Abstract
The human dorsolateral prefrontal cortex (dlPFC) is crucial for monitoring and manipulating information in working memory, but whether such contributions are domain-specific remains unsettled. Neuroimaging studies have shown bilateral dlPFC activity associated with working memory independent of stimulus domain, but the causality of this relationship cannot be inferred. Repetitive transcranial magnetic stimulation (rTMS) has the potential to test whether the left and right dlPFC contribute equally to verbal and spatial domains, however this is the first study to investigate the interaction of task domain and hemisphere using offline rTMS to temporarily modulate dlPFC activity. In separate sessions, twenty healthy right-handed adults received 1Hz-rTMS to left dlPFC, right dlPFC, plus the vertex as a control site. Working memory performance was assessed pre- and post-rTMS using both verbal-‘letter’ and spatial-‘location’ versions of the 3-back task. Response times were faster post-rTMS, independent of task domain or stimulation condition, indicating the influence of practice or other nonspecific effects. For accuracy, rTMS of the right dlPFC, but not the left dlPFC or vertex, led to a transient dissociation: reducing spatial, but increasing verbal accuracy. A post-hoc correlation analysis found no relationship between these changes indicating the substrates underlying verbal and spatial domains are functionally independent. Collapsing across time, there was a trend towards a double dissociation, suggesting a potential laterality in functional organization of verbal and spatial working memory. At a minimum, these findings provide human evidence for domain-specific contributions of the dlPFC to working memory and reinforce the potential of rTMS to ameliorate cognition.
Keywords: rTMS, dlPFC, working memory, functional specialization, functional neuroanatomy
1 Introduction
Working memory refers to the use and manipulation of retained information to guide behavior (Courtney et al. 1998). The crucial role of the prefrontal cortex in working memory is supported by invasive research in nonhuman primates (Bauer and Fuster 1976; Funahashi et al. 1993), and human lesion (Kumar et al. 2013), neuroimaging (D’Esposito et al. 1995) and noninvasive brain stimulation studies (Mottaghy et al. 2000; Postle et al. 2006). In particular, the dorsolateral prefrontal cortex (dlPFC) is associated with monitoring and updating information (D’Esposito et al. 1999; Postle et al. 2000), is critical for tasks with complex demands and high-load conditions (du Boisgueheneuc et al. 2006; Kumar et al. 2013), and has been posited as the source of top-down signals that bias activity in posterior association cortices (Feredoes et al. 2011; Lee and D’Esposito 2012).
Presently there is little consensus whether dlPFC function is dissociable by working memory domain. Meta-analyses of normative neuroimaging data (Owen et al. 2005; Nee et al. 2013) reveal the left inferior frontal gyrus and right caudal superior frontal sulcus show selectivity for verbal and spatial information, respectively; however both domains show relatively equivalent activity within the region that most closely corresponds to the dlPFC—the intermediate middle frontal gyrus at the putative junction of Brodmann areas 9 and 46. While inferences from neuroimaging are limited to correlations, transcranial magnetic stimulation (TMS) can probe the causality of brain-behavior relationships (Pascual-Leone et al. 1999). However, only a few TMS studies have directly investigated content-selectivity in the dlPFC, either by comparing the effect of left versus right stimulation on a single domain (Mull and Seyal 2001; Mottaghy, Pascual-Leone, et al. 2003), or assessing the impact of stimulating one hemisphere on multiple domains (Mottaghy et al. 2002; Feredoes et al. 2011). Owing in part to their relatively small sample sizes and diverse approaches, these studies offer only tepid support for the lateralization of verbal and spatial domains.
One study (Sandrini et al. 2008) found evidence of lateralized dlPFC function from directly comparing left and right stimulation on verbal and spatial n-back tasks. The authors found a double-dissociative interaction between hemisphere and working memory domain, but only when the task required suppression of features from the opposing domain. Furthermore, their use of TMS to disrupt ongoing processes is suboptimal as TMS side-effects can potentially confound concomitant cognitive processes (Abler et al. 2005). An alternative approach exploits the potential of repetitive TMS (rTMS) to modulate activity beyond the duration of stimulation; however no study has yet applied this approach to investigate the intersection of hemisphere and domain. The present study aims to fill this gap. By independently modulating left and right dlPFC activity and assessing both verbal and spatial working memory tasks, the study will directly test the hypothesis that the dlPFC is functionally organized by domain. Differential effects of left and right dlPFC modulation will be taken as evidence of hemispheric specialization, while opposing changes to verbal and spatial working memory will be interpreted as evidence of domain selectivity.
2 Materials and Methods
2.1 Ethics Statement
The experiments in this study were conducted on adult human participants. All forms and procedures used in the experiment conformed to the Declaration of Helsinki and received appropriate approval by the Institutional Review Board at Boston University School of Medicine. All participants provided written consent upon enrollment in the study and were compensated for their time proportional to their involvement.
2.2 Participants
The present study consisted on a primary experiment (Experiment 1) conducted on a group of 20 healthy adults (three male, 17 female), of mean age 20.8 years (range = 18.3 – 25.5), and a secondary control experiment (Experiment 2) conducted on a group of 11 healthy adults (seven male, three female), of mean age 27.5 years (range = 21.4 – 32.4), including one crossover from the primary experiment (see Table 1). All participants were right-handed and fluent English speakers and none had any known history of neurological disease. Prior to each TMS or MRI procedure, participants were thoroughly screened for safety against known exclusion criteria (Keel et al. 2001).
Table 1.
