Abstract
Left paraduodenal hernia (LPDH) is a retrocolic internal hernia of congenital origin that develops through the fossa of Landzert, and extends into the descending mesocolon and left portion of the transverse mesocolon. It carries significant overall risk of mortality, yet delay in diagnosis is not unusual due to subtle and elusive features. Familiarisation with the embryological and anatomical features of this rare hernia is essential for surgical management. This is especially important with respect to vascular anatomy as major mesenteric vessels form intimate relationships with the ventral rim and anterior portion of the hernia. As an illustrative case, we describe our experience with a striking example of LPDH, particularly focusing on the inherent diagnostic challenges and associated critical vascular anatomy. We advocate the role of diagnostic laparoscopy; however caution that decision to safely proceed with laparoscopic repair must occur only with confident identification of the vascular anatomy involved.
Background
An internal hernia is classically defined as herniation of a viscus through a normal or abnormal aperture within the boundaries of the peritoneal cavity.1 Paraduodenal herniae (PDH) historically constitute a 30–53% majority proportion of internal herniae,2–4 although recent literature suggests an epidemiological shift is occurring with rising incidence of transmesenteric herniae contributed by increasing volume of Roux-en-Y gastric bypass procedures.5 6
Two types of PDH exist, the left PDH (LPDH) and right PDH. The former is three times more common, and each should be considered as separate entities based on embryological and anatomical features.4 7 LPDH develop through the paraduodenal fossa of Landzert and extend in a retrocolic fashion into the descending mesocolon and left portion of the transverse mesocolon.1 The fossa of Landzert is located lateral to the fourth part of the duodenum, posterior to the inferior mesenteric vein (IMV) and the ascending branch of the ascending left colic artery (LCA), directly beneath the posterior parietal peritoneum.1 8 Most authors seem to agree that the defect is purely congenital, resultant from malrotation of the mid-gut and failure of the inferior mesentery to fuse with the posterior parietal peritoneum in early gestation.8 9 The small bowel is consequently entrapped beneath the developing colon and remains interposed between the left mesocolon and the posterior abdominal wall.2 4 An alternative theory is that the hernia is acquired by gradual mechanical pressure of peristaltic bowel loops enlarging a potential space formed through a pre-existing congenital abnormality (fossa of Landzert) present in 2% of the population.1 4 8
As an illustrative case we describe our encounter with LPDH, particularly focusing on the inherent diagnostic challenges and associated critical vascular anatomy.
Case presentation
We present the case of a 55-year-old man with a history of recurrent acute episodes of abdominal pain occurring over a 3-year period. Initial features were mild and suggestive of biliary colic. Ultrasound confirmed cholelithiasis prompted a laparoscopic cholecystectomy. Over the next 26 months, episodic symptoms persisted and changed in nature, with eight subsequent hospital presentations. Characteristic clinical features evolved to be that of progressively decreasing bowel motions leading to obstipation, vomiting, left-sided abdominal swelling and severe colicky abdominal pain that localised to the left iliac fossa. In several occasions, a soft non-pulsatile mass was transiently palpable in the left lower quadrant that was tender and with focal signs of peritonism. All episodes were self-limiting and spontaneously resolved within 12 h.
Investigations
Routine blood test findings were consistently normal. Diagnostic imaging workup consisted of plain abdominal films, ultrasound scans, contrast-enhanced CT scans and interval colonoscopies. These repeated investigations remained largely inconclusive with the exception of a CT scan undertaken during the final acute presentation that indicated features of an internal hernia (figures 1 and 2). Based on this, the patient was electively readmitted for diagnostic laparoscopy. At this time, a giant capacious retrocolic hernia was observed in the left abdomen that contained the entire small bowel from duodenojejunal flexure to distal ileum (figure 3). The hernia orifice was approximately 60 mm in length, located in the left upper quadrant and extending obliquely in an inferior direction from 10 cm inferolateral to the ligament of Treitz towards the root of the small bowel mesentery at the midline. The free edge of the hernia sac (ventral rim) was formed by a peritoneal duplication fold and appeared as a fibrous band (figure 3). Running parallel and closely adjacent to this structure was a large leash of mesenteric vessels, now understood to represent the IMV and ascending LCA (figure 3A).
Figure 1.
