Abstract
Introduction
GATA2 was recently described as a critical survival factor and therapeutic target for KRAS mutant non-small cell lung cancer (NSCLC). However, whether this role is affected by epigenetic repression of GATA2 in lung cancer is unclear.
Methods
GATA2 expression and promoter CpG island methylation were evaluated using human and mouse NSCLC cell lines and tumor-normal pairs. In vitro assays were used to study GATA2 repression on cell survival and during tobacco carcinogen-induced transformation.
Results
GATA2 expression in KRAS wild-type (n=15) and mutant (n=10) NSCLC cell lines and primary lung tumors (n=24) was significantly lower, 1.3–33.6-fold (p=2.2×10−9), compared to corresponding normal lung. GATA2 promoter was unmethylated in normal lung (0/10) but frequently methylated in lung tumors (96%, 159/165) and NSCLC cell lines (97%, 30/31). This highly prevalent aberrant methylation was independently validated using TCGA data for 369 NSCLC tumor-normal pairs. In vitro studies using an established carcinogen-induced pre-malignancy model revealed that GATA2 expression was initially repressed by chromatin remodeling followed by cytosine methylation during transformation. Similarly, expression of Gata2 in NNK-induced mouse lung tumors (n=6) and cell lines (n=5) was 5-fold and 100-fold lower, respectively, than normal mouse lung. Finally, siRNA-mediated knockdown of GATA2 in KRAS mutant [human (n=4) and murine (n=5)] and wild-type [human (n=4)] NSCLC cell lines showed that further reduction of expression (up to 95%) does not induce cell death.
Conclusion
GATA2 is epigenetically repressed in human and mouse lung tumors and its further inhibition is not a valid therapeutic strategy for KRAS mutant lung cancer.
Keywords: GATA-2, epigenetics, K-RAS, mutation, carcinogenesis
Introduction
Lung cancer remains the most common and the leading cause of cancer mortality world-wide with approximately 1.6 million new cases and 1.4 million deaths a year.1 Currently, there is no established screening protocol for lung cancer, thus most patients are diagnosed at an advanced stage of the disease with poor prognosis.2 Lung tumors in approximately 50% of patients are driven by specific mutations to genes such as KRAS (20–30%), EGFR (10–20%), BRAF (<5%), PIK3CA (<5%), and EML4-ALK translocation (5–15%).3 Targeted inhibition of these key cancer-driver mutations for the treatment of lung cancer has produced encouraging results leading to a growing optimism that personalized therapy could significantly improve treatment responses and patients’ survival. Drugs targeting the EGFR pathway (e.g. gefitinib, erlotinib) have already been approved for frontline therapy of EGFR mutant lung tumors and have dramatically improved the response rate.4–6 Clinical trials using inhibitors of ALK, PI3K, BRAF, HER2, and AKT are currently underway.7–12 However, targeting the RAS family of oncogenes, the most common cancer-driver mutations in lung cancer, has not been successful. Kumar et al.13 recently took a different approach to indirectly target these oncogenes and showed that KRAS mutant lung tumors depend on GATA2 expression for survival.
GATA2 is a member of the GATA family of zinc finger proteins that serves as a transcriptional activator or repressor of multiple genes regulating diverse cellular processes during development and carcinogenesis. Six genes (GATA1–6) constitute the GATA family in human. GATA1–3 are expressed mainly in the hematopoietic cell lineage while GATA4–6 are found in many organs including the heart, lung, liver, gastrointestinal tract and are implicated in regulating epithelial cell differentiation.14 GATA2 is considered the master regulator of hematopoietic stem cells (HSC) and progenitor cells (HPC) and its role during development and malignancy of the hematopoietic system has been well established.15, 16 It is abundantly expressed and indispensable for the proliferation, maintenance, and function of HSC and HPC.17–19 GATA2 expression decreases during HSC/HPC differentiation, and its down-regulation is key for terminal differentiation.20 GATA2 null mice show defective hematopoiesis and die of anemia at day 10–11 of gestation.18 Overexpression and mutations of GATA2 have been demonstrated in myelodysplastic syndrome, acute, and chronic myeloid leukemia.21–23 In contrast, the level of GATA2 expression and its role in normal lung and during lung carcinogenesis is unclear.
Epigenetic regulation of the GATA family genes via promoter CpG island methylation has been demonstrated for GATA4 and 5 in lung, colorectal, gastric, and ovarian cancers.24, 25 Our previous transcriptome array based genome-wide screen for epigenetically silenced genes in NSCLC cell lines identified GATA2 as one of the repressed genes.26 Similarly, our study using cumene-induced lung tumors in B6C3F1 mice also showed that Gata2 was epigenetically repressed. However, the promoter CpG island of Gata2 was not methylated in the mouse tumors indicating other epigenetic changes such as chromatin modifications could be responsible for Gata2 repression.27 Given the recent excitement about targeting GATA2 for the treatment of KRAS mutant lung cancer,13 we further characterized the role of epigenetic regulation of GATA2 during lung carcinogenesis and its impact on the survival of KRAS mutant lung cancer.
The objective of this study was to answer the following key questions. 1) Does epigenetic regulation of GATA2 in lung cancer differ by KRAS mutation status? 2) Why would a gene critically required for the survival of KRAS mutant lung cancer cells be epigenetically repressed in lung cancer? Since the study by Kumar et al. did not evaluate GATA2 in lung cancer patients, we first determined the level of GATA2 expression in lung tumor-normal pairs and compared it with KRAS mutant and wild-type NSCLC cell lines. Methylation of the GATA2 promoter CpG island was evaluated in lung tumor-normal pairs and NSCLC cell lines and the results were validated using publicly available methylation data for lung tumor-normal pairs from The Cancer Genome Atlas (TCGA) project. The timing for aberrant epigenetic changes of GATA2 during early lung carcinogenesis was established using the in vitro transformation model developed in our laboratory.28, 29 Expression and methylation of the mouse Gata2 gene was also evaluated using normal lung and NNK (4-methylnitrosamino-1-(3-pyridyl)-1-butanone)-induced lung tumors in A/J mice and mouse NSCLC cell lines derived from these tumors. Finally, the impact of siRNA mediated knockdown of GATA2 on the survival of KRAS wild-type and mutant lung cancer cells was investigated using human and mouse NSCLC cell lines.