Study Demographics
| Sex | Age (y) | Sessions (in order)a | |
|---|---|---|---|
| Participant 1 | F | 20 | F3, F4, Cz, MRI |
| Participant 2 | F | 19 | F4, F3, Cz, |
| Participant 3 | F | 20.3 | F3, F4, Cz, MRI |
| Participant 4 | F | 23.4 | F4, F3, Cz |
| Participant 5 | F | 20 | F3, F4, Cz |
| Participant 6 | F | 19.3 | F4, F3, Cz, MRI |
| Participant 7 | F | 20.1 | F3, F4, Cz, MRI |
| Participant 8 | F | 18.3 | F4, F3, Cz, MRI |
| Participant 9 | F | 19.9 | F3, F4, Cz, MRI |
| Participant 10 | M | 18.5 | F4, F3, Cz, MRI |
| Participant 11 | M | 25.5 | F3, Cz, F4 |
| Participant 12 | F | 20 | F4, Cz |
| Participant 13 | F | 23.7 | F3, Cz, F4, MRI |
| Participant 14 | F | 19.3 | F4, Cz, F3 |
| Participant 15 | F | 21.3 | MRI, F3, Cz, F4 |
| Participant 16 | F | 20.6 | F4, Cz, F3 |
| Participant 17 | F | 20.8 | F3, Cz, F4, MRI |
| Participant 18 | F | 18.9 | F4, Cz, F3 |
| Participant 19 | M | 24.2 | MRI, Cz, F3, F4 |
| Participant 20 | F | 22.9 | NT, MRI, F4, Cz, F3 |
| Participant 21 | M | 31.3 | NT |
| Participant 22 | M | 26.5 | NT |
| Participant 23 | M | 27.4 | NT |
| Participant 24 | F | 21.4 | NT |
| Participant 25 | F | 28.7 | NT |
| Participant 26 | M | 32.4 | NT |
| Participant 27 | F | 27 | NT |
| Participant 28 | M | 29.6 | NT |
| Participant 29 | M | 26.5 | NT |
| Participant 30 | M | 28.5 | NT |
F3 = Left dlPFC, F4 = Right dlPFC, Cz = Vertex, NT = No TMS
2.3 The 3-back Task of Working Memory
Verbal and spatial domains of working memory were assessed separately, using different versions of the 3-back task. The 3-back is a high-load condition (Barr et al. 2009) of the classic n-back task (Gevins and Cutillo 1993). Each trial of the 3-back task requires the participant to monitor sequentially presented stimuli, remember the most recent three stimuli, compare each new stimulus (n) to the oldest member of the set (n – 3), respond “yes” or “no” by pressing one of two buttons, and then mentally shift the set over by one for the next trial.
Participants performed the task while seated in a chair with a button box accessible to their hand (Figure 1A). Stimuli were displayed on a 19-inch Diamond Pro (Mitsubishi Electric, Tokyo, Japan) CRT monitor at a distance of approximately 65 cm. In the verbal version, single letters (‘A-J’) were presented one at a time in pseudorandom order in white 78-point Arial font (subtending 1.1° of visual angle horizontally and 2° vertically) in the center of a black screen. Letters were presented as either upper- or lowercase characters, chosen randomly for each trial. Participants were instructed to ignore the case of the letter (i.e., to treat both cases of the same letter as a match), thus requiring them to encode the verbal identity of the letter instead of its shape. In the spatial condition, the stimulus was a one-inch diameter white dot (subtending 2° of visual angle horizontally and vertically) that appeared in one of 10 locations arranged in a rectangular grid (covering approximately 22° of visual angle horizontally and 17.5° vertically) around the center of a black screen. This arrangement was chosen to reduce the ability of participants to verbalize the locations and therefore contaminate the spatial variant with verbal-based strategies. Participants provided feedback as they learned the tasks, confirming that attempts to verbalize the spatial locations used in the present study was a counterproductive strategy.
Figure 1. Schematic of 3-back Tasks and Experimental Protocol.
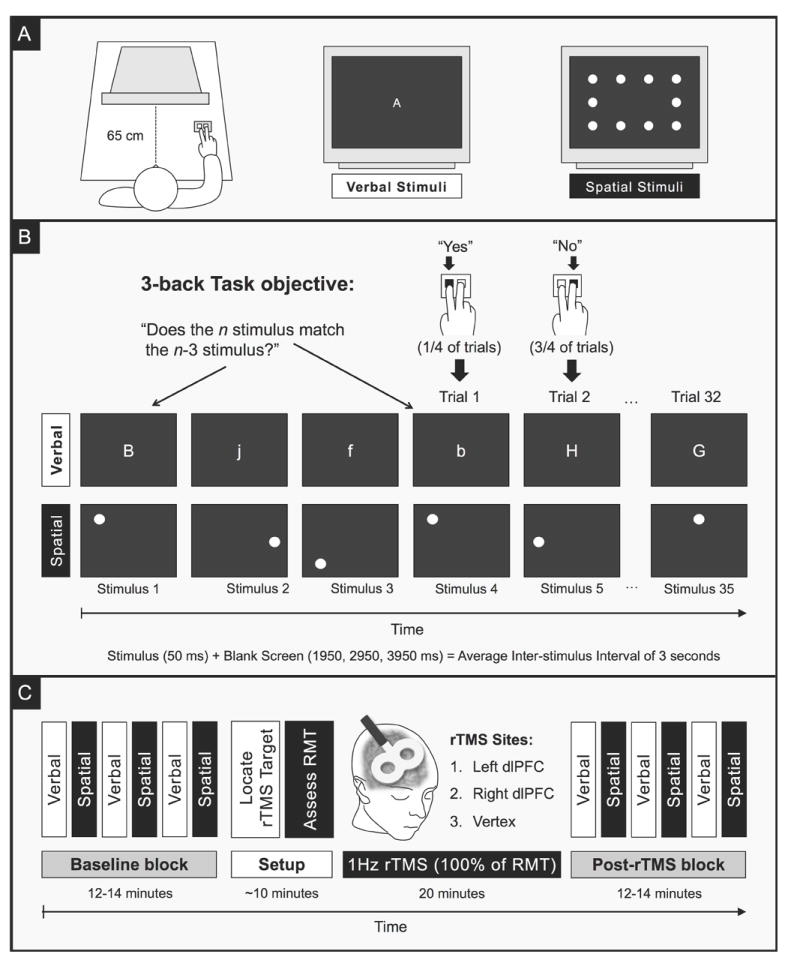
A. Verbal and spatial versions of the 3-back task were administered as participant as sat in front of a computer screen with a response box in their right hand. The verbal stimuli consisted of single letters (‘A-J’) that were presented in the center of the screen. Letters were randomly presented in either upper- or lowercase, and participants had to treat both cases as matching stimuli. In the spatial version, participants had to remember the visuotopic position of a dot that appeared in one of ten locations. B. For each trial, the participant had to remember the previous three stimuli, determine whether the next stimulus (n) matched the oldest member of the set (n – 3), respond “yes” or “no” by pressing one of two buttons, and then shift the set forward by one for the next trial. C. Each participant completed three experimental sessions in which a different site was targeted for repetitive transcranial magnetic stimulation (rTMS). All sessions followed the same format: (1) working memory abilities were assessed at baseline with alternating blocks of the verbal and spatial 3-back tasks; (2) the target site was determined based on scalp landmarks; (3) the resting motor threshold (RMT) was assessed; (4) a 1Hz train of rTMS was applied to the target site for 20 minutes at 100% of the RMT; (4) immediately after rTMS ended, working memory abilities were reassessed with alternating blocks of the verbal and spatial 3-back tasks. Task order was consistent throughout each session, but counterbalanced between sessions. Individual sessions were separated by at least 48 hours.
