Abstract
Hepatocellular carcinoma (HCC) is a malignant tumor associated with a generally poor prognosis and a high rate of recurrence. HCC usually develops in the context of chronic liver diseases, and long-lasting premalignant conditions precede cancer development. A promising therapeutic approach is to eliminate precancerous cells, which are considered as the precursors of cancer stem cells, to prevent further malignant transformation. In this study, we identified a subpopulation of precancerous cells in a rat liver carcinogenesis model, which were enriched in CD133+CD44+CD45−HIS49− cells that formed part of the hepatic oval cells fraction. Prospective isolation of the precancerous cells using flow cytometry identified stem cell properties such as the ability to expand clonally and differentiate into bi-lineage cell types. Furthermore, an acyclic retinoid, which was recently shown to improve overall survival after HCC resection, directly inhibited the extensive expansion of the isolated precancerous cells in vitro and decreased the emergence of the precancerous cells and their progeny in vivo. Long-term follow-up after the acyclic retinoid treatment confirmed reduction in precancerous changes, ultimately resulting in suppression of HCC development. These findings, together with data from recent clinical trials showing marked reduction in intrahepatic recurrence, suggest that acyclic retinoid directly prevents de novo HCC by inhibiting the development of precancerous cells. Given recent advances in diagnostic techniques and the establishment of surveillance programs, the targeting of precancerous cells may have a huge impact on preventative cancer therapies.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent primary cancers in the world. The high incidence of intrahepatic recurrence and low survival rates remain major clinical challenges [1]. Prevention of recurrence is clearly essential for improving overall survival rates after HCC resection.
Numerous studies have shown that several cancers are initiated from cancer stem cells in various solid tumors [2–8]. Since the precancerous lesions or changes are observed in almost all types of cancers [9], at this very early stage of cancer development, the precancerous cells, also referred to as precancerous stem cells or progenitors, are considered as the precursors of cancer stem cells [10] and are promising therapeutic targets for early cancer detection and prevention [11,12]. The cellular pathology of precancerous cells exhibits abnormal development, which is indicative of an early precancerous change at the cellular level. The natural history of cancer remains unclear, but multistep neoplastic progression probably involves multiple somatic mutations. It is possible to transform normal hepatic stem cells into cancer stem cells with the potential to form tumors [13]. Thus, it is hypothesized that the transformation of normal cells to cancer cells accompanies precancerous changes in epithelial neoplasms, such as uterine cervical, HCC, gastric, colorectal, pancreatic, cutaneous, and oral carcinomas (summarized in Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). The concept of precancerous cells in uterine cervical cancer diagnosis may provide unique insight into their development, thereby facilitating earlier diagnosis, treatment, and prevention of other types of cancers such as HCC. As in the case of cervical caners, precancerous cells are often linked with chronic infection. Human papilloma virus infection is often involved in cervical cancer development [14]. Similarly, the chronic viral infection with hepatitis C virus and/or hepatitis B virus is also associated with the majority of HCC cases [15]. In previous studies on humans and experimental animal models, the development of a subset of HCC cases was found to involve hepatic oval cells [16,17], the activation of which is correlated with the grade and stage of disease in chronic viral infection [18,19]. On the basis of this evidence, we hypothesized that some oval cells would behave as precancerous cells contributing to HCC development. Therefore, inhibiting these precancerous cells might help to prevent HCC development. Importantly, putative cancer stem cells in HCC can be identified using the markers CD133 [8], CD44 [20], OV6 [21], and EpCam [22]. Given that precancerous cells are intermediates between normal and cancer cells, these markers may also be expressed in precancerous oval cells [23–25], and therefore, might be useful to isolate this cell population and assess cancer development.
A prospective clinical study showed that oral administration of acyclic retinoids significantly inhibits HCC recurrence [26,27]. The inhibitory effect of acyclic retinoids was supported by the finding of lower vitamin A (all-trans retinol) levels in human HCC samples compared with surrounding tissues [28]. Studies in rats and mice have also shown similar results [29], and a causal relationship between the hepatic loss of retinoic acid function and the onset of liver tumors [30]. Furthermore, acyclic retinoids persistently inhibit HCC development even after they are no longer administered, which is potentially a result of the clonal deletion of latent malignant cells [31]. Thus, acyclic retinoid-based therapy is a promising treatment option for HCC.
However, the cells targeted by acyclic retinoids and their therapeutic mechanism of action remain unclear, which is a hindrance to the adoption of this approach in clinical practice. The elucidation of the cellular origin of human HCC and development of novel therapeutic targets is obviously critical. In this study, we attempted to identify the subpopulation of precancerous cells that contribute to HCC and the mechanism by which acyclic retinoids suppress HCC development.
