Abstract
As one of the main serotonergic (5HT) projections to the forebrain, the dorsal raphe nucleus (DRN) has been implicated in disorders of anxiety and depression. Although the nucleus contains the densest population of 5HT neurons in the brain, at least 50% of cells within this structure are non-serotonergic, including a large population of nitric oxide synthase (NOS) containing neurons. The DRN has a unique topographical efferent organization and can also be divided into sub-regions based on rostro-caudal and medio-lateral dimensions. NOS is co-localized with 5HT in the midline DRN but NOS-positive cells in the lateral wing (LW) of the nucleus do not express 5HT. Interestingly, the NOS LW neuronal population is immediately rostral to and in line with the cholinergic lateral dorsal tegmental nucleus (LDT). We used immunohistochemical methods to investigate the potential serotonergic regulation of NOS LW neurons and also the association of this cell grouping to the LDT. Our results indicate that >75% of NOS LW neurons express the inhibitory 5HT1A receptor and are cholinergic (> 90%). The findings suggest this assembly of cells is a rostral extension of the LDT, one that it is subject to regulation by 5HT release. As such the present study suggests a link between 5HT signaling, activation of cholinergic/NOS neurons, and the stress response including the pathophysiology underlying anxiety and depression.
Keywords: Serotonin, Dorsal raphe, Nitric oxide synthase, Lateral dorsal tegmental nucleus, acetylcholine
1. Introduction
The dorsal raphe nucleus is one of the main sources of serotonin (5HT) to the forebrain (O’Hearn and Molliver, 1984; Vertes, 1991) and has been implicated in both depression and anxiety disorders (Chauloff, 1993; Graeff et al., 1996; Lowry, 2002; Lowry et al., 2005; Underwood et al., 1999). Although the nucleus contains the densest concentration of 5HT neurons in the brain (Palkovits et al., 1974), at least 50% of the cells within this structure are non-serotonergic (non-5HT; Descarries et al. 1982; Steinbusch et al., 1980). One of the more prominent non-5HT phenotypes present are cells that express neuronal nitric oxide synthase (NOS; Johnson and Ma, 1993; Wotherspoon et al., 1994), the enzyme responsible for the synthesis of the gaseous transmitter nitric oxide (NO). Neurons containing NOS have a unique topographical distribution and co-localization pattern across the DRN (see Vasudeva et al., 2011 for review).
Both NOS and tryptophan hydroxylase (TrpH, the enzyme that synthesizes 5HT), co-localize across the midline (ML) DRN in approximately 75% of 5HT cells, but neurons expressing both enzymes are never found in the lateral wing (LW) subdivision of the nucleus (Okere and Waterhouse, 2006a,b). NOS-positive cells increase in number along the rostrocaudal extent of the nucleus, beginning with little to no expression in the rostral DRN and increasing numbers of NOS-positive cells concentrated in the caudal end of the nucleus, specifically within a region designated in previous studies as the LW (Johnson and Ma, 1993; Lu et al., 2010; Okere and Waterhouse, 2006a,b). Interestingly, the number of NOS-positive cells in this region of the brainstem and the intensity of immunohistochemical staining in these neurons is increased following restraint (Okere & Waterhouse, 2006a,b), thus linking these nitrergic cells to the stress response.
Because of their morphology and location within the brainstem, caudal and lateral NOS-expressing cells are typically associated with the LW sub-region of the DRN (Okere and Waterhouse, 2006a,b; Vasudeva et al., 2011). However, it is important to note the juxtaposition of the LW to the lateral dorsal tegmental nucleus (LDT). The LW is in line with and immediately rostral to the LDT (Paxinos and Watson, 1998), and forms a continuous column of cells that, in Nissl stained coronal sections, exhibit morphology that is indistinguishable from the LDT (Paxinos and Watson, 1998; Vasudeva et al., 2011). The LDT consists mainly of neurons that express both NOS and choline acetyl transferase (ChAT), the enzyme responsible for acetylcholine synthesis (Vincent et al., 1986), but the literature is not clear about the expression of ChAT within the DRN (Houser et al., 1983; Satoh et al., 1983; Wang et al., 2000; Woolf and Butcher, 1982). Given the well-established role of 5HT and the DRN in anxiety and stress responding, and the more recent findings of NOS-cell involvement in the DRN stress response (Okere and Waterhouse, 2006a,b), it is of interest to further investigate the receptor and neurochemical phenotype of this nitrergic cell population.
Historically, neurons have been assigned to the DRN based on their serotonergic content and/or their localization with respect to other 5HT-containing cell groups within this structure. Similarly, neurons have been assigned to the LDT based on their nitrergic (NOS-containing) or cholinergic content. Because of the juxtaposition of NOS-positive and 5HT cells in the border region between the caudal LW and rostral LDT, we postulated that NOS-containing cells in this area are also cholinergic and may be subject to regulation by 5HT release. Serotonergic regulation of NOS cells within this region could influence local circuit operations in response to stressor exposure. Prior investigations have demonstrated weak expression of the excitatory 5HT2A receptor on cells of the LDT (Fay and Kubin, 2000), but beyond this study the 5HT receptor complement of this structure is not clear (Bonnavion et al., 2010).
Long considered a defining feature of 5HT cells, the 5HT1A receptor is found on the soma and dendrites of 5HT neurons but is also expressed post-synaptically by non-5HT and/or cholinergic cells in DRN terminal fields (Kia et al., 1996a,b). The receptor has also been demonstrated on non-5HT cells within the DRN (Day et al, 2004; Kirby et al., 2003). Although 5HT1A receptors have yet to be identified on NOS neurons associated with the caudal LW, the presence of this receptor on both 5HT-NOS co-localized cells and non-5HT cells of the midline (Kirby et al., 2003; Okere and Waterhouse, 2006a) suggests the possibility that NOS only cells within the LW may also express 5HT1A receptors. Clark and colleagues (2006) have in fact demonstrated the presence of 5HT1A receptor protein within the DRN LW and extending into the LDT (termed “extra DRN wings” in their study), providing support for the possibility that neurons within this structure express the 5HT1A receptor. Serotonin has also been shown to inhibit LDT cells in vivo, indicating the presence of an inhibitory 5HT receptor (Koyama and Kayama, 1993).
The goals of the present study were to: 1) define the anatomical relationship between NOS-containing cells and cholinergic neurons within the caudal LW-LDT transition zone, and 2) determine if NOS-positive neurons in this region express the 5HT1A receptor. Further characterization of the NOS-containing cell group associated with the DRN will provide insight regarding the role of this class of neurons in the operation of the DRN circuitry as it relates to the stress response.
