Abstract
AIMS. Immunohistochemical evaluation of hormone receptors (ER, PR) and correlation of immunohistochemical and morpho-clinical data. METHOD. The study was performed on paraffin-embedded and HE stained tissues originating from 100 cases of invasive mammary carcinoma. Monoclonal antibodies anti-estrogen and anti progesterone receptors were used for the immunohistochemical study. The detection system was EnVision HRP and the visualization system was 3-3’ diaminobenzidine tetrahydrochloride (DAB). The evaluation of the result was performed using the Allred score. REZULTS. The majority of the studied cases (57%) expressed both types of hormone receptors and in 32% of the cases the hormone receptors were completely absent. The rest of the cases presented a heterogeneous phenotype: 7% presented the ER-/PR+ type and 4%, the ER+/PR- type. Compared with the classical phenotype (ER+/PR-), ER+/PR- tumors were more frequent at patients over 50 years. The tumors with ER+/PR- were larger than the ER+/PR+ and they were of the invasive ductal carcinoma type with an Allred score for ER under 6. CONCLUSION. The predictive value is amplified when the ER status is correlated with the PR status because the heterogeneous phenotypes are identified, especially the ER+/PR- phenotype which have an aggressive behavior and the lowest response to tamoxifen therapy.
Keywords: mammary carcinoma, hormone receptors, immunohistochemistry, predictive factors
Introduction
Hormone receptors for estrogen (ER) and for progesterone (PR) are biomarkers with pronostic snd predictive value in mammary carcinoma therapy. ER and PR are commonly used for more than 30 years to conduct the therapy of mammary carcinoma [1].
Estrogens produce celulary responses acting on 2 types of estrogenic receptors ERα and ERβ. Estrogen receptors are members of a larger class of nuclear receptors called ligand-inducible transcription factors [1]. The factors wich modulates transcriptional activity of alfa receptors are used today for the therapy of various diseases such as mammary carcinoma, osteoporosis and cardiovascular diseases [8]. Synthetic ligands such as tamoxifen and raloxifen belong to a group of molecules known as selective modulators for estrogenic receptors that act as estrogen antagonists [13]. The discovery of the second receptor, known as Erβ, indicates that the estrogens’ acting mechanism is more complex than anticipated. The human receptor Erβ has a very similar structure to Erα. Erβ is expressed in the normal mammary epithelium and in most mammary carcinoma. [10]. The vast majoriy of Erβ positive mammary carcinoma are also ERα and PR-positive, without ganglionic metastasis, well defferentiated and with low proliferative activity [11].
The progesterone receptors belong to the same class of nuclear receptors, ligand-inducible transcription factors. There are two forms PR-B and PR-A, transcription products of the same gene, but by using different promoters [5]. Mollecular analisys have proven, that although some genes are regulated through both isoforms, the majority of genes are regulated through only one isoform, predominantly through PR-B [5].
The quantification of ER and PR is a controversial problem [1,4,14,15). Initial studies that validated the evaluation of estrogenic receptors through imunohistochemistry established a level of 10% positive cells that corelate with 10fmol/mg of biochemicaly detedcted protein. The positivity level of 10 %, irrespective of the imunomarker intensity, has been accepted and has been the most used level to imunohistochemically interpret ER and PR. [7]. Despite this, following studies have shown that patients with tumors that express ER in more than 1% of neoplazic cells, with moderate or bold intensity are responsive to anti-estrogenic therapy. [13]
The score recomended now to interpret the hormonal receptors imunomarks is the one Allred had suggested, according to which the cases that have a total score of ≥ 3 are considered positive.
