developed mitochondria.
We observed lysosomes only occasionally in the terminal cells, but more frequently in the peripheral and middle cells (Fig. 4B, G, and I). Phagocytic vacuoles were not prominently visible in any of the cell types (Fig. 4A, H); in contrast, the digestive vacuoles were observed frequently in all cell types (Fig. 4B, F, H). Residual bodies were infrequently observed in the three cell types; however, these bodies were encountered most often in the middle cells of the EBs (Fig. 4A, F, I). Occasionally we observed autophagocytic vacuoles in the three cell types (Fig. 4A, G, H).
Most nuclei in the three cell types were large and oval in shape; however, the nuclei of the middle cells exhibited membrane folding and low-density euchromatin (Fig. 4C, F, H). The nuclei contained one to three reticular nucleoli. The cytoplasmic-to-nuclear ratio was noticeably low in all cell types (Fig. 4C, F, H). Some features of cell division were evident in the middle cells (Fig. 4I).
We observed junction complexes between the EB cells; however, there were no obvious cell–cell junctions between the terminal and the peripheral cells, because they were separated by the matrix-like structure (Fig. 4C). Tight junctions were observed between the cells of the terminal and peripheral layers (Fig. 4B, E). Desmosome-like junctions were prominent, occurring between the cells of all three layers (Fig. 4D, I). Some gap junctions were observed to occur between the cells in the peripheral layer (Fig. 4E). Furthermore, lipid droplets were frequently observed in all EB cell types (Fig. 4A, G, I). The Golgi apparatus was observed only infrequently, whereas the smooth endoplasmic reticulum was not prominent in the cytoplasm of any of the EB cells.
The mitochondria in the peripheral and middle cells were observed only in a well-developed form and were infrequently observed in the terminal cells (Fig. 4C, E, G, I). Small, poorly developed mitochondrial forms were prominently present in the terminal cells (Fig. 4B).
Long stalks of ribosome-studded rER were observed in all peripheral and middle cells, but not in the terminal cells (Fig. 4C, F, H). Dilated profiles of the rER were only evident in the terminal cells (Fig. 4C). The cytoplasm of all cell types was very rich in free ribosomes and polysomes (Figs. 4B, F, G).
Ultrastructural analysis of C57BL/6 EBs
TEM examination of the C57BL/6 EBs showed that EBs were composed of two cell layers arranged as peripheral cells surrounding the middle cells. There was no comparable basement-like membrane separating the peripheral and the middle cells (Fig. 5A). The middle cells of these EBs were rounded in shape (Fig. 5E), whereas the peripheral layer was composed of overlapping flattened cells (Fig. 5A). On the surface of the peripheral cells, very few short microvilli were observed (Fig. 5A); furthermore, microvilli were only rarely observed in the middle cells (Fig. 5E).
FIG. 5.
TEM of cells in C57BL/6 EBs. (A) m, well-developed mitochondria. (B) (Arrowheads) Desmosomal-like junction. (C) (Arrowheads) Desmosomal-like junction; m, poorly developed mitochondria. (D) m1, juvenile mitochondria; m2, elongated mitochondria; (arrowheads) tight junction. (E) m, well-developed mitochondria.
Lysosomes were abundant in the middle cells, but were infrequently encountered in the peripheral cells (Fig. 5D). Phagocytic vacuoles were infrequently observed in all cell types (Fig. 5F). In contrast, digestive vacuoles were observed frequently in all cell types (Fig. 5A, C). Residual bodies occurred more frequently in the middle cells (Fig. 5C). Occasional autophagocytic vacuoles were encountered in the middle cells, although not often in the peripheral cells (Fig. 5D).
The nuclear shape varied depending on the cell shape. The middle cells' nuclei were large and rounded (Fig. 5A), whereas those of the peripheral cells were oval in shape (Fig. 5C). Furthermore, the nuclei showed low euchromatin density and contained between one and three reticular nucleoli. The cytoplasmic-to-nuclear ratio was distinctly low in all cell types (Figs. 5A, C). Some features of cell division were evident in the middle cells (Fig. 5D). Features of cell degradation were evidenced, such as nuclear deformation, chromatin condensation, and nuclear envelope disruption, with this being notable mainly in the middle cells (Fig. 5E).
