Abstract
Electrical cardioversion (EC) for atrial fibrillation (AF) is a common procedure performed in an attempt to restore normal sinus rhythm (NSR). Many factors predict long‐term maintenance of NSR and the risk of AF recurrence. The duration of AF, cardiac size and function, rheumatic heart disease, significant mitral valve disease, left atrial enlargement, and older age are among the most common recognized factors. A number of interventions can potentially decrease the AF recurrence rate. Identifying and treating reversible causes and the use of antiarrhythmic medications in certain situations can help decrease the risk of AF recurrence. The role of the newer anticoagulants is expanding, and wider application is expected in the near future. We hope that this summary will serve as a guide to physicians and healthcare providers to address the question of who should undergo cardioversion, as there are patients who are most likely to benefit from this procedure and others that will revert back into AF within a short period. To identify who would benefit most from EC and have a reasonable chance of long‐term maintenance of NSR, a thorough evaluation of each individual patient should be performed to tailor the best therapy to each individual.
Introduction
Preservation of sinus rhythm after successful electrical cardioversion (EC) for atrial fibrillation (AF) remains a challenge for clinicians. Despite high rates of successful cardioversion (>90%) and restoration of normal sinus rhythm (NSR) for the treatment of AF, less than half remain in sinus rhythm after 1 year.1, 2 AF is a common problem affecting approximately 2.3 million individuals in the United States alone.3 Factors that help maintain patients in sinus rhythm after successful cardioversion have been investigated, as well as the predictors of AF recurrence after successful EC. These factors are important to recognize, as they can help guide the physician's decision regarding the optimal treatment strategy for AF and which patients would benefit most from restoration of NSR. We believe that many cardioversions could be avoided if clear guidelines identify the patients who may or may not have a reasonable chance of long‐term maintenance of NSR.
Predictors of Maintenance of Sinus Rhythm After Successful Cardioversion
Delays in conversion and prolonged episodes of AF promote electrical atrial remodeling that makes restoration of sinus rhythm more difficult and increases the likelihood of postcardioversion AF recurrence.4 Maintenance of sinus rhythm at 1 month is related to short duration of AF before cardioversion.5 In one study, it was shown that AF will likely recur after cardioversion if AF has been present for 3 or more years,1 whereas another study demonstrated that the presence of AF for <1 month is a significant predictor for cardioversion success.6 The presence of underlying heart disease, especially rheumatic heart disease, was associated with increased risk of recurrence of AF after successful cardioversion.2
Atrial remodeling plays an essential role in early AF recurrence, and the use of intracellular calcium‐lowering medications (calcium channel blockers) during AF appeared to reduce recurrences, possibly due to a reduction of this adverse remodeling.7
There is strong evidence that the renin‐angiotensin‐aldosterone (RAS) system has a role in AF. The effects of treatment with angiotensin‐converting enzyme inhibitors (ACE‐Is) or angiotensin receptor blockers (ARBs), in the primary or secondary prevention of AF, showed a significant reduction in the risk for AF recurrence after cardioversion with the use of ACE‐Is or ARBs, reducing the odds for secondary AF by 33%.8 A recent meta‐analysis of 3972 patients with AF showed that inhibition of RAS is effective, safe, and well tolerated for preventing the recurrence of AF.9
The left atrial (LA) diameter is a powerful predictor of AF recurrence as shown in more than one AFFIRM (Atrial Fibrillation Follow‐up Investigation of Rhythm Management) analysis.10, 11 AF recurrence is more likely to occur within 6 months after a successful cardioversion if left atrial enlargement is present, especially when the (LA) diameter exceeds 5.0 cm.5, 6
A recent prospective study assessed the predictive value of the left atrial volume index (LAVI) in EC, and observed the recurrence rate of AF after a successful EC in patients with nonvalvular AF. Lower LAVI values (<30 mL/m2) before the EC were strong and independent predictors of the success of the EC and the maintenance of NSR after a successful EC.12 The average LAVI for patients who maintained NSR by the end of the first month was 30.8 ± 6.2 mL/m2, whereas the average LAVI was 46.8 ± 13.9 mL/m2 for those who reverted back to AF.
