Abstract
Objective
Traditional predictors suboptimally predict cardiovascular disease (CVD) in individuals with chronic kidney disease (CKD). This study compared five non-traditional cardiac and kidney markers regarding the improvement of cardiovascular prediction among those with CKD.
Approach and Results
Among 8,622 participants aged 52–75 years in the ARIC Study, cardiac troponin T [cTnT], N-terminal pro-B-type natriuretic peptide [NT-proBNP], cystatin C, β2-microglobulin, and β-trace protein were compared for improvement in predicting incident CVD after stratifying by CKD status (940 participants with CKD [kidney dysfunction or albuminuria]). During a median follow-up of 11.9 years, there were 1,672 CVD events including coronary disease, stroke, and heart failure (336 cases in CKD). Every marker was independently associated with incident CVD in participants with and without CKD. The adjusted HRs (per 1 SD) were larger for cardiac markers than kidney markers, particularly in CKD (1.61 [95% CI, 1.43–1.81] for cTnT, 1.50 [1.34–1.68] for NT-proBNP, and <1.26 for kidney markers). Particularly in CKD group, cardiac markers compared to kidney markers contributed to greater c-statistic increment (0.032–0.036 vs. 0.012–0.015 from 0.679 with only conventional predictors in CKD and 0.008–0.011 vs. 0.002–0.010 from 0.697 in non-CKD) and categorical net reclassification improvement (0.086–0.127 vs. 0.020–0.066 in CKD and 0.057–0.077 vs. 0.014–0.048 in non-CKD). The superiority of cardiac markers was largely consistent in individual CVD outcomes.
Conclusions
A greater improvement in cardiovascular prediction was observed for cardiac markers than kidney markers in persons with CKD. These results suggest that cTnT and NT-proBNP are useful for better CVD risk classification in this population.
Keywords: Chronic kidney disease, cardiovascular disease, cardiovascular disease risk factors, risk prediction, biomarkers
Chronic kidney disease (CKD), defined as presence of kidney dysfunction or damage, is a major public health problem due to its prevalence of 10–15% in many parts of the world and its contribution to adverse outcomes.1, 2 The prevention of cardiovascular disease (CVD) is crucial, particularly in individuals with CKD, as they are more likely to die of CVD than to reach dialysis or die due to kidney failure.2 A recent report suggests that CKD is a health condition equivalent to coronary heart disease (CHD) in terms of high mortality risk.3
Nevertheless, individuals with CKD show substantial variation in CVD risk.1, 2 However, neither conventional CVD risk factors4 nor conventional CKD measures capture the CVD risk gradient effectively,5, 6 resulting in controversies as to how to classify CVD risk in persons with CKD. 7, 8 Several studies demonstrate that non-traditional biomarkers of cardiac and kidney dysfunction or damage can improve risk classification in this population.9, 10 However, it is unclear whether either of cardiac or kidney markers contributes more to improvement in CVD prediction in persons with CKD. Thus, we compared several non-traditional cardiac and kidney markers regarding their improvement in CVD risk prediction among individuals with and without CKD.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Out of 8,622 participants, 10.9% (n=940) had CKD with eGFRcre <60 ml/min/1.73m2 or ACR ≥ 30 mg/g. Those with CKD generally had a worse CVD risk profile (e.g., older age, higher prevalence of hypertension and diabetes) as compared to those without (Table 1). As compared to those without CKD, individuals with CKD stage 1–2 were likely to be current smokers, but there were fewer current smokers in CKD stage 3–5. The proportion of blacks was higher in CKD stage 1–2 as compared to non-CKD or CKD stage 3–5. The median value for every cardiac and kidney marker was higher in those with CKD compared to those without.
Table 1.
