Abstract
Drug metabolism and transport processes in the liver, intestine and kidney that affect the pharmacokinetics and pharmacodynamics of therapeutic agents have been studied extensively. In contrast, comparatively little research has been conducted on these topics as they pertain to the eye. Recently, however, catalytic functions of ocular cytochrome P450 enzymes have gained increasing attention, in large part due to the roles of CYP1B1 and CYP4V2 variants in primary congenital glaucoma and Bietti’s corneoretinal crystalline dystrophy, respectively. In this review, we discuss challenges to ophthalmic drug delivery, including Phase I drug metabolism and transport in the eye, and the role of three specific P450s, CYP4B1, CYP1B1 and CYP4V2 in ocular inflammation and genetically determined ocular disease.
Keywords: CYP4B1, CYP1B1, CYP4V2, cytochrome P450, drug metabolism, drug transporters, genetic eye disease
Introduction
Tissue-specific effects on the disposition of drugs, xenobiotics and endogenous compounds are critical to understanding their pharmacological and toxicological activities. While much is now known about drug metabolism and transport in the liver, intestine and kidney, there is a paucity of such information for most extra-hepatic tissues. This is especially true for the eye. From a therapeutic perspective, anatomical and physiological constraints associated with the eye make it challenging to continuously achieve appropriate levels of drug exposure. To overcome the problem, effective but invasive ways to deliver drugs have been developed, such as intra-vitreal injection. However, this treatment route is often associated with complications. Therefore, non-invasive, orally and topically administered drugs are more desirable (Thrimawithana et al., 2011).
While ocular drug-metabolizing enzymes and transporters have been studied in animal models, the catalytic activities, tissue localization and substrate specificities of drug-metabolizing enzymes and transporters may differ from those in humans; thus, caution is necessary when extrapolating such data from animals to man. In short, our present knowledge about ocular transporters and drug-metabolizing enzymes is insufficient for a full understanding of xenobiotic and endobiotic disposition in human ocular tissues. Nevertheless, at least three cytochrome P450 (P450) enzymes – CYP1B1, CYP4B1 and CYP4V2 – play important physiological roles in the eye. CYP4B1 is associated with neovascularization in animal models after hypoxic insult (Mastyugin et al., 1999, 2001, 2004), whereas human CYP1B1 and CYP4V2 are causally linked to primary congenital glaucoma and Bietti’s corneoretinal dystrophy, respectively (Li et al., 2004; Suri et al., 2009; Vasiliou & Gonzalez, 2008).
This review will discuss (i) barriers to ophthalmic drug delivery, (ii) drug transport and drug metabolizing activity in the eye and (iii) the role of the three P450s described above in ocular inflammation and genetically determined diseases with an emphasis on the signaling pathways that may connect their catalytic functions with pathophysiological changes in the eye.
Eye disease and associated challenges of drug delivery
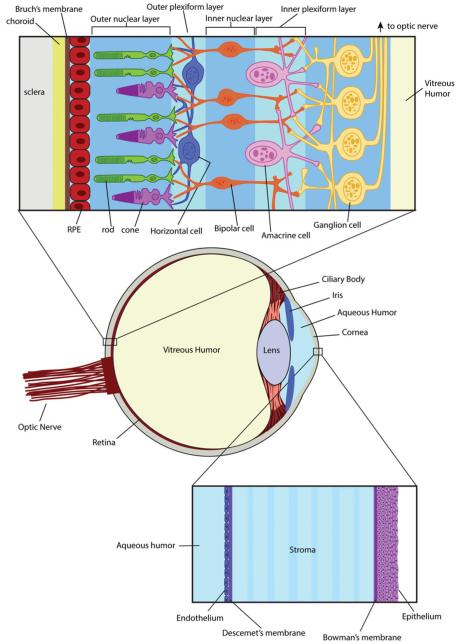
Although eye diseases are not generally accompanied by severe systemic problems or life-threatening symptoms, they can still be quite serious and significantly impact quality of life. An estimated 1 in 28 Americans older than 40 will suffer from impaired vision in 2020 based on demographics from the 2000 U.S. Census (Congdon et al., 2004) with the leading causes of blindness and impaired vision being age-related macular degeneration, glaucoma and cataracts. Although the etiology of these diseases has been studied for decades, available treatments are not curative. From the drug-based treatment perspective, the challenges are not only the development of effective agents, but also the development of delivery systems that provide therapeutic drug concentrations in the target tissues. As for most tissues, important determinants of ocular drug distribution include lipophilicity and drug transporter affinity. The optimal logP value for permeation of the cornea is reported to be 2–3 (Huang et al., 1983), because a drug must cross both the lipophilic cornea epithelium and the more hydrophilic stroma (Figure 1).
Figure 1.
Anatomical structure of the retina and cornea. The retina (upper blowout panel) is a complex tissue comprised of layers of cells built on the structural foundation of the sclera, choroid and Bruch’s membrane. The retinal pigmented epithelial (RPE) cells provide a base layer to support the structure and function of photoreceptor cells (rods and cones) located in the outer nuclear layer. When light enters the eye, it penetrates the retinal tissue and is absorbed by photoreceptor cells where it is converted into an electrical signal. A series of nerve-type cells (horizontal, bipolar and amacrine cells) form the outer plexiform, inner nuclear and inner plexiform layers, and are responsible for transmitting the electrical signal to the ganglion cells in the innermost layer of the retina. Ganglion cells connect to the optic nerve and transmit the visual signal to the brain. The cornea (lower blowout panel) consists of an endothelial layer of cells in contact with the aqueous humor of the anterior chamber. The stroma is located between Descement’s membrane on the endothelial side, and Bowman’s membrane on the epithelial side. The epithelial layer of cells makes up the surface of the cornea.
Typically, the treatment of eye diseases involves topical administration of eye drops or ointments. However, eye drops and ointments have low bioavailability for several reasons. First, tears can wash away topically administered drugs. Second, the low permeability of the corneal epithelium blocks absorption and prevents drugs from entering the anterior part of the eye (Macha et al., 1993). Third, drug-metabolizing enzymes and efflux transporters, such as those, that will be described in the next section, can rapidly eliminate the drug. Fourth, other anatomical and physiological constraints associated with the eye result in a negligible amount of topically applied drug reaching the posterior part of eye, specifically the retina.
Oral delivery is the next most common mode of ocular drug administration, but drug distribution to the eye from the systemic circulation is also challenging. Oral drugs targeted to the eye are often limited by low bioavailability due to the blood–retinal barrier (BRB), which is composed of the inner BRB (also referred to as blood–aqueous barrier) and the outer BRB (Figure 2). Similar to the situation at the blood–brain barrier, the inner and outer BRBs contain tight junctions between endothelial cells and retinal pigmented epithelial cells (RPE) to separate and protect the multilayered retinal neuronal cells from substances present in the blood (Campbell & Humphries, 2012). These tight junctions limit the entrance of xenobiotics to the retinal cells, and, in addition, RPE expresses drug-metabolizing enzymes and transporters that facilitate elimination of drugs (Zhang et al., 2008). Hepatic and intestinal drug metabolism also can significantly reduce the circulating drug concentration, further compounding the difficulty in targeting oral drugs to the retina and other posterior portions of the eye. Therefore, in addition to the conventional drug delivery methods, eye-specific drug delivery devices and procedures have become available at eye clinics.
Figure 2.
Localization of uptake and efflux transporters in the corneal epithelium, outer blood retinal barrier (BRB) and inner BRB. The outer BRB includes the retinal pigmented epithelium (RPE) that is bound by tight junctions to provide a physical barrier between systemic circulation and the photoreceptor cells and neural retina. The inner BRB is formed by the vasculature that serves the neural retina and acts as a gatekeeper for nutrient delivery, toxin removal and prevention of endogenous and exogenous compounds that do not belong in the eye from reaching the sensitive retinal tissue. Approximate cellular localization is illustrated, along with proposed functional direction of transport. Transporters that have been identified to be present in ocular tissue, but for which exact localization or function has not been confirmed are placed within the cell without directional arrows. Uptake transporters: Amino-acid transporter B0,+ (ATB0,+), nucleoside transporters (CNT, ENT), creatinine transporter (CRT), gamma-aminobutyric acid transporter (GAT), facilitative glucose transporter (GLUT), large, neutral amino-acid transporter (LAT), multidrug and toxin exclusion transporters (MATE), monocarboxylate transporter (MCT) Na+-taurocholate cotransporting polypeptide (NTCP), organic anion transporter (OAT), organic anion transporting peptide (OATP), organic cation transporters (OCT), organic cation/carnitine transporter (OCTN), peptide transporter (PEPT), reduced folate transporter (RFT), taurine transporter (TAUT), Na+-independent glutamate/cysteine exchange transporter (Xc−). Efflux transporters: breast cancer resistance protein (BCRP), excitatory amino-acid transporter (EAAC1), multidrug resistance-associated protein (MRP), monocarboxylate transporter (MCT), multiple drug resistance protein/P-glycoprotein (P-gp).
Intra-vitreal injection with aflibercept has FDA approval for the treatment of wet age-related macular degeneration (AMD) and macular edema following central retinal vein occlusion. Although a valuable pharmacological tool for many patients, potential complications include cataracts, inflammation, retinal detachment and hemorrhaging. Also, due to rapid drug elimination, intra-vitreal injection requires multiple treatments (Schultz et al., 2011; Thrimawithana et al., 2011). Nevertheless, biologic drugs such as Lucentis for wet AMD are considered standard of care despite the invasive nature of their administration (Ventrice et al., 2013). Other delivery options include the implantation of biodegradable or non-biodegradable devices into the intra-vitreal space. In terms of less-invasive options, hydrogel contact lenses (Xinming et al., 2008) and iontophoresis (Eljarrat-Binstock et al., 2005) to deliver the drug deep into the cornea are also available, but the contact lenses may cause discomfort and both methods require multiple treatments to maintain a sufficient drug concentration at the cellular/tissue target. Newer promising procedures, such as micro-/nano-particle injections, have been developed, but they are not yet widely available at clinics because the procedure requires specialized techniques. In summary, there is ongoing need for methods that can ensure that ocular drugs are delivered to the target cells or tissues in therapeutic concentrations that can be maintained over desired period of time. To this end, a thorough knowledge of how drugs are metabolized and transported within eye tissues is important.
