Abstract
Objective
To determine whether additional supplementation of tryptophan (Trp) and tyrosine (Tyr) improve serotonin and dopamine metabolism in individuals with phenylketonuria treated with large neutral amino acid (LNAA) tablets.
Study design
Ten adult individuals with phenylketonuria participated in a randomized, double-blind, placebo-controlled cross-over study consisting of three 3-week phases:washout,treatment with LNAA tablets plus supplementation with either Trp and Tyr tablets or placebo, and LNAA tablets plus the alternate supplementation. An overnight protocol to measure blood melatonin, a serotonin metabolite in the pinealocytes, and urine 6-sulfatoxymelatonin and dopamine in first-void urine specimens was conducted after each phase.
Results
Serum melatonin and urine 6-sulfatoxymelatonin and dopamine levels were increased in the LNAA phase (LNAA plus placebo) compared with the washout phase. Serum melatonin and urine 6-sulfatoxymelatonin were not increased in the active phase (LNAA plus Trp + Tyr) compared with the LNAA phase, although plasma Trp:LNAA was increased compared with the LNAA phase. Among 7 subjects with a plasma Trp/LNAA >0.03, a negative correlation between urine 6-sulfatoxymelatonin and plasma phenylalanine levels was observed (r = −0.072). Urine dopamine levels and plasma Tyr:LNAA were increased in the active phase compared with the LNAA phase.
Conclusion
Melatonin levels were not increased with the higher dose of Trp supplementation, but dopamine levels were increased with the higher dose of Tyr supplementation. Serotonin synthesis appears to be suppressed by high phenylalanine levels at the Trp hydroxylase level. (J Pediatr 2014;165:184–89).
The dietary treatment of phenylketonuria (PKU) has been based primarily on restricting phenylalanine (Phe) intake to maintain blood Phe levels within the recommended range. Blood Phe levels of 2–6 mg/dL (120–360 μM) for children age <12 years and <15 mg/dL (<900 μM) for individuals age >12 years are widely recommended.1 Neuropsychological studies of individuals with PKU who are diagnosed early and well controlled have shown a higher prevalence of characteristic deficits, such as decreased executive functioning and internalizing disorders.2,3 Abnormal metabolism of neurotransmitters, particularly serotonin and dopamine, is frequently observed in individuals with PKU and is believed to be involved in these psychological and psychiatric symptoms. A direct correlation between blood Phe level and neurotransmitter levels in the central nervous system (CNS) has not been established. Because melatonin is synthesized from serotonin in the pinealocytes in the CNS, in our previous study4 we measured this metabolite as a biomarker to reflect serotonin synthesis in the CNS.
The transport of tryptophan (Trp) from blood into the pinealocytes is achieved through the monocarboxylate transporter 10 or T-type amino acid transporter 1.5 Both Trp and tyrosine (Tyr) are transported into the brain by a shared large neutral amino acid transporter 1 (LAT1) at the blood-brain barrier, and their uptake is inhibited by high levels of competing large neutral amino acids (LNAAs) such as Phe. Trp concentration is the most important single metabolic determinant in brain serotonin synthesis, because Trp hydroxylase (TPH; EC 1.14.16.4) is unsaturated at the physiological concentration of Trp in the brain.6 In our previous study, we found low 6-sulfatoxymelatonin and dopamine levels in first-void urine in the study subjects with PKU compared with controls.4 When diets were supplemented with LNAA tablets (PheBloc Applied Nutrition, Los Angeles, California), providing 30 mg/kg/day of Trp and 100 mg/kg/day of Tyr, these levels increased and Trp:LNAA and Tyr:LNAA improved, although they were significantly lower than those in controls.4 Although these 2 urine biomarker levels were increased after increases in plasma Trp:LNAA and Tyr:LNAA after supplementation of LNAA tablets, they did not reach the control levels. In this study, additional dosages of Trp and Tyr were provided to individuals with PKU, along with LNAA tablets, to determine whether further increases in 6-sulfatoxymelatonin and dopamine levels, likely indicating improvement in the CNS serotonin and dopamine metabolism, could be seen.