2. Methods
Animals
Male Long Evans rats (n = 7) ranging in weight from 300 – 450g (Charles River Laboratories, Wilmington, MA) were group housed in standard facilities and maintained on a 12:12 light-dark schedule with access to rat chow and water ad libitum. All animals used in these studies were treated in accordance with the published National Institute of Health Guide for Care and Use of Laboratory Animals. All protocols and procedures were approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee.
Immunohistochemical controls
For all immunohistochemical procedures, control sections of brain were processed as described above but with elimination of the primary and/or secondary antibody. Elimination of either of these antibodies would reveal any potential non-specific staining or fluorescence. When available, control peptides were included with the primary antibody incubation to verify specificity.
2.1. NOS, TrpH, GAD, and VAChT expression
Perfusion and DRN sectioning
Animals (n = 3) were overdosed with 5% isoflurane and perfused through the left ventricle with 0.1 M phosphate buffer (PB, pH 7.3 – 7.4), followed by 4% paraformaldehyde (PFA) in 0.1 M PB (pH 7.4). Brains were removed and post fixed in 4% PFA overnight, then transferred to 30% sucrose solution in 0.1 M PB for 3 – 5 days. Dorsal raphe sections 30 μm thick were collected in 0.1 M phosphate buffered saline (PBS), and sets of sections at 180 μm intervals across the DRN and LDT were processed for all immunohistochemical investigations.
Immunohistochemistry
The cholinergic profile of cells within the DRN and LDT was assessed with an antibody against the vesicular acetylcholine transporter (VAChT,), a marker for cholinergic cells (n = 3; Ichikawa et al., 1997). Serotonergic phenotype was determined by tryptophan hydroxylase-2 (TrpH2) staining, the rate limiting enzyme necessary for synthesis of 5HT. In addition to investigating the cholinergic content of NOS neurons, the cells were assayed for GABA decarboxylase (GAD, the enzyme responsible for the synthesis of GABA) to confirm that NOS lateral wing neurons did not co-localize this inhibitory neurotransmitter as described in the literature for the LDT (Boucetta and Jones, 2009; Wang et al., 1997). All sections were washed in PBS (5 x 5 min) followed by PBS + 0.03% Triton-X 100 (PBS-T), except for VAChT. Tissue was blocked in 10% donkey serum before incubating in the following primary antibodies for 36 – 48 hours at 4° Celsius followed by a two hour incubation period in corresponding sets of secondary antibodies at room temperature:
-
! NOS-GAD:
1:1000 mouse anti-nNOS (Sigma Inc., St. Louis, MO) + 1:500 rabbit anti-GAD65 (Sigma Inc., St. Louis, MO) or 1:500 rabbit anti-GAD67 (ImmunoStar Inc., Hudson, WI) in PBS-T
1:500 FITC donkey anti-mouse (green) + 1:500 AlexaFluor 594 donkey anti-rabbit (red, Invitrogen Corporation, Carlsbad, CA) in PBS
-
NOS-TrpH-VAChT
1:1000 rabbit anti-nNOS (ImmunoStar Inc.) + 1:1000 mouse anti-tryptophan hydroxylase 2 (Millipore, Billerica, MA) + 1:500 goat anti-VAChT (ImmunoStar Inc.) in PBS
1:500 AlexaFluor 594 donkey anti-rabbit (red) + 1:500 AlexaFluor 488 donkey anti-mouse (green) + 1:250 AlexaFluor 350 donkey anti-goat (blue) in PBS
At the completion of the secondary antibody incubation, all sections were washed in PBS (5 x 5 min), mounted on gelatin-coated microscope slides, dehydrated overnight, and cover slipped with Slowfade Gold (Invitrogen Corporation). In addition, one set of horizontal brain sections was processed for VAChT.
An additional set of horizontal sections (n = 1) was stained for NOS (red) and TrpH2 (green) following the procedure as described above in order to demonstrate the interaction of these neurochemicals in another perspective across the lateral wing.
2.2 5HT1A and NOS expression
Perfusion and sectioning
Naïve animals (n = 3) were overdosed with 5% isoflurane gas anesthesia and perfused through the left ventricle with 0.1 M PB (pH 7.3) followed by 4% PFA in 0.1 M PB with 0.05% gluteraldehyde (pH 7.4). Brains were post fixed in 4% PFA for 12 – 24 hours before being immersed in 30% sucrose in 0.1M PB (pH 7.4; without sodium azide) for 3 –7 days. Sections 20 μm thick were collected in 0.01 M PBS (pH 7.4), and sets of sections 120 μm apart were processed for immunohistochemistry.
Immunohistochemistry
Due to the sensitivity of the 5HT1A receptor antibody, the concentration and pH of buffer solutions were carefully monitored and the use of detergents such as Triton-X 100 was avoided as described by the manufacturer (ImmunoStar Inc.). Brain sections were washed in 0.01 M PBS (pH 7.4; 5 x 5 min) before blocking for one hour at room temperature with 10% goat serum. Sections were then incubated with 1:500 rabbit anti-5HT1A receptor (ImmunoStar Inc.) and 1:1000 mouse anti-nNOS (Sigma Inc.) for 48 hours at 4 ° Celsius. The tissue was then washed with 0.01 M PBS (5 x 5 min) before incubating in 1:500 AlexaFluor Fa’B fragment 594 goat anti-rabbit (red) and 1:1000 AlexaFluor 488 goat anti-mouse (green; Invitrogen Corporation) at room temperature for two hours. The sections were then washed in 0.01 M PBS (5 x 5 min), mounted on gelatin-coated microscope slides, dehydrated overnight, and cover slipped using Slowfade Gold (Invitrogen Corporation).
2.3 Imaging and Quantification
Imaging
All fluorescent immunohistochemical images were collected with a Leica DM RBE microscope affixed with an MTI 3CCD camera and MicroColor image converting system (model RGB-MS-C, CRI, Inc., Boston, MA). Images were generated utilizing IPLab computer software for Mac OS 9.2 (Beckton, Dickson and Company, Franklin Lakes, NJ) on a Power Macintosh G4 computer (Apple Inc., Cupertino, CA) and saved in TIF format. Files were edited for brightness and contrast using Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA).
