Abstract
A major barrier of vaccines as cancer treatment is their failure to activate and maintain a complete cancer-specific CD8+ effector T cell repertoire. Low avidity T cells are more likely to escape clonal deletion in the thymus when compared to higher avidity T cells, and therefore comprise the major population of effector T cells available for activation in cancer patients. However, low avidity T cells fail to traffic into the tumor microenvironment and function in eradicating tumor under optimal vaccination conditions as opposed to higher avidity T cells that escape clonal deletion and function in tumor-killing. We utilized high and low avidity TCR transgenic CD8+ T cells specific for the immunodominant epitope HER-2/neu (RNEU420-429) to identify signaling pathways responsible for the inferior activity of the low avidity T cells. Adoptive transfer of these cells into tumor-bearing vaccinated mice identified members of apoptosis pathways that are upregulated in low avidity T cells. The increased expression of pro-apoptotic proteins by low avidity T cells promoted their own cell death and also that of other tumor-specific CD8+ T cells within their local environment. Importantly, we show that this pro-apoptotic effect can be overcome using a strong costimulatory signal that prevents activation-induced cell death and enables low avidity T cells to traffic into the tumor and assist in tumor clearance. These findings identify new therapeutic opportunities for activating the most potent anticancer T cell responses.
Keywords: T cell repertoire, TNFR agonists, AICD, cancer vaccine, immune tolerance
Introduction
Cancer vaccines that activate cytotoxic CD8+ T cells are emerging as a promising treatment for patients with cancer (1). Vaccines are most effective when they induce a large higher-avidity T cell repertoire with populations specific for multiple epitopes of relevant tumor antigens (2). However, one barrier to effective immunization is the predominant establishment of lower avidity and therefore less potent T cells instead of the high avidity T cells typically found in non tumor-bearing hosts. This scarcity of high avidity T cells is due to clonal deletion during thymic selection, from which the low avidity T cells are capable of escaping (3, 4). This results in a population of low avidity T cells available in the periphery to mount a potential antitumor response (3, 4). While thymic deletion is an important mechanism to protect the host from the activation of self-reactive T cells, the remaining lower avidity T cells have a higher threshold of activation and are greatly impaired by the peripheral tolerance mechanisms (5–7).
A second barrier to effective activation of both high and low avidity T cells are the many cell types within the tumor microenvironment, including the tumor cells themselves, which inactivate vaccine-induced tumor-targeted T cells. Both high avidity and low avidity T cells are regulated by multiple peripheral tolerance mechanisms including suppression by regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and intrinsic T cell tolerance mechanisms such as anergy that result in increased expression of co-inhibitory molecules including cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death receptor-1 (PD-1) (6, 8–10). Low avidity T cells are also subject to peripheral deletion triggered by the chronic presence of small amounts of antigen, but can either escape deletion when no antigen is present or be protected against deletion through activation or anergy if they receive a strong enough TCR signal (6). While high and low avidity T cells may be regulated by similar mechanisms, emerging evidence suggest that there are also signaling pathways that predominantly regulate low versus high avidity populations within the T cell repertoire (8, 11–15). Agents are being developed that can modulate a number of these T cell regulatory pathways leading to enhanced antitumor activity in preclinical models and in patients with cancer (11, 12, 16–19). The first such agent, ipilimumab (Yervoy), was recently approved for use by the US FDA for its ability to activate T cells by down-regulating the T cell inhibitory signal CTLA-4 in patients with metastatic melanoma (20). It is still not clear which signaling pathways regulate low versus high avidity T cells and whether it is possible to convert a low avidity T cell into one that functions with high avidity potency. Thus, understanding the activation signaling differences between high and low avidity T cells will facilitate the discovery of additional drugs that activate a more complete T cell repertoire.
We previously reported the development of high and low avidity CD8+ T cell populations specific for the same HER-2/neu-expressing (21) immunodominant epitope, RNEU420-429 (22). These TCR transgenic mice were used to identify a sub-population of Tregs that inhibit high avidity T cells from functioning within the tumor. In addition, immune-modulating doses of cyclophosphamide (23) given with a neu-targeted, GM-CSF-secreting vaccine inhibit these Tregs and allow for complete eradication of neu-expressing mammary tumors by adoptively transferred high avidity T cells (24). In contrast, adoptively transferred low avidity T cells fail to traffic into and eradicate the tumor under the same conditions (22). Using these two TCR transgenic T cell populations, we show for the first time that low avidity T cells have increased expression of the pro-apoptotic proteins TNFRSF10B (DR5), FasL, and CD24; and the expression of these proteins correlates with reduced T cell function and increased T cell death. In addition, the presence of FasL-expressing low avidity T cells also causes death of high avidity CD8+ T cells when co-cultured in vitro. These studies establish that blocking activation-induced cell death (AICD) with TNF-receptor agonists (anti-OX40 and 41BB antibodies) allows low avidity T cells to traffic into the tumor and enhance tumor clearance. Therefore, these data identify the death receptors as a new mechanism responsible for regulating low avidity CD8+ T cell populations, providing new opportunities for developing another class of immune-modulating agents that can enhance the activity of cancer vaccines.
Materials and Methods
Mice
HER-2/neu (neu-N) transgenic mice were purchased from Jackson Laboratory (FVB-Tg(MMTV-Erbb2)NK1Mul/J), and bred and housed at the Johns Hopkins animal facility. Experiments were performed using female mice between 6 and 12 weeks old and protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. High and low avidity T cell receptor (TCR) transgenic mice were generated from RNEU420-429 specific T cell clones as described (22). Dilutional tetramer staining was used to verify the avidity of each mouse before use.
Cell lines and Media
The NT2.5 tumor cell line, 3T3neuGM vaccine, 3T3GM mock vaccine, and T2-Dq T cell target cell line were created and maintained as previously reported (25). GM-CSF was verified by ELISA and neu levels were verified by flow cytometry bi-annually (25).