Methods
Ten adult individuals (2 females and 8 males) with classical PKU were enrolled after providing informed consent. The study protocol was approved by the University of Southern California’s Health Science Institutional Review Board. Study subjects were recruited from our outpatient population and ranged in age from 21 to 51 years (mean ± SD, 29.4 ± 9.4 years). The study was conducted in three 3-week phases. After an initial washout phase without use of any medical food products, the subjects were given LNAA tablets during the next two 3-week phases. They were randomly assigned in a double-blind manner to take tablets consisting of either additional Trp and Tyr or placebo during the next 3 weeks, and then cross over to the alternate supplementation for the final 3 weeks. They consumed a regular diet but avoided high-protein foods, and no dietary changes were made throughout the study. The number of LNAA tablets (PheBloc; Applied Nutrition, Cedar Knolls, New Jersey) taken daily, following the manufacturer’s recommendation, was based on the subject’s weight (body weight in kg × 0.5; maximum total daily intake, 45 tablets/day) and provided the following doses of LNAAs: Trp, 30.6 mg/kg/day; Tyr, 98.4 mg/kg/day; histidine, 15.6 mg/kg/day; isoleucine, 15.7 mg/kg/day; leucine, 15.4 mg/kg/day; methionine, 24.8 mg/kg/day; threonine, 16.4 mg/kg/day; valine, 16 mg/ kg/day; and Phe, 0 mg/kg/day. Trp/Tyr tablets and placebo tablets were manufactured by Applied Nutrition for this study. During the active phase, subjects were further supplemented with Trp/Tyr tablets providing an additional 69.4 mg/kg/day of Trp and 101.6 mg/kg/day of Tyr, to receive a total of 100 mg/kg/day of Trp and 200 mg/kg/day of Tyr.
The study subjects stayed overnight at the Clinical Trials Unit at the University of Southern California’s University Hospital at the end of each phase. All subjects received the same protein-controlled meal during the overnight evaluation. Serum melatonin was measured every 2 hours from 7:00 p.m. to 7:00 a.m., and first-void urine specimens were collected at 7:00 a.m. to measure dopamine and 6-sulfatoxymelatonin, to which 80%−90% of melatonin is metabolized and excreted into urine.6 Specimens for plasma amino acid measurements were obtained before dinner at 7:00 p.m. Serum and urine specimens were processed and kept in a freezer (−20°C) until analysis. Serum melatonin and urine 6-sulfatoxymelatonin were measured as described elsewhere,7 and plasma amino acids were measured by high-performance liquid chromatography at the Special Chemistry Laboratory of Children’s Hospital Los Angeles. Urine dopamine was measured by high-performance liquid chromatography at Quest Diagnostics (San Juan Capistrano, California). Blood specimens for serum melatonin measurements were obtained and processed under dim light after 11:00 p.m. to keep the subjects’ eyes from exposure to bright light, which can inhibit melatonin synthesis.
Statistical Analyses
Serial serum blood melatonin levels measured during the overnight stay were summarized as area under the curve (AUC) of melatonin vs time, calculated by the trapezoidal formula, and maximum concentration. Additional outcome measures included levels of Phe, Trp, Tyr, and other amino acids in blood and urine levels of dopamine and 6-sulfatoxymelatonin and the ratios of Phe, Trp, and Tyr to the sum of all LNAAs. Before analysis, study variables were log-transformed as needed to normalize distributions. Comparisons among the 3 phases were done with repeated-measures ANOVA, with post hoc contrasts between the washout and LNAA + placebo (LNAA) phases and between the LNAA and LNAA with Trp/Tyr supplementation (LNAA + TT) phases. Associations between blood Phe and urine 6-sulfatoxymelatonin levels were examined with Pearson correlation analysis. Statistical tests were 2-sided at a significance level of P < .05. Analyses were performed using SAS/STAT version 9 (SAS Institute, Cary, North Carolina).
Results
Two subjects failed to complete the LNAA + TT phase owing to discomfort, including dizziness and nausea, attributed to the Trp/Tyr tablets. For these subjects, Trp/Tyr supplementation was reduced to administer 65 mg/kg/day of Trp and 150 mg/kg/day of Tyr for the remainder of the LNAA + TT phase. One subject failed to complete the LNAA + TT phase because of poor compliance, and 1 subject accidentally took the study tablets just before a blood draw in the LNAA + TT phase, thereby invalidating plasma amino acid analysis. These data were removed from the statistical analysis. All subjects (n = 10) completed the washout and LNAA phases (LNAA and placebo supplement), 6 subjects completed the study following the protocol, and 2 subjects completed the study with a reduced amount of Trp/Tyr supplementation in the LNAA + TT phase.