Baseline characteristics according to CKD status
| Non-CKD | CKD stage 1–2 | CKD stage 3–5 | |
|---|---|---|---|
| N | 7682 | 464 | 476 |
| Age, year | 62 (6) | 63 (6) | 66 (5) |
| Female, % | 59 | 58 | 61 |
| White, % | 80 | 64 | 80 |
| Current smoker, % | 14 | 22 | 11 |
| Hypertension, % | 40 | 70 | 65 |
| Antihypertensive drugs, % | 34 | 56 | 64 |
| Renin-angiotensin system inhibitors, % | 10 | 20 | 23 |
| Beta blockers, % | 8 | 13 | 18 |
| Diuretics, % | 14 | 26 | 33 |
| Calcium channel blockers, % | 9 | 22 | 19 |
| Alpha blockers, % | 4 | 5 | 6 |
| Systolic blood pressure, mmHg | 126 (18) | 141 (23) | 131 (21) |
| Diastolic blood pressure, mmHg | 71 (10) | 75 (12) | 70 (11) |
| Lipid lowering medication, % | 10 | 14 | 18 |
| Statins, % | 8 | 11 | 13 |
| Total cholesterol, mmol/L | 5.2 (0.9) | 5.2 (1.1) | 5.4 (1.0) |
| HDL cholesterol, mmol/L | 1.3 (0.4) | 1.3 (0.5) | 1.2 (0.4) |
| Diabetes, % | 13 | 38 | 20 |
| Fasting glucose, mmol/L | 5.9 (1.8) | 7.4 (3.4) | 6.2 (2.1) |
| Body mass index, kg/m2 | 28 (5) | 29 (6) | 29 (5) |
| eGFRcre, ml/min/1.73m2 | 87 (12) | 87 (15) | 52 (9) |
| ACR (IQR), mg/g | 3.4 (1.6–6.2) | 66.5 (43.4–148.2) | 4.5 (2.0–18.3) |
| cystatin C (IQR), mg/dL | 0.77 (0.69–0.86) | 0.80 (0.71–0.91) | 1.06 (0.92–1.28) |
| B2M (IQR), mg/dL | 0.19 (0.17–0.22) | 0.20 (0.17–0.24) | 0.28 (0.23–0.34) |
| BTP (IQR), mg/dL | 0.65 (0.57–0.74) | 0.68 (0.59–0.80) | 0.87 (0.77–1.06) |
| cTnT (IQR), ng/L | 4.0 (1.5–7.0) | 6.0 (3.0–11.0) | 7.0 (4.0–12.0) |
| NT-proBNP (IQR), pg/mL | 61 (30–113) | 81 (44–168) | 110 (53–218) |
| hsCRP (IQR), mg/L | 2.2 (1.0–5.1) | 3.1 (1.4–7.1) | 3.3 (1.2–6.5) |
CKD: chronic kidney disease, HDL: high-density lipoprotein, eGFR: estimated glomerular filtration rate, ACR: urinary albumin-to-creatinine ratio, B2M: β2 microglobulin, BTP: β-trace protein, cTnT: cardiac troponin T, NT-proBNP: N-terminal pro–B-type natriuretic peptide, hsCRP: high-sensitivity C-reactive protein.
CKD stage 3–5 and 1–2 was defined as eGFRcre <60 ml/min/1.73m2 and ACR ≥ 30 mg/g + eGFRcre ≥ 60, respectively.
All variables differed across the groups with a p-value <0.0001 except for % Female (p=0.635) and HDL-C (p=0.0007)
Values are mean (SD), %, or median (IQR)
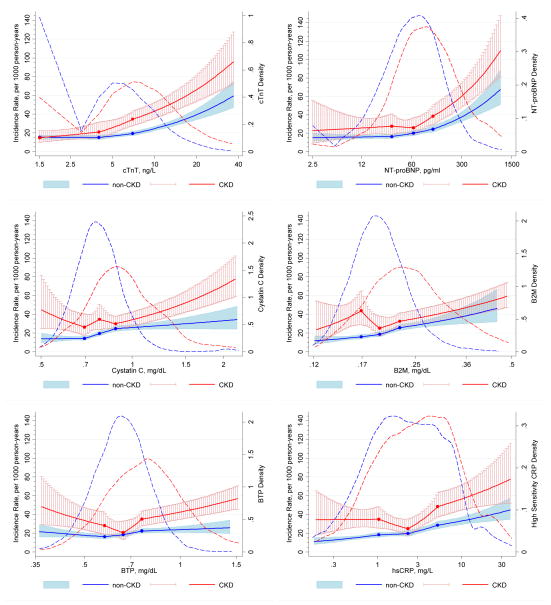
During a median follow-up of 11.9 years, there were 1,672 CVD events (336 events in CKD) (872 CHD cases, 310 stroke cases, and 526 HF cases with 36 concurrent cases of two subtypes [mostly CHD and HF]). Cumulative incidences of composite CVD and each individual CVD outcome are shown in Figure I. The overall incidence rate of CVD was 18.1 per 1,000 person-years (38.0 per 1,000 person-years in CKD and 16.0 per 1,000 person-years in non-CKD). Those with CKD had higher incidence rate of CVD than those without, as the red line located above the blue line at any given level of non-traditional markers (Figure). Although there was generally a graded positive association between each marker and CVD risk, the risk gradient tended to be steeper for cardiac markers than for kidney or inflammatory markers, regardless of CKD status.