Peptides and Antibodies
The immunodominant RNEU420-429 (PDSLRDLSVF) peptide and LCMV NP118-126 (RPQASGVYM) negative control peptide were synthesized at >95% purity in the Johns Hopkins Biosynthesis and Sequencing Facility. The anti-OX40 antibody was produced from the OX86 hybridoma, a gift from the Weinberg lab. The hybridoma was grown in protein-free hybridoma media (Gibco) and purified over a protein G column (BD Pharmingen). Purified rat IgG was used as an irrelevant control (Jackson Laboratories). Antibodies for flow cytometry were: anti-DR5-PE, anti-CD24-APC, anti-AnnexinV-Pacific Blue, anti-Thy1.2-PerCP, anti-Thy1.2-Pacific Blue, anti-CD8-FITC, anti-Bcl-2-APC (Biolegend); anti-FasL-PerCP-efluor710 (eBioscience); anti-AnnexinV-PE, anti-Thy1.2-FITC, anti-IFNγ-PE, anti-TNFα-APC, and purified anti-Fas (BD Pharmingen); anti-Survivin-PE (Cell Signaling Technology); purified anti-CD24 and polyclonal rabbit IgG (Santa Cruz).
Microarrays
Microarrays were performed at the Johns Hopkins Deep Sequencing and Microarray Core Facility using the NuGen amplification system and an Affymetrix Exon 1.0 ST array (GEO accession: GSE54020). Cells were adoptively transferred into tumor-bearing, Cy-treated, vaccinated neu-N mice before being extracted from the tumor-draining node on day 3. RNA was extracted using Qiagens RNeasy mini kit.
Tumor, Cy, vaccine, adoptive transfer, CD4+ depletion, and OX40 and 41BB antibody administration procedures
1×106 NT2.5 tumor cells were injected subcutaneously (s.c.) into the right mammary fat pad. One week after tumor injection mice received 100mg/kg Cy intraperitoneally (i.p.). Twenty-four hours later mice received three s.c. injections of the 3T3neuGM vaccine (1×106 cells per injection) into the left upper limb and both lower limbs. One day after vaccination 6×106 high or low avidity CD8+ T cells were adoptively transferred into the mice. T cells were isolated from splenocytes of high and low avidity TCR transgenic mice using a Dynal mouse CD8-negative isolation kit (Invitrogen). Proliferation was assessed using the CellTrace CFSE cell proliferation kit (Invitrogen). 300μg of OX40 or 41BB antibody (R&D) was given i.p. with adoptive transfer three and seven days after vaccination. 300ug of CD4+ T cell-depletion antibody (Biolegend) was given i.p. three days before adoptive transfer. All experiments described in this paper were repeated at least twice with a minimum of 3 mice per group.
Intracellular cytokine staining (26) and flow cytometry
Three days after adoptive transfer, intracellular staining was performed on cells from tumor-draining lymph nodes using the mouse intracellular staining kit (BD Biosciences). 1×106 extracted cells were incubated with 2×105 peptide-pulsed T2-Dq cells for 5 hours at 37°C with Brefeldin A (BD Biosciences) as described in Weiss et al. (22). Extracellular surface staining was performed overnight in FACS buffer. Samples were read on an LSR-II flow cytometer and analyzed using FACSDiva software (BD Biosciences). Tumor-infiltrating lymphocytes were analyzed by surface staining 5 days after adoptive transfer. Tumors were mashed and digested using 1mg/ml collagenase (Gibco) and 25μg/ml hyaluronidase (Sigma) before being stained and analyzed by flow cytometry.
Tumor-treatment studies
5×104 NT2.5 cells were injected s.c. into the right mammary fat pad two days before treatment with Cy, vaccination, and adoptive transfer as described above. 300μg of OX40 or IgG was given i.p. 3 and 7 days after vaccination. Tumor length and width were measured every 5 days. Mice were sacrificed when tumor size exceeded 1cm2.
Apoptosis studies
T cells were isolated as described above from tumor-draining lymph nodes on day 3 and stained for surface markers before staining with Annexin V and 7-AAD (7-amino-actinomycin D) as described in BD Biosciences’ Apoptosis Detection Kit. Crosslinking apoptosis studies were performed in vitro on splenocytes from high and low avidity TCR transgenic mice by adding 0.1μg/ml purified Fas antibody, 500ng/ml CD24 antibody, or 500ng/ml IgG to 5×105 cells/ml in a 96-well plate incubated at 37°C for three hours with T2-Dq cells pulsed with 10ng of peptide. Following incubation, T cells were washed and stained as described previously.
Procedure for low avidity T cell killing of high avidity T cells
Following lysis of the red blood cells using ACK buffer (Ammonium-Chloride-Potassium buffer, Gibco), splenocytes from high avidity TCR transgenic mice were mixed with CD8+ isolated low avidity T cells at a ratio of 1:4 before incubating with peptide-pulsed (20μg) T2-Dq cells at 37°C for 24 hours. Apoptosis staining was performed as described above using Vβ4 TCR staining to differentiate the high avidity T cells from the Vβ2 low avidity T cells.
Statistics
Student’s t tests (paired and unpaired) were performed using GraphPad Prism software. Differences were considered statistically significant if a value of p<0.05 was found.
Results
Cell death proteins are upregulated in low avidity CD8+ T cells relative to high avidity T cells in tolerant mice
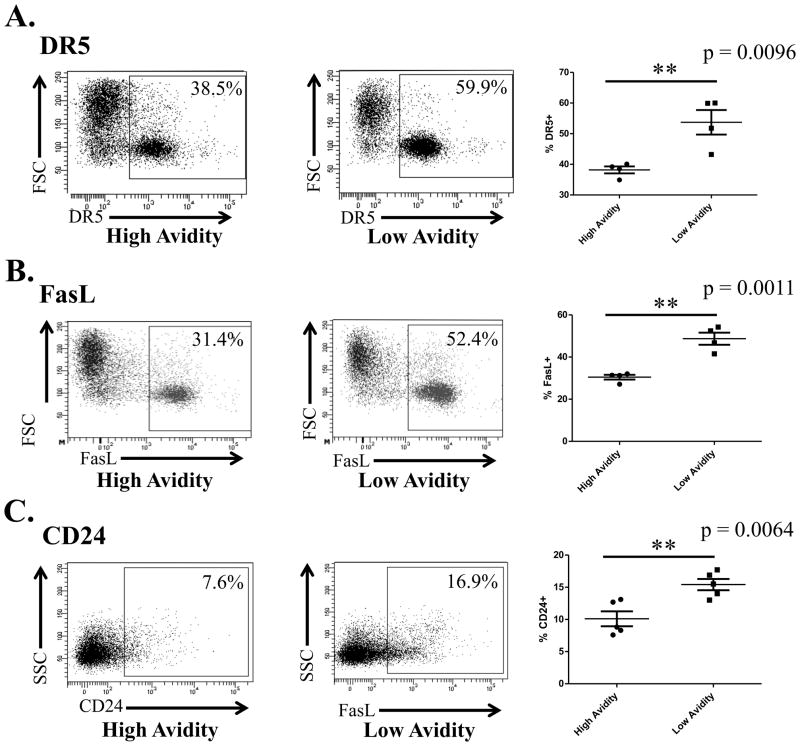
We previously reported that vaccinated, Cy-treated low avidity RNEU420-429-specific TCR transgenic T cells adoptively transferred into a tolerant environment do not clear tumor, whereas similarly treated high avidity T cells also specific for RNEU420-429 are capable of clearing large burdens of tumor (22). Low avidity T cells also do not secrete effector cytokines characteristic of fully activated T cells nor do they traffic to the tumor (22). To explain this disparity in functionality we adoptively transferred naive low and high avidity TCR transgenic T cells into neu-N transgenic mice to identify differences in protein expression between high and low avidity T cells in a tolerant environment. CD8+ T cells were adoptively transferred into tumor-bearing neu-N mice treated with vaccine+Cy and isolated from the tumor-draining lymph nodes of the neu-N mice 3 days after adoptive transfer (22). mRNA was extracted from these cells to perform microarray analysis of gene expression (Fig. S1). Ingenuity analysis of microarray data identified cell death pathways significantly increased in low versus high avidity T cells. Flow cytometry studies further confirmed that the pro-death proteins DR5, FasL, and CD24 are expressed at higher levels on low avidity cells than high avidity cells (Fig. 1A–C). These initial studies led to the hypothesis that higher expression of pro-death proteins on low avidity T cells may explain the difference in function between low and high avidity T cells in a tolerant tumor microenvironment.
