SUMMARY
Positive autoregulation is an effective mechanism for the long-term maintenance of a transcription factor’s expression. This strategy is widely deployed in cell lineages, where the autoregulatory factor controls the activity of a battery of genes that constitute the differentiation program of a post-mitotic cell type. In Drosophila, the Notch pathway transcription factor Suppressor of Hairless activates its own expression specifically in the socket cell of external sensory organs, via an autoregulatory enhancer called the ASE. Here we show that the ASE is composed of several enhancer sub-modules, each of which can independently initiate weak Su(H) autoregulation. Cross-activation by these sub-modules is critical to ensuring that Su(H) rises above a threshold level necessary to activate a maintenance sub-module, which then sustains long-term Su(H) autoregulation. Our study reveals the use of interlinked positive feedback loops to control autoregulation dynamically, and provides mechanistic insight into initiation, establishment, and maintenance of the autoregulatory state.
INTRODUCTION
Positive autoregulation by a gene encoding a DNA-binding transcription factor is a widely utilized mechanism for insuring the long-term maintenance of the factor’s expression, well after the signals and other factors that initiated this activity are no longer present (Crews and Pearson, 2009; Hobert, 2011b). One common setting in which such prolonged, stable expression of a transcription factor may be especially advantageous is a post-mitotic, differentiated cell type. Here, the autoregulatory factor can function as a “terminal selector”, responsible for driving the co-expression of a battery of downstream genes that constitute the cell’s differentiation program (Hobert, 2011a).
Suppressor of Hairless [Su(H)] is an ancient, highly conserved protein that acts as the transducing transcription factor for the Notch cell-cell signaling pathway (Fortini and Artavanis-Tsakonas, 1994; Schweisguth and Posakony, 1992). Functioning in this role, which dates at a minimum to the last common ancestor of demosponges and eumetazoa (Richards and Degnan, 2012), Su(H) participates in a huge variety of conditional cell fate specification events in virtually all metazoans.
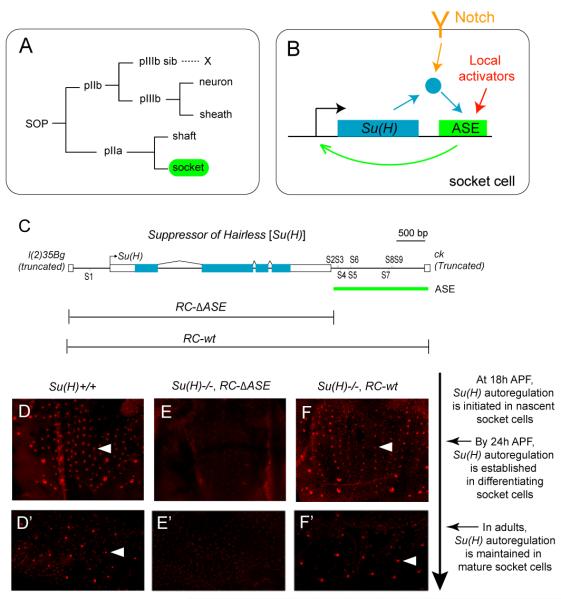
It came as a surprise, then, to find that Su(H) has been co-opted in Drosophila for a very different role: Acting as an essential regulator of the differentiation of the socket cell, a non-neuronal component of external sensory organs in the fly (Figure 1A) (Barolo et al., 2000). Regulation of Notch pathway target genes by Su(H) requires only a low basal level of the protein, which is present broadly or ubiquitously. But in Drosophila, Su(H) is in addition very highly expressed specifically in socket cells, beginning soon after the birth of the cell via the division of the pIIa secondary precursor and continuing stably thereafter (Figures 1A and 1D-1D’) (Barolo et al., 2000; Miller et al., 2009). This high level of transcript and protein accumulation from Su(H) is driven by a dedicated transcriptional control module, the autoregulatory socket enhancer (ASE). The ASE lies downstream of the gene, includes eight high-affinity Su(H) binding sites, and mediates a direct positive autoregulation activity specifically in socket cells (Figures 1B-1C and 1E-1F’).
Figure 1. The ASE Controls Transcriptional Autoactivation of Su(H) in the Socket Cell.
(A) Lineage of Drosophila adult external mechanosensory organs. The socket cell is highlighted in green. SOP, sensory organ precursor cell.
(B) Su(H) autoactivates its expression specifically in the socket cell via a dedicated cis-regulatory module, the autoregulatory socket enhancer (ASE) (Barolo et al., 2000). The socket cell-specific activation of the ASE is dependent on synergies between Notch signaling, via Su(H), and inputs from other activators in the sensory organ lineage (Barolo et al., 2000).
(C) Diagram of the Su(H) gene. The ASE is included within a 1.9-kb genomic segment located downstream of Su(H), and contains eight high-affinity Su(H) binding sites (S2-S9) (Barolo et al., 2000). The RC-wt genomic DNA fragment fully rescues all known functions of Su(H) when placed in a Su(H) null mutant background; RC-ΔASE lacks the ASE, and hence the autoregulatory activity of the gene, but rescues all other functions, including the broad basal level of Su(H) expression (see D-F and D’-F’) (Barolo et al., 2000).
(D-F’) Anti-Su(H) antibody (red) marks the high level of the protein in socket cells in the pupal notum at 24 hours after puparium formation (APF) (D-F) and in the abdominal epithelium of adult flies (D’-F’). Note the lack of strong staining in the Su(H)−/−; RC-ΔASE genotype (E-E’). Individual socket cells are indicated by arrowheads. Su(H)−/− refers to the null genotype Su(H)AR9/Su(H)SF8 (Barolo et al., 2000; Schweisguth and Posakony, 1992). The relationship between the imaging timepoints and the development of microchaete socket cells is described to the right of these panels.
While the fate of the socket cell is specified by Notch signaling, the ASE play s no role in this — indeed, the enhancer’s activity does not commence until after the cell’s fate has already been determined. Rather, mechanosensory organs in a fly lacking the ASE display severely impaired mechanotransduction, evidently due to defects in socket cell differentiation (Barolo et al., 2000). In addition, Su(H)’s socket cell autoregulatory activity, acting in concert with the socket cell-specific transcription factor Sox15, is also required to prevent deployment of an alternative differentiative program, that of the shaft cell, the socket cell’s sister (Figure 1A) (Miller et al., 2009). This is accomplished by repressing expression of shaven (sv), which encodes a high-level regulator of shaft cell differentiation (Kavaler et al., 1999).
A previous study from our laboratory (Barolo et al., 2000) revealed that the ASE is initially activated by the Notch signaling event that specifies the socket cell fate. Here, a low level of Notch-stimulated Su(H) functions cooperatively with certain “local activators” that are expressed in both the socket and shaft cells (Figures 1A-1B). By contrast, the long-term maintenance of high-level Su(H) autoregulation was found to be independent of Notch.
Despite the prevalence of positive autoregulation by transcription factor -encoding genes as a developmental control strategy, the specific mechanisms by which the autoregulatory state is initiated, established, and maintained have not been studied in detail. We have chosen the Su(H) ASE as a model for investigating this question. Dissecting a direct transcriptional autoregulatory activity necessitates separately analyzing the associated enhancer’s ability to respond to the normal wild -type context, with its normal level of the autoregulatory factor (e.g., by examining the expression of reporter transgene variants in a wild -type background) and its ability to establish the autoregulatory state (e.g., by examining levels of the autoregulatory factor generated by genomic rescue construct variants in a genetic background lacking the function of the autoregulatory gene). We have studied these two capabilities in detail in exploring the ASE’s mechanism of operation.