Plasma Amino Acids, Serum Melatonin, and Urine 6-Sulfatoxymelatonin and Dopamine
In the 6 subjects who completed the study, levels of plasma amino acids, including Phe, Trp, and Tyr, serum melatonin, urine 6-sulfatoxymelatonin, and urine dopamine, were compared among the 3 study phases (washout, LNAA, and LNAA + TT). Phe levels were not statistically different among the 3 phases. Trp levels and Trp:LNAA were not different between the washout phase and the LNAA phase (P = .3628 and .1657, respectively), but were significantly higher in the LNAA + TT phase compared with the LNAA phase (P = .0003 and .0001). Tyr levels were higher in the LNAA phase compared with the washout phase, although not significantly so (P = .0588). Tyr levels were higher in the LNAA + TT phase compared with the LNAA phase (P = .0010). Tyr:LNAA were significantly higher in the LNAA phase compared with the washout phase, as well as in the LNAA + TT phase compared with the LNAA phase (P = .0177 and .0006, respectively).
The AUC of serum melatonin level was significantly higher after the LNAA phase than after washout (P = .0158). There was no statistically significant difference in AUC between the LNAA and LNAA + TT phases (P = .56).
The 6-sulfatoxymelatonin levels in first-void urine were significantly higher after the LNAA phase than after the washout phase (P = .0082). Three weeks of supplementation with Trp/Tyr did not increase urine 6-sulfatoxymelatonin levels compared with LNAA alone (P = .3894). Dopamine levels were significantly higher after the LNAA phase than after the washout phase (P < .0009). Supplementation with Trp/Tyr in addition to LNAA increased urine dopamine levels compared with LNAA alone (P = .0052) (Figure 1; available at www.jpeds.com).
Figure 1.
Urine 6-sulfatoxymelatonin and dopamine in subjects with PKU (n=6).Error bars represent SE.Urine6-sulfatoxymelatonin was significantly increased in the LNAA phase compared with the washout phase (P = .0158); however, there was no significant difference between the LNAA phase and the LNAA + TT phase. Urine dopamine was significantly increased in the LNAA phase compared with the washout phase (P = .0009), and was further increased in the LNAA + TT phase compared with the LNAA phase (P = .0052).
Urine 6-Sulfatoxymelatonin vs Trp/LNAA and Urine Dopamine vs Tyr/LNAA
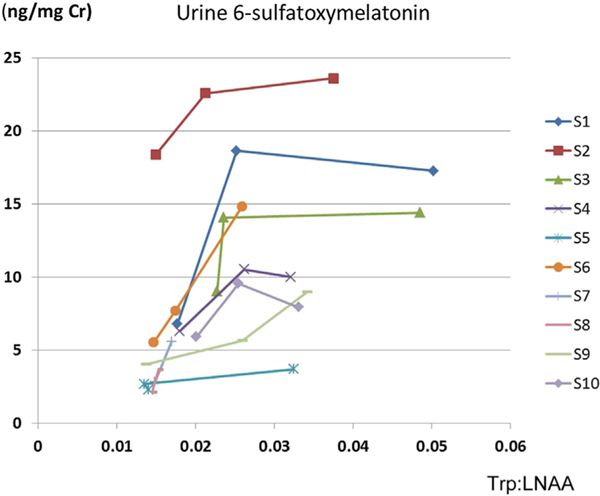
Urine 6-sulfatoxymelatonin levels were increased corresponding to Trp/LNAA up to ~0.03 and plateaued thereafter. Urine dopamine levels were increased with Tyr/LNAA but did not plateau at a certain Tyr/LNAA.