Quantification
The dorsal raphe was divided into nine sub-regions for immunohistochemical analysis. In the rostrocaudal dimension: rostral (−7.3 – −7.8 mm Bregma), intermediate (−7.8 – −8.3 mm Bregma), and caudal (−8.3 – −8.8 mm Bregma) -levels and, within each dimension: dorsomedial (immediately below the cerebral aqueduct), ventromedial (between and immediately dorsal to the medial longitudinal fasciculus), and lateral wings (flanking both midline groupings) to describe specific sub-regions (see Vasudeva et al., 2011, 2012 for specific delineation of each rostral-caudal zone).
Three to four coronal sections per DRN level (rostral, intermediate, caudal) were quantified per animal. The LDT was examined solely for the presence or absence of the 5HT1A receptor and was not divided into sub-regions with the exception of the caudal LW - rostral LDT transition zone. The number of various cell types in dorsomedial, ventromedial, and lateral wing regions were then averaged across all sample sections. All images were quantified at 40x magnification, corresponding to approximately 150 μm2 of tissue. This magnification allowed the clear discrimination of neurons (approximately 15 – 20 μm in size) and punctate receptor staining across the three different wavelengths of fluorescence used. Labeled cells were quantified using individual grayscale color channels to ensure accuracy.
3. Results
3.1 VAChT expression in NOS DRN cells
Choline acetyl transferase (ChAT) is the requisite enzyme for ACh production and is considered a definitive marker for cholinergic cells in the CNS. Alternatively, the vesicular acetylcholine transporter (VAChT) can also be used to detect cholinergic neurons, as this transporter protein is necessary for the intracellular movement of ACh into secretory vesicles in the axon. Interestingly, the gene for VAChT is located within an intron of the gene for ChAT, thus immunohistochemical localization of either is sufficient evidence for establishing the cholinergic content of central neurons (Ichikawa et al., 1997; Wang et al., 1998).
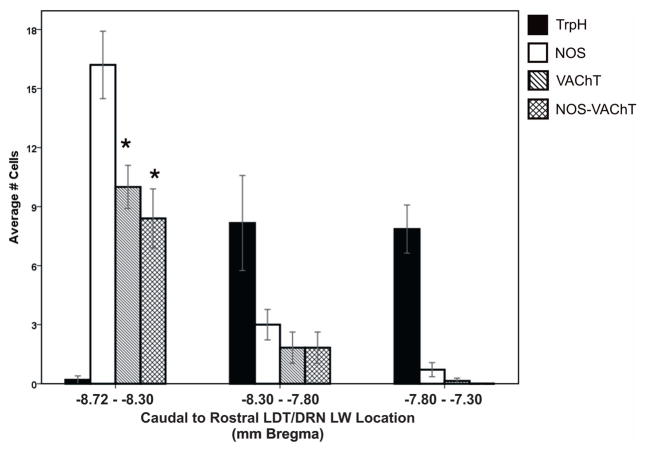
Upon examination of horizontal and coronal sections through the DRN LW, a striking relationship between 5HT, NOS, and VAChT expressing cells was revealed (Figures 1 and 2, respectively). Cell counts are represented in Table 1. Cholinergic neurons were not found in any midline DRN regions but were identified in substantial numbers in the intermediate and caudal regions of the DRN at the LW/LDT border. Within these locations, NOS was co-expressed in 80 – 100% of VAChT-positive cells. In contrast, VAChT was never expressed in TrpH-positive (i.e. 5HT) cells in any portion of the DRN. A multiple comparisons ANOVA showed a significant effect of sub-region on VAChT expression (F(8,44) = 12.02; p < .001) and on VAChT-NOS expression (F(8,44) = 4.22; p < .001). Post-hoc Bonferroni tests revealed that VAChT and VAChT-NOS expression in the caudal LW – LDT transition zone were significantly different than all other DRN sub-regions (Figure 3; p < .001).
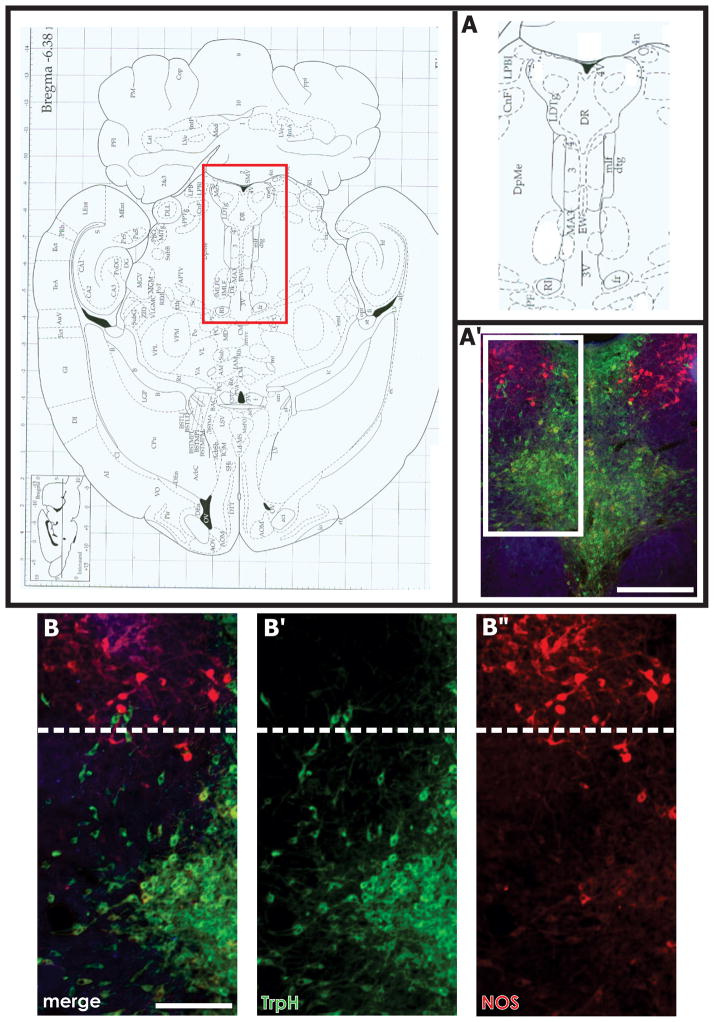
Figure 1. Horizontal Sections of NOS and VAChT Staining across the DRN.