Counter to the expectation that the ASE functions as a single modular enhancer unit, our investigation reveals that it is instead composed of several overlapping structural and functional elements that we refer to as enhancer sub-modules, each of which can independently become active in the differentiating socket cell. Moreover, because each of the ASE’s sub-modules contains one or a few Su(H) binding sites, together they form several interlinked positive feedback loops with the Su(H) gene. Interestingly, not all of the ASE’s autoregulatory sub-modules respond to Su(H) equally: While some are activated by a low level of Su(H), others require much higher levels. As a result, the different sub-modules are deployed in succession, first to initiate a low-level autoregulatory activity, then to establish the autoregulatory state by exceeding a threshold level of Su(H), and finally to “lock down” a permanent high -level maintenance function. We propose a coherent model that explains how the ASE rapidly translates an initiating Notch pathway input signal into a highly elevated and irreversible level of Su(H) expression specifically in the developing socket cell. We suggest that enhancer sub-functionalization via enhancer sub-modules may be a generalizable mechanism for integrating inputs from a suite of dynamically expressed trans-regulators into a stable gene expression state.
RESULTS
Molecular Dissection of the ASE
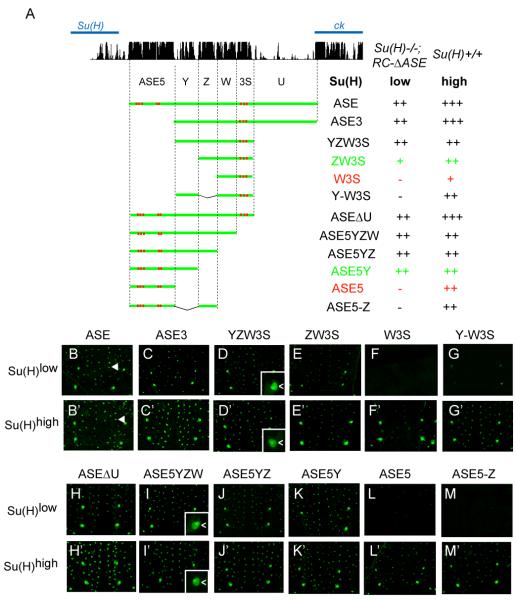
We began the detailed analysis of the ASE’s function by asking if its initial activity in the nascent socket cell is dependent on the ASE-Su(H) autoregulatory feedback loop. To address this question, we used a transgenic reporter gene assay to examine the ASE’s activity in a Su(H) null mutant background that also carries a genomic DNA rescue construct (RC-ΔASE) capable of rescuing only the low basal level of Su(H) expression (Barolo et al., 2000) (see Figures 1C-1F’). As shown in Figures 2A-2B, an ASE-GFP reporter is active at substantial levels specifically in the nascent socket cell, despite the low level of Su(H) in these flies [Su(H)−/−; RC-ΔASE, or Su(H)low (see Figure 1E)]. This result indicates that the initial phase of the ASE’s activity is independent of its autoregulatory function.
Figure 2. Identification of Functional Sequence Elements of the ASE in the Nascent Socket Cell.
(A) The pattern of conservation of ASE sequences in Drosophila species, as displayed by the UCSC genome browser (genome.ucsc.edu), is shown at the top. Diagrams of ASE fragments (green lines) tested in GFP reporter transgene constructs are shown below. Previously identified Su(H) binding sites are marked in red. Observed levels of GFP expression in nascent socket cells are summarized at right. Reporter gene activities were assayed in two genetic backgrounds: Wild type [Su(H)+/+ or Su(H)high] and Su(H)−/−; RC-ΔASE [only the basal level of Su(H), or Su(H)low]. GFP levels were scored using the following semi-quantitative system: strong +++, moderate ++, weak +, weak stochastic −/ +, and negative −. Two comparisons (ZW3S/ W3S and ASE5Y/ ASE5) are highlighted in green/ red type.
(B-M) GFP expression in the pupal notum at 24 hours APF; Su(H)low background, lacking the autoregulatory activity of Su(H).
(B’-M’) GFP expression in the pupal notum at 24 hours APF; wild-type [Su(H)high] background.
Arrowheads in B and B’ mark the positions of single mechanosensory organs. Insets in D, D’, I, and I’ show socket cell (<) specificity of GFP expression.
Next, to determine which part(s) of the ASE is required for its early activity, we tested the cis-regulatory activities of a series of deletion constructs in the Su(H)low mutant background (Figure 2A). Interestingly, we find that the ASE contains two non-overlapping fragments — ZW3S and ASE5Y (Figure 2A) — that can independently become active in the nascent socket cell (Figures 2A, 2E-2E’, and 2K-2K’). Consistent with an important functional role for these fragments, the sequences of both ZW3S and ASE5Y are highly conserved among Drosophila species (Figure 2A).
In an earlier study, a small fragment called ASE5, which is part of ASE5Y (Figure 2A), was shown to be strongly active in the socket cell in a wild-type background [Su(H)+/+, or Su(H)high (see Figures 1D-1D’)] (Barolo et al., 2000). However, ASE5 is completely silent in the Su(H)low background (Figure 2L), which suggests that its activity must rely on a certain threshold level of Su(H). On the other hand, that ASE5Y, but not ASE5, is active in the Su(H)low background suggests that fragment Y mediates important activator input(s) (compare Figures 2K and 2L). However, unlike ASE5, Y alone shows no detectable activity even in the wild -type Su(H)high background (Figures 3A-3B), suggesting that Y does not respond to Su(H). Indeed, Y contains no sequence motif that resembles a Su(H) binding site, whereas ASE5 contains five (S2-S6; see Figures 1C and 2A) (Barolo et al., 2000).
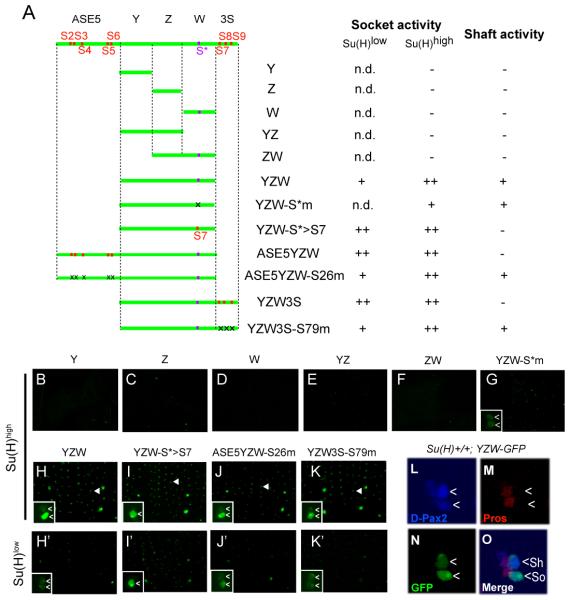
Figure 3. The ASE is Activated by the Synergistic Function of a Low Level of Su(H) and Weak Local Activators.
(A) Diagrams of ASE fragments (green lines) tested in GFP reporter transgene constructs in the pupal notum at 24 hours APF. Previously identified Su(H) binding sites (S2-S9) are marked in red; a Su(H) binding site, S*, identified here is marked in purple. Mutated Su(H) binding sites are indicated by “x”. Observed levels of GFP expression are summarized at right, using the same semi-quantitative scoring system as in Figure 2. “n.d.” means not determined.
(B-K) GFP expression in the pupal notum at 24 hours APF in the wild-type [Su(H)high] background. Arrowheads in H-K mark the positions of single mechanosensory organs. (H’-K’) GFP expression in the pupal notum at 24 hours APF in the mutant [Su(H)low] background [Su(H)−/−; RC-ΔASE] lacking the autoregulatory activity of Su(H). Insets in G-K and H’-K’ show higher-magnification views of GFP expression at single mechanosensory organ positions. Cells displaying GFP signal are marked (<).
