Abstract
Background
Discordance of monozygotic twins for thyroid dysgenesis suggests that epigenetic mechanisms may underlie defects in thyroid gland development. This prompted us to evaluate whether differentially methylated regions (DMRs) can be found between human thyroids (either eutopic or ectopic) and matched leukocytes.
Methods
To compare the genome-wide methylation profile of thyroids and leukocytes, immunoprecipitated methylated DNA was interrogated on human promoter plus CpG island tiling arrays. In addition, the methylation profile of the human FOXE1, PAX8, and NKX2.1 promoter was examined using bisulfite sequencing. Finally, the functional impact of CpG methylation of the promoter on FOXE1 expression was assessed with luciferase assays.
Results
Genome-wide methylation profiling and bisulfite sequencing of CpG islands of PAX8 and NKX2.1 promoters revealed no DMR between thyroid and leukocytes. However, bisulfite sequencing revealed that the methylation level of two consecutive CpG dinucleotides (CpG14 and CpG15, which were not covered by the genome-wide array) in one CpG island of the FOXE1 promoter (−1600 to −1140 from the transcription start site) is significantly higher in leukocytes than in eutopic or ectopic thyroid tissues, suggesting that methylation of this region may decrease FOXE1 gene expression. Indeed, luciferase activities were decreased when FOXE1 promoter constructs were methylated in vitro. Moreover, derepression of luciferase activity was observed when the methylation of CpG14 and CpG15 was prevented by mutations.
Conclusion
We report a tissue-dependent DMR in the FOXE1 promoter. This DMR contains two consecutive CpG dinucleotides, which are epigenetic modifiers of FOXE1 expression in nontumoral tissues.
The transcription factor forkhead box E1 (FOXE1) is a member of the forkhead/winged-helix family and plays an essential role in thyroid morphogenesis (1, 2). In humans, FOXE1 mutations have been identified in a few syndromic cases of athyreosis associated with spiky hair, cleft palate, and sometimes with choanal atresia and bifid epiglottis (3–5). Animal studies have pointed to the critical role of Foxe1 in the embryonic migration of the thyroid. Homozygous Foxe1 knockout mice have either ectopic or absent thyroid gland at embryonic day 1.5, whereas the thyroid is completely absent at birth in all (2). In addition, evidence suggests that the migration of the thyroid bud is a cell-autonomous event requiring the paired box transcription factor 8 (Pax8)-dependent expression of Foxe1in the migrating thyroid cells of mouse embryos (6). In humans, failure of the thyroid precursor cells to migrate from their origin in the primordial pharynx to their final anatomical location (the anterior part of the neck) results in thyroid ectopy (lingual or sublingual), whereas the complete absence of the thyroid (athyreosis) may result either from lack of differentiation or from disappearance of the thyroid before birth.
Ectopy and athyreosis are generally grouped under the term thyroid dysgenesis, which is the most common cause of congenital hypothyroidism. The incidence of congenital hypothyroidism due to thyroid dysgenesis (CHTD) is 1 in 4000 live births (7). Germline mutations in thyroid-related transcription factors NKX2.1 (8, 9), FOXE1 (4), PAX8 (10), and NKX2.5 (11) have been identified by candidate gene screening in a small subset (3%) of patients with sporadic CHTD (12). Linkage analysis has excluded these genes in rare multiplex families with CHTD (13). Indeed, CHTD is predominantly not inherited [98% of cases are nonfamilial (14)], has a high discordance rate of 92% in monozygotic twins, and has a female and ethnic (ie, Caucasian) predominance (15, 16). This, together with evidence of nonpenetrance of mutations in close relatives of patients [eg, NKX2.5 (11)], suggests that modifiers, possibly additional somatic epigenetic or genetic events, are associated with CHTD.
CpG island hypermethylation is reported in many cancers and some differentially methylated regions (DMRs) in cancers are found in regions that are also differentially methylated among different nontumoral tissues (17). Consequently, because FOXE1 CpG islands are known to be hypermethylated in cancers of the skin, pancreas, and breast (18–20), it is plausible that a FOXE1 DMR would account for differential FOXE1 expression in nontumoral tissues. However, our recent integrative molecular analysis of ectopic thyroids did not find any alteration of genomic structure and methylation profile when compared with eutopic thyroids, even though the expression profile differed (21). To find no DMR in a single tissue (thyroid) differing only in its location (ectopic vs eutopic) may be expected, but DMRs are likely to exist between the thyroid and leukocytes, two tissues with different expression profiles. Moreover, CpGs and DMRs are genetic and epigenetic mutational hot spots (22, 23). Thus, finding a DMR within the upstream regulatory region of thyroid-related transcription factors might pave the way for further studies in which cases with CHTD are screened for genetic variants within this DMR. Therefore, in the present study, we set out to determine whether the promoter methylation profile was different between the thyroid and leukocytes using genome-wide and candidate gene approaches (ie, FOXE1, PAX8, and NKX2.1).
Materials and Methods
Detailed descriptions of all experimental protocols are available in the Supplemental Data.
Participant characteristics and tissue collection
We obtained three ectopic and four eutopic thyroids as described previously (21). Because the methylomes of ectopic and eutopic thyroids are similar (21), they were treated as one group for the present analysis. For controls, we used matched leukocytes (when available) from the above-mentioned cases and additional leukocytes from normal subjects (Supplemental Table 1). This study was approved by the Ethics Committee of the Centre Hospitalier Universitaire S Sainte-Justine. All the parents and participants gave written informed consent.