Drug transporters
Despite the lack of availability (in most cases) of monospecific antibodies, access to gene sequence information and to RT-PCR techniques has facilitated measurement of the transcriptional levels of drug transporters in the eye. A recent study used high-throughput RT-PCR techniques to screen ocular tissues for expression of genes from the solute carrier (SLC) and ATP binding cassette (ABC) families of transporters, in addition to several metabolic enzymes (Dahlin et al., 2013). Overall, expression was highest in retinal and corneal tissues compared to other substructures, and in both tissues SLC family transporters were the most prevalent with 68% of the total evaluated gene expression in the cornea and 75% in the retina attributed to SLC transporters. Among the most highly expressed genes, more nutrient transporters were present in the retina compared to cornea (46 versus 38%, respectively), and more drug transporters were identified in the cornea (29%) compared to the retina (13%). In the cornea, ABC transporters represented 16% of expressed transporters, nuclear receptors and transcription factors accounted for 9%, and Phase I and II enzymes represented 7% of the evaluated gene expression. In the retina, ABC transporters represented 9% of expressed transporters, nuclear receptors and transcription factors accounted for 6%, and Phase I and II enzymes represented 10% of the evaluated gene expression (Dahlin et al., 2013).
The human cornea expresses both uptake and efflux transporters. RT-PCR studies have detected transcripts for numerous uptake transporters, including several SLC, the amino-acid transporter B0,+ (ATB0,+) and the large, neutral, amino-acid transporter 1 (LAT1), and their functional activities have been confirmed in transporting l-arginine and l-phenylalanine across isolated rabbit cornea (Jain-Vakkalagadda et al., 2003, 2004). Figure 2 illustrates the localization of transporter proteins in ocular tissues as determined by RT-PCR quantitation of gene transcripts. The uptake transporters from the family of solute carriers expressed in the cornea include peptide transporters 1 and 2 (PEPT1 and PEPT2), organic cation transporters 1, 2 and 3 (OCT1, OCT2 and OCT3), organic cation/carnitine transporter 2 (OCTN2), organic anion transporters 1 and 3 (OAT1 and OAT3), multidrug and toxin exclusion transporters 1 and 2 (MATE1 and MATE2), organic anion transporting peptide 1A2, 1B1, 1B3 and 2B1 (OATP1A2, OATP1B1, OATP1B3 and OATP2B1) and Na+-taurocholate cotransporting polypeptide (NTCP). In terms of efflux, the ATP-binding cassette transporters B1 (ABCB1, multiple drug resistance 1 or P-glycoprotein), C1 (ABCC1 or multidrug resistance-associated protein 1, MRP1), C2 (ABCC2 or MRP2), C3 (ABCC3 or MRP3), C4 (ABCC4 or MRP4), C5 (ABCC5 or MRP5), C6 (ABCC6 or MRP6) and breast cancer resistance protein (BCRP) are expressed in human cornea and in Statens Seruminstitut rabbit corneal cells (Chen et al., 2013; Dahlin et al., 2013; Dey et al., 2003; Karla et al., 2007a,b). However, the quantitative data obtained from these corneal expression studies often varies widely for a given transporter, due possibly to differences in experimental methods, but perhaps more likely to the varying clinical background and post-mortem status of the particular tissue samples that were used. There is very limited information available regarding subcellular localization of transporters within ocular tissues; however, a recent study examined cultured rabbit primary corneal epithelial cells and determined the efflux transporter P-glycoprotein and PEPT1 were present in the mitochondrial membranes (Barot et al., 2013).
Retinal tissue is highly vascularized, and the sensitive tissues are protected from systemic endogenous and exogenous compounds by the BRB. Figure 2 illustrates transporters localized in the inner and outer BRB. In the RPE cells of the outer BRB, the high affinity excitatory amino acid transporter 1 (EAAC1) facilitates removal of extracellular glutamate, an excitatory neurotransmitter used by cells of the neural retina that is neurotoxic in high concentrations. Other amino acid transporters localized in the RPE include neurotransmitter transporters from the SLC6 family (TAUT, system β) that are responsible for transport of taurine, the most abundant retinal amino acid with transport direction dependent upon taurine and ion concentration gradients. Creatinine transporter (CRT) has been identified in rat retinal endothelium, and GABA transporter T3 (GAT3), the most abundant inhibitory neurotransmitter found in retinal tissues. The ATB0,+ has been detected in RPE cells, but the function is unknown at the time of this review. The large, neutral, amino-acid transporter 1 (LAT1) is involved in uptake of l-phenylalanine in ARPE-19 cells, and LAT2 is believed to mediate transport of leucine. The Na+-independent glutamate/cysteine exchange transporters (Xc−) are present in outer BRB as well as inner BRB to support glutamate homeostasis. MCT1, MCT2, MCT3 and MCT4 facilitate uptake of lactate in ARPE-19 cells and isolated bovine RPE, and OCT1, OCT2 and OCT3 have been identified in RPE cells, which may affect drug transport. Folate uptake is mediated by reduced folate transporter 1 (RFT-1). Equilibrate nucleoside transporters 1 and 2 (ENT1 and ENT2) and concentrative nucleoside transporters 1 and 2 (CNT1 and CNT2) have been identified in an immortalized retinal capillary endothelial cells (TR-iBRB), as well as organic anion transporting polypeptides OATP2, OATPE and OATP12. Efflux transporters expressed in the outer BRB include MDR, MRP1, MRP4, MRP5 and BCRP (Mannermaa et al., 2006).
In the inner BRB, transporters are located in the vascular endothelium of vessels in the neural retina. The retinal capillary system includes many of the same transporters that have been identified in the outer BRB. d-Glucose is transported by facilitative glucose transporter 1 (GLUT1) from the blood as the primary energy source for retinal tissue. CRT has been identified in rat retinal vessels, as well as LAT1. MCT1 facilitates movement of lactate in between blood and retina through the inner BRB. The Na+-independent glutamate/cysteine exchange transporters (Xc−) are also present in inner BRB and OATP2, and OATP14 were detected in rat retinal vessels. Efflux transporters expressed in the inner BRB include MDR1, OAT3 and BCRP (Hosoya & Tachikawa, 2009; Hosoya & Tomi, 2005; Mannermaa et al., 2006).
In the retina, MRP1, PEP2, OCT1 and OCTN1 and OCTN2 exhibited higher expression levels than in the liver, whereas the expression levels of MDR1 and BCRP were approximately 4-fold lower than those in liver (Zhang et al., 2008). Interestingly, there is a good consensus on MDR1 and BCRP expression levels across multiple reports (Chen et al., 2013; Dahlin et al., 2013) although, compared to the data for the cornea, limited numbers of comparator studies are available. In addition, MRPs and LRP retinal expression of MRPs were observed (Chen et al., 2013; Dahlin et al., 2013; Zhang et al., 2008). In ARPE-19 cells, transcripts for the uptake transporters, EAAC1 TAUT, LAT2, creatinine transporter (xCT), peptide histidine transporter 1 (PHT1), MCT1, MCT3 and MCT4, RFT and OCT3 have all been detected (Mannermaa et al., 2006).
It seems plausible that efflux and uptake transporters in the eye could have evolved to perform both an ocular barrier function and to control both endobiotic and xenobiotic disposition because certain transporters show mRNA expression levels comparable to those in the liver, small intestine and kidney (Zhang et al., 2008), organs that play significant roles in drug disposition. Clearly, transporters are likely to have an important role in ocular drug absorption, distribution and clearance, but the degree of involvement by each transporter for a given drug substrate and the subcellular localization of most of these proteins remains to be elucidated. This requires more comprehensive functional studies together with robust transporter protein quantitation data that are becoming increasingly easy to obtain using mass spectrometry techniques (Prasad et al., 2014).
Drug-metabolizing enzymes
Historically, due to limited access to human eye tissue, the majority of ocular drug metabolism studies were carried out in animal models such as the cow, rat and rabbit. In an early study of bovine ocular drug-metabolizing enzymes, benzopyrene hydroxylase activity – presumably catalyzed by CYP1 family enzymes – was demonstrated in tissue homogenates prepared from the ciliary body, retina, cornea and iris (Shichi & Nebert, 1982). These researchers also found Phase II drug-metabolizing enzyme activities, including glutathione S-transferase and N-acetyltransferase to be present in the bovine ciliary body, RPE and choroid.
Ocular esterases, a major class of Phase I enzymes, were investigated intensively between the 1960s and 1980s. Retinal esterase protein was first detected by immunohistochemical staining (Esilae, 1964), and acetylcholine esterase activity was found to be present at high levels in the inner retina compared to the outer retina of the C57BL/6 mouse (Ross et al., 1975). Subsequently, human carboxylesterase activity in sub-retinal fluid was reported (Lam et al., 1977). As acetylcholine is a neurotransmitter at the inner and outer synaptic cell layers in the vertebrate retina, research on ocular acetylcholinesterase was conducted (Hutchins, 1987; Hutchins & Hollyfield, 1987). Recently, phosphodiesterase 10A (PDE10A) has been identified in the retina. PDE10A was found in a subcortical part of the forebrain – the striatum – and is considered an attractive drug target for certain psychiatric disorders (Grauer et al., 2009; Smith et al., 2013).
Numerous studies of corneal esterase in animal models have been performed from the viewpoint of using the enzyme as a possible pro-drug-activating enzyme in the eye (Esilae, 1963, 1964; Lee, 1983; Lee et al., 1982a,b). Lee and colleagues investigated corneal esterase expression based on a functional assay of 1- or 2-naphthyl ester hydrolysis. These authors separated rabbit and bovine eye tissues into corneal epithelium, corneal endothelium, stroma and iris-ciliary and prepared mitochondrial, microsomal and cytoplasmic fractions. The highest to lowest esterase activities were found in the iris-ciliary body, corneal epithelium and stroma (Figure 3). The majority of esterase activity was found in the microsomal fractions (80% of total activity), with some residual activity present in the cytoplasmic fractions. Mitochondrial fractions did not exhibit esterase activity. Because 1- or 2-naphthyl esters were used as functional probes and enzyme activity was largely microsomal, the esterase activities were probably those of carboxylesterase(s), but this remains to be determined.
Figure 3.
Localization of ocular cytochrome P450 and esterase enzymes in ocular tissues. CYP1B1 is expressed in the retinal pigment epithelium, Mullar cells, ciliary epithelium, corneal epithelium and corneal keratocytes, while CYP4V2 is expressed most highly in the retinal pigment epithelium (**) with lesser expression in the corneal epithelium (*). Weak expression of CYP4V2 was also observed in ganglion cells and internal and external nuclear layers of the retina. CYP4B1 is inducible (^) and expression in the corneal epithelium increases under hypoxia. CYP3A4, CYP2A6, CYP2C8, CYP2D5, CYP2E1 and CYP3A4 have been detected at low levels in both corneal and retinal tissue, and CYP2J2 has also been detected in ARPE-19 retinal cells. Esterases have also been detected in the cornea and ciliary body, and phosphodiesterase 10A (PDE10A) is expressed in the retina.