(L-O) The YZW fragment of the ASE drives weak expression in both the shaft (Sh) and socket (So) cells (denoted by <). Shown is a single developing mechanosensory organ in the pupal notum at 20 hours APF in the wild-type background. (L) The cells of the mechanosensory organ lineage (see Figure 1A) are marked by anti-D-Pax2 antibody (blue); the strongest signal is in the shaft cell (Kavaler et al., 1999). (M) The cells of the pIIb branch of the lineage are marked by anti-Prospero (Pros) antibody (red). (N) Anti-GFP antibody (green) marks the activity of the YZW-GFP transgene. (O) Merged three-channel image. The socket and shaft cells are distinguished by their enlarged nuclei (due to endoreplication) and lack of anti-Prospero reactivity.
See also Figures S1, S2, and S3.
Remarkably, ZW3S shares with ASE5Y a similar bipartite organization. It includes a small fragment, called W3S (Figure 2A), which by itself is active in the wild-type Su(H)high background, but is silent in the mutant Su(H)low background (Figures 2F-2F’). The remaining portion of ZW3S, fragment Z (Figure 2A), though required for generating ZW3S’s activity in the Su(H)low background (compare Figures 2E and 2F), is silent in the socket cell even in the wild-type Su(H)high background (Figures 3A and 3C). Thus, W3S acts like ASE5 in responding to Su(H) in a dose-dependent manner, and Z functions like Y in mediating weak Su(H)-independent activator input(s). As in the case of ASE5 and Y, this functional difference between W3S and Z is also likely because W3S contains a cluster of three Su(H) binding sites (S7-S9; see Figures 1C and 2A), while Z contains none.
Despite the similarities between ASE5Y and ZW3S in their structure and function, we found that their components are not interchangeable, inasmuch as two synthetic constructs, one consisting of artificially linked ASE5 and Z (ASE5-Z; Figure 2A) and the other of Y and W3S (Y-W3S; Figure 2A), show little activity in the socket cell in the Su(H)low background (Figures 2G and 2M). Thus, ASE5Y and ZW3S act as distinct enhancer modules in the socket cell, with their cis-regulatory activities being respectively dependent on specific synergies between ASE5 and Y, and between Z and W3S.
These results suggest that four fragments — ASE5, ASE5Y, W3S, and ZW3S — represent distinct enhancer sub-modules, each making a unique contribution to the temporal and quantitative activity of the ASE in the socket cell.
Fragment YZW Responds to Weak Activator Inputs in the Shaft and Socket Cells
While the ASE fragments described above all show socket cell-specific cis-regulatory activities, another fragment called YZW — which includes all sequences between the two clusters of Su(H) binding sites (S2-S6 and S7-S9; see Figures 1C and 2A) — is active in both the socket cell and the shaft cell in the pupal-stage notum (Figures 3H and 3L-3O; see also Figures 1A and S1). We divided YZW into three smaller pieces (Y, Z, and W), and found that none of them showed any detectable enhancer activity (Figures 3A-3D); the same result was obtained with the YZ and ZW combinations (Figures 3A and 3E-3F). Thus, the dual shaft/ socket activity of YZW derives from the synergistic action of weak activator inputs that are distributed among the three separate segments (see also Figure S2).
Curiously, although no Su(H) binding site was previously identified in YZW, this fragment’s socket cell activity is significantly greater in the Su(H)high background than in the Su(H)low background, whereas its shaft cell activity does not exhibit such a difference (Figures 3H-3H’). Two lines of evidence indicate that the Su(H) dose dependence of YZW’s socket cell activity is mediated by a Su(H) binding motif (CCTGAGAA) we found in the W fragment (Figure 3A). First, this motif, which we call S*, binds a purified 6XHis-Su(H) fusion protein in vitro in a sequence-specific manner (see Figure S3F). Second, when S* is mutated, YZW’s socket cell activity in the wild -type [Su(H)high] background is severely weakened, so that it now functions at similarly low levels in both the shaft cell and the socket cell (compare Figures 3G and 3H; see also Figures S3A-S3E). This effect is essentially the same as reducing the Su(H) level in the socket cell; that is, by placing YZW in the Su(H)low background (Figure 3H’). We conclude that S* responds directly to a high level of Su(H) to promote YZW’s activity in the socket cell.
In an earlier study, it was shown that Su(H) binding sites S2-S9 in the ASE (see Figure 1C) mediate a transcriptional repression function of Su(H) in the shaft cell (where Notch is “off”) (Barolo et al., 2000), but the activity of YZW in the shaft cell indicates that S* does not function in this way. In support of this interpretation, we find that simply replacing S* with S7, the nearest Su(H) site that conforms to the canonical motif definition YGTGDGAA, results in nearly complete silencing of YZW in the shaft cell (compare Figures 3H-3H’ and Figures 3I-3I’). Furthermore, it is notable that the S*-to-S7 mutation also resulted in an increased activity of YZW in the socket cell under Su(H)low conditions (compare Figures 3H’ and 3I’). Together, these observations indicate that, in comparison with S7, S* is a functionally w eaker Su(H) binding site: It does not mediate significant repression in the shaft cell, and does not mediate a strong activation function in the socket cell. The weakness of S* is likely due to the fact that its sequence (CCTGAGAA) differs by one base (underlined) from the high-affinity Su(H) binding motif definition (YGTGDGAA) (Bailey and Posakony, 1995; Barolo et al., 2000; Nellesen et al., 1999; Tun et al., 1994). We note that the functional importance of the S* site is supported by its conservation in all of the original 12 Drosophila species with sequenced genomes, and by the observed effect of mutating it in the context of the full-length Su(H) rescue construct (see Figures S3G and S3H).
In summary, then, these experiments indicate that YZW includes binding sites for a pIIa lineage-specific factor or factors that generate comparably weak cis-regulatory activity in the shaft and socket cells, along with a weakened Su(H) binding site S*, which can promote the socket cell activity of YZW, but only when Su(H) is present at high levels.
The Basal Level of Su(H) Synergizes with Weak Local Activators to Trigger Strong Activation of the ASE in the Nascent Socket Cell
As shown above, fragment ASE5, which contains Su(H) binding sites S2-S6, is active in the wild-type [Su(H)high] background, but is completely silent in the Su(H)low background. Although the latter result may suggest that S2-S6 do not respond to basal levels of Su(H), we observed that ASE5YZW — which includes the sequences of both ASE5 and YZW — is substantially more active than YZW in the socket cell when both are assayed in the Su(H)low background (compare Figures 2I and 3H’). Importantly, this difference is abolished by mutation of sites S2-S6 (Figures 3H’ and 3J’), suggesting that the Su(H) input via S2-S6 in ASE5 can synergize with inputs on YZW to promote the latter’s socket cell activity, even when Su(H) is present at low levels. In a similar vein, we find that Su(H) binding sites S7-S9 can likewise promote the socket cell activity of YZW in the Su(H)low background [compare YZW3S (Figure 2D) and YZW3S-S79m (Figure 3K’)].
Together, these experiments reveal the cis-regulatory logic underlying the initiation of the ASE’s activity in the socket cell: It is combinatorially activated by the basal level of Su(H), which acts upon two separate clusters of high-affinity Su(H) binding sites (S2-S6 and S7-S9), and by certain weak “local activators”, which act upon the YZW fragment to generate — independent of Su(H) — a baseline level of activity in both the shaft cell and the socket cell. The basal level of Su(H) acts cooperatively with the “local activators” in the socket cell, while at the same time repressing their function in the shaft cell, resulting in a socket cell-specific initial activity of ASE.