Figure 1. Expression of pro-apoptotic proteins DR5, FasL, and CD24 are increased on low avidity relative to high avidity CD8+ T cells.
6×106 high or low avidity T cells were adoptively transferred into neu-N mice treated with Cy and vaccine. Three days later, adoptive transfer cells were extracted from the tumor-draining node of the neu-N mice and protein expression was assessed by flow cytometry. These experiments were repeated at least 5 times (n=3–5 mice/group). A. DR5 expression compared between low avidity CD8+ T cells and high avidity CD8+ T cells. B. FasL expression compared between low avidity CD8+ T cells and high avidity CD8+ T cells. C. CD24 expression compared between low avidity CD8+ T cells and high avidity CD8+ T cells.
DR5, FasL, and CD24 expression correlates with reduced low avidity CD8+ T cell function in a tolerant microenvironment
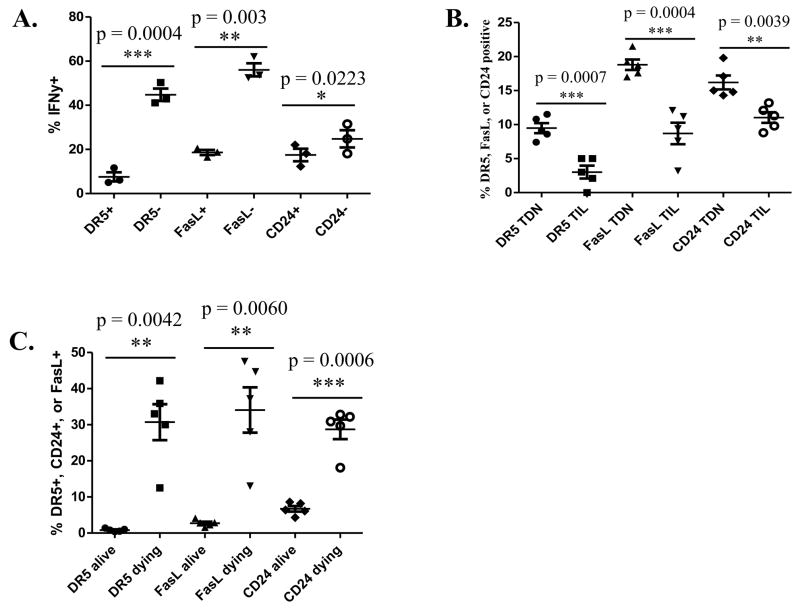
These studies demonstrate a correlation between the expression of DR5, FasL, and CD24 on T cells and avidity. Next, we evaluated whether increased expression of death pathway proteins also correlates with reduced function of low avidity CD8+ T cells. Low and high avidity T cells, adoptively transferred into tumor-bearing neu-N mice treated with low dose Cy and vaccine, were evaluated for cytokine secretion and trafficking into the tumor. The principal cytokine produced by activated RNEU420-429-specific CD8+ T cells is IFNγ, and low avidity RNEU420-429-specific CD8+ T cells produce much less IFNγ than high avidity T cells when adoptively transferred into a tolerant environment (22). To determine the effect of DR5, FasL, and CD24 on the function of low avidity T cells we first compared the expression of these proteins with IFNγ secretion. Low avidity T cells that express DR5, FasL, and CD24 secrete even less IFNγ than low avidity T cells that do not express these proteins (Fig. 2A). T cell trafficking into tumors is another important function of activated cancer-specific T cells. As mentioned before, low avidity T cells do not traffic into the tumor even in mice treated with Cy plus vaccine, whereas high avidity T cells do traffic into and eradicate large burdens of neu-expressing tumors in mice treated with Cy plus vaccine. We therefore evaluated whether there is differential expression of death-associated proteins on high avidity T cells that traffic into the tumor versus those that do not. Analysis of these high avidity tumor-infiltrating lymphocytes showed significantly lower expression of DR5, FasL, and CD24 on cells that traffic into the tumor when compared to cells that remained in the tumor-draining lymph nodes (Fig. 2B). These proteins are also more highly expressed on low avidity T cells activated in vivo or in vitro than naïve cells (Fig. S2). Annexin V and 7AAD staining confirmed that DR5, FasL, and CD24 protein expression is upregulated on apoptosing T cells (Fig. 2C). The finding that T cells expressing DR5, FasL, and CD24 secrete less IFNγ and are less likely to traffic into tumors indicate that T cells expressing these death receptor proteins are less functional as antitumor effector cells than cells that do not express these proteins.
Figure 2. Expression of DR5, CD24, and FasL is correlated with reduced T cell function and increased apoptosis.