Materials and Methods
Tissue samples and cell lines
Lung tumor-normal pairs from 165 adenocarcinoma patients were obtained from frozen tumor banks at University of New Mexico (UNM) and Johns Hopkins. Additional lung tumor-normal pairs from an independent group of 56 NSCLC patients were obtained from the Mayo Clinic. Normal human bronchial epithelial cells (NHBEC) collected through diagnostic bronchoscopy30 and peripheral blood mononuclear cells (PBMC) were obtained from cancer-free smokers (UNM). All samples were obtained with written informed consent from patients, and the study was approved by the institute’s Ethics Committee. Five HBEC lines (HBEC1, 2, 3, 13, and 14) immortalized as described31 were obtained from Drs. Shay and Minna, Southwestern Medical Center, Dallas, TX. NSCLC cell lines obtained from and authenticated by the American Type Culture Collection were used. This include, H23, H358, H441, H1435, H1568, H1734, H1993, H2009, H2023, H2085, H2228, Calu-3, Calu-6, SKLU1, A549, H1650, H1838, H1975, HCC827, HCC4006, H3255, PC9, SW900, SKMES1, H520, H522, H2170, H460, H1299, H1869, and H1770. Experiments were conducted in cell lines passed for a maximum of 6 months post-resuscitation. Normal lung tissues, NNK-induced lung tumors, and lung adenocarcinomas cell lines (IO33, CL13, CL20, CL25, and CL30) derived from NNK-induced lung tumors all from A/J mice were obtained from previously described study.32, 33
Carcinogens exposures and epigenetic drug treatments
HBECs exposed to the tobacco carcinogens methylnitrosourea (MNU) and benzo(a)pyrene-diolepoxide (BPDE) for 4–12 weeks, and transformed HBECs selected for growth in soft-agar following 12 weeks exposure were obtained from previous experiments.28, 29 Cell lines were treated with vehicle (0.6 µl ethanol in 10 ml medium), the class I and II histone deacetylase (HDAC) inhibitor trichostatin A, TSA (300 nM for 18 h [Sigma; stock solution 5 mM in ethanol]), or the DNA methyltransferases (DNMTs) inhibitor 5-Aza-2’-deoxycytidine, DAC (500 nM every 12 h for 96 h [Sigma; stock solution 100 mM in PBS]) as described.34
DNA methylation and Gene expression Analysis
Combined Bisulfite Modification and Restriction Analysis (CoBRA) and Methylation Specific PCR (MSP) assays were performed as described35 to evaluate GATA2 methylation in lung tumors (n = 165) and distant normal lung tissue, DNLT (n = 10) from the adenocarcinoma patients, NHBEC (n = 10), HBEC (n = 5), PBMC (n = 10), and NSCLC cell lines (n = 31). Primer sequences and amplification conditions are described in supporting Table S1. Lung tumors (n = 56) and DNLT pairs (n = 21) from the independent group of 56 NSCLC cases were used for quantitative methylation analysis using the Infinium HumanMethylation450 beadchip (HM450K) from Illumina (San Diego, CA) as recommended.36 The TCGA HM450K data was obtained from http://genome.ucsc.edu/cgi-bin/hgTracks?hgsid=311019695.
Gene expression analysis was performed for 24 lung tumor-normal pairs from the adenocarcinoma cases as well as NSCLC and control cell lines using SYBR Green (for primers and amplification conditions see Table S1) and TaqMan assays as described.37–39 For human gene expression analysis, TaqMan assays for GATA2 all transcripts (Hs00231119_m1), Var2 (Hs00925057_m1), Var1 and 2 (Hs00925060_m1), Var1 and 3 (Hs00927739_m1), Var3 (Hs00927741_m1), and the housekeeping gene BETA-ACTIN (4310881E) all from Applied Biosystems were used. Expressions in the mouse samples were evaluated using TaqMan assays (Applied Biosystems) for Gata2 (Mm00492301), beta-actin (4352341E), and Gapdh (4352339E). All samples were analyzed at least twice in duplicate and relative expression levels were calculated using ΔΔCT method as described.40
GATA2 Knockdown and Cell Death Assays
Cells were transfected with siControl (negative control #1) or GATA2 specific human [siGATA2-#1 (s5598), siGATA2-#2 (s5597)] or mouse [siGata2-#1 (s66477), siGata2-#2 (s66478)] siRNAs (each at 20 pmol concentration) from Applied Biosystems (Foster City, CA) using Lipofectamine 2000 (Invitrogen, Santa Clara, CA) as described.39 Live and dead cells were counted by trypan blue viability assay using the Countess™ automated cell counter (Invitrogen) and gene expression was evaluated as above. Each experiment was conducted in triplicate and cell survival was determined as a percentage to the total cell count.
Immunoblotting
Human and mouse NSCLC cell lines without or 72h post-siRNA transfection were washed with ice-cold phosphate-buffered saline and lysed with RIPA Lysis Buffer containing 50mM Tris pH8, 1% Np-40, 1M NaCl, 1mM EDTA, and 0.25% Na-deoxycholate and a 1× cocktail of protease inhibitors (Sigma, Milwaukee, WI). Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA), 65µg protein per sample was fractionated on a 10% sodium dodecyl sulfate–polyacrylamide gel, and transferred to a PVDF membrane (Immobilon, Millipore, Bedford, MA). The membranes were blocked in Tween-20/Tris buffered saline (TTBS) and probed with the polyclonal anti-GATA2 (H-116, 1:200) and the monoclonal anti-beta-actin (C4, sc-47778 at 1:5000) primary antibodies that recognize both human and mouse proteins and with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies all from Santa Cruz Biotechnology Inc. (Dallas, TX). Antibody staining was visualized using the enhanced chemiluminescence (ECL) detection kit (Amersham Pharmacia Biotech, Inc., NJ).
Chromatin Immunoprecipitation (ChIP)
Methylation of histone-3 (H3) lysine residues were examined using ChIP.34 ChIP grade antibodies specific for H3 and H3 di-methyl-K9 (H3K9m2) from Abcam (Cambridge, MA) and H3 di-methyl-K4 (H3K4m2) and tri-methyl-K27 (H3K27m3) from Cell Signaling (Danvers, MA) were used to capture protein–DNA complexes. Rabbit and mouse IgGs were used for isotype control. The ChIP-DNA was quantified by SYBR Green PCR using GATA2 promoter specific primers and amplification conditions described in Table S1.