Nucleic acid isolation
Genomic DNA and/or total cellular RNA were isolated from cell lines, thyroid tissues, and matched leukocytes using a pureLink genomic DNA minikit (Life Technologies) and an RNeasy minikit (QIAGEN), respectively.
Methylation profiling by methylated DNA immunoprecipitation (MeDIP) and MeDIP-chip
The MeDIP-chip was performed using pairs of enriched methylated fraction and normal fraction of genomic DNA (gDNA) from four thyroids and five leukocytes (four matched; see Supplemental Table 1). The methylated fraction of gDNA was enriched using the MeDIP assay (24) and interrogated on human promoter plus CpG island tiling arrays (Roche NimbleGen) as described previously (21).
Cell culture
The human thyroid cancer cell line WRO was a gift from Dr H. Mircescu (University of Montréal), the human leukemia cell lines Jurkat (T cell acute lymphoblastic leukemia), K562 (chronic myelogenous leukemia), and REH (acute lymphoblastic leukemia of the non-T, non-B type) were a gift from Dr A. Ahmad (University of Montréal), the rat follicular thyroid PCCL3 cell line was a gift from Dr F. Miot (Université Libre de Bruxelles, Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire, Brussels, Belgium) and the immortalized human thyroid Nthy-ori 3–1 cells were obtained from Sigma. Culture media and conditions are explained in the Supplemental Data.
Bisulfite gDNA sequencing and methylation analysis
Bisulfite treatment of gDNA was performed using the Zymo EZ DNA methylation-gold kit (Zymo Research). The targeted promoter region of FOXE1 [−1600 to −125 from transcription start site (TSS)], PAX8 (−635 to −471 from ATG), and NKX2.1 (−343 to −107 from ATG) were subsequently amplified with a nested PCR protocol in which two sets of forward and reverse primers were used. Primers designed using the MethPrimer software (www.urogene.org/methprimer/), reagents, and PCR conditions are listed in the Supplemental Table 2.
Construction of luciferase reporter vectors
A pGL3-Basic plasmid incorporating a 2.38-kb fragment that includes the 5′-upstream regulatory region of the human FOXE1 (hFOXE1) gene from −1934 to +446 relative to the transcriptional start site (+1) was a generous gift from Dr T. Eichberger (University of Salzburg, Salzburg, Austria). A deletion construct missing CpG island 1 was generated by restriction enzyme digestion using AflII/NsiI followed by blunt-end ligation. The two CpGs 14 and 15 within CpG island 1 located at −1417 and −1412 relative to TSS were mutated using the QuikChange site-directed mutagenesis kit (Stratagene). Primers are reported in the Supplemental Data.
In vitro global methylation assay
For the in vitro methylation of pGL3-Basic and the different human FOXE1 promoter constructs, whole plasmids were methylated using the site-specific CpG methyltransferase M.SssI (New England Biolabs). Mock-methylated plasmids were subjected to the same treatment in the absence of M.SssI. After the methylation, the plasmids were purified using the EZ-10 spin column DNA gel extraction kit (Bio Basic). The extent of the methylation was subsequently verified by digestion with the CpG methylation-sensitive restriction enzyme HpaII (New England Biolabs) and its isoschizomer the CpG methylation-insensitive MspI (Thermo Scientific). Only completely methylated (M.SssI+) and mock-methylated (M.SssI−) plasmids were then used in the transfection experiments.
In vitro regional (patch) methylation assay
To allow the integration of methylated fragment of CpG island 1 in unmethylated plasmids, NdeI and NsiI restriction sites were engineered into both the wild-type and point-mutated FOXE1 promoter fragments using the QuikChange site-directed mutagenesis kit (Stratagene). The efficiency of ligation and equivalence of incorporated DNA into the methylated and mock-methylated constructs were confirmed by agarose gel electrophoresis as published elsewhere (25). In addition, the ligation reactions were transformed into chemically competent Escherichia coli to assess the ligation efficiency. To obtain optimal readouts, luciferase assays were performed in the immortalized human thyroid Nthy-ori 3–1 cells. See Supplemental Figure 1 and the detailed protocol listed in the Supplemental Data.
Transient transfection and luciferase reporter gene assay
Rat follicular thyroid PCCL3 cells were transiently transfected with polyethylenimine (PEI; Polysciences) in triplicate in 24-well tissue culture plates (Corning). The volume of PEI used is based on a 4:1 ratio of PEI (micrograms): to total plasmid DNA (micrograms). Cells were seeded at a density of 0.3 × 105 cells/well 48 hours prior to transfection. Cells were transfected with 0.32 μg/well of the methylated or mock-methylated reporter vector pGL3-Basic (Promega) as well as the reporter plasmids including the different FOXE1 promoter inserts. To correct for transfection efficiency and variation in cell viability between wells, cells were cotransfected with 0.005 μg/well Renilla luciferase reporter vector (pRL-TK; Promega) as an internal control. The activities of the firefly luciferase and the Renilla luciferase were measured in cell lysates 24 hours after transfection using the dual-luciferase reporter assay system (Promega). Luminescence was detected by the 2104 EnVision multilabel plate reader (PerkinElmer). For the regional methylation, each of the religated methylated and mock methylated wild-type and point mutated plasmids (0.75 μg DNA) were transfected in Nthy-ori 3–1 cells (0.1 × 105 cells/well), plated 48 hours before transfection, using X-tremeGENE 9 (Roche Diagnostics). In each experiment performed in triplicate, the pRL-TK plasmid (0.25 μg) was cotransfected for normalization purposes. Luminescence was measured 48 hours after transfection using the dual-luciferase reporter assay system (Promega).
