Abstract
Patients undergoing dialysis are particularly vulnerable to methicillin-resistant Staphylococcus aureus (MRSA) infections. We performed a meta-analysis of published studies to estimate the prevalence of MRSA colonization in dialysis patients, time trends, and long-term risk of subsequent MRSA infections. Our search of the PubMed and Embase databases returned 5743 nonduplicate citations, from which we identified 38 relevant studies that included data on 5596 dialysis patients. The estimated prevalence of MRSA colonization was 6.2% (95% confidence interval [95% CI], 4.2% to 8.5%). The prevalence increased over time but remained stable after 2000. Stratification of patients according to dialysis modality and setting revealed that 7.2% (95% CI, 4.9% to 9.9%) of patients on hemodialysis were colonized with MRSA compared with 1.3% (95% CI, 0.5% to 2.4%) of patients on peritoneal dialysis (P=0.01), and that a statistically significant difference existed in the percentage of colonized inpatients and outpatients (14.2% [95% CI, 8.0% to 21.8%] versus 5.4% [95% CI, 3.5% to 7.7%], respectively; P=0.04). Notably, the risk of developing MRSA infections increased among colonized hemodialysis patients compared with noncolonized patients (relative risk, 11.5 [95% CI, 4.7 to 28.0]). The long-term (6–20 months) probability of developing a MRSA infection was 19% among colonized hemodialysis patients compared with only 2% among noncolonized patients. In summary, 6.2% of dialysis patients are MRSA colonized, and the average prevalence of colonization has remained stable since 2000. Colonization in hemodialysis patients is associated with increased risk of MRSA infection.
Invasive methicillin-resistant Staphylococcus aureus (MRSA) infections are associated with mortality that is as high as 30%.1 ESRD patients have a 100-fold higher risk of MRSA infection compared with the general population.2 Among 80,461 invasive MRSA infections in 2011, 15,169 (18.9%) were among dialysis patients.3 Although a significant proportion of S. aureus infections are of endogenous origin,4 the relative risk of MRSA infections in colonized patients in this population is largely unknown. Our aim is to comprehensively assess the available data and give a global picture of MRSA colonization among dialysis patients. In this systematic review and meta-analysis, we estimate the prevalence of MRSA colonization among ESRD patients on dialysis treatment and study the significance of MRSA colonization in this population.
Results
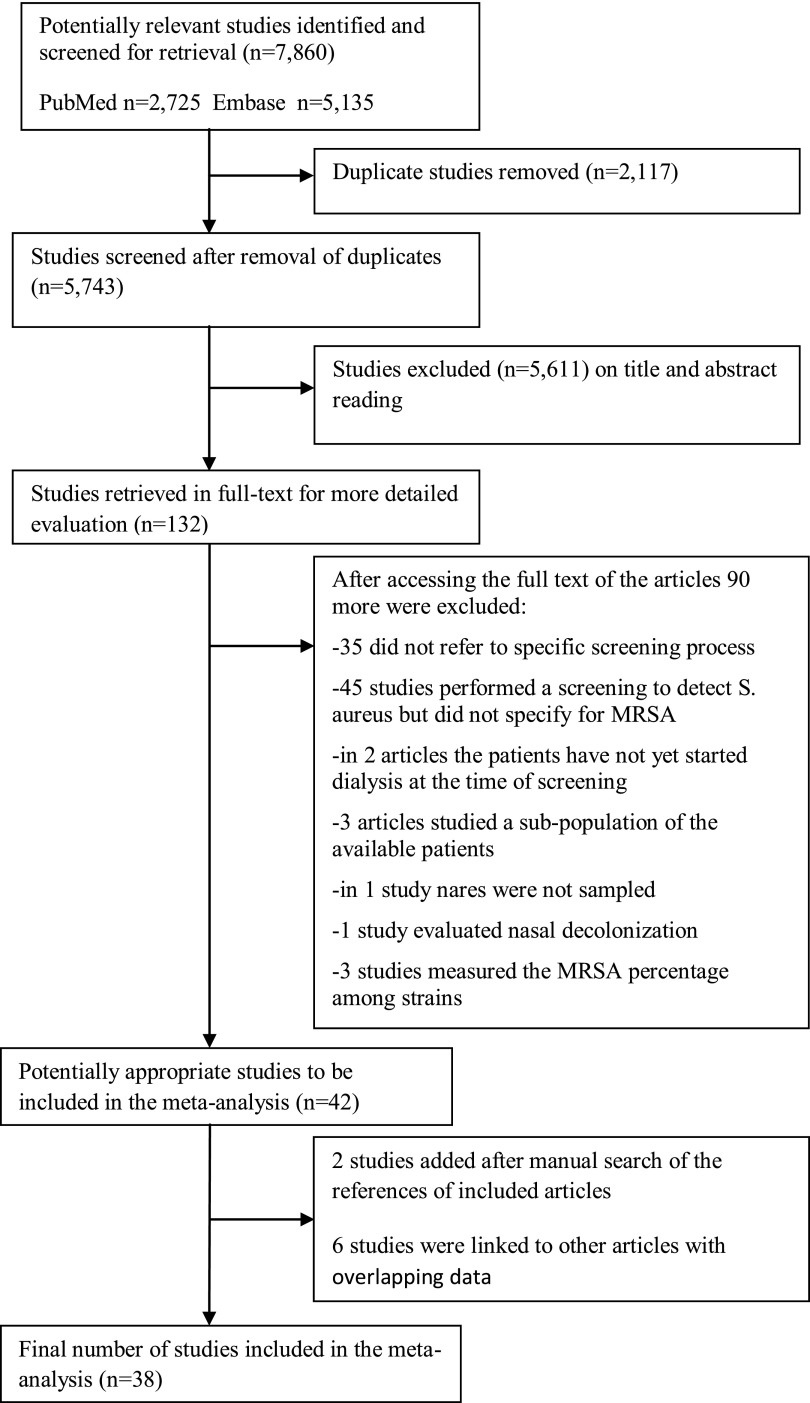
The electronic database search yielded 2725 articles from PubMed (from 1922 to October 2013) and 5135 from Embase (from 1958 to October 2013). After removing 2117 duplicate citations, 5743 remained for evaluation. Our database search was last updated to include citations published in PubMed and Embase up to October 19, 2013. After scrutinizing the titles and abstracts of retrieved articles, we identified 132 potentially relevant studies, which were reviewed in full text. Of these, 42 studies met our inclusion criteria and were subjected to the meta-analysis. Thirty-five studies were excluded because they did not refer to a screening process performed for detection of MRSA colonization among dialysis patients. In addition, 45 studies did not specify the number of MRSA colonized patients among those who were positive for S. aureus, and 3 studies reported data on a specific subpopulation of available dialysis patients (e.g., aged≥65) and were also excluded, as were 3 articles that measured the percentage of MRSA isolates among strains and not among patients. We also excluded two articles in which the screening was performed before the first dialysis session5,6 and one study in which the patients were treated with nasal decolonization.7 Finally, one study did not include nasal swabs in its protocol and was excluded.8 Two additional studies were identified through hand-searching of the reference list of eligible studies (Figure 1).
Figure 1.
Flow diagram of meta-analysis. Number of studies screened, assessed for eligibility, and included in the meta-analysis with reasons for exclusion at each stage.