Matherial and Method
This study had been conducted on a number of 100 invasive mammary carcinoma cases. The tisues were fixed in 10% neutral formol and included in paraffin blocks. Serial sections , initially colored HE, were made that were later imunohistochemically processed. The imunohistochemical tehnique was applied to 4 µm thick sections that were layed on superfrost slides. It was followed by deparaffination in 3 xilen baths of 5 minutes each, a hydration with succesive baths of absolute alcohol 96%, 90% and 75% of 5 minutes each and a distilated water bath for 5 minutes. The antigenic exposure was done in the microwaves with an 8 ph EDTA buffer, for 20 minutes. This stage was followed by the inhibition of the endogenous peroxidase by running it through 6% oxygenated water for 5 minutes. After washing them with plenty of water the sections were washed for 5 minutes with PBS, the next stage consisting in incubating them with the primary antibody for 1 hour at 37 degrees Celsius. The primary antibodies used were ER (monoclonal mouse anti-human estrogen receptor α, 1D5 clone; DAKOCytomation, Denmark) and PR (monoclonal mouse anti-human progesterone receptor, Pgr 636 clone; DAKOCytomation, Denmark) in 1:50 dilution. After washing them with PBS/Tween the sections were incubated with the En Vision HRP detection system for 30 minutes in envinroment temperature. After being washed with water, the signal visualising was performed with 3-3’ diaminobenzidine DAB. The countercolouring was done with Mayer hematoxiline, then the products were dehidrated in ethanol, clarified and mounted with Canadian balm. In each determination there were included products that had both positice and negative external control.
Method of evaluation
To evaluate imunohistochemical results only the nuclear marking was taken into consideration. . To quantify the hormonal status the Allred score was used. This takes into consideration both the proportion of marked cells and the medium intensity of the nuclear marking. The Allred score is the sum of the proportion score (proportion of marked cells) and the intensity score ( marking intensity) (See tablebelow).
Table-.
Allred Score
| Positive cells proportion | Proportion score |
| 0 | 0 |
| 0-1% | 1 |
| 1%-10% | 2 |
| 10%-1/3 | 3 |
| 1/3-2/3 | 4 |
| 2/3-100% | 5 |
| Marking intensity | Intensity score |
| Lack of marking | 0 |
| Low intensity | 1 |
| Moderate intensity | 2 |
| High intensity | 3 |
The tumors that had an Allred score ≤ 2 were considered negative, and the ones that had an Allred > 2 score were positive.
Results
In the present paper 100 cases of invasive mammary carcinoma were analised. The patients were aged between 22 and 75 (average age 53). From these, 37 % were under 50 years old and 63 % were older or 50 years old. The sizes of primitive mammary tumors were smaller or equal with 2 cm in 35 % of cases and larger than 2 cm in 65% of patients. Examining the sections of tumor under the optical microscope, with the usual hematoxiline-eozine coloration, led to the identifying of 90 cases of invasive ductal mammary carcinoma and 10 cases of invasive lobular mammary carcinoma (Table 1). From the 90 cases of invasive ductal mammary carcinoma, 47 had areas of intraductal carcinoma. The evaluation of hormonal receptors was performed according to the specifications in spacialty literature only at the level of invasive carcinoma areas.
Table 1.
Characteristics of patients and tumors
| Characteristics | No. Of patients | Percentage(%) |
|
Age under 50 years ≥ 50 years |
37 67 |
37% 67% |
|
Size of tumor Under or 2 cm > 2 cm |
35 65 |
35% 65% |
|
Histological type Invasive ductal carcinoma Invasive lobular carcinoma |
90 10 |
90% 10% |
The estrogenic receptors (ER) were positive ( Allred score ≥ 3) in 63% of the cases, and the progesterone receptors (PR) in 64 % of the cases. Most cases have expressed the hormonal receptors in a heterogenous manner, thus a very careful evaluation of the entire histological product being required. Therefore, in the same case the tumor cells had a nuclear marking that was different in intensity from one area to another, and the percentage of positive cells also varied from area to area. The imunomarking heterogenity was more pregnant in the case of progesterone receptors.