Horizontal sections of the rat brainstem at the level of the DRN and lateral dorsal tegmental nucleus (LDT) reveal the lateral wing sub-region of the DRN and the LDT (A′, white box) form a continuous column of cells along the rostral-caudal dimension. The caudal region of the LDT is bordered by the fourth ventricle (top of figure A and A′). Tryptophan hydroxylase (TrpH) positive neurons (B′, green) clearly intermingle with NOS positive cells (B″, red) along the caudal lateral wing and rostral LDT border (B, B′, B″, white dashed line). Scale bars: A′ = 500 μm, B = 250 μm. Abbreviations in A: 3-oculomotor nucleus, 3V- third ventricle, 4- trochlear nucleus, 4n- trochlear nerve root, 4V- fourth ventricle, CnF- cuneiform nucleus, DpMe- deep mesencephalic nucleus, DR- dorsal raphe nucleus, dtg-dorsal tegmental bundle, EW- nucleus of Edinger Westphal, fr- fasciculus retroflexus, LDTg- lateral dorsal tegmental nucleus, mlf- medial longitudinal fasciculus, PF- parafascicular thalamic nucleus, RI- rostral interstitial nucleus of the mlf.
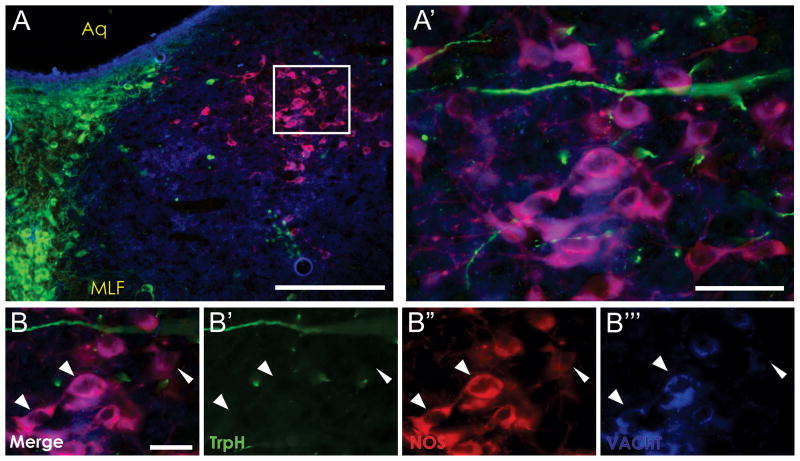
Figure 2. Juxtaposition of NOS, TrpH, and VAChT in the lateral wing of the DRN.
NOS-positive, tryptophan hydroxylase (TrpH)-negative cells located within the caudal portion of the DRN lateral wing (A, A′) express the vesicular acetylcholine transporter (VAChT). High magnification images (B – B‴, arrowheads) show NOS-positive neurons (B″, red) co-localize with VAChT (B‴, blue) but not TrpH (B′, green). This finding indicates that NOS-positive, 5HT-negative cells within the DRN lateral wing are actually the rostral-most extension of the cholinergic lateral dorsal tegmental nucleus. Scale bars: A = 250 μm, A′ = 50 μm, B = 30 μm. Aq- cerebral aqueduct, MLF- medial longitudinal fasciculus.
Table 1.
Cell counts per DRN sub-region at 40X magnification.
|
Cellular Phenotype
| |||||||
|---|---|---|---|---|---|---|---|
| DRN Location | NOS | TrpH | 5HT1A | VAChT | NOS + TrpH | NOS + 5HT1A | NOS + VAChT |
| rDM | 4.25 (1.47) | 11.88 (1.76) | 13.50 (2.41) | 0 (0.44) | 2.63 (0.86) | 1.50 (1.44) | 0 (0.41) |
| rVM | 5.75 (1.47) | 12.25 (1.76) | 15.50 (2.41) | 0 (0.34) | 3.50 (0.86) | 3.75 (1.44) | 0 (0.41) |
| rLW | 0.71 (1.56) | 7.86 (1.88) | 8.67 (2.79) | 0.14 (0.37) | 0 (0.92) | 0.67 (1.67) | 0 (0.41) |
| iDM | 7.17 (1.67) | 13.33 (2.03) | 15.43 (1.83) | 0 (0.47) | 4.50 (0.99) | 5.29 (1.09) | 0 (0.48) |
| iVM | 9.17 (1.66) | 14.50 (2.03) | 19.25 (1.71) | 0.17 (0.40) | 7.33 (0.99) | 10.88 (1.02) | 0 (0.48) |
| iLW | 3.00 (1.66) | 8.17 (2.03) | 11.22 (1.61) | 1.83 (0.40) | 0 (0.99) | 5.67 (0.96) | 1.83 (0.48) |
| cDM | 1.80 (1.82) | 5.40 (2.23) | 11.00 (2.16) | 0 (0.40) | 1.40 (1.09) | 2.80 (1.27) | 0 (0.52) |
| cVM | 10.60 (1.82) | 14.40 (2.23) | 13.75 (1.71) | 0 (0.40) | 8.00 (1.09) | 6.00 (1.02) | 0 (0.52) |
| cLW/LDT | 16.20* (1.82) | 0.20 (2.23) | 14.31 (1.21) | 10.00* (0.40) | 0 (1.09) | 8.94 0.72 |
8.40* (0.52) |
Standard error is depicted below each value in parenthesis.
= p < .001 compared to all other DRN sub-regions.
r = rostral, i = intermediate, c = caudal. DM = dorsomedial, VM = ventromedial, LW = lateral wing. LDT = lateral dorsal tegmental area.
Figure 3. Distribution and cell counts of NOS, TrpH, and VAChT cells across the DRN.