Of the 44 eligible studies identified, 6 contained overlapping data.9–14 For these studies, only a single set of data was used in the meta-analysis, leaving 38 suitable studies for data analysis (coded from 44 articles). The 38 studies meeting inclusion criteria provided screening data on 5596 dialysis patients. Of note is that the vast majority (4463 patients) was from studies published within the last decade. The characteristics of the eligible studies are summarized in Table 1.15–52
Table 1.
Characteristics of eligible studies
| Study | Index Year | Location | Screeninga | Outpatient or Inpatient | Type of Dialysis (HD, PD, or Both) | Screening Sites | Evaluable Sample, n | MRSA Colonized, % |
|---|---|---|---|---|---|---|---|---|
| Europe | ||||||||
| Celik G15 | 2010 | Turkey | Multiple, two positives | Outpatient | HD | N | 184 | 4.9 |
| Schmid H16 | 2007 | Germany | Multiple, single positive | Outpatient | HD | N | 289 | 11.8 |
| Bogut A,13,17 | 2004 | Poland | Once | Inpatient | HD | N, V | 43 | 2.3 |
| Lederer SR18 | 2004 | Germany | Multiple, single positive | Outpatient | HD | N | 136 | 11.8 |
| Duran N19 | 2004 | Turkey | Once | Outpatient | HD | N | 261 | 20.7 |
| Mountricha A20 | 2004 | Greece | <24 h | Inpatient | HD | N | 9 | 33.3 |
| 2004 | Greece | Once | Outpatient | HD | N | 41 | 2.4 | |
| Koziol-Montewka21 | 2004 | Poland | Once | Outpatient | HD | N | 43 | 2.3 |
| Peña C22 | 2000 | Spain | Multiple, single positive | Outpatient | HD | N | 71 | 4.2 |
| Nouwen JL23 | 1998 | Netherlands | Multiple, single positive | Outpatient | PD | N, C | 52 | 0 |
| Nouwen J24 | 1996 | Netherlands | Multiple, single positive | Outpatient | PD | N, C | 98 | 0 |
| Kluytmans JA25 | 1992 | Netherlands | Multiple, single positive | Outpatient | HD | N | 174 | 0 |
| Boelaert JR26 | 1990 | Belgium | Multiple, two positives | Outpatient | HD | N | 150 | 0 |
| Oh J27 | NR | Northern Europe | Multiple, single positive | Outpatient | PD | N | 92 | 2.2 |
| Aktaş E28 | NR | Turkey | Multiple, two positives | Outpatient | HD | N, A, P | 30 | 3.3 |
| NR | Turkey | Multiple, two positives | Outpatient | PD | N, A, P | 40 | 0 | |
| Cavdar C10,11,29 | NR | Turkey | Multiple, single positive | Outpatient | PD | N, A, G, C | 36 | 0 |
| North America | ||||||||
| Patel G30 | 2007 | United States | Multiple, single positive | Outpatient | HD | N, A, V | 102 | 16.7 |
| Alexander EL31 | 2007 | United States | Once | Outpatient | HD | N | 157 | 6.4 |
| Mermel LA32 | 2006 | United States | Once | Outpatient | HD | N | 208 | 14.9 |
| Johnson LB33 | 2006 | United States | <3 d of admission | Inpatient | Both | N | 120 | 21.7 |
| Pop-Vicas A34 | 2005 | United States | Once | Outpatient | HD | N | 67 | 4.5 |
| Vas SI14,35 | 1998 | Canada | Once | Outpatient | PD | N, A, G, C | 167 | 1.2 |
| Kirmani N36 | 1978 | United States | Once | Outpatient | HD | N, T, S | 50 | 0 |
| Watanakunakorn C37 | NR | United States | Multiple, single positive | Outpatient | HD | N | 52 | 0 |
| Hadley AC38 | NR | United States | Once | Outpatient | HD | N, C | 197 | 5.6 (5.6)b |
| Holton DL39 | NR | Canada | Multiple, two positives | Outpatient | HD | N | 68 | 1.5 |
| Berman DS40 | NR | United States | Once | Outpatient | HD | N, T, S | 54 | 9.3 |
| Price41 | NR | United States | <48 h | Inpatient | Both | N | 118 | 11.0 |
| Asia | ||||||||
| Kang YC42 | 2011 | Taiwan | Once | Outpatient | HD | N | 116 | 5.2 |
| 2011 | Taiwan | Once | Outpatient | HD | N | 129 | 2.3 | |
| Yeoh LY43 | 2010 | Singapore | ≤24 h of admission | Inpatient | HD | N, A, G, W | 179 | 15.1 |
| Uehara Y44 | 2009 | Japan | Once | Outpatient | HD | N | 112 | 8.9 |
| Wang CY12,45 | 2007 | Taiwan | Once | Outpatient | HD | N | 541 | 5.9 |
| Lai CF46 | 2007 | Taiwan | Multiple, single positive | Outpatient | HD | N | 306 | 9.5 |
| Ghasemian R47 | 2006 | Iran | Once | Outpatient | HD | N | 84 | 27.4 |
| Lu PL48 | 2002 | Taiwan | Multiple, single positive | Outpatient | HD | N | 509 | 2.4 |
| 2002 | Taiwan | Multiple, single positive | Outpatient | PD | N | 83 | 2.4 | |
| Saxena AK9,49 | 1998 | Saudi Arabia | Multiple, two positives | Outpatient | HD | N | 208 | 9.6 |
| Aminzadeh Z50 | NR | Iran | Once | Outpatient | HD | N | 96 | 45.8 |
| Africa | ||||||||
| Oumokhtar B51 | 2010 | Morocco | Once | Outpatient | HD | N | 70 | 1.4 |
| Souly K52 | 2008 | Morocco | Multiple, single positive | Outpatient | HD | N | 54 | 5.6 |
Data are stratified by location and mid-year of each study. HD, hemodialysis; PD, peritoneal dialysis; N, nose; V, vascular access site; C, catheter exit site; NR, not reported; A, axillae; P, perineum; G, groin; T, throat; S, skin; W, wound.
Once: screening was performed once. Multiple, single positive: multiple screenings were performed; one positive result was enough for a patient to be considered a carrier. Multiple, two positives: multiple screenings were performed; two positive results were needed for a patient to be considered a carrier. In hospitalized patients, the time from admission to screening is reported.
Data on nasal colonization are in parentheses.