Semiquantitative RT-PCR
The forkhead box E1 gene (FOXE1, NCBI reference sequence NT_008470.19) mRNA expression was examined by semiquantitative RT-PCR in two ectopic lingual thyroids, three orthotopic thyroids (flash frozen healthy tissue adjacent to papillary thyroid carcinomas), and leukocytes from normal subjects. In addition, FOXE1 mRNA expression was determined in the thyroid cancer WRO cell line and the human leukemia cell lines Jurkat, K562, and REH. Primer sequences are reported in the Supplemental Data.
Statistical analysis
All data are reported as means ± SEM. The data were subjected to Fisher’s exact test or unpaired two-tailed Student’s t test, with correction for multiple comparisons with the Holm-Sidak method, when appropriate. A value of P ≤ .05 or lower was considered statistically significant.
Results
Lack of tissue-specific methylation differences in CpG islands of PAX8 and NKX2.1 promoter
No methylation profile differences were observed in the CpG-rich region of the PAX8 (−635 to −471 from ATG) and NKX2.1 (−343 to −107 from ATG) promoters using either MeDIP arrays or bisulfite sequencing (Supplemental Figure 2).
A CpG island of the FOXE1 promoter is differentially methylated in human thyroid tissue when compared with matched leukocyte DNA
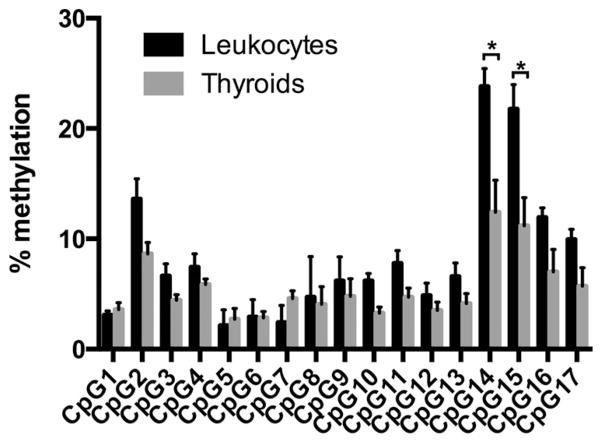
To determine whether the CpG-rich 5′-flanking region of the FOXE1 gene (−1600 to −125 from transcription start site; see Figure 1) show differences in methylation between tissues with different levels of FOXE1 expression, we used bisulfite sequencing of five thyroids, four of which were paired with leukocytes from the same donors. Findings were validated with bisulfite pyrosequencing on four thyroids and leukocyte tissues (three pairs) (Supplemental Table 1). Bisulfite sequencing revealed the presence of a DMR at CpG island 1 (−1600 to −1140 from TSS, +1) that was globally more methylated in leukocytes (10%) compared with thyroids (3%, P < .001, two sided exact Fischer’s test). Indeed, we found two consecutive CpG dinucleotides [ie, CpG14 and CpG15, located −1417 and −1412 relative to TSS (+1)] with a significantly higher methylation rate in leukocytes (51% for CpG14 and 43% for CpG15) when compared with that in thyroids (ie, 5%; P < .001, two sided exact Fischer’s test) (Figure 2). These results were validated with bisulfite pyrosequencing, which showed an average methylation rate of 24% for GpC14 and 22% for GpC15 in leukocytes, whereas the thyroid methylation rate was 12% for GpC14 and 11% for GpC15 (P < .05, two sided paired t test, corrected for multiple comparison with Holm-Sidak method) (Figure 3). The other CpG island (CpG island 2; see Figure 1) encompassing the transcription start site of FOXE1 promoter showed no differential methylation (see Supplemental Figure 3), and no differences were observed at methylation-sensitive restriction enzyme sites close to the ATG (not shown). Of note, the dinucleotide stretches CpG7-GpC9 and CpG14-GpC17 were not covered by the probes of the CpG island tiling array, which explains why these subtle differences were not detected by this technology.
Figure 1.
CpG methylation profile of the human FOXE1 promoter. A, CpG methylation profile was determined using European Molecular Biology Open Software Suite (EMBOSS) CpGblot (http://www.ebi.ac.uk/tools/emboss/). B, Schematic representation of the FOXE1 promoter, numbered from the TSS (+1), with the CpG island 1 (−1600 to −1140 from TSS). C, Nucleotide sequence of CpG island 1, with CpG dinucleotides numbered; CpG14 and CpG15 are highlighted in a box.
Figure 2.
DNA methylation status of CpG island 1 (−1600 to −1140 from the TSS) in the 5′-untranslated region of the FOXE1 gene using bisulfite sequencing. A and B, CpG island of FOXE1 (−1600 to −1140 from TSS, +1) is globally more methylated in leukocytes (10%) compared with the thyroids (3%, P < .001). In addition, two CpG dinucleotides (ie, CpG14 and CpG15, located −1417 and −1412 relative to TSS) show higher methylation in leukocytes (51% for CpG14 and 43% for CpG15) when compared with thyroids (5%; P < .001). C, Genomic DNA was extracted from four different human cell lines and subjected to sodium bisulfite sequencing to analyze the methylation profile of CpG island 1 of the FOXE1 promoter. Seven (REH cells) to 14 (other cell lines) different clones were sequenced. All leukemia cell lines showed significantly higher CpG island 1 methylation (Jurkat, 51%; K562, 15%; and REH, 86%) when compared with the thyroid cell line WRO (4.4% methylation; P < .001 when compared with each leukemia cell line); this methylation difference is even more pronounced for CpG14 and CpG15 (Jurkat, 100%; K562 39%; REH 100% compared with 0% in WRO). Each line represent the sequencing results of distinct tissues or cell lines. Circles represent the 33 CpG dinucleotides of CpG island 1, which are labeled as follows: black, methylation greater than 75%; dark gray, methylation of 50%–75%; gray, methylation of 10%–49%; white, methylation less than 10%. Numbers of clones analyzed for each tissue or cell line are listed on the right of the figure.