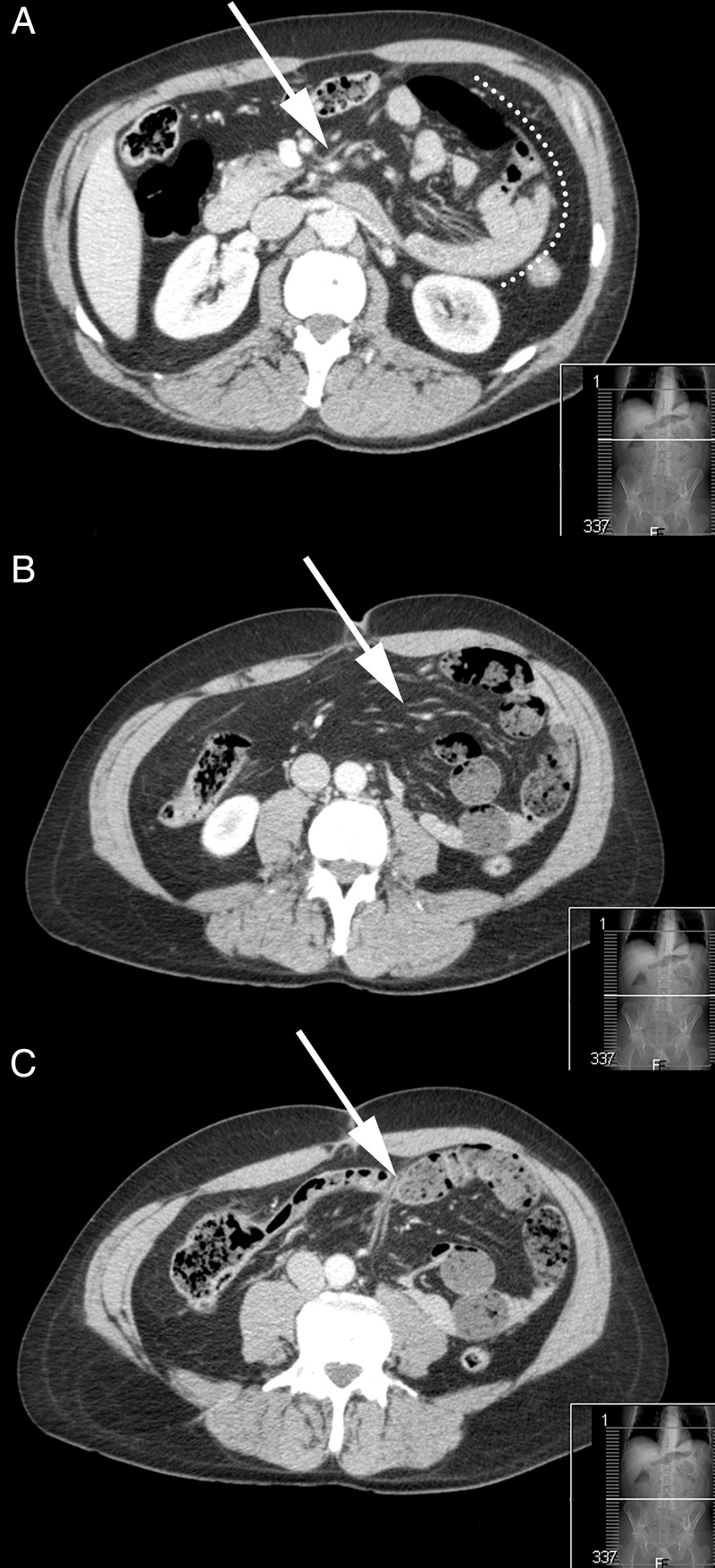
Axial CT slices taken during the most recent symptomatic episode prior to surgical intervention. Several pathognomonic radiological features of left paraduodenal hernia (LPDH) are visible. (5 13) A large sac-like mass of clustered small bowel loops can be seen in the left abdomen with a well-circumscribed lateral convex border (A, dotted line). The inferior mesenteric vein runs at the anteromedial border of the hernia, a hallmark of LPDH. There is crowding and congestion of mesenteric vessels (A, arrow). A characteristic ‘whirl’ pattern of engorged mesenteric vessels is present (B, arrow). A transition zone of partial obstruction can be seen at the site of the efferent distal ileum exiting the hernia neck (C, arrow). At this site, a double-layered wall of peritoneal fold is also visible (superior duodenal plica), representing the ventral free edge of the hernia.
Figure 2.