Figure.
Adjusted incidence rate of CVD in those with and without CKD according to novel cardiac and kidney markers and their distributions. Adjusted incidence rate of CVD in those with and without CKD according to cardiac and kidney markers and their distributions. The solid lines (red for CKD and blue for non-CKD) show estimated incidence rates of CVD (per 1,000 person-years) and 95% CIs (whiskers and shaded area) with spline (knots at thresholds defining quartiles) for (A) cTnT, (B) NT-proBNP, (C) cystatin C, (D) B2M, (E) BTP, and (F) hsCRP. The incidence rate was adjusted to mean age, men, and whites, and the plot was truncated at 0.5th and 99.5th percentile of each marker. The dash lines (red for CKD and blue for non-CKD) show the kernel density estimate for each marker.
When we further adjusted for other conventional factors, HRs of CVD per 1 SD increment were larger for cardiac markers than for kidney markers, particularly in CKD population (Table 2). The results were much the same when we specifically adjusted for renin-angiotensin inhibitors and beta blockers or further adjusted for the use of statin (data not shown). Further adjustment for the interaction of these non-traditional markers with CKD status on CVD risk was significant for cTnT (p=0.019) and B2M (p=0.008). Among CVD types, the HRs for both cardiac markers were highest for HF followed by CHD. Similar results were observed when CHD cases based only on coronary revascularization and hard CHD cases of myocardial infarction and fatal CHD were analyzed separately (data not shown). The associations of cystatin C and B2M with CVD outcomes were similar to or slightly stronger than that of hsCRP in persons with CKD. B2M demonstrated similar HRs of CVD outcomes as the cardiac markers in those without CKD. The HRs for cTnT and NT-proBNP remained significant for composite CVD even when they were simultaneously included in the model along with conventional factors (data not shown). The higher HRs of CVD for cardiac markers compared to kidney markers in CKD were similarly observed, when every marker was modeled by its quartiles (Table I).
Table 2.