Statistical Analysis
Methylation array data were processed with the Methylation Module of GenomeStudio Software using HumanMethylation450 manifest v1.1 and after the necessary quality check and normalization processes was analyzed as described.36 β-values were compared using Fisher’s exact test and t-test. GATA2 expression between tumor-normal pairs was compared using paired t-test. The effects of siRNAs on gene expression were determined by one way analysis of variance (ANOVA) using nonparametric Wilcoxon Rank-sum test to control the impact of potential outliers. Tukey’s and Dunnett’s methods were used for pair wise and treatment control comparison adjustments, respectively. All analyses were conducted in SAS 9.2.
Results
GATA2 expression is markedly reduced in lung cancer
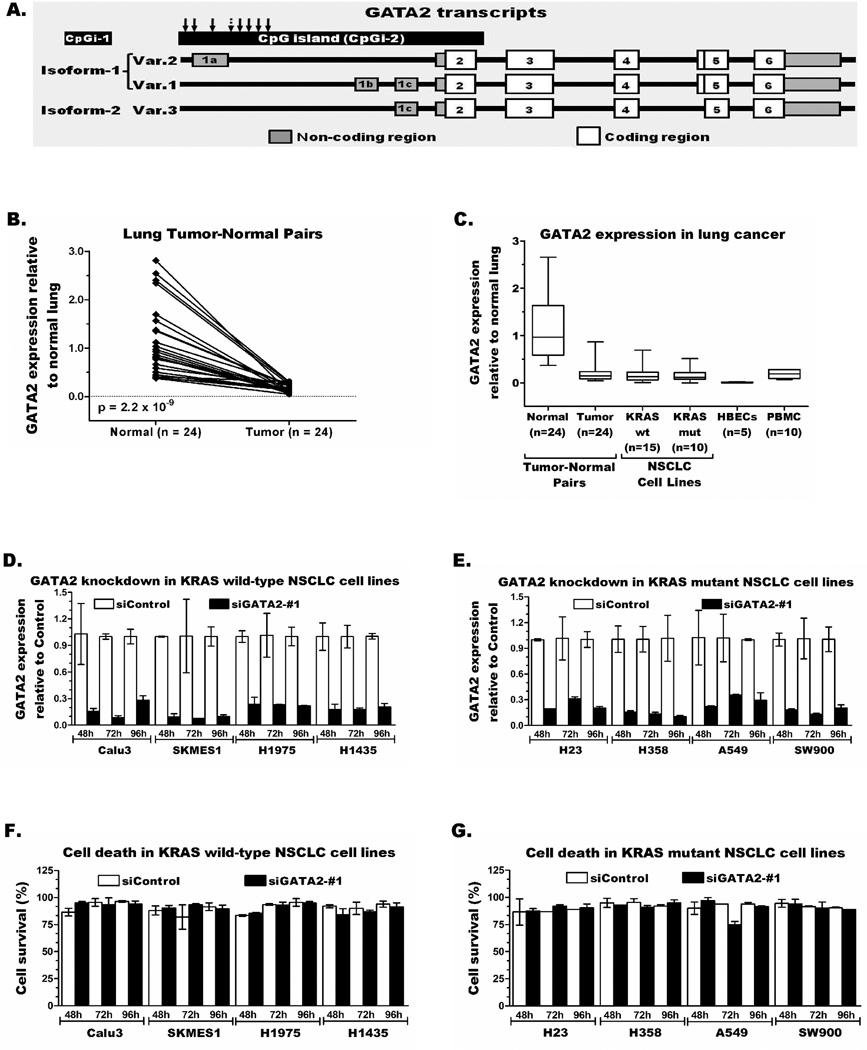
Kumar et al.13 indicated that GATA2 expression is key for the survival of KRAS mutant lung cancer cells, while our previous screenings showed epigenetic repression of GATA2 in human and mouse lung cancer.26, 27 To resolve these discrepancies, we first measured GATA2 expression in lung tumor-normal pairs and KRAS wild-type and mutant NSCLC cell lines. The human GATA2 has three transcript variants (Var.1, 2, and 3) encoding for two distinct GATA2 proteins, Isoform-1 (encoded by Var.1 and Var.2) and Isoform-2 (encoded by Var.3) (Figure 1A). TaqMan and SYBR green assays that could discriminate these transcripts showed comparable expression of Var.1 and Var.3 in normal lung and to a lesser extent in lung tumors and most NSCLC cell lines. In contrast, Var.2 was not detected in any of these samples (not shown). Evaluation of the total GATA2 transcripts in 24 lung tumor-normal pairs revealed 1.3–33.6-fold reduced expression (p = 2.2 × 10−9) in each tumor (average 8.5-fold) compared to the corresponding normal lung (Figure 1B). Both KRAS wild-type (n=15) and mutant (n=10) NSCLC cell lines also showed comparably reduced expression (Figure 1C). Similarly, NNK-induced lung tumors (n=6) and derived cell lines (n=5) from A/J mice respectively showed 5-fold and 100-fold lower expression compared to normal lung from these mice (Figure S1A–B).
Figure 1. GATA2 expression is significantly reduced in lung cancer and does not provide survival advantage to lung cancer cells.
(A) A schematic representation of the three transcript variants of GATA2. Expression of total GATA2 in (B) tumor-normal pairs and (C) KRAS wild-type and mutant NSCLC cell lines was compared using a TaqMan assay that detects all transcripts. Transient knockdown of GATA2 in KRAS wild-type (D) and mutant (E) NSCLC cell lines does not increase cell death (F–G).