Urine 6-Sulfatoxymelatonin vs Plasma Phe Levels
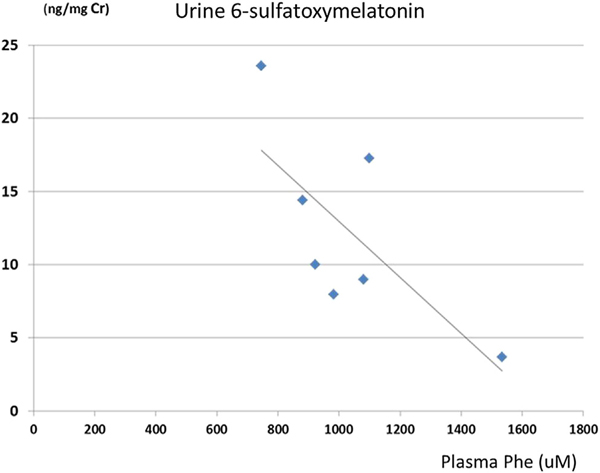
To investigate the roles of plasma Phe levels on the plateauing of urine 6-sulfatoxymelatonin response to increasing Trp/LNAA levels (Figure 2), we analyzed data for subjects with a Trp:LNAA >0.03. Among 8 subjects (6 who completed the full dose of Trp/Tyr supplementation and 2 with reduced-dose Trp/Tyr supplementation), Trp:LNAA was >0.03 in 7 subjects. One subject (S6) showed a continuous increase of urine 6-sulfatoxymelatonin with increasing Trp:LNAA, with the highest Trp/LNAA ratio <0.03. Figure 3 shows a negative correlation between urine 6-sulfatoxymelatonin levels and plasma Phe levels (r = −0.721).
Figure 2.
Urine 6-sulfatoxymelatonin and Trp:LNAA. All 10 study subjects are plotted, 8 of whom completed the LNAA and LNAA + TT phases. In all subjects, Trp:LNAA were increased in the LNAA + TT phase compared with the LNAA phase, although 6-sulfatoxymelatonin did not significantly increase. Subject S6 showed a continuous increase in urine 6-sulfatoxymelatonin with increasing Trp/LNAA (the highest ratio was <0.03). The other 7 subjects had a Trp/LNAA >0.03.
Figure 3.
Urine 6-sulfatoxymelatonin and plasma Phe levels for the 7 subjects with a Trp:LNAA >0.03 in the LNAA + TT phase. Urine 6-sulfatoxymelatonin level was negatively correlated with plasma Phe level (r = −0.72).
Urine Dopamine vs Plasma Phe Levels
In all 10 study subjects, urine dopamine level was not correlated with plasma Phe level in either the washout phase or LNAA phase. No correlation was established in the LNAA + TT phase in the 6 subjects who tolerated the Trp/Tyr supplementation protocol.
Discussion
We recently reported low melatonin levels in the blood during the nighttime and low 6- sulfatoxymelatonin and dopamine levels in first-void urine in individuals with PKU.4 Melatonin, a serotonin metabolite in the pineal body, is released into the bloodstream during the night and subsequently excreted into urine as 6-sulfatoxymelatonin.8 The low melatonin levels, indicating decreased serotonin synthesis in the brain, in individuals with PKU are consistent with previous reports.9,10 Supplementation of LNAA led to increased melatonin and dopamine levels in individuals with PKU, although at levels still significantly lower than those in controls.4 Based on the positive correlation of melatonin levels and Trp:LNAA, as well as of dopamine and Tyr:LNAA, reported previously, we supplied larger doses of Trp and Tyr in the present study, expecting further improvement or even normalization of these levels in the study subjects. The lack of further improvement in melatonin levels in the LNAA + TT phase with significantly increased Trp:LNAA compared with the LNAA phase suggests involvement of a mechanism other than the transporter in melatonin synthesis.