Adjusted hazard ratio (95% CI) of CVD for cardiac, kidney, and conventional predictors among participants with and without CKD
| Risk Factors | CVD | CHD | Stroke | HF | ||||
|---|---|---|---|---|---|---|---|---|
| CKD 336 events HR (95%CI) |
Non-CKD1,336 events HR (95%CI) |
CKD 164 events HR (95%CI) |
Non-CKD 802 events HR (95%CI) |
CKD 94 events HR (95%CI) |
Non-CKD 277 events HR (95%CI) |
CKD 200 events HR (95%CI) |
Non-CKD 538 events HR (95%CI) |
|
| cTnT | 1.61 (1.43–1.81) | 1.32 (1.24–1.40) | 1.44 (1.22–1.69) | 1.27 (1.17–1.38) | 1.22 (0.99–1.51) | 1.21 (1.06–1.39) | 1.91 (1.64–2.23) | 1.59 (1.44–1.76) |
| NT-proBNP | 1.50 (1.34–1.68) | 1.40 (1.32–1.50) | 1.43 (1.23–1.67) | 1.26 (1.16–1.36) | 1.31 (1.07–1.61) | 1.58 (1.37–1.82) | 1.68 (1.45–1.94) | 1.77 (1.60–1.95) |
| Cystatin C | 1.25 (1.16–1.34) | 1.22 (1.15–1.29) | 1.19 (1.08–1.32) | 1.15 (1.06–1.24) | 1.16 (1.01–1.33) | 1.18 (1.03–1.34) | 1.36 (1.25–1.48) | 1.33 (1.23–1.44) |
| B2M | 1.22 (1.16–1.29) | 1.35 (1.27–1.44) | 1.15 (1.06–1.25) | 1.24 (1.13–1.35) | 1.16 (1.04–1.30) | 1.30 (1.13–1.49) | 1.33 (1.25–1.42) | 1.51 (1.38–1.66) |
| BTP | 1.21 (1.13–1.28) | 1.13 (1.07–1.21) | 1.15 (1.06–1.26) | 1.16 (1.08–1.26) | 1.18 (1.05–1.32) | 1.04 (0.91–1.20) | 1.32 (1.23–1.42) | 1.17 (1.07–1.29) |
| hsCRP | 1.22 (1.09–1.36) | 1.25 (1.18–1.33) | 1.04 (0.89–1.22) | 1.22 (1.13–1.32) | 1.13 (0.93–1.38) | 1.18 (1.04–1.34) | 1.36 (1.18–1.57) | 1.33 (1.21–1.45) |
cTnT: cardiac troponin T, NT-proBNP: N-terminal pro–B-type natriuretic peptide, B2M: β2 microglobulin, BTP: β-trace protein, hsCRP: high-sensitivity C-reactive protein.
1 SD for log of the continuous variables: cTnT (0.84), NT-proBNP (1.12), cystatin C (0.23), B2M (0.24), BTP (0.24), hsCRP (1.11). HRs shown above correspond to a multiplicative change for an exponential function of base e with an exponent of the respective 1SD for each non-traditional predictor (e1SD). For example, the HR shown in the row of cTnT above is for 2.3 fold (=e0.84) higher cTnT.
In the CKD group, c-statistics for composite CVD significantly improved, from a base of 0.679 with only conventional predictors, for 0.036 (95% CI, 0.016–0.056) with added NT-proBNP, 0.032 (0.009–0.054) with cTnT, and 0.015 (0.001–0.026) with B2M (Table 3). In this group, the categorical NRI for CVD was significant for both cardiac markers (0.127 [95% CI, 0.046–0.206] for cTnT and 0.086 [0.010–0.153] for NT-proBNP) but only for cystatin C among kidney markers (0.066 [0.005–0.130]) (Table 4). hsCRP did not contribute to significant difference in c-statistic or categorical NRI for CVD in CKD (Tables 3 and 4). The contribution of both cardiac markers to better CVD risk discrimination was largely consistent between CKD stage 1–2 and 3–5, whereas that of kidney markers was more evident in stage 3–5 than in stage 1–2 (Table II), although confidence intervals were wide due to small sample size in each stage. The prediction improvement by cTnT and NT-proBNP was consistent even when eGFRcre was added in the base model (Tables III and IV) and was further enhanced when these cardiac markers were taken into account together (Tables 3 and 4). Consistent, but more evident, improvement was observed for continuous NRI and IDI (Tables V and VI). When we directly compared cTnT or NT-proBNP with every kidney marker for CVD prediction, there was at least one statistic demonstrating significantly better prediction by cardiac markers in CKD (Table 5).
Table 3.