The average number of cells expressing tryptophan hydroxylase (TrpH, black bars), nitric oxide synthase (NOS, white bars), vesicular acetylcholine transferase (VAChT, diagonal bars), or NOS and VAChT (hashed bars) are compared caudal to rostral across the rostral LDT/caudal LW border (−8.72 to −8.30 Bremga) through to the rostral LW subregion of the DRN (−7.80 to −7.30 Bregma). The LDT/LW border region (left side data bars; −8.72 - −8.30 Bregma) contains significantly higher numbers of VAChT and NOS-VAChT expressing cells than more rostral regions of the LW or any DRN midline subregions. (* p < .001)
3.2 5HT1A expression in the DRN and LDT
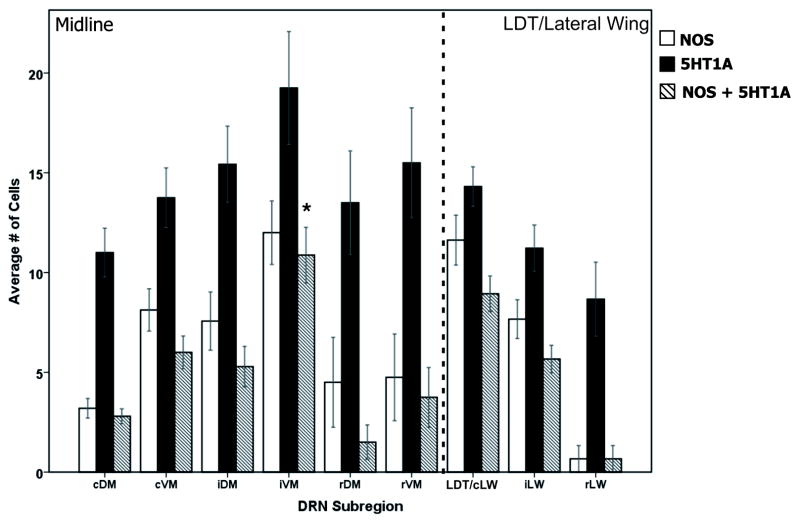
The presence of the 5HT1A receptor was indicated by punctate staining surrounding cell bodies (Figure 4) at all DRN levels (rostral, intermediate, and caudal) and in all sub-regions (dorsomedial, ventromedial, and LW) of the nucleus (Figure 5; Table 1). With the exception of the rostral dorsomedial region (rDM) and the rostral LW (rLW) (neither of which contain NOS-positive cells), greater than 70% of DRN NOS-positive neurons expressed the 5HT1A receptor, including the NOS-only cells within the LW of the DRN. A multiple comparisons ANOVA revealed a significant effect of sub-region on the expression of the 5HT1A receptor (F(8,55) = 2.39, p < .05), NOS (F(8,55) = 5.62, p < .001), and double labeled cells (F(8, 55) = 8.50, p < .001). Post- hoc Bonferroni tests indicated significant differences between several sub-regions for NOS, 5HT1A, and double labeled cells. Expression of 5HT1A receptor-positive cells was significantly higher in the intermediate ventromedial (iVM) sub-region compared to the intermediate LW (iLW; p < .05), and, as expected, higher numbers of NOS cells were present in the caudal versus rostral LW sub-regions (p < .05). The caudal LW – LDT transition zone had higher numbers of double labeled cells (NOS + 5HT1A) compared to the dorsomedial sub-region (p < .005). Interestingly, with the exception of the caudal LW – LDT transition zone, the intermediate ventromedial sub-region contained more double labeled cells compared to all other DRN subregions (p < .05). Results are shown graphically in Figure 5.
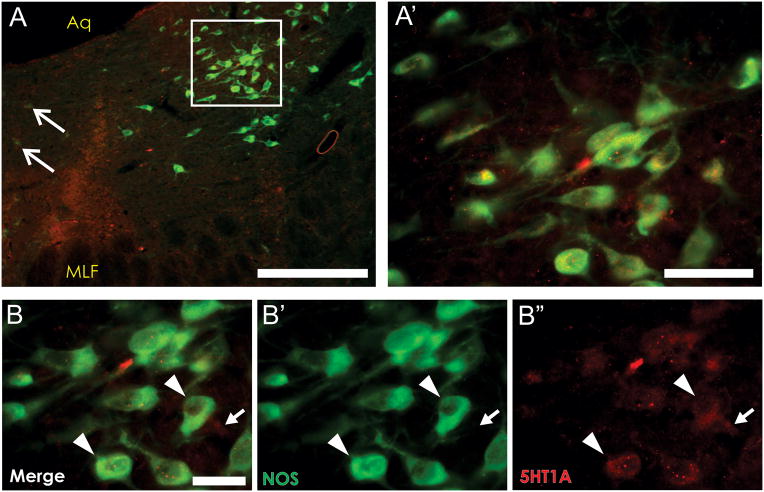
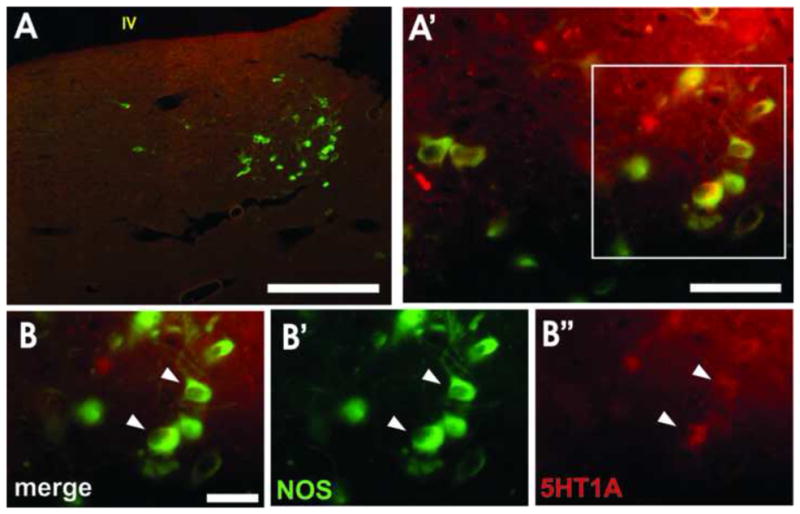
Figure 4. Co-localization of the 5HT1A receptor and NOS on lateral wing neurons in the caudal DRN.
NOS-positive neurons located in the lateral wing DRN- rostral LDT border (A, A′) express the 5HT1A receptor. White arrows in (A) indicates NOS-positive cells in the DRN midline to confirm the caudal DRN location (Vasudeva et al., 2011). High magnification (B) demonstrates clear co-expression of NOS (green, B′) and the 5HT1A receptor (red, B″) on the same cells (arrowheads). A non-NOS cell expressing the 5HT1A receptor can also be seen (B-B″, white arrow). Scale bars: A = 500 μm, A′ = 50 μm, B = 30 μm. Aq- cerebral aqueduct, MLF= medial longitudinal fasciculus.
Figure 5. Distribution and cell counts of NOS cells and 5HT1A expression across the DRN.
The average number of NOS (white bars), 5HT1A receptor (black bars) and double labeled cells (hashed bars) are shown across the midline and lateral wing DRN. Both 5HT1A-only cells and double labeled cells were seen in every DRN sub-region. With the exception of the caudal lateral wing (cLW)/LDT border, the intermediate ventromedial sub-region (iVM) contained the highest number of double labeled cells compared to all other subregions (* = p < .05). cDM- caudal dorsomedial, cLW-caudal lateral wing, cVM- caudal ventromedial, iDM- intermediate dorsomedial, iLW- intermediate lateral wing, iVM- intermediate ventromedial, LDT- lateral dorsal tegmental area, rDM- rostral dorsomedial, rLW- rostral lateral wing, rVM- rostral ventromedial.