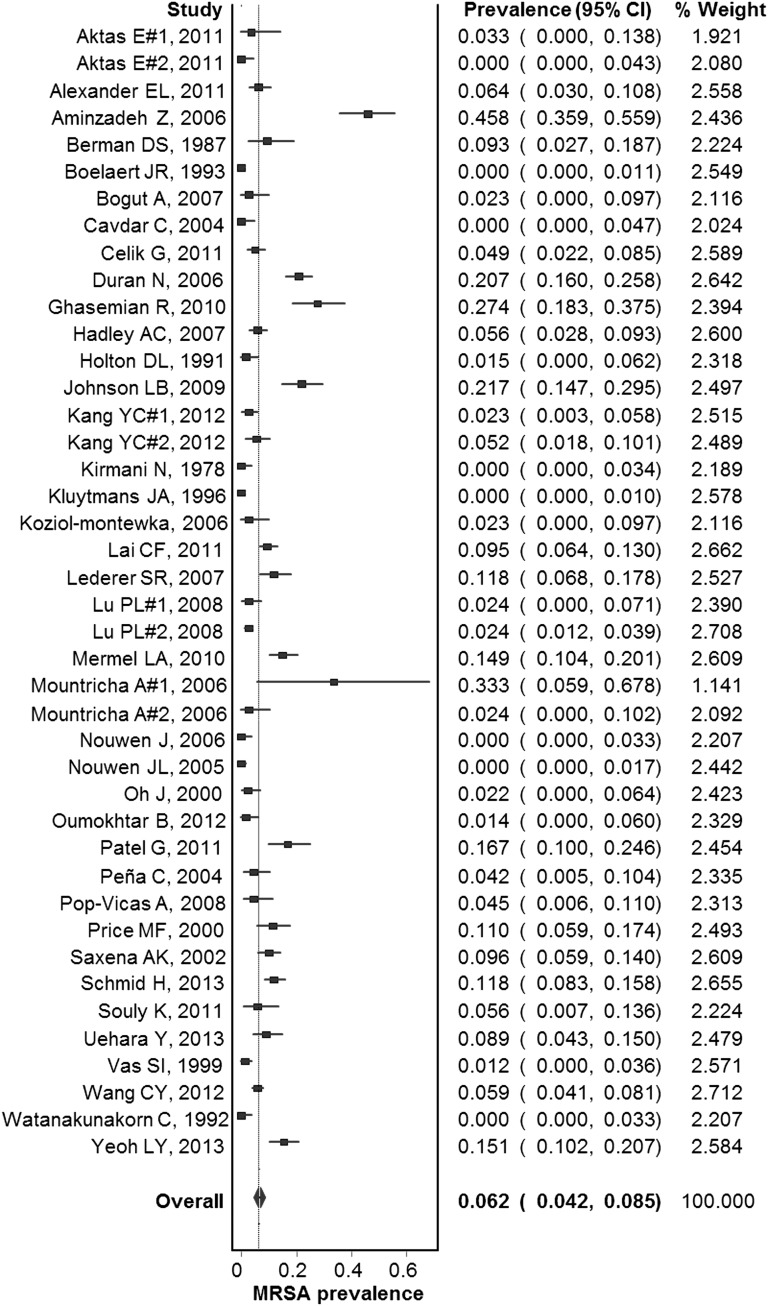
Most of the studies were prospective or cross-sectional (37 of 38; 97%) and only one study was retrospective. On the basis of the Newcastle-Ottawa scale, all studies were deemed of high quality. Fifteen studies were conducted in Europe, 12 in North America, 9 in Asia, and 2 in Africa. No studies from South America and Oceania were identified. The most common country of origin was the United States with 10 studies, followed by Taiwan with 4. The summary prevalence estimates are presented in Table 2. Among the included studies, the estimated prevalence of MRSA colonization in dialysis patients was 6.2% (95% confidence interval [95% CI], 4.2% to 8.5%) (Figure 2) and there was no evidence of publication bias (Egger’s bias, −0.78; P=0.58).
Table 2.
Summary estimates of included studies
| MRSA Colonization | Studies (Stratified Data Sets), n | Patients at Risk, n | Combined Prevalence, % (95% CI) | τ2 | P Value |
|---|---|---|---|---|---|
| All studies | 38 (42) | 5596 | 6.2 (4.2 to 8.5) | 0.072 | |
| Screening site | |||||
| Nares only | 26 (29) | 4428 | 7.1 (4.6 to 10.0) | 0.072 | Ref |
| Nares plus additional sites | 12 (13) | 1168 | 4.4 (1.6 to 8.6) | 0.083 | 0.28 |
| Geographic region | |||||
| United States | 10 (10) | 1125 | 7.9 (4.4 to 12.3) | 0.045 | Ref |
| Europe | 15 (17) | 1749 | 4.0 (1.5 to 7.7) | 0.096 | 0.19 |
| Asia | 9 (11) | 2363 | 10.3 (5.7 to 16.0) | 0.077 | 0.51 |
| Type of dialysis | |||||
| Hemodialysis | 31 (33) | 4790 | 7.2 (4.9 to 9.9) | 0.069 | Ref |
| Peritoneal dialysis | 7 (7) | 568 | 1.3 (0.5 to 2.4) | 0.00 | 0.01 |
| Setting | |||||
| Inpatient | 5 (5) | 469 | 14.2 (8.0 to 21.8) | 0.034 | Ref |
| Outpatient | 34 (37) | 5127 | 5.4 (3.5 to 7.7) | 0.070 | 0.04 |
| Screening of outpatientsa | |||||
| Once | 16 (17) | 2393 | 8.2 (4.6 to 12.7) | 0.087 | Ref |
| Multiple (single positive)b | 13 (14) | 2054 | 3.8 (1.7 to 6.6) | 0.051 | 0.07 |
| Multiple (two positives)c | 5 (6) | 680 | 3.0 (0.6 to 7.3) | 0.049 | 0.10 |
Ref, referent subgroup for comparison
All inpatients were screened once for MRSA.
Multiple screenings were performed per patient, but only one positive result was needed to define a carrier.
Multiple screenings were performed per patient, but two positive results were needed to define a carrier.
Figure 2.
Forest plot of included studies. Individual and combined estimates of prevalence of MRSA colonization.
Across the 34 studies in the outpatient setting, 13 performed multiple screenings per patient to identify MRSA colonization. Notably, the prevalence of MRSA colonization among these studies was not significantly different from the 16 studies that performed a single screening per patient (P=0.07). Across the remaining five studies, the authors also performed multiple screenings per patient, but required two positive culture results to define the carrier state. Again, this policy did not significantly affect the rate of MRSA colonization compared with studies in which only one screening was performed (P=0.10) (Table 2). All inpatients were screened once (five studies). Moreover, all studies used culture methods for isolation of MRSA and 26 of 38 studies performed the screening only by nasal swabs, whereas in the remaining 12 studies the authors also evaluated extranasal sites (e.g., axilla, groin, perineum, rectum, vascular access site, and catheter exit site). The prevalence of MRSA among patients who had only their nares swabbed was 7.1% (95% CI, 4.6% to 10.0%), and it was not different from studies that also included sampling of extranasal sites (P=0.28).
The pooled MRSA prevalence from European studies was 4.0% (95% CI, 1.5% to 7.7%) and it was lower than the prevalence among the United States studies (7.9%; 95% CI, 4.4% to 12.3%), whereas the corresponding figure among Asian studies was 10.3% (95% CI, 5.7% to 16.0%). Across 5 of 38 studies that focused on hospitalized patients, the estimated prevalence of MRSA colonization upon admission was 14.2% (95% CI, 8.0% to 21.8%), which was significantly higher compared with nonhospitalized chronic renal patients (5.4%; 95% CI, 3.5% to 7.7%; P=0.04).