GATA2 knockdown in lung cancer does not increase cell death
It is plausible that even low level GATA2 expression could play a key survival role in KRAS mutant lung cancer. This premise was rigorously tested through transient knockdown studies in KRAS wild-type (n=4) and mutant (n=4) NSCLC cell lines. GATA2 expression was reduced by 70–95% in siGATA2-#1 transfected cells compared to the corresponding cells transfected with siControl siRNA (Figure 1D–E). However, these further reductions in GATA2 expression did not increase cell death in either KRAS wild-type or mutant cells (Figure 1F–G). Furthermore, TaqMan and SYBR Green expression assays demonstrated that the KRAS mutant NSCLC cell line H441 naturally survives and proliferates with barely detectable GATA2 transcripts [ΔCt=23 (H441) vs. mean ΔCt=8 (normal lung)]. Among the human and mouse NSCLC cell lines evaluated for GATA2 mRNA and protein levels, GATA2 protein was detected by western blot only in H1435, a KRAS wt human lung adenocarcinoma cell line in which the GATA2 mRNA level was also comparable to some normal lung tissues (Figure S1C). Transfection of this cell line with the siGATA2-#1 and #2 siRNAs resulted in 65–85% reduction in transcription and similarly reduced GATA2 protein levels (Figure S1D) with no difference in cell survival from the control cells (Figures 1D and 1F). Similarly, reducing GATA2 expression in KRAS wt and mut human NSCLC cell lines by 60–85% using a second siRNA targeting a different exon (siGATA2-#2) and mouse Gata2 expression by 50–80% in five mouse lung cancer cell lines transfected with siGata2 (siGata2-#1 and siGata2-#2) compared to siControl transfected cells showed no significant difference in cell death (Figure S2A–F).
GATA2 methylation is highly prevalent and unique to human lung cancer
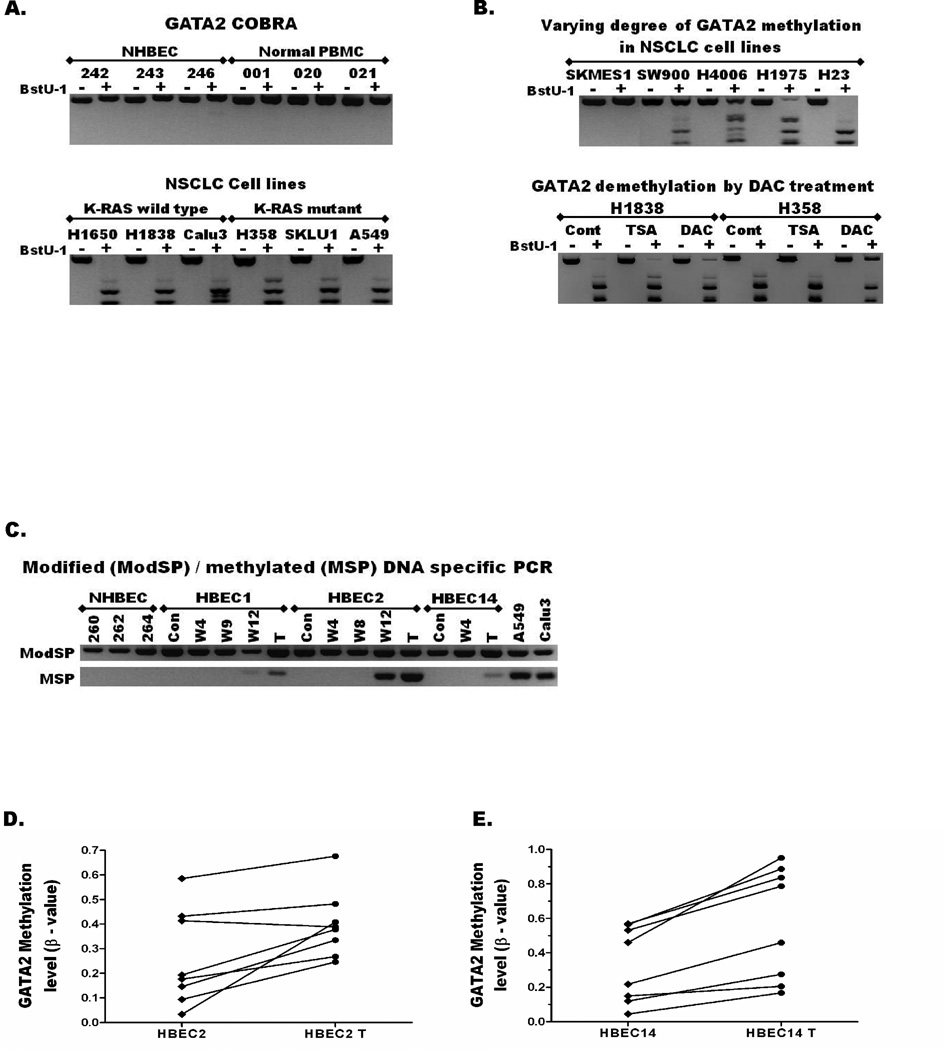
The transcription start sites (TSS) of both human and mouse GATA2 are located within CpG islands. The human GATA2 CpG island was unmethylated in NHBECs and PBMCs obtained from cancer-free donors (n=10), and immortalized HBECs (n=5), but methylated in 30/31 (97%) NSCLC cells regardless of KRAS mutation status (Table 1, Figure 2A). Similarly, GATA2 was methylated in 159/165 (96%) of primary lung adenocarcinomas regardless of patients’ smoking status (Table 1), but unmethylated in distant normal lung (0/10) obtained from a subset of these patients. The different levels of GATA2 methylation seen among NSCLC cell lines (Figure 2B, top panel) and levels of demethylation following treatment with epigenetic drugs (Figure 2B, bottom panel) were estimated by the semi-quantitative methylation assay CoBRA. In contrast, the mouse Gata2 CpG island was not methylated in any NNK-induced lung tumors, lung cancer cell lines, or normal lung tissues from the A/J mice (Figure S3A).
Table 1.
GATA2 methylation status in normal tissue and lung cancer.
| Sample | Number | Methylated, n (%) |
|---|---|---|
| CELLS AND CELL LINES | ||
| NHBECs (cancer free smokers) | 10 | 0 (0%) |
| HBEC cell lines | 5 | 0 (0%) |
| PBMC (Healthy donors) | 10 | 0 (0%) |
| NSCLC cell lines | 31 | 30 (97%) |
| PRIMARY TISSUE | ||
| Distant normal lung | 10 | 0 (%) |
| Lung Adenocarcinoma | 165 | 159 (96%) |
| Current smokers | 34 | 31 (91%) |
| Former smokers | 56 | 53 (95%) |
| Never smokers | 75 | 75 (100%) |
Figure 2. Aberrant methylation of GATA2 is common in lung cancer and occurs early during lung carcinogenesis.