Figure 3.
Confirmation of DNA methylation status by bisulfite pyrosequencing. The methylation profile of the first 17 CpG dinucleotides in CpG island 1 was analyzed using sodium bisulfite pyrosequencing. The figure represents the means ± SEM percentage of DNA methylation of the 17 CpG dinucleotides in leukocytes (in black) vs thyroid tissues (in gray). *, P < .05, two-tailed paired t test, corrected for multiple comparisons with the Holm-Sidak method.
The methylation status of this DMR was also analyzed in bisulfite-treated DNA from the human leukemia cell lines (Jurkat, K562, and REH) as well as from the thyroid cancer cell line WRO. The human leukemia cell lines Jurkat and REH exhibited a high percentage of DMR methylation (51% and 86%, respectively), whereas a moderate percentage of methylation (15%) was detected in K562 cells. On the other hand, the DMR in the thyroid cancer WRO cells was almost unmethylated (4%, P < .001), which represented a typical and significant decrease of methylation when compared with each of the leukemia cell lines (Figure 2).
No expression of FOXE1 was detected in normal leukocytes and human leukemia cell lines
Because altering DNA methylation in CpG islands is an essential mechanism involved in the regulation of gene expression, we examined whether the methylation pattern of the detected DMR in thyroids and leukocytes is implicated in the transcriptional status of FOXE1. Interestingly, the lack of FOXE1 expression was observed in leukocytes that exhibited a global hypermethylation of the DMR in comparison to thyroids (Figure 4A). As expected from the findings in normal leukocytes, no expression of FOXE1 was detected in the three leukemia cell lines (Jurkat, K562, and REH) used in the present study (Figure 4B). On the other hand, FOXE1 expression was detected in all thyroid tissues (mean FOXE1 to γ-actin ratio of 1.06, range 0.95–1.32, normalized to that of ectopic thyroid tissue number 3) (Figure 4A). In contrast, in the dedifferentiated thyroid cancer WRO cells, which show a globally unmethylated DMR (Figure 2C), FOXE1 expression was still detected but was about 2.5-fold lower than in thyroid tissues (Figure 4A), which is consistent with previous work (21, 26) and implies that the low expression levels of FOXE1 in WRO can be attributed to other controlling mechanisms, such as partial repression through the polycomb repressive complex 2 (PRC2), as suggested by our chromatin immunoprecipitation (ChIP) assays (Supplemental Figure 4). Altogether, methylation of CpG sites within the DMR correlates with the expression of FOXE1.
Figure 4.
Semiquantitative RT-PCR analysis of FOXE1 expression. FOXE1 RT-PCR in normal leukocytes and thyroid tissues (A), in human leukemia cell lines (Jurkat, K562, and REH), and in thyroid cancer WRO cells (B). The numbers denote the ratio of FOXE1: γ-actin values, normalized to that of ectopic thyroid tissue number 3 (set as 1.0).
DNA methylation decreases transcription from the FOXE1 promoter
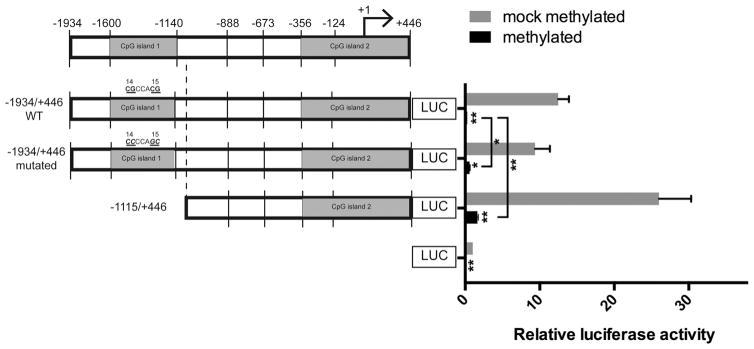
To investigate whether the methylation of the 5′-upstream region of FOXE1 gene has an impact on transcription from this promoter, a 5′ deletion reporter gene construct (the −1115/+446 bp deletion construct, from which CpG island 1 was deleted), and constructs with wild-type and mutated CpG14-CpG15 were in vitro methylated using the CpG methyltransferase M.SssI (Figures 1 and 5). First, transfection of the mock-methylated construct with deletion of the CpG island 1 showed a marked increase of the luciferase expression (Figure 5), suggesting that transcriptional repressor may target the CpG island 1, which was confirmed by our ChIP assays targeting SUZ12 (a core protein of the PRC2) in immunoprecipitated chromatin of WRO cells (Supplemental Figure 3). Then transfection with the methylated and mock-methylated promoter constructs showed a profound decrease of luciferase expression levels upon methylation (Figure 5). Notably, the methylated constructs in which CpG14 and CpG15 were either point mutated or deleted (−1115/+446 bp deletion construct) exhibited a significant increase in luciferase activity (0.6- and 1.6-fold of the basic construct activity, respectively) compared with that of the methylated wild-type construct (0.27-fold of the basic construct activity, P < .05).
Figure 5.