Junction complexes were observed between the EB cells. Tight junctions were observed between the cells of the middle layer (Fig. 5D), whereas some desmosome-like junctions were obvious between the peripheral cells and between some of the middle cells (Fig. 5B, C). Lipid droplets were frequently observed in the middle cells compared with the peripheral cells (Fig. 5A, F). The Golgi apparatus was rarely observed, whereas the smooth endoplasmic reticulum was not prominent in the cytoplasm of any of the EB cells.
Different mitochondrial forms were observed in the middle cells, which may indicate different stages of development. These forms included juvenile (Fig. 5D), poorly developed forms (Fig. 5C), elongated forms (Fig. 5D) and well-developed forms (Fig. 5E). In the peripheral cells, only the well-developed form was observed (Fig. 5A). Long stalks of ribosome-studded rER were frequently observed in the middle cells, but infrequent in the peripheral cells (Fig. 5E). The cytoplasm of all cell types was rich in free ribosomes and polysomes.
Within the middle layer, another type of cell was seen, although at a low frequency. These dense cells presented with some features of differentiation, characterized mainly by their dilated rER profiles and high content of free ribosomes within the cytoplasm. They also possessed dense nuclei with no clear nucleoli (Fig. 5F).
Ultrastructural analysis of 129 W9.5 EBs
TEM examination showed that the morphology of 129 W9.5 EBs was similar to that of BALB/c EBs, except that the terminal cells were not continuous around the whole EBs (Fig. 6C, D).
FIG. 6.
TEM analysis of the terminal and peripheral cells from 129 W9.5 EBs. (A) m, juvenile mitochondria, basement-like structure (*). (B) tj, tight junction; m, poorly developed mitochondria. (D) m, elongated mitochondria. (F) m, well-developed mitochondria. (G) m, poorly developed mitochondria; desmosomal like junction (arrowheads). (H) Lipid plaques (*).
The 129 W9.5 EBs were composed of three cell types arranged as noncontinuous terminal cells along the edges and peripheral cells surrounding the middle cells (Fig. 6A). A basement-like membrane surrounded the entire EB and separated the terminal and peripheral cells (Fig. 6A). In general, the 129 W9.5 EB cells were rounded; however, the terminal cells had a columnar shape (Fig. 6C). Numerous microvilli of varying lengths were observed on the surface of terminal cells (Fig. 6C). Microvilli occurred rarely on the peripheral and middle cells (Figs. 6E and 7B).
FIG. 7.

TEM analysis of the middle cells in 129 W9.5 EBs. (A) m, well-developed mitochondria. (B) Dividing cell (*). (C) Indication of cell necrosis (*).
Lysosomes were infrequently observed in the terminal cells, although they were commonly present in the peripheral and middle cells (Figs. 6F and 7A). Phagocytic vacuoles were frequently observed in all cell types, although most predominantly in the terminal cells (Figs. 6C and 7A). In contrast, digestive vacuoles were common among all cell types (Figs. 6E, F and 7B). Residual bodies were occasionally observed in all of the three cell types (Fig. 7B). Autophagocytic vacuoles were negligible in all three cell types (Fig. 6F). There was also evidence of cell necrosis in the middle cell layer (Fig. 7C).
The nuclei in all the three cell types were large and rounded and contained one to two reticular-shaped nucleoli. The cytoplasmic-to-nuclear ratio was obviously depleted in all cell types (Figs. 6B, C and 7A). The terminal cell nuclei were highly similar to the peripheral cell nuclei. Some cell division characteristics were evident in the peripheral and middle cells (Fig. 7B).
Junction complexes were infrequently observed between the EB cells. However, no cell–cell junctions were observed between the terminal and peripheral cells, because these cells were separated by the basement-like membrane. Desmosome-like junctions were observed between the peripheral and terminal cells (Fig. 6G), whereas some tight junctions were observed between the terminal cells (Fig. 6B). Lipid droplets were frequently observed in all EB cell types, and lipid plaques were recognized in some terminal cells (Fig. 6H). Golgi apparatus was rarely observed, whereas sER was not prominent in the cytoplasm of any of the EB cells.
Well-developed mitochondria were observed in all cell types (Figs. 6F and 7A). Some poorly developed and elongated forms were observed infrequently in the terminal and peripheral cells (Figs. 6D, G). Conversely, juvenile mitochondria were common in the terminal cells (Fig. 6A). Short stalks of ribosome-studded rER were visible in the peripheral and middle cells (Figs. 6A, G and 7C). Dilated profiles of rER were only prominent in the terminal cells (Fig. 6D). The cytoplasm of all cell types was especially rich in free ribosomes and polysomes.