The presence of left ventricular dysfunction, as well as mitral valve disease, are also risk factors for AF recurrence.13
Cardioversion for AF is less successful in the presence of obstructive sleep apnea (OSA), and treatment of OSA has been shown to improve control of AF.14 However, a stronger relationship between OSA treatment and success of AF cardioversion needs to be established.
In a substudy done on subjects from the AFFIRM study,10 it was noted that P‐wave duration of >135 ms is a risk factor for the early recurrence of AF within 2 months of cardioversion. In this study, 563 patients were randomized to rate control, and 730 patients were randomized to rhythm control strategy. The rate control arm consisted of patients who were in sinus rhythm at the time of randomization. NSR could have been achieved by either electrical or chemical cardioversion. In the rhythm control arm, patients were put on antiarrhythmic medications and had repeated cardioversions when AF recurrence was noted. Risk factors for having 2 or more cardioversions in this arm were found to be LA enlargement of >4.5 cm and mitral valve thickening. One major limitation of this study is that the results would be relevant to the AFFIRM study population (age >65 years and presence of risk factors for stroke or death), and may not be applicable to the general population with AF.
Predictors of recurrence in another retrospective study were age (patients <65 years old), paroxysmal AF, and alcohol consumption.15 Another prospective study found that short duration of AF, treatment with β‐blockers or verapamil/diltiazem, and right atrial dimension <37 mm are independent predictors for maintenance of sinusrhythm.16
Some investigators advocated for an aggressive policy toward persistent AF by means of repetitive electrical cardioversions for early AF recurrence. They found this approach (repeat cardioversion for early AF recurrence compared to medical therapy alone) useful in maintaining sinus rhythm after 12 months.17 Data from the Metrix (InControl, Redmond, WA) automatic implantable atrial defibrillator trial showed clear evidence that in patients with symptomatic, recurrent, and drug refractory AF, recurrent ECs are safe and effective in restoring NSR.18 They also noticed that the frequency of symptomatic AF episodes decreased, the time between episodes requiring therapy increased, and the risk of having an episode requiring treatment decreased with serial cardioversions with each AF recurrence.19, 20
In another study, it was shown that serial cardioversions were successful in maintaining sinus rhythm only in young patients who have good exercise tolerance and duration of AF of less than 36 months but not in older (>56 years old) patients who have poor exercise tolerance and >36 months duration of AF.21 Other investigators have suggested that cardioversion should be avoided in patients older than 80 years because the risks outweigh the benefits in this population.13 Table 1 summarizes the major findings and studies regarding the various predictors.
Table 1.
Predictors of AF Recurrence After Successful Electrical Cardioversion
| Predictor | Authors | Sample Size (Population) | Major Finding |
|---|---|---|---|
| Time in AF | Dittrich et al5 | 85 patients | Maintenance of NSR is related to a short duration of AF |
| Dalzell et al6 | 80 patients | AF duration <1 month is a significant predictor of EC long‐term success | |
| Rheumatic heart disease | Van Gelder et al2 | 246 patients | Rheumatic heart disease decreases EC long‐term success |
| Left atrial size | Olshansky et al 11 | 2472 patients | LA diameter >4.5 cm predicts AF recurrence |
| Akdemir et al12 | 80 patients | LAVI <30 mL/m2 is a strong predictor of EC success | |
| LV dysfunction | Raitt et al10 | 1293 patients | LV ejection fraction <0.50 is a risk factor for AF recurrence |
| P wave duration | Raitt et al10 | 1293 patients | P‐wave duration >135 ms is a risk factor for AF recurrence |
| Antiarrhythmic drugs | Lafuente‐Lafuente et al22 | 11 322 patients (systematic review of 44 trials) | Antiarrhythmic drugs significantly reduce AF recurrence |
Abbreviations: AF, atrial fibrillation; EC, electrical cardioversion; LA, left atrium; LAVI, left atrial volume index; LV, left ventricle; NSR, normal sinus rhythm.