Synergistic Inputs on the ASE Are Required to Initiate Su(H) Autoregulation
The experiments described thus far delineate three segments of the ASE (ASE5Y, YZW, and ZW3S) that display cis-regulatory activities in the nascent socket cell that do not require an above-basal level of Su(H). Because the role of the ASE in development is to form a strong positive feedback loop with Su(H), we reasoned that these fragments may be involved in initiating this loop.
To determine more specifically how the ASE-Su(H) feedback loop is established, we placed several ASE truncations downstream of the minimum Su(H) rescue construct (RC-ΔASE), and asked if they are able to drive expression of the transgene in a Su(H) null background [Su(H)−/−] (Figure 4A). In this Su(H) rescue assay, we first focused on YZW and ASE5, which respond primarily to the socket/ shaft activators and Su(H), respectively. As summarized in Figure 4C, neither YZW nor ASE5 is able to drive significant Su(H) transgene expression in the nascent socket cell (Figures 4E-4F). By contrast, ASE5YZW, which includes the sequences of both ASE5 and YZW, supports strong Su(H) transgene activity in the nascent socket cell (Figure 4G; see also Figures S4B and S4E), suggesting that synergistic interaction between ASE5 and YZW is required to initiate high levels of Su(H) expression in this cell.
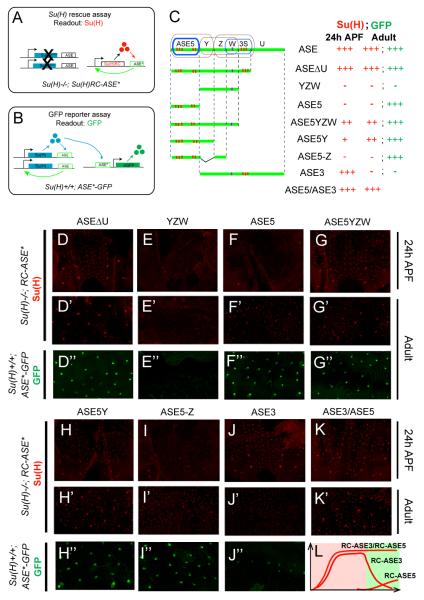
Figure 4. The ASE’s Sub-modules Dynamically Control Su(H) Autoregulation.
(A) The Su(H) transgene rescue assay. Left: The endogenous Su(H) autoregulatory loop is disrupted by Su(H) null mutations (X’s). Right: The Su(H) gene [Su(H)RC] and ASE* (wild-type ASE or fragment thereof) carried in the rescue transgene construct form a positive feedback loop. Anti-Su(H) antibody staining is used as the readout, indicating whether ASE* is sufficient to establish Su(H) autoregulation in the socket cell.
(B) GFP reporter transgene assay in the wild-type background. Once the endogenous ASE-Su(H) feedback loop is established (left), its output [Su(H)] acts on the ASE fragment (ASE*) carried in the reporter transgene construct to drive GFP expression (right). GFP fluorescence in adult flies is used as the readout, indicating whether ASE* is able to respond to the high maintenance levels of Su(H).
(C) Diagrams of ASE fragments (green lines) tested in both the Su(H) transgene rescue and GFP reporter transgene assays. Sub-modules of the ASE are encircled: Brown indicates a sub-module active in the Su(H)low background in pupal stages; blue indicates a sub-module active only in the Su(H)high background in pupal stages. Observed levels of Su(H) and GFP expression are summarized at right, using the same semi-quantitative scoring system as in Figure 2.
(D-K) Su(H) rescue assay: Anti-Su(H) antibody staining in the pupal notum at 24 hours APF.
(D’-K’) Su(H) rescue assay: Anti-Su(H) antibody staining in the adult abdominal epithelium.
(D”-J”) GFP reporter assay: GFP expression in socket cells in the adult abdominal epithelium.
(L) Cartoon illustration of Su(H) expression in the Su(H) rescue assays using RC-ASE3, RC-ASE5, or RC-ASE3/RC-ASE5. Pink panel indicates pupal stage; green panel indicates adult stage; red lines indicate levels of Su(H) expression (relative levels are arbitrary).
See also Figure S4.
Next, to verify the specificity of interaction between certain cis-elements of the ASE in establishing Su(H) autoregulation, we also compared the activities of the fragment ASE5Y and the synthetic construct ASE5-Z in the Su(H) rescue assay (Figures 4H-4I). We found that ASE5Y can drive weak, but significant, Su(H) transgene expression in the nascent socket cell (Figure 4H; see also Figures S4C and S4F), while the synthetic construct ASE5-Z almost completely failed to generate significant above-basal levels of Su(H) (Figure 4I). Thus, consistent with the earlier experiments using a GFP reporter assay (see Figures 2K and 2M), ASE5 selectively synergizes with Y but not Z to contribute to the initiation of Su(H) autoregulation.
Only ASE5 is Responsible for Maintaining Su(H) Autoregulation
While the socket/ shaft factors acting upon YZW are required for the ASE’s initial activity in the nascent socket cell, we find that this fragment displays no detectable activity in the adult socket cell (Figures 4E’-4E”), indicating that some or all of the socket/ shaft activators are only transiently expressed in the socket cell and may thus be required only for the initiation, but not for the maintenance, of the ASE’s activity in this cell.
As mentioned earlier, the ASE contains two Su(H) response elements, ASE5 and W3S (Figure 2A), both of which require above-threshold levels of Su(H) for activation. Because both ASE5 and W3S can drive significant reporter gene activity in the adult socket cell (Figure 4F” and Figures S7C and S7F), we reasoned that they may be required to maintain the autoregulation of Su(H) in this cell, in which Su(H) is expressed at high levels. Unexpectedly, we find that deleting ASE5 from the ASE (ASE3; Figure 4C) results in almost complete loss of the above-basal level of Su(H) expression in the adult socket cell (Figures 4J’-4J”). By contrast, two rescue constructs, RC-ASE5Y and RC-ASE5-Z, can both drive significant Su(H) transgene expression in adult socket cells, even though they lack the W3S segment (Figures 4H’-4H” and 4I’-4I”). Thus, even though both ASE5 and W3S can function as Su(H) response elements, only ASE5 is strictly required for the autoregulated expression of Su(H) in adults.
Consistent with an essential role for ASE5 in mediating the ASE’s function in adult socket cells, we find that, in the GFP reporter gene assay, the cis-regulatory activity of ASE5 is comparable to that of the full ASE in adults, whereas ASE3, which includes all of the ASE except ASE5, is almost silent in adults (Figures 4D”, 4F”, and 4J”; see Figures S7B-S7D). These observations suggest that the ASE’s activity in adults can be attributed primarily to one Su(H) response element, ASE5. In addition, they suggest that the cis-regulatory activity of the other Su(H) response element, W3S, is repressed in adults in the context of the full ASE, presumably because of negative inputs mediated by nearby sequences (see Figures S7D-S7F).
Taken together, these experiments indicate that ASE5 is both necessary and sufficient to account for the ASE’s activity in the adult socket cell. We conclude that the ASE’s function in adults — long-term maintenance of Su(H) autoregulation — is carried out by ASE5.
The ASE’s Sub-modules are Functionally Interlinked
One notable finding from the above experiments is that two non -overlapping components of the ASE, ASE3 and ASE5, exhibit temporally distinct cis-regulatory activities during development. On the one hand, while ASE3 can stimulate strong Su(H) autoregulation in the socket cell in the early pupal stage (see Figure 4J), its activity decreases over time (see Figures 4J’-4J”). On the other hand, although ASE5 cannot initiate Su(H) autoregulation in the pupal stage (see Figure 4F), it is required for maintaining the late phase of the ASE’s activity in adults. Thus, these two separate parts of the ASE appear to be involved in mediating Su(H) autoregulation at different stages of development.