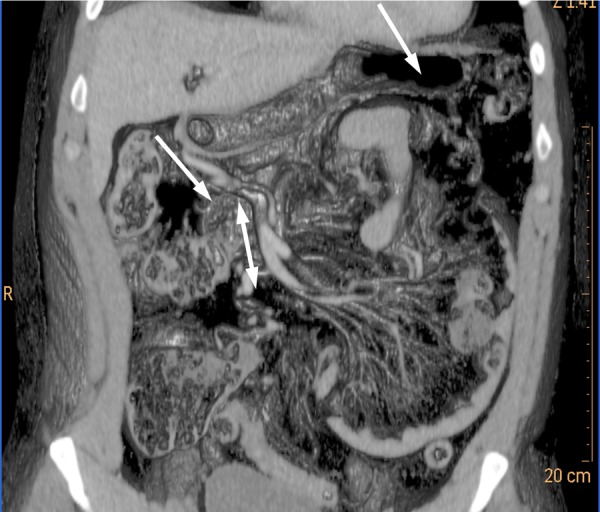
Coronal volume rendering CT of the abdomen. A large sac-like mass of clustered small bowel loops can be seen in the left side of the abdomen. This structure is between the pancreas and the stomach (solid arrows). Engorged and crowded vessels are running towards the centre of the mass from the superomedial rim of the hernia mass, which represents the site of the fossa of Landzert (double-headed arrow).
Figure 3.
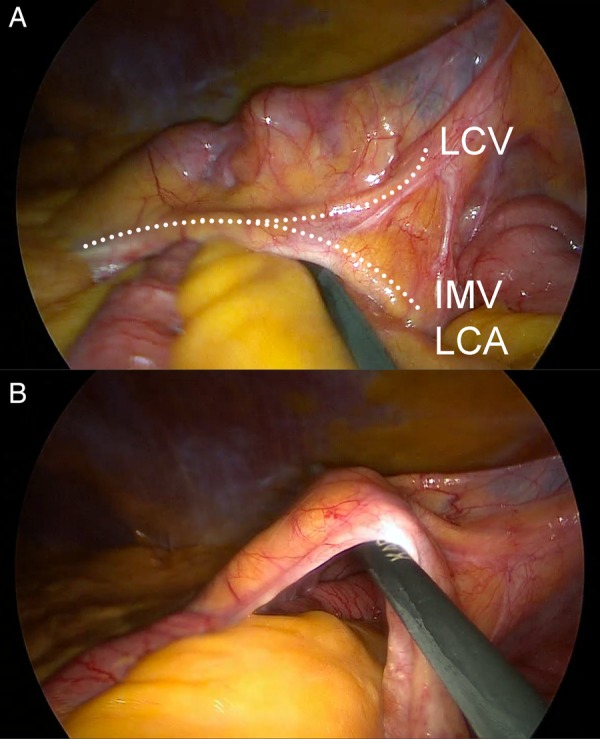
Laparoscopic view of left paraduodenal hernia with laparoscopic instrument probing the fossa of Landzert (A). Small bowel herniates through the cavernous fossa posteriorly and downward towards the left iliac fossa (B). The ventral surface of the hernia (descending mesocolon) was tented to the lateral abdominal wall peritoneum by dense adhesions (A, top right). Relevant vascular anatomy is indicated in (A) by dotted lines (IMV, inferior mesenteric vein; LCA, left colic artery; LCV, left colic vein).
Treatment
Decision was made to convert to laparotomy due to uncertain vascular anatomy and difficulty reducing distended bowel through the tight orifice. Intraoperative Doppler ultrasound was used to accurately identify the relevant vascular anatomy, with a strong pulsatile signal at the site indicated in figure 3A. All the remaining incarcerated intestinal segments were reduced. The thin ventral surface (descending mesocolon) was opened in an avascular plane lateral to the IMV/LCA for inspection of the hernia cavity (figure 3A). In light of the significant vessels in the free edge of the sac, this was not divided, but reflected and transfixed it to its normal anatomical position in the adjacent retroperitoneum.
Following repair of the hernia, a well-demarcated 35 mm wedge of small bowel and mesentery was noted to be acutely ischaemic (figure 4B). This was not observed at the time of reduction. A small superficial serosal surface incision confirmed some viability by the presence of fresh capillary bleeding; however, the affected portion failed to demonstrate signs of improvement and was therefore resected, with side-to-side stapled anastomosis to restore continuity.
Figure 4.