High or low avidity CD8+ T cells were adoptively transferred into Cy- and vaccine-treated neu-N mice. Cells are taken from the tumor-draining node (TDN) on day 3 for all except for figure 2B where cells were taken on day 5 from the tumor-draining node and tumor. These experiments were repeated at least 3 times (n=3–5 mice/group). A. IFNγ secretion on low avidity T cells without DR5, FasL, and CD24 expression when compared with low avidity T cells expressing these proteins. B. DR5, FasL, and CD24 expression on high avidity T cells from the tumor-draining node compared to tumor-infiltrating lymphocytes. C. Dying low avidity T cells were identified through Annexin V+ and 7AAD− staining and levels of DR5, FasL, and CD24 protein expression were compared.
FasL expression on apoptosis-sensitive low avidity T cells also causes increased apoptosis of high avidity T cells
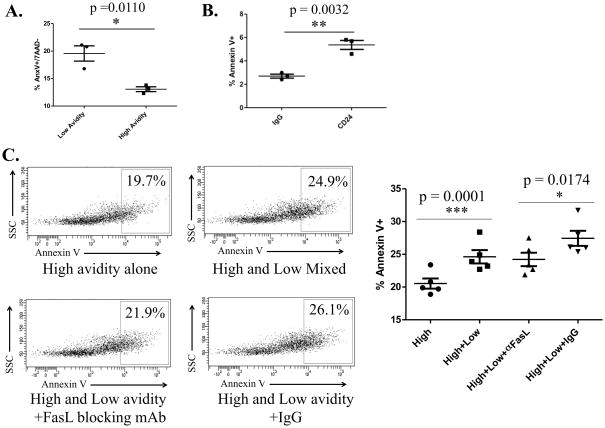
To determine whether low avidity T cells expressing these death receptors are more susceptible to cell death as our initial studies suggest we used an anti-Fas receptor antibody. 1×104 naïve high and low avidity T cells from TCR transgenic mice were plated with 0.1ug of anti-Fas receptor antibody for three hours. Death was determined by staining for 7AAD and Annexin V. Low avidity T cells demonstrated increased cell death following Fas receptor binding when compared with high avidity T cells further confirming that low avidity T cells are more susceptible to Fas-induced cell death than high avidity T cells (Fig. 3A). CD24-mediated low avidity T cell death was also verified by antibody crosslinking of CD24 (Fig. 3B).
Figure 3. Low avidity T cells are more susceptible to Fas-induced apoptosis than high avidity cells; CD24-crosslinking leads to cell death; and low avidity T cells are able to cause death of high avidity T cells.
Low and high avidity T cells extracted from TCR transgenic mice were treated in culture plated at a concentration of 1×105 cells/mL and incubated at 37°C before death was assessed by flow cytometry by staining for Annexin V and 7AAD. These experiments were repeated at least 3 times (n=3–5 mice/group). A. High and low avidity T cells were plated at and treated with 0.1μg/mL Fas-crosslinking antibody and peptide-pulsed T2 cells for three hours before analysis. B. Cells were incubated at 1mg/mL of CD24 crosslinking antibody for 24 hours. C. Low and high avidity T cells were mixed at a ratio of 3:1 and incubated for 24 hours. FasL blocking antibody was added at a concentration of 10μg/mL.
Since vaccination induces a polyclonal T cell repertoire with a range of avidities specific for a tumor antigen, we wanted to address whether low avidity T cells could negatively affect the life span of high avidity T cells. We conducted an in vitro experiment to determine if apoptosis would increase in high avidity T cells when mixed with low avidity T cells. High avidity T cells were stimulated with T2-Dq cells pulsed with RNEU420-429 peptide, with and without low avidity T cells. We found that apoptosis does increase in high avidity T cells when stimulated in the presence of low avidity T cells (Fig. 3C). In addition, we found that blocking the Fas/FasL interaction on high avidity T cells with a FasL blocking antibody prevented the increase in high avidity T cell apoptosis. This indicates that low avidity T cells cause death of high avidity T cells in a Fas-dependent manner. These studies demonstrate that not only are low avidity T cells more susceptible to death themselves but they are also able to induce cell death in other tumor-specific T cell populations.
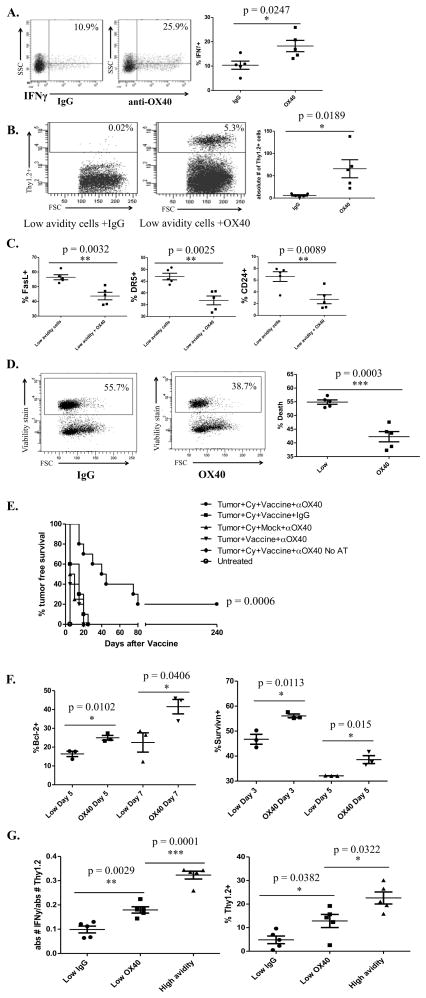
Blocking AICD with OX40 antibody allows low avidity T cells to secrete increased IFNγ and traffic into the tumor
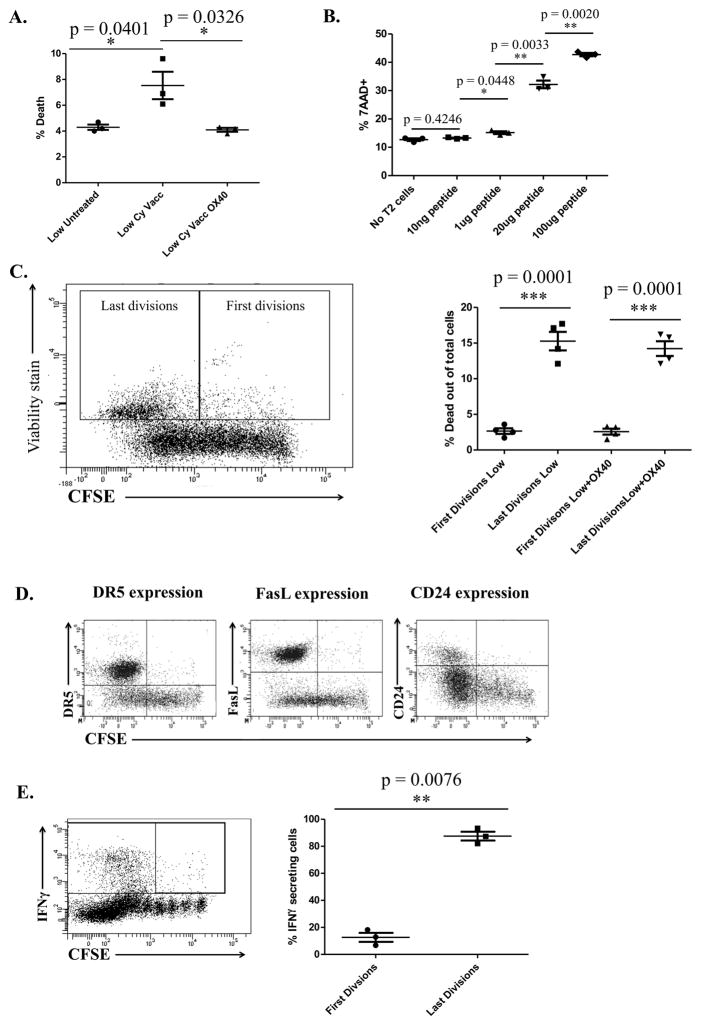