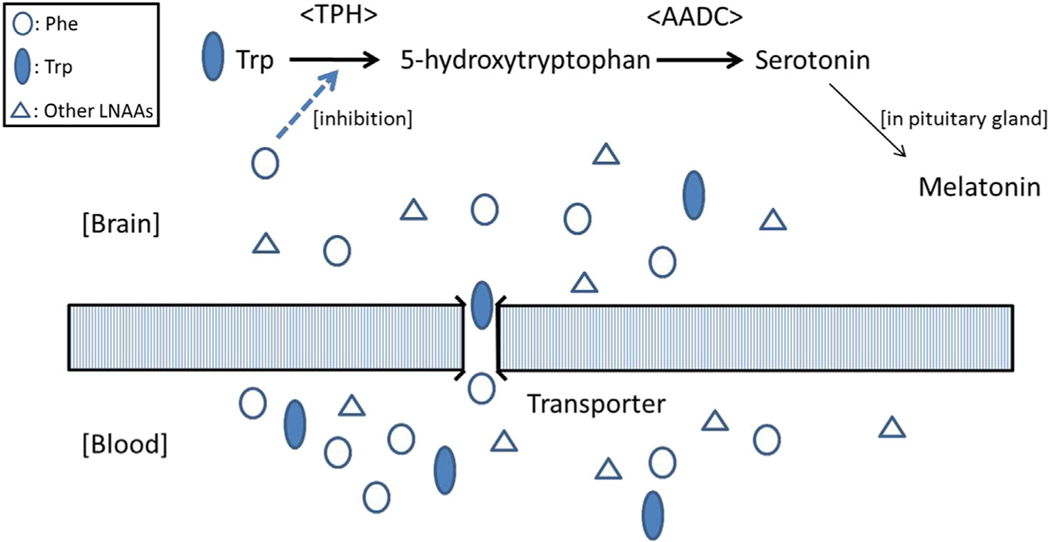
Trp is transported through the LAT1 into the brain, and hydroxylated to 5-hydroxytryptophan by TPH, and subsequently decarboxylated by aromatic L-amino acid decarboxylase (EC 4.1.1.28), forming 5-hydroxytryptamine (serotonin). TPH is the rate-limiting enzyme in the biosynthesis of serotonin.6 Trp uptake from blood into the pinealocytes is thought to be achieved through T-type amino acid transporter 1.5 Because TPH is unsaturated with regard to Trp, serotonin synthesis is thought to be dependent on blood Trp levels6; consequently, Trp:LNAA should be the main determining factor for Trp uptake at the transporter level. Based on the fact that no further improvement was observed in melatonin synthesis in the LNAA + TT phase even though the Trp:LNAA were higher (Table), the Trp:LNAA likely reached saturation levels in transporting Trp into the CNS (Figure 4).
Table.
Phe, Trp, Tyr, Trp:LNAA, Tyr:LNAA, serum melatonin, and urine 16-sulfatoxymelatonin and dopamine levels at the end of each phase
| Variable (n = 6) | Phase | Paired comparison | ||
|---|---|---|---|---|
|
| ||||
| Washout (W) | LNAA (L) | LNAA + TT (T) | ||
|
| ||||
| Phe, μM | 1388.66 ± 362.24 | 1271.50 ± 370.32 | 1148.83 ± 353.53 | L vs W: P = NS; L vsT: P = NS |
| Trp, μM | 32.33 ± 4.07 | 38.17 ± 7.45 | 70.67 ± 18.77 | L vs W: P = NS; L vsT: P = .0003 |
| Tyr, μM | 45.33 ± 7.99 | 81.67 ± 36.09 | 159.67 ± 39.73 | L vs W: P = NS; L vsT: P = .001 |
| Trp:LNAA | 0.0170 ± 0.0030 | 0.0212 ± 0.0045 | 0.0378 ± 0.0089 | L vs W: P = NS; L vsT: P = .0001 |
| Tyr:LNAA | 0.0234 ± 0.0024 | 0.0464 ± 0.0197 | 0.0859 ± 0.0204 | L vs W: P = .0177; L vsT: P = .0006 |
| Serum melatonin AUC | 254.24 ± 143.35 | 361.185 ± 141.13 | 389.12 ± 194.67 | L vs W: P = .0158; L vsT: P = NS |
| Urine 6-sulfatoxymelatonin, ng/mg Cr | 8.05 ± 5.02 | 12.69 ± 6.64 | 13.97 ± 6.13 | L vs W: P = .0082; L vsT: P = NS |
| Urine dopamine, μg/g Cr | 64.33 ± 16.79 | 93.5 ± 20.80 | 116.67 ± 29.17 | L vs W: P = .0009; L vsT: P = .0052 |
Cr, creatinine; NS, not significant.
Data are mean ± SD. P values are from repeated-measures ANOVA for within-group comparisons of subjects with PKU after each phase, with post hoc contrasts between LNAA and washout phases and between active and LNAA phases.
Figure 4.
Trp transporter and melatonin synthesis. Decreased serotonin synthesis in the CNS involves at least 2 steps. The first step is inhibition of transport of the precursor amino acid Trp by other LNAAs through competitive inhibition. The second step is inhibition of TPH by high Phe levels. AADC, aromatic L-amino acid decarboxylase.