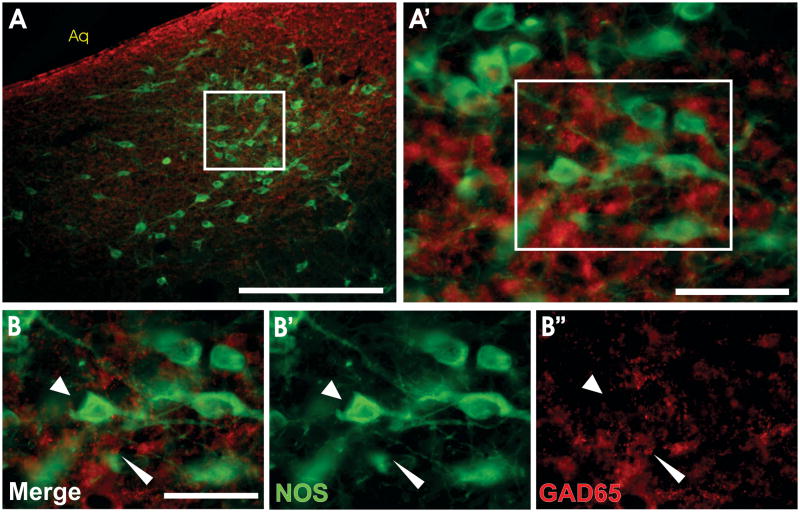
Examination of the rostral and intermediate levels of the LDT revealed expression of the 5HT1A receptor in many NOS-positive neurons within this nucleus (Figure 6). Given the extent of co-localization of NOS and VAChT in the LDT, we postulate that many of the NOS cells expressing the 5HT1A receptor in this structure are also cholinergic (Grant and Highfield, 1991; Kayama and Ogawa, 1987; Leonard et al., 1995; Satoh el al., 1983). In support of previous findings (Wang et al., 1997), we further demonstrated that NOS positive neurons in the DRN lateral wing do not co-localize with GABA (Figure 7).
Figure 6. Expression of NOS and the 5HT1A receptor in the LDT.
NOS-positive, 5HT-lacking neurons of the lateral dorsal tegmental nucleus (LDT; A, A′) express the 5HT1A receptor. High magnification (B, arrowheads) shows NOS-positive cells (B′, green) co-expressing the 5HT1A receptor (B″, red). Scale bars: A = 500 μm, A′ = 50 μm, B = 30 μm. IV- fourth ventricle.
Figure 7. NOS LW cells are not GABAergic.
NOS-positive cells in the DRN lateral wing-LDT transition zone (A, A′) are not GABAergic cells. High magnification (B) clearly shows NOS-positive cells (B′, green, arrowheads) are separate from GAD65-positive neurons (B″, red, pointed arrowheads). Scale bars: A = 250 μm, A′ = 50 μm, B = 50 μm. Aq- cerebral aqueduct.
4. Discussion
The major findings of the present study can be summarized as follows:
In rat brain the LDT is in line with and caudal to the DRN LW, forming a nearly continuous column of morphologically similar cells within the brainstem (see Figure 1).
The cells of the rostral LDT and caudal LW intermingle to a limited extent at their borders (see Figures 1, 2). Previous studies may have mistaken LDT cells as LW cells because of this proximity.
NOS cells located within the caudal LW – LDT transition zone are cholinergic and thus belong to the LDT (see Figures 2, 3). These cells are caudal to serotonin neurons in the LW.
NOS cells within the LDT and midline DRN express 5-HT1A receptors (see Figures 4, 5, and 6).
The presence of nitric oxide producing cells within the borders of the DRN has been firmly established (Johnson and Ma, 1993; Simpson et al., 2003; Wang et al., 1997; Wotherspoon et al., 1994) but, beyond the evidence of co-localization of NOS and 5HT in the midline DRN and segregation of NOS and 5HT laterally, not much is known about the receptor complement or co-localization of other transmitters in these cells. The relationship between NOS and 5HT within the DRN LW and the juxtaposition of the cholinergic/nitrergic LDT to this portion of the DRN calls into question the boundary between these two structures and the functional relationships between these classes of cells. In the present study we provided evidence that the NOS-only neurons previously included as part of the caudal LW of the DRN (Okere and Waterhouse, 2006a,b; Vasudeva et al., 2011) are, by virtue of their distribution and neurochemical phenotype, the rostral most extension of the LDT. Nevertheless, because of the expression of 5HT1A receptors, the activity of these cells is subject to modulation by 5HT release. These findings and the assignment of cells to DRN LW vs LDT are critically important for interpreting the outcomes of electrophysiological recording and behavioral studies aimed at elucidating the role of this region of the brainstem in the stress response and the pathogenesis of anxiety.
The LDT is composed mainly of acetylcholine- (ACh) and NOS-containing neurons. These cells are distributed throughout the nucleus, expressing one or both transmitters, and are considered a defining feature of the structure (Grant and Highfield, 1991; Kayama and Ogawa, 1987; Koyama et al., 1994; Leonard et al., 1995; Satoh et al., 1983; Vincent et al., 1986). The DRN LW is aligned with and immediately rostral to the LDT, yet the anatomical or neurochemical boundaries separating the two regions have not been made clear in the literature. Many studies associate the NOS containing cells within the vicinity of the caudal LW with the DRN, while others designate these cells as part of the LDT (Halberstadt and Balaban, 2008; Lee et al., 2008; Okere and Waterhouse, 2006b; Satoh and Filbiger, 1986; Simpson et al., 2003). These conflicting assignments confound the interpretation of electrophysiological, pharmacological and behavioral characterizations of the cells in this region since the DRN and LDT are associated with different functions. While both structures are involved in the regulation of arousal and sleep (Fibiger et al., 1991; Kayama et al., 1992; Koyama and Sakai, 2000; Leonard and Lydic, 1995; Lowry et al., 2008a; Nabeshima, 1993; Urbain et al., 2006), the DRN is also associated with the stress response and the pathophysiology underlying anxiety, depression, aggressive behavior, substance abuse, and obsessive-compulsive disorder (Abrams et al., 2005; Blier and de Montigny, 1999; Graeff et al., 1996, 1997; Molliver et al., 1990; Starr et al., 2008; Wilson et al., 1993).