Effect of global CpG methylation on FOXE1 promoter activity. FOXE1 promoter activity was tested in PCCL3 cells using either M.SssI methylated constructs (black bars) or mock methylated constructs (gray bars). Values are expressed as fold of the basic empty vector. Data represent means ± SEM of three independent experiments, each in triplicate. *, P <.05; **, P < .01 (Student’s t test). All methylated constructs showed significant decreased luciferase activity. Methylated constructs with CpG14-CpG15 mutations and with CpG island 1 deletion showed a significant derepression when compared with the methylated WT construct.
Although these results indicate that the promoter activity of FOXE1 can be suppressed by DNA methylation, the observed decrease in luciferase activities could be due in part to methylation within the pGL3-Basic vector itself (27, 28) (Figure 5). Therefore, experiments in which only CpG island 1 (either wild type or mutated) was methylated (ie, regional patch methylation) and ligated into unmethylated pGL3-Basic vector were carried out to confirm the impact of methylation of CpG island 1 on FOXE1 transcription. Indeed, regional methylation of CpG island 1 induced a significant decrease in luciferase activity (a decrease of 33%, from 3.27- to 2.19-fold of the basic construct, P < .05; 48% if corrected for the background obtained for transfections with empty basic vector), whereas regional methylation of the CpG island 1 construct with mutated CpG14 and CpG15 did not affect FOXE1 expression (Figure 6).
Figure 6.
Effect of regional methylation of the DMR on FOXE1 promoter activity. FOXE1 promoter activity was tested in Nthy-ori 3–1 cells using either M.SssI regionally methylated constructs (black bars) or mock methylated constructs (gray bars). Values are expressed as fold of the basic empty vector. Data represent means ± SEM of two independent experiments, each in triplicate. *, P < .05; **, P < .01 (n.s., nonsignificant; Student’s t test). Regional methylation of CpG island 1 encompassing the wild-type CpG14 and CpG15 (at −1417 and −1412) leads to a significant decrease by reducing porter gene activity by 33% when compared with mock methylated control (48% if corrected for the background obtained for transfections with empty basic vector). Regional methylation of the CpG island 1 construct with mutated CpG14 and CpG15 induced no difference between methylated and mock methylated constructs.
Discussion
Herein we examined the impact of the tissue-dependent differential methylation on the expression of FOXE1. We found a DMR in the FOXE1 promoter when comparing thyroid and leukocytes tissues. We then showed that the FOXE1 expression is repressed upon methylation of this DMR and that the methylation of two specific consecutive CpG dinucleotides suffices to decrease FOXE1 expression.
The role of DNA methylation in the regulation of tissue-specific gene expression has been previously reported (29, 30). Several genome-wide studies have shown that distinct regions of the mammalian genome exhibit a tissue-dependent pattern of DNA methylation and are increasingly reported to be associated with tissue-specific gene activity (31–36). These tissue-specific DMRs are found in either CpG-rich or -poor DNA sequences (37). In a recent attempt to identify DMRs in humans, it was shown that DMRs are enriched in promoter regions of genes exhibiting tissue-specific functions (promoter-like DMRs) (38). Hence, DNA methylation of promoters can be implicated in major cell lineage determination. On the other hand, DMRs enriched in enhancer elements (enhancer-like DMRs) are identified as cell type-specific DMRs. Thus, DNA methylation is a possible mechanism, allowing cells to attain final lineage commitment or maintain a distinct cell type. Collectively a tight association between differences in DNA methylation involving gene regulatory elements (promoters and enhancers) and gene activity has been established (38). Of note, transcriptional repression is not linearly related to methylation (39). Indeed, a tissue-dependent and gene-specific methylation threshold is required to attenuate gene expression (40), which explains why low levels of methylation of FOXE1 DMR did not hamper FOXE1 expression in thyroid tissue (Figures 3 and 4).
Abnormal methylation patterns of the FOXE1 gene has been previously reported in primary pancreatic carcinomas as well as in pancreatic cancer cell lines. Expression of FOXE1 was induced upon treatment with the demethylating agent 5-aza-2′-deoxycytidine in pancreatic cell lines and not in unmethylated nonneoplastic cells (19). Moreover, FOXE1 hypermethylation in tumor-derived DNA released into the bloodstream of patients with breast cancer has also been described (20). Kuang et al (41) determined the methylation status of FOXE1 in Jurkat, K562, and REH cells, among other leukemia cell lines, using methylated CpG island amplification coupled to representational differential analysis or a DNA promoter microarray and validated their findings using bisulfite pyrosequecing. FOXE1 was found to be aberrantly hypermethylated in leukemia cell lines when compared with normal peripheral lymphocytes (controls). The genomic region within the FOXE1 promoter that was found to be hypermethylated in the above-mentioned study is located within the region from −673 to −124 from the TSS. According to our data, this region was not differentially methylated between leukocytes and thyroid. For that reason, we have not determined the methylation status of the region from −673 to −124 from TSS in leukemia cell lines. On the other hand, the DMR that we identified (−1600 to −1140 from the TSS or CpG island 1) was not covered on the proximal promoter microarray, covering −1.0 kb upstream and +0.3 kb downstream from the TSS, used in the study of Kuang et al (41). Recently Venza et al (18) have shown that hypermethylation of CpG islands located within the promoter of FOXE1 gene is frequent among patients with cutaneous simple squamous carcinoma. Although these authors did not specify the site of promoter methylation related to transcriptional repression, both the concordance between the methylation status of the FOXE1 promoter and its mRNA expression together with the reactivation of expression upon treatment with 5-Aza-dc point to the involvement of DNA methylation in the transcriptional regulation of FOXE1 in simple squamous carcinoma (18). Moreover, a marked hypermethylation was found at the TSS of the FOXE1 gene in adenoid cystic carcinoma of the salivary glands, suggesting the association of FOXE1 aberrant methylation with the development and progression of adenoid cystic carcinoma (42).