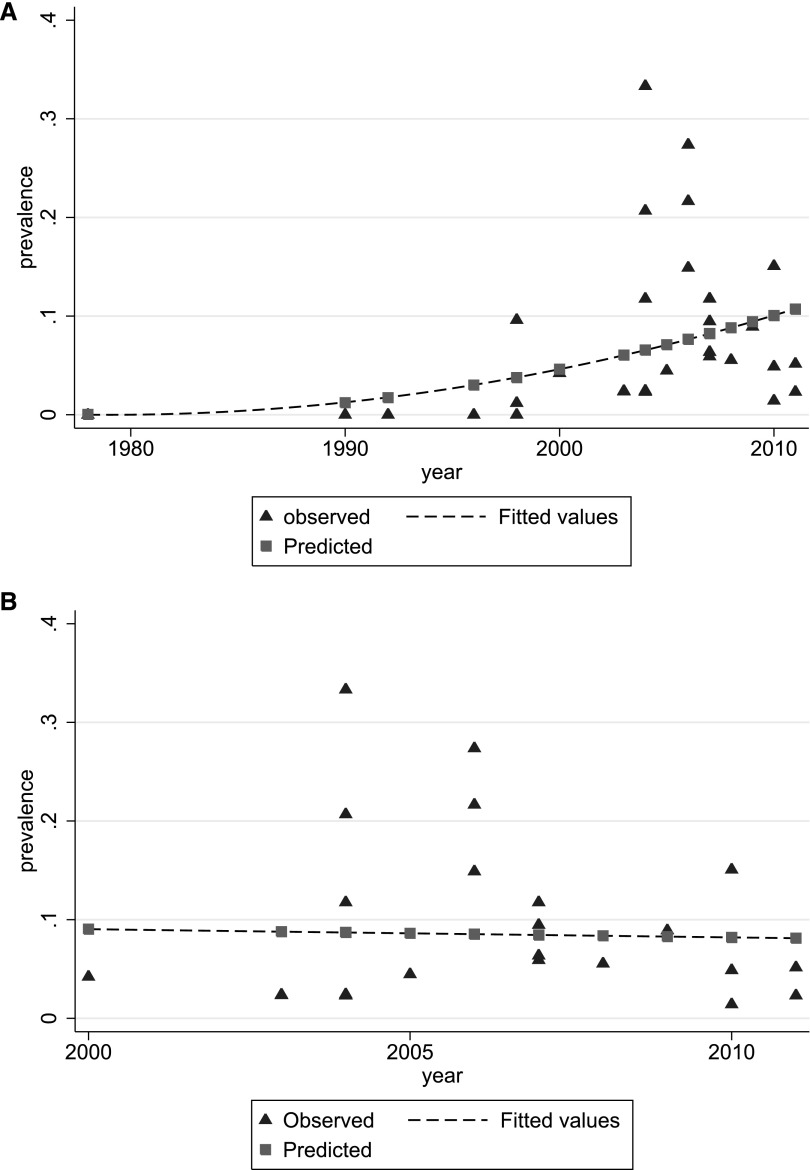
The index year of all eligible articles was used to study the time trends of MRSA colonization among dialysis patients. An increasing trend was observed over the years among all studies (Figure 3A). Of note is that nine studies did not report the time frame of the screening process and could not be included in modeling the course of MRSA prevalence over time.27–29,37–41,50 For the sensitivity analysis, we excluded the studies that were conducted before 2000 and we found that the trend of MRSA colonization has stabilized thereafter (Figure 3B).
Figure 3.
MRSA colonization trends over time. (A) Observed (triangles) and fitted (dashed line) MRSA prevalence estimates (all studies), by study mid-year. (B) Observed (triangles) and fitted (dashed line) MRSA prevalence estimates, by study mid-year, for studies conducted after 2000.
Twenty-nine of 38 studies included data exclusively from hemodialysis patients, whereas 5 of 38 studies reported data exclusively on peritoneal dialysis patients and 2 of 38 studies included stratified data on both hemodialysis and peritoneal dialysis patients.28,48 The two remaining studies reported nonstratified data and were not included in this subanalysis.33,41 Interestingly, the estimated MRSA colonization was 7.2% (95% CI, 4.9% to 9.9%) among hemodialysis patients and 1.3% (95% CI, 0.5% to 2.4%) among peritoneal dialysis patients (P=0.01).
A total of 6 of 38 studies included in our meta-analysis reported data on the MRSA infection rate among MRSA colonized and noncolonized hemodialysis patients.28,30,33,42,43,46 Two of these studies reported the MRSA infection rate during hospitalization of inpatient individuals,33,43 whereas four studies included data after a long-term follow-up period of outpatient individuals.28,30,42,46 Because we were interested in the long-term risk of acquiring a MRSA infection, only the data from the latter four studies were used and the data from each study are summarized in Table 3. The estimated risk of MRSA infection was 3.1% (95% CI, 1.9% to 4.7%). The duration of follow-up among these studies was between 6 and 20 months. The relative risk for MRSA colonized hemodialysis patients to develop a MRSA infection (compared with noncolonized patients) was estimated at 11.5 (95% CI, 4.7 to 28.0; τ2=0). The combined sensitivity of screening for MRSA infections was 0.55 (95% CI, 0.32 to 0.77), whereas the combined specificity was 0.92 (95% CI, 0.88 to 0.95). Overall, the positive and negative predictive values of MRSA colonization were 0.19 (95% CI, 0.11 to 0.26) and 0.98 (95% CI, 0.92 to 1.0) respectively, for prior probabilities of infection ranging from 1.9% to 4.7%. Taken together, these numbers suggest that 19% of hemodialysis patients who are MRSA colonized will develop a MRSA infection within 6–20 months of screening compared with only 2% of noncolonized patients. There were no data to pool for the risk of infection among peritoneal dialysis patients.
Table 3.
Individual hemodialysis study data included in the diagnostic meta-analysis
| Study | Country | Screening | Follow-Up Period | TP | FP | TN | FN | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|
| Kang YC42 | Taiwan | N | 6 mo | 1 | 10 | 149 | 1 | 0.50 (0.01–0.99) | 0.94 (0.89–0.97) |
| Aktaş E28 | Turkey | N, A, P | 6 mo | 1 | 0 | 29 | 0 | 1.00 (0.03–1.00) | 1.00 (0.88–1.00) |
| Lai CF46 | Taiwan | N | 613 d | 5 | 24 | 271 | 6 | 0.45 (0.17–0.77) | 0.92 (0.88–0.95) |
| Patel G30 | United States | N, A, V | 12 mo | 3 | 14 | 84 | 1 | 0.75 (0.19–0.99) | 0.86 (0.77–0.92) |
All studies used culture methods to identify MRSA. TP, true positive; FP, false positive; TN, true negative; FN, false negative; N, nasal; A, axilla; P, perineum; V, vascular access site.
Discussion
Bloodstream and other infectious complications are a leading cause of death among individuals requiring chronic dialysis and MRSA is one of the major pathogens that cause infections in this population.53 The purpose of this study was to investigate the significance of MRSA colonization among patients on dialysis, estimate the prevalence of MRSA colonization in this population, and measure the effect of this colonization on MRSA infections. The overall prevalence of MRSA colonization in this patient population was 6.2%, has increased over time, and seems to have stabilized after 2000. Interestingly, the prevalence among hospitalized patients was 3 times higher than among outpatients. In addition, we stratified the patients according to the modality of dialysis and found an association between the modality and the prevalence of MRSA colonization (7.2% in hemodialysis patients versus 1.3% in peritoneal dialysis patients). Importantly, we found a relative risk of 11.5 in developing MRSA infections among colonized hemodialysis patients compared with the noncolonized hemodialysis patients. We estimated that the probability of developing a MRSA infection within 6–20 months is 19% among MRSA colonized patients compared with just 2% among hemodialysis patients that are not colonized.