Antiarrhythmic Drugs and Their Role in Maintenance of Sinus Rhythm
Antiarrhythmic agents initiated prior to EC have been shown to increase the likelihood of a successful EC as well as the long‐term maintenance of NSR.4 Because many antiarrhythmic medications have potentially serious side effects, including proarrhythmic effects, they should be only prescribed by physicians familiar with their use. Patient characteristics along with the side effect profile of each antiarrhythmic agent should be considered prior to initiating these medications. Amiodarone, dofetilide, flecainide, propafenone, and sotalol are the most commonly used antiarrhythmics to maintain NSR. It was shown in more than one study that amiodarone is associated with the highest chance of maintaining NSR but with also the greatest risk of long‐term toxicities.22, 23
The presence of ischemic or structural heart disease, paroxysmal vs persistent AF, adrenergically mediated AF, and the concern for proarrhythmia are other factors that influence which antiarrhythmic medication should be used. For example, dofetilide is rarely used in paroxysmal AF due to its reduced efficacy when compared to amiodarone in this setting,24 β‐blockers may be used when proarrhythmia is a concern or if AF is thought to be adrenergically mediated, and a screen for coronary heart disease should be done prior to initiating class 1C agents (flecainide and propafenone).24 In the presence of heart failure, amiodarone is preferred over dofetilide, and the use of flecainide, propafenone, and dronedarone is relatively contraindicated per the current American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines.24, 25, 26
Ibutilide, a class III agent, which is available only as an intravenous medication, is useful for the acute conversion of AF, has been used in patients who are resistant to EC, and was shown to lower the cardioversion energy requirement.27, 28 However, ibutilide prolongs repolarization and the QT interval, with the potential of provoking torsade de pointes and nonsustained monomorphic ventricular tachycardia, and patients should be observed with continuous electrocardiograph monitoring after the infusion of this medication for 4 hours or until the QT interval has normalized.27 In 3 large series, the rate of torsade de pointes ranged between 1.7% and 3.6%.27, 29, 30 It was shown that pretreatment with intravenous magnesium prior to ibutilide infusion decreases the risk of torsade de pointes significantly and improves the conversion efficacy of this medication.31
Anticoagulation Before and After Cardioversion
All patients with AF duration longer than 48 hours or of unknown duration undergoing EC should be anticoagulated prior to the procedure due to the increased risk of thromboembolic events associated with conversion from AF to NSR. It has been shown that most embolic events occur within 10 days of cardioversion, and mainly occur in patients who are not effectively anticoagulated in the pericardioversion period.32 The risk of thromboembolism has decreased significantly (<1%) if anticoagulation has been used for 4 weeks prior, and 4 weeks after cardioversion.32, 33 The rationale for precardioversion anticoagulation is that left atrial thrombi might have formed, and the resolution of such thrombi with proper anticoagulation decreases the risk of embolization and stroke events.34 The rationale for postcardioversion anticoagulation, is largely related to atrial stunning, which is defined as a transient atrial contractile dysfunction that develops after cardioversion and lasts up to 4 weeks, until the complete recovery of atrial mechanical function.35 Current guidelines recommend a goal international normalized ratio (INR) of 2.0 to 3.0 for nonvalvular AF when warfarin is used as an anticoagulant.36
The safety of anticoagulation with warfarin in older patients has been demonstrated even in those patients receiving dual antiplatelet therapy in addition to warfarin.37 However, a recent study by Hess et al suggests that that many older patients with AF who qualify for anticoagulation are being managed without warfarin therapy.38
In recent years, new oral, nonwarfarin, anticoagulants have been introduced, with the potential benefit of eliminating both monitoring during therapy (serial INRs) and frequent dose adjustments as compared to warfarin. For example, dabigatran, an oral direct thrombin inhibitor, was evaluated and compared to warfarin in the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulant Therapy) trial in over 18 000 patients.39 It was shown that the lower dose of dabigatran (110 mg twice daily) was similar to, and the higher dose (150 mg twice daily) was superior to, warfarin for the prevention of chronic thromboembolism and stroke. Similar observations were noted when a subpopulation of the RE‐LY trial who underwent EC were studied. No significant difference in the rate of stroke within 30 days of cardioversion was noted between patients who received dabigatran or warfarin,40 which makes dabigatran a reasonable alternative to warfarin for patients who require anticoagulation around the EC period. The oral factor Xa inhibitor anticoagulants, rivaroxaban and apixaban, have been studied as well and were compared to warfarin. It was shown that these agents are at least equal to warfarin for the prevention of thromboembolism in patients with AF.41, 42 However, unlike dabigatran, these agents have not been studied and evaluated in the pericardioversion period, which limits their use for the prevention of thromboembolic events prior to or immediately after cardioversion until more studies are conducted.