Discussion
This present study is the first to demonstrate an ultrastructural comparison between three different mESC lines and their predifferentiation stage as EBs. The colony formation rate was highest in 129 W9.5 ESCs and lowest in BALB/c ESCs, whereas the rate was moderate in the C57BL/6 mESC line, thus indicating that 129 W9.5 ESCs have a greater tendency to differentiate than BALB/c and C57BL/6 mESCs. Conversely, the number of EBs formed was the highest in BALB/c ESCs and the lowest in C57BL/6 cells, indicating that BALB/c ESCs differentiate at a higher rate than the other cell lines (Baharvand and Matthaei, 2003).
Our mESC colonies were comprised of undifferentiated cells, which were confirmed by immunostaining of mESCs with anti-Oct4, anti-Sox2, and anti-SSEA-1 antibodies. However, we discovered that some of the cells on the edges of the colonies exhibited weak staining for pluripotent markers, thus indicating the start of differentiation (Avilion et al., 2003; Schöler et al., 1989; Solter and Knowles, 1978). The mESC colonies consisted mostly of undifferentiated cells, whereas it is known that hESC colonies consisted of a mixture of differentiated, differentiating, and undifferentiated cells (Sathananthan et al., 2001).
Extended processes on the cell surface were detected in BALB/c and 129 W9.5 ESCs but not in the C57BL/6 ESCs; the extended processes may result from a higher sensitivity of BALB/c and 129 W9.5 ESCs to the PBS that was used when washing the cells (Talbot and Garrett, 2001). Microvilli were observed on the cell surfaces with different distribution degrees and patterns, depending on the ESC line, which could indicate that these cells had a high metabolic activity that resulted in an increase in the absorptive areas of the cells. BALB/c and 129 W9.5 ESCs showed few microvilli compared to the C57BL/6 ESCs, which could indicate that these cells were preparing for cell division. The microvilli on the mESC surfaces have also been observed in isolated hESCs (Sathananthan et al., 2001; Thomson and Marshall, 1998) and mouse blastocysts (Cech and Sedlácková, 1983; Ducibella et al., 1977; Maro et al., 1985; Mohr and Trounson, 1982; Nadijcka and Hillman, 1974; Shalgi and Sherman, 1979) among others.
Phagolysosomes and digestive vacuoles were encountered frequently, and their presence could indicate highly active metabolic cells, a feature found in mESCs that are very active and highly proliferative. Vacuoles and lysosomes are also commonly found in the blastocysts of many mammalian species (Mohr and Trounson, 1981; Mohr and Trounson, 1982; Plante and King, 1994; Wintenberger-Torres and Flechon, 1974). The presence of autophagic vacuoles in all three ESC lines suggests that autophagy has important housekeeping or protective functions (Eskelinen, 2005), or indicates cell death, and may be a normal feature of colony development (Mohr and Trounson, 1982).
Large and deeply infolded euchromatin nuclei with one to three reticular-shaped nucleoli were generally seen in all three ESC lines, indicating highly mitotically active cells. These observations were similar to those observed previously in the ICM of mouse and human blastocysts (Mohr and Trounson, 1982; Nadijcka and Hillman, 1974) and in hESCs (Sathananthan et al., 2001). In both C57BL/6 and BALB/c ESCs, but not in 129 W9.5 ESCs, the characteristics of cell division were evident, which may indicate a high proliferation rate in 129 W9.5 ESCs.
Lipid droplets of moderate electron density are rich in unsaturated fatty acids, and have been documented in pig ESC-like cells (Talbot and Garrett, 2001), murine embryonic cells (Potts, 1968), and bovine cells (Abe et al., 1999); however, to date no reports have confirmed the presence of lipid droplets in hESCs (Sathananthan et al., 2001). In our study, we frequently observed lipid droplets in C57BL/6 ESCs, and with a lower frequency in BALB/c and 129 W9.5 ESCs. The presence of lipid droplets indicates high metabolic activity in the cells. Furthermore, the cells use lipid droplets as storage material.
The Golgi apparatus and sER did not occur prominently in the cytoplasm of any of the ESC lines, possibly indicating a low activity of these organelles. Low organelle activity could lead to reduced protein secretion. Reportedly, ESCs require high protein levels (Wassarman and Josefowicz, 1978).