(A) Combined Bisulfite Modification and Restriction Analysis (CoBRA) assay shows GATA2 promoter CpG island is not methylated (PCR products are not digested into smaller fragments by BstU1 enzyme) in NHBECs and PBMCs obtained from cancer-free donors (top panel), but methylated in most KRAS wild-type and mutant NSCLC cell lines (bottom panel). (B) The degree of methylation across the GATA2 promoter could be estimated based on the proportion of undigested and digested fragments. Top panel: Among the NSCLC cell lines evaluated, GATA2 was not methylated in SKMES1 (0%), partially methylated in SW900 (~25%), HCC4006 (~50%), and H1975 (~75%), and completely methylated (100%) in H23 and the remaining cell lines as also shown in the bottom panel of Figure 2A. Bottom panel: The higher proportion of undigested bands in the BstU1+ lanes in the DAC than TSA and Vehicle (Cont) treated KRAS wild-type (H1838) and mutant (H358) NSCLC cell lines indicates demethylation of GATA2 by DAC treatment. (C) The CoBRA primers that amplify bisulfite modified GATA2 promoter CpG island regardless of its methylation were used as modified DNA specific PCR (ModSP). Methylation Specific PCR (MSP) assays using primers that specifically amplify only the methylated promoter show that GATA2 is unmethylated in NHBECs and HBECs (no band). However, methylation was detected in HBEC2 after 12 weeks of exposure to the tobacco carcinogens MNU and BPDE and in the transformed HBEC1, 2, and 14 that were exposed to these carcinogens for 12 weeks and further selected for growth in soft agar. Quantitative methylation data from HM450K arrays show increased methylation of eight probes located within the GATA2 promoter CpG island during transformation of (D) HBEC2 and (E) HBEC14. These eight probes were not methylated in normal lung but aberrantly hypermethylated in nearly all lung tumors.
Quantitative assays validate GATA2 hypermethylation in independent lung tumors
Genome-wide methylation (HM450K array) was conducted on 56 lung tumors and 21 corresponding normal pairs from independent NSCLC cases. Eight probes (depicted by arrows in Figure 1A) within the CpG island extending across the TSS of all GATA2 transcripts (CpGi-2) were found to discriminate tumors from their corresponding normal tissues (Table 2). The average β-value of each probe in the 21 normal lung tissues ranged from 0.06–0.27 compared to 0.32–0.52 in the corresponding 56 tumors. On average, each probe showed 20–25% increased methylation in the tumors (β-value increase of 0.19–0.26) compared to the corresponding normal (Table 2). Because the average β-value of each probe was ≤ 0.27 in normal lung and ≥ 0.32 in the tumors, β ≥ 0.30 was used as a cut-off to define hypermethylation. Based on this cut-off, all eight probes showed very significant hypermethylation in tumors compared to normal lung (p < 0.0001, Table 2).
Table 2.
Comparison of differentially methylated probes within the GATA2 promoter CpG island between lung tumor-normal pairs from NSCLC patients.
| HM450K Probe ID |
Normal lung (N1 = 21) | NSCLC (N = 56) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| M2 | β-value | M* | β-value | Fisher exact test |
t-test | |||
| n3 (%) | Mean ± SD | Min - Max | n (%) | Mean ± SD | Min - Max | |||
| cg00241663 | 3 (14) | 0.26 ± 0.04 | 0.19 – 0.35 | 47 (84) | 0.48 ± 0.17 | 0.14 – 0.86 | 2.37E-08 | < 0.0001 |
| cg22801992 | 0 (0) | 0.17 ± 0.03 | 0.12 – 0.22 | 35 (63) | 0.37 ± 0.16 | 0.11 – 0.75 | 1.69E-07 | < 0.0001 |
| cg03839949 | 4 (19) | 0.27 ± 0.03 | 0.24 – 0.32 | 53 (95) | 0.50 ± 0.13 | 0.20 – 0.81 | 1.14E-10 | < 0.0001 |
| cg22978620 | 0 (0) | 0.06 ± 0.03 | 0.02 – 0.12 | 29 (52) | 0.32 ± 0.20 | 0.02 – 0.80 | 8.27E-06 | < 0.0001 |
| cg16674492 | 0 (0) | 0.09 ± 0.06 | 0.02 – 0.24 | 32 (57) | 0.34 ± 0.25 | 0.02 – 0.87 | 1.03E-06 | < 0.0001 |
| cg06490988 | 0 (0) | 0.20 ± 0.07 | 0.09 – 0.29 | 35 (63) | 0.38 ± 0.18 | 0.06 – 0.76 | 1.69E-07 | < 0.0001 |
| cg19638477 | 0 (0) | 0.15 ± 0.04 | 0.10 – 0.23 | 34 (61) | 0.37 ± 0.20 | 0.08 – 0.84 | 2.78E-07 | < 0.0001 |
| cg13483882 | 5 (24) | 0.26 ± 0.05 | 0.13 – 0.36 | 50 (89) | 0.52 ± 0.16 | 0.14 – 0.87 | 6.72E-08 | < 0.0001 |
‘N’ indicates the number of samples, normal lung tissue of tumors from NSCLC used.
The mean β-value for each of the 8 probes was < 0.3 in normal lung and > 0.3 in lung tumors. Thus, β = 0.3 was used as a cut-off to define the prevalence for methylation each probes.
‘n’ indicates the number of samples out of N that show a mean β-value ≥ 0.3 and considered methylated.
HM450K array data generated by TCGA is publicly available at https://tcga-data.nci.nih.gov for 369 lung tumors and 74 corresponding normal lung. Evaluation of this data for the eight GATA2 probes using the above criteria validated that GATA2 methylation is significantly higher in lung tumors compared to the corresponding normal lung (p < 0.0001, Table 3). Furthermore, the large sample size for TCGA data allowed comparison of GATA2 methylation between adenocarcinoma (AC) and squamous cell carcinoma (SCC). GATA2 hypermethylation was highly prevalent in both of these NSCLC sub-types, and the degree of methylation of some probes (e.g. cg03839949) showed significant differences between AC and SCC (Table S2–S4).
Table 3.