Discussion and Conclusion
Many factors predict the maintenance of sinus rhythm following EC, which include but are not limited to the duration of AF, cardiac size and function, underlying heart disease, rheumatic heart disease, significant mitral valve disease, left atrial enlargement, age, the New York Heart Association functional class, and the timing and number of AF recurrences. Unstable hemodynamics and worsening symptoms ascribed to AF are clear indications to pursue EC (Figure 1). We also believe that at least 1 attempt at cardioversion is warranted in the majority of patients with a first‐ever episode of AF; however, it seems advisable to give up even the first attempt at cardioversion in special populations like the minimally symptomatic or asymptomatic patients who are very old, in patients with AF episodes dating back more than 24 to 36 months, and in those with severe valvular heart disease, severe left ventricular dysfunction, or marked left atrial enlargement. We do not recommend serial cardioversions if AF recurs after the second EC, because <14% of patients remain in NSR for a prolonged period after a third EC.17 Initiation and maintenance of antiarrhythmic agents around EC help restore NSR and prevent AF recurrence. However, their use should be discouraged in patients with their first episode of AF who are at low risk of recurrence or have an easily reversible cause of their AF such as hyperthyroidism, stimulant use, or alcohol intoxication.
Figure 1.
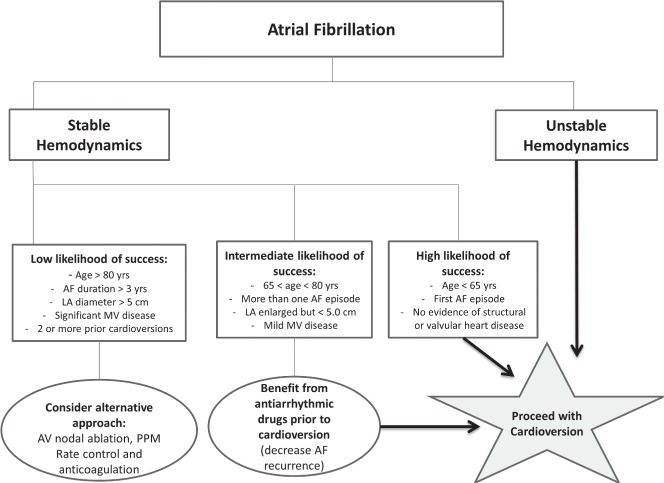
Suggested algorithm for atrial fibrillation management. Abbreviations: AF, atrial fibrillation; AV, atrioventricular; LA, left atrium; MV, mitral valve; PPM, permanent pacemaker.
The use of intracellular calcium‐lowering medications reduced AF recurrences, possibly due to a reduction of adverse electrical remodeling of the atrium.7 However, β‐blockers are more commonly used as first‐line agents to prevent AF recurrence.43 It would be interesting to compare the efficacy of these 2 agents for the prevention of AF recurrence in a randomized, controlled trial. Inhibition of the RAS system is another area of possible future research. It was shown that the use of an ACE‐I or ARB decreases AF recurrence by 33%.8 The predictive value of the P wave duration has been studied,10 and future work may focus on its value for prediction of future AF recurrences.