This analysis highlights the high prevalence of MRSA colonization in this population, which is comparable to that reported among other high-risk populations such as critically ill patients in the intensive care unit (ICU).54 Of note is that the estimated prevalence might underestimate the actual burden of MRSA in dialysis patients, because all studies included in this analysis used culture to screen for MRSA colonization, which has lower sensitivity compared with PCR that is now frequently used.55 Interestingly, our study indicates that MRSA colonization in dialysis patients has increased over time, similar to patients in the ICU.54 The trend seems to stabilize after 2000, a result that is in line with the relatively stable, and in some studies decreasing, trend of nasal carriage among healthy individuals and non-ICU hospitalized patients.56,57
MRSA colonization is significantly higher among hospitalized patients on dialysis compared with outpatients (14.2% versus 5.4%), indicating that the contact with the hospital environment plays a significant role in the colonization of the dialysis patients. This is not surprising because the rehospitalization rate in this population within 30 days is as high as 36%.53 In addition, hospitalized patients may differ from outpatients in many ways, such as comorbidities and exposure to antibiotics. Interestingly, the prevalence of MRSA colonization in our analysis varied significantly between hemodialysis and peritoneal dialysis patients (7.2% versus 1.3% respectively). This difference could be a result of the difference in comorbidities between the two patient populations. For example, Miskulin et al. report that the number of comorbidities of ESRD patients, before the onset of dialysis treatment, was significantly lower in patients who later started peritoneal dialysis, and this was independent of other factors that could influence the modality selection.58 The difference in comorbidities and the fact that hemodialysis requires frequent contact between patients and the health care system could explain the transmission of antibiotic-resistant pathogens.
Remarkably, we estimated that 19% of MRSA colonized hemodialysis patients would develop a MRSA infection in the following 6–20 months compared with only 2% of noncolonized hemodialysis patients, and the risk of developing an MRSA infection in this population is 11.5 times higher among patients who are MRSA colonized compared with patients that are not colonized. This finding is current because it is based on four different studies published after 2011.28,30,42,46 The high long-term risk of MRSA infection among colonized patients has also been demonstrated among hospitalized patients (another high-risk population), in which Datta et al. showed that 23% of hospitalized patients who were MRSA carriers developed a MRSA infection during the following year.59 It is encouraging that even with this stable trend in colonization after 2000 and the fact that MRSA colonization is closely associated with infection,60 the rate of MRSA infections among dialysis patients in the United States over the last 8 years has been declining.61,62 This decrease in the infection rate seems to be multifactorial and correlates with the decreasing use of central venous catheters (CVCs) as a vascular access for dialysis (nine reporting areas in the United States showed a consistent reduction in the proportion of hemodialysis patients with a CVC from 27.8% in 2005 to 18.8% in 2011)61 and the use of aseptic techniques for catheter insertion and maintenance.63 These interventions (decreasing use of CVCs and improved aseptic techniques) seem to reduce the risk of infection in both colonized and noncolonized patients and along with the strict compliance to infection control policies (including the possible increase in the use of decolonization methods) may further decrease the infection rate in the future.
Of note is that the risk of developing MRSA infection among colonized patients can be affected by several factors that may be specific to the particular patient, provider, or facility (e.g., comorbidities, use of antibiotics for prophylaxis or treatment, and infection control practices). These factors may also change significantly over time and may be very different in different parts of the world. Our estimated risk of infection combines the risk from different settings, patient populations, and healthcare practices and may not apply to a specific center where local epidemiology, infection control policies, and patients’ characteristics may affect MRSA infection. The method used for culture (e.g., chromogenic media, nonchromogenic selective media, or enrichment broth) as well as the sites screened in each study can have a significant effect on the detection of MRSA, and can represent an additional source of heterogeneity.64,65 However, we were not able to show a significant difference of MRSA prevalence based on the screening strategy (nasal or nasal plus extranasal site screening). Nevertheless, our findings provide an important overall estimation and they are based on recently published studies.
In addition to chronic or persistent MRSA colonization, there are also intermittent carriers.66 We cannot be sure that patients were persistently colonized during the 6–20 months of follow-up until they developed the MRSA infection and there is the theoretical possibility that these patients lost and reacquired MRSA close to their infection.67–69 In addition, because different studies had different follow-up periods, we were unable to determine the exact risk of infection over time. By summarizing the reported effect of MRSA colonization in the development of MRSA infection, we highlighted the importance of colonization in this population and provided an estimation of risk of infection over 6–20 months (the duration of follow-up observation in the studies included in our meta-analysis).
Our data underscore the association of MRSA colonization with MRSA infections. Implementing a uniform policy for managing MRSA colonization is challenging, given the unique characteristics of the dialysis population (frequent contact with the health care system, long duration of dialysis treatment, and high frequency of hospitalizations), which raise the concerns of recolonization70 and of the emergence of mupirocin-resistant strains.71,72 However, the MRSA colonization rate among dialysis patients is approximately 6.2% and it is associated with an 11.5-fold increase in the risk of MRSA infections among hemodialysis patients. Overall, one-fifth of colonized hemodialysis patients will develop a MRSA infection during the following 6–20 months. Because invasive MRSA infection is associated with a >30% mortality,1 there is a need for strict compliance with the recommendations of the US Centers for Disease Control and Prevention and National Kidney Foundation73,74 and for a systematic effort to combat MRSA colonization in this population.
Concise Methods
Study Selection
We performed a PubMed and Embase literature search to identify all studies on MRSA colonization among patients undergoing dialysis treatment. The search terms were as follows: (MRSA OR Staphylococcus OR (methicillin AND resistant)) AND (dialysis OR hemodialysis OR peritoneal). Retrieved citations were reviewed by title and abstract and all potentially relevant studies were accessed in full text. The search was complemented by scanning the reference lists of all included articles. A restriction for English literature was imposed. We did not consider abstracts, conference proceedings, and unpublished material. This meta-analysis follows the Meta-Analysis of Observational Studies in Epidemiology guidelines.75
Inclusion and Exclusion Criteria
Studies were included in the meta-analysis if they reported prevalence data on MRSA nasal colonization among individuals with ESRD who had been undergoing regular dialysis treatment. Because S. aureus primarily colonizes the nares,57,66,76 we did not include studies in which nares were not sampled. Many studies provide evidence that MRSA colonization rates are not equally distributed among different groups of dialysis patients.77 For this, we excluded studies that focused on a specific subpopulation of the available dialysis patients (e.g., studies that included only individuals aged≥65 years, patients with diabetes, etc.). We also excluded studies that evaluated colonization rates after implementing nasal decolonization protocols.
Outcomes of Interest
The primary outcome of interest was the prevalence of MRSA nasal colonization among dialysis patients. Prevalence was calculated as the proportion of patients with a positive screening result among the patients “at risk” (i.e., those that were screened for MRSA colonization). Effects were adjusted for geographical region, dialysis modality (hemodialysis versus peritoneal dialysis), dialysis setting (inpatient versus outpatient), and sampling process (nasal versus nasal and extranasal sampling, one-time screening versus multiple screenings). The secondary outcome of interest was the relative risk of colonized patients compared with noncolonized patients to acquire a MRSA infection.
Data Extraction
Using standardized forms, two reviewers (I.M.Z., F.N.Z.) independently extracted relevant information from the text, tables, and figures of eligible articles. Extrapolated information of included articles was summarized using a spreadsheet. Data from trials published in duplicate were included only once, and the maximum of relevant information was extracted. Consensus was reached if there were any discrepancies between the reviewers.
The following data were extracted. First, we extracted the characteristics of each study, including the study design (prospective versus retrospective), the country of origin, and the study period. Second, information on the patient population, including the population screened, the number of MRSA colonized patients, the type of dialysis treatment used (hemodialysis, peritoneal dialysis), and the dialysis setting (inpatient, outpatient) was extracted. Moreover, the details of the screening process, such as the anatomic sites screened (nasal or nasal plus extranasal sites), the method of MRSA isolation (culture or PCR), the definition of carriage state, and the time frame of screening process were extrapolated. Finally, we extracted the information relevant to the follow-up, including the duration of follow-up and the number of MRSA infections.