In both versions of the task (Figure 1B), each stimulus was presented for 50 ms and followed by a blank screen for a randomly selected duration of 1950, 2950, or 3950 ms (for an average inter-stimulus interval of 3 seconds). A variable inter-stimulus interval decreases the predictability of stimulus onset, and this has been shown to both increase the attentional demands of task and reduce automatic responses (Mottaghy et al. 2002). Participants were instructed to respond to each stimulus as quickly and accurately as possible by pressing one of two buttons on a button box with their right index or middle finger: index finger for a matching target and middle for a nonmatching target. A typical run contained exactly 32 trials (35 stimuli) and lasted approximately 100 seconds. Baseline and post-rTMS blocks each consisted of three verbal and three spatial runs in alternating sequence.
Practice session
Every participant was given the opportunity to practice the 3-back tasks for approximately 30 minutes on a separate visit prior to any of the experimental sessions. This served to acclimate participants to the verbal and spatial versions of the task and to reduce variability and training effects (i.e., achieve a more consistent performance) prior to their use in subsequent sessions in combination with noninvasive brain stimulation.
2.4 Transcranial Magnetic Stimulation
Transcranial Magnetic Stimulation (TMS) was applied to participants using an air-cooled 70 mm figure-of-eight focal coil (Magstim Co. Ltd., Dyfeld, Wales, UK) attached to a Magstim biphasic stimulator (either the Rapid or the SuperRapid). All stimulation parameters used in the study were well within accepted guidelines for the safe application of TMS (Machii et al. 2006; Rossi et al. 2009). The resting motor threshold (RMT) was measured for each participant on each stimulation session using a standard protocol (Fried et al. 2011). The RMT was used as an individually-referenced value of cortical excitability for determining the safe and appropriate stimulator output for repetitive stimulation (Pascual-Leone et al. 1993).
Repetitive TMS (rTMS) was administered using a typical off-line protocol (Figure 1C). Stimulation was applied to the participant while he or she was seated comfortably in a chair with eyes opened. The impact of rTMS on 3-Back task performance was measured immediately after rTMS ended and compared to a pre-rTMS assessment. The pattern of stimulation consisted of a continuous 1Hz train, which has been shown to temporarily reduce cortical excitability and metabolism (Boroojerdi et al. 2000; Muellbacher et al. 2000; Valero-Cabré et al. 2007). The sequence of pulses was programmed and initiated using proprietary Magstim software to deliver a total of 1200 pulses over 20 minutes at an intensity of 100% of the RMT.
2.5 Identification of rTMS Targets
Three scalp locations were identified that corresponded to coordinates F3, F4 and Cz of the International “10-20” system for EEG electrode placement (Klem et al. 1999). Coordinates F3 and F4 are commonly used as reference points on the scalp for the left and right dlPFC, respectively (Mottaghy et al. 2000; Fregni et al. 2005; Kim et al. 2007). Coordinate Cz, which corresponds to the vertex of the scalp, was used as a control stimulation site to account for non-specific effects of TMS. The use of EEG coordinates to guide TMS placement over functional brain areas represents an economical and practical tradeoff over complex neuroimaging-based methods (Herwig et al. 2003), especially when MRI data are not available for all participants. All sites were marked on a snug-fitting Lycra™ swim cap worn by the participant. The Beam F3 System (Beam et al., 2009) was used to accurately locate coordinates F3 and F4.
To identify the targeted brain region with greater precision, a T1-weighted anatomical magnetic resonance imaging scan was obtained in twelve of the 20 participants on a separate visit from the behavioral sessions. A magnetization prepared rapid gradient echo sequence was employed with the following parameters: 150 sagittal-oriented slices for whole-brain coverage; field-of-view = 256 mm (FH) × 240 mm (AP) × 180 mm (RL); native resolution = 1.0 mm × 1.0 mm × 1.2 mm voxel; flip angle = 8°; TE = 3.1 ms; TR = 6.8 ms; total scan duration = 314 seconds. Prior to scanning, vitamin D capsules were placed on the same scalp locations targeted for stimulation: F3, F4 and Cz. Each T1 image was loaded into Brainsight™ (Rogue Research, Inc., Montreal, Quebec, Canada), which allowed precise identification of the region of cortex directly underneath each EEG site. This method provided confirmation that locations F3 and F4 overlaid the center of the middle frontal gyrus, whereas location Cz was over the medial longitudinal fissure near the precentral gyrus (Figure 2, right panel). The average (± standard deviation) coordinates (in MNI space) of the targets were: -41.5 (± 3), 41.1 (± 6), 33.4 (± 7) for the left dlPFC; 42.5 (± 4), 41.3 (± 5), 34.0 (± 6) for the right dlPFC; and -2.2 (± 5), -9.3 (± 6), 76.2 (± 2) for the vertex.