Finally, by better tailoring the specific treatment to the individual patient, we can hope to apply these therapies to those most likely to benefit and avoid the cost and potential morbidity in those least likely to maintain sinus rhythm.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Resnekov L, McDonald L. Appraisal of electroconversion in treatment of cardiac dysrhythmias. Br Heart J. 1968;30:786–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Gelder IC, Crijns HJ, Van Gilst WH, et al. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct‐current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol. 1991;68:41–46. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, et al., Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 4. Naccarelli GV, Dell'Orfano JT, Wolbrette DL, et al., Cost‐effective management of acute atrial fibrillation: role of rate control, spontaneous conversion, medical and direct current cardioversion, transesophageal echocardiography, and antiembolic therapy. Am J Cardiol. 2000;85: 36D–45D. [DOI] [PubMed] [Google Scholar]
- 5. Dittrich HC, Erickson JS, Schneiderman T, et al. Echocardiographic and clinical predictors for outcome of elective cardioversion of atrial fibrillation. Am J Cardiol. 1989;63:193–197. [DOI] [PubMed] [Google Scholar]
- 6. Dalzell GW, Anderson J, Adgey AA. Factors determining success and energy requirements for cardioversion of atrial fibrillation. Q J Med. 1990;76:903–913. [PubMed] [Google Scholar]
- 7. Tieleman RG, Van Gelder IC, Crijns HJ, et al. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation‐induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–173. [DOI] [PubMed] [Google Scholar]
- 8. Schneider MP, Hua TA, Bohm M, et al. Prevention of atrial fibrillation by Renin‐Angiotensin system inhibition a meta‐analysis. J Am Coll Cardiol. 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 9. Li TJ, Zang WD, Chen YL, et al. Renin‐angiotensin system inhibitors for prevention of recurrent atrial fibrillation: a meta‐analysis. Int J Clin Pract. 2013;67:536–543. [DOI] [PubMed] [Google Scholar]
- 10. Raitt MH, Volgman AS, Zoble RG, et al; AFFIRM Investigators. Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2006;151:390–396. [DOI] [PubMed] [Google Scholar]
- 11. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–2033. [DOI] [PubMed] [Google Scholar]
- 12. Akdemir B, Altekin RE, Kucuk M, et al. The significance of the left atrial volume index in cardioversion success and its relationship with recurrence in patients with non‐valvular atrial fibrillation subjected to electrical cardioversion: a study on diagnostic accuracy. Anadolu Kardiyol Derg. 2013;13:18–25. [DOI] [PubMed] [Google Scholar]
- 13. Camm AJ, Lip GY, De Caterina R, et al., 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 14. Goyal SK, Sharma A. Atrial fibrillation in obstructive sleep apnea. World J Cardiol. 2013;5:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuppahally SS, Foster E, Shoor S, et al. Short‐term and long‐term success of electrical cardioversion in atrial fibrillation in managed care system. Int Arch Med. 2009;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frick M, Frykman V, Jensen‐Urstad M, et al. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin Cardiol. 2001;24:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertaglia E, D'Este D, Zerbo F, et al. Success of serial external electrical cardioversion of persistent atrial fibrillation in maintaining sinus rhythm; a randomized study. Eur Heart J. 2002;23:1522–1528. [DOI] [PubMed] [Google Scholar]
- 18. Geller JC, Reek S, Timmermans C, et al. Treatment of atrial fibrillation with an implantable atrial defibrillator—long term results. Eur Heart J. 2003;24:2083–2089. [DOI] [PubMed] [Google Scholar]
- 19. Timmermans C, Levy S, Ayers GM, et al. Spontaneous episodes of atrial fibrillation after implantation of the Metrix Atrioverter: observations on treated and nontreated episodes. Metrix Investigators. J Am Coll Cardiol. 2000;35:1428–1433. [DOI] [PubMed] [Google Scholar]