Herein upon transfection with different globally methylated FOXE1 promoter constructs, the luciferase activities were significantly reduced compared with the corresponding mock-methylated constructs. Given that the luciferase gene in the plasmids can also be methylated by M.SssI methylase (27, 28) and that regions outside CpG island 1 (ie, the T-DMR of the FOXE1 promoter) were mainly unmethylated in leukocytes (Supplemental Figure 3), further regional (patch) methylation was carried out to assess the specific impact of CpG island 1 methylation on the FOXE1 expression. In this regard, the regional methylation of CpG island 1 encompassing wild-type CpG14 and CpG15 (at −1417 and −1412) leads to a significant decrease in reporter gene activity when compared with mock-methylated control.
In contrast, no significant difference in the activity was observed upon point mutating the two CpG dinucleotides of interest, which indicates their role in regulating the expression of the FOXE1 gene. This is consistent with the previously reported involvement of site-specific methylation in tissue or cell-specific gene expression. Grant et al (43) have shown that the methylation status of three promoter CpG dinucleotides (−22, −54, and −455) is altered in a tissue-specific manner and that lactoferrin expression was detected in tissues exhibiting at least two of three unmethylated CpG dinucleotides. Similarly, Boatright et al (44) have shown that tissue- and site-specific methylation of the two CpG dinucleotides (−725 and −115) of the murine interphotoreceptor retinoid binding protein (IRBP) promoter decreased the promoter activity in vitro and correlated with IRBP expression in vivo. Moreover, repression of transcription mediated via single-site methylation in promoter regions has been previously reported within the promoter region of the Herpes simplex virus thymidine kinase (tk) gene (45), the calcium-binding protein gene S100A2 (46), the p16 gene in human bladder cell lines (47), the alternative reading frame (ARF) gene promoter (48), and the p53 gene promoter (49). Collectively, these observations underline the ability of methylation at specific CpG sites to efficiently repress transcriptional activity.
Generally, CpG methylation contributes to transcriptional suppression by directly preventing ubiquitous transcriptional regulators from binding to their target gene promoters (50) or by binding of methyl-CpG-binding proteins that subsequently recruit repressive complexes such as histone deacetylases that lead to chromatin compaction and in turn transcriptional repression of the gene (51). Among the methyl-CpG-binding protein, methyl-CpG-binding protein 2 has been shown to bind to as few as one to three methylated cytosines (52), thus supporting the notion that site-specific methylation of CpG dinucleotides is involved in transcriptional repression. Whether the mechanism via which CpG14 and CpG15 mediate the differential expression of FOXE1 involves the recruitment of methyl-CpG-binding proteins or direct blocking of transcription factors binding needs further investigation.
Our luciferase assay also suggests that the unmethylated FOXE1 CpG island 1 binds to a transcriptional repressor (Figure 5). ChIP assays revealed that this repressor is the polycomb repressive complex 2, in which suppressor of zeste 12 (SUZ12, target of ChIP) is a core component (Supplemental Figure 4A). This result is consistent with SUZ12-ChIP sequencing data from ENCODE (http://genome.ucsc.edu/), showing SUZ12 binding in this region of the FOXE1 promoter (Supplemental Figure 4B). Unmethylayed CpG islands have a key role in polycomb complex recruitment, and most PRC2 target genes actually remain constitutively unmethylated throughout development (53, 54). Some genes are de novo repressed by PRC2-mediated methylation on H3K27 (such as WRO), but subsequently only a subset of these genes become definitively methylated and lose their epigenetic plasticity (54). This suggests that there are additional (as yet unknown) factors required for definitive DNA methylation (54).
In conclusion, the main outcome of the present study supports the concept that DNA methylation plays a role in the differential expression of the thyroid transcription factor FOXE1 in normal leukocytes (cells that do not express FOXE1) and in the thyroid a tissue that abundantly expresses FOXE1. Evidence for the role of DNA methylation mainly depends on the CpG methylation profiling of human FOXE1 that exhibited the presence of a DMR in its 5′-flanking regulatory region. One limitation of the present study is the low number of thyroid samples included that were obtained from female in the age range of 8–18 years. Tissue-specific, age-related DMRs have been reported in humans (55). Of note, over the 490 age-related DMRs found through epigenome-wide scans by Bell et al (55), none were found in FOXE1, PAX8, and NKX2.1. Moreover, within this limited age range (8–18 y), we saw no difference in methylation pattern among the different thyroid tissues investigated. In addition to age, sex influences genome-wide methylation in humans (56). Indeed, Liu et al (56) assessed the genome-wide methylation profile of 20 493 CpG sites and found 690 sex-related DMRs in 432 genes (421 on X chromosome, 11 genes on autosomes); none were found in FOXE1, PAX8, and NKX2.1. Consequently, there is currently no evidence that age or sex might have an impact on our results. However, further studies will determine whether these results are observed in a wider range of tissues (in terms of age and sex) and will assess whether rare genetic variants in the FOXE1 DMRs are associated with congenital hypothyroidism due to thyroid ectopy.
Supplementary Material
Acknowledgments
We thank the patients and their parents for their cooperation and Jean Paquette for technical assistance.