Figure 2. Direct Impact of rTMS on 3-back Accuracy.
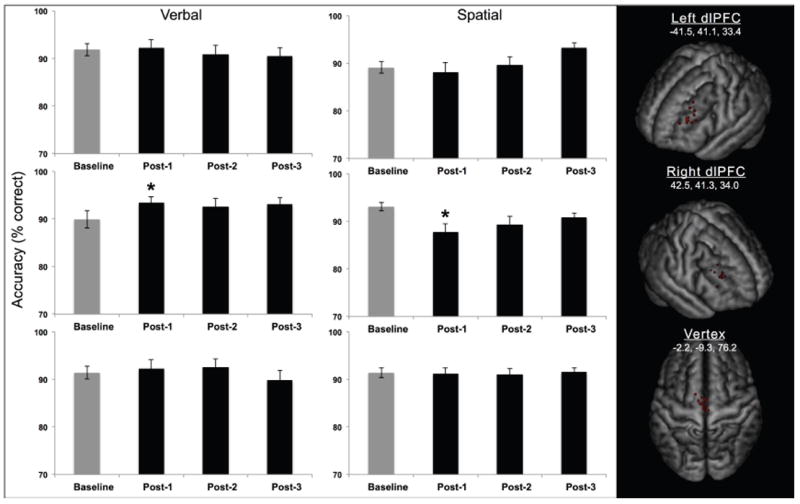
The mean accuracy (percent correct) for both tasks (verbal, spatial) and all three rTMS conditions (left dlPFC, right dlPFC, vertex) for Experiment 1. Error bars represent standard error. *p < 0.05. Right panel. An MRI was obtained in a twelve participants with vitamin D capsules in place over the stimulation sites.
2.6 Experimental Sessions
Experiment 1
The primary experiment consisted of four visits per participant, including one practice session and three experimental sessions. Each of the experimental sessions lasted approximately one hour and followed the same general procedure. The participant began the experiment by practicing the 3-back task, alternating between verbal and spatial runs. Once the participant achieved a relatively consistent accuracy across three runs for each task, as indicated by a standard deviation of less than 10%, those runs were designated as the baseline block. The participant then donned a swim-cap and measurements of his or her head were taken and entered into the Beam F3 system to determine the location of the stimulation site. The three sites, left dlPFC, right dlPFC, vertex, were determined for each individual based on the scalp position of EEG coordinates F3, F4, and Cz, respectively. The RMT was assessed for the hemisphere that was targeted for rTMS. For the vertex, the RMT of the left hemisphere was referenced. A 1Hz rTMS train was delivered for 20 minutes at 100% of RMT. The coil was kept fixed in place for the duration of stimulation with the assistance of a multi-joint adjustable Magic Arm (Manfrotto, Italy). Throughout the stimulation, the participant sat awake, with eyes opened, in a comfortable chair. As soon as stimulation ceased, the participant completed six more runs of the 3-back task, alternating between verbal and spatial versions. These runs constituted the post-rTMS block of the task. Task order was maintained throughout each experimental session, but was counterbalanced across subjects and sessions. The relative session order between the left and right dlPFC was also counterbalanced across subjects. Experimental sessions were separated by at least two days to reduce the likelihood of carryover effects from the previous session (Maeda et al. 2000a; Valero-Cabré et al. 2008).
Experiment 2
In addition to the vertex-stimulation control condition in Experiment 1, a separate control experiment was run with sham stimulation. A separate group of participants (Table 1) completed a single session that followed the same procedure as the primary experiment, with the exception that rTMS ran in the background and thus participants did not receive any stimulation. Sham rTMS is typically administered by tilting the coil 45-90° and placing its outer edge against the participant’s scalp; however, as this arrangement can still induce intra-cerebral currents (Loo et al. 2000; Lisanby et al. 2001), it was suboptimal for the purpose of establishing average performance in the absence of stimulation. To simulate the overall environment of rTMS without any inducing any current in the brain or musculature of the scalp, the pattern of the background stimulation was matched to the real stimulation: a 1Hz train for 20 minutes at 80% of maximum stimulator output. During the stimulation, participants wore earplugs and a swim cap and remained seated comfortably with eyes opened, with the TMS stimulator and coil positioned approximately one meter behind the participant.
2.7 Data Analysis
Performance on the verbal and spatial 3-back tasks was assessed in terms of accuracy (percent correct), and the mean response time of correct trials. Response times that fell outside two standard deviations from the mean were excluded (Mottaghy, Gangitano, et al. 2003; Sandrini et al. 2008). To account for the possibility of a speed-accuracy trade off, a parallel analysis was conducted for Experiment 1 using a diffusion model approach (Wagenmakers et al. 2007). The diffusion model combines response time and accuracy to provide information about the “drift rate,” or the participant’s sensitivity to the relevant stimulus. This approach has been used in at least two other TMS studies (Cohen Kadosh et al. 2010; Soto et al. 2012). Performance measures for the first three runs of each task were averaged to yield an overall baseline, while post-rTMS runs were treated as individual time points as the effects of prefrontal rTMS have been shown to be transient (Mottaghy et al. 2002; Eisenegger et al. 2008). Statistical analyses were performed using the software package JMP Pro version 10.0 (SAS Institute Inc., Cary, NC). Data from Experiments 1 and 2 were each analyzed using a linear mixed model (LMM) approach, which accounts for the inter-individual variance in repeated-measures designs with crossed random effects for subjects and independent variables (Baayen et al. 2008). Data points outside of the interquartile range for each condition were excluded. The models were fit by restricted maximum likelihood.