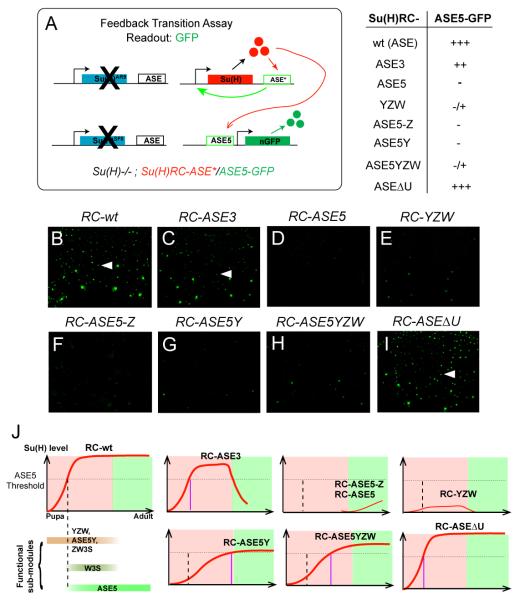
Because ASE3 can transiently drive strong Su(H) expression in the socket cell in early pupae, we hypothesized that this early activity of ASE3 may function to provide the necessary threshold level of Su(H) required to activate ASE5. In support of this hypothesis, although ASE5-GFP is not expressed at any stage in a Su(H)low background [Su(H)−/−; RC-ΔASE], we found that replacing RC-ΔASE with RC-ASE3 in the Su(H) null background resulted in significant ASE5-GFP expression in the socket cells of the pupal notum, starting from as early as 28 hours APF (Figures 5A and 5C).
Figure 5. The Transition From Initiation to Maintenance of Su(H) Autoregulation Depends on Interlinked Functions of the ASE’s Sub-modules.
(A) Diagram of the feedback transition assay. One copy each of a Su(H) rescue construct and the ASE5-GFP reporter construct, both on the third chromosome, are placed into the Su(H) null background. Observed levels of GFP expression are summarized at right, using the same semi-quantitative scoring system as in Figure 2.
(B-I) Activity of the ASE5-GFP reporter in the pupal notum at 28 hours APF in the Su(H)−/−; RC-ASE* background. Arrowheads in B, C, and I indicate single microchaete socket cells that express high levels of GFP.
(J) Cartoon illustrations of the time window s in which fragments of the ASE are active in development. Pink panels indicate pupal stage; green panels indicate adult stage; red lines represent levels of Su(H) expression. Su(H) autoregulation is established in the pupal notum between 18 and 24 hours APF, and persists into adulthood. The dashed vertical line marks the time (24 hours APF) when ASE5 is normally activated by an above-threshold level of Su(H); this threshold is indicated by the dashed horizontal line. In each illustration, the purple vertical line marks the suggested time (if ever) when the indicated Su(H) rescue transgene reaches the threshold required to activate ASE5.
See also Figures S5 and S6.
To explore this further, we placed one copy each of RC-ASE5 and RC-ASE3 in the Su(H) null background, and examined the resulting level of Su(H) in the socket cell. We found that flies of this genotype [Su(H)−/−; RC-ASE3/RC-ASE5] strongly express Su(H) in the socket cell at both pupal and adult stages (Figures 4K-4K’). Because neither RC-ASE5 alone nor RC-ASE3 alone is able to establish robust high levels of Su(H) expression in adults (Figures 4F’ and 4J’), and given that ASE3 exhibits little GFP reporter gene activity in adults (Figure 4J”), the appearance of high levels of Su(H) in adult flies of the Su(H)−/−; RC-ASE3/RC-ASE5 genotype demonstrates an ability of these two non-overlapping elements to communicate and cooperate even when they are separate rather than adjacent (Figure 4L). This finding provides strong support to the conclusion that autoregulatory sub-modules of the ASE are functionally interlinked.
Control of the Timing of the Initiation-Maintenance Transition in Su(H) Autoregulation
The above experiments demonstrate an intriguing feature of the ASE’s functional organization: Initiation of its activity requires a significantly larger part of the enhancer than its maintenance. Specifically, initiation in the nascent socket cell is dependent on several seemingly redundant enhancer sub-modules (ASE5Y, YZW, and ZW3S), but maintenance in the adult socket cell is dependent only on ASE5, which is but a small part of the ASE.
Because a certain high level of Su(H) is required to activate ASE5 (Figures 2L-2L’), we reasoned that the involvement of multiple initiation sub-modules may be important to insure that Su(H) reaches the required threshold level for this activation. To test this idea more systematically, we examined the expression of the ASE5-GFP reporter gene at the pupal stage in various Su(H) rescue genotypes (Figure 5A). We found a strong correlation between the activity of ASE5-GFP and the extent of the enhancer sequences included in the Su(H) rescue construct. In particular, although five rescue constructs can drive significant Su(H) expression in the nascent socket cell, only three — RC-ASE, RC-ASE3, and RC-ASEΔU — can consistently stimulate strong ASE5-GFP activity in the pupal notum at 28 hours APF (Figures 5B-5I; see also Figure S5 and Figure S6). By contrast, even though both RC-ASE5Y and RC-ASE5YZW are also able to generate significant Su(H) expression in the nascent socket cell (see Figures 4G-4H; see also Figure S4), the levels of rescued Su(H) in these genotypes do not appear to be high enough to activate ASE5-GFP robustly in socket cells at this stage (Figures 5G-5H).
These experiments suggest that the minimum requirement for stable activation of the ASE5-GFP reporter in early pupae is for the rescue construct to include the sequences of the two initiation sub-modules YZW and ZW3S (shared by ASEΔU and ASE3; see Figure 4C), even though neither of them is essential for the late activity of ASE5 or for the maintenance of Su(H) autoregulation in adults. Therefore, although many of the functionally important components of the ASE are only transiently active in development, they contribute quantitatively to the initial increase of Su(H) expression in the nascent socket cell, and thus are involved in controlling the timing of the transition into the maintenance phase of Su(H) autoregulation (Figure 5J).
Combinatorial Activation of ASE5 in the Pupal Stage is Required to Establish Su(H) Autoregulation in Adults
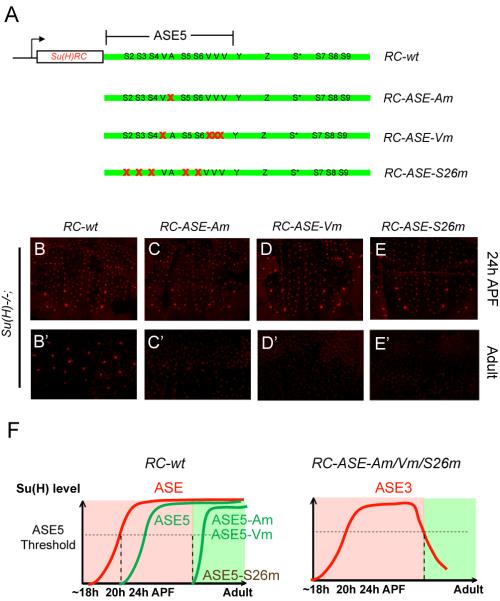
In a previous study (Liu and Posakony, 2012), we analyzed the functional architecture of ASE5, and found that its activity in the socket cell relies not only on the five Su(H) binding sites (S2-S6; Figure 6A), but also on two other types of sequence motif. The first is a single 11-bp sequence element (AACGCGAAGCT, or A motif), and the other comprises four high-affinity binding sites for the POU-homeodomain factor Ventral veins lacking (Vvl) (Figure 6A). The A motif and the Vvl sites are both required for ASE5’s activation in the pupal stage, but they become somewhat redundant in adults. Thus, mutation of either the A motif or the Vvl binding sites each resu lts in severe reduction of ASE5’s activity in the pupal stage, while having relatively little effect on its function in adults (Liu and Posakony, 2012).
