Abstract
The majority of chromosome rearrangements are balanced reciprocal and Robertsonian translocations. It is now known that such abnormalities cause no phenotypic effect on the carrier but lead to increased risk of producing unbalanced gametes. Here, we report the inheritance of a translocation between chromosomes 3 and 21 in a family with one of two fetuses with Down Syndrome carrying the same translocation and the other also carrying the same translocation without the additional chromosome 21. Chromosomal analysis from fetal amniotic fluid and peripheral blood lymphocytes from the family were performed at the Çukurova University Hospital at Adana, Turkey. We assessed a family in which the translocation between chromosomes 3 and 21 segregates: one of the three progenies carried the 47,XX,+21,t(3;21)(q21;q22) karyotype and presented with Down Syndrome; another of the three progenies carried the 46,XX,t(3;21) (q21;q22) karyotype and the third had the 46,XY karyotype. Their mother is phenotypically normal. Apparently this rearrangement occurred due to an unbalanced chromosome segregation of the mother [t(3;21)(q21;q22)mat]. This family will enable us to explain the behavior of segregation patterns and the mechanism for each type of translocation from carrier to carrier and their effects on reproduction and numerical aberrations. These findings can be used in clinical genetics and may be used as an effective tool for reproductive guidance and genetic counseling.
Keywords: Cytogenetics, Diagnosis of reciprocal translocation, Fetal amniotic fluid, Down Syndrome
INTRODUCTION
Balanced reciprocal translocations result from exchange of fragments between two chromosomes, without any gain or loss of genetic material, and are a common form of chromosomal abnormalities, occurring in about 1 in every 625 newborns [1–4]. Although, these translocation carriers usually do not exhibit any particular phenotypes, there is a balanced complement of genes. These are responsible for a high incidence of infertility, pregnancy loss, mental retardation, behavioral abnormalities, morbidity and mortality. Carriers of reciprocal translocations have reduced fertility and thus form an increased risk of having a spontaneous abortion or an unbalanced karyotype in their offspring [1,3,5–11]. Pure trisomy due to non disjunction of chromosome 21 is responsible for 96.0% of Down Syndrome with a recurrence risk of less than 1.0%. Parental karyotype is not required in non disjunction type of trisomies [12–15].
The population risk for trisomy 21 is 1 in 700 births but some couples are at a much higher risk owing to parental translocation or mosaicism [2]. Trisomy 21 due to reciprocal translocations are caused by exchange of euchromatic regions of chromosome 21 with the euchromatin regions of different autosomes or gonosomes. In addition to trisomic regions in various lengths and location of chromosome 21, unbalanced forms also show partial mono-somy for the exchanged regions of the other translocation chromosome. As a result, the phenotype in the carriers of an unbalanced translocation is not consistent [6]. Since these translocations rarely occur, there are no reliable data for their incidence, but their frequency is assumed to be less than 1:1000 in standard trisomy cases. According to reports in previous literature, the most common partners for a reciprocal translocation seem to be chromosomes 18 and 22 [11].
The true mechanisms responsible for structural rearrangement at segregation remain unknown. There is some evidence that considers chromosomal translocations as a risk factor for aneuploidy, therefore, translocations have to be considered in combination with aneuploidy analysis [10]. In one of our recent studies we assessed a family in which the translocation between chromosomes 12 and 16 segregates; one of the eight progenies carried the 47,XY, +21,t(12;16)(q24;q24) karyotype and presented with Down Syndrome [10]. His mother was phenotypically normal, one brother and one sister also carried the same translocation. Apparently, this rearrangement occurred due to the unbalanced chromosome segregation of the mother [t(12;16)(q24;q24)mat].
Here, we present a segregation of a balanced translocation between chromosomes 3 and 21 [t(3;21) (q21; q22] of a phenotypically normal mother that led to one offspring with a viable balanced translocation and Down Syndrome, one child with a normal karyotype and one phenotypically normal fetus with the translocation. This rearrangement apparently originated from the mother [t(3;21)(q21;q22)mat].