In order to model the time trends of MRSA colonization among dialysis patients, an index year of each eligible study was determined. For this purpose, we used the year that the study was conducted and not the year of publication. If the study extended for more than 1 calendar year, the year that the sampling took place was assumed to be the index year of the study. If the sampling period lasted over 1 calendar year, a mid-year was calculated. The surveillance period after the initial sampling was not considered.
Quality Assessment
Two reviewers (I.M.Z., F.N.Z.) independently assessed the methodologic quality of eligible studies using the Newcastle-Ottawa Quality Assessment Scale.78 According to the requirements of the scale, studies received stars in the context of the representativeness of the exposed cohort, ascertainment of exposure, assessment of outcome, adequacy of follow-up time for outcomes to occur, and adequacy of follow-up of cohorts. Included studies could receive a maximum of five stars because the fields “selection of the nonexposed cohort,” “demonstration that the outcome of interest was not present at the start of the study,” and “comparability between cohorts” were not applicable to our studies. Studies that received at least four stars were deemed of high quality. Details of the quality assessment of all eligible studies are provided in the Supplemental Appendix.
Statistical Analyses
We used the Freeman–Tukey arcsine methodology in order to deal with stabilizing variances.79 The meta-analysis was performed using a random-effects model to estimate the pooled (combined) prevalence and the 95% CIs, using DerSimonian and Laird weights.80 Heterogeneity was assessed using the between-study variance τ2 estimation.80,81 Egger’s test for publication bias was used to address small study effects.82 Furthermore, we incorporated a subgroup and meta-analysis regression technique to adjust for possible sources of heterogeneity. For time trends, the estimated coefficients were retransformed to prevalence and fitted values were drawn against the index year.54,83
To assess the effect of MRSA nasal colonization on MRSA infection among dialysis patients, we performed a diagnostic meta-analysis using a bivariate, mixed-effects binomial regression model to account for within-study and between-study variability.54,84,85 We followed this methodology because it is considered more suitable when variability beyond the threshold effect is documented.86,87
Statistical analysis was performed using the Stata v11 software package (StataCorp, LP, College Station, TX) and MetaXL (EpiGear International, Ltd., QLD, Australia). The MIDAS (Meta-analytical Integration of Diagnostic Accuracy Studies) set of commands in Stata was used to implement the diagnostic meta-analysis.88,89 The significance threshold was set at 0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
The Brown University Infectious Diseases Program in Outcomes Research is supported through funding from the Warren Alpert School of Brown University, the Department of Medicine, and the Division of Infectious Diseases.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091028/-/DCSupplemental.
References
- 1.Kaye KS, Anderson DJ, Choi Y, Link K, Thacker P, Sexton DJ: The deadly toll of invasive methicillin-resistant Staphylococcus aureus infection in community hospitals. Clin Infect Dis 46: 1568–1577, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) : Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients—United States, 2005. MMWR Morb Mortal Wkly Rep 56: 197–199, 2007 [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC): Active Bacterial Core Surveillance Report: Emerging Infections Program Network, Methicillin- Resistant Staphylococcus aureus, 2011. Available at: http://www.cdc.gov/abcs/reports findings/survreports/mrsa11.pdf Accessed September 3, 2013
- 4.von Eiff C, Becker K, Machka K, Stammer H, Peters G, Study Group : Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 344: 11–16, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Saxena AK, Panhotra BR, Al-hafiz AA, Sundaram DS, Abu-Oyun B, Al Mulhim K: Cefotaxime-heparin lock prophylaxis against hemodialysis catheter-related sepsis among Staphylococcus aureus nasal carriers. Saudi J Kidney Dis Transpl 23: 743–754, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Lye WC, Leong SO, Lee EJ: Methicillin-resistant Staphylococcus aureus nasal carriage and infections in CAPD. Kidney Int 43: 1357–1362, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Al-Hwiesh AK, Abdul Rahman IS: Prevention of staphylococcal peritonitis in CAPD patients combining ablution and mupirocin. Saudi J Kidney Dis Transpl 19: 737–745, 2008 [PubMed] [Google Scholar]
- 8.Embil JM, Kabani A, Zhanel G, Nicolle LE: Low prevalence of gastrointestinal colonization with antimicrobial-resistant bacteria in high risk units in a Canadian tertiary care centre. Can J Infect Dis Med Microbiol 7: 307–312, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena AK, Panhotra BR, Chopra R: Advancing age and the risk of nasal carriage of Staphylococcus aureus among patients on long-term hospital-based hemodialysis. Ann Saudi Med 24: 337–342, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavdar C, Atay T, Zeybel M, Celik A, Ozder A, Yildiz S, Gulay Z, Camsari T: Emergence of resistance in staphylococci after long-term mupirocin application in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 20: 67–70, 2004 [PubMed] [Google Scholar]
- 11.Cavdar C, Saglam F, Sifil A, Celik A, Atay T, Gungor O, Ozder A, Gulay Z, Camsari T: Effect of once-a-week vs thrice-a-week application of mupirocin on methicillin and mupirocin resistance in peritoneal dialysis patients: Three years of experience. Ren Fail 30: 417–422, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Wu VC, Wang WJ, Lin YF, Lin YH, Chen YM, Su CT, Wang JY, Wu KD, Hsueh PR: Risk factors for nasal carriage of methicillin-resistant Staphylococcus aureus among patients with end-stage renal disease in Taiwan. J Formos Med Assoc 111: 14–18, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bogut A, Kozioł-Montewka M, Baranowicz I, Józwiak L, Al-Doori Z, Morrison D, Kaczor D, Ksiazek A: Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) in Poland: Further evidence for the changing epidemiology of MRSA. New Microbiol 31: 229–234, 2008 [PubMed] [Google Scholar]
- 14.Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, Jassal V, Oreopoulos D: Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 21: 554–559, 2001 [PubMed] [Google Scholar]
- 15.Celik G, Gülcan A, Dikici N, Gülcan E: Prevalence of nasal Staphylococcus aureus carriage in the patients undergoing hemodialysis and evaluation of risk factors and laboratory parameters. Ren Fail 33: 494–498, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Schmid H, Romanos A, Schiffl H, Lederer SR: Persistent nasal methicillin-resistant staphylococcus aureus carriage in hemodialysis outpatients: A predictor of worse outcome. BMC Nephrol 14: 93, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogut A, Kozioł-Montewka M, Baranowicz I, Jóźwiak L, Ksiazek A, Al-Doori Z, Morrison D, Kaczor D, Paluch-Oleś J: Characterisation of Staphylococcus aureus nasal and skin carriage among patients undergoing haemodialysis treatment. New Microbiol 30: 149–154, 2007 [PubMed] [Google Scholar]
- 18.Lederer SR, Riedelsdorf G, Schiffl H: Nasal carriage of meticillin resistant Staphylococcus aureus: The prevalence, patients at risk and the effect of elimination on outcomes among outclinic haemodialysis patients. Eur J Med Res 12: 284–288, 2007 [PubMed] [Google Scholar]