Figure 6. ASE5 is Combinatorially Activated in Pupal Stages to Maintain Su(H) Autoregulation in Adults.
(A) Diagrams of Su(H) rescue constructs carrying point mutations in the ASE5 sub-module of the ASE. Note that the ASE5 region has been expanded out of proportion to the rest of the ASE in order to illustrate the sequence motif composition of ASE5. Red X’s indicated mutated motifs.
(B-E) Anti-Su(H) antibody staining in the pupal notum at 24 hours APF.
(B’-E’) Anti-Su(H) antibody staining in the adult abdominal epithelium.
(F) (Left) Cartoon summary of the effects of mutating the A motif or the Vvl sites on the function of an ASE5-GFP reporter (green lines) in a wild -type background (Liu and Posakony, 2012). In the presence of normal levels of Su(H) (red line), ASE5 normally becomes active in the socket cell of the microchaetes in the pupal notum between 20 and 24 hours APF; ASE5-Am and ASE5-Vm are not active until pre-pharate adult stages; and ASE5-S26m is silent throughout development. (Right) Based on these prior results, on those shown in Figures 4J-4K’, and on those shown in B-E’, we suggest that Su(H) expression directed by RC-ASE-Am, RC-ASE-Vm, and RC-ASE-S26m is due to ASE3 (red line). Like RC-ASE3 (see Figures 4J-4J’), these constructs all generate very substantial levels of Su(H) in pupal stages (C-E), but this early expression is not maintained into adults (C’-E’). We suggest that this is because by the time ASE3 becomes inactive, ASE5 has not been activated in time (RC-ASE-Am and RC-ASE-Vm) or has not been activated at all (RC-ASE-S26m).
To investigate the possible roles of the A motif and the Vvl sites in Su(H) autoregulation, we mutated them separately in the context of the wild -type Su(H) rescue construct (RC-wt; Figure 6A). We found that these two types of mutation had little effect on the ability of RC-wt to establish high levels of Su(H) expression in the pupal stage, but in both cases, the early high level of Su(H) is lost in the adult socket cell (Figures 6B-6B’, 6C-6C’, and 6D-6D’). This is similar to the effect of mutating all five Su(H) binding sites in ASE5 (S2-26) in the context of RC-wt (Figures 6E-6E’).
Based on the “phase transition” model described above, we suggest that these results can be explained by the failure of ASE5 to be activated in a timely manner in the differentiating socket cell. That is, the apparently normal rescue of Su(H) expression observed in the pupal stage in these genotypes is driven by an early phase of the ASE’s activity, which is mediated by the sequences outside of ASE5 (i.e., ASE3). However, in the absence of input via the A motif, the Vvl sites, or the Su(H) binding sites, ASE5 remains silent at the time ASE3’s activity declines and disappears, so that the transient expression of high levels of Su(H) cannot be maintained in adults (Figure 6F).
DISCUSSION
A Dynamic Model of the Control of Su(H) Autoregulation
In this study, we have systematically dissected the functional organization of the autoregulatory socket enhancer (ASE) of the Drosophila Su(H) gene, which controls the long-term transcriptional autoactivation of Su(H) specifically in the socket cell of external sensory organs (Barolo et al., 2000). Based on these experiments, we propose here a dynamic model to explain how Su(H) autoregulation is controlled (Figure 7).
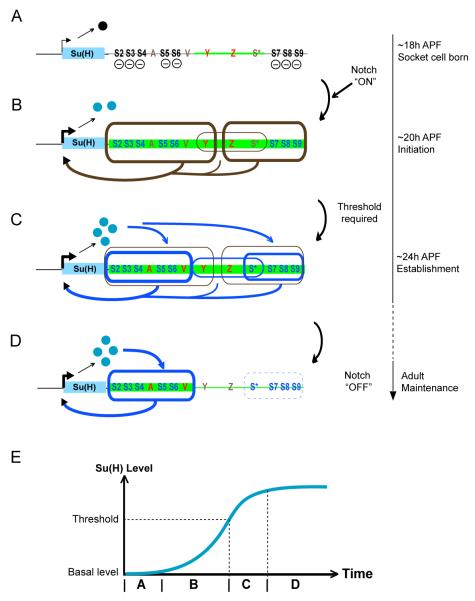
Figure 7. Model of How the ASE Integrates Multiple Feedback Loops to Control Su(H) Autoregulation in the Differentiating Socket Cell.
(A-D) Diagrams depicting the dynamic interaction between the ASE and its functional inputs in the socket cell. The timeline at right refers to the developmental stages of microchaete socket cells in the pupal notum. The body of the Su(H) gene is represented as a blue box; the accompanying arrow shows the direction of transcription, with its thickness denoting the intensity of transcriptional activity in the socket cell. The ASE is shown downstream of the gene, with functionally important sequence motifs and sub-regions labeled. Segments of the ASE receiving local activator inputs are highlighted in green, with the input level indicated by the thickness of the green line. The repressor form of Su(H) is shown as a black ball, the activator form as blue balls. (A) Shortly after the socket cell is born, the ASE receives local activator inputs via the sequences in YZW (thin green line, red Y and Z), but is silenced by the default repression function of Su(H), acting via binding sites S2-S9 (—).
(B) Activation of the Notch pathway in the socket cell converts Su(H) into a transcriptional activator (Barolo et al., 2000). Su(H) and various local activator inputs selectively synergize to activate two sub-modules, ASE5Y and ZW3S (brown boxes, thick lines), which drive an increase in Su(H) transcription (thick brown arrows). YZW (brown box, thin line) may also contribute independently to this activity (thin brown arrow).
(C) Once Su(H) expression rises above a threshold level, two Su(H) response elements, ASE5 and W3S (blue boxes, thick lines), are activated to fully establish the ASE-Su(H) feedback loop (blue arrows). Activation of ASE5 and W3S depend s in part on other local activator inputs; in particular, ASE5 receives two activator inputs via a single A motif (red A) and multiple binding sites for Vvl (red V), a POU-homeodomain transcription factor (Liu and Posakony, 2012).
(D) As the socket cell differentiates, the local activator inputs on YZW disappear (brown Y and Z) and W3S is silenced (blue box, dashed line), but the activator inputs on ASE5 persist into adulthood, keeping ASE5 active to maintain Su(H) autoregulation (blue arrows). See also Figure S7.
(E) The dynamic pattern of activation of the ASE’s sub-modules progressively establishes Su(H) autoregulation in the socket cell. Letters on the time axis refer to stages A-D above.
We suggest that, within a short time window (0-2 hours) after the division of the pIIa secondary precursor cell (see Figure 1A), the ASE receives certain “local activator” inputs in both postmitotic daughter cells via the YZW fragment; at the same time, the ASE is silenced by the repressive activity of basal levels of Su(H), via the two flanking clusters of high-affinity Su(H) binding sites, S2-S6 and S7-S9 (Figure 7A).
Next, the incoming Notch signal that specifies the socket cell fate converts Su(H) into a transcriptional activator in this cell (Barolo et al., 2000); Su(H) then synergizes with the early inputs on YZW to activate two enhancer sub-modules, ASE5Y and ZW3S, to trigger a rapid rise in Su(H) transcription. Simultaneously, YZW may contribute independently to the activity of Su(H). The activation of these three sub-modules marks the initiation of the ASE-Su(H) autoregulatory loop (Figure 7B).