Mixed forms of mitochondria have been reported in mouse blastocysts and in late morula mouse embryos (Calarco and Brown, 1969; Mohr and Trounson, 1982; Nadijcka and Hillman, 1974). We found that the frequency of small poorly developed mitochondria in the three ESC lines was high; in addition, the mitochondria were similar to those found in cells of the ICM of mouse and bovine blastocysts, as reported by Mohr and Trounson (Mohr and Trounson, 1982). Juvenile mitochondria, elongated mitochondria, and reticular-shaped mitochondria were also encountered in all ESC lines, all of which indicate rapidly dividing cells (Stern et al., 1971). Long stalks of rER, free ribosome-rich cytoplasm, and polysomes were observed in all ESC lines, a finding similar to what has been reported previously in mammalian embryos (Calarco and McLaren, 1976; Mohr and Trounson, 1981; Mohr and Trounson, 1982; Nadijcka and Hillman, 1974; Sathananthan et al., 1990; Vanblerkoma et al., 1973). A dilated profile of rER occurred most prominently only in BALB/c and 129 W9.5 ESC lines, and it was not seen in C57BL/6 ESCs. This discrepancy indicates that BALB/c and 129 W9.5 ESCs are highly active cells. In addition, it is possible that BALB/c and 129 W9.5 ESCs were undergoing differentiation and thus not fully undifferentiated (Baharvand and Matthaei, 2003).
Evans and Kaufman (1981) first reported the pluripotency of ESCs following observation of their in vitro differentiation capability as EBs. Subsequently, ESCs were identified by their similar morphologies and differentiation capabilities and characteristics similar to embryonic carcinoma cells (Sell, 2004).
We observed varying cellular arrangements in the EBs, which may indicate different stages of embryonic development. The morphology of BALB/c and 129 W9.5 EBs was different from that of C57BL/6 EBs. H&E staining on day 11 EB of C57BL/6 samples showed a comparable morphology to day-4 EBs from BALB/c and 129 W9.5, which indicates a slower differentiation rate of C57BL/6 EBs. The basement-like membrane that separated the terminal cells from the peripheral cells was an indication of endodermal cell migration (Hoa et al., 2010; Li et al., 2002). This feature was similar to the distinctive outer layer of endodermal cell differentiation reported in day-2 EBs of B6D2-F1 (C57BL/6J ♀×DBA/2J ♂) (Tanaka et al., 2006), day-3 EBs derived from HM-1 cells (Mittmann et al., 2002), and mouse cystic EBs formed from the 1E6/2 cell line (Wang et al., 1992).
In our study, microvilli were observed on the surfaces of the terminal cells on the EBs from the three ESC lines, presumably increasing the surface absorption areas of the cells. This phenomenon indicates highly metabolically active cells. Microvilli have been reported in EBs from embryonic carcinomas (Pierce and Beals, 1964). In addition, the presence of microvilli has been reported previously to occur on endodermal cells in mouse cystic EBs formed from a 1E6/2 cell line (Wang et al., 1992). This finding may support our determination that only the 129 W9.5 and BALB/c ESC lines formed a visceral endodermal layer of cells, whereas the morphology of C57BL/6 was more primitive similar to the endodermal cells found in earlier stage of EB development (Turksen, 2006).
Furthermore, we found that EBs had a tendency toward undergoing neural differentiation because the EBs were more positive for βIII tubulin than vimentin throughout their differentiation phase. The increase in expression levels of specific markers from all three germ layers in EBs compared to mESCs indicates differentiation into cells from all three germ layers.
The observation that there was a more frequent presence of lysosomes in the middle cells than in the terminal or peripheral cells was an indication of high cellular activity that required hydrolysis enzymes to digest the vacuoles. The presence of digestive vacuoles was higher in all cell types in EBs—terminal, peripheral, and middle cells—from all three ESC lines. A low frequency of residual bodies that we documented herein may indicate a rapid cleaning system within all cell types. Some indication for cell necrosis observed in the middle layer cells of 129 W9.5 EBs could indicate the beginning of cyst formation, which is similar to the structure of a proamniotic cavity (Turksen, 2006).
In most of the EB cells, we observed folded nuclei with two to three nucleoli. These features have been reported in EBs that were formed from embryonic carcinoma cells (Pierce and Beals, 1964). Cell division was detected mostly among the cells comprising the middle area of the EBs in the three cell lines, which could indicate a high proliferation rate and therefore be evidence that EBs are expanding from the core. Cell degradation within the middle cells of C57BL/6 could be another indication of the beginning of cyst formation. A previous study on PSA4 EC cells reported that cellular cavities were first observed after 4–5 days as areas of cellular debris on the cells formed EBs (Boyd et al., 1984).