Change in C-statistic for CVD outcomes by adding non-traditional predictors
| CVD | CHD | Stroke | HF | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictors | CKD† | Non-CKD | CKD† | Non-CKD | CKD† | Non-CKD | CKD† | Non-CKD |
| Cardiac markers | ||||||||
| cTnT | 0.032 (0.009–0.054) | 0.008 (0.002–0.014) | 0.023 (−0.004–0.050) | 0.005 (−0.001–0.011) | 0.008 (−0.007–0.023) | 0.006 (−0.002–0.014) | 0.053 (0.020–0.085) | 0.020 (0.007–0.034) |
| NT-proBNP | 0.036 (0.016–0.056) | 0.011 (0.004–0.018) | 0.022 (−0.004–0.049) | 0.004 (−0.001–0.009) | 0.027 (0.004–0.049) | 0.019 (0.0003–0.037) | 0.049 (0.021–0.078) | 0.029 (0.013–0.044) |
| Two combined | 0.045 (0.020–0.069) | 0.015 (0.007–0.024) | 0.033 (0.003–0.063) | 0.008 (0.001–0.015) | 0.026 (0.004–0.049) | 0.020 (0.002–0.039) | 0.067 (0.032–0.103) | 0.041 (0.023–0.058) |
| Kidney markers | ||||||||
| Cystatin C | 0.013 (−0.002–0.028) | 0.005 (0.001–0.010) | 0.011 (−0.006–0.027) | 0.001 (−0.002–0.004) | 0.000 (−0.017–0.017) | 0.005 (−0.002–0.011) | 0.027 (0.006–0.049) | 0.015 (0.006–0.023) |
| B2M | 0.015 (0.001–0.030) | 0.010 (0.004–0.016) | 0.010 (−0.005–0.024) | 0.003 (−0.001–0.008) | −0.003 (−0.021–0.015) | 0.010 (0.001–0.020) | 0.035 (0.013–0.057) | 0.019 (0.007–0.031) |
| BTP | 0.012 (−0.001–0.026) | 0.002 (−0.001–0.004) | 0.008 (−0.006–0.023) | 0.002 (−0.001–0.005) | 0.006 (−0.012–0.023) | −0.0008 (−0.003–0.001) | 0.027 (0.005–0.049) | 0.001 (−0.004–0.006) |
| Three combined | 0.015 (0.001–0.030) | 0.010 (0.004–0.017) | 0.011 (−0.005–0.027) | 0.004 (−0.001–0.008) | 0.004 (−0.012–0.020) | 0.014 (0.004–0.025) | 0.035 (0.013–0.056) | 0.022 (0.009–0.035) |
| Inflammatory marker | ||||||||
| hsCRP | 0.003 (−0.007–0.014) | 0.007 (0.002–0.012) | −0.001 (−0.005–0.002) | 0.006 (0.002–0.011) | −0.000 (−0.010–0.010) | 0.003 (−0.005–0.011) | 0.018 (−0.001–0.036) | 0.010 (0.000–0.019) |
cTnT: cardiac troponin T, NT-proBNP: N-terminal pro–B-type natriuretic peptide, B2M: β2 microglobulin, BTP: β-trace protein, hsCRP: high-sensitivity C-reactive protein.
CKD was defined as eGFRcre <60 ml/min/1.73m2 or ACR ≥ 30 mg/g.
C-statistic with only conventional predictors was 0.679 and 0.697 for CVD, 0.673 and 0.721 for CHD, 0.733 and 0.712 for Stroke, 0.702 and 0.718 for HF, in CKD and in non-CKD, respectively.
Table 4.