A good example of a tissue-targeted pro-drug is valacyclovir, the l-valine peptidomimetic prodrug of acyclovir (Anand & Mitra, 2002; Anand et al., 2004). Acyclovir is a first-line drug for the treatment of herpes simplex and herpes simplex keratitis is one of the leading causes of blindness in the U.S. (Turner et al., 2003). However, the low solubility of acyclovir prevents formulation of a topical version of the drug, which requires a relatively high concentration (1–3% w/v) for efficacy. Covalently linking acyclovir to two valine residues provides better solubility and facilitates formulation for topical administration, whereas the presence of peptide transporters in the eye with broad substrate specificity increases overall bioavailability of the drug (Bras et al., 2001; Dias et al., 2002). Presumably the corneal esterase, biphenyl-like hydrolase protein (Kim et al., 2003), hydrolyzes valacyclovir to generate acyclovir intra-ocularly. Treatment of herpes simplex virus infection in the rabbit eye with topical valacyclovir appeared promising (Katragadda et al., 2008). Although a 1-year suppression treatment study showed that valacyclovir (500 mg daily) was as effective as acyclovir (400 mg twice daily; Miserocchi et al., 2007), the disposition of the drug in human eye tissues has not been examined.
Cytochromes P450 (CYPs) constitute a superfamily of enzymes that carry out the majority of Phase I oxidative metabolism in mammals. The CYP1, CYP2 and CYP3 families, collectively known as the “drug-metabolizing P450s”, typically oxidize xenobiotics to generate more polar products, a first step in their elimination and detoxification, although some P450 reactions bio-activate pro-carcinogens and generate reactive metabolites that covalently bind DNA and proteins to cause toxicity (Loannides & Lewis, 2004). Zhang et al. (2008) utilized total RNA isolated from various sections of the human eye, liver, small intestine and kidney to measure the transcriptional levels of 10 P450s and 21 efflux or uptake transporters. These workers detected CYP2A6, CYP3A4, CYP2C8, CYP2D6 and CYP2E1 genes in both human cornea and retina/choroid tissues (Figure 3), but the expression levels were low compared to transcript levels measured in human liver. The P450s that were selected for this study were those that are highly expressed in the liver and so are considered to be major drug-metabolizing enzymes. In the following sections, we focus on three P450s: CYP1B1, CYP4V2 and CYP4B1. None of these three are considered to be important drug metabolizing hepatic P450s, but they each have important ocular roles because functionally disrupting mutations in CYP1B1 and CYP4V2 give rise to eye diseases in humans, whereas disruption of CYP4B1 in animal models causes neovascularization problems in the cornea. These effects and their relationship to altered metabolism of endogenous compounds are discussed below.
Ocular CYP4B1 and its role in the defense system
In terms of understanding the biochemical mechanism of self-defense, endobiotic-metabolizing P450s in the eye have been largely overlooked. An exception is ocular CYP4B1, which appears to generate bioactive mediators of oxidative stress in the cornea under hypoxic conditions (Bonazzi et al., 2000; Mastyugin et al., 1999, 2001, 2004; Vafeas et al., 1998). Typical substrates of CYP4 enzymes are endogenous saturated and unsaturated fatty acids, but CYP4B1 also metabolizes numerous xenobiotics (Baer & Rettie, 2006; Hardwick, 2008). CYP4B1, which is predominately expressed in extra-hepatic tissues, such as lung and kidney, bio-activates a range of pro-toxins that often exert tissue-specific toxicological effects (e.g. 4-ipomeanol; Parkinson et al., 2013). The majority of CYP4B1 research has been performed with the recombinantly expressed rabbit enzyme because the expression of the recombinant human CYP4B1 native form (wild-type CYP4B1) has been challenging. In fact, human CYP4B1 expressed in insect cells was inactive, but an S427P mutation introduced into the gene “restored” activity (Zheng et al., 1998). A conserved proline residue at position 427 may be essential for CYP4B1 enzyme activity because proper incorporation of the heme prosthetic group appears to require the presence of an intact meander region Pro-X-Arg motif (Zheng et al., 2003). In the absence of readily available functional human CYP4B1, the closely related rabbit ortholog has been used extensively to probe the function of this enzyme in vitro and in vivo.
The preferred saturated fatty-acid substrates of CYP4B1 have short- to medium-carbon-chain lengths that are preferentially hydroxylated at the thermodynamically unfavored ω-terminus (Fisher et al., 1998). A more physiologically interesting substrate is the ω6 polyunsaturated fatty acid (PUFA), arachidonic acid, from which CYP4B1 appears to generate inflammatory mediators following hypoxic injury. Hypoxia is a low-oxygen condition that occurs when the eyes are closed or when the individual wears contact lenses for long periods. Hypoxic injury initiates the inflammatory response with cyclooxygenases and lipoxygenases generating prostaglandins and leukotrienes. In addition, rabbit CYP4B1 has been reported to generate the very powerful inflammatory mediators 12(R)-hydroxyeicosatetraenoic acid (12(R)-HETE) and 12-hydroxyeicosatrienoic acid (12-HETrE) from arachidonic acid in an NADPH-dependent manner (Conners et al., 1995a,b; Stoltz et al., 1994).
The majority of ocular studies involving 12(R)-HETE and 12-HETrE have been performed in bovine and rabbit cornea epithelium. These studies suggest that CYP4B1 in the corneal epithelium is responsible for generating these bioactive compounds, because an anti-rabbit CYP4B1 antibody inhibited their formation (Mastyugin et al., 1999). In addition, CYP4B1 expression increased in the corneal epithelium during hypoxic injury in vivo, and CYP4B1 expression correlated well with the progression of inflammation in the anterior part of the eye. In terms of its physiological effects, 12(R)-HETE is a potent inhibitor of Na+/K+-ATPase. 12(R)-HETrE is a vasodilator and a chemotactic and angiogenic factor that is generated from both 12(R)-HETE and 12(S)-HETE via an oxidation–keto reduction-generated intermediate (Nishimura et al., 1991; Yamamoto et al., 1994). 12-Lipoxygenase (12-LO) also generates 12-HETE, but only the S-enantiomer, and it is unknown whether either CYP4B1 or 12-LO has a greater influence on the production of 12(R)-HETrE.
Ocular CYP4B1 generates bioactive eicosanoids that are mediators of oxidative stress, at least in animal models, and one symptomatic response to oxidative stress in the cornea is neovascularization. The precise signaling pathway by which 12(R)-HETrE influences corneal neovascularization is uncertain, however, some insights have been derived from the rabbit eye hypoxia model developed by Laniado-Schwartzman and colleagues (Baragatti et al., 2009; Mastyugin et al., 2004; Mezentsev et al., 2005; Seta et al., 2007). These researchers have suggested that vascular endothelial growth factor (VEGF) expression depends on 12(R)-HETrE levels and that CYP4B1 expression parallels VEGF expression in the cornea. Moreover, CYP4B1-dependent VEGF induction was sensitive to the CYP4 inhibitor 17-octadecynoic acid, and CYP4B1 gene knock-down by siRNA negatively influenced VEGF expression (Seta et al., 2007). Collectively, these data prompted the hypothesis that CYP4B1 generates 12(R)-HETE, which activates pathways that induce expression of VEGF to initiate neovascularization. While the extent to which the animal models translate to humans is uncertain, it seems clear that CYP4B1 has a physiological role in the rabbit eye.
Role of CYP1B1 in the ocular genetic disease glaucoma
CYP1B1 localization and disease-associated mutations
It is now well established that CYP1B1 mutations are a risk factor for several types of glaucoma (Vasiliou & Gonzalez, 2008). CYP1B1 is expressed widely in the eye, kidney, spleen, thymus, prostate, lung, ovary, small intestine, colon, uterus, mammary gland and liver (Doshi et al., 2006; Shimada et al., 1996; Stoilov et al., 1998). Ocular CYP1B1 expression is localized at the intra-cytoplasmic segment of the ciliary epithelium, cornea epithelium, corneal keratocytes, Mullar cells and RPE (Bejjani et al., 2002; Doshi et al., 2006), and is highly expressed in the fetal state compared to the adult (Doshi et al., 2006). These data suggest that CYP1B1 may be involved in the early stages of ocular development and likely functions in a tissue- and/or cell-specific manner.
Glaucoma is the second leading cause of blindness in the world, according to the World Health Organization (Kingman, 2004), causing progressively higher intraocular pressures that lead to gradual peripheral vision loss. Currently, more than 2 million Americans over the age of 40 have glaucoma, half of who are not aware they have this condition. Unfortunately, there is no cure for glaucoma, so treatment has been focused on controlling intraocular pressure with drugs and surgery, with early diagnosis especially important for preventing vision loss.
Subtypes of glaucoma are categorized based on the mechanism underlying high intraocular pressure, the most common being primary open-angle glaucoma (POAG) and primary congenital glaucoma (PCG, gene symbol: GLC3), whose population frequency is 1:10,000–20,000 (Chakrabarti et al., 2006). As PCG is an autosomal recessive disease, the disease locus was investigated. Chromosome 2q21, the location of CYP1B1, was found to be genetically linked to PCG, along with two other loci: GLC3B at chromosome 1q36 and GLC3C at chromosome 14q24.3–q31.1 (Sarfarazi et al., 1995). Further studies showed that CYP1B1 is a causative gene in PCG, a modifier gene in POAG (Bar-Yosef et al., 2010; Chakrabarti et al., 2010; Fuse et al., 2010; Hilal et al., 2010; Pasutto et al., 2010), a contributor to juvenile open-angle glaucoma (Bayat et al., 2008; Su et al., 2012) and, most recently, a modifier gene in coloboma/microphthalmia (Prokudin et al., 2013). Finally, the CYP1B1 variant, L432V; CYP1B1*3, is a relatively common gene mutation in the healthy population [17%, n = 929 (Karypidis et al., 2006)] that has been linked to other types of glaucoma, including Peter’s anomaly (Vincent et al., 2002, 2006).