An increase in rostral LDT NADPH-d staining following stressor exposure in the rat is indicative of increased NOS activity and NO production (Okere and Waterhouse, 2006a,b), but it does not necessarily reflect an increased discharge of these neurons. However, Lu et al. (2010) demonstrated the expression of NOS along axons arising from this region of the DRN, suggesting the possibility of an increase in NO release in LDT terminal fields following stressor exposure. Moreover, NO release has been shown to increase the activity of GABA-containing cells (Bains and Ferguson, 1997; Yang et al., 2007), thus an increase in NO production locally at the level of the LDT may activate neighboring GABAergic cells in the LDT itself, the DRN, and/or other neighboring brainstem nuclei and may also influence inhibitory tone in more remote terminal fields. The putative role of concomitant release of acetylcholine in this group of NADPH-d positive cells can only be inferred from the functions of the neural circuits that receive input from these cells. Because of their projections, it appears that activation of this group of NOS-producing cells may impact sensory signal processing during stressor presentation (Simpson et al., 2003; Vincent et al., 1986).
The results described in this report, in conjunction with the results of other prior studies, provide evidence of non-5HT cell populations within the DRN that express the 5HT1A receptor (Clark et al., 2006; Day et al, 2004; Kirby et al, 2003). Stimulation of the 5HT1A receptor suppresses cell firing via Gi/o G-protein signaling, which inhibits cyclic-AMP (cAMP) in heteroreceptor configurations but not in raphe autoreceptors (Clarke et al., 1996). A difference in the expression of specific Gi/o subunit subtypes in different brain regions is believed to be responsible for this functional distinction (Mannoury la Cour et al., 2006). In addition to cAMP modulation, activation of the 5HT1A receptor opens potassium (K+) channels (specifically G-protein coupled inwardly rectifying potassium (GIRK) channels), and modifies calcium (Ca2+) channel conductance and likely triggers other downstream effects (Penington and Kelly, 1990; Polter and Li, 2010; Valdizan et al., 2009). Activation of this receptor via 5HT or agonist administration ultimately results in hyperpolarization of the neuron and a dose-dependent decrease in cell firing (Martin et al., 1999). However, the different G-protein subunit subtypes associated with anatomical distribution of 5HT1A receptors (auto- versus heteroreceptors) may result in different time courses of inhibitory action (Mannoury la Cour et al., 2006; Polter and Li, 2010; Valdizan et al., 2009). Thus, it is reasonable to predict that following activation by 5HT, 5HT1A receptors expressed on LDT NOS neurons (heteroreceptors) may differ in sensitivity and mechanistic action compared to those on DRN cells (autoreceptors). This hypothesis can be tested by recording the extracellular responses of these cells to 5HT1A agonists, applied either iontophoretically or systemically.
Implications
These data suggest that anxiolytic and antidepressant drugs that exert their effects through the 5HT1A receptor, most notably the selective serotonin reuptake inhibitors (SSRIs) which elevate extracellular 5HT and 5HT1A partial agonists such as buspirone, may have a prominent impact on not only serotonergic but also cholinergic and nitrergic cell functions. Investigating the sensitivity of 5HT1A receptors on DRN 5HT vs LDT NOS-positive neurons would provide insight into the interaction of these two structures during pharmacological treatments for anxiety and depression. This anatomical relationship may also underlie specific roles for the DRN LW versus the midline with regard to differential signaling in response to acute versus chronic stressors.
Highlights.
The relationship of NOS cells of the DRN to the lateral dorsal tegmental nucleus (LDT) is examined.
Immunohistochemistry shows DRN lateral wing (LW) NOS cells have 5HT1A receptors and are cholinergic.
LW NOS cells appear to be a rostral extension of the cholinergic LDT and not part of the DRN.
Studies grouping NOS neurons with the DRN LW did not consider the cholinergic content of these cells.
A re-examination of prior physiological and behavioral DRN data with regard to anxiety is needed.
Abbreviations
- 5HT
serotonin
- 5HT1A
serotonin-1A receptor
- ACh
acetylcholine
- cAMP
cyclic adenosine monophosphate
- ChAT
choline acetyl transferase
- DRN
dorsal raphe nucleus
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- LDT
lateral dorsal tegmental nucleus
- LW
lateral wing
- ML
midline
- NO
nitric oxide
- NOS
nitric oxide synthase
- TrpH
tryptophan hydroxylase
- VAChT
vesicular acetylcholine transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rani K. Vasudeva, Email: rani.vasudeva@temple.edu.
Barry D. Waterhouse, Email: Barry.Waterhouse@drexelmed.edu.
References
- Abrams JK, et al. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol. 1997;499 (Pt 3):733–746. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Bonnavion P, et al. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518:2744–2770. doi: 10.1002/cne.22331. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29:4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Clark MS, et al. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- Clarke WP, et al. Lack of 5-hydroxytryptamine1A-mediated inhibition of adenylyl cyclase in dorsal raphe of male and female rats. J Pharmacol Exp Ther. 1996;277:1259–1266. [PubMed] [Google Scholar]
- Day HE, et al. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, et al. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- Fibiger HC, et al. Behavioral pharmacology and biochemistry of central cholinergic neurotransmission. Adv Exp Med Biol. 1991;295:399–414. doi: 10.1007/978-1-4757-0145-6_23. [DOI] [PubMed] [Google Scholar]
- Graeff FG, et al. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Graeff FG, et al. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Highfield DA. Extracellular characteristics of putative cholinergic neurons in the rat laterodorsal tegmental nucleus. Brain Res. 1991;559:64–74. doi: 10.1016/0006-8993(91)90287-6. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Balaban CD. Selective anterograde tracing of nonserotonergic projections from dorsal raphe nucleus to the basal forebrain and extended amygdala. J Chem Neuroanat. 2008;35:317–325. doi: 10.1016/j.jchemneu.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, et al. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266:97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, et al. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: in situ hybridization histochemistry and immunohistochemistry. J Chem Neuroanat. 1997;13:23–39. doi: 10.1016/s0891-0618(97)00021-5. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ma PM. Localization of NADPH diaphorase activity in monoaminergic neurons of the rat brain. J Comp Neurol. 1993;332:391–406. doi: 10.1002/cne.903320402. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ogawa T. Electrophysiology of ascending, possibly cholinergic neurons in the rat laterodorsal tegmental nucleus: comparison with monoamine neurons. Neurosci Lett. 1987;77:277–282. doi: 10.1016/0304-3940(87)90512-x. [DOI] [PubMed] [Google Scholar]