- 20. Daoud EG, Timmermans C, Fellows C, et al. Initial clinical experience with ambulatory use of an implantable atrial defibrillator for conversion of atrial fibrillation. Metrix Investigators. Circulation. 2000;102:1407–1413. [DOI] [PubMed] [Google Scholar]
- 21. Van Gelder IC, Crijns HJ, Tieleman RG, et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med. 1996;156:2585–2592. [DOI] [PubMed] [Google Scholar]
- 22. Lafuente‐Lafuente C, Mouly S, Longas‐Tejero MA, et al. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166:719–728. [DOI] [PubMed] [Google Scholar]
- 23. McNamara RL, Tamariz LJ, Segal JB, et al. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. 2003;139:1018–1033. [DOI] [PubMed] [Google Scholar]
- 24. Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–242. [DOI] [PubMed] [Google Scholar]
- 25. American College of Cardiology Foundation, American Heart Association, European Society of Cardiology, Heart Rhythm Society ; Wann LS, Curtis AB, Ellenbogen KA, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:1916–1926. [DOI] [PubMed] [Google Scholar]
- 26. European Heart Rhythm Association, European Association for Cardio‐Thoracic Surgery , Camm AJ, Kirchhof P, Lip GY, et al; ESC Committee for Practice Guidelines. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 27. Abi‐Mansour P, Carberry PA, McCowan RJ, et al. Conversion efficacy and safety of repeated doses of ibutilide in patients with atrial flutter and atrial fibrillation. Study Investigators. Am Heart J. 1998;136:632–642. [DOI] [PubMed] [Google Scholar]
- 28. Oral H, Souza JJ, Michaud GF, et al. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–1854. [DOI] [PubMed] [Google Scholar]
- 29. Giudici MC, Fischer WJ III, Cervantes DC, et al. Ibutilide therapy for atrial fibrillation. 5‐year experience in a community hospital. J Cardiovasc Nurs. 2008;23:484–488. [DOI] [PubMed] [Google Scholar]
- 30. Ellenbogen KA, Stambler BS, Wood MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose‐response study. J Am Coll Cardiol. 1996;28:130–136. [DOI] [PubMed] [Google Scholar]
- 31. Patsilinakos S, Christou A, Kafkas N, et al. Effect of high doses of magnesium on converting ibutilide to a safe and more effective agent. Am J Cardiol. 2010;106:673–676. [DOI] [PubMed] [Google Scholar]
- 32. Gentile F, Elhendy A, Khandheria BK, et al. Safety of electrical cardioversion in patients with atrial fibrillation. Mayo Clin Proc. 2002;77:897–904. [DOI] [PubMed] [Google Scholar]
- 33. Pritchett EL. Management of atrial fibrillation. N Engl J Med. 1992;326:1264–1271. [DOI] [PubMed] [Google Scholar]
- 34. Collins LJ, Silverman DI, Douglas PS, et al. Cardioversion of nonrheumatic atrial fibrillation. Reduced thromboembolic complications with 4 weeks of precardioversion anticoagulation are related to atrial thrombus resolution. Circulation. 1995;92:160–163. [DOI] [PubMed] [Google Scholar]
- 35. Manning WJ, Leeman DE, Gotch PJ, et al. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13:617–623. [DOI] [PubMed] [Google Scholar]
- 36. Fuster V, Ryden LE, Cannom DS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 37. Rubboli A, Schlitt A, Kiviniemi T, et al; for the AFCAS Study Group. One‐year outcome of patients with atrial fibrillation undergoing coronary artery stenting: an analysis of the AFCAS Registry [published online ahead of print January 30, 2014]. Clin Cardiol. doi: 10.1002/clc.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hess PL, Greiner MA, Fonarow GC, et al. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2012;35:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 40. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131–136. [DOI] [PubMed] [Google Scholar]
- 41. Patel MR, Mahaffey KW, Garg J, et al., Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 42. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 43. Dagres N, Lewalter T, Lip GY, et al; Scientific Initiatives Committee, European Heart Rhythm Association. Current practice of antiarrhythmic drug therapy for prevention of atrial fibrillation in Europe: The European Heart Rhythm Association survey. Europace. 2013;15:478–481. [DOI] [PubMed] [Google Scholar]