The tight junction formation that we observed between the terminal cells of BALB/c EBs may indicate the strong junction between these cells and the manner in which they achieved physical continuity in contrast to the 129 W9.5 EB terminal cells that lacked tight junctions. Presence of lipid plaques formed in the terminal 129 W9.5 EB cells could be an indication of higher metabolic activity than that found in the peripheral and middle cells. The Golgi apparatus was rarely observed in any of the EB cells compared to the ESCs, in which the Golgi apparatus was not prominent. This finding may reflect the need for greater proteins quantities required by EB cells for achieving proliferation.
Presence of mature mitochondria in the EBs was a clear indication of differentiation. We saw a dilated profile of the rER in the BALB/c and 129 W9.5 terminal cells, indicating the high activity of these cells (Pierce and Beals, 1964). The terminal cells of C57BL/6 EBs were unformed, and the dilated profile of the rER was not prominent in this cell line. The ER stalks have been reported previously in undifferentiated cells, and the dilated profile was also observed in the differentiated cells of the EBs that were formed from embryonic carcinoma cells (Pierce and Beals, 1964). The cytoplasm of all examined EB cell types from the three ESC lines reported herein were highly rich in free ribosomes and polysomes, indicating highly metabolically active cells.
Conclusions
We have shown that ESCS, when grown as colonies, are undifferentiated, as determined by the expression levels of the undifferentiated marker. Accordingly, the differentiated ESCs as EBs expressed different differentiated proteins. In general, the ultrastructure analyses of the ESCs identified microvilli on the cell surfaces, large and deeply infolded nuclei, low cytoplasm-to-nuclear ratios, frequent lipid droplets, nonprominent Golgi apparatus and sER, prominent juvenile mitochondria, and ribosome- and polysome-rich cytoplasm with some changes depending on the cell line. On the other hand, the ultrastructural analyses of the EBs showed varying cell arrangements, which may indicate different stages of EB development. The morphologies of BALB/c and 129 W9.5 EBs at day 4 were very similar, whereas that of C57BL/6 differed in many ways. These observations showed that EBs tend to differentiate in the same pattern but not at the same rate. This disparate differentiation characteristic may indicate a slower differentiation rate for C57BL/6 EBs than for BALB/c and 129 W9.5 EBs. Another explanation could be that C57BL/6 ESCs were fully undifferentiated when cultured as colonies, and therefore, upon EB formation, the differentiation rate was slower. Conversely, we believe that some of the BALB/c and 129 W9.5 ESCs had already started to differentiate when grown as colonies. The ultrastructural analysis of these ESCs revealed some differentiating features, such as a dilated profile of rER. In addition, we also observed low expression levels of the undifferentiated marker genes on the outer cells of the 129 W9.5 and BALB/c colonies, which therefore indicated a greater and faster differentiation potentiality as EBs.
Supplementary Material
Acknowledgments
This work was supported by a grant (no. 10-BIO1304-02) from the National Plan for Sciences and Technology Program, King Saud University, and partially supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Abe H., Yamashita S., Itoh T., Satoh T., and Hoshi H. (1999). Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: Comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 53, 325–335 [DOI] [PubMed] [Google Scholar]
- Avilion A.A., Nicolis S.K., Pevny L.H., Perez L., Vivian N., and Lovell-Badge R. (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharvand H., and Matthaei K.I. (2003). The ultrastructure of mouse embryonic stem cells. Reprod. BioMed. Online 7, 330–335 [DOI] [PubMed] [Google Scholar]
- Baharvand H., Ashtiani S.K., Valojerdi M.R., Shahverdi A., Taee A., and Sabour D. (2004). Establishment and in vitro differentiation of a new embryonic stem cell line from human blastocyst. Differentiation 72, 224–229 [DOI] [PubMed] [Google Scholar]