MATERIALS AND METHODS
Subjects
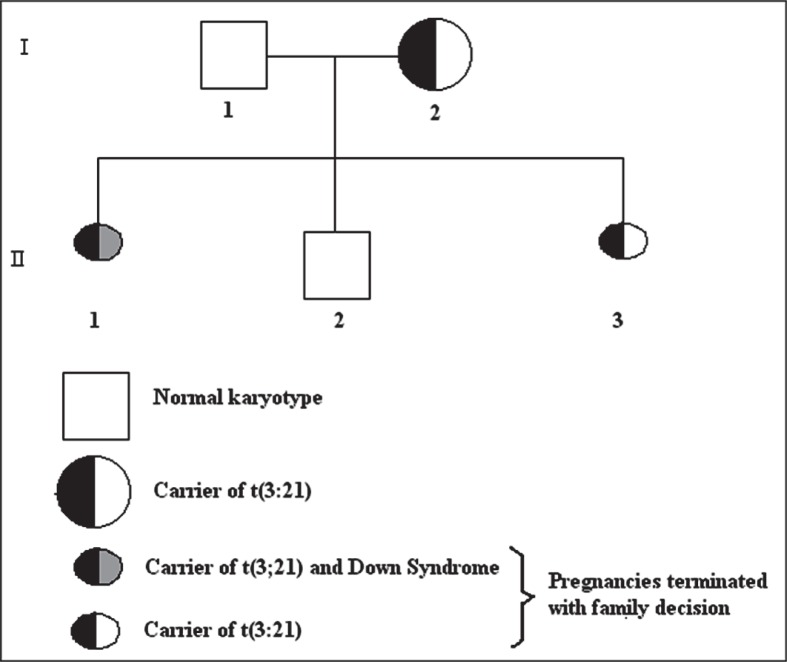
A 30-year-old woman with a history of two previous pregnancies was referred by the Department of Obstetrics and Gynecology, Çukurova University, Adana, Turkey, to our genetics laboratory for prenatal diagnosis due to having a terminated pregnancy because of a fetus with Down Syndrome and a risk of positive triple test screening at 16 weeks of gestation. The biopsy of the terminated fetus was performed by the pathology department of our faculty for confirmation. The woman and her 35-year-old husband were healthy and phenotypically normal. Both parents were subjected to chromosomal analysis, based on standard blood lymphocyte culture and G-banding techniques. Twenty metaphases were microscopically analyzed for the parents. They were not consanguineous and the mother had become pregnant a total of three times, as can be seen in the pedigree of the family (Figure 1). Of these three pregnancies, only one resulted in the birth of a phenotypically and karyotypically normal male baby.
Figure 1.
Pedigree of the family.
Cytogenetic Analyses and Findings
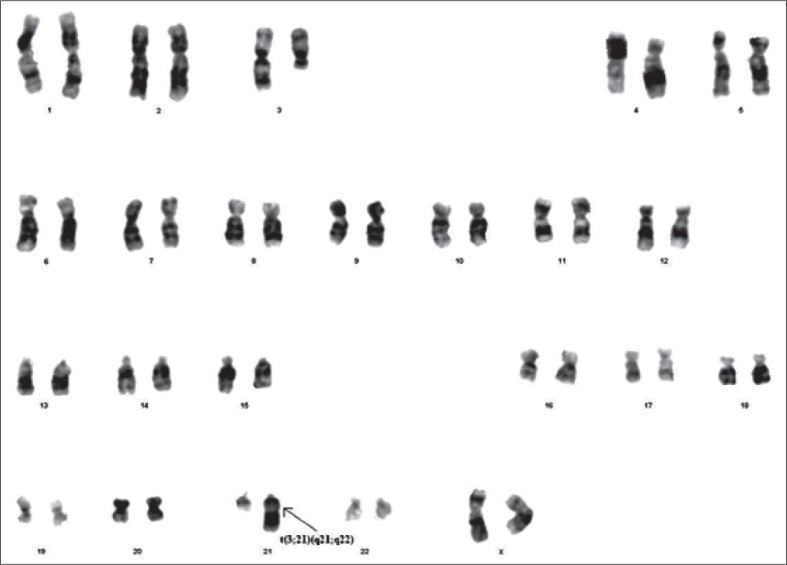
Karyotypes of the fetuses of the second generation of the family’s pedigree were performed on fetal cells that were obtained from the amniotic fluid sample by using a long-term cell culture. After adequate growth, cultures were harvested after an average 8 to 10 days. Karyotyping was routinely performed by G-banding using the trypsin-Giemsa staining technique. At least 20 metaphases were analyzed. Chromosome analysis confirmed that all cells of the mother had a translocation between chromosomes 3 and 21; her karyotype was 46,XX,t(3;21)(q21;q22) (Figure 2). The father’s karyotype was normal. In the mother’s first pregnancy, the fetus was a carrier of the translocation between chromosomes 3 and 21 with trisomy 21; the karyotype of these fetus was 47,XX,+21,t(3;21)(q21;q22) (Figure 3). The family decided to terminate the pregnancy in the second trimester. The diagnosis of Down Syndrome was verified at the clinical examination of the terminated fetus. Bilateral telecanthus (+), nose origin flatness (+), back of neck was short and abundant nuchal skin with cystic hygroma residues (Figure 4A, 4B and 4C). Brachydactylia was also seen on the right hand’s third finger (+) and clinodactyly of both right and left hands’ fifth finger (+) (Figure 4A, 4B and 4C). Simian line was observed on both right and left hands (Figure 4A, 4B and 4C). The fetus was phenotypic-ally female. Of these pregnancies, only the second resulted with the birth of phenotypically and karyotypically normal male baby. In the third pregnancy, the fetus was a carrier of the translocation between chromosomes 3 and 21; the karyotype of this fetus was 46,XX,t(3;21)(q21;q22) (Figure 5). This pregnancy was also terminated in the second trimester at the family decision.