To test the hypothesis that rTMS altered task performance, the independent variables, rTMS condition (left dlPFC, right dlPFC, vertex), task domain (verbal, spatial), and time (baseline, post-1, post-2, post-3) were entered as fixed effects into a 3 × 2 × 4 full factorial design with a 95% confidence interval (α = 0.05). Post-hoc comparisons of each post-rTMS run to baseline were performed using Tukey’s HSD tests to reduce Type 1 errors. To assess the relationship between conditions in which a significant effect from rTMS was observed, Pearson’s correlation coefficients were computed on the change in accuracy (calculated by subtracting the baseline score from that of the relevant post-rTMS time point).
Based on the results from the LMM (see Results below), a follow-up analysis was conducted to compare the average net effects of rTMS on verbal and spatial accuracy for each rTMS stimulation site. Scores at baseline were subtracted from post-rTMS blocks and this average net change was analyzed using a LMM. The factors rTMS condition and task domain were entered into a 3 × 2 full-factorial model (using α = 0.05). For each rTMS stimulation site, planned pairwise comparisons between verbal and spatial tasks were made using paired-samples Student’s t tests with a Bonferroni-corrected 98.3% confidence interval (α/3 = 0.0167).
To analyze data from Experiment 2, a 2 × 4 full factorial model was fit with task domain and time as fixed effect factors (using α = 0.05). As with Experiment 1, Tukey’s tests were used for post-hoc comparisons of each post-rTMS run to baseline.
3 Results
Behavioral data (representing mean ± standard error of response times and accuracy scores) from all conditions are listed in Table 2. All participants tolerated TMS with no side effects. Data from the left dlPFC condition could not obtained in one participant who moved away before completing the study.
Table 2.
Response Time (RT) and Accuracy
| Verbal 3-Back Task | Spatial 3-Back Task | |||
|---|---|---|---|---|
| RT ± SE (ms) | Score ± SE (% correct) | RT ± SE (ms) | Score ± SE (% correct) | |
| Left dlPFC (n = 19) | ||||
| Baseline | 705 ± 25 | 91.9 ± 1.3 | 726 ± 26 | 89.1 ± 1.2 |
| Post-1 | 684 ± 29 | 92.3 ± 1.6 | 676 ± 27 | 88.2 ± 2.0 |
| Post-2 | 674 ± 27 | 90.9 ± 1.8 | 735 ± 28 | 89.7 ± 1.7 |
| Post-3 | 671 ± 19 | 90.5 ± 1.8 | 677 ± 23 | 93.3 ± 1.0 |
| Right dlPFC (n = 20) | ||||
| Baseline | 728 ± 32 | 89.9 ± 1.8 | 724 ± 32 | 93.1 ± 0.9 |
| Post-1 | 700 ± 36 | 93.4 ± 1.3 | 695 ± 30 | 87.7 ± 1.8 |
| Post-2 | 694 ± 26 | 92.6 ± 1.7 | 705 ± 33 | 89.3 ± 1.7 |
| Post-3 | 703 ± 29 | 93.1 ± 1.4 | 704 ± 23 | 90.9 ± 0.8 |
| Vertex (n = 20) | ||||
| Baseline | 706 ± 24 | 91.4 ± 1.3 | 715 ± 28 | 91.4 ± 1.0 |
| Post-1 | 679 ± 28 | 92.3 ± 1.8 | 659 ± 27 | 91.2 ± 1.3 |
| Post-2 | 668 ± 25 | 92.6 ± 1.8 | 680 ± 29 | 91.1 ± 1.1 |
| Post-3 | 657 ± 26 | 89.9 ± 2.0 | 657 ± 35 | 91.5 ± 1.0 |
| No TMS (n = 11) | ||||
| Baseline | 853 ± 57 | 89.8 ± 2.0 | 818 ± 54 | 90.3 ± 2.2 |
| Post-1 | 810 ± 71 | 88.1 ± 3.0 | 796 ± 25 | 91.5 ± 2.9 |
| Post-2 | 781 ± 63 | 87.2 ± 2.8 | 703 ± 42 | 90.4 ± 2.6 |
| Post-3 | 746 ± 61 | 87.8 ± 3.3 | 717 ± 49 | 90.9 ± 2.6 |
Accuracy
With regard to the direct impact of rTMS, the LMM for Experiment 1 yielded no significant main effects (all F’s < 1.1, all p’s > 0.3). However, there were significant interactions between the factors task domain and time, F(3,303.4) = 4.975, p = 0.0022, and between the factors rTMS condition, task domain, and time F(6,303.5) = 2.477, p = 0.0236, thus rejecting the null hypothesis that there was no difference in accuracy across conditions. Post-hoc Tukey’s tests revealed that rTMS of the right dlPFC, but not the left dlPFC or the vertex, had a transient, dissociative impact on task accuracy: immediately after rTMS (post-1), accuracy declined on the spatial task, p = 0.0183, but increased on the verbal task, p = 0.0249 (Figure 2). A Pearson’s correlation analysis revealed no relationship between the two tasks in terms of the immediate effects of right dlPFC stimulation, r(18) = -.0109, p = 0.674, suggesting that verbal and spatial domains have substrates that are independent from one another. No other time points were significantly different from baseline, all p’s > 0.05. For Experiment 2, the LMM yielded no significant main effects or interactions between them (all F’s < 4.6, all p’s > 0.05) thus confirming the null hypothesis of that the no TMS control experiment did not impact accuracy.