Next, we wanted to address whether low avidity T cells would become more functional in clearing tumor if they were able to survive longer. An agonistic OX40 antibody was used because of the known role of OX40 in preventing AICD. Tumor-bearing Cy and vaccine-treated mice were treated with anti-OX40 antibody or rat IgG on the day of adoptive transfer. Intracellular staining of low avidity T cells taken from the tumor-draining nodes of anti-OX40 antibody-treated mice on day 3 showed a significant increase in IFNγ secretion over IgG treated mice (Fig. 4A). Since anti-OX40 antibody induces function in low avidity T cells, we next tested whether OX40 treatment had the ability to facilitate low avidity T cell trafficking into the tumors of neu-N mice and facilitate tumor rejection. Low avidity T cells adoptively transferred into tumor-bearing mice treated with Cy, vaccine and anti-OX40 mAb did traffic into the tumor whereas low avidity T cells adoptively transferred into tumor-bearing mice treated with Cy, vaccine, and IgG failed to traffic into the tumors (Fig. 4B). Low avidity T cells from the tumor-draining nodes of mice treated with anti-OX40 antibody also had decreased expression of DR5, CD24, and FasL (Fig. 4C). The percent of dead cells was also significantly lower in the anti-OX40-treated group in comparison to the IgG-treated group (Fig. 4D). Furthermore, increased cytokine activity and trafficking function exhibited by low avidity T cells treated with Cy+vaccine+anti-OX40 antibody resulted in increased tumor-free survival when compared with mice treated with either Cy+vaccine+IgG, anti-OX40+vaccine alone without Cy, or anti-OX40+Cy+mock vaccine (Fig. 4E). Anti-OX40+vaccine+Cy-treated mice were also able to eradicate 10% of established tumors (Fig. S3).
Figure 4. Anti-OX40 agonistic antibody treatment leads to increased function of low avidity T cells and reduced expression of FasL, DR5, and CD24.
Cy- and vaccine-treated neu-N mice were adoptively transferred with low avidity T cells and also treated with 300ug of OX40 agonistic antibody or IgG the day of adoptive transfer. OX40 antibody or IgG was also given three days following adoptive transfer for trafficking experiments. These experiments were repeated at least 3 times (n=3–5 mice/group). A. Comparison of IFNγ production between mice treated with anti-OX40 antibody or IgG. B. Comparison of Thy1.2+ T cell trafficking between mice treated with anti-OX40 antibody or IgG. C. Comparison of pro-apoptotic protein expression between mice treated with or without anti-OX40 antibody. D. Cell death comparison between mice treated with anti-OX40 antibody or IgG. E. Tumor-free survival comparison between Cy-, vaccine- and/or anti-OX40 antibody-treated mice, with and without low avidity adoptive T cell transfer. P value indicates the difference between the Tumor+Cy+Vacc+OX40 group and the next closest group, Tumor+Cy+Vacc+IgG. F. Comparison of Bcl-2 and survivin expression with and without anti-OX40 antibody on days 5 and 7 for Bcl-2 and days 3 and 5 for survivin. G. Comparison of low avidity T cell activity in mice treated with or without anti-OX40 antibody as compared to high avidity T cell activity.
Two mechanisms by which treatment with anti-OX40 antibody prevents cell death are increased expression of anti-apoptotic proteins Bcl-2 and survivin (Fig. 4F). Survivin expression is significantly increased in anti-OX40+Cy+vaccine-treated mice on Day 3 and Day 5, while Bcl-2 expression is significantly increased on Days 5 and 7. The increase in function exhibited by low avidity T cells treated with Cy+vaccine+anti-OX40, although significantly increased over mice not receiving OX40 antibody, is not as functional as Cy+vaccine-treated high avidity T cells (Fig. 4G). However, this increase in IFNγ secretion, trafficking, and tumor-free survival demonstrates that preventing AICD can enhance the function of low avidity T cells.