The association of a mutation in the CYP1B1 gene with PCG was first reported in 1997 (Stoilov et al., 1997). In the last 15 years, more than 140 distinct mutations, two thirds of which are mis-sense polymorphisms, have been identified in over 500 PCG patients (Li et al., 2011). Across all populations studied, G61E is the most common mutation identified in PCG patients; other prominent mutations are R368H and R469W and collectively these three coding-region changes account for ~30% of known CYP1B1 mutations in PCG (Li et al., 2011).
CYP1B1 mutants and altered catalytic function
CYP1B1 can bioactivate a wide range of procarcinogenic compounds, such as polycyclic aromatic hydrocarbons, heterocyclic amines and aromatic amines (Guengerich, 2005). Although CYP1B1 expression in the liver is as low as ~1 pmol/mg of microsomal protein (Kim et al., 2004), the study of CYP1B1 as a xenobiotic-metabolizing enzyme has been quite intensive. The role of CYP1B1 in cancer initiation and progression in the kidney and sex organs has been studied extensively in the past few years (Beuten et al., 2008; Divi et al., 2012; Gajjar et al., 2012; Han et al., 2010; Murray et al., 2010). CYP1B1 is also known to metabolize endogenous compounds including melatonin (Ma et al., 2005), steroids, fatty acids and retinoids (see below). Since CYP1B1 null mice exhibited abnormalities in their trabecular meshwork and ocular drainage structures similar to those reported for PCG patients (Libby et al., 2003), a reasonable hypothesis was that mutations in human CYP1B1 disrupted the metabolism of key endogenous substrates for the enzyme (Vasiliou & Gonzalez, 2008).
Steroids
When the Rotterdam study highlighted an association between early menopause and open-angle glaucoma (Hulsman et al., 2001), endogenous steroids became a focus of attention as possible pathogenic CYP1B1 substrates. Wild-type CYP1B1 generates predominantly the 6β- and 16α-hydroxylated metabolites from testosterone and progesterone and the major, 4-hydroxy and 2-hydroxy metabolites of estradiol (Jansson et al., 2001). Early functional studies showed that the CYP1B1 G61E and R469W disease-causing mutants reduced catalytic activity towards each of these substrates by 50–75%, with only modest changes in the regioselectivities for hydroxylation (Jansson et al., 2001). These workers further suggested that the loss of functional activity for the G61E mutation, at least, was due to protein instability.
Arachidonic acid
CYP1B1 hydroxylates arachidonic acid (AA) to produce two series’ of regioisomeric HETEs (70% of products) and EETs (30%; Choudhary et al., 2008). Currently, the physiological role of 5-HETE, one of the major metabolites formed by CYP1B1, is unknown, and a lack of information about other HETE metabolites’ stereochemistry further hampers analysis of their ocular significance. While the stereochemistry of 12-HETE generated by CYP1B1 is unknown, rat hepatic microsomal P450s and human skin preferentially form the 12(R)-HETE stereoisomer (Capdevila et al., 1986; Woollard, 1986). 12(R)-HETE exhibits enantiomer-specific inhibition of Na+/K+-ATPase activity in rabbit cornea (Masferrer et al., 1990). It is known that Na+/K+-ATPase activity regulates corneal transparency via pressure-induced hydration (Stiemke et al., 1991), the inhibition of which might plausibly promote the corneal clouding associated with glaucoma. However, human CYP1B1 has a much higher catalytic efficiency (16.5 mM−1min−1) towards arachidonic acid hydroxylation than mouse CYP1B1 (0.3 mM−1min−1), 12(R)-HETE is neither a major metabolite of either the mouse or rabbit enzymes, nor are the animal orthologs as catalytically efficient at its production as the human form (Choudhary et al., 2004). Therefore, because the Cyp1b1-null mouse recapitulates features of the human congenital disease (Libby et al., 2003), it seems unlikely that arachidonic acid is the endogenous substrate whose metabolism is deranged by CYP1B1 mutations in glaucoma.
CYP1B1 mutants and gene regulation in the retinoic-acid signaling pathway
Retinoic acid (RA), a ligand of the retinoid signaling pathway, regulates gene expression that induces morphogenesis and differentiation during embryogenesis and during development of stem cells in tissues. RA is the oxidized form of retinol (vitamin A) formed via two oxidation reactions catalyzed by retinol dehydrogenases (RDH) and retinal dehydrogenases (RALDH), respectively. However, CYP1B1 also will carry out these two oxidation steps (Choudhary et al., 2004). The catalytic efficiency (Kcat/KM) of RDH enzymes isolated from human liver for the conversion of all-trans retinol to all-trans retinal ranged up to 4.5 μM−1min−1 (Han et al., 1998) and the RALDH2-catalyzed conversion of retinal to retinoic acid was also an efficient process, exhibited a K0.5 of 0.3 μM and a Vmax of 82 nmol/mg protein/min (Paik et al., 2014). By comparison, the catalytic efficiencies for CYP1B1 in the conversion of retinol to retinal and retinal to RA were also high at 8.3 and 90.8 μM−1min−1, respectively. Therefore, CYP1B1 may contribute significantly to RA formation, especially in tissues where RDH and RALDH enzymes are poorly expressed (Chambers et al., 2007).
Schenkman and coworkers analyzed the effect of the relatively common CYP1B1 R368H mutation on metabolism of retinol and retinal (Choudhary et al., 2008). In contrast, to its moderate effect on estradiol metabolism, this mutation greatly reduced (>90%) metabolism of retinal and retinoic acid relative to the wild-type enzyme. Substrate-dependent alterations in catalytic efficiency are well recognized for certain P450 polymorphic variants (Bogni et al., 2005). These workers also found that several other mutations found in PCG either abolished or greatly attenuated CYP1B1 function which could result in a disruption of RA signaling, although more studies are required to elucidate the importance of this pathway in PCG.
In summary, many of the CYP1B1 coding region mutations identified in PCG patients cause a functional deficit, due either to reduced protein stability or intrinsic enzymatic activity (Choudhary et al., 2008; Chavarria-Soley et al., 2008). A complete mechanistic analysis of the functional consequences of the CYP1B1 mutations found worldwide has yet to be performed, although this might be facilitated, in part, by interrogation of the recently solved crystal structure of human CYP1B1 (Wang et al., 2011). Regardless, additional work is needed to identify the physiological substrate(s), if one exists, whose regulation is impacted by deleterious mutations in the CYP1B1 gene.
The orphan P450, CYP4V2, and Bietti’s corneoretinal dystrophy
CYP4V2, a relatively new member of the family of human cytochrome P450 enzymes, has been termed an “orphan” P450 due to its unknown substrate specificity and physiological role (Stark & Guengerich, 2007). Of the 57 functional cytochrome P450 genes that have been identified in the human genome (Nelson et al., 2004), approximately a dozen P450 enzymes can be described as orphans. A partial CYP4V2 gene was first identified and listed as CYP4AH1 in 1998, but a full-length clone of CYP4V2 was not reported until 2003. CYP4V2, along with 11 other enzymes, belongs to the CYP4 family and seven of the CYP4 enzymes metabolize fatty acids. CYP4V2 is located on human chromosome 4, separate from the CYP4ABXZ and CYP4F gene clusters on chromosomes 1 and 19, respectively (Hsu et al., 2007). CYP4V2 has low sequence identity to other CYP4 proteins, ~35% (Rettie & Kelly, 2008), which initially raised the question of whether CYP4V2 should belong to a new P450 family (see “Substrate specificity and tissue localization of CYP4V2” section).
As CYP4V2 gene mutations are closely associated with the development of the ocular disease, Bietti’s corneoretinal crystalline dystrophy (BCD; Li et al., 2004), this orphan P450 is receiving increasing attention from the ophthalmology research community. The following sections will discuss BCD, the physiology of the retina and CYP4V2 tissue localization and substrate specificity.
CYP4V2 mutations in BCD
BCD is a type of retinitis pigmentosa, a group of inherited diseases that cause retinal degeneration. It is progressive in nature, leading to atrophy of the retina, which causes a constriction of the visual field and night blindness similar to that found in glaucoma patients. BCD is an autosomal recessive disease characterized by small, yellow, sparkling crystals scattered throughout the eye (Lin et al., 2005). The Italian ophthalmologist Dr. Gian Battista Bietti first reported three patients exhibiting these symptoms more than 75 years ago (Bietti, 1937), and numerous clinical case reports have been published worldwide (Meyer et al., 2004; Sarraf et al., 2003; Usui et al., 2001; Welch, 1977). The chemical composition of the crystal deposits has not yet been determined, although their appearance has been reported as resembling cholesterol or cholesterol esters in complex lipid inclusions (Wilson et al., 1989).
Clues to the etiology of BCD were practically non-existent until Hejtmancik and colleagues at the National Eye Institute found that BCD was strongly associated with mutations in the CYP4V2 gene (Li et al., 2004). Since the initial 2004 article, many additional mutations of CYP4V2 have been identified, and currently more than 30 are described (Garcia-Garcia et al., 2013; Gekka et al., 2005; Haddad et al., 2012; Jin et al., 2006; Lee et al., 2005; Li et al., 2004; Lin et al., 2005; Mamatha et al., 2011; Manzouri et al., 2012; Nakamura et al., 2006; Rossi et al., 2011, 2013; Shan et al., 2005; Song et al., 2013; Wada et al., 2005; Wang et al., 2012; Xiao et al., 2011; Yokoi et al., 2010, 2011; Zenteno et al., 2008). As reviewed in Kelly et al. (2011), the most common mutation in BCD results in deletion of exon 7. A non-sense mutation, W340X, and a mis-sense mutation, H331P, have also been identified in multiple patient groups. Hejtmancik and colleagues suggested that these (and other) Bietti’s mutations render CYP4V2 non-functional based on homology modeling of CYP4V2 that used an X-ray crystal structure for CYP102A1 (PDB ID: 2IJ2) as a template (Li et al., 2004). We reported recently that the mis-sense mutation, H331P, confers loss of activity to CYP4V2 due likely to protein instability of the mutant (Nakano et al., 2012). The catalytic activity of the other known CYP4V2 coding-region variants remains to be determined.
Physiology and composition of the photoreceptor outer segments in relation to BCD
The retina has the most important function within the eye: capturing information about light and colors to send to the brain. Consequently, disruption of retinal function causes blindness. Within the retina, photoreceptor cells – rods and cones – receive dim light (rods) and colors and brighter light (cones) and relay these signals to adjacent retinal neurons, which eventually lead to the brain via ganglion cells. Photoreceptor cells are either cylindrical (rods) or conical (cones), and are composed of a synaptic neuron, a cell body, an inner segment and an outer segment, the last of which contains rhodopsin-enriched disk membranes.