In all cases a nuclear positivity was noticed at the level of normal ductal epithelial cells that were adjacent to the tumor (internal control), this validating the corectness of the technique used and the results that were obtained.
In relation to the histological type, the invasive ductal carcinoma expressed estrogenic receptors in 53 cases (58,88%), and progesterone receptors in 57 cases (63,33%), while the invasive lobular carcinomas expressed estrogenic receptors in 8 cases (80%), and progesterone receptors in 7 cases (70%).
Most cases, (57%), presented both types of receptors with a ER positive /Pr positive fenotype (fig. 1, 2,3, 4). 32% of the cases had no hormonal receptors with a ER negative /PR negative fenotype (fig.5 and fig. 6). The rest of the cases (11%) had a heterogenous fenotype. Thus, 7 % of cases were ER negative/ PR positive(fig. 7 and fig. 8), and 4 % of cases were ER positive /PR negative (fig. 9 and fig. 10) (Table 2).
Figure 1.

Fenotype ER +/ PR +:ER positive in tumor, x200
Figure 2.

Fenotype ER +/ PR +:PR positive in tumor (heterogenous marking), x200
Figure 3.

Fenotype ER +/ PR +:ER positive in tumor (other case), x200
Figure 4.

Fenotype ER +/ PR +:PR positive in tumor (other case), x200
Figure 5.
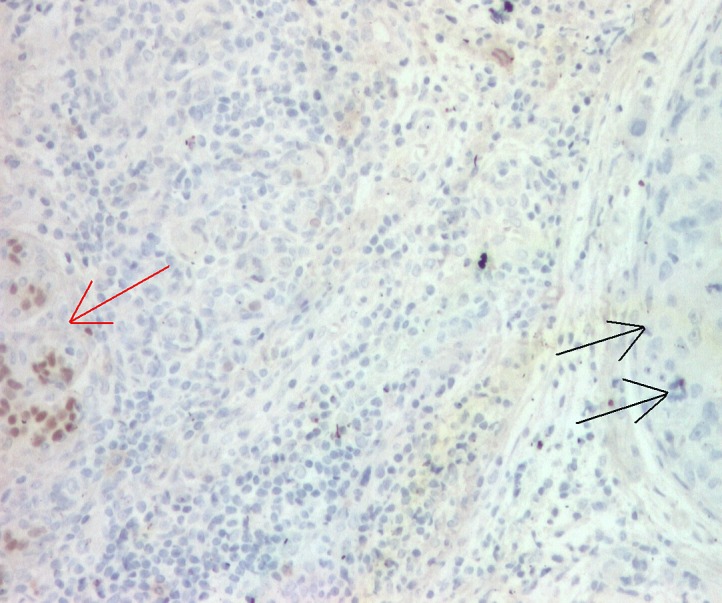
Fenotype ER -/ PR -:ER negative in tumor (double arrow), positive in internal control ( normal ducts- simple arrow), x100
Figure 6.

Fenotype ER -/ PR -:PR negative in tumor (double arrow), positive in internal control (normal ducts- simple arrow), x100
Figure 7.

Fenotype ER -/ PR +:ER negative in tumor, positive in normal ducts, x100
Figure 8.

Fenotype ER -/ PR +:PR positive in tumor, x100
Figure 9.

Fenotypul ER +/ PR -:ER positive in tumor and internal control, x200
Figure 10.
Fenotype ER +/ PR -:PR negative in tumor, positive in internal control ( normal ducts), x200
Table 2.