- Bongso A., and Richards M. (2004). History and perspective of stem cell research. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 827–842 [DOI] [PubMed] [Google Scholar]
- Boyd S., Hooper M., and Wyllie A. (1984). The mode of cell death associated with cavitation in teratocarcinoma-derived embryoid bodies. J. Embryol. Exp. Morph. 80, 63–74 [PubMed] [Google Scholar]
- Calarco P., and McLaren A. (1976). Ultrastructural observations of preimplantation stages of the sheep. J Embryol Exp Morphol. 36, 609–622 [PubMed] [Google Scholar]
- Calarco P.G., and Brown E.H. (1969). An ultrastructural and cytological study of preimplantation development of the mouse. J. Exp. Zool. 171, 253–283 [DOI] [PubMed] [Google Scholar]
- Cech S., and Sedlácková M. (1983). Ultrastructure and morphometric analysis of preimplantation mouse embryos. Cell Tissue Res. 230, 661–670 [DOI] [PubMed] [Google Scholar]
- Dani C., Smith A.G., Dessolin S., Leroy P., Staccini L., Villageois P., Darimont C., and Ailhaud1 G. (1997). Differentiation of embryonic stem cells into adipocytes in vitro. J. Cell Sci. 110, 1279–1285 [DOI] [PubMed] [Google Scholar]
- Doetschman T., Eistetter H., Katz M., Schmidt W., and Kemler R. (1985). The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 87, 27–45 [PubMed] [Google Scholar]
- Ducibella T., Ukena T., Karnovsky M., and Anderson E. (1977). Changes in cell surface and cortical cytoplasmic organization during early embryogenesis in the preimplantation mouse embryo. J. Cell Biol. 74, 153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen E.-L. (2005). Maturation of autophagic vacuoles in mammalian cells. Autophagy 1, 1–10 [DOI] [PubMed] [Google Scholar]
- Evans M.J., and Kaufman M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 [DOI] [PubMed] [Google Scholar]
- Festag M., Viertel B., Steinberg P., and Sehner C. (2007). An in vitro embryotoxicity assay based on the disturbance of the differentiation of murine embryonic stem cells into endothelial cells. II. Testing of compounds. Toxicol. In Vitro 21, 1631–1640 [DOI] [PubMed] [Google Scholar]
- Fraichard A., Chassande O., Bilbaut G., Dehay C., Savatier P., and Samarut J. (1995). In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J. Cell. Sci. 108, 3181–3188 [DOI] [PubMed] [Google Scholar]
- Friel R., Sar S.V.D., and Mee P.J. (2005). Embryonic stem cells: Understanding their history, cell biology and signalling. Adv. Drug Deliv. Rev. 57, 1894–1903 [DOI] [PubMed] [Google Scholar]
- Hoa H.-Y., Moffata R.C., Patela R.V., Awaha F.N., Balouea K., and Crowe D.L. (2010). Embryoid body attachment to reconstituted basement membrane induces a genetic program of epithelial differentiation via jun N-terminal kinase signaling. Stem Cell Res. 5, 144–156 [DOI] [PubMed] [Google Scholar]
- Horwitz E.M. (2003). Stem cell plasticity: The growing potential of cellular therapy. Arch. Med. Res. 34, 600–606 [DOI] [PubMed] [Google Scholar]
- Kawagoe S., Higuchi T., Meng X.L., Shimada Y., Shimizu H., Hirayama R., Fukuda T., Chang H., Nakahata T., Fukada S., Ida H., Kobayashi H., Ohashi T., and Eto Y. (2011). Generation of induced pluripotent stem (iPS) cells derived from a murine model of Pompe disease and differentiation of Pompe-iPS cells into skeletal muscle cells. Mol. Genet. Metab. 104, 123–128 [DOI] [PubMed] [Google Scholar]
- Kramer J., Steinhoff J., Klinger M., Fricke L., and Rohwedel J. (2006). Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation 74, 91–104 [DOI] [PubMed] [Google Scholar]
- Kruse C., Jennifer K., Petschnik A.E., Maab A., Klink E., Rapoport D.H., and Wedel T. (2006). Adult pancreatic stem/progenitor cells spontaneously differentiate in vitro into multiple cell lineages and form teratoma-like structures. Ann. Anat. 188, 503–517 [DOI] [PubMed] [Google Scholar]
- Kruse F., and Völcker H. (1997). Stem cells, wound healing, growth factors, and angiogenesis in the cornea. Curr. Opin. Ophthalmol. 8, 46–54 [DOI] [PubMed] [Google Scholar]
- Kuai X.L., Shao N., Lu H., Xiao S.D., and Zheng Q. (2014). Differentiation of nonhuman primate embryonic stem cells into hepatocyte-like cells. J. Digest. Dis. 15, 27–34 [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U., and Verfaillie C. (2005). Stem cell plasticity. Blood Rev. 19, 29–38 [DOI] [PubMed] [Google Scholar]