Materials and Methods
Animals and study design
Eight-week-old male Fischer 344/N Slc rats were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were performed according to protocols approved by the Laboratory Animal Resource Center of Yokohama City University (No. 10–34). To induce the development of hepatic oval cells and hepatocarcinogenesis, the animals were fed a diet containing 2-acetylaminofluorene (2-AAF) at a dose of 12 mg/kg body weight/day. Partial hepatectomy (PH) was performed, and all animals were subjected to surgical removal of two-thirds of the liver after treatment with 2-AAF for 1 week. PH was performed under inhalational anesthesia using Escain isoflurane (Mylan, Osaka, Japan). The acyclic retinoid peretinoin (Kowa, Tokyo, Japan) was administered intragastrically each day. Rats were randomly assigned to one of four treatment groups, and peretinoin was administered at a dose of 0, 40, 60, or 80 mg/kg body weight for 240 days. The dose was temporarily decreased to 40 mg/kg for 1 week after PH in the group that received 80 mg/kg for more than 2 weeks (Supplementary Fig. S2).
Immunohistochemistry
Cryostat sections and cells cultured in dishes were fixed using methanol:acetone (1:1), and cytospin analysis was performed with sorted cells, which were fixed in acetone before immunohistochemical staining. Primary antibodies specifically detected cytokeratin 19 (CK19; Progen, Heidelberg, Germany), cytokeratin 7 (CK7; Dako; Glostrup, Denmark), glutathione S-transferase placental form (GSTP; MBL, Nagoya, Japan), OV-6 (R&D Systems, Minneapolis, MN), CD44 (BD Biosciences, Franklin Lakes, NJ), CD133 (Abcam, Cambridge, UK), EpCam (Abcam), or Ki67 (Abcam). Alexa Fluor 488-, Alexa Fluor 555-, or Alexa Fluor 647-conjugated goat anti-mouse IgG, IgG2b, or goat anti-rabbit IgG (Invitrogen Molecular Probes, Eugene, OR) were used as secondary antibodies. GSTP-positive foci were counted with WinROOF software (ver. 6.1; Mitani, Tokyo, Japan).
Real-time RT-PCR
Total RNA was extracted from freshly sorted cells or liver tissue using Isogen reagent (Nippon Gene, Toyama, Japan). One milligram of total RNA was converted into cDNA using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). cDNA was amplified with SYBR Premix ExTaqII (Takara Bio, Otsu, Shiga, Japan) using an ABI PRISM 7700 System (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using ABI TaqMan Gene Expression Assays (Applied Biosystems) for α-fetoprotein (Afp; assay ID: Rn00560661_m1) and with other primer sets from Takara Bio (Table 1).
Table 1.
Primers Used for Real-Time Quantitative Polymerase Chain Reaction
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Dlk1 | CTGGCTGTGTTAATGGACTCTGTGA | GGTAGAGGTGCAAGCCCGAATA |
| Krt19 | TCAGTATGAGGCCATGGCAGAG | CCTTACGTCGGAGTTCCGTGA |
| Gpc3 | CTCTGGTGACGGCATGATGATGAA | GCATCGTCCACATCCAGATCATA |
| Epcam | GAATGAGAATGGTGAATGCCAGTG | CTCCCAGACTTGCTGTGAGTCATC |
| Gstp1 | ATGCCACCGTACACCATTG | AGGGTGAGGTCTCCATCTTCA |
| 18S rRNA | AAGTTTCAGCACATCCTGCGAGTA | TTGGTGAGGTCAATGTCTGCCTTTC |
Cell isolation and flow cytometric sorting
Hepatic nonparenchymal cells were isolated from rats administered 2-AAF 7 days after PH using a standard 2-step collagenase perfusion method [32]. Antibodies were used to detect CD45 (BD Biosciences), erythroid cells/HIS49 (HIS49 clone; BD Biosciences), and CD133 (Abcam). Fluorescein isothiocyanate (FITC)-conjugated CD44 (BD Biosciences), Alexa 647-conjugated anti-rabbit IgG (Invitrogen), and phycoerythrin (PE)-conjugated streptavidin (Invitrogen) were also obtained. Analysis and sorting were performed using a MoFlo sorter (Beckman Coulter, Glostrup, Denmark).
Clonal colony assay in vitro
We used a low-density clonogenic culture system [33,34]. Cells were treated with the acyclic retinoid peretinoin after the first 24 h of culture. In all cultures, the final concentrations were 30 μg/L peretinoin and 0.03% dimethyl sulfoxide (the same concentration was used in the controls). We assessed clonogenesis and proliferation in the fractionated cells by assessing the colony size and the relative frequency of colony formation after 1 week of culture (only colonies containing >20 cells were counted).
Statistical analyses
All data are expressed as the mean±SD or SE. The results were compared using Mann–Whitney U-tests for two groups, with Bonferroni correction when multiple groups were compared. P-values less than 0.05 were considered significant.
Results
Development of hepatic oval cells is accompanied by emergence of precancerous cells
Hepatic stem/progenitor cells contribute to liver regeneration if hepatocyte proliferation is impaired. To induce this process, the rats were treated with 2-AAF and subjected to PH, after which responses to liver injury were examined. Oval and ductal cells were identified using CK19 in and around the periportal region (Fig. 1A and Supplementary Fig. S3A). We also identified foci positive for GSTP, a marker protein for precancerous changes in the liver that is undetectable in normal rat livers [35–37]. Foci were observed from day 28 (Fig. 1A).
FIG. 1.