- 19.Duran N, Ocak S, Eskiocak AF: Staphylococcus aureus nasal carriage among the diabetic and non-diabetic haemodialysis patients. Int J Clin Pract 60: 1204–1209, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mountricha A, Platsouka E, Pappas C, Vourli S, Velonakis E, Hadjiconstantinou V, Vatopoulos A, Paniara O: S. aureus nasal carriage among hemodialysis (HD) patients. Clin Nephrol 65: 229–230, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kozioł-Montewka M, Szczepanik A, Baranowicz I, Jóźwiak L, Ksiazek A, Kaczor D: The investigation of Staphylococcus aureus and coagulase-negative staphylococci nasal carriage among patients undergoing haemodialysis. Microbiol Res 161: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Peña C, Fernández-Sabe N, Domínguez MA, Pujol M, Martinez-Castelao A, Ayats J, Gudiol F, Ariza J: Staphylococcus aureus nasal carriage in patients on haemodialysis: Role of cutaneous colonization. J Hosp Infect 58: 20–27, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Nouwen JL, Fieren MW, Snijders S, Verbrugh HA, van Belkum A: Persistent (not intermittent) nasal carriage of Staphylococcus aureus is the determinant of CPD-related infections. Kidney Int 67: 1084–1092, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Nouwen J, Schouten J, Schneebergen P, Snijders S, Maaskant J, Koolen M, van Belkum A, Verbrugh HA: Staphylococcus aureus carriage patterns and the risk of infections associated with continuous peritoneal dialysis. J Clin Microbiol 44: 2233–2236, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluytmans JA, Manders MJ, van Bommel E, Verbrugh H: Elimination of nasal carriage of Staphylococcus aureus in hemodialysis patients. Infect Control Hosp Epidemiol 17: 793–797, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Boelaert JR, Van Landuyt HW, Godard CA, Daneels RF, Schurgers ML, Matthys EG, De Baere YA, Gheyle DW, Gordts BZ, Herwaldt LA: Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteraemias in haemodialysis patients. Nephrol Dial Transplant 8: 235–239, 1993 [PubMed] [Google Scholar]
- 27.Oh J, von Baum H, Klaus G, Schaefer F, European Pediatric Peritoneal Dialysis Study Group (EPPS) : Nasal carriage of Staphylococcus aureus in families of children on peritoneal dialysis. Adv Perit Dial 16: 324–327, 2000 [PubMed] [Google Scholar]
- 28.Aktaş E, Pazarli O, Külah C, Cömert F, Külah E, Sümbüloğlu V: Determination of Staphylococcus aureus carriage in hemodialysis and peritoneal dialysis patients and evaluation of the clonal relationship between carriage and clinical isolates. Am J Infect Control 39: 421–425, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Cavdar C, Zeybel M, Atay T, Sifil A, Sanlidag C, Gulay Z, Camsari T: The effects of once- or thrice-weekly mupirocin application on mupirocin resistance in patients on continuous ambulatory peritoneal dialysis—first 6 months’ experience. Adv Perit Dial 20: 62–66, 2004 [PubMed] [Google Scholar]
- 30.Patel G, Jenkins SG, Mediavilla JR, Kreiswirth BN, Radbill B, Salgado CD, Calfee DP: Clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus among patients in an ambulatory hemodialysis center. Infect Control Hosp Epidemiol 32: 881–888, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Alexander EL, Morgan DJ, Kesh S, Weisenberg SA, Zaleskas JM, Kaltsas A, Chevalier JM, Silberzweig J, Barrón Y, Mediavilla JR, Kreiswirth BN, Rhee KY: Prevalence, persistence, and microbiology of Staphylococcus aureus nasal carriage among hemodialysis outpatients at a major New York Hospital. Diagn Microbiol Infect Dis 70: 37–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mermel LA, Eells SJ, Acharya MK, Cartony JM, Dacus D, Fadem S, Gay EA, Gordon S, Lonks JR, Perl TM, McDougal LK, McGowan JE, Maxey G, Morse D, Tenover FC: Quantitative analysis and molecular fingerprinting of methicillin-resistant Staphylococcus aureus nasal colonization in different patient populations: A prospective, multicenter study. Infect Control Hosp Epidemiol 31: 592–597, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Johnson LB, Jose J, Yousif F, Pawlak J, Saravolatz LD: Prevalence of colonization with community-associated methicillin-resistant Staphylococcus aureus among end-stage renal disease patients and healthcare workers. Infect Control Hosp Epidemiol 30: 4–8, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Pop-Vicas A, Strom J, Stanley K, D’Agata EM: Multidrug-resistant gram-negative bacteria among patients who require chronic hemodialysis. Clin J Am Soc Nephrol 3: 752–758, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vas SI, Conly J, Bargman JM, Oreopoulos DG: Resistance to mupirocin: No indication of it to date while using mupirocin ointment for prevention of Staphylococcus aureus exit-site infections in peritoneal dialysis patients. Perit Dial Int 19: 313–314, 1999 [PubMed] [Google Scholar]
- 36.Kirmani N, Tuazon CU, Murray HW, Parrish AE, Sheagren JN: Staphylococcus aureus carriage rate of patients receiving long-term hemodialysis. Arch Intern Med 138: 1657–1659, 1978 [PubMed] [Google Scholar]
- 37.Watanakunakorn C, Brandt J, Durkin P, Santore S, Bota B, Stahl CJ: The efficacy of mupirocin ointment and chlorhexidine body scrubs in the eradication of nasal carriage of Staphylococcus aureus among patients undergoing long-term hemodialysis. Am J Infect Control 20: 138–141, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Hadley AC, Karchmer TB, Russell GB, McBride DG, Freedman BI: The prevalence of resistant bacterial colonization in chronic hemodialysis patients. Am J Nephrol 27: 352–359, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Holton DL, Nicolle LE, Diley D, Bernstein K: Efficacy of mupirocin nasal ointment in eradicating Staphylococcus aureus nasal carriage in chronic haemodialysis patients. J Hosp Infect 17: 133–137, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Berman DS, Schaefler S, Simberkoff MS, Rahal JJ: Staphylococcus aureus colonization in intravenous drug abusers, dialysis patients, and diabetics. J Infect Dis 155: 829–831, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Price MF, Carlini M, Houston S, Gentry LO: Prevalence of nasal colonization with methicillin-resistant Staphylococcus aureus in selected patient populations. Infect Control Hosp Epidemiol 21: 603–605, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Kang YC, Tai WC, Yu CC, Kang JH, Huang YC: Methicillin-resistant Staphylococcus aureus nasal carriage among patients receiving hemodialysis in Taiwan: Prevalence rate, molecular characterization and de-colonization. BMC Infect Dis 12: 284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeoh LY, Tan FL, Willis GC, Ooi ST: Methicillin-resistant Staphylococcus aureus carriage in hospitalized chronic hemodialysis patients and its predisposing factors. Hemodial Int 18: 142–147, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Uehara Y, Kuwahara-Arai K, Hori S, Kikuchi K, Yanai M, Hiramatsu K: Investigation of nasal meticillin-resistant Staphylococcus aureus carriage in a haemodialysis clinic in Japan. J Hosp Infect 84: 81–84, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Wang CY, Wu VC, Chen YM, Su CT, Wu KD, Hsueh PR: Nasal carriage of methicillin-resistant Staphylococcus aureus among patients with end-stage renal disease. Infect Control Hosp Epidemiol 30: 93–94, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Lai CF, Liao CH, Pai MF, Chu FY, Hsu SP, Chen HY, Yang JY, Chiu YL, Peng YS, Chang SC, Hung KY, Tsai TJ, Wu KD: Nasal carriage of methicillin-resistant Staphylococcus aureus is associated with higher all-cause mortality in hemodialysis patients. Clin J Am Soc Nephrol 6: 167–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghasemian R, Najafi N, Makhlough A, Khademloo M: Frequency of nasal carriage of Staphylococcus aureus and its antimicrobial resistance pattern in patients on hemodialysis. Iran J Kidney Dis 4: 218–222, 2010 [PubMed] [Google Scholar]