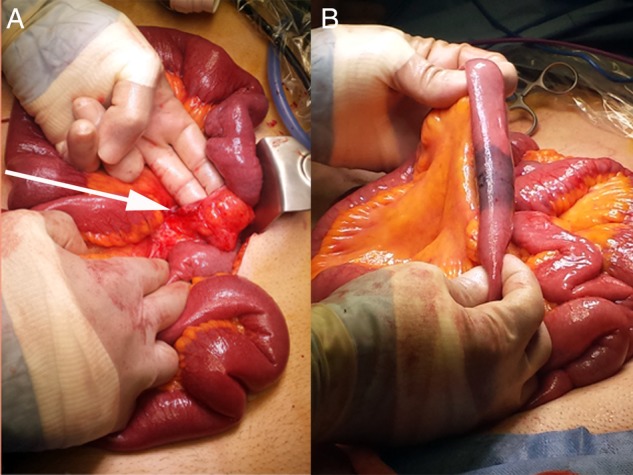
Appearance following complete reduction of herniated bowel content. Gentle manual traction was applied to enlarge the hernia orifice and facilitate reduction (A). The inferior apex of the hernia orifice is visible at the root of small bowel mesentery (arrow). A small, well-demarcated segment of acutely ischaemic small bowel was observed following reduction of hernia content (B).
Outcome and follow-up
On suspicion of a mesenteric thromboembolic event, CT angiography was performed in the immediate postoperative period; however no evidence of thrombus was identified in main mesenteric vessels. Postoperatively, the patient passed melaena stool and required blood transfusion. This was likely due to an early and brief luminal staple-line bleeding. The remainder of the early postoperative course was otherwise uneventful and the patient was discharged on the sixth postoperative day.
Discussion
In many ways, this case is somewhat typical of LPDH. This pathology usually presents in patients between their fourth and sixth decades of life and occurs three times more frequently in men.2 10 With the benefit of hindsight, we now realise that recurrent acute presentations in this case were likely due to transient obstruction of herniated small bowel content. Almost half of patients with LPDH present with symptoms related to various degrees of bowel obstruction or mesenteric vascular compromise, while the other half remain asymptomatic and are diagnosed incidentally.3 The size of the hernia orifice in LPDH predisposes to incarceration or strangulation, as this is consistently small unlike the hernia volume that is more variable.1 11 Only several previous reports have described subtotal herniation of the small bowel within the fossa of Landzert, as seen in this case.1–3 9 12 13
PDH is a diagnostic challenge. Presenting symptoms are highly variable, ranging from vague digestive symptoms such as postprandial pain or indigestion, to severe acute abdominal pain. Features on clinical examination are typically non-specific unless the exceptionally infrequent sign of a transient large left-sided mass is present.7 Identification of LPDH on imaging studies can be rather elusive with pathognomonic characteristics often only apparent during acute symptomatic episodes. The most favourable imaging modality is consensually acknowledged as CT. Given the rarity of this pathology, diagnostic heuristics often falls victim to Occam's razor. More easily suspected differential diagnoses include gastritis, biliary colic, ileitis, diverticulitis, inflammatory bowel disease, irritable bowel syndrome, volvulus, adhesive small bowel obstruction and postcholecystectomy syndrome.1 7 It is not surprising therefore that the larger case series and reviews describe time to diagnosis averaging a prolonged 21–23 months.4 14
The acute focal mesenteric ischaemia seen in this case is curious. As many as 20% of patients with LPDH present with acute bowel necrosis,7 although in this case the ischaemia appeared de novo at the time of surgery. Despite an absence of imaging findings to support our theory, we postulate that this was the result of an intraoperative thromboembolic event. There is an inherent risk of thrombosis in LPDH as stasis of blood flow with vascular compression is not uncommon.1 Manual reduction of the hernia and local dissection is suspected to have disturbed a pre-existing thrombus present in a neighbouring mesenteric vessel. If the opportunity and resources permit, we feel there is a future role for preoperative CT angiography and multiplanar reconstruction in these cases to delineate the important and often distorted vascular anatomy, and to screen for mesenteric thrombosis that would alter the management plan.
There are two approaches to definitive surgical repair of LPDH following reduction of hernia content—either direct closure or wide opening of the orifice.4 15 Management is generally via laparotomy in both emergency and non-emergency settings. A small number of cases have been reported as being successfully repaired laparoscopically and these almost invariably occurred with exact preoperative imaging diagnosis.16–18 In the largest identified series of laparoscopic PDH hernia repairs, mesenteric vessels were intentionally divided or unintentionally injured in 50% of cases (2/4).15 The free ventral edge of the hernia orifice normally contains the IMV and LCA, with various tributaries of these vessels extending across the anteromedial wall of the hernia (figures 2 and 3A).1 Correct identification and careful dissection around these vessels is the operative step that carries highest risk and this must not be compromised. While we feel there is a strong role for diagnostic laparoscopy in these cases, we caution that proceeding to laparoscopic repair must occur only if there is absolute confidence and familiarity with the vascular anatomy.