Development of HCC in a rat model. Development of hepatic oval cells, GSTP-positive cells, and foci, and HCC in rats treated with 2-AAF and subjected to PH. (A) Immunohistochemical lineage tracing during development of hepatic oval cells and formation of GSTP-positive foci in the livers of normal model rats. (B) Localizations of hepatic oval cells and GSTP-positive cells. (C) Localizations of hepatic oval cells and GSTP-positive foci. (D) Histopathology detected HCC phenotype on day 300. CV, central vein; GSTP, glutathione S-transferase placental; HCC, hepatocellular carcinoma; PV, portal vein. Scale bars, 200 μm (A) and 100 μm (B–D). Color images available online at www.liebertpub.com/scd
Immunocytochemical tracing was performed to determine whether the emergence of hepatic oval cells and GSTP-positive cells and foci were correlated. In CK19-expressing oval cells, GSTP-positivity was cytologically distinct with a relatively low frequency (25.4%±3.8%, Fig. 1B) after initial treatment with 2-AAF and PH on day 7. In addition to oval cell development, high-intensity GSTP-positive foci emerged, and they frequently overlapped or were adjacent to oval cells expressing CK19 (Fig. 1C). After GSTP-positive foci emerged on day 28, a significantly higher percentage of the foci were adjacent to oval cells compared with that on day 42 (80.0%±9.5% vs. 67.8%±11.0%, P<0.05; Table 2). Furthermore, the spatial relation images produced using serial sections showed that most of the GSTP-positive foci colocalized and/or made contact with hepatic oval cells (Supplementary Fig. S3B), suggesting that the GSTP-positive foci were probably derived from the GSTP-positive subpopulation of hepatic oval cells.
Table 2.
GSTP-Positive Foci and Hepatic Oval Cells in the 2-AAF/PH Rat Model
| Days after PH | Number of tissue samples | GSTP-positive foci | GSTP-positive foci adjacent to oval cells | Percentage of GSTP-positive foci adjacent to oval cells (%) |
|---|---|---|---|---|
| 28 | 6 | 90 | 72 | 80.0±9.5a |
| 42 | 4 | 174 | 118 | 67.8±11.0 |
P<0.05, Mann–Whitney U-test.
GSTP, glutathione S-transferase placental.
Pathological diagnoses of hepatocarcinogenesis were confirmed on the basis of architectural and cytological features in rats at 300 days after PH (Fig. 1D and Supplementary Fig. S3C). The results suggested that hepatic precancerous changes and HCC followed GSTP-positive hepatic oval cell development in this rat model.
Hepatic precancerous cell isolation and characterization
One week after PH, several OV-6-positive cells (also recognized as CK19 [38]), which are rare in normal livers, also expressed CD133, CD44, and EpCam. Furthermore, most of the CD44-positive cells expressed the hepatic oval cell marker OV-6 (Supplementary Fig. S4).
We isolated and characterized the new cell population in the precancerous, changed livers using flow cytometric cell sorting with hepatic nonparenchymal cells and the surface markers CD133 and CD44 after hematopoietic cells were removed with antibodies specific for CD45 and HIS49 (Fig. 2A). One week after PH, the percentage of CD133+CD44+CD45−HIS49− cells was 1.00%±0.61% in livers from rats treated with 2-AAF and subjected to PH, which was significantly higher than that observed in normal rat livers (0.05%±0.03%, P<0.01). We determined the cell population that was hepatic precancerous cell-enriched by assessing the gene expression profiles of perfused total liver cells, liver parenchymal cells, liver nonparenchymal cells, and the subpopulations isolated using flow cytometry (CD133−CD44−CD45−HIS49−, CD133+CD44−CD45−HIS49−, and CD133+CD44+CD45−HIS49−; Fig. 2A). Compared with nonparenchymal liver cells, CD133+CD44+CD45−HIS49− cells had significantly higher expression levels of oval cell markers such as Afp, Krt19, Dlk1, Epcam, Gstp1, and Gpc3 (Fig. 2B). Furthermore, immunocytochemical examination of freshly sorted cells indicated that >90% of the CD133+CD44+CD45−HIS49− cells coexpressed the hepatic oval cell markers EpCam and OV-6 at significantly higher levels than all other liver cell populations (P<0.01; Fig. 2C, D and Supplementary Fig. S5A).
FIG. 2.
Isolation of hepatic precancerous cells among oval cells. (A) Flow cytometric analysis and sorting. Note the enhanced CD133+CD44+CD45−HIS49− subpopulation in liver samples from the 2-AAF/PH group. (B) The expression levels of oval cell marker genes were higher in the CD133+CD44+CD45−HIS49− cells than in the nonparenchymal cells. (C–F) Cytospin analysis showed that cells coexpressing EpCam (C) or GSTP (E) and OV-6 (C, E) were predominant among the CD133+CD44+CD45−HIS49− cells. Cells positive for EpCam and OV-6 comprised 92.3% of the samples (D), while those positive for GSTP and OV-6 comprised 77.2% of the samples (F). **P<0.01, *P<0.05; n=5–16 (A), n=3 (B, D), and n=6–7 (F). Scale bars, 50 μm. HIS49, erythroid cells; PC, hepatic parenchymal cells; NPC, hepatic nonparenchymal cells. Color images available online at www.liebertpub.com/scd
Cells positive for EpCam, CK19, and/or OV-6 were also detected in CD133+CD44+CD45−HIS49− cell-derived colonies (Supplementary Fig. S5B), which indicated that hepatic oval cells were present in this cell population. Cells with the capability to form clonal colonies (representative cell tracing results are shown in Fig. 3A) in in vitro cell culture at a significantly high frequency were restricted to the CD133+CD44+CD45−HIS49− subpopulation (P<0.01; Fig. 3B). Immunocytochemistry showed that the clonal colonies also contained albumin- and CK7- or CK19-positive cells, in addition to cells that were positive for both proteins, which suggested that the cells could differentiate into at least 2 cell types (Fig. 3C).