- Kayama Y, et al. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- Kia HK, et al. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996a;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kia HK, et al. Serotonin1A receptors are expressed by a subpopulation of cholinergic neurons in the rat medial septum and diagonal band of Broca--a double immunocytochemical study. Neuroscience. 1996b;74:143–154. doi: 10.1016/0306-4522(96)00087-5. [DOI] [PubMed] [Google Scholar]
- Kirby LG, et al. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Kayama Y. Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by ionophoresis of these transmitters in rat brainstem nuclei. Neuroscience. 1993;55:1117–1126. doi: 10.1016/0306-4522(93)90325-a. [DOI] [PubMed] [Google Scholar]
- Koyama Y, et al. Sensory responsiveness of “broad-spike” neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–1031. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Sakai K. Modulation of presumed cholinergic mesopontine tegmental neurons by acetylcholine and monoamines applied iontophoretically in unanesthetized cats. Neuroscience. 2000;96:723–733. doi: 10.1016/s0306-4522(00)00004-x. [DOI] [PubMed] [Google Scholar]
- Lee SB, et al. The collateral projection from the dorsal raphe nucleus to whisker-related, trigeminal sensory and facial motor systems in the rat. Brain Res. 2008;1214:11–22. doi: 10.1016/j.brainres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Leonard CS, et al. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- Leonard TO, Lydic R. Nitric oxide synthase inhibition decreases pontine acetylcholine release. Neuroreport. 1995;6:1525–1529. doi: 10.1097/00001756-199507310-00015. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, et al. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, et al. The dorsal raphe nucleus and median raphe nucleus: organization and projections. In: Monti JM, Jacobs BL, Pandi-Perurnal, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser Verlag; Boston, PA: 2008. pp. 25–68. [Google Scholar]
- Lu Y, et al. Coexpression of serotonin and nitric oxide in the raphe complex: cortical versus subcortical circuit. Anat Rec (Hoboken) 2010;293:1954–1965. doi: 10.1002/ar.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoury la Cour C, et al. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–1021. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- Martin LP, et al. Electrophysiological comparison of 5-Hydroxytryptamine1A receptor antagonists on dorsal raphe cell firing. J Pharmacol Exp Ther. 1999;288:820–826. [PubMed] [Google Scholar]
- Molliver ME, et al. Neurotoxicity of MDMA and related compounds: anatomic studies. Ann N Y Acad Sci. 1990;600:649–661. doi: 10.1111/j.1749-6632.1990.tb16916.x. discussion 661-644. [DOI] [PubMed] [Google Scholar]
- Nabeshima T. Behavioral aspects of cholinergic transmission: role of basal forebrain cholinergic system in learning and memory. Prog Brain Res. 1993;98:405–411. doi: 10.1016/s0079-6123(08)62424-3. [DOI] [PubMed] [Google Scholar]
- O’Hearn EMM. Organization of raphe-cortical projections in the rat: a quantitative retrograde study. Brain Research Bulletin. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD. Activity-dependent heterogeneous populations of nitric oxide synthase neurons in the rat dorsal raphe nucleus. Brain Res. 2006a;1086:117–132. doi: 10.1016/j.brainres.2006.02.107. [DOI] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD. Acute restraint increases NADPH-diaphorase staining in distinct subregions of the rat dorsal raphe nucleus: implications for raphe serotonergic and nitrergic transmission. Brain Res. 2006b;1119:174–181. doi: 10.1016/j.brainres.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Palkovits M, et al. Serotonin content of the brain stem nuclei in the rat. Brain Res. 1974;80:237–249. doi: 10.1016/0006-8993(74)90688-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Penington NJ, Kelly JS. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron. 1990;4:751–758. doi: 10.1016/0896-6273(90)90201-p. [DOI] [PubMed] [Google Scholar]
- Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010;22:1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, et al. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983;11:693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Simpson KL, et al. Differential expression of nitric oxide in serotonergic projection neurons: neurochemical identification of dorsal raphe inputs to rodent trigeminal somatosensory targets. J Comp Neurol. 2003;466:495–512. doi: 10.1002/cne.10912. [DOI] [PubMed] [Google Scholar]
- Starr MA, et al. MDMA (3,4-methylenedioxymethamphetamine)-mediated distortion of somatosensory signal transmission and neurotransmitter efflux in the ventral posterior medial thalamus. J Pharmacol Exp Ther. 2008;327:20–31. doi: 10.1124/jpet.108.139337. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, et al. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci Lett. 1980;19:137–142. doi: 10.1016/0304-3940(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Underwood MD, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Urbain N, et al. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdizan EM, et al. Agonist-dependent modulation of G-protein coupling and transduction of 5-HT1A receptors in rat dorsal raphe nucleus. Int J Neuropsychopharmacol. 2009:1–9. doi: 10.1017/S1461145709990940. [DOI] [PubMed] [Google Scholar]
- Vasudeva RK, et al. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J Chem Neuroanat. 2011;41:281–293. doi: 10.1016/j.jchemneu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Vasudeva RK, et al. Cellular Physiology of the DRN: Implications for Anxiety and Depression. In: Hall FS, editor. Serotonin: Biosynthesis, Regulation, and Health Implications. NOVA; New York: 2012. pp. 97–112. [Google Scholar]
- Vertes RP. A Pha-L analysis of ascending projections of the dorsal raphe nucleus in the rat. The Journal of Comparative Neurology. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vincent SR, et al. Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience. 1986;17:167–182. doi: 10.1016/0306-4522(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Wang QP, et al. Electron microscopic study of GABAergic synaptic innervation of nitric oxide synthase immunoreactive neurons in the dorsal raphe nucleus in the rat. Synapse. 1997;25:24–29. doi: 10.1002/(SICI)1098-2396(199701)25:1<24::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wang QP, et al. An electron microscopic observation of the vesicular acetylcholine transporter-immunoreactive fibers in the rat dorsal raphe nucleus. Brain Res Bull. 1998;46:555–561. doi: 10.1016/s0361-9230(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Wang QP, et al. Synaptic contacts between serotonergic and cholinergic neurons in the rat dorsal raphe nucleus and laterodorsal tegmental nucleus. Neuroscience. 2000;97:553–563. doi: 10.1016/s0306-4522(99)00605-3. [DOI] [PubMed] [Google Scholar]
- Wilson MA, et al. Reactions of 5-HT neurons to drugs of abuse: neurotoxicity and plasticity. NIDA Res Monogr. 1993;136:155–178. doi: 10.1037/e495922006-009. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1982;8:751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, et al. Serotonin and NADPH-diaphorase in the dorsal raphe nucleus of the adult rat. Neurosci Lett. 1994;173:31–36. doi: 10.1016/0304-3940(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Yang Q, et al. Kv1.1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience. 2007;149:315–327. doi: 10.1016/j.neuroscience.2007.08.007. [DOI] [PubMed] [Google Scholar]