Learning points.
Left paraduodenal hernia is a rare pathology that typically presents a diagnostic challenge.
Imaging workup should occur at the time of acute symptoms to capture somewhat elusive diagnostic radiological features.
Preoperative CT angiography should be considered to aid operative planning with respect to the vascular anatomy involved.
Diagnostic laparoscopy has a clear role; however, laparoscopic repair should only be approached with confident understanding of pathological anatomy, especially when mesenteric vessels are involved.
Footnotes
Contributors: All the authors contributed in the conception, design, writing and final approval of the manuscript. TPC and MH obtained and interpreted the CT images. TPC, ANDM and AD obtained and interpreted the operative images.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Meyers MA. Paraduodenal hernias. Radiologic and arteriographic diagnosis. Radiology 1970;95:29–37 [DOI] [PubMed] [Google Scholar]
- 2.Falk GA, Yurcisin BJ, Sell HS. Left paraduodenal hernia: case report and review of the literature. BMJ Case Rep 2010;2010,pii:bcr0420102936. 10.1136/bcr.04.2010.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbulut S. Unusual cause of intestinal obstruction: left paraduodenal hernia. Case Rep Med 2012;2012:529246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brigham RA, Fallon WF, Saunders JR, et al. Paraduodenal hernia: diagnosis and surgical management. Surgery 1984;96:498–502 [PubMed] [Google Scholar]
- 5.Blachar A, Federle MP, Dodson SF. Internal hernia: clinical and imaging findings in 17 patients with emphasis on CT criteria. Radiology 2001;218:68–74 [DOI] [PubMed] [Google Scholar]
- 6.Ghiassi S, Nguyen SQ, Divino CM, et al. Internal hernias: clinical findings, management, and outcomes in 49 nonbariatric cases. J Gastrointest Surg 2007;11:291–5 [DOI] [PubMed] [Google Scholar]
- 7.Zonca P, Maly T, Mole DJ, et al. Treitz's hernia. Hernia 2008;12:531–4 [DOI] [PubMed] [Google Scholar]
- 8.Peltier J, Gars DL, Page C, et al. The duodenal fossae: anatomic study and clinical correlations. Surg Radiol Anat 2005;27:303–7 [DOI] [PubMed] [Google Scholar]
- 9.Tireli M. Left paraduodenal hernia. Br J Surg 1982;69:114. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly LF, Rencken IO, deLorimier AA, et al. Left paraduodenal hernia leading to ileal obstruction. Pediatr Radiol 1996;26:534–6 [DOI] [PubMed] [Google Scholar]
- 11.Tang V, Daneman A, Navarro OM, et al. Internal hernias in children: spectrum of clinical and imaging findings. Pediatr Radiol 2011; 41:1559–68 [DOI] [PubMed] [Google Scholar]
- 12.Frediani S, Almberger M, Iaconelli R, et al. An unusual case of congenital mesocolic hernia. Hernia 2010;14:105–7 [DOI] [PubMed] [Google Scholar]
- 13.Schaffler GJ, Groell R, Kammerhuber F, et al. Anterior and upward displacement of the inferior mesenteric vein: a new diagnostic clue to left paraduodenal hernias? Abdom Imaging 1999;24:29–31 [DOI] [PubMed] [Google Scholar]
- 14.Tong RS, Sengupta S, Tjandra JJ. Left paraduodenal hernia: case report and review of the literature. ANZ J Surg 2002;72:69–71 [DOI] [PubMed] [Google Scholar]
- 15.Palanivelu C, Rangarajan M, Jategaonkar PA, et al. Laparoscopic management of paraduodenal hernias: mesh and mesh-less repairs. A report of four cases. Hernia 2008;12:649–53 [DOI] [PubMed] [Google Scholar]
- 16.Khalaileh A, Schlager A, Bala M, et al. Left laparoscopic paraduodenal hernia repair. Surg Endosc 2010;24:1486–9 [DOI] [PubMed] [Google Scholar]
- 17.Nam SH, Kim KW, Kim JS, et al. Laparoscopic treatment of left paraduodenal hernia in two cases of children. Int J Surg Case Rep 2012; 3:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong GA, Cho GS, Kim HC, et al. Laparoscopic repair of paraduodenal hernia: comparison with conventional open repair. Surg Laparosc Endosc Percutan Tech 2008;18:611–15 [DOI] [PubMed] [Google Scholar]