FIG. 3.
Stem cell characteristics and in vitro proliferative inhibition of precancerous cells. Freshly isolated CD133+CD44+CD45−HIS49− oval cells after 6 days (or 7 days) in culture. (A) Single cells were traced and viewed during clonal colony expansion. (B–D) CD133+CD44+CD45−HIS49− cells were significantly more likely to form colonies than CD45−HIS49− nonparenchymal cells (B). On day 7, the single cell-derived colonies exhibited a bipotential differential capability, expressing CK7 or CK19 and albumin single- and dual-positive (C), while they were also GSTP- and OV-6-positive (D). (E) Inhibited colony formation and cell expansion compared with results observed in the DMSO-treated control group. (F) Colony size and frequency of colony formation are decreased under the acyclic retinoid peretinoin treatment. Data are presented as mean±SD in B (n=3) and SE in F (n=6–13). *P<0.05, **P<0.01. Scale bars, 100 μm. Color images available online at www.liebertpub.com/scd
Immunocytochemical examination also showed that GSTP-positive cells were enriched at a high frequency in the freshly sorted CD133+CD44+CD45−HIS49− subpopulation (P<0.05; Fig. 2E, F and Supplementary Fig. S5C) and in the single cell-derived colonies after 1 week of culture (Fig. 3D). Thus, GSTP-positive precancerous cells were enriched in and were developed from CD133+CD44+CD45−HIS49− cells, which showed characteristics of hepatic oval cells.
Expansion inhibition of hepatic precancerous cells in vitro
We investigated whether hepatic precancerous cells could be inhibited in vitro after treatment with the acyclic retinoid peretinoin because this drug has been reported to suppress HCC [26,27], and to verify the relationship between oval cells and HCC [39–41]. In the present study, we found that GSTP-positive precancerous cells comprised a small subpopulation of oval cells. To evaluate the effects of acyclic retinoid on cell expansion and the colony formation capabilities of isolated hepatic precancerous cells in vitro, we performed a single cell-based assay, as described previously [33,34]. Phase-contrast images showed that the acyclic retinoid markedly inhibited the clonal expansion of CD133+CD44+CD45−HIS49− cells (Fig. 3E). After 6 days of culture, the hepatic precancerous oval cell-derived colonies in the group treated with the acyclic retinoid were half the size of the control colonies (49.3%±9.1%, P<0.05; Fig. 3F), and the efficiency of clonal colony formation was also significantly lower than that of controls (43.9%±6.4%, P<0.05; Fig. 3F). These data showed that the acyclic retinoid inhibited the stem-like characteristics of hepatic precancerous oval cells in vitro, including clonal expansion and colony formation.
Inhibition of the development of hepatic oval cells and precancerous cells in vivo
Immunohistochemical experiments were performed to examine the effects of the acyclic retinoid during the initial stages of hepatic oval cell generation and precancerous subpopulation formation in vivo. Significantly fewer oval cells expressing CK19 were detected on day 7 in the groups that received the acyclic retinoid (Fig. 4A). Peretinoin at a dose of 60 mg/kg or 80 mg/kg significantly reduced the percentage of CK19+ oval cells compared with the control group (P<0.01 for both comparisons; Fig. 4B). Reduction in oval cells led to a decline in the subpopulation of GSTP-positive precancerous cells, while more importantly, the precancerous cell subpopulation frequency decreased (P<0.01; Fig. 4C, D). Quantitative PCRs for the oval cell-related genes Afp, Krt19, Dlk1, Epcam, and Gpc3 showed that the expression levels of these genes tended to decrease with increasing peretinoin doses. The control group had significantly higher Epcam and Gpc3 expression levels compared with the peretinoin-treated groups (P<0.01) and significantly higher expression levels of Krt19 compared with the group treated with 80 mg/kg peretinoin (P<0.05; Fig. 4E).
FIG. 4.