Because Phe inhibition on TPH is known,11,12 we studied the correlation of urine 6-sulfatoxymelatonin levels and blood Phe levels to evaluate whether Phe level has any effect on melatonin synthesis. Seven of the 8 subjects who received additional Trp and Tyr in the LNAA + TT phase showed a minimal increase in urine 6-sulfatoxymelatonin despite a significantly increased Trp:LNAA (Figure 2). One subject (S6) had a Trp:LNAA of 0.026 in the LNAA + TT phase and no plateau of 6-sulfatoxymelatonin. To minimize the effects of Trp:LNAA on 6-sulfatoxymelatonin levels, we focused on data with a Trp:LNAA >0.03 (n = 7). Figure 3 shows a negative correlation between urine 6-sulfatoxymelatonin and plasma Phe levels (r = −0.72). Aromatic L-amino acid decarboxylase was reportedly not affected by high Phe levels.13 Low melatonin levels likely directly reflect low serotonin synthesis in the pinealocytes because inhibition by Phe on the two enzymes involved in melatonin synthesis from serotonin (ie, aralkylamine N-acetyltransferase [EC 2.3.1.87] and acetylserotonin O-methyltransferase [EC 2.1.1.4]) is not known.
Humans have 2 forms of TPH, TPH1 and TPH2, which are encoded by 2 different TPH genes, TPH1 and TPH2, located on chromosome 11 and chromosome 12, respectively. These forms have an overall sequence identity of 71%14 and have different kinetic properties and tissue expression. Compared with TPH2, TPH1 has an approximately 3-fold higher Vmax (maximal velocity) values with Trp and 50% lower Km (Michaelis constant) values. TPH2 has a lower affinity for Trp and a more stringent amino acid substrate specificity than TPH1, suggesting a higher substrate preference for Trp. This implies that TPH2 is relatively “protected” against inhibition by high blood Phe levels compared with TPH1. Studies of TPH1 and TPH2 expression in different regions of the human brain in postmortem studies showed the highest TPH1 expression in the hypothalamus and amygdala and the highest TPH2 expression in the raphe nuclei.15 Patel et al16 reported predominant messenger RNA expression of TPH1 and TPH2 in the rat pineal gland and the raphe nuclei, respectively. Decreased serotonin concentrations in various brain regions, including the prefrontal cortex, amygdala, hippocampus, and striatum, have been reported in the PKU mouse model.2
Our previous study showed that dopamine levels in first-void urine were low in individuals with PKU, were increased after supplementation of LNAA including Tyr, and were positively correlated with Tyr:LNAA. Although urine dopamine levels likely reflect neuronal cell metabolism not only in the CNS, but also in other organs, including the kidneys and gastrointestinal tract, they still may represent dopamine metabolism in the CNS, given the similarity of urine dopamine and 6-sulfatoxymelatonin profiles.4 Low dopamine levels in individuals with PKU can be caused by similar inhibitory effects of high blood Phe levels as seen with serotonin synthesis, first at the transporter level and then at the enzyme level, that is, Tyr hydroxylase (TH; EC 1.14.16.2). TH is the rate-limiting enzyme in the synthesis of dopamine,17 and Phe showed mixed-type inhibition of TH (more competitive and less noncompetitive).11 TH has the lowest affinity for Phe among TH, TPH1, and TPH2,11,14 and Phe does not demonstrate substrate inhibition on TH.17 In the present study, in contrast to urine 6-sulfatoxymelatonin, urine dopamine levels were further increased with increasing Tyr:LNAA in the LNAA + TT phase.
The finding of no correlation between urine dopamine level and blood Phe level in any of the 3 study phases is consistent with the fact that TH has the lowest affinity for Phe, suggesting that high blood Phe levels have a minimal effect at the level of TH in dopamine synthesis in individuals with PKU. It seems that inhibition of dopamine synthesis in individuals with PKU is primarily at the level of the LAT1 transporter, which is expressed in the systemic tissues including the CNS.