- Li S., Harrison D., Carbonetto S., Fässler R., Smyth N., Edgar D., and Yurchenco P.D. (2002). Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. Cell Biol. 157, 1279–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K.C., Chuang W.W., and Lamb D.J. (2003). Stem cell research: The facts, the myths and the promises. J. Urol. 170, 2453–2458 [DOI] [PubMed] [Google Scholar]
- Maltseva V.A., Rohwedela J., Heschelerb J., and Wobus A.M. (1993). Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech. Dev. 44, 41–50 [DOI] [PubMed] [Google Scholar]
- Maro B., Johnson M.H., and Pickering S.J. (1985). Changes in the distribution of membranous organelles during mouse early development. J. Embryol. Exp. Morphol. 90, 287–309 [PubMed] [Google Scholar]
- Martin G.R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma. Proc. Natl. Acad. Sci. USA. 78, 7634–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Hance W.C., Lacorbiere M., Fuller S.J., Evans S.M., Lyons G., Schmidt C., Robbins J., and Chien K.R. (1993). In vitro chamber specification during embryonic stem cell cardiogenesis. Expression of the ventricular myosin light chain_2 gene is independent of heart tube formation. J. Biol. Chem. 268, 25244–22552 [PubMed] [Google Scholar]
- Mittmann J., Kerkis I., Kawashima C., Sukoyan M., Santos E., and Kerkis A. (2002). Differentiation of mouse embryonic stem cells and their hybrids during embryoid body formation. Genet. Mol. Biol. 25, 103–111 [Google Scholar]
- Mohr L., and Trounson A. (1981). Structural changes associated with freezing of bovine embryos. Biol. Reprod. 25, 1009–1025 [DOI] [PubMed] [Google Scholar]
- Mohr L.R., and Trounson A.O. (1982). Comparative ultrastructure of hatched human, mouse and bovine blastocysts. J. Reprod. Fertil. 66, 499–504 [DOI] [PubMed] [Google Scholar]
- Nadijcka M., and Hillman N. (1974). Ultrastructural studies of the mouse blastocyst substages. Embryol. Exp. Morphol. 32, 675–695 [PubMed] [Google Scholar]
- Pierce G.B., and Beals T.F. (1964). The ultrastructure of primordial germinal cells of the fetal testes and of embryonal carcinoma cells of mice. Cancer Res. 24, 1553–1567 [PubMed] [Google Scholar]
- Plante L., and King W. (1994). Light and electron microscopic analysis of bovine embryos derived by in vitro and in vivo fertilization. J. Assist. Reprod. Genet. 11, 515–529 [DOI] [PubMed] [Google Scholar]
- Potts D.M. (1968). The ultrastructure of implantation in the mouse. J. Anat. 103, 77–90 [PMC free article] [PubMed] [Google Scholar]
- Sampath P., Pritchard D.K., Pabon L., Reinecke H., Schwartz S.M., Morris D.R., and Murry C.E. (2008). A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2, 448–460 [DOI] [PubMed] [Google Scholar]
- Sanders J.R.C., Slayton W.B., Cogle C.R., Fisher R.C., and Scott E.W. (2006). Stem cell research. Paediatr. Resp. Rev. 7, 135–140 [DOI] [PubMed] [Google Scholar]
- Sathananthan H., Bongso A., Ng S.-C., Ho J., Mok H., and Ratnam S. (1990). Ultrastructure of preimplantation human embryos co-cultured with human ampullary cells. Hum. Reprod. 5, 309–318 [DOI] [PubMed] [Google Scholar]
- Sathananthan H., Pera M., and Trounson A. (2001). The fine structure of human embryonic stem cells. Reprod. BioMed. Online. 4, 56–61 [DOI] [PubMed] [Google Scholar]
- Sathananthan H., Gunasheela S., and Menezes J. (2003). Critical evaluation of human blastocysts for assisted reproduction techniques and embryonic stem cell biotechnology. Reprod. BioMed. Online. 7, 219–227 embryonic stem cells. Reprodu. BioMed. Online. 4, 56–61 [DOI] [PubMed] [Google Scholar]
- Schlafke S., and Enders A.C. (1963). Observations on the fine structure of the rat blastocyst. J. Anat. Lond. 97, 353–360 [PMC free article] [PubMed] [Google Scholar]
- Schmitt R.M., Bruyns E., and Snodgrass H.R. (1991). Hematopoietic development of embryonic stem cells in vitro: Cytokine and receptor gene expression. Genes Dev. 5, 728–4 [DOI] [PubMed] [Google Scholar]