Figure 2.
Karyotype of the mother: 46,XX,t(3;21)(q21;q22).
Figure 3.
Karyotype of the fetus of the first (terminated) pregnancy: 47,XX,+21,t(3;21)(q21;q22).
Figure 4.
A: Abundant nuchal skin of terminated first fetus of the family. B: Facial appearance of the first terminated fetus of the family. C: Clinodactyly at left hand’s fifth finger and simian line.
Figure 5.
Karyotype of the fetus of the third (terminated) pregnancy: 46,XX,t(3;21)(q21;q22).
DISCUSSION
Autosomal reciprocal translocations have been proposed as the most common chromosomal changes in couples who have recurrent pregnancy loss (RPL). In parallel, reciprocal translocations were the most common abnormalities (2.9%) in our series as reported in the literature, and all the chromosomes that are involved in these reciprocal translocations were found in autosomes [10]. Although reciprocal translocations are balanced rearrangements, they are important for the offspring of carriers who have increased risk of a chromosomal imbalance during gameto-genesis due to unequal meiotic segregation. Especially when one of the parents is a carrier of a balanced reciprocal translocation, pregnancy may result in one of the three different types of offspring: a child with a normal karyotype, a child with a balanced reciprocal translocation, or a conceptus with an unbalanced karyotype that may lead to a spontaneous miscarriage or a live-born child with malformations and mental retardation. Cytogenetic findings do not only lead to RPL but also increase the frequency of bearing a malformed child, therefore, genetic counseling for subsequent pregnancies of couples who have balanced translocation is important [1,3].
It is generally accepted that balanced rearrangements lead to increased non disjunction of other chromosomes during meiosis. Carriers of balanced translocations may be apparent because of recurrent miscarriages with or without healthy and/or affected children [5].
Prenatal diagnosis is essential if there are recurrent miscarriages for couples with a known chromosome rearrangement and if there is advanced maternal age. Maternal age is the only well-established risk factor for Down Syndrome, and the associated risk increases exponentially at age 35 years and over [7,8,16]. As a result, amniocentesis is regularly recommended for women at age 35 and over. It is also recommended that case reports of clinically normal subjects with balanced karyotypes should be published so that an informed decision can be made prenatally when a similar rearrangement is identified.