The expression of hormonal receptors depending on the histological type
| Invasive ductal carcinoma No. % |
Invasive lobular carcinoma No. % |
Total cases No. % | |
| ER+/PR+ | 50 55,55 | 7 70 | 57 57 |
| ER-/PR- | 30 33,33 | 2 20 | 32 32 |
| ER-/PR+ | 7 7,77 | - - | 7 7 |
| ER+/PR- | 3 3,33 | 1 10 | 4 4 |
The ER positive /Pr positive fenotype was seen in 55,55%(50 cases) of the invasive ductal carcinomas vs. 70%(7 cases)of the invasive lobular carcinomas, and the ER negative /PR negative fenotype was present in 33,33% (30 cases) of the ductal carcinomas vs. 20% (2 cases) of the lobular carcinomas. The heterogenous fenotypes that had ER negative /PR negative were detected in 7,77% (7 cases) of the invasive ductal carcinomas vs. no case of invasive lobular carcinomas, and the one with ER positive /Pr negative was present in only 3,33% (3 cases) of the ductal carcinomas vs. 10 % (1 case ) of lobular carcinomas. We can see that in lobular carcinomas the estrogenic receptors are epressed at a much higher rate than in the ductal ones (80% of cases vs. 58,88%) , while the epression of progesterone receptors was relatively similar in the two histological types (70% vs. 63,32%).
A particular fenotype regarding the results to anti-hormonal therapy, the evolution and prognosis is the ER positive /Pr negative one, a fact due to which we have analised this fenotype in relation to the classical ER positive /Pr positive fenotype depending on the characteristics of the patients and tumors that were included in the study.
Thus, the ER positive /Pr negative fenotype was more commonly seen in patients over the age of 50 years, in comparison to the ER positive /Pr positive fenotype (75% din cases vs. 68,42%). ER positive /Pr negative tumors were larger (over 2 cm ) than the ER positive /Pr positive tumors (50% of cases vs. 42,11%) (Table 3).
Table 3.
Characteristics of cases with a ER+/PR+ and ER+/PR-fenotype
| Characteristics | ER+/PR+ 57 cases |
ER+/PR- 4 cases |
|
Age under 50 years ≥ 50 years |
18 31,58% 39 68,42% |
1 25% 3 75% |
|
Size of tumors Under or 2 cm > 2 cm |
33 57,89% 24 42,11% |
2 50% 2 50% |
|
Histological types Invasive ductal carcinoma Invasive lobular carcinoma |
50 87,72% 7 12,28% |
3 75% 1 25% |
Also, the majority of ER positive /Pr negative tumors were invasive ductal carcinoma type, these expressing much more frequently the ER positive /Pr negative fenotype than the lobular carcinomas (75% of cases vs. 25 % of cases). All ER positive /Pr negative cases had low Allred score values for estrogens, this score being below 6.
Discussion
Because the hormonal receptors are well-known predictive factors of the response to the hormonal therapy in mammary carcinoma, their evaluation through the actual imunohistochemical methods is absolutely necessary.
In this study 61% of invasive mammary carcinomas had estrogenic receptors, and the progesterone receptors were detected in 64 % of cases, this being in accordance to the recent data in literature that states the presence of ER in 63 % of patients and of PR in 65% of these [18].
Both types of receptors had, in most cases, a heterogenous marking. The presence of the heterogenity of the imunomarking seems to partially explain the weak response to the hormonal therapy of some tumors with present hormonal receptors. Thus, it is known that 30-40% of the mammary carcinoma do not respond to therapy. The absence of response is insufficiently understood , but it seems that the steroid-depending growth factors (ex. via Her2-neu), the deficitary functioning of ER and tumoral heterogenity are involved [9]. As we have seen in this study, the heterogenity of the imunomarking was more pregnant in the case of progesterone receptors. The nuclear marking for PR is generally more heterogenous than the one for ER and can be a source of false negative results [6].
The lobular carcinomas analised have expressed ER in a much greater proportion than the ductal carcinomas (80% vs. 58,88%). In accordance with the observations from the literature, around 70-95% of lobular carcinomas are ER positive, the rate of positivity being greater than the one of 70-80% seen in invasive ductal carcinomas, and the positivity for progesterone is of 60-70% in both histological types. [17].