While the chemical composition of the crystals found in the retina and lymphocytes with BCD is unknown, several research groups have suggested that the crystalline deposits may be due to abnormal lipid metabolism (Kaiser-Kupfer et al., 1994; Lee et al., 2001). The most abundant classes of lipids in these disk membranes are phosphatidyl cholines and phosphatidyl ethanolamines, and the most abundant fatty acids comprising these phospholipids are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1) and docosahexaenoic acid (C22:6, DHA) (Anderson, 1970).
There is a physiological requirement for efficient lipid recycling systems in photoreceptor cells and the RPE to regenerate disk membranes given that rod and cone outer segments are constantly shed, occurring at a rate of 10% per day in primates (Young, 1967, 1971, 1976). The precise mechanisms of outer membrane regulation of the synthesis and disposal of disk membranes are not well understood (Guisto et al., 2000). However, it is known that the tip of the outer segment of photoreceptors is shed, the shed membranes are phagocytized, and lipids from processed membranes enter into RPE cells by endocytosis (Gordon & Bazan, 1993). The lipids are then transferred from RPE cells to photoreceptor inner segments for use in the biosynthesis of new disk membranes. Also, polyunsaturated fatty acids (PUFA), including DHA formed in the liver from precursor ω3 fatty acids such as linolenic acid (C18:3), are taken up by RPE cells and further transferred to photoreceptor inner segments (Scott & Bazan, 1989). In addition, whether mice are fed a DHA-supplemented or DHA-deficient diet, DHA levels in ROS remain constant, whereas the concentration of this PUFA in the liver changes markedly (Nishizawa et al., 2003). These data indicate tight regulation of DHA homeostasis in RPE cells. The DHA-derived compounds, protectin D1, neuroprotectin D1 and resolvin D1, have been identified as anti-inflammatory lipid mediators (Bazan et al., 2010; Mukherjee et al., 2004; Serhan et al., 2004). Therefore, the well-recognized role of CYP4 enzymes in fatty acid metabolism and the suspected abnormal lipid metabolism in BCD raises the possibility that a deficiency in the PUFA-hydroxylase catalytic function of CYP4V2 might play a role in BCD (Kelly et al., 2011).
Substrate specificity and tissue localization of CYP4V2
Despite its low-sequence homology to other CYP4 enzymes, recombinant CYP4V2 expressed in insect cells displays catalytic properties similar to other CYP4 enzymes. First, CYP4V2 was found to be a selective medium-chain-length fatty-acid ω-hydroxylase (Nakano et al., 2009). CYP4V2 also exhibited ω-hydroxylase activity towards eicosapentaenoic acid (C20:5) and DHA, and the rates of ω-hydroxylation were similar to CYP4F2, an established hepatic PUFA hydroxylase (Fer et al., 2008; Nakano et al., 2012). These data demonstrate that CYP4V2 is an efficient ω-hydroxylase of both saturated and unsaturated fatty acids. In addition, like several other CYP4 enzymes (Miyata et al., 2001; Sato et al., 2001; Seki et al., 2005), CYP4V2 activities were potently inhibited by HET0016 at low nanomolar concentrations (Nakano et al., 2012). Since CYP4V2 is a ω-hydroxylase of DHA, a major constituent of ocular membranes (Fliesler & Anderson, 1983), the protein-level localization of CYP4V2 in the eye is a critical element in the puzzle for understanding the role of the enzyme in BCD.
Following the development of a selective polyclonal antibody to purified recombinant CYP4V2, the CYP4V2 protein was localized to the endoplasmic reticulum in ARPE-19 cells and found at a level of ~6 pmol/mg protein (Nakano et al., 2012). In the same studies, the expression of the CYP4V2 protein in paraffin-fixed human ocular tissues from healthy individuals was analyzed by immunohistochemical staining. Strong staining was observed only in RPE cells, with weak staining of ganglion cells and internal and external nuclear layers in the retina and moderate reactivity in corneal epithelial cells. Finally, RT-PCR analysis of mRNA isolated from ARPE-19 cells demonstrated that CYP4V2 is the only CYP4 enzyme that is transcribed and its concentration is similar to that of the established ocular P450, CYP1B1 (Nakano et al., 2012). These localization studies demonstrate that CYP4V2 is expressed in ocular tissues that are the target for BCD and that CYP4V2 is a functional fatty acid ω-hydroxylase in RPE cells. Interestingly, the CYP4V2 gene is expressed ubiquitously in human tissues, including the brain, placenta, lung, liver and kidney (Li et al., 2004; Nakano et al., 2012), yet clinical symptoms of BCD occur only in the eyes.
Conclusions and prospects for future work
Whereas hepatic drug-metabolizing enzymes and transporters have been studied in detail, metabolism and transport in the eye is a developing area. The expression of several ocular P450s and drug transporters have been characterized at the mRNA and protein levels, but much remains to be learned about substrate specificity and the application of this information to the prediction of ocular drug disposition and the rational development of ocular pro-drugs.
A strong case can be made that human CYP4B1 is functionally defective (vide infra), yet the gene persists. An intriguing possibility is that the protein has some other, non-monooxygenase, function. Examples of such pseudoenzymes include the recently discovered iRhoms (Adrain & Freeman, 2012). The recent development of a Cyp4b1-null mouse may aid in the evaluation of other potential non-metabolic functions for CYP4B1 (Parkinson et al., 2013).
Whereas CYP1B1 gene mutations alone does not cause PCG, disruptive mutations of CYP4V2 are the sole cause of BCD. In the last 5 years, significant progress has been made on the expression, purification, substrate specificity and tissue localization of CYP4V2 – the latter two characteristics supporting a key functional role for the enzyme in BCD. However, the exact molecular mechanism(s) underlying CYP4V2 involvement in crystal accumulation and atrophic damage in the eye remain to be elucidated. Early biochemical tracer studies indicated a potential cellular defect in the anabolism of ω-3 PUFAs by BCD patients (Lee et al., 2001). More recently, analysis of total fatty acids in the plasma of BCD patients relative to control subjects suggested a defect in the synthesis of oleic acid from stearic acid, but the link between altered lipid profiles and development of BCD-associated ocular crystals is unclear (Lai et al., 2010). A Cyp4v3 null mouse has been created in our laboratories (Lockhart, submitted for publication) and BCD-like phenotype and lipidomic analyses are underway with this animal model that may shed some light on the biochemical mechanisms that underlie this debilitating disease.
Acknowledgments
This work was supported in part by the National Institutes of Health (Grant GM 49054) and the UW School of Pharmacy’s Drug Metabolism, Transport and Pharmacogenetics Research Fund and by the Center for Ecogenetics and Environmental Health (Grant ES007033).
Abbreviations
- P450
cytochrome P450
- BRB
blood–retina barrier
- RPE
retinal pigmented epithelium
- SLC
solute carrier
- ABC
ATP binding cassette
- ATB0,+
amino-acid transporter B0,+
- PEP
peptide transporter
- OCT
organic cation transporter
- OCTN
organic cation/carnitine transporter
- OAT
organic anion transporter
- MATE
multidrug and toxin exclusion transporter
- OATP
organic anion transporting peptide
- NTCP
Na+-taurocholate cotransporting polypeptide
- MRP
multidrug resistance-associated protein
- EAAC
excitatory amino acid transporter
- TAUT
taurine transporter
- CRT
creatinine transporter
- GAT
GABA transporter
- LAT
large neutral amino acid transporter
- Xc−
Na+-independent glutamate/cysteine exchange transporter
- MCT
monocarboxylate transporter
- RFT
reduced folate transporter
- ENT
equilibrative nucleoside transporter
- CNT
concentrative nucleoside transporter
- BCRP
breast cancer resistance protein
- GLUT
glucose transporter
- ARPE-19
immortalized human retinal pigmented epithelial cells
- SIRC
Statens Seruminstitut rabbit corneal cells
- PDE10A
phosphodiesterase 10A
- PUFA
polyunsaturated fatty acid
- COX
cyclooxygenase
- LO
lipoxygenase
- 12(R)-HETE
12(R)-hydroxyeicosatetraenoic acid
- 12-HETrE
12-hydroxyeicosatrienoic acid
- VEGF
vascular endothelial growth factor
- POAG
primary open-angle glaucoma
- PCG
primary congenital glaucoma
- AA
arachidonic acid
- EET
epoxyeicosatrienoic acid
- RA
retinoic acid
- RDH
retinol dehydrogenase
- RALDH
retinal dehydrogenase
- BCD
Bietti’s corneoretinal crystalline dystrophy
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
Footnotes
Declaration of interest
The authors declare no conflicts of interest.