In the rare group of parents carrying a balanced chromosome rearrangement affecting whole or partial 21q, the risk of having a child with a complete or partial trisomy 21 is relatively high, with about 20.0% in females and 10.0% in males. If the translocation chromosomes and their normal homologous show pairing difficulties in meiosis I, the additional risk of a 3:1 segregation has to be taken into account, leading to a recurrence risk of up to 30.0% [6].
We concluded that carriers of reciprocal translocations including chromosome 21 are at increased risk of having offspring with trisomy 21. Thus, the t(3;21) could promote formation of trisomy 21 in the offspring. Recent studies quite clearly indicate that interchromosomal effects (ICEs) do exist [9,17–19]. It has been hypothesized that a familial translocation frequently induces errors of pairing of het-erologous chromosomes in the prophase of meiosis I leading to an aneuploid gamete (ICE). Recent investigations could not prove this hypothesis. Kovaleva [20] found that carriers of balanced reciprocal translocations or inversions but not a Robertsonian translocation, are at increased risk of bearing a trisomy 21 offspring. According to this researcher, these data do not support the existence if ICE in its common sense, i.e., as an effect of rearrangement on another chromosomes’ segregation at the carrier’s meiosis. Nowadays, it is assumed that the reduced fertility of the translocation carriers is the reason for pregnancies at increased maternal age that leads to an elevated risk for pregnancies with trisomy 21 [6,15,16]. Translocation of chromosome 21 (4.0% of Down Syndrome) recurrence risk varies between 10.0–25.0%, if one parent is a carrier of a translocation comprising chromosome 21 [12]. The risk of unbalanced translocation in the offspring will depend on both the type of translocation in the parents, and which parent is affected and whether the translocation is between homologous or non homologous chromosomes. If the parents are carriers of balanced translocation, risk for unbalanced translocation in the fetus is high and all subsequent pregnancies require prenatal sampling.
Once an unbalanced translocation in the fetus/ child has been identified, parental karyotype is essential. More than 50.0% of the translocations in a fetus are de novo. So if parents have a normal karyotype, no matter what type of translocation in the fetus, recurrence risk is minimal <1.0% [12]. We may then expect carriers of balanced parental structural rearrangements to be at a greater risk of having an offspring with a distinct aneuploidy.
REFERENCES
- 1.Beyazyurek C, Ekmekçi CG, Sağlam Y, Çinar Ç, Kahraman S. Preimplantation genetic diagnosis (PGD) for extremes—successful birth after PGD for a consanguineous couple carrying an identical balanced reciprocal translocation. Fertil Steril. 2010;93(7):2413.e1–2413.e5. doi: 10.1016/j.fertnstert.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Conn CM, Cozzi J, Harper JC, Winston ML, Delhanty JDA. Preimplantation genetic diagnosis for couples at high risk of Down syndrome pregnancy owing to parental translocation or mosaicism. J Med Genet. 1999;36(1):45–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Çirakoğlu A, Yilmaz S, Kuru D, Tarkan-Arguden Y, Guven GS, Deviren A, et al. Structural Chromosomal Abnormalities in Couples with Recurrent Pregnancy Loss. Turkiye Klinikleri J Med Sci. 2010;30(4):1185–1188. [Google Scholar]
- 4.De Braekeleer M, Dao TN. Cytogenetic studies in male infertility: a review. Hum Reprod. 1991;6(2):245–250. [PubMed] [Google Scholar]