Most mammary carcinoma have expressed both types of hormonal receptors, with a ER+/PR+ (57% of cases) fenotype, being followed in frequency by the tumors without hormonal receptors and a ER-/PR- (32% of cases) fenotype. The speciality studies quote that aprox. 50% of invasive mammary carcinomas express both types of hormonal receptors, and 25 % have no estrogenic or progesterone receptors. [3].
The cases that have a heterogenous fenotype , in which one of the types of receptors was absent, were met in 11% of cases, of which 7% had a ER-/PR+ fenotype, and 4 % a ER+/PR- fenotype.
Being known the fact that the presence of estrogenic receptors is necessary for the progesterone receptors to be positive , it seems that the appearance of the ER-/PR+ fenotype is due to the fact that the estrogenic receptors are incapable of linking the circulating hormone or to be recognised by the monoclonal antibodies used in imunohistochemical techniques, but that they can still be functional in regard to the stimulation of the forming of progesterone receptors. Also, it is possible for the estrogenic receptors to be present at a level below the detectable threshold, with IHC methods [3].
The cases with a heterogenous fenotype are still widely debated now because the benefit of hormone-therapy diminishes almost by half in the cases in which there is one lacking receptor, in comparison to the ones that have both. The ER+/PR- fenotype is a sub-group of mammary carcinomas, because they posess agresive clinical and biological features, benefiting less than the other phenotypes from the hormonal therapy. [2]
In the present study, the ER+/PR- fenotype was detected in 4% of the tumors. It seems that the loss of the progesterone receptors is caused by the loss of the activity of estrogenic receptors(or by a low blood level of estrogen in some older women, or or dur to non-functioning of intracelular paths of estrogenic receptors). . This theory does not, however, explain why some ER+/PR- fenotype tumors respond to the endocrine therapy, even though the response is diminished compared to the ER+/PR+ fenotype [2]
It was later proven that the status of hormone receptors is not a stable fenotype and can be modified during the natural evolution of the disease or as a consequence of endocrine therapy. During the tamoxifen treatment the levels of estrogenic and progesterone receptors diminish, but the one of progesterone drops, and almost half of the tumors lose the PR expression and become tamoxifen-resistant. In such cases, the loss of PR expression leads to a more agresive evolution suggesting other alterations of the tumor growth process accompany the loss of PR [2]. The cumulated data suggests that the loss of PR could be a marker of excessive activation of the growth factors(Her-1 and Her-2), which leads to the resistance to tamoxifen.
In comparison to the ER+/PR + fenotype, the ER+/PR- fenotype was more frequent in patients over 50 years, with tumors larger than 2 cm, this being in accordance to the results of a major study performed on 40 000 patients with mammary carcinoma.
As we have seen in the present paper, the ER+/PR- fenotype cases have had low values on the Allred scale (below 6), the results being similar to those obtained through other methods (dextran coated charcoal-DCC), according to which the average level of estrogenic receptors in ER+/PR- tumors is only half of the one in ER+/PR+ tumors. [2]
Conclusions
The correlated evaluation of theestrogen and progesterone receptors imunoexpression improves their predictive value by identifying the tumors that have a heterogenous fenotype.
In comparison with the classical ER+/PR + fenotype, a distinctive sub-group of invasive carcinomas is the ER+/PR – fenotype, this being more frequent for the patients over 50 years of age and with tumors larger than 2 cm, invasive ductal carcinoma with lower than 6 Allred score.
The detection of ER+/PR- fenotype tumors allows the selection of cases that have both clinical and biological characteristics, that will have the fewest benefits from the hormonal therapy.