References
- Adrain C, Freeman M. New lives for old: Evolution of psuedoenzyme function illustrated by iRhoms. Nat Rev Mol Cell Biol. 2012;13:489–498. doi: 10.1038/nrm3392. [DOI] [PubMed] [Google Scholar]
- Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: Targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19:1194–1202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- Anand BS, Katragadda S, Mitra AK. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: Intestinal absorption and liver metabolism. J Pharmacol Exp Ther. 2004;311:659–667. doi: 10.1124/jpet.104.069997. [DOI] [PubMed] [Google Scholar]
- Anderson RE. Lipids of ocular tissues. IV. A comparison of the phospholipids from the retina of six mammalian species. Exp Eye Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- Baer BR, Rettie AE. CYP4B1: An enigmatic P450 at the interface between xenobiotic and endobiotic metabolism. Drug Metab Rev. 2006;38:451–476. doi: 10.1080/03602530600688503. [DOI] [PubMed] [Google Scholar]
- Baragatti B, Schwartzman ML, Angeloni D, et al. EDHF function in the ductus arteriosus: Evidence against involvement of epoxyeicosatrienoic acids and 12S-hydroxyeicosatetraenoic acid. Am J Physiol Heart Circ Physiol. 2009;297:H2161–H2168. doi: 10.1152/ajpheart.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Pal D, Mitra AK. Mitochondrial localization of P-glycoprotein and peptide transporters in corneal epithelial cells – Nocel stratefies for intracellular drug targeting. Exp Eye Res. 2013;106:47–54. doi: 10.1016/j.exer.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Yosef U, Levy J, Elbedour K, et al. Congenital glaucoma: CYP1B1 mutations in Israeli Bedouin kindreds. J Glaucoma. 2010;19:35–38. doi: 10.1097/IJG.0b013e3181a98b6f. [DOI] [PubMed] [Google Scholar]
- Bayat B, Yazdani S, Alavi A, et al. Contributions of MYOC and CYP1B1 mutations to JOAG. Mol Vis. 2008;13:508–517. [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani BA, Xu L, Armstrong D, et al. Expression patterns of cytochrome P4501B1 (Cyp1b1) in FVB/N mouse eyes. Exp Eye Res. 2002;75:249–257. [PubMed] [Google Scholar]
- Beuten J, Gelfond JA, Byrne JJ, et al. CYP1B1 variants are associated with prostate cancer in non-Hispanic and Hispanic Caucasians. Carcinogenesis. 2008;29:1751–1757. doi: 10.1093/carcin/bgm300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bietti G. Ueber familiaeres vorkommen von“retinitis punctata albescens” (verbunden mit”dystrophia marginalis cristallinea cormeae”), glitzern des glaskoerpers und anderen degenerativen augenveraenderungen. Klin Mbl Augenheilk. 1937;99:737–757. [Google Scholar]
- Bogni A, Monshouwer M, Moscone A, et al. Substrate specific metabolism by polymorphic cytochrome P450 2D6 alleles. Toxicol In Vitro. 2005;19:621–629. doi: 10.1016/j.tiv.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bonazzi A, Mastyugin V, Mieyal PA, et al. Regulation of cyclooxygenase-2 by hypoxia and peroxisome proliferators in the corneal epithelium. J Biol Chem. 2000;275:2837–2844. doi: 10.1074/jbc.275.4.2837. [DOI] [PubMed] [Google Scholar]
- Bras AP, Sitar DS, Aoki FY. Comparative bioavailability of acyclovir from oral valacyclovir and acyclovir in patients treated for recurrent genital herpes simplex virus infection. Can J Clin Pharmacol. 2001;8:207–211. [PubMed] [Google Scholar]
- Campbell M, Humphries P. The blood-retina barrier: Tight juctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- Capdevila J, Yadagiri P, Manna S, Falck JR. Absolute configuration of the hydroxyeicosatetraenoic acids (HETEs) formed during catalytic oxygenation of arachidonic acid by microsomal cytochrome P-450. Biochem Biophys Res Commun. 1986;141:1007–1011. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Ghanekar Y, Kaur K, et al. A polymorphism in the CYP1B1 promoter is functionally associated with primary congenital glaucoma. Hum Mol Genet. 2010;19:4083–4090. doi: 10.1093/hmg/ddq309. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Kaur K, Kaur I, et al. Globally, CYP1B1 mutations in primary congenital glaucoma are strongly structured by geographic and haplotype backgrounds. Invest Ophthalmol Vis Sci. 2006;47:43–47. doi: 10.1167/iovs.05-0912. [DOI] [PubMed] [Google Scholar]
- Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryo-genesis by CYP1B1. Development. 2007;134:1369–1383. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- Chavarria-Soley G, Sticht H, Aklillu E, et al. Mutations in CYP1B1 cause primary congenital glaucoma by reduction of either activity or abundance of the enzyme. Hum Mutat. 2008;29:1147–1153. doi: 10.1002/humu.20786. [DOI] [PubMed] [Google Scholar]
- Chen P, Chen H, Zang X, et al. Expression of efflux transporters in human ovular tissues. Drug Metab Dispos. 2013;42:1934–1948. doi: 10.1124/dmd.113.052704. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, et al. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Sarfarazi M, Schenkman JB. Characterization of the biochemical and structural phenotypes of four CYP1B1 mutations observed in individuals with primary congenital glaucoma. Pharmacogenet Genomics. 2008;18:665–676. doi: 10.1097/FPC.0b013e3282ff5a36. [DOI] [PubMed] [Google Scholar]
- Congdon N, O’colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Conners MS, Stoltz RA, Davis KL, et al. A closed eye contact lens model of corneal inflammation. Part 2: Inhibition of cytochrome P450 arachidonic acid metabolism alleviates inflammatory sequelae. Invest Ophthalmol Vis Sci. 1995a;36:841–850. [PubMed] [Google Scholar]
- Conners MS, Stoltz RA, Webb SC, et al. A closed eye contact lens model of corneal inflammation. Part 1: Increased synthesis of cytochrome P450 arachidonic acid metabolites. Invest Ophthalmol Vis Sci. 1995b;36:828–840. [PubMed] [Google Scholar]
- Dahlin A, Geier E, Stocker SL, et al. Gene expression profiling of transporters in the solute carrier and ATP-binding cassette superfamilies in human eye substructures. Mol Pharm. 2013;10:650–663. doi: 10.1021/mp300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Patel J, Anand BS, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002;25:243–252. doi: 10.1076/ceyr.25.4.243.13488. [DOI] [PubMed] [Google Scholar]
- Divi RL, Luch A, Verma M, Mahadevan B. CYP1B1 detection. Curr Protoc Toxicol. 2012;51:4.31.1–4.31.26. doi: 10.1002/0471140856.tx0438s51. [DOI] [PubMed] [Google Scholar]
- Doshi M, Marcus C, Bejjani BA, Edward DP. Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp Eye Res. 2006;82:24–32. doi: 10.1016/j.exer.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106:386–390. doi: 10.1016/j.jconrel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Esilae R. Histochemical and electrophoretic properties of cholinesterases and non-specific esterases in the retina of some mammals, including man. Acta Ophthalmol Suppl. 1963;77:1–113. [PubMed] [Google Scholar]
- Esilae R. Histochemistry of retinal esterases. Acta Ophthalmol (Copenh) 1964;42:435–437. doi: 10.1111/j.1755-3768.1964.tb03634.x. [DOI] [PubMed] [Google Scholar]
- Fer M, Corcos L, Dreano Y, et al. Cytochromes P450 from family 4 are the main omega hydroxylating enzymes in humans: CYP4F3B is the prominent player in PUFA metabolism. J Lipid Res. 2008;49:2379–2389. doi: 10.1194/jlr.M800199-JLR200. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Zheng YM, Rettie AE. Positional specificity of rabbit CYP4B1 for omega-hydroxylation1 of short-medium chain fatty acids and hydrocarbons. Biochem Biophys Res Commun. 1998;248:352–355. doi: 10.1006/bbrc.1998.8842. [DOI] [PubMed] [Google Scholar]
- Fliesler S, Anderson RE. Chemistry and metabolism of lipids in the vertebrate. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fuse N, Miyazawa A, Takahashi K, et al. Mutation spectrum of the CYP1B1 gene for congenital glaucoma in the Japanese population. Jpn J Ophthalmol. 2010;54:1–6. doi: 10.1007/s10384-009-0769-1. [DOI] [PubMed] [Google Scholar]
- Gajjar K, Martin-Hirsch PL, Martin FL. CYP1B1 and hormone-induced cancer. Cancer Lett. 2012;324:13–30. doi: 10.1016/j.canlet.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia GP, Lopez-Garrido MP, Martinez-Rubio M, et al. Genotype-phynotype analysis of Bietti crystalline dystrophy in a family with the CYP4V2 Ile111Thr mutation. Cornea. 2013;32:1002–1008. doi: 10.1097/ICO.0b013e31828a27bc. [DOI] [PubMed] [Google Scholar]
- Gekka T, Hayashi T, Takeuchi T, et al. CYP4V2 mutations in two Japanese patients with Bietti’s crystalline dystrophy. Ophthalmic Res. 2005;37:262–269. doi: 10.1159/000087214. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG. Visualization of [3H]docosahexaenoic acid trafficking through photoreceptors and retinal pigment epithelium by electron microscopic autoradiography. Invest Ophthalmol Vis Sci. 1993;34:2402–2411. [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, et al. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther. 2009;331:574–590. doi: 10.1124/jpet.109.155994. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz De Montellano PR, editor. Cytochrome P450 structure, mechanism, and biochemistry. 3rd ed Kluwer Academic/Plenum Publisher; New York: 2005. pp. 377–530. [Google Scholar]
- Guisto NM, Pasquare SJ, Salvador GA, et al. Lipid metabolism in vertebrate retinal rod outer segments. Prog Lipid Res. 2000;39:315–391. doi: 10.1016/s0163-7827(00)00009-6. [DOI] [PubMed] [Google Scholar]
- Haddad NM, Waked N, Bejjani R, et al. Clinical and molecular finding s in three Lebanese families with Bietti crystalline dystrophy: Report on a noval mutation. Mol Vis. 2012;18:1182–1188. [PMC free article] [PubMed] [Google Scholar]
- Han CL, Liao CS, Wu CW, et al. Contribution to first-pass metabolism of ethanol and inhibition by ethanol for retinol oxidation in human alcohol dehydrogenase family – Implications for etiology of fetal alcohol syndrome and alcohol-related diseases. Eur J Biochem. 1998;254:25–31. doi: 10.1046/j.1432-1327.1998.2540025.x. [DOI] [PubMed] [Google Scholar]
- Han EH, Kim HG, Hwang YP, et al. Prostaglandin E2 induces CYP1B1 expression via ligand-independent activation of the ERalpha pathway in human breast cancer cells. Toxicol Sci. 2010;114:204–216. doi: 10.1093/toxsci/kfq013. [DOI] [PubMed] [Google Scholar]
- Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Hilal L, Boutayeb S, Serrou A, et al. Screening of CYP1B1 and MYOC in Moroccan families with primary congenital glaucoma: Three novel mutations in CYP1B1. Mol Vis. 2010;16:1215–1226. [PMC free article] [PubMed] [Google Scholar]