Low avidity T cell apoptosis is antigen-dependent and cell death is most prevalent among divided cell populations
Peripheral deletion occurs when T cells are exposed to a weak TCR signal without the proper costimulation (7). T cells that do not encounter antigen survive without being activated and are non-functional, whereas T cells that receive a weak TCR signal are susceptible to peripheral deletion and T cells that receive a strong TCR signal are activated. We confirmed this finding by comparing cell survival between untreated, vaccinated, and OX40-treated mice (Fig. S4). Because low avidity T cells are more likely to be present in the periphery of tumor-bearing mice than high avidity T cells but were identified as being more susceptible to cell death, we wanted to determine if the amount of antigen exposure was contributing to the death of low avidity T cells. To test this, we compared untreated mice with Cy+vaccine-treated tumor-bearing mice where the vaccine provides an antigen source for low avidity T cells. We found that the percent of apoptosing T cells was increased in the spleen of tumor-bearing treated mice when compared to non-tumor-bearing untreated mice (Fig. 5A). We also found that giving the Cy+vaccine-treated neu-N mice anti-OX40 antibody reduced the amount of low avidity T cell death to equivalent levels observed in untreated non-tumor-bearing mice (Fig. 5A). These data suggest that low avidity T cell apoptosis is facilitated by the presence of T cell-specific antigens in the context of a T cell-alerting vaccine. In vitro, low avidity T cells incubated with T2 cells pulsed with increasing amounts of peptide demonstrated that increased peptide stimulation led to increased low avidity T cell death (Fig. 5B). We also found that low avidity T cell death increases upon proliferation (likely a consequence of the T cell-alerting vaccine), with increased proliferation leading to increased cell death (Fig. 5C). This proliferating population of T cells most likely to apoptose is also the population with the highest expression of pro-apoptotic proteins and potential to secrete IFNγ (Fig. 5D and 5E), both are likely consequences of the vaccine’s attempt to activate these T cells. These studies demonstrate that low avidity T cells are susceptible to apoptosis after vaccine activation, and that the terminally divided group of low avidity T cells most likely to be functionally active is also the group most likely to express pro-apoptotic proteins and be susceptible to cell death. OX40 treatment can rescue this low avidity T cell population from cell death allowing these T cells to sustain functional activity.
Figure 5. Low avidity T cell apoptosis is antigen-dependent in the periphery and the most highly activated T cells are undergoing apoptosis; express high levels of CD24, DR5, and FasL; and secrete IFNγ.
Three days after adoptive transfer, low avidity T cells were extracted from tumor-bearing, Cy-, and vaccine-treated mice. Cells were CFSE stained by incubating at 37°C with 1.5mM CFSE for 8 minutes. Death was assessed by Annexin V and 7AAD staining. These experiments were repeated at least 3 times (n=3–5 mice/group). A. Comparison of cell death between low avidity T cells from tumor-bearing, Cy-treated, and vaccinated neu-N mice with and without anti-OX40 antibody, and untreated mice. B. Amount of low avidity T cell death over increasing amounts of peptide (20μg = 17.39μM). C. Comparison of low avidity T cell death between early and late divisions. D. Demonstration of DR5, FasL, and CD24 expression in divided cells. E. Demonstration of IFNγ secretion in divided cells.
Increase in low avidity T cell function by anti-OX40 treatment is independent of CD4+ T cell help
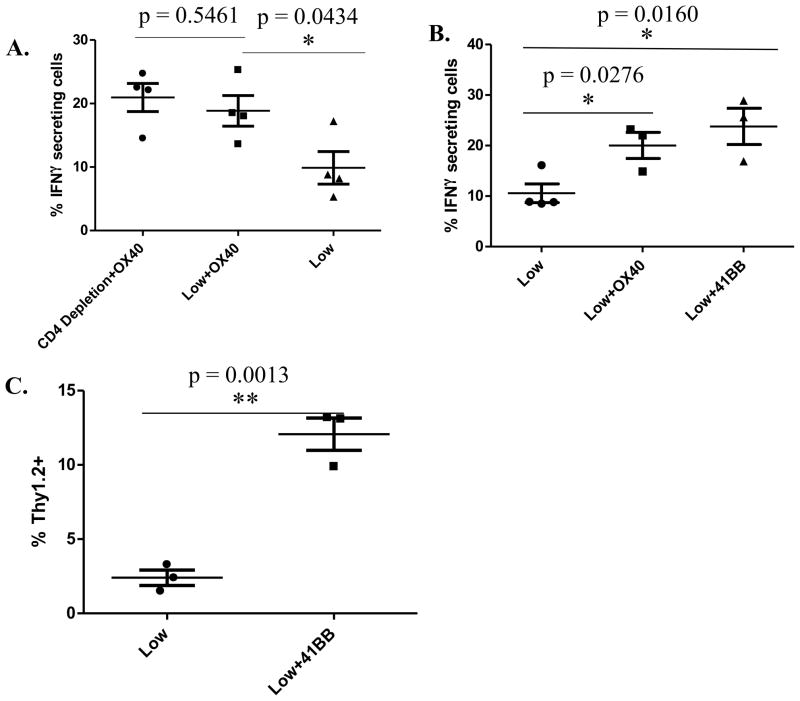
To further evaluate the mechanism by which anti-OX40 agonistic antibody augments low avidity T cell function, we evaluated whether CD4+ T cells are necessary for anti-OX40 activity. A CD4-depletion antibody was used to deplete CD4+ T cells in vitro prior to assessing CD8+ low avidity T cell function. CD8+ low avidity T cells were isolated from mice treated with Cy+vaccine+anti-OX40 antibody or control antibody as in figure 4A. CD4+ T cell depletion was verified by flow cytometry (data not shown). CD4+ depletion did not affect IFNγ secretion by low avidity CD8+ T cells (Fig. 6A). To confirm that anti-OX40 treatment lead to increased function of low avidity T cells through increased co-stimulation of the TNF receptor (death receptor) pathway, agonistic 41BB-specific co-stimulation was also tested since it is also a member of this family of death receptor agonists. Cy-treated and vaccinated neu-N mice given anti-41BB also demonstrated increased low avidity T cell function as measured by IFNγ secretion and tumor-trafficking (Fig. 6B–C). Anti-41BB was also able to eradicate established tumors (Fig. S3). These results demonstrate that the increased rate of death and decreased function seen by low avidity T cells can be overcome with strong costimulation through the TNF receptor pathway when a Treg-depleting agent is given with a T cell-activating vaccine.
Figure 6. Enhanced low avidity T cell function observed with anti-OX40 antibody treatment is not CD4+ T cell-dependent; costimulation with the agonistic 41BB antibody also increases low avidity T cell function.
Tumor-bearing, Cy- and vaccine-treated neu-N mice were given anti-OX40, anti-OX40 and CD4-depleting antibody, or 41BB antibody and cells were extracted for analysis three days after low avidity T-cell adoptive transfer for analysis of cytokine secretion and five days after adoptive transfer for trafficking information. OX40 and 41BB were both given at doses of 300μg on the day of adoptive transfer with OX40 again being given three days after adoptive transfer to mice being analyzed for trafficking. These experiments were repeated at least 3 times (n=3–5 mice/group). A. Comparison of IFNγ secretion in anti-OX40-treated cells with and without CD4 depletion. B–C. Comparison of IFNγ secretion and Thy1.2+ T cell trafficking with and without 41BB treatment.