- 48.Lu PL, Tsai JC, Chiu YW, Chang FY, Chen YW, Hsiao CF, Siu LK: Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol Dial Transplant 23: 1659–1665, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Saxena AK, Panhotra BR, Venkateshappa CK, Sundaram DS, Naguib M, Uzzaman W, Al Mulhim K: The impact of nasal carriage of methicillin-resistant and methicillin-susceptible Staphylococcus aureus (MRSA & MSSA) on vascular access-related septicemia among patients with type-II diabetes on dialysis. Ren Fail 24: 763–777, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Aminzadeh Z, Farhani MF, Gachkar L: Prevalence of Staphylococcus aureus carriage in patients on hemodialysis and the pattern of antibacterial resistance. Iran J Clin Infect Dis 1: 55–68, 2006 [Google Scholar]
- 51.Oumokhtar B, Elazhari M, Timinouni M, Bendahhou K, Bennani B, Mahmoud M, El Ouali Lalami A, Berrada S, Arrayhani M, Squalli Houssaini T: Staphylococcus aureus nasal carriage in a Moroccan dialysis center and isolates characterization. Hemodial Int 17: 542–547, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Souly K, Ait el kadi M, Lahmadi K, Biougnach H, Boughaidi A, Zouhdi M, Benasila S, Elyoussefi Z, Bouattar T, Zbiti N, Skalli Z, Rhou H, Ouzeddoun N, Bayahia R, Benamar L: Epidemiology and prevention of Staphylococcus aureus nasal carriage in hemodialyzed patients. Med Mal Infect 41: 469–474, 2011 [DOI] [PubMed] [Google Scholar]
- 53.US Renal Data System: 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End- Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 54.Ziakas PD, Anagnostou T, Mylonakis E: The Prevalence and significance of methicillin-resistant Staphylococcus aureus colonization at admission in the general icu setting: A meta-analysis of published studies. Crit Care Med 42: 433–444, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Luteijn JM, Hubben GA, Pechlivanoglou P, Bonten MJ, Postma MJ: Diagnostic accuracy of culture-based and PCR-based detection tests for methicillin-resistant Staphylococcus aureus: A meta-analysis. Clin Microbiol Infect 17: 146–154, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Denkinger CM, Grant AD, Denkinger M, Gautam S, D’Agata EM: Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 56: 227–230, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL: The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5: 751–762, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Miskulin DC, Meyer KB, Athienites NV, Martin AA, Terrin N, Marsh JV, Fink NE, Coresh J, Powe NR, Klag MJ, Levey AS: Comorbidity and other factors associated with modality selection in incident dialysis patients: The CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis 39: 324–336, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Datta R, Huang SS: Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis 47: 176–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallen AJ, Jernigan JA, Patel PR: Decolonization to prevent infections with Staphylococcus aureus in patients undergoing hemodialysis: A review of current evidence. Semin Dial 24: 533–539, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Nguyen DB, Lessa FC, Belflower R, Mu Y, Wise M, Nadle J, Bamberg WM, Petit S, Ray SM, Harrison LH, Lynfield R, Dumyati G, Thompson J, Schaffner W, Patel PR, Active Bacterial Core Surveillance (ABCs) MRSA Investigators of the Emerging Infections Program : Invasive methicillin-resistant Staphylococcus aureus infections among patients on chronic dialysis in the United States, 2005-2011. Clin Infect Dis 57: 1393–1400, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, Ray SM, Harrison LH, Lynfield R, Dumyati G, Townes JM, Schaffner W, Patel PR, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators of the Emerging Infections Program : Health care-associated invasive MRSA infections, 2005-2008. JAMA 304: 641–648, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C: An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355: 2725–2732, 2006 [DOI] [PubMed] [Google Scholar]
- 64.French GL: Methods for screening for methicillin-resistant Staphylococcus aureus carriage. Clin Microbiol Infect 15[Suppl 7]: 10–16, 2009 [DOI] [PubMed] [Google Scholar]
- 65.McKinnell JA, Huang SS, Eells SJ, Cui E, Miller LG: Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 34: 161–170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluytmans J, van Belkum A, Verbrugh H: Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10: 505–520, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC: Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis 32: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Larsson AK, Gustafsson E, Nilsson AC, Odenholt I, Ringberg H, Melander E: Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: A four-year experience from southern Sweden. Scand J Infect Dis 43: 456–462, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Robicsek A, Beaumont JL, Peterson LR: Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis 48: 910–913, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Gilpin DF, Small S, Bakkshi S, Kearney MP, Cardwell C, Tunney MM: Efficacy of a standard methicillin-resistant Staphylococcus aureus decolonisation protocol in routine clinical practice. J Hosp Infect 75: 93–98, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Patel JB, Gorwitz RJ, Jernigan JA: Mupirocin resistance. Clin Infect Dis 49: 935–941, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Pérez-Fontán M, Rosales M, Rodríguez-Carmona A, Falcón TG, Valdés F: Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 39: 337–341, 2002 [DOI] [PubMed] [Google Scholar]
- 73.US Centers for Disease Control and Prevention : Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 50: 1–43, 2001 [PubMed] [Google Scholar]
- 74.National Kidney Foundation : III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: Update 2000. Am J Kidney Dis 37[Suppl 1]: S137–S181, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC: Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis 193: 172–179, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Forster AJ, Oake N, Roth V, Suh KN, Majewski J, Leeder C, van Walraven C: Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: A systematic review. Am J Infect Control 41: 214–220, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed December 5, 2013
- 79.Fazel S, Khosla V, Doll H, Geddes J: The prevalence of mental disorders among the homeless in western countries: Systematic review and meta-regression analysis. PLoS Med 5: e225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 81.Rücker G, Schwarzer G, Carpenter JR, Schumacher M: Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 8: 79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ziakas PD, Thapa R, Rice LB, Mylonakis E: Trends and significance of VRE colonization in the ICU: A meta-analysis of published studies. PLoS ONE 8: e75658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH: Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58: 982–990, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA: A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 8: 239–251, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Poulou LS, Ziakas PD, Ziogas DC, Doxani C, Xyla V, Vakrinos G, Voulgarelis M, Thanos L: FDG-PET for detecting local tumor recurrence of ablated liver metastases: A diagnostic meta-analysis. Biomarkers 17: 532–538, 2012 [DOI] [PubMed] [Google Scholar]
- 87.Moses LE, Shapiro D, Littenberg B: Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat Med 12: 1293–1316, 1993 [DOI] [PubMed] [Google Scholar]
- 88.Dwamena BA: Evidence-based radiology: Step 3—diagnostic systematic review and meta-analysis (critical appraisal). Semin Roentgenol 44: 170–179, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Ziakas PD, Poulou LS, Voulgarelis M, Thanos L: The Gordian knot of interim 18-fluorodeoxyglucose positron emission tomography for Hodgkin lymphoma: A meta-analysis and commentary on published studies. Leuk Lymphoma 53: 2166–2174, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.