- Hosoya KI, Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: Establishment of cell lines and transport functions. Biol Pharm Bull. 2005;28:1–8. doi: 10.1248/bpb.28.1. [DOI] [PubMed] [Google Scholar]
- Hosoya KI, Tachikawa M. Inner blood-retinal barrier transporters: Role of retinal drug delivery. Pharm Res. 2009;26:2055–2065. doi: 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. Human cytochrome p450 family 4 enzymes: Function, genetic variation and regulation. Drug Metab Rev. 2007;39:515–538. doi: 10.1080/03602530701468573. [DOI] [PubMed] [Google Scholar]
- Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. J Pharm Sci. 1983;72:1272–1279. doi: 10.1002/jps.2600721109. [DOI] [PubMed] [Google Scholar]
- Hulsman CA, Westendorp IC, Ramrattan RS, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemol. 2001;154:138–144. doi: 10.1093/aje/154.2.138. [DOI] [PubMed] [Google Scholar]
- Hutchins JB. Acetylcholine as a neurotransmitter in the vertebrate retina. Exp Eye Res. 1987;45:1–38. doi: 10.1016/s0014-4835(87)80075-1. [DOI] [PubMed] [Google Scholar]
- Hutchins JB, Hollyfield JG. Acetylcholinesterase in the human retina. Brain Res. 1987;400:300–311. doi: 10.1016/0006-8993(87)90629-9. [DOI] [PubMed] [Google Scholar]
- Jain-Vakkalagadda B, Dey S, Pal D, Mitra AK. Identification and functional characterization of a Na+-independent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest Ophthalmol Vis Sci. 2003;44:2919–2927. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- Jain-Vakkalagadda B, Pal D, Gunda S, et al. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol Pharm. 2004;1:338–346. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Effect of two mutations of human CYP1B1, G61E and R469W, on stability and endogenous steroid substrate metabolism. Pharmacogenetics. 2001;11:793–801. doi: 10.1097/00008571-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Jin ZB, Ito S, Saito Y, et al. Clinical and molecular findings in three Japanese patients with crystalline retinopathy. Jpn J Ophthalmol. 2006;50:426–431. doi: 10.1007/s10384-006-0350-0. [DOI] [PubMed] [Google Scholar]
- Kaiser-Kupfer MI, Chan CC, Markello TC, et al. Clinical biochemical and pathologic correlations in Bietti’s crystalline dystrophy. Am J Ophthalmol. 1994;118:569–582. doi: 10.1016/s0002-9394(14)76572-9. [DOI] [PubMed] [Google Scholar]
- Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007a;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007b;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karypidis AH, Soderstrom T, Nordmark A, et al. Association of cytochrome P450 1B1 polymorphism with first-trimester miscarriage. Fertil Steril. 2006;86:1498–1503. doi: 10.1016/j.fertnstert.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: Evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359:15–24. doi: 10.1016/j.ijpharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EJ, Nakano M, Rohatgi P, et al. Finding homes for orphan cytochrome P450s: CYP4V2 and CYP4F22 in disease states. Mol Interv. 2011;11:124–132. doi: 10.1124/mi.11.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Chu XY, Kim S, et al. Identification of a human valacyclovirase: Biphenyl hydrolase-like protein as valacyclovir hydrolase. J Biol Chem. 2003;278:25348–25356. doi: 10.1074/jbc.M302055200. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sherman ME, Curriero FC, et al. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol Appl Pharmacol. 2004;199:210–219. doi: 10.1016/j.taap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kingman S. In focus: Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888. [PMC free article] [PubMed] [Google Scholar]
- Lai TY, Chu KO, Chan KP, et al. Alterations in serum fatty acid concentrations and desaturase activities in Bietti crystalline dystrophy unaffected by CYP4V2 genotypes. Invest Ophthalmol Vis Sci. 2010;51:1092–1097. doi: 10.1167/iovs.09-3665. [DOI] [PubMed] [Google Scholar]
- Lam KW, Feman SS, Ray GS, Van Heuven WA. A biochemical characterization of the carboxyl esterases in human subretinal fluid – A study comparing the enzymes in serum, in leukocytes, and in subretinal fluid. Exp Eye Res. 1977;24:467–477. doi: 10.1016/0014-4835(77)90268-8. [DOI] [PubMed] [Google Scholar]
- Lee J, Jiao X, Hejtmancik JF, et al. The metabolism of fatty acids in human Bietti crystalline dystrophy. Invest Ophthalmol Vis Sci. 2001;42:1707–1714. [PubMed] [Google Scholar]
- Lee KY, Koh AH, Aung T, et al. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations. Invest Ophthalmol Vis Sci. 2005;46:3812–3816. doi: 10.1167/iovs.05-0378. [DOI] [PubMed] [Google Scholar]
- Lee VH. Esterase activities in adult rabbit eyes. J Pharm Sci. 1983;72:239–244. doi: 10.1002/jps.2600720310. [DOI] [PubMed] [Google Scholar]
- Lee VH, Iimoto DS, Takemoto KA. Subcellular distribution of esterases in the bovine eye. Curr Eye Res. 1982a;2:869–876. doi: 10.3109/02713688209020024. [DOI] [PubMed] [Google Scholar]
- Lee VH, Morimoto KW, Stratford RE., Jr Esterase distribution in the rabbit cornea and its implications in ocular drug bioavailability. Biopharm Drug Dispos. 1982b;3:291–300. doi: 10.1002/bdd.2510030402. [DOI] [PubMed] [Google Scholar]
- Li A, Jiao X, Munier FL, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet. 2004;74:817–826. doi: 10.1086/383228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhou Y, Du L, et al. Overview of cytochrome P450 1B1 gene mutations in patients with primary congenitalglaucoma. Exp Eye Res. 2011;93:572–579. doi: 10.1016/j.exer.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Libby RT, Smith RS, Savinova OV, et al. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science. 2003;299:1578–1581. doi: 10.1126/science.1080095. [DOI] [PubMed] [Google Scholar]
- Lin J, Nishiguchi KM, Nakamura M, et al. [last accessed 16 May 2014];Recessive mutations in the CYP4V2 gene in East Asian and Middle Eastern patients with Bietti crystalline corneoretinal dystrophy. J Med Genet. 2005 doi: 10.1136/jmg.2004.029066. [online] Available from: http://jmg.bmj.com/content/42/6/e38.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loannides C, Lewis DF. Cytochrome P450 in the bioactivation of chemicals. Curr Top Med Chem. 2004;4:1767–1788. doi: 10.2174/1568026043387188. [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- Macha S, Mitra AK, Hughes PH. Ophthalmic drug delivery systems. Marcel Dekker; New York: 1993. [Google Scholar]
- Mamatha G, Umashankar V, Kasinathan N, et al. Moleculat screening of the CYP4V2 gene in Bietti crystalline dystrophy that is associated with choroidal neovascularization. Mol Vis. 2011;17:1970–1977. [PMC free article] [PubMed] [Google Scholar]
- Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Manzouri B, Sergouniotis PI, Robson AG, et al. Bietti crystalline retinopathy: Report of retinal crystal deposition in make adolescent siblings. Arch Ophthalmol. 2012;130:1470–1473. doi: 10.1001/archophthalmol.2012.1567. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Rios AP, Schwartzman ML. Inhibition of renal, cardiac and corneal (Na(+)-K+)ATPase by 12(R)-hydroxyeicosatetraenoic acid. Biochem Pharmacol. 1990;39:1971–1974. doi: 10.1016/0006-2952(90)90617-t. [DOI] [PubMed] [Google Scholar]
- Mastyugin V, Aversa E, Bonazzi A, et al. Hypoxia-induced production of 12-hydroxyeicosanoids in the corneal epithelium: Involvement of a cytochrome P-4504B1 isoform. J Pharmacol Exp Ther. 1999;289:1611–1619. [PubMed] [Google Scholar]
- Mastyugin V, Mosaed S, Bonazzi A, et al. Corneal epithelial VEGF and cytochrome P450 4B1 expression in a rabbit model of closed eye contact lens wear. Curr Eye Res. 2001;23:1–10. doi: 10.1076/ceyr.23.1.1.5422. [DOI] [PubMed] [Google Scholar]
- Mastyugin V, Mezentsev A, Zhang WX, et al. Promoter activity and regulation of the corneal CYP4B1 gene by hypoxia. J Cell Biochem. 2004;91:1218–1238. doi: 10.1002/jcb.20018. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Rodrigues EB, Mennel S, Schmidt JC. Optical coherence tomography in a case of Bietti’s crystalline dystrophy. Acta Ophthalmol Scand. 2004;82:609–612. doi: 10.1111/j.1600-0420.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- Mezentsev A, Mastyugin V, Seta F, et al. Transfection of cytochrome P4504B1 into the cornea increases angiogenic activity of the limbal vessels. J Pharmacol Exp Ther. 2005;315:42–50. doi: 10.1124/jpet.105.088211. [DOI] [PubMed] [Google Scholar]
- Miserocchi E, Modarati G, Galli L, Rama P. Efficacy of valavyvlovir vs acyclovir for the prevention of recurrent herpes simplex virus eye disease: A pilot stydy. Am J Ophthalmol. 2007;144:547–551. doi: 10.1016/j.ajo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Miyata N, Taniguchi K, Seki T, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GI, Patimalla S, Stewart KN, et al. Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010;57:202–211. doi: 10.1111/j.1365-2559.2010.03606.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Lin J, Nishiguchi K, et al. Bietti crystalline corneoretinal dystrophy associated with CYP4V2 gene mutations. Adv Exp Med Biol. 2006;572:49–53. doi: 10.1007/0-387-32442-9_8. [DOI] [PubMed] [Google Scholar]
- Nakano MJ, Kelly EJ, Wiek C, et al. CYP4V2 in Bietti’s crystalline dystrophy: Ocular localization, metabolism of ω3 polyun-saturated fatty acids and functional deficit of the H331P variant. Mol Pharmacol. 2012;82:679–686. doi: 10.1124/mol.112.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase. Drug Metab Dispos. 2009;37:2119–2122. doi: 10.1124/dmd.109.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, et al. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Schwartzman ML, Falck JR, et al. Metabolism of 12(R)-hydroxy-5,8,10,14-eicosatetraenoic acid (12(R)-HETE) in corneal tissues: Formation of novel metabolites. Arch Biochem Biophys. 1991;290:326–335. doi: 10.1016/0003-9861(91)90548-w. [DOI] [PubMed] [Google Scholar]
- Nishizawa C, Wang JY, Sekine S, Saito M. Effect of dietary DHA on DHA levels in retinal rod outer segments in young versus mature rats. Int J Vitam Nutr Res. 2003;73:259–265. doi: 10.1024/0300-9831.73.4.259. [DOI] [PubMed] [Google Scholar]
- Paik J, Haenisch M, Muller CH, et al. [last accessed 16 May 2014];Inhibition of retinoic acid biosynthesis by WIN 18,466 markedly suppresses spermatogenesis and alters retinoid metabolism in mice. J Biol Chem. 2014 doi: 10.1074/jbc.M113.540211. [online] Available from: http://www.jbc.org/content/early/2014/04/07/jbc.M113.540211.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson OT, Liggitt HD, Rettie AE, Kelly EJ. Generation and characterization of a Cyp4b1 null mouse and the role of CYP4B1 in the activation and toxicity of Ipomeanol. Toxicol Sci. 2013;134:243–250. doi: 10.1093/toxsci/kft123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, Chavarria-Soley G, Mardin CY, et al. Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8, CRYGC, PAX6 and CYP1B1. Science. 2010;299:1578–1581. [Google Scholar]