Comparison of methylated probes within the GATA2 promoter CpG island between lung tumor-normal pairs from NSCLC patients (TCGA data).
| HM450K Probe ID |
Normal lung (N = 74) | NSCLC (N = 369) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| M | β-value | M | β-value | Fisher exact test |
t-test | |||
| n (%) | Mean ± SD | Min - Max | n (%) | Mean ± SD | Min - Max | |||
| cg00241663 | 4 (5) | 0.24 ± 0.05 | 0.15 – 0.38 | 310 (84) | 0.50 ± 0.19 | 0.04 – 0.90 | 3.75E-40 | < 0.0001 |
| cg22801992 | 0 (0) | 0.16 ± 0.03 | 0.10 – 0.24 | 238 (65) | 0.37 ± 0.17 | 0.05 – 0.81 | 3.02E-29 | < 0.0001 |
| cg03839949 | 0 (0) | 0.20 ± 0.03 | 0.13 – 0.29 | 319 (86) | 0.46 ± 0.14 | 0.11 – 0.83 | 4.66E-51 | < 0.0001 |
| cg22978620 | 0 (0) | 0.05 ± 0.02 | 0.02 – 0.10 | 215 (58) | 0.33 ± 0.20 | 0.02 – 0.85 | 7.25E-25 | < 0.0001 |
| cg16674492 | 0 (0) | 0.04 ± 0.02 | 0.02 – 0.12 | 183 (50) | 0.30 ± 0.25 | 0.02 – 0.88 | 5.56E-20 | < 0.0001 |
| cg06490988 | 0 (0) | 0.14 ± 0.04 | 0.04 – 0.23 | 225 (61) | 0.36 ± 0.20 | 0.02 – 0.82 | 8.04E-27 | < 0.0001 |
| cg19638477 | 0 (0) | 0.09 ± 0.03 | 0.04 – 0.21 | 186 (50) | 0.31 ± 0.20 | 0.03 – 0.91 | 1.88E-20 | < 0.0001 |
| cg13483882 | 3 (4) | 0.21 ± 0.05 | 0.13 – 0.37 | 267 (72) | 0.45 ± 0.19 | 0.06 – 0.88 | 4.64E-30 | < 0.0001 |
Aberrant GATA2 methylation occurs early during lung carcinogenesis
An in vitro HBEC transformation model that we have developed to characterize early pre-malignancy changes during lung carcinogenesis28, 29 was used to establish the timing of GATA2 methylation. MSP assay revealed that GATA2 was unmethylated in HBEC1, 2, and 14 before or during the initial 8 wks exposure to MNU and BPDE (Figure 2B). However, methylation was detected in HBEC2 after 12 wks of exposure and in all three transformed HBEC lines (HBEC-Ts, selected after growth in soft agar) (Figure 2C). Comparison of the eight GATA2 probes using HM450K array data available for control and transformed HBECs showed increased β-values for 7 probes in HBEC2-T vs. HBEC2, and all 8 probes in HBEC14-T vs. HBEC14 (Figure 2D–E, Table S5). Among these probes, cg16674492 (dotted arrow in Figure 1A) interrogated 3 CpGs in common with the reverse MSP primer. Concurring with the strong MSP signal seen in HBEC2-T, the β-value of this probe increased from 0.033 (HBEC2) to 0.408 (HBEC2-T), and from 0.045 (HBEC14) to 0.167 (HBEC14-T). This indicates a 38% (3 to 41%) and 12% (5 to 17%) increase in methylation upon transformation of HBEC2 and HBEC14, respectively (Table S5, Figure 2C–E).
GATA2 repression is epigenetically mediated
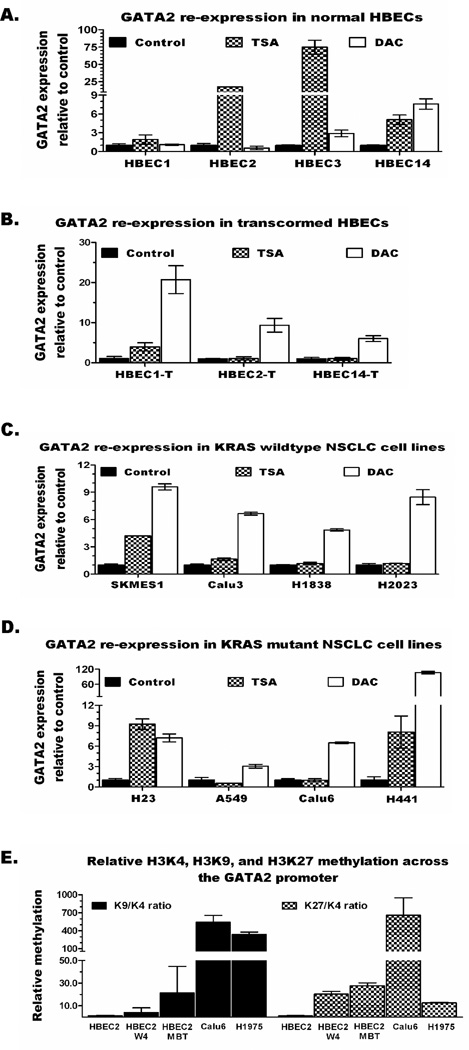
Despite the absence of GATA2 methylation in HBECs prior to transformation (Figure 2C) its expression in these cells is already silenced or significantly reduced (Figure 1C). The responsiveness of GATA2 repression to the HDAC inhibitor TSA or the DNMT inhibitor DAC was investigated in vitro. As shown in Figure 3A, GATA2 expression in HBECs was primarily induced by TSA (2–75-fold increase in each HBEC) and to a lesser extent by DAC (3–8-fold increase in 2 out of 4 HBECs). In contrast, expression of GATA2 in the HBEC-Ts and NSCLC cell lines (KRAS wild-type or mutant) was mainly induced by DAC (Figures 3B–D). Overall, DAC treatment increased GATA2 expression by 6–21-fold in the HBEC-Ts and 2–10-fold in NSCLC cell lines (> 100-fold in H441). GATA2 expression in the primary lung tumors and NSCLC cell lines was approximately > 5-fold lower than in normal lung tissue (Figures 1B-C and S1A). Thus, re-expression by ≥ 5-fold in DAC/TSA treated NSCLC cell lines compared to vehicle treated control of each cell line suggests restoration of GATA2 expression to levels seen in normal lung. Similarly, expression of Gata2 in mouse lung cancer cell lines was increased by up to 26-fold and 33-fold following TSA or DAC treatments, respectively (Figure S3B).