Decreased cerebrospinal fluid levels of homovanillic acid and 5-hydroxyindoleacetic acid, metabolites of dopamine and serotonin, respectively, were reported in individuals with PKU under dietary treatment.18 Deficiency of dopamine was noted to be less severe compared with serotonin deficiency in the cerebrospinal fluid in individuals with PKU.9,10,13 This likely is related to the catalytic activity of TH on Phe, leading to the synthesis of dopamine.13,19
The dietary treatment of PKU has long been focused on restricting Phe intake and blood Phe levels. It is well known that individuals with PKU who are treated early and well controlled often develop psychological and psychiatric problems, including depression, anxiety disorders, and attention deficit disorder,20 as well as lower executive function including working memory, inhibitory control, and cognitive flexibility.21 It is likely that abnormal neurotransmitter metabolism, including serotonin and dopamine deficiency, is involved in these neuropsychological problems, based on previous reports.22–30
It is also known that some individuals with PKU do well with no dietary treatment and chronic high blood Phe levels above the recommended range. They may have normal or close to normal 6-sulfatoxymelatonin and dopamine levels in first-void urine. We have analyzed 19 adult individuals with PKU and identified 2 with normal intellectual function with a long history of high blood Phe levels who had normal levels of these 2 urine biomarkers. These individuals may have specific polymorphisms in TPH1, leading to lower affinity for Phe. Another possibility is that these individuals may have decreased monoamine oxidase activities, which decrease both serotonin and dopamine degradation. We are currently testing these hypotheses.
Because blood Phe levels cannot be used to evaluate serotonin and dopamine metabolism in the CNS, evaluation of 6-sulfatoxymelatonin and dopamine in first-void urine may provide important information. Serotonin and dopamine metabolism defects are involved in neuropsychological symptoms, and there may be different neurotransmitters involved in PKU. Further studies are needed to establish the relationship between neuropsychological disorders and serotonin and dopamine metabolism in the CNS in individuals with PKU. Establishing reference ranges for various age groups of these 2 biomarkers is essential for evaluating neurotransmitter metabolism along with the Phe level to provide the best clinical management for optimal outcome.
Acknowledgments
Funded by Applied Nutrition and the National Institutes of Health (NIH)/National Center for Research Resources (SC-CTSI UL1 RR031986). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glossary
- AUC
Area under the curve
- CNS
Central nervous system
- LAT1
Large neutral amino acid transporter 1
- LNAA
Large neutral amino acid
- LNAA + TT
Large neutral amino acid with tryptophan/tyrosine supplementation
- Phe
Phenylalanine
- PKU
Phenylketonuria
- TH
Tyrosine hydroxylase
- TPH
Tryptophan hydroxylase
- Trp
Tryptophan
- Tyr
Tyrosine
Footnotes
The authors appreciate Frank Stanczyk, MD (Obstetrics/Gynecology, Keck School of Medicine, University of Southern California), for performing laboratory analysis and Jennifer Doan (Research Assistant, Genetics, Pediatrics, Keck School of Medicine, University of Southern California) for her assistance in the preparation of this manuscript.
The authors declare no conflicts of interest.
References
- 1.National Institutes of Health. Phenylketonuria (PKU): screening and management. October 16–18, 2000. Pediatrics 2001;108:972–82. [DOI] [PubMed] [Google Scholar]
- 2.De Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab 2010;99(Suppl 1):S86–9. [DOI] [PubMed] [Google Scholar]
- 3.Sharman R, Sullivan KA, Young RM, McGill JJ. Tyrosine monitoring in children with early and continuously treated phenylketonuria: results of an international practice survey. J Inherit Metab Dis 2010;33(Suppl 3): S417–20. [DOI] [PubMed] [Google Scholar]
- 4.Yano S, Moseley K, Azen C. Large neutral amino acid supplementation increases melatonin synthesis in phenylketonuria: a new biomarker. J Pediatr 2013;162:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutiérrez CI, Urbina M, Obregion F, Glykys J, Lima L. Characterization of tryptophan high-affinity transport system in pinealocytes of the rat: day-night modulation. Amino Acids 2003;25: 95–105. [DOI] [PubMed] [Google Scholar]
- 6.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science 1971;173:149–52. [DOI] [PubMed] [Google Scholar]