- 5.Demirhan O, Pazarbaşi A, Guzel AI, Taştemir D, Yilmaz B, Kazap M, et al. The reliability of maternal serum triple screening for the prenatal diagnosis of fetal chromosomal abnormalities in Turkish women. Genet Test Molec Biomarkers. 2011;15(10):701–707. doi: 10.1089/gtmb.2010.0171. [DOI] [PubMed] [Google Scholar]
- 6.Eggermann T, Schwanitz G. Genetics of Down syndrome. In: Dey S, editor. Genetics and Etiology of Down Syndrome, Part 1. Rijeka, Croatia: InTech; 2011. pp. 5–22. [Google Scholar]
- 7.Hassold TJ, Jacobs PA. Trisomy in man. Ann Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, et al. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down Syndrome. Am J Hum Genet. 2000;67(3):623–630. doi: 10.1086/303055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovaleva NV. Trisomy 21 in the offspring of carriers of balanced non-contributing autosomal rearrangements: examination of interchromosomal effect and non-homologous meiotic co-orientation. In: van den Bosch A, Dubois E, editors. New Developments in Down Syndrome Research. New York, USA: Nova Science Publishers Inc; 2012. pp. 149–176. (ISBN: 978-1-62081-893-0. Available from: https://www.novapublishers.com/catalog/product_info.ph;?products_id=376). [Google Scholar]
- 10.Pazarbaşi A, Demirhan O, Turgut M, Guzel AI, Taştemir D. Inheritance of a translocation between chromosomes 12 and 16 in a family with recurrent miscarriages and a newborn with Down Syndrome carrying the same translocation. Genet Counsel. 2008;19(3):301–308. [PubMed] [Google Scholar]
- 11.Schinzel A. Catalogue of Unbalanced Chromosome Aberrations in Man. 2nd ed. Berlin, Germany: De Gruyter; 2001. [Google Scholar]
- 12.Banzal V, Suresh S, Suresh I, Jagadeesh S, Fazal GJ. Genetic counseling in chromosomal abnormalities. J Prenat Diagn Ther. 2010;1(1):14–19. [Google Scholar]
- 13.Vorzanova SG, Iourov IY, Beresheva AK, Demidova IA, Monakhov VV, Kravetz VS, et al. Non-disjunction of chromosome 21, alphoid DNA variation, and sociogenetic features of Down Syndrome. Tsitol Genet. 2005;39(6):30–36. [PubMed] [Google Scholar]
- 14.Hultén MA, Jonasson J, Iwarsson E, Uppal P, Vorsanova SG, Yurov YB, et al. Trisomy 21 mosaicism: we may all have a touch of Down Syndrome. Cytogenet Genome Res. 2013;139(3):189–192. doi: 10.1159/000346028. [DOI] [PubMed] [Google Scholar]
- 15.Hultén MA, Patel SD, Tankimanova M, Westgren M, Papadogiannakis N, Jonsson AM, et al. On the origin of trisomy 21 Down syndrome. Mol Cytogenet. 2008;1:21. doi: 10.1186/1755-8166-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultén MA, Patel S, Jonasson J, Iwarssom E. On the origin of the maternal age effect in trisomy 21 DS: the Oocyte Mosaicism Selection model. Reproduction. 2010;139(1):1–9. doi: 10.1530/REP-09-0088. [DOI] [PubMed] [Google Scholar]
- 17.Anton E, Vidal F, Blanco J. Role of sperm FISH in the genetic reproductive advice of structural reorganization carriers. Hum Reprod. 2007;22(8):2088–2092. doi: 10.1093/humrep/dem152. [DOI] [PubMed] [Google Scholar]
- 18.Gianaroli L, Magli MC, Ferraretti AP, Dunne S, Balicchia B, Escudero T, et al. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17(12):3201–3207. doi: 10.1093/humrep/17.12.3201. [DOI] [PubMed] [Google Scholar]
- 19.Pujol A, Benet J, Staessen C, van Assche E, Campillo M, Egozcue J, et al. The importance of aneu-ploidy screening in reciprocal transloca tion carrier. Reproduction. 2006;131(6):1025–1035. doi: 10.1530/rep.1.01063. [DOI] [PubMed] [Google Scholar]
- 20.Kovaleva NV. Increased risk of trisomy 21 off-spring in carriers of balanced non-contributing autosomal rearrangements is not explained by interchromosomal effect. Russian J Genet. 2013;49(2):259–268. doi: 10.7868/s0016675812110045. (in Russian). [DOI] [PubMed] [Google Scholar]