With regard to the average change in accuracy from baseline, the LMM yielded a significant interaction between the factors rTMS condition and task domain, F(2,37.6) = 8.47, thus rejecting the null hypothesis that there was no difference in the effect of rTMS across conditions (Figure 3). Bonferroni-corrected, paired-samples Student’s t tests revealed a significant difference between verbal and spatial tasks after rTMS was applied to the right dlPFC, t(19) = 3.03, p = 0.0068, two-tailed, consistent with the direct effects of rTMS observed in the preliminary analysis. In addition, there was a trend towards a significant difference between verbal and spatial tasks following rTMS of the left dlPFC, t(18) = 1.84, p = 0.0823, two-tailed, reflecting a 1.10% (± 1) decrease in verbal and a 1.04% (± 1) increase in spatial accuracy. By comparison, there was no difference between tasks in the vertex rTMS condition, t(19) = 0.57, p = 0.5737, two-tailed. These results suggest a potential double dissociation in task accuracy that was not captured by the evaluation of post-rTMS scores relative to baseline within each condition.
Figure 3. Interaction Between rTMS Condition and Task Domain for 3-back Accuracy.
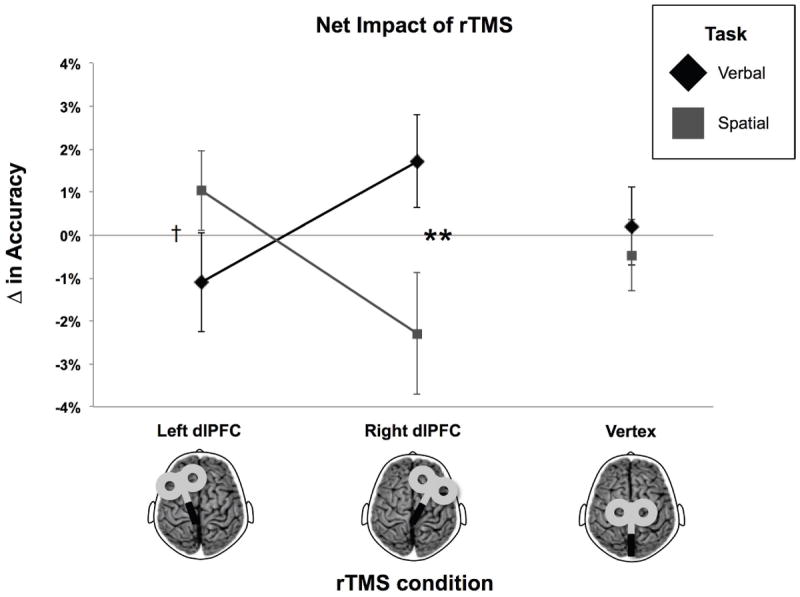
Net change in accuracy (% correct) calculated by subtracting baseline from post-rTMS scores for Experiment 1. Error bars represent standard error of the mean. **p < 0.01, †p < 0.1.
Response Time
For Experiment 1, the LMM yielded significant variance in response times by time, F(3,57) = 9.448, p < 0.0001, indicating a change in performance speed that was not specific to task domain or rTMS condition. No other main effects or interactions were significant (all F’s < 1.8, all p’s > 0.1). Post-hoc Tukey’s tests revealed that responses for all three post-rTMS time points (post-1, post-2, post-3) were quicker on average than at baseline, all p’s < 0.02, indicating the influence of non-specific effects (Figure 4). A similar finding was observed for the no TMS condition in Experiment 2: the LMM yielded a significant main effect of time, F(3,28.3) = 14.6, p < 0.0001, indicating that regardless of the task domain, responses became quicker in the absence of a direct intervention.
Figure 4. Nonspecific Impact of rTMS on 3-back Task Response Time.

Response times (ms) averaged across tasks and rTMS conditions for Experiment 1. Error bars represent standard error of the mean. *p < 0.05.
Drift Rate
The LMM yielded no main effects, however there was a significant interaction between the factors of rTMS condition, task domain, and time, F(6,313.8) = 2.316, p = 0.0334, confirming the pattern seen with accuracy alone. Post-hoc Tukey’s tests revealed that the participant’s sensitivity to memory matches for the spatial 3-back task was significantly worsened following rTMS of the right dlPFC, p = 0.0331. All other comparisons were non-significant (p’s > 0.1). That there was a null result for the verbal 3-back task in the same condition suggests that rTMS of the right dlPFC may have induced a speed-accuracy trade-off that was not captured in the individual analyses of accuracy and response time.
4 Discussion
In the study of the functional neuroanatomy of working memory, there has been a persistent debate as to whether the dlPFC is dissociable with respect to the content of information in working memory. The origins of this debate can be traced to early work by Sperry and colleagues (Gazzaniga et al. 1965) and Ungerleider and Mishkin (Ungerleider LG and Mishkin M 1982) demonstrating hemispheric specialization and the segregation of visual pathways, respectively. Neuroimaging studies (Owen et al. 2005; Nee et al. 2013) investigating content-based selectivity in the prefrontal cortex yield relatively higher activation for verbal and spatial tasks in the vicinity of Broca’s area (specifically, the left inferior frontal gyrus, pars triangularis) and the right frontal eye field (specifically, the caudal superior frontal sulcus), respectively, but relatively equivalent activity across tasks within the dlPFC (specifically the middle frontal gyrus at the putative junction of Brodmann areas 9 and 46). One interpretation of these studies is that the dlPFC contributes equally to working memory regardless of domain. If this were the case, it would follow that modulation of dlPFC activity would have a similar impact on verbal and spatial working memory tasks. On the contrary, the current study demonstrated that applying low-frequency rTMS to the right dlPFC of intact adult humans had opposing effects on their ability to accurately perform verbal and spatial versions of the 3-back task of working memory. Specifically, accuracy was transiently impaired relative to baseline on the spatial task, but enhanced on the verbal task. The 1Hz pattern of rTMS has been shown to reduce cortical excitability and metabolism beyond the duration of stimulation in animal models (Valero-Cabré et al. 2007), as well as in normal human motor (Muellbacher et al. 2000; Romero et al. 2002) and visual cortex (Boroojerdi et al. 2000; Fried et al. 2011). Assuming that 1Hz rTMS has a similar suppressive impact on the activity of the dlPFC, the present findings can be interpreted as confirmation that the dlPFC is a critical substrate for working memory that can be functionally dissociated by the type of information it processes.