- 7.Hsing AW, Meyer TE, Niwa S, Quraishi SM, Chu LW. Measuring serum melatonin in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2010;19:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chojnacki C, Poplawski T, Klupinska G, Blasiak J, Chojnacki J, Reiter RJ. Secretion of melatonin and 6-sulfatoxymelatonin urinary excretion in functional dyspepsia. World J Gastroenterol 2011;17: 2646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen JB, Lou HC, Gu€ttler F. Effects of diet discontinuation and dietary tryptophan supplementation on neurotransmitter metabolism in phenylketonuria. Brain Dysfunction 1988;1:51–6. [Google Scholar]
- 10.Lykkelund C, Nielsen JB, Lou HC, Rasmussen V, Gerdes AM, Christensen E, et al. Increased neurotransmitter biosynthesis in phenylketonuria induced by phenylalanine restriction or by supplementation of unrestricted diet with large amounts of tyrosine. Eur J Pediatr 1988;148: 238–45. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Ichinose H. Effect of metals and phenylalanine on the activity of human tryptophan hydroxylase-2: comparison with that on tyrosine hydroxylase activity. Neurosci Lett 2006;401:261–5. [DOI] [PubMed] [Google Scholar]
- 12.Kowlessur D, Kaufman S. Cloning and expression of recombinant human pineal tryptophan hydroxylase in Escherichia coli: purification and characterization of the cloned enzyme. Biochim Biophys Acta 1999;1434:317–30. [DOI] [PubMed] [Google Scholar]
- 13.Yuwiler A, Geller E, Slater G. On the mechanism of the brain serotonin depletion in experimental phenylketonuria. J Biol Chem 1965;240: 1170–4. [PubMed] [Google Scholar]
- 14.McKinney J, Knappskog PM, Haavik J. Different properties of the central and peripheral forms of human tryptophan hydroxylase. J Neurochem 2005;92:311–20. [DOI] [PubMed] [Google Scholar]
- 15.Zill P, Büttner A, Eisenmenger W, Möller HJ, Ackenheil M, Bondy B . Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J Psychiatr Res 2007;41:168–73. [DOI] [PubMed] [Google Scholar]
- 16.Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry 2004; 55:428–33. [DOI] [PubMed] [Google Scholar]
- 17.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr 2007;137:1539S–47S. [DOI] [PubMed] [Google Scholar]
- 18.Burlina AB, Bonafé L, Ferrari V, Suppiej A, Zacchello F, Burlina AP. Measurement of neurotransmitter metabolites in the cerebrospinal fluid of phenylketonuric patients under dietary treatment. J Inherit Metab Dis 2000;23:313–6. [DOI] [PubMed] [Google Scholar]
- 19.Katz I, Lloyd T, Kaufman S. Studies on phenylalanine and tyrosine hydroxylation by rat brain tyrosine hydroxylase. Biochim Biophys Acta 1976;445:567–78. [DOI] [PubMed] [Google Scholar]
- 20.Brumm VL, Bilder D, Waisbren SE. Psychiatric symptoms and disorders in phenylketonuria. Mol Genet Metab 2010;99:S59–63. [DOI] [PubMed] [Google Scholar]
- 21.Christ S, Huijbregts SC, de Sonneville LM, White DA. Executive function in early- treated phenylketonuria: profile and underlying mechanisms. Mol Genet Metab 2010;99:S22–32. [DOI] [PubMed] [Google Scholar]
- 22.Sun HS, Tsai HW, Ko HC, Chang FM, Yeh TL. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes Brain Behav 2004;3:328–36. [DOI] [PubMed] [Google Scholar]
- 23.Andre K, Kampman O, Viiki M, Illi A, Setälä-Soikkeli E, Poutanen O, et al. TPH1 A218C polymorphism and temperament in major depression. BMC Psychiatry 2013;13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinney J, Johansson S, Halmoy A, Dramsdahl M, Winge I, Knappskog PM, et al. A loss-of-function mutation in tryptophan hydroxylase 2 segregating with attention- deficit/hyperactivity disorder. Mol Psychiatry 2008;13:365–7. [DOI] [PubMed] [Google Scholar]
- 25.Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, et al. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. J Child Psychol Psychiatry 2009;50:317–25. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase2 identified in unipolar major depression. Neuron 2005;45:11–6. [DOI] [PubMed] [Google Scholar]
- 27.Tsai SJ, Hong CJ, Liou YJ, Yu YW, Chen TJ, Hou SJ, et al. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:637–41. [DOI] [PubMed] [Google Scholar]
- 28.Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry 2004;9:1030–6. [DOI] [PubMed] [Google Scholar]
- 29.Posner J, Gorman D, Nagel B. Tyrosine supplements for ADHD symptoms with comorbid phenylketonuria. J Neuropsychatry Clin Neurosci 2009;21:229–30. [DOI] [PubMed] [Google Scholar]
- 30.Diamond A. Evidence for the importance of dopamine for prefrontal cortex functions early in life. Philos Trans R Soc Lond B Biol Sci 1996; 351:1483–94. [DOI] [PubMed] [Google Scholar]