Figure 3. Epigenetic repression of GATA2 in early lung carcinogenesis is mediated by chromatin modification and progresses to a more stable repression by adding cytosine-DNA methylation upon transformation.
(A) GATA2 expression responds better to the HDAC inhibitor TSA, while transformed HBECs (B), KRAS wild-type (C) and mutant (D) NSCLC cell lines show better response to the DNMTs inhibitor DAC. (E) Chromatin marks for transcriptional repression were increased during transformation of HBEC2 and the highest repression marks were seen in NSCLC cell lines, Calu6 (KRAS mutant) and H1975 (KRAS wild-type).
Chromatin remodeling drives initial GATA2 repression during lung carcinogenesis
GATA2 in HBECs was unmethylated and mainly re-expressed by TSA, whereas in HBEC-Ts and NSCLC cell lines it was methylated and re-expressed the most by DAC. This suggests that transcriptional repression of GATA2 progresses during carcinogen-induced transformation and carcinogenesis from the more dynamic chromatin remodeling to the more stable repression by adding cytosine-DNA methylation. This premise was tested by comparing the levels of active (H3K4m2) and repressive (H3K9m2 and H3K27m3) chromatin marks across the GATA2 promoter using quantitative ChIP assays. HBEC2, HBEC2-wk4 (exposed to MNU and BPDE for 4 weeks), HBEC2-T, and KRAS mutant (Calu-6) and KRAS wild-type (H1975) NSCLC cell lines were evaluated. After adjustment to total histone-3, the level of H3K9m2 relative to H3K4m2 in HBEC2 was increased by 4-, 21-, 539-, and 338-fold in HBEC2-wk4, HBEC2-T, Calu6, and H1975, respectively (Figure 3E). Similarly, the level of H3K27m3 relative to H3K4m2 in HBEC2 was increased by 20-, 28-, 659-, and 13-fold, respectively (Figure 3E).
Discussion
The recently described addiction of KRAS mutant lung tumors to GATA2 has raised a lot of excitement about the potential of targeting this most common, but elusive cancer-driving mutation for the treatment of lung cancer.13, 41 However, this study reports for the first time that GATA2 is commonly repressed in human and murine lung tumors via distinct epigenetic mechanisms, and further knock-down of the gene does not increase cell death regardless of KRAS mutation status. The promoter CpG island of human GATA2 is unmethylated in normal lung but aberrantly hypermethylated in nearly all primary lung tumors and NSCLC cell lines. After validating this highly prevalent hypermethylation in lung cancer using publically available large, independent, and quantitative methylation data from TCGA, subsequent studies established that this epigenetic modification occurs early during tobacco carcinogen-induced transformation. In contrast, the mouse Gata2 promoter CpG island is unmethylated in NNK-induced lung tumors and cancer cell lines despite a dramatic reduction in expression of the gene compared to normal lung, suggesting chromatin-based repression. Most importantly, in clear contrast to Kumar et al.,13 our data demonstrated that GATA2 is already repressed in both human and murine lung cancer and further reducing up to 95% of its expression does not increase cell death in KRAS mutant or wild-type lung cancer cells. The use of different experimental models in the two studies and the fact that Kumar et al.13 did not evaluate epigenetic regulation of GATA2, or study primary human tumors or normal lung tissue may account for some of the observed differences. However, the results from our focused and comprehensive studies clearly demonstrate that it is highly unlikely that GATA2 suppression will offer any clinical benefit in the treatment of KRAS mutant lung cancer.
The normal expression profile of GATA2 in lung tissue and its role in lung carcinogenesis is not well characterized. Previous studies have shown that the different transcripts of GATA2 are expressed in a tissue-specific manner. Transcript variant-2 (often described as the IS transcript) is specific for hematopoietic and neuronal cells, whereas transcript variants-1 and -3 (IG transcripts) are expressed in most tissues.16, 42, 43 In agreement with those findings, our assays did not detect expression of Var.2 in any of the primary lung tumor-normal pairs or NSCLC cell lines. In contrast, comparable levels of Var.1 and Var.3 were expressed primarily in the normal lung tissue and to a lesser extent in the primary tumors and NSCLC cell lines.
Earlier screenings from our group and others have indicated that GATA2 is potentially epigenetically repressed in lung cancer. Over a decade ago Eickhoff et al., reported that GATA2 is one of the genes induced by TSA treatment of human lung adenocarcinoma cells.44 Similarly, genome-wide unmasking of epigenetically silenced genes in NSCLC cell lines using TSA and DAC treatments identified GATA2 as one of the repressed genes in lung cancer.26 Validating these earlier observations, this study first showed that GATA2 methylation is specific to tumors (not seen in normal lung tissue and bronchial epithelial cells) and is highly prevalent among NSCLC cell lines (97%) and primary lung adenocarcinomas (96%) from current, former, and never smokers. These results were validated using quantitative methylation analysis of two independent sets of NSCLC samples (ours and TCGA). Moreover, the HBEC transformation model developed in our laboratory28, 29 showed that although the GATA2 promoter is unmethylated in HBECs, its expression is repressed and primarily re-expressed by TSA indicating epigenetic silencing through histone modifications. The GATA2 promoter becomes methylated during transformation and re-expression in the transformed HBECs and NSCLC cell lines is mainly induced by DAC indicating the establishment of a more stable repression through promoter CpG island hypermethylation. The fact that aberrant methylation of GATA2 occurs early during transformation also explains its very high prevalence in NSCLC cell lines and primary lung tumors.
We previously reported that Gata2 expression is reduced in cumene-induced K-ras mutant lung tumors in mice, while its promoter CpG island remained unmethylated.27 Similarly, this study shows that Gata2 expression is dramatically reduced in NNK-induced K-ras mutant lung tumors in A/J mice and cancer cell lines without methylation of its promoter CpG island. Thus, it appears that epigenetically driven silencing of this gene in mice occurs largely by chromatin remodeling and does not involve cytosine DNA methylation as seen in human lung tumors. However, irrespective of the factors underlying transcriptional repression, further knockdown of Gata2 in murine K-ras mutant tumors did not affect survival of these malignant cells. These conclusive findings coupled with identical results in human KRAS wild-type as well as mutant tumor-derived lines do not support GATA2 as a target for lung cancer therapy.