This work was supported by a joint scholarship from the Egyptian Ministry of Higher Education and the University of Montréal and by a doctoral grant from the Fondation des Étoiles and the Sainte Justine University Hospital Foundation (to R.A.-K.) and by Grant MOP-130390 from the Canadian Institutes of Health Research (to J.D.); Research in Pediatric Thyroid Diseases at Centre Hospitalier Universitaire Sainte Justine is supported by the Girafonds/Fondation du Centre Hospitalier Universitaire Sainte Justine (to J.D. and G.V.V.).
Abbreviations
- ChIP
chromatin immunoprecipitation
- CHTD
congenital hypothyroidism due to thyroid dysgenesis
- DMR
differentially methylated region
- FOXE1
forkhead box E1
- gDNA
genomic DNA
- MeDIP
methylated DNA immunoprecipitation
- Pax8
paired box transcription factor 8
- PEI
polyethylenimine
- PRC2
polycomb repressive complex 2
- TSS
transcription start site
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R. Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn. 2002;224:450–456. doi: 10.1002/dvdy.10118. [DOI] [PubMed] [Google Scholar]
- 2.De Felice M, Ovitt C, Biffali E, et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–398. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- 3.Bamforth JS, Hughes IA, Lazarus JH, Weaver CM, Harper PS. Congenital hypothyroidism, spiky hair, and cleft palate. J Med Genet. 1989;26:49–51. doi: 10.1136/jmg.26.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifton-Bligh RJ, Wentworth JM, Heinz P, et al. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet. 1998;19:399–401. doi: 10.1038/1294. [DOI] [PubMed] [Google Scholar]
- 5.Castanet M, Park SM, Smith A, et al. A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum Mol Genet. 2002;11:2051–2059. doi: 10.1093/hmg/11.17.2051. [DOI] [PubMed] [Google Scholar]
- 6.Parlato R, Rosica A, Rodriguez-Mallon A, et al. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol. 2004;276:464–475. doi: 10.1016/j.ydbio.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Deladoey J, Ruel J, Giguere Y, Van Vliet G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Quebec. J Clin Endocrinol Metab. 2011;96:2422–2429. doi: 10.1210/jc.2011-1073. [DOI] [PubMed] [Google Scholar]
- 8.Pohlenz J, Dumitrescu A, Zundel D, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krude H, Schutz B, Biebermann H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2–1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macchia PE, Lapi P, Krude H, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19:83–86. doi: 10.1038/ng0598-83. [DOI] [PubMed] [Google Scholar]
- 11.Dentice M, Cordeddu V, Rosica A, et al. Missense mutation in the transcription factor NKX2–5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91:1428–1433. doi: 10.1210/jc.2005-1350. [DOI] [PubMed] [Google Scholar]
- 12.Narumi S, Muroya K, Asakura Y, Adachi M, Hasegawa T. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J Clin Endocrinol Metab. 2010;95:1981–1985. doi: 10.1210/jc.2009-2373. [DOI] [PubMed] [Google Scholar]
- 13.Castanet M, Sura-Trueba S, Chauty A, et al. Linkage and mutational analysis of familial thyroid dysgenesis demonstrate genetic heterogeneity implicating novel genes. Eur J Hum Genet. 2005;13:232–239. doi: 10.1038/sj.ejhg.5201321. [DOI] [PubMed] [Google Scholar]
- 14.Castanet M, Lyonnet S, Bonaiti-Pellie C, Polak M, Czernichow P, Leger J. Familial forms of thyroid dysgenesis among infants with congenital hypothyroidism. N Engl J Med. 2000;343:441–442. doi: 10.1056/NEJM200008103430614. [DOI] [PubMed] [Google Scholar]
- 15.Perry R, Heinrichs C, Bourdoux P, et al. Discordance of monozygotic twins for thyroid dysgenesis: implications for screening and for molecular pathophysiology. J Clin Endocrinol Metab. 2002;87:4072–4077. doi: 10.1210/jc.2001-011995. [DOI] [PubMed] [Google Scholar]
- 16.Stoppa-Vaucher S, Van Vliet G, Deladoey J. Variation by ethnicity in the prevalence of congenital hypothyroidism due to thyroid dysgenesis. Thyroid. 2011;21:13–18. doi: 10.1089/thy.2010.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varley KE, Gertz J, Bowling KM, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23:555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venza I, Visalli M, Tripodo B, et al. FOXE1 is a target for aberrant methylation in cutaneous squamous cell carcinoma. Br J Dermatol. 2010;162:1093–1097. doi: 10.1111/j.1365-2133.2009.09560.x. [DOI] [PubMed] [Google Scholar]
- 19.Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–3742. [PubMed] [Google Scholar]
- 20.Weisenberger DJ, Trinh BN, Campan M, et al. DNA methylation analysis by digital bisulfite genomic sequencing and digital Methy-Light. Nucleic Acids Res. 2008;36:4689–4698. doi: 10.1093/nar/gkn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Khudir R, Paquette J, Lefort A, et al. Transcriptome, methylome and genomic variations analysis of ectopic thyroid glands. PLoS One. 2010;5:e13420. doi: 10.1371/journal.pone.0013420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaudet AL, Jiang YH. A rheostat model for a rapid and reversible form of imprinting-dependent evolution. Am J Hum Genet. 2002;70:1389–1397. doi: 10.1086/340969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper DN, Mort M, Stenson PD, Ball EV, Chuzhanova NA. Methylation-mediated deamination of 5-methylcytosine appears to give rise to mutations causing human inherited disease in CpNpG tri-nucleotides, as well as in CpG dinucleotides. Hum Genomics. 2010;4:406–410. doi: 10.1186/1479-7364-4-6-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber M, Davies JJ, Wittig D, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 25.McGowan PO, Sasaki A, D’Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Staveren WC, Solis DW, Delys L, et al. Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. Cancer Res. 2007;67:8113–8120. doi: 10.1158/0008-5472.CAN-06-4026. [DOI] [PubMed] [Google Scholar]