Discussion
Our data support four novel findings that link the control of tumor-specific low avidity T cell activity to the apoptosis pathway. First, this study shows that low avidity T cells are non-functional and have limited survival in tumor-bearing hosts. Second, nonfunctional low avidity T cells have increased expression of the pro-apoptotic proteins DR5, CD24, and FasL that are associated with reduced T cell survival. Third, expression of these pro-apoptotic proteins by low avidity T cells also induces cell death in neighboring high avidity T cells. Fourth, co-stimulation of low avidity T cells with TNF receptor (death receptor; TNFR) family member agonists prevents low avidity T cell death and enhances low avidity T cell function and trafficking into tumors.
We recently reported that adoptively transferred high avidity T cells require both an antigen-specific vaccination and Treg inhibitors to achieve long-term eradication of progressing murine tumors in neu-N mice (22). That study also reported that low avidity T cells were ineffective under these conditions. This paper demonstrates that this lack of function can be overcome with TNFR agonists, which has important implications for the treatment of cancer patients.
The TNFR agonists used in this study to improve the function of low avidity T cells were agonistic anti-OX40 and -41BB antibodies. OX40 is a costimulatory molecule that is able to protect cells from AICD (27–30). Both OX40 and 41BB have been shown in neu-N mice to be able to elicit an antitumor response from endogenous T cells when combined with vaccination of pulsed dendritic cells (31). Hombach et al. showed that chimeric antigen receptor (CAR) engineered CCR7 negative T cells are susceptible to AICD and that combined CD28 and OX40 stimulation rescued the T cells, enabling them to provide a more efficient antitumor response (32). This is in direct agreement with our findings that OX40 agonists can allow non-trafficking tumor specific T cells to traffic to the tumor. It has also been shown that OX40 can help T cells overcome tolerance and reverse anergy (33, 34). Our previously published work demonstrated an improved antitumor response in neu-N mice when an OX40 agonist is combined with vaccine treatment (33). That study looked at the endogenous population of CD8+ T cells as opposed to using adoptively transferred T cells of known avidities as we did in this work. In addition to OX40 agonists, 41BB agonists have also been shown to increase T cell function and survival (35–38). Hernandez-Chacon et al. demonstrated that 41BB agonists could protect melanoma TILs from AICD (36). While this study again is unrelated to avidity, it supports our study results demonstrating that T cells trafficking into the tumor benefit from 41BB agonists. Eliminating established tumors with OX40 and 41BB was performed to determine the mechanism that increases the function of low avidity T cells and not to compare the efficacy of 41BB agonists to OX40 agonists. The eventual reestablishment of tumors is not surprising since only one dose was given. While many studies show the benefits of TNFR agonists on tumor-specific T cells, our study is the first to elucidate a specific mechanism showing a direct effect on low avidity antigen-specific T cells.
41BB and OX40 antibody treatments have been shown to increase the expression of the anti-apoptotic protein Bcl-2 (36, 39). OX40 has also been shown to increase the expression of another anti-apoptotic protein, survivin, resulting in T cell proliferation and expansion (30). These findings are important because they demonstrate the link between the TNFR agonists and the mechanism these death receptors are using to prevent death. As in those studies, our study found that Bcl-2 and survivin expression were increased on low avidity T cells with OX40 mAb treatment, overcoming the effects of the pro-apoptotic proteins highly expressed on neu-specific low avidity T cells. Our data also show that OX40 is not affecting CD8+ T cells indirectly through its effects on CD4+ T cells, but rather, alters the CD8+ T cell function and trafficking directly through the death receptor pathways.
The discovery that TNFR agonists enable low avidity T cells to effectively eradicate tumor was a direct result of the major novel finding in this study that low avidity T cells are ineffective at tumor-trafficking and -killing due to their early cell death upon activation. This finding is significant because the first step to engaging low avidity T cell populations in eradicating tumors is to identify the mechanism(s) that cause them to be nonfunctional. In this study, low avidity T cells were found to be specifically vulnerable to cell death due to the increased expression of three pro-apoptotic proteins: DR5, CD24, and FasL. Our study found that TNFR agonists reduce the expression of these pro-apoptotic proteins. Due to early upregulation of the death receptors upon T cell activation in our mouse model we were unable to alter the function of low avidity T cells with drugs targeting FasL and DR5 alone in Cy+vaccine-treated mice or in conjunction with OX40 treatment (data not shown). Published work has shown that monoclonal antibody treatment of breast cancer with a DR5 receptor agonist results in decreased tumor burden, which was increased further with additional use of anti-Erbb-2 mAb (40). Therefore, systemic blockage of DR5, although possibly protective of tumor-specific T cells, may inhibit death of tumor cells. While these death proteins do affect the survival of the T cells, targeting them specifically was ineffective as an anticancer therapeutic intervention. Blocking antibodies against CD24 led to non-CD8+ T cell-dependent death of the mice due to possible anaphylaxis when given with vaccine (data not shown). CD24 has also been shown to be involved in apoptosis in B cells and tumor cells as well as T cells indicating that had CD24-blocking antibody not been lethal in those conditions it may have had side effects counterproductive to tumor elimination (41–44).
The increased expression of FasL on low avidity T cells that was found by our study is noteworthy because death by ligation of Fas/FasL is the main pathway for AICD, the mechanism for peripheral deletion (45–47). In addition to causing autonomous death of the T cell itself, FasL expression also causes death in Fas-expressing neighboring T cells (48). Thus, another novel finding by our study was that not only were low avidity T cells more susceptible to cell death, but the presence of low avidity tumor-specific T cells led to Fas-dependent cell death of high avidity tumor-specific T cells. This finding indicates that low avidity T cells could potentially be regulating high avidity T cells and be an additional factor contributing to the suppressive environment causing a lack of tumor immunogenicity. These data also suggest that agents that only aim to activate high avidity T cells may not achieve optimal activity if low avidity T cells are not also activated. Thus, understanding the different mechanisms of T cell suppression regulating sub-populations of effector T cells should allow the optimal design of the most effective immunotherapies.