- Prasad B, Evers R, Gupta A, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and p-glycoprotein (ABCB1) protein expression: Quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88. doi: 10.1124/dmd.113.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokudin I, Simons C, Grigg JR, et al. [last accessed 16 May 2014];Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8, CRYGC, PAX6 and CYP1B1. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.268. [online] Available from: http://www.nature.com/ejhg/journal/vaop/ncurrent/pdf/ejhg2013268a.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettie AE, Kelly EJ. The CYP4 family. In: Ioannides C, editor. Cytochome P450: Role in the metabolism and toxicity of drugs and other xenobiotics. Royal Society of Chemistry; Cambridge, UK: 2008. pp. 385–407. [Google Scholar]
- Ross D, Cohen AI, Mcdougal DB., Jr Choline acetyltransferase and acetylcholine esterase activities in normal and biologically fractionated mouse retinas. Invest Ophthalmol. 1975;14:756–761. [PubMed] [Google Scholar]
- Rossi S, Testa F, Li A, et al. An atypicla form of Bietti crystalline dystrophy. Ophthalmic genet. 2011;32:118–121. doi: 10.3109/13816810.2011.559653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Testa F, Li A, et al. Clinical and genetic features in Italian Bietti crystalline dystrophy patients. Br J Ophthalmol. 2013;97:174–179. doi: 10.1136/bjophthalmol-2012-302469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M, Akarsu AN, Hossain A, et al. Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995;30:171–177. doi: 10.1006/geno.1995.9888. [DOI] [PubMed] [Google Scholar]
- Sarraf D, Ceron O, Rasheed K, et al. West African crystalline maculopathy. Arch Ophthalmol. 2003;121:338–342. doi: 10.1001/archopht.121.3.338. [DOI] [PubMed] [Google Scholar]
- Sato M, Ishii T, Kobayashi-Matsunaga Y, et al. Discovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorg Med Chem Lett. 2001;11:2993–2995. doi: 10.1016/s0960-894x(01)00614-x. [DOI] [PubMed] [Google Scholar]
- Schultz C, Breaux J, Schentag J, Morck D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin Exp Optom. 2011;94:212–218. doi: 10.1111/j.1444-0938.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci USA. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Wang MH, Miyata N, Laniado-Schwartzman M. Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N’-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull. 2005;28:1651–1654. doi: 10.1248/bpb.28.1651. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- Seta F, Patil K, Bellner L, et al. Inhibition of VEGF expression and corneal neovascularization by siRNA targeting cytochrome P450 4B1. Prostaglandins Other Lipid Mediat. 2007;84:116–127. doi: 10.1016/j.prostaglandins.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Dong B, Zhao X, et al. Novel mutations in the CYP4V2 gene associated with Bietti crystalline corneoretinal dystrophy. Mol Vis. 2005;11:738–743. [PubMed] [Google Scholar]
- Shichi H, Nebert DW. Genetic differences in drug metabolism associated with ocular toxicity. Environ Health Perspect. 1982;44:107–117. doi: 10.1289/ehp.8244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, et al. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Smith SM, Uslaner JM, Cox CD, et al. The novel phosphodiesterase 10A inhibitor THPP-1 has antipsychotic-like effects in rat and improves cognition in rat and rhesus monkey. Neuropharmacology. 2013;64:215–223. doi: 10.1016/j.neuropharm.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Song Y, Mo G, Yin G. A novel mutation in the CYP4V2 gene in a Chinese patient with Bietti’s crystalline dystrophy. Int Ophthalmol. 2013;33:269–276. doi: 10.1007/s10792-012-9686-2. [DOI] [PubMed] [Google Scholar]
- Stark K, Guengerich FP. Characterization of orphan human cytochromes P450. Drug Metab Rev. 2007;39:627–637. doi: 10.1080/03602530701467708. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Edelhauser HF, Geroski DH. The developing corneal endothelium: Correlation of morphology, hydration and Na/K ATPase pump site density. Curr Eye Res. 1991;10:145–156. doi: 10.3109/02713689109001742. [DOI] [PubMed] [Google Scholar]
- Stoilov I, Akarsu AN, Alozie I, et al. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet. 1998;62:573–584. doi: 10.1086/301764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- Stoltz RA, Conners MS, Dunn MW, Schwartzman ML. Effect of metabolic inhibitors on arachidonic acid metabolism in the corneal epithelium: Evidence for cytochrome P450-mediated reactions. J Ocul Pharmacol. 1994;10:307–317. doi: 10.1089/jop.1994.10.307. [DOI] [PubMed] [Google Scholar]
- Su CC, Liu YF, Li SY, et al. Mutations in the CYP1B1 gene may contribute to juvenile-onset open-angle glaucoma. Eye (Lond) 2012;26:1369–1377. doi: 10.1038/eye.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri F, Yazdani S, Narooie-Nejhad M, et al. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009;116:2101–2109. doi: 10.1016/j.ophtha.2009.04.045. [DOI] [PubMed] [Google Scholar]
- Thrimawithana TR, Young S, Bunt CR, et al. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Turner KR, McFarland W, Kellogg TA, et al. Incidence and prevalence of herpes simplex virus type 2 infection in persons seeking repeat HIV counseling and testing. Sex Transm Dis. 2003;30:331–334. doi: 10.1097/00007435-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Usui T, Tanimoto N, Takagi M, et al. Rod and cone a-waves in three cases of Bietti crystalline chorioretinal dystrophy. Am J Ophthalmol. 2001;13:2395–2402. doi: 10.1016/s0002-9394(01)00963-1. [DOI] [PubMed] [Google Scholar]
- Vafeas C, Mieyal PA, Urbano F, et al. Hypoxia stimulates the synthesis of cytochrome P450-derived inflammatory eicosanoids in rabbit corneal epithelium. J Pharmacol Exp Ther. 1998;287:903–910. [PubMed] [Google Scholar]
- Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–358. doi: 10.1146/annurev.pharmtox.48.061807.154729. [DOI] [PubMed] [Google Scholar]
- Ventrice P, Leporini C, Aloe JF, et al. Anti-vascular endothelial growth factor drugs safety and efficacy in opthalmic diseases. J Pharmacol Pharmacother Suppl. 2013;1:S38–S42. doi: 10.4103/0976-500X.120947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AL, Billingsley G, Buys Y, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Billingsley G, Priston M, et al. Further support of the role of CYP1B1 in patients with Peters anomaly. Mol Vis. 2006;12:506–510. [PubMed] [Google Scholar]
- Wada Y, Itabashi T, Sato H, et al. Screening for mutations in CYP4V2 gene in Japanese patients with Bietti’s crystalline corneoretinal dystrophy. Am J Ophthalmol. 2005;139:894–849. doi: 10.1016/j.ajo.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Wang A, Savas U, Stout CD, Johnson EF. Structural characterization of the complex between alpha-naphthoflavone and human cytochrome P450 1B1. J Biol Chem. 2011;286:5736–5743. doi: 10.1074/jbc.M110.204420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo L, Cai SP, et al. Exome sequencing identifies compound heterozygous mutations in CYP4V2 in a pedigree with retinitis pigmentosa. PLoS One. 2012;7:e33673. doi: 10.1371/journal.pone.0033673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RB. Bietti’s tapetoretinal degeneration with marginal corneal dystrophy crystalline retinopathy. Trans Am Ophthalmol Soc. 1977;75:164–197. [PMC free article] [PubMed] [Google Scholar]
- Wilson DJ, Weleber RG, Klein ML, et al. Bietti’s crystalline dystrophy: A clinicopathologic correlative study. Arch Ophthalmol. 1989;107:213–221. doi: 10.1001/archopht.1989.01070010219026. [DOI] [PubMed] [Google Scholar]
- Woollard PM. Stereochemical difference between 12-hydroxy-5,8,10,14-eicosatetraenoic acid in platelets and psoriatic lesions. Biochem Biophys Res Commun. 1986;136:169–176. doi: 10.1016/0006-291x(86)90891-0. [DOI] [PubMed] [Google Scholar]
- Xiao X, Mai G, Li S, et al. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy. Biochem Biophys Res Commun. 2011;409:181–186. doi: 10.1016/j.bbrc.2011.04.112. [DOI] [PubMed] [Google Scholar]
- Xinming L, Yingde C, Lloyd AW, et al. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Cont Lens Anterior Eye. 2008;31:57–64. doi: 10.1016/j.clae.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura M, Conners MS, et al. Oxidation and keto reduction of 12-hydroxy-5,8,10,14-eicosatetraenoic acids in bovine corneal epithelial microsomes. Biochim Biophys Acta. 1994;1210:217–225. doi: 10.1016/0005-2760(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Yokoi Y, Nakazawa M, Mizukoshi S, et al. Crystal deposits on the lens capsules in Bietti crystalline corneoretinal dystrophy associated with a mutation in the CYP4V2 gene. Acta Ophthalmol. 2010;88:607–609. doi: 10.1111/j.1755-3768.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- Yokoi Y, Sato K, Aoyagi H, et al. A novel compound heterozygous mutation in the CYP4V2 gene in a Japanese patient with Bietti’s crystalline corneoretinal dystrophy. Case Rep Ophthalmol. 2011;2:296–301. doi: 10.1159/000331885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971;34:190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–725. [PubMed] [Google Scholar]
- Zenteno JC, Ayala-Ramirez R, Graue-Wiechers F. Novel CYP4V2 gene mutation in a Mexican patient with Bietti’s crystalline corneoretinal dystrophy. Curr Eye Res. 2008;33:313–318. doi: 10.1080/02713680801983217. [DOI] [PubMed] [Google Scholar]
- Zhang T, Xiang CD, Gale D, et al. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: Implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Fisher MB, Yokotani N, et al. Identification of a meander region proline residue critical for heme binding to cyto-chrome P450: Implications for the catalytic function of human CYP4B1. Biochemistry. 1998;37:12847–12851. doi: 10.1021/bi981280m. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Henne KR, Charmley P, et al. Genotyping and site-directed mutagenesis of a cytochrome P450 meander Pro-X-Arg motif critical to CYP4B1 catalysis. Toxicol Appl Pharmacol. 2003;186:119–126. doi: 10.1016/s0041-008x(02)00028-5. [DOI] [PubMed] [Google Scholar]