- Schöler H.R., Hatzopoulos A.K., Balling R., Suzuki N., and Gruss P. (1989). A family of octamer-specific proteins present during mouse embryogenesis: Evidence for germline-specific expression of an Oct factor. EMBO J. 8, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. (2004). Stem Cells Handbook. (Humana Press, Totowa, New Jersey: ) [Google Scholar]
- Shalgi R., and Sherman M.I. (1979). Scanning electron microscopy of the surface of normal and implantation-delayed mouse blastocysts during development in vitro. Dev. Biol. 210, 69–80 [DOI] [PubMed] [Google Scholar]
- Shirasawa S., Yoshie S., Yue F., Ichikawa H., Yokoyama T., Nagai M., Tomotsune D., Hirayama M., and Sasaki K. (2011). Pancreatic exocrine enzyme-producing cell differentiation via embryoid bodies from human embryonic stem cells. Biochem. Biophys. Res. Commun. 410, 608–613 [DOI] [PubMed] [Google Scholar]
- Smith A.G., Heath J.K., Donaldson D.D., G.G. Wong J.M., and Rogers M.S.a.D. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336688–690 [DOI] [PubMed] [Google Scholar]
- Solter D., and Knowles B.B. (1978). Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75, 5565–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Biggers J.D., and Anderson E. (1971). Mitochondria and early development of the mouse. J. Exp. Zool. 176, 179–191 [DOI] [PubMed] [Google Scholar]
- Taha M.F., Valojerdi M.R., Hatami L., and Javeri A. (2012). Electron microscopic study of mouse embryonic stem cell-derived cardiomyocytes. Cytotechnology 64, 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot N.C., and Garrett W.M. (2001). Ultrastructure of the embryonic stem cells of the 8-day pig blastocyst before and after in vitro manipulation: Development of junctional apparatus and the lethal effects of PBS mediated cell–cell dissociation. Anat. Rec. 264, 101–113 [DOI] [PubMed] [Google Scholar]
- Tanaka N., Takeuchi T., Neri Q.V., Sills E.S., and Palermo G.D. (2006). Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: Applications and preliminary results in a murine model. J. Transl. Med. 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J., and Marshall V. (1998). Primate embryonic stem cells. Curr. Top. Dev. Biol. 38, 133–165 [DOI] [PubMed] [Google Scholar]
- Tielens S., Verhasselt B., Liu J., Dhont M., Van Der Elst J., and Cornelissen M. (2006). Generation of embryonic stem cell lines from mouse blastocysts developed in vivo and in vitro: Relation to Oct-4 expression. Reproduction 132, 59–66 [DOI] [PubMed] [Google Scholar]
- Turksen K. (2006). Embryonic Stem Cell Protocols: Volume I: Isolation and Characterization (Methods in Molecular Biology). (Humana Press Inc., Totowa, New Jersey: ) [Google Scholar]
- Vanblerkoma J., Manesa C., and Daniel JC. (1973). Development of preimplantation rabbit embryos in vivo and in vitro: An ultrastructural comparison. Dev. Biol. 35, 262–282 [DOI] [PubMed] [Google Scholar]
- Vittet D., Prandini M.-H., Berthier R., Schweitzer A., Martin-Sisteron H., Uzan G., and Dejana E. (1996). Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood 88, 3424–3431 [PubMed] [Google Scholar]
- Wang R., Clark R., and Bautch V. (1992). Embryonic stem cell-derived cystic embryoid bodies form vascular channels: An in vitro model of blood vessel development. Development 114, 303–316 [DOI] [PubMed] [Google Scholar]
- Wassarman P.M., and Josefowicz W.J. (1978). Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J. Morphol. 156, 209–235 [DOI] [PubMed] [Google Scholar]
- Williams R.L., Hilton D.J., Pease S., Willson T.A., Stewart C.L., Gearing D.P., Wagner E.F., Metcalf D., Nicola N.A., and Gough N.M. (1988). Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687 [DOI] [PubMed] [Google Scholar]
- Wintenberger-Torres S., and Flechon J.-E. (1974). Ultrastructural evolution of the trophoblast cells of the pre-implantation sheep blastocyst from day 8 to day 18. J. Anat. 118, 143–153 [PMC free article] [PubMed] [Google Scholar]
- Wobus A.M. (2001). Potential of embryonic stem cells. Mol. Aspects Med. 22, 149–164 [DOI] [PubMed] [Google Scholar]
- Wobus A.M., Wallukat G., and Hescheler J. (1991). Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 48, 173–182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.