Also important to the clarity of these findings is to demonstrate that low avidity T cells are dying upon exposure to antigen. We found that low avidity T cells have an increased rate of death when given Cy+vaccine versus no treatment, in tumor-bearing or non-tumor-bearing mice, and that low avidity T cells have increased death upon exposure to increased amounts of peptide. These data confirm and expand the results of Redmond and colleagues demonstrating increased numbers of surviving T cells when adoptively transferred without peptide transfer (6, 7). Interestingly, our data showed that this death is in the population of divided cells, which may express pro-apoptotic proteins or secrete IFNγ. These results are significant because they suggest that tumor-specific low avidity T cells are dying upon exposure to antigen and initial activation signals, which has not been reported. In fact, one study reported that lower avidity CD4+ GAD-specific autoreactive T cells were actually less susceptible to AICD (49), but that study was performed under completely different activation conditions than ours.
In conclusion, these studies establish that low avidity T cells are not inherently ineffective, and that blocking AICD with costimulatory signals through the TNF receptor (death receptor) pathway enables low avidity T cell activation in the presence of a simultaneous antigen-specific signal. In addition, low avidity T cells committed to AICD may adversely affect the function and survival of other antigen-specific T cell populations not typically under the control of AICD. These findings support combinatorial immunotherapies that alter the function of multiple effector T cell populations to achieve optimal immunotherapy of cancer.
Supplementary Material
Acknowledgments
Financial Support: R01CA122081 NIH/NCI grant, P50CA062924 NIH/NCI Spore grant in Gastrointestinal Cancer, P30CA006973 Cancer Center Grant
The authors would like to thank the Johns Hopkins Flow Cytometry Core including Ada Tam and Lee Blosser for their technical assistance on this paper. We also thank the Johns Hopkins Deep Sequencing and Microarray Core including Conover Talbot and Haiping Hao. Dr. Jaffee is the first recipient of the Dana and Albert “Cubby” Chair in Oncology.
Footnotes
Conflicts of interest: Through a licensing agreement between JHU and Aduro Biotech, Dr. Jaffee has the potential to receive royalties on the sale of the human vaccine form of GVAX.
References
- 1.Durrant LG, Spendlove I. Cancer vaccines entering Phase III clinical trials. Expert opinion on emerging drugs. 2003;8:489–500. doi: 10.1517/14728214.8.2.489. [DOI] [PubMed] [Google Scholar]
- 2.Uram JN, Black CM, Flynn E, Huang L, Armstrong TD, Jaffee EM. Nondominant CD8 T cells are active players in the vaccine-induced antitumor immune response. Journal of immunology. 2011;186:3847–57. doi: 10.4049/jimmunol.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Ohashi PS. Immunological function of a defined T-cell population tolerized to low-affinity self antigens. Nature. 1995;374:68–9. doi: 10.1038/374068a0. [DOI] [PubMed] [Google Scholar]
- 5.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. The Journal of experimental medicine. 1996;184:485–92. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–84. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. Journal of immunology. 2005;174:2046–53. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 8.Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert review of vaccines. 2010;9:89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. The Journal of experimental medicine. 2001;194:707–17. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. Journal of immunology. 2005;174:4098–104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. Journal of immunology. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, et al. High Avidity T cells are Preferentially Tolerized in the Tumor Microenvironment. Cancer research. 2012 doi: 10.1158/0008-5472.CAN-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.De Visser KE, Schumacher TN, Kruisbeek AM. CD8+ T cell tolerance and cancer immunotherapy. Journal of immunotherapy. 2003;26:1–11. doi: 10.1097/00002371-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 20.Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 21.Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:624–30. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 22.Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PloS one. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499–509. doi: 10.1182/blood-2011-01-325266. [DOI] [PubMed] [Google Scholar]
- 24.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. The Journal of experimental medicine. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, et al. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. Journal of immunology. 2003;170:4273–80. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 26.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Current opinion in immunology. 2013;25:230–7. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. Journal of immunology. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 28.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. Journal of immunology. 2004;172:4821–5. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 29.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. Journal of immunology. 1998;161:6510–7. [PubMed] [Google Scholar]
- 30.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–31. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. International journal of cancer Journal international du cancer. 2005;116:934–43. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 32.Hombach AA, Chmielewski M, Rappl G, Abken H. Adoptive Immunotherapy with Redirected T Cells Produces CCR7(−) Cells That Are Trapped in the Periphery and Benefit from Combined CD28-OX40 Costimulation. Human gene therapy. 2013;24:259–69. doi: 10.1089/hum.2012.247. [DOI] [PubMed] [Google Scholar]
- 33.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. Journal of immunology. 2006;176:974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz RH. T cell anergy. Annual review of immunology. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 35.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. International immunology. 2002;14:1155–67. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. Journal of immunotherapy. 2011;34:236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KH, Choi BK, Kim JD, Kim YH, Lee SK, Suh JH, et al. 4-1BB signaling breaks the tolerance of maternal CD8+ T cells that are reactive with alloantigens. PloS one. 2012;7:e45481. doi: 10.1371/journal.pone.0045481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Molecular cancer therapeutics. 2012;11:1062–70. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 39.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 40.Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16254–9. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hitsumoto Y, Song DS, Okada M, Hamada F, Saheki S, Takeuchi N. Enhancement of CD3-mediated thymocyte apoptosis by the cross-linkage of heat-stable antigen. Immunology. 1996;89:200–4. doi: 10.1046/j.1365-2567.1996.d01-741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung KC, Park WS, Kim HJ, Choi EY, Kook MC, Lee HW, et al. TCR-independent and caspase-independent apoptosis of murine thymocytes by CD24 cross-linking. Journal of immunology. 2004;172:795–802. doi: 10.4049/jimmunol.172.2.795. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Kiyokawa N, Taguchi T, Sekino T, Katagiri YU, Fujimoto J. CD24 induces apoptosis in human B cells via the glycolipid-enriched membrane domains/rafts-mediated signaling system. Journal of immunology. 2001;166:5567–77. doi: 10.4049/jimmunol.166.9.5567. [DOI] [PubMed] [Google Scholar]
- 44.Kim JB, Ko E, Han W, Lee JE, Lee KM, Shin I, et al. CD24 cross-linking induces apoptosis in, and inhibits migration of, MCF-7 breast cancer cells. BMC cancer. 2008;8:118. doi: 10.1186/1471-2407-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunological reviews. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 46.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 47.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 48.Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, Chavez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cellular & molecular immunology. 2009;6:15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallone R, Kochik SA, Laughlin EM, Gersuk VH, Reijonen H, Kwok WW, et al. Differential recognition and activation thresholds in human autoreactive GAD-specific T-cells. Diabetes. 2004;53:971–7. doi: 10.2337/diabetes.53.4.971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.