Later, about 4 hours after the socket cell is born, the accumulated level of Su(H) rises above a certain threshold and feeds back on the ASE to activate two Su(H) response elements, ASE5 and W3S, the activities of which are strictly Su(H) concentration-dependent. Moreover, at least for ASE5, Su(H) must synergize with two other activator inputs that are mediated by the A-motif and several high-affinity Vvl binding motifs (Liu and Posakony, 2012). The activation of ASE5 and W3S marks the time when the ASE-Su(H) autoregulatory loop is fully established (Figure 7C).
Finally, as the socket cell differentiates, the factors acting upon Y and Z disapp ear from the socket cell, leading to the inactivation of the three sub-modules — ASE5Y, YZW, and ZW3S — that are responsible for the initiation of Su(H) autoregulation. However, one of the Su(H) response elements, ASE5, remains strongly active and maintains the autoregulatory loop into adulthood (Figure 7D). The other Su(H) response element, W3S, is silent at this stage, evidently due to a late repression function of the adjacent sequences (see Figure S7).
Another important dynamic element is embodied in the above model. Su(H) is seen to play an essential role in all three phases of Su(H) autoregulation in the socket cell — initiation, establishment, and maintenance. In the first step, initiation, Su(H) is already present at its low basal level; here, it is fully dependent on Notch signaling, which converts it from a repressor to an activator (Figures 7A-7B). But we have shown previously that, in the long-term maintenance phase (Figure 7D), when Su(H) is present at a high level in the socket cell, it acts independently of Notch, possibly employing a distinct co-activator (Barolo et al., 2000). Thus, the transition from low -level initiation to high-level maintenance in Su(H) autoregulation involves a transition from a Notch-dependent to a Notch-independent mode of action for Su(H) and the ASE.
The ASE Consists of Interlocking Enhancer Sub-modules
An enhancer module is typically defined as a discrete genomic fragment capable of driving a specific pattern of gene transcription (with respect to location, time, and level). Most studies of enhancer function have accordingly treated each such module as a unit, and have focused on its integrative capacity; namely, the enhancer’s ability to synthesize multiple transcription factor inputs, both positive and negative, into a single transcriptional output.
Our detailed functional analysis of the ASE has revealed a more complex picture that emphasizes enhancer substructure. We have found that different component fragments of the full ASE have distinct functional roles to play in the initiation, establishment, and maintenance of cell type-specific Su(H) autoregulation. One might perhaps conceive of these component fragments as discrete enhancer modules themselves, but as we have shown, their boundaries often overlap (for example, ASE5 and W3S are respectively embedded within ASE5Y and ZW3S), and in any case they all help generate the same output — continuous, elevated expression of Su(H) in the socket cell. Therefore, we suggest that it is conceptually more useful to think of the ASE as a single enhancer composed of several enhancer sub-modules that are functionally distinct but not clearly separable physically. In a highly dynamic manner (Figure 7), each sub-module makes an important contribution to the overall spatial, temporal, and quantitative cis-regulatory activity of the full enhancer. Conversely, the ASE’s architecture gives it the ability to integrate these multiple regulatory contributions into a stably progressing temporal pattern of Su(H) expression in the differentiating socket cell (Figure 7E), a context in which both the external stimuli and the internal gene expression profile undergo dramatic changes. Such a sophisticated integration capacity has been observed previously for the control of some genes by a cohort of separate enhancer modules (Ben-Tabou de-Leon and Davidson, 2010; Wahl et al., 2009; Yuh et al., 1998; Yuh et al., 2001), but our study shows that even a single enhancer may employ this strategy using a compact set of sub-modules.
Finally, we suggest that dividing the ASE’s various activities among multiple functionally connected, and partially redundant, sub-modules confers on the enhancer considerable evolutionary flexibility to fine-tune almost every aspect of its function (time, space, and level). The successive employment of distinct sub-modules allows the ASE’s activity at different stages to be modified separately by mutational changes within individual sub-modules. Likewise, the partially redundant functions of the ASE5Y and ZW3S initiation sub-modules allow each to undergo separate changes in a context in which the ASE’s activity is buffered by the other sub-module.
Interlinked Positive Feedback Loops Create a Stable “Lockdown” Switch
A particularly notable feature of the ASE is that each of its sub-modules contains one or a few Su(H) binding sites. Because each sub-module can activate Su(H) transcription independently, and at the same time can respond to direct activation by Su(H), the reciprocal interaction between the ASE and Su(H) comprises not one, but several, interlinked positive feedback loops (Figure 7). Furthermore, due in part to the requirement for different levels of Su(H) for sub-module activation, the feedback loops between the ASE and Su(H) are interlinked in three distinct layers. The first linkage occurs among the ASE’s three initiation sub-modules (ASE5Y, YZW, and ZW3S), which cooperate to drive Su(H) expression above its basal level (Figure 7B). The second linkage is between the initiation sub-modules and the two Su(H) response elements (ASE5 and W3S), since the activities of the former group are responsible for generating the high level of Su(H) required to activate the latter (Figure 7C). The third linkage occurs between the two Su(H) response elements themselves: When they are activated by high levels of Su(H), each then acts as a discrete Su(H) autoregulatory enhancer to reinforce the other’s activity via the feedback input from Su(H) (Figure 7C). The logic of this system is evident: Once a certain threshold level of Su(H) expression is established by the linked initiation sub-modules of the ASE, it can be quickly “locked down” by the linked autoregulatory functions of the Su(H) response elements.
Recent studies have suggested that, in comparison with single positive feedback loops, interlinked positive feedback loops with different time constants are less sensitive to background noise and more effective at mediating rapid irreversible gene activation (Brandman et al., 2005). We suggest that the ASE represents a transcriptional implementation of this paradigm. For example, when the function of one of the ASE’s initiation sub-modules is removed, Su(H) autoregulation can still be established, but the process is either fast-and-reversible (e.g., RC-ASE3) or slow-and-irreversible (e.g., RC-ASE5Y). Thus, while a simpler module might indeed be capable of establishing Su(H) autoregulation per se, the interlinked feedback loops of the ASE insure that this happens rapidly and robustly, in a switch-like manner.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
The sequences of the ASE, Su(H) RC-wt, and Su(H) RC-ΔASE were defined previously (Barolo et al., 2000). To make GFP reporter transgene constructs, the full ASE and its variants were amplified by PCR and cloned into the EcoRI and BamHI sites of the plasmid vector pH-Stinger-attB (Steven W. Miller, UCSD).
Su(H) rescue constructs were generated in three steps. First, the ϕC31 attB recognition sequence (Huang et al., 2009) was amplified by PCR and cloned into the HindIII site of the P element-based plasmid vector pNot-CaSpeR to generate the vector attB-pNot-CaSpeR. Second, the genomic DNA fragment Su(H) RC-ΔASE (Barolo et al., 2000) was amplified by PCR and cloned into the NotI and BamHI sites of the attB-pNot-CaSpeR vector. Third, the BamHI site in the attB-pNot-CaSpeR/Su(H) RC-ΔASE plasmid was used to insert the ASE or its variants to generate the rescue constructs described in this study. Only constructs containing inserts matching the orientation of the wild -type ASE with respect to the Su(H) coding sequences were used to generate transgenic flies.
Plasmid constructs were confirmed by DNA sequencing (GENEWIZ San Diego, La Jolla, CA). PCR primers used for the above experiments are described in Supplemental Experimental Procedures.