The second major finding was a nonspecific quickening of response times. Given that this improvement was observed in both the active (vertex stimulation) and passive (no TMS) control conditions, the reduction in response times can be attributed to residual learning or practice effects rather than a nonspecific effect of the 1Hz stimulation per se. In fact, the results of the diffusion model approach demonstrate that the influence of these effects was less generalized following rTMS of the right dlPFC, when the greatest changes in accuracy were observed. The influence of nonspecific effects is a likely factor in the high inter-individual variability reported in many rTMS studies (Maeda et al. 2000b), which in turn may have also contributed to the small effect sizes for the impact of rTMS on accuracy. Furthermore, the young age (18.3 – 25.5 years) and relatively high education (all were enrolled or had recently graduated from college) of the present cohort coincides with the peak of working memory development (Grady and Craik 2000) and cognitive reserves (Stern et al. 2005). Whether alone or in combination, these factors could have mitigated some of the presumed modulatory effect of 1Hz rTMS on dlPFC activity and working memory abilities.
It is notable that stimulation of the left dlPFC did not significantly alter accuracy on either the verbal or spatial 3-back task. While this is not the only study to report a lack of change in n-back accuracy from stimulating the left dlPFC (Sandrini et al. 2008; Barr et al. 2009), the null finding was nevertheless surprising given that lesions of the left middle and superior frontal cortices are associated with working memory impairments (Barbey et al. 2013), and several prior studies have reported changes in working memory abilities from stimulating the left dlPFC with single pulse TMS (Mull and Seyal 2001; Mottaghy, Gangitano, et al. 2003), rTMS (Mottaghy et al. 2000, 2002), and transcranial direct current stimulation (Fregni et al. 2005; Zaehle et al. 2011). It is possible that the reduced impact of left dlPFC stimulation relative to the right could be accounted for by hemispheric asymmetries related to language dominance and handedness that have been shown to manifest in the left dlPFC tending to be larger and/or having a more variable organization than the right dlPFC in right-handed individuals (Hervé et al. 2006). However, inspection of the stimulation sites for the twelve participants who received structural MRIs yielded no obvious differences in the relationship between the scalp position and the anatomy of the underlying cortex that would indicate coordinate F4 was a more consistent target for the right dlPFC than F3 was for the left dlPFC. A more plausible alternative concerns the impact of rTMS on the broader bihemispheric working memory network. Modulating cortical excitability in a given brain region can alter intrinsic network connectivity (Eldaief et al. 2011) and impact activity in non-stimulated, but connected regions (Mottaghy et al. 2000), Furthermore, asymmetries in the net effects of modulating left and right homologues, including compensatory mechanisms in non-stimulated regions, have been reported both in the context of working memory (Mottaghy, Pascual-Leone, et al. 2003) and mental imagery (Sack et al. 2005). Thus it is possible that the right hemisphere was better able to compensate following suppression of the left dlPFC than vice versa, and that this asymmetry could account for the discrepancy in the behavioral effects of left and right dlPFC conditions.
In sum, the present study demonstrated a dissociation of verbal and spatial working memory following modulation of the right dlPFC. These results support a systems-based model of working memory driven by domain-specific storage buffers (Baddeley and Hitch 1974; Baddeley 2000) over state-based models that depict the fluid control of activation states by general executive functions that are context- rather than content-dependent (Larocque et al. 2014). Further, the absence of a significant correlation between the immediate effects of right dlPFC stimulation on verbal and spatial accuracy indicates that the mechanisms that led to the these changes were independent. It has been suggested that unilateral rTMS may act by shifting the balance of hemispheric activity (Rossini et al. 2010) via excitatory callosal projections onto assemblies of inhibitory interneurons. In this context, the impact of 1Hz rTMS would be predicted to reduce excitability on that side that received stimulation and indirectly increase excitability in the contra-stimulated hemisphere. The results of the present experiment are also consistent with a more nuanced account of dlPFC function (Sreenivasan et al. 2014), which posits a role in maintaining abstract and goal-directed representations (Lee et al. 2013), and as a source of top-down signals that bias activity in extrastriate visual areas (Feredoes et al. 2011; Lee and D’Esposito 2012). While these and other studies (Sandrini et al. 2008) have highlighted the ability of the dlPFC to select relevant information amid irrelevant or distracting features, the present results suggest the dlPFC might mediate activity in posterior association areas even in a working memory task that does not require suppressing irrelevant features. At a minimum, the fact that rTMS of the dlPFC had different effects on verbal and spatial 3-back task accuracy strongly suggests that processes for manipulating verbal and spatial information have a dissociable underlying functional organization. Lastly, the facilitation of verbal working memory is further evidence of the potential of noninvasive brain stimulation to improve cognition and could serve as the basis for future translational research.
Acknowledgments
The contributions of Dr. Fried were supported by the Department of Anatomy and Neurobiology and the Division of Graduate Medical Sciences at Boston University School of Medicine. The contributions of Drs. Rushmore and Valero-Cabré were supported by the National Institutes of Health (NS062317). The contributions of Dr. Pascual-Leone were supported by a grant from the National Institutes of Health – Harvard Clinical and Translational Science Center/Harvard Catalyst (UL1 RR025758). Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI).
Abbreviations
- dlPFC
dorsolateral prefrontal cortex
- LMM
linear mixed model
- rTMS
repetitive transcranial magnetic stimulation
- RMT
resting motor threshold
- TMS
transcranial magnetic stimulation
Footnotes
The authors declare no competing financial interests.