References
- 1.ALLRED C, HARVEY JM, BERADO M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1999;11:155–168. [PubMed] [Google Scholar]
- 2.ARPINO G, WEISS H, LEE AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 3.BARDOU VJ, ARPINO G, ELLEDGE RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 4.BARNES DM, MILLIS RR, BEEX LV, et al. Increased use of immunohistochemistry for estrogen receptor measurement in mammary carcinoma: the need for quality assurance. Eur J Cancer. 1998;34:1677–1682. doi: 10.1016/s0959-8049(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 5.ENMARK E, GUSTAFSSON J-A. strogen receptor β: a novel receptor opens up new possibilities for cancer diagnosis and treatment. Endocr Rel Cancer. 1998;5:213–222. [Google Scholar]
- 6.FARID M, editor. Essentials of Diagnostic Breast Pathology. A Practical Approach. Berlin: Springer; 2007. Immunohistochemistry for Prognostic or Predictive Factors in Breast Carcinoma: Hormone Receptors; pp. 475–486. [Google Scholar]
- 7.FITZGIBBONS PL, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 8.GOUVEA AP, et al. Selecting antibodies to detect HER2 overexpression by immunohistochemistry in invasive mammary carcinomas. Appl Immunohistochem Mol Morphol. 2006;14(1):103–108. doi: 10.1097/01.pai.0000155794.64525.11. [DOI] [PubMed] [Google Scholar]
- 9.GOBBI H, ROCHA RM, BUZELIN C. Predictive factors of breast cancer evaluated by immunohistochemistry. J Bras Patol Med Lab. 2008;44(2):131–140. [Google Scholar]
- 10.HUANG Z, ZHU W, MENG Y. Novel rabbit monoclonal antibody to estrogen receptor (clone SP1): no heat pretreatment but effective on paraffin embedded tissue. Appl Immunohistoch Mol Morphol. 2005;13:91–95. doi: 10.1097/00129039-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 11.LENASI H, HUDNIK-PLEVNIK T, RAKAR S, RAINER S. Distribution of progesterone receptors between the cytosol and nuclear fraction in normal and neoplastic human endometrium. J Steroid Biochem. 1999;26(4):457–462. doi: 10.1016/0022-4731(87)90056-2. [DOI] [PubMed] [Google Scholar]
- 12.MALEEVA A, MILKOV V. Clinical significance of analysis of estrogen and progesterone receptors in human uterine tissues. Akush Ginekol (Mosk) 2004;5:55–57. [PubMed] [Google Scholar]
- 13.MOSKALUK , CA Standardization of clinical immunohistochemistry: why, how and by whom? Am J Clin Pathol. 2002;118(5):669–671. doi: 10.1309/KM95-6LVL-UNLB-R3RH. [DOI] [PubMed] [Google Scholar]
- 14.OGAWA Y, MORIYA T, KATO Y, et al. Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: analysis for a cut-off point as the predictor for endocrine therapy. Breast Cancer. 2004;11:267–275. doi: 10.1007/BF02984548. [DOI] [PubMed] [Google Scholar]
- 15.REGITNIG P, REINER A, DINGES HP, et al. Quality assurance for detection of estrogen and progesterone receptors by immunohistochemistry in Austrian pathology laboratories. Virchows Arch. 2002;441:328–334. doi: 10.1007/s00428-002-0646-5. [DOI] [PubMed] [Google Scholar]
- 16.RHODES A, JASANI B, BALATON AJ, et al. tudy of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe.Documentation of poor reliability and identification of insufficient microwave antigen retrieval times as a major contributory element of unreliable assays. Am J Clin Pathol. 2001;115:44–58. doi: 10.1309/H905-HYC1-6UQQ-981P. [DOI] [PubMed] [Google Scholar]
- 17.ZAFRANI B, AUBRIOT MH, MOURET E, et al. High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology. 2000;37(6):536–545. doi: 10.1046/j.1365-2559.2000.01006.x. [DOI] [PubMed] [Google Scholar]
- 18.ZHOU B, YANG DQ, XIE F. Biological markers as predictive factors of response to neoadjuvant taxanes and anthracycline chemotherapy in breast carcinoma. Chinese Medical Journal. 2008;121(5):387–391. [PubMed] [Google Scholar]