- 27.Deng G, Chen A, Pong E, Kim YS. Methylation in hMLH1 promoter interferes with its binding to transcription factor CBF and inhibits gene expression. Oncogene. 2001;20:7120–7127. doi: 10.1038/sj.onc.1204891. [DOI] [PubMed] [Google Scholar]
- 28.DiNardo DN, Butcher DT, Robinson DP, Archer TK, Rodenhiser DI. Functional analysis of CpG methylation in the BRCA1 promoter region. Oncogene. 2001;20:5331–5340. doi: 10.1038/sj.onc.1204697. [DOI] [PubMed] [Google Scholar]
- 29.Futscher BW, Oshiro MM, Wozniak RJ, et al. Role for DNA methylation in the control of cell type specific maspin expression. Nat Genet. 2002;31:175–179. doi: 10.1038/ng886. [DOI] [PubMed] [Google Scholar]
- 30.Ching TT, Maunakea AK, Jun P, et al. Epigenome analyses using BAC microarrays identify evolutionary conservation of tissue-specific methylation of SHANK3. Nat Genet. 2005;37:645–651. doi: 10.1038/ng1563. [DOI] [PubMed] [Google Scholar]
- 31.Song F, Smith JF, Kimura MT, et al. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proc Natl Acad Sci USA. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckhardt F, Lewin J, Cortese R, et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006;38:1378–1385. doi: 10.1038/ng1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khulan B, Thompson RF, Ye K, et al. Comparative isoschizomer profiling of cytosine methylation: the HELP assay. Genome Res. 2006;16:1046–1055. doi: 10.1101/gr.5273806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitamura E, Igarashi J, Morohashi A, et al. Analysis of tissue-specific differentially methylated regions (TDMs) in humans. Genomics. 2007;89:326–337. doi: 10.1016/j.ygeno.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Illingworth R, Kerr A, Desousa D, et al. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maunakea AK, Nagarajan RP, Bilenky M, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohgane J, Yagi S, Shiota K. Epigenetics: the DNA methylation profile of tissue-dependent and differentially methylated regions in cells. Placenta. 2008;29(suppl A):S29–S35. doi: 10.1016/j.placenta.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Zhou Y, Lin N, et al. Functional DNA methylation differences between tissues, cell types, and across individuals discovered using the M&M algorithm. GenomeRes. 2013;23:1522–1540. doi: 10.1101/gr.156539.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curradi M, Izzo A, Badaracco G, Landsberger N. Molecular mechanisms of gene silencing mediated by DNA methylation. Mol Cell Biol. 2002;22:3157–3173. doi: 10.1128/MCB.22.9.3157-3173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JS, Kim KH, Jeon YK, et al. Promoter hypermethylation of the ADAM23 gene in colorectal cancer cell lines and cancer tissues. Int J Cancer. 2009;124:1258–1262. doi: 10.1002/ijc.24023. [DOI] [PubMed] [Google Scholar]
- 41.Kuang SQ, Tong WG, Yang H, et al. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leukemia. 2008;22:1529–1538. doi: 10.1038/leu.2008.130. [DOI] [PubMed] [Google Scholar]
- 42.Bell A, Bell D, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117:2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant DJ, Shi H, Teng CT. Tissue and site-specific methylation correlates with expression of the mouse lactoferrin gene. J Mol Endocrinol. 1999;23:45–55. doi: 10.1677/jme.0.0230045. [DOI] [PubMed] [Google Scholar]
- 44.Boatright JH, Nickerson JM, Borst DE. Site-specific DNA hypomethylation permits expression of the IRBP gene. Brain Res. 2000;887:211–221. doi: 10.1016/s0006-8993(00)02990-5. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Hattar J, Jiricny J. Methylation of single CpG dinucleotides within a promoter element of the Herpes simplex virus tk gene reduces its transcription in vivo. Gene. 1988;65:219–227. doi: 10.1016/0378-1119(88)90458-1. [DOI] [PubMed] [Google Scholar]
- 46.Wicki R, Franz C, Scholl FA, Heizmann CW, Schafer BW. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium. 1997;22:243–254. doi: 10.1016/s0143-4160(97)90063-4. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalgo ML, Hayashida T, Bender CM, et al. The role of DNA methylation in expression of the p19/p16 locus in human bladder cancer cell lines. Cancer Res. 1998;58:1245–1252. [PubMed] [Google Scholar]
- 48.Robertson KD, Jones PA. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol Cell Biol. 1998;18:6457–6473. doi: 10.1128/mcb.18.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pogribny IP, Pogribna M, Christman JK, James SJ. Single-site methylation within the p53 promoter region reduces gene expression in a reporter gene construct: possible in vivo relevance during tumorigenesis. Cancer Res. 2000;60:588–594. [PubMed] [Google Scholar]
- 50.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 51.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 52.Nan X, Meehan RR, Bird A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993;21:4886–4892. doi: 10.1093/nar/21.21.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch MD, Smith AJ, De Gobbi M, et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate Polycomb complex recruitment. EMBO J. 2012;31:317–329. doi: 10.1038/emboj.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 55.Bell JT, Tsai PC, Yang TP, et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012;8:e1002629. doi: 10.1371/journal.pgen.1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS One. 2010;5:e10028. doi: 10.1371/journal.pone.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.