Inhibition of the development of GSTP-positive precancerous oval cells in vivo. Rats were examined on day 7. (A) Histological and immunocytochemical analyses of hepatic oval cells. Oval cell in the periportal region is around with dot lines Individual expression of CK19 (red) declined as the dose increased. The cell counts are shown in (B). (C) GSTP (red)-positive precancerous cells in CK19 (green)-expressing oval cells with peretinoin (80 mg/kg, right) and vehicle treatment (0 mg/kg, left). The cell counts are shown in (D). (E) Gene expression levels in liver tissue analyzed using real-time PCR. (F) The cell counts for Ki67-positive cells in CK19-hepatic oval cells in Supplementary Fig. S6. PV, portal vein. *P<0.05; **P<0.01. Data are presented as mean±SD. n=3–6 (D), n=3 (E, F), and n=5–6 (Gstp1). Scale bar, 100 μm. Color images available online at www.liebertpub.com/scd
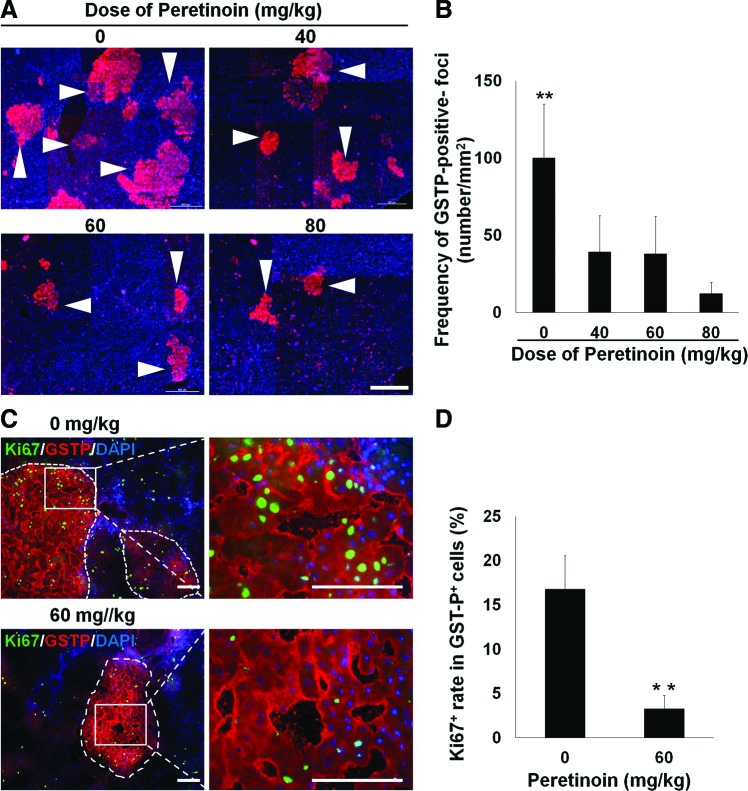
To examine the effects of the acyclic retinoid on oval cell proliferation after PH, tissues were stained with antibodies specific to Ki67 and CK19. Fewer proliferating oval cells were detected following the acyclic retinoid (peretinoin) treatment (Fig. 4F and Supplementary Fig. S6). Immunohistochemical analysis showed that the percentage of Ki67-positive oval cells relative to the total number of oval cells expressing CK19 was reduced at all peretinoin doses after 7 days (P<0.01; Fig. 4F).
GSTP-positive foci have been reported as a marker of precancerous changes in the liver [35–37]. Our results suggested a relationship between GSTP-positive cells, foci, and oval cells. Indeed, the oval cell subpopulation could be the source of GSTP-positive foci. Immunohistochemical analysis showed that peretinoin at doses of 40–80 mg/kg significantly reduced the number of GSTP-positive foci on day 42 (P<0.01 compared with control samples; Fig. 5A, B). Quantitative PCR analyses showed that peretinoin (80 mg/kg) significantly reduced the Gstp1 mRNA expression levels (P<0.05 vs. control group; Fig. 4E). The proliferative activity of GSTP-positive foci in the Ki67-positive cell assay was also significantly reduced by peretinoin treatment (P<0.01; Fig. 5C, D). These data suggest that the acyclic retinoid peretinoin inhibits oval cell proliferation and development at an early stage, thus suppressing the generation of oval cell progeny and precancerous cells in vivo.
FIG. 5.
Therapeutic blocking precancerous oval cell also inhibits the development of GSTP-positive foci and HCC. Rats were examined on day 42 (A–D) or day 240 (E–H). (A) Immunohistochemical detection of GSTP-positive foci (arrowheads) in samples treated with different peretinoin doses. (B) Reduced GSTP-positive foci development in response to the acyclic retinoid peretinoin. (C) Reduced Ki67-expressing cells in GSTP-positive foci in response to the acyclic retinoid. The cell counts are shown in (D). (E) Immunohistochemistry demonstrated the lower expression of Ki67 (green) in the 80 mg/kg peretinoin group compared with the 0 mg/kg vehicle control group. The corresponding results for the Ki67-positive cells are shown in F. (G) Development of HCC. (H) Significantly reduced development of HCC regions in the group treated with 60 mg/kg or 80 mg/kg. *P<0.05; **P<0.01. Data are presented as mean±SD. n=3 (B, D, F) and n=4–16 (H). Scale bar, 100 μm and 1.0 cm (for the macro images in G). Color images available online at www.liebertpub.com/scd
Direct suppression of precancerous cells also inhibits HCC in vivo
To further assess the consequences of GSTP-positive precancerous cell inhibition, we conducted long-term observations for 240 days. Immunohistochemical analysis of Ki67 showed that the number of proliferative liver cells was significantly reduced by treatment with the acyclic retinoid peretinoin at doses of 60 mg/kg or 80 mg/kg (P<0.01; Fig. 5E, F). HCC regions were identified on the basis of pathological diagnoses (Fig. 5G). Compared with the control group, the peretinoin-treated groups showed dose-dependent reductions in the number of HCC regions per unit area (Fig. 5H). There were significant differences at doses of 60 mg/kg (P<0.05) and 80 mg/kg (P<0.01). These data suggest that the generation of hepatic oval cells is inhibited by the acyclic retinoid, including the subpopulation of precancerous cells. Thus, HCC cell proliferation is reduced, which suppresses HCC formation.