Supplementary Material
Acknowledgments
Data generated by The Cancer Genome Atlas (TCGA) pilot project established by the NCI and NHGRI was used to validate part of our findings. The dbGaP accession number for TCGA data is phs000178.v8.p7. Information about TCGA and the investigators and institutions that constitute the TCGA research network can be found at http://cancergenome.nih.gov/.
Funding
This work was supported by the National Institutes of Health [grant numbers R01 ES008801, R01CA089551, and R01CA095568g, all to S.A.B.].
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Dominioni L, Imperatori A, Rovera F, et al. Stage I nonsmall cell lung carcinoma: analysis of survival and implications for screening. Cancer. 2000;89:2334–2344. doi: 10.1002/1097-0142(20001201)89:11+<2334::aid-cncr4>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 5.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24:3340–3346. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 6.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ. Treatment of ALK-positive non-small cell lung cancer. Arch Pathol Lab Med. 2012;136:1201–1204. doi: 10.5858/arpa.2012-0246-RA. [DOI] [PubMed] [Google Scholar]
- 8.Eder JP, Vande Woude GF, Boerner SA, et al. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–1636. doi: 10.1097/JTO.0b013e3181e8b3a3. [DOI] [PubMed] [Google Scholar]
- 10.Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012;23:2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 11.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 13.Kumar MS, Hancock DC, Molina-Arcas M, et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–655. doi: 10.1016/j.cell.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD. The zinc finger-containing transcription factors GATA-4-5, and-6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 15.Bresnick EH, Martowicz ML, Pal S, et al. Developmental control via GATA factor interplay at chromatin domains. J Cell Physiol. 2005;205:1–9. doi: 10.1002/jcp.20393. [DOI] [PubMed] [Google Scholar]
- 16.Vicente C, Conchillo A, Garcia-Sanchez MA, et al. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Ling KW, Ottersbach K, van Hamburg JP, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 19.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- 20.Persons DA, Allay JA, Allay ER, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- 21.Fadilah SA, Cheong SK, Roslan H, et al. GATA-1 and GATA-2 gene expression is related to the severity of dysplasia in myelodysplastic syndrome. Leukemia. 2002;16:1563–1565. doi: 10.1038/sj.leu.2402517. [DOI] [PubMed] [Google Scholar]
- 22.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama Y, Watkins N, Suzuki H, et al. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23:8429–8439. doi: 10.1128/MCB.23.23.8429-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo M, Akiyama Y, House MG, et al. Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res. 2004;10:7917–7924. doi: 10.1158/1078-0432.CCR-04-1140. [DOI] [PubMed] [Google Scholar]
- 26.Tessema M, Klinge DM, Yingling CM, et al. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene. 2010;29:5159–5170. doi: 10.1038/onc.2010.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakamatsu N, Collins JB, Parker JS, et al. Gene expression studies demonstrate that the K-ras/Erk MAP kinase signal transduction pathway and other novel pathways contribute to the pathogenesis of cumene-induced lung tumors. Toxicol Pathol. 2008;36:743–752. doi: 10.1177/0192623308320801. [DOI] [PubMed] [Google Scholar]
- 28.Damiani LA, Yingling CM, Leng S, et al. Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bronchial epithelial cells. Cancer Res. 2008;68:9005–9014. doi: 10.1158/0008-5472.CAN-08-1276. [DOI] [PubMed] [Google Scholar]
- 29.Tellez CS, Juri DE, Do K, et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62:2370–2377. [PubMed] [Google Scholar]
- 31.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 32.Belinsky SA, Devereux TR, Foley JF, et al. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;52:3164–3173. [PubMed] [Google Scholar]
- 33.Vuillemenot BR, Pulling LC, Palmisano WA, et al. Carcinogen exposure differentially modulates RAR-beta promoter hypermethylation, an early and frequent event in mouse lung carcinogenesis. Carcinogenesis. 2004;25:623–629. doi: 10.1093/carcin/bgh038. [DOI] [PubMed] [Google Scholar]
- 34.Tessema M, Yu YY, Stidley CA, et al. Concomitant promoter methylation of multiple genes in lung adenocarcinomas from current, former and never smokers. Carcinogenesis. 2009;30:1132–1138. doi: 10.1093/carcin/bgp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessema M, Willink R, Do K, et al. Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Res. 2008;68:1707–1714. doi: 10.1158/0008-5472.CAN-07-6325. [DOI] [PubMed] [Google Scholar]
- 36.Bibikova M, Barnes B, Tsan C, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Tessema M, Yingling CM, Thomas CL, et al. SULF2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-I inhibitors via induction of ISG15. Oncogene. 2012;31:4107–4106. doi: 10.1038/onc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng S, Bernauer AM, Hong C, et al. The A/G allele of rs16906252 predicts for MGMT methylation and is selectively silenced in premalignant lesions from smokers and in lung adenocarcinomas. Clin Cancer Res. 2011;17:2014–2023. doi: 10.1158/1078-0432.CCR-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessema M, Yingling CM, Grimes MJ, et al. Differential epigenetic regulation of TOX subfamily high mobility group box genes in lung and breast cancers. PLoS One. 2012;7:e34850. doi: 10.1371/journal.pone.0034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Barbacid M. Opening a new GATAway for treating KRAS-driven lung tumors. Cancer Cell. 2012;21:598–600. doi: 10.1016/j.ccr.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minegishi N, Ohta J, Suwabe N, et al. Alternative promoters regulate transcription of the mouse GATA-2 gene. J Biol Chem. 1998;273:3625–3634. doi: 10.1074/jbc.273.6.3625. [DOI] [PubMed] [Google Scholar]
- 43.Pan X, Minegishi N, Harigae H, et al. Identification of human GATA-2 gene distal IS exon and its expression in hematopoietic stem cell fractions. J Biochem. 2000;127:105–112. doi: 10.1093/oxfordjournals.jbchem.a022570. [DOI] [PubMed] [Google Scholar]
- 44.Eickhoff B, Ruller S, Laue T, et al. Trichostatin A modulates expression of p21waf1/cip1, Bcl-xL, ID1, ID2, ID3, CRAB2, GATA-2, hsp86 and TFIID/TAFII31 mRNA in human lung adenocarcinoma cells. Biol Chem. 2000;381:107–112. doi: 10.1515/BC.2000.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.