Drosophila Stocks and Crosses
Drosophila embryo injections were performed according to standard protocols (Rubin and Spradling, 1982). Both the GFP reporter gene and Su(H) rescue constructs were integrated into the attP2 docking site on the third chromosome (68A4) using the ϕC31 integrase system (Bischof et al., 2007). Transgenic lines were backcrossed to the w1118 strain to establish homozygous stocks.
The flies used for Su(H) rescue experiments are of the following genotype: Su(H)AR9/Su(H)SF8; p[w+; RC-ASE*] (ASE* indicates the ASE or its variants). Su(H)AR9 and Su(H)SF8 are both null alleles (Schweisguth and Posakony, 1992).
To assay GFP reporter gene expression in the Su(H)low background, the following crosses were performed: Su(H)AR9/Cyo, Kr>GFP; p[ASE*-GFP] X Su(H)AR9/Su(H)SF8; p[RC-ΔASE]. Progeny of the genotype Su(H)AR9/Su(H)SF8; p[RC-ΔASE]/p[ASE*-GFP] were distinguished by the lack of Kr>GFP expression in the pupal trunk (Casso et al., 2000).
For the feedback transition assay, the following crosses were performed: Su(H)AR9/Cyo, Kr>GFP; p[ASE5-GFP] X Su(H)AR9/Su(H)SF8; p[RC-ASE*]. Progeny of the genotype Su(H)AR9/Su(H)SF8; p[RC-ASE*]/ p[ASE5-GFP] were distinguished by the lack of Kr>GFP expression in the pupal trunk (Casso et al., 2000).
All crosses were carried out at 25°C. For timed dissection, white prepupae (0 hours APF) were handpicked and cultured in a humidified chamber until the desired time point for dissection.
Immunohistochemistry and Confocal Microscopy
Pupal nota and adult abdominal epithelia were dissected in PBT (1XPBS, 0.1% Triton-X100) and fixed in 4% paraformaldehyde in 1XPBT for 30 minutes at room temperature. After three washes in PBT, tissues were used for direct visualization of GFP fluorescence or for immunohistochemistry. Primary antibodies used were: Guinea pig anti-D-Pax2 polyclonal antibody (1:1000; Steven W. Miller, UCSD); rabbit anti-GFP polyclonal antibody (1:1000; Invitrogen); mouse anti-Prospero monoclonal antibody (1:10; Developmental Studies Hybridoma Bank) (Spana and Doe, 1995); rabbit anti-Su(H) polyclonal antibody (1:1000; Santa Cruz Biotechnology, Inc.). Secondary antibodies used were: Anti-rabbit-Alexa488, anti-rabbit-Alexa555, anti-mouse-Alexa555, and anti-guinea pig-Alexa647, all diluted 1:200 (Molecular Probes).
Fluorescent images were collected with a Leica TCS SP2 confocal microscope (Leica Microsystems). Each image was generated as an average projection of a series of Z-section images taken at 2-μm intervals. All images were collected using the same magnification and gain settings. Care was taken to select representative images from each experiment, though little variation was apparent between repetitions. Experiments to be directly compared were conducted, and the results imaged, at the same time.
GFP levels were scored using the following semi-quantitative system: strong +++, moderate ++, weak +, weak stochastic −/+, and negative −.
Supplementary Material
HIGHLIGHTS.
An autoregulatory enhancer is composed of functionally separable sub-modules.
Autoregulation is subject to dynamic combinatorial control.
Interlinked positive feedback loops establish rapid and irreversible autoregulation.
A threshold level of the factor is essential to creating the autoregulatory state.
ACKNOWLEDGEMENTS
We are grateful to Steve Miller for providing the pH-Stinger-attB vector and for numerous suggestions during the course of this work. We thank Steve Miller and Sui Zhang for generating the anti-D-Pax2 antibody. Steve Miller and Jenny Wilson-Connelly provided very useful comments on the manuscript. This work was supported by National Institute of General Medical Sciences grant R01 GM046993 to J.W.P.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes seven figures, Supplemental Experimental Procedures, and one Supplemental Reference and can be found with this article online at.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bailey AM, Posakony JW. Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW. A Notch-independent activity of Suppressor of Hairless is required for normal mechanoreceptor physiology. Cell. 2000;103:957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- Ben-Tabou de-Leon S, Davidson EH. Information processing at the foxa node of the sea urchin endomesoderm specification network. Proc Natl Acad Sci USA. 2010;107:10103–10108. doi: 10.1073/pnas.1004824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Ferrell JEJ, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casso D, Ramirez-Weber F, Kornberg TB. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech. Dev. 2000;91:451–454. doi: 10.1016/s0925-4773(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Crews ST, Pearson JC. Transcriptional autoregulation in development. Curr Biol. 2009;19:R241–R246. doi: 10.1016/j.cub.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME, Artavanis-Tsakonas S. The Suppressor of Hairless protein participates in Notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol. 2011a;27:681–696. doi: 10.1146/annurev-cellbio-092910-154226. [DOI] [PubMed] [Google Scholar]
- Hobert O. Maintaining a memory by transcriptional autoregulation. Curr Biol. 2011b;21:R146–R147. doi: 10.1016/j.cub.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhou W, Dong W, Hong Y. Targeted engineering of the Drosophila genome. Fly (Austin) 2009;3:274–277. doi: 10.4161/fly.9978. [DOI] [PubMed] [Google Scholar]
- Kavaler J, Fu W, Duan H, Noll M, Posakony JW. An essential role for the Drosophila Pax2 homolog in the differentiation of adult sensory organs. Development. 1999;126:2261–2272. doi: 10.1242/dev.126.10.2261. [DOI] [PubMed] [Google Scholar]
- Liu F, Posakony JW. Role of architecture in the function and specificity of two Notch-regulated transcriptional enhancer modules. PLoS Genet. 2012;8:e1002796. doi: 10.1371/journal.pgen.1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SW, Avidor-Reiss T, Polyanovsky A, Posakony JW. Complex interplay of three transcription factors in controlling the tormogen differentiation program of Drosophila mechanoreceptors. Dev. Biol. 2009;329:386–399. doi: 10.1016/j.ydbio.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellesen DT, Lai EC, Posakony JW. Discrete enhancer elements mediate selective responsiveness of Enhancer of split Complex genes to common transcriptional activators. Dev. Biol. 1999;213:33–53. doi: 10.1006/dbio.1999.9324. [DOI] [PubMed] [Google Scholar]
- Richards GS, Degnan BM. The expression of Delta ligands in the sponge Amphimedon queenslandica suggests an ancient role for Notch signaling in metazoan development. Evodevo. 2012;3:15. doi: 10.1186/2041-9139-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992;69:1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–3195. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- Tun T, Hamaguchi Y, Matsunami N, Furukawa T, Honjo T, Kawaichi M. Recognition sequence of a highly conserved DNA binding protein RBP-Jk. Nucleic Acids Res. 1994;22:965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl ME, Hahn J, Gora K, Davidson EH, Oliveri P. The cis-regulatory system of the tbrain gene: Alternative use of multiple modules to promote skeletogenic expression in the sea urchin embryo. Dev Biol. 2009;335:428–441. doi: 10.1016/j.ydbio.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh CH, Bolouri H, Davidson EH. Genomic cis-regulatory logic: experimental and computational analysis of a sea urchin gene. Science. 1998;279:1896–1902. doi: 10.1126/science.279.5358.1896. [DOI] [PubMed] [Google Scholar]
- Yuh CH, Bolouri H, Davidson EH. Cis-regulatory logic in the endo16 gene: switching from a specification to a differentiation mode of control. Development. 2001;128:617–629. doi: 10.1242/dev.128.5.617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.