Discussion
A comprehensive understanding of the origin of HCC is required to develop clinical strategies that target precancerous cells and prevent recurrence after initial therapy. Oval cells have been described as multipotent stem/progenitor cells that can give rise to hepatocytes and cholangiocytes. They have also been shown to replicate after liver injury and reside in the canals of Hering [42]. Previous reports suggest that HCC might originate from CK19-positive progenitor cells [43]. The number of oval cells increases with the severity of human chronic liver disease [44], which supports a causal role for oval cells in hepatocarcinogenesis. However, direct evidence for this relationship is still lacking. Indeed, most studies have relied on phenotypic analyses or analyses performed at a single time point [39–41]. Furthermore, in our experiments, oval cells could be induced to emerge in all model animals but not in all the animals suffering from HCC disease. Only a small subpopulation of oval cells expressed the precancerous marker GSTP, which suggests that some event had occurred to transform a subpopulation of oval-like cells so that they had the appearance of precancerous cells, which could in turn increase the risk of their developing into HCC cells. To demonstrate the capability of precancerous cells to proliferate, differentiate, and transform, we combined in vitro precancerous cell isolation and characterization with in vivo dynamic tracing of cell fate to obtain relevant supporting evidence.
In the present study, liver carcinogenesis was traced and examined to identify the cellular origins of hepatic precancerous cells and HCC. GSTP-positive foci localized with oval cells in the animal model used in the present study, which demonstrated the link between oval cells and HCC development. GSTP-positive foci have been associated with hepatic precancerous changes [35], and the degree of hepatic precancerous change correlates with the incidence of HCC [45]. Furthermore, we found that GSTP-positive cells can be isolated from nonhematopoietic fractions containing hepatic oval cells that express CD133+CD44+, whereas GSTP-positive foci more frequently overlapped or were adjacent to oval cells. We also noted that only some of the hepatic oval cells expressed GSTP, and that the GSTP-positive cells expanded over time both in vivo and in vitro. The acyclic retinoid dose-dependently inhibited oval cell expansion and the subpopulation of precancerous cells in the livers of rats treated with 2-AAF and subjected to PH of liver tissue, which reflected the reduced numbers of GSTP-positive foci and HCC regions. Overall, our data show that GSTP-positive cells and foci are associated with hepatic oval cells and HCC occurrence. These results may have clinical significance for HCC if these precancerous cells or their progeny also can be found in humans, and thus therapeutically targeted.
The acyclic retinoid was shown to reduce the rate of HCC recurrence in clinical trials [26,27,31]. However, the therapeutic targets and mechanisms of action of acyclic retinoids remain unclear. Clonal deletion refers to the removal of latent malignant (or premalignant) cells from an organ in a hypercarcinogenic state [46,47]. Our results suggest that the therapeutic effects of the acyclic retinoid may reflect the deletion of precancerous cells. Acyclic retinoids suppress the clonal expansion of the hepatic oval cell subpopulation, which may further inhibit precancerous changes and prevent de novo carcinogenesis. Indeed, the acyclic retinoid inhibited the clonal expansion of CD133+CD44+CD45−HIS49− cells in vitro and the expression of stem or oval cell markers in vivo. As expected, a follow-up study based on our data showed that peretinoin inhibited HCC formation.
In a phase II/III clinical trial, the acyclic retinoid peretinoin significantly reduced the risk of HCC recurrence and death compared with placebo in patients with Child–Pugh grade A impairment and a major tumor diameter <20 mm prior to curative therapy, although there was no significant improvement in the study population as a whole [48]. These data suggest that the acyclic retinoid provides early benefits in preventing HCC recurrence in patients with preserved liver function who have undergone curative therapy for small tumors. HCC can arise from multiple cellular populations, and current therapies have limited efficacy for small tumors. Thus, our results suggest a possible effect of the acyclic retinoid peretinoin during the precancerous stage of liver cancer development, which mainly comprises precancerous cells and their progeny.