References
- Abler B, Walter H, Wunderlich A, Grothe J, Schönfeldt-Lecuona C, Spitzer M, Herwig U. Side effects of transcranial magnetic stimulation biased task performance in a cognitive neuroscience study. Brain Topogr. 2005;17:193–196. doi: 10.1007/s10548-005-6028-y. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- Baddeley The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working Memory. Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex J Devoted Study Nerv Syst Behav. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rusjan PM, Chen R, Fitzgerald PB, Daskalakis ZJ. Potentiation of Gamma Oscillatory Activity through Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex. Neuropsychopharmacology. 2009;34:2359–2367. doi: 10.1038/npp.2009.79. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol. 1976;90:293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Muggleton N, Silvanto J, Walsh V. Double dissociation of format-dependent and number-specific neurons in human parietal cortex. Cereb Cortex N Y N 1991. 2010;20:2166–2171. doi: 10.1093/cercor/bhp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain J Neurol. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Treyer V, Fehr E, Knoch D. Time-course of “off-line” prefrontal rTMS effects--a PET study. NeuroImage. 2008;42:379–384. doi: 10.1016/j.neuroimage.2008.04.172. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci U S A. 2011;108:17510–17515. doi: 10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MTA, Paulus W, Pascual-Leone A. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Fried PJ, Elkin-Frankston S, Rushmore RJ, Hilgetag CC, Valero-Cabre A. Characterization of visual percepts evoked by noninvasive stimulation of the human posterior parietal cortex. PloS One. 2011;6:e27204. doi: 10.1371/journal.pone.0027204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci Off J Soc Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS, Bogen JE, Sperry RW. Observations on visual perception after disconnexion of the cerebral hemispheres in man. Brain J Neurol. 1965;88:221–236. doi: 10.1093/brain/88.2.221. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Hervé P-Y, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. NeuroImage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim S, Joo E, Tae W, Choi S, Hong S. Cortical localization of scalp electrodes on three-dimensional brain surface using frameless stereotactic image guidance system. Neurol Asia. 2007;12:84. [Google Scholar]
- Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Kumar S, Rao SL, Chandramouli BA, Pillai S. Reduced contribution of executive functions in impaired working memory performance in mild traumatic brain injury patients. Clin Neurol Neurosurg. 2013 doi: 10.1016/j.clineuro.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Larocque JJ, Lewis-Peacock JA, Postle BR. Multiple neural states of representation in short-term memory? It’s a matter of attention. Front Hum Neurosci. 2014;8:5. doi: 10.3389/fnhum.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kravitz DJ, Baker CI. Goal-dependent dissociation of visual and prefrontal cortices during working memory. Nat Neurosci. 2013;16:997–999. doi: 10.1038/nn.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TG, D’Esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J Neurosci Off J Soc Neurosci. 2012;32:15458–15466. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry. 2000;47:325–331. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2000a;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale. 2000b;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Krause BJ, Pascual-Leone A. Chronometry of parietal and prefrontal activations in verbal working memory revealed by transcranial magnetic stimulation. NeuroImage. 2003;18:565–575. doi: 10.1016/s1053-8119(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex N Y N 1991. 2002;12:369–375. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Krause BJ, Kemna LJ, Töpper R, Tellmann L, Beu M, Pascual-Leone A, Müller-Gärtner HW. Modulation of the neuronal circuitry subserving working memory in healthy human subjects by repetitive transcranial magnetic stimulation. Neurosci Lett. 2000;280:167–170. doi: 10.1016/s0304-3940(00)00798-9. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Pascual-Leone A, Kemna LJ, Töpper R, Herzog H, Müller-Gärtner H-W, Krause BJ. Modulation of a brain-behavior relationship in verbal working memory by rTMS. Brain Res Cogn Brain Res. 2003;15:241–249. doi: 10.1016/s0926-6410(02)00196-9. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Mull BR, Seyal M. Transcranial magnetic stimulation of left prefrontal cortex impairs working memory. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2001;112:1672–1675. doi: 10.1016/s1388-2457(01)00606-x. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cereb Cortex N Y N 1991. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of “virtual lesions”. Philos Trans R Soc B Biol Sci. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, Valls-Solé J, Brasil-Neto JP, Wassermann EM, Cohen LG. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol. 1993;89:120–130. doi: 10.1016/0168-5597(93)90094-6. [DOI] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci. 2006;18:1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D’Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc. 2000;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini D, Lucca A, Magri L, Malaguti A, Smeraldi E, Colombo C, Zanardi R. A Symptom-Specific Analysis of the Effect of High-Frequency Left or Low-Frequency Right Transcranial Magnetic Stimulation over the Dorsolateral Prefrontal Cortex in Major Depression. Neuropsychobiology. 2010;62:91–97. doi: 10.1159/000315439. [DOI] [PubMed] [Google Scholar]
- Sack AT, Camprodon JA, Pascual-Leone A, Goebel R. The dynamics of interhemispheric compensatory processes in mental imagery. Science. 2005;308:702–704. doi: 10.1126/science.1107784. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Rossini PM, Miniussi C. Lateralized contribution of prefrontal cortex in controlling task-irrelevant information during verbal and spatial working memory tasks: rTMS evidence. Neuropsychologia. 2008;46:2056–2063. doi: 10.1016/j.neuropsychologia.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Soto D, Llewelyn D, Silvanto J. Distinct causal mechanisms of attentional guidance by working memory and repetition priming in early visual cortex. J Neurosci Off J Soc Neurosci. 2012;32:3447–3452. doi: 10.1523/JNEUROSCI.6243-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan KK, Curtis CE, D’Esposito M. Revisiting the role of persistent neural activity during working memory. Trends Cogn Sci. 2014;18:82–89. doi: 10.1016/j.tics.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex N Y N 1991. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle D, editor. Analysis of visual behavior. Cambridge Mass.: MIT Press; 1982. [Google Scholar]
- Valero-Cabré A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, van der Maas HLJ, Grasman RPPP. An EZ-diffusion model for response time and accuracy. Psychon Bull Rev. 2007;14:3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jäncke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12:2. doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