However, a recent report demonstrated distinctly different HCC progenitors from the oval cell-derived precancerous cells suggested in this study. The cells residing in dysplastic foci in DEN-mice for 3 months only developed into cancer cells when transplanted in a certain liver environment [49]. Although the cells shared several markers with hepatic stem cells/oval cells, the original cells were most likely derived from dedifferentiated hepatocytes in zone 3. These findings suggest multiple sources of HCC. The initiation of liver tumor formation is a very complex area of study, made even more complex by the fact that the markers, cell types, and initiating factors, and the microenvironment, considerably differ between different rodent models and human tumors. Furthermore, the effects on rodent oval cells do not necessarily translate directly into effects on human hepatic progenitor cells. Thus, conclusions based on rodent models should be interpreted with caution when extrapolating mechanisms to describe tumorigenesis in humans. Future clinical trials should examine cells in patient specimens directly when evaluating the prevention of HCC recurrence using the acyclic retinoid. If precancerous cells can be detected and evaluated prior to treatment during clinical practice, it may be possible to increase the confidence associated with achieving a specified exposure level to a medicinal product, such as the acyclic retinoid peretinoin, with desired therapeutic outcomes in patients. In conclusion, it is clear that a subpopulation of hepatic oval cells is precancerous and that these cells can lead to the development of HCC. Suppression of precancerous cell development by the acyclic retinoid peretinoin in rats treated with 2-AAF and PH inhibited HCC formation. Thus, precancerous cells that represent a transformed subpopulation of oval cells can be considered as therapeutic targets of the acyclic retinoid peretinoin. HCC is a complex disease in humans; thus, multiple solutions are probably required. The acyclic retinoid peretinoin may prevent de novo HCC carcinogenesis by targeting precancerous cells and their progeny, thereby providing a potential avenue for treatment and prevention in patients with HCC.
Supplementary Material
Acknowledgments
We thank A. Tanaka and H. Sato for performing the FACS analysis, and T. Yazawa and Y. Nagashima for providing diagnoses of hepatic pathology. We also thank M. Kimura, K. Takiguchi, and H. Tanaka for experimental assistance. This work was supported by Grants-in-Aid (18591421, 20591531, and 23591872) for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and grants for the Strategic Promotion of Innovative Research and Development (S-innovation, 62890004) from the Japan Science and Technology Agency (JST).
Author Disclosure Statement
The authors disclose that N. Ishibashi and M. Imajima are employees of Kowa Co. (Japan). The remaining authors have no conflicts of interest to disclose.
References
- 1.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N. and Makuuchi M. (1996). Recurrence of hepatocellular carcinoma after surgery. Br J Surg 83:1219–1222 [PubMed] [Google Scholar]
- 2.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD. and Dirks PB. (2004). Identification of human brain tumour initiating cells. Nature 432:396–401 [DOI] [PubMed] [Google Scholar]
- 3.O'Brien CA, Pollett A, Gallinger S. and Dick JE. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445:106–110 [DOI] [PubMed] [Google Scholar]
- 4.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AT, Berry PA, Hyde C, Stower MJ. and Maitland NJ. (2005). Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951 [DOI] [PubMed] [Google Scholar]
- 6.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, et al. (2008). Identification of cells initiating human melanomas. Nature 451:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ. and Heeschen C. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323 [DOI] [PubMed] [Google Scholar]
- 8.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ. and Guan XY. (2007). Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 132:2542–2556 [DOI] [PubMed] [Google Scholar]
- 9.Berman JJ, Albores-Saavedra J, Bostwick D, Delellis R, Eble J, Hamilton SR, Hruban RH, Mutter GL, Page D, et al. (2006). Precancer: a conceptual working definition—results of a Consensus Conference. Cancer Detect Prev 30:387–394 [DOI] [PubMed] [Google Scholar]
- 10.Gao JX. (2008). Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med 12:67–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, Wen J, Zimmerer J, Wang Y, et al. (2007). Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS One 2:e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng YW, Tsuchida T. and Taniguchi H. (2012). A novel concept of identifying precancerous cells to enhance anti-cancer therapies. J Hepatobiliary Pancreat Sci 19:621–625 [DOI] [PubMed] [Google Scholar]
- 13.Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, et al. (2007). Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology 133:937–950 [DOI] [PubMed] [Google Scholar]
- 14.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ. and Munoz N. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19 [DOI] [PubMed] [Google Scholar]
- 15.Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, Lok AS, Hussain KB, Gish R, et al. (2003). Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol 98:2060–2063 [DOI] [PubMed] [Google Scholar]
- 16.Roskams T. (2006). Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 25:3818–3822 [DOI] [PubMed] [Google Scholar]
- 17.Sell S. and Dunsford HA. (1989). Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol 134:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- 18.Eleazar JA, Memeo L, Jhang JS, Mansukhani MM, Chin S, Park SM, Lefkowitch JH. and Bhagat G. (2004). Progenitor cell expansion: an important source of hepatocyte regeneration in chronic hepatitis. J Hepatol 41:983–991 [DOI] [PubMed] [Google Scholar]
- 19.Fotiadu A, Tzioufa V, Vrettou E, Koufogiannis D, Papadimitriou CS. and Hytiroglou P. (2004). Progenitor cell activation in chronic viralhepatitis. Liver Int 24:268–274 [DOI] [PubMed] [Google Scholar]
- 20.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y. and Wang TC. (2009). Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27:1006–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, et al. (2008). Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 68:4287–4295 [DOI] [PubMed] [Google Scholar]
- 22.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, et al. (2009). EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136:1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C. and Dabeva MD. (2008). Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 47:636–647 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H. and Taniguchi H. (2008). Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology 48:1964–1978 [DOI] [PubMed] [Google Scholar]
- 25.Yin L, Sun M, Ilic Z, Leffert HL. and Sell S. (2002). Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology 35:315–324 [DOI] [PubMed] [Google Scholar]
- 26.Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, et al. (1996). Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med 334:1561–1567 [DOI] [PubMed] [Google Scholar]
- 27.Muto Y, Moriwaki H. and Saito A. (1999). Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med 340:1046–1047 [DOI] [PubMed] [Google Scholar]
- 28.Muto Y, Omori M. and Sugawara K. (1979). Demonstration of a novel cellular retinol-binding protein, F-type, in hepatocellular carcinoma. Gann 70:215–222 [PubMed] [Google Scholar]
- 29.Muto Y. and Moriwaki H. (1984). Antitumor activity of vitamin A and its derivatives. J Natl Cancer Inst 73:1389–1393 [PubMed] [Google Scholar]
- 30.Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, Hashiguchi K, Kanbe T, Saeki T, et al. (2004). Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology 40:366–375 [DOI] [PubMed] [Google Scholar]
- 31.Takai K, Okuno M, Yasuda I, Matsushima-Nishiwaki R, Uematsu T, Tsurumi H, Shiratori Y, Muto Y. and Moriwaki H. (2005). Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. Updated analysis of the long-term follow-up data. Intervirology 48:39–45 [DOI] [PubMed] [Google Scholar]
- 32.Seglen PO. (1976). Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H. and Taniguchi H. (2000). Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 32:1230–1239 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H. and Taniguchi H. (2002). Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol 156:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh K, Kitahara A, Soma Y, Inaba Y, Hatayama I. and Sato K. (1985). Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A 82:3964–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satoh K, Yamakawa D, Sugio H, Kida K, Sato T, Hosoi K. and Hayakari M. (2008). Bile duct-bound growth of precursor cells of preneoplastic foci inducible in the initiation stage of rat chemical hepatocarcinogenesis by 2-acetylaminofluorene. Jpn J Clin Oncol 38:604–610 [DOI] [PubMed] [Google Scholar]
- 37.Noguti J, Barbisan LF, Cesar A, Seabra CD, Choueri RB. and Ribeiro DA. (2012). In vivo models for measuring placental glutatione-S-transferase (GST-P 7–7) levels: a suitable biomarker for understanding cancer pathogenesis. In Vivo 26:647–650 [PubMed] [Google Scholar]
- 38.Bisgaard HC, Parmelee DC, Dunsford HA, Sechi S. and Thorgeirsson SS. (1993). Keratin 14 protein in cultured nonparenchymal rat hepatic epithelial cells: characterization of keratin 14 and keratin 19 as antigens for the commonly used mouse monoclonal antibody OV-6. Mol Carcinog 7:60–66 [DOI] [PubMed] [Google Scholar]
- 39.Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F. and Bannasch P. (1994). Oval cell lines OC/CDE 6 and OC/CDE 22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest 71:700–709 [PubMed] [Google Scholar]
- 40.Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER. and Thorgeirsson SS. (1992). Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology 16:1327–1333 [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG. and Zhang BX. (2008). Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology 52:224–232 [DOI] [PubMed] [Google Scholar]
- 42.Fausto N. and Campbell JS. (2003). The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120:117–130 [DOI] [PubMed] [Google Scholar]
- 43.Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A. and Thorgeirsson SS. (2010). Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology 51:1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowes KN, Brennan BA, Yeoh GC. and Olynyk JK. (1999). Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 154:537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogiso T, Tatematsu M, Tamano S, Tsuda H. and Ito N. (1985). Comparative effects of carcinogens on the induction of placental glutathione S-transferase-positive liver nodules in a short-term assay and of hepatocellular carcinomas in a long-term assay. Toxicol Pathol 13:257–265 [DOI] [PubMed] [Google Scholar]
- 46.Moriwaki H, Yasuda I, Shiratori Y, Uematsu T, Okuno M. and Muto Y. (1997). Deletion of serum lectin-reactive alpha-fetoprotein by acyclic retinoid: a potent biomarker in the chemoprevention of second primary hepatoma. Clin Cancer Res 3:727–731 [PubMed] [Google Scholar]
- 47.Kojima S, Okuno M, Matsushima-Nishiwaki R, Friedman SL. and Moriwaki H. (2004). Acyclic retinoid in the chemoprevention of hepatocellular carcinoma (review). Int J Oncol 24:797–805 [PubMed] [Google Scholar]
- 48.Okusaka T, Makuuchi M, Matsui O, Kumada H, Tanaka K, Kaneko S, Moriwaki H, Izumi N, Ohashi Y, Okita K. and Group PS. (2011). Clinical benefit of peretinoin for the suppression of hepatocellular carcinoma (HCC) recurrence in patients with Child-Pugh grade A (CP-A) and small tumor: A subgroup analysis in a phase II/III randomized, placebo-controlled trial. J Clin Oncol 29(Suppl. 4):abstr 165 [Google Scholar]
- 49.He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y, Shalapour S, Seki E, Yost SE, et al. (2013). Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell 155:384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.