Categorical NRI for CVD outcomes by adding non-traditional predictors
| CVD | CHD | Stroke | HF | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictors | CKD† | Non-CKD | CKD† | Non-CKD | CKD† | Non-CKD | CKD† | Non-CKD |
| Cardiac markers | ||||||||
| cTnT | 0.127 (0.046–0.206) | 0.057 (0.033–0.080) | 0.094 (−0.000–0.203) | 0.026 (−0.007–0.059) | 0.025 (−0.068–0.135) | NA | 0.168 (0.063–0.264) | 0.116 (0.074–0.166) |
| NT-proBNP | 0.086 (0.010–0.153) | 0.077 (0.049–0.105) | 0.008 (−0.094–0.107) | 0.039 (0.006–0.069) | −0.030 (−0.125–0.072) | 0.078 (0.024–0.129) | 0.124 (0.026–0.224) | 0.121 (0.070–0.176) |
| Two combined | 0.136 (0.047–0.218) | 0.098 (0.068–0.130) | 0.060 (−0.043–0.161) | 0.029 (−0.005–0.066) | 0.035 (−0.076–0.129) | 0.100 (0.048–0.152) | 0.237 (0.124–0.347) | 0.177 (0.123–0.230) |
| Kidney markers | ||||||||
| Cystatin C | 0.066 (0.005–0.130) | 0.026 (0.008–0.045) | −0.028 (−0.116–0.049) | 0.019 (−0.004–0.040) | −0.000 (−0.069–0.081) | NA | 0.130 (0.034–0.218) | 0.012 (−0.021–0.043) |
| B2M | 0.032 (−0.026–0.094) | 0.048 (0.024–0.072) | −0.024 (−0.111–0.044) | 0.023 (−0.005–0.052) | 0.003 (−0.083–0.093) | 0.012 (−0.006–0.041) | 0.117 (0.021–0.207) | 0.067 (0.020–0.115) |
| BTP | 0.020 (−0.046–0.080) | 0.014 (−0.000–0.030) | 0.016 (−0.063–0.084) | 0.015 (−0.009–0.039) | 0.008 (−0.073–0.099) | NA | 0.093 (0.011–0.182) | 0.021 (−0.004–0.047) |
| Three combined | 0.034 (−0.023–0.092) | 0.050 (0.024–0.075) | −0.027 (−0.116–0.048) | 0.028 (0.001–0.058) | 0.008 (−0.071–0.083) | 0.012 (−0.007–0.039) | 0.114 (0.024–0.204) | 0.077 (0.035–0.129) |
| Inflammatory marker | ||||||||
| hsCRP | −0.019 (−0.080–0.035) | 0.035 (0.010–0.057) | −0.029 (−0.070--0.007) | 0.035 (0.008–0.064) | −0.001 (−0.079–0.074) | NA | 0.016 (−0.072–0.107) | 0.057 (0.015–0.099) |
cTnT: cardiac troponin T, NT-proBNP: N-terminal pro–B-type natriuretic peptide, B2M: β2 microglobulin, BTP: β-trace protein, hsCRP: high-sensitivity C-reactive protein.
CKD was defined as eGFRcre <60 ml/min/1.73m2 or ACR ≥ 30 mg/g.
NA: NRI was not available due to no reclassification to higher or lower risk categories in some bootstrap samples.
Table 5.
Comparison of a model with a cardiac marker plus traditional predictors and another model with a kidney marker plus traditional predictors for CVD
| Model with kidney marker | Model with cardiac markers
|
|||
|---|---|---|---|---|
| CKD† | non-CKD | |||
|
| ||||
| cTnT | NT-proBNP | cTnT | NT-proBNP | |
| Cystatin C | ||||
| Δc-statistic | 0.019 (−0.003, 0.040) | 0.023 (0.003, 0.043) | 0.002 (−0.004, 0.009) | 0.005 (−0.002, 0.013) |
| Categorical NRI | 0.052 (−0.025, 0.133) | 0.016 (−0.059, 0.081) | 0.034 (0.007, 0.060) | 0.052 (0.021, 0.080) |
| Continuous NRI | 0.235 (0.092, 0.370) | 0.039 (−0.097, 0.186) | 0.138 (0.075, 0.200) | 0.132 (0.067, 0.200) |
| IDI | 0.025 (0.014, 0.038) | 0.017 (0.004, 0.028) | 0.006 (0.003, 0.009) | 0.012 (0.008, 0.015) |
| B2M | ||||
| Δc-statistic | 0.017 (−0.005, 0.039) | 0.021 (0.001, 0.041) | −0.002 (−0.010, 0.005) | 0.001 (−0.007, 0.009) |
| Categorical NRI | 0.084 (0.004, 0.160) | 0.048 (−0.030, 0.117) | 0.010 (−0.019, 0.040) | 0.029 (−0.05, 0.056) |
| Continuous NRI | 0.158 (0.015, 0.300) | −0.026 (−0.172, 0.114) | 0.044 (−0.024, 0.102) | 0.026 (−0.040, 0.091) |
| IDI | 0.021 (0.008, 0.035) | 0.012 (−0.001, 0.025) | 0.001 (−0.002, 0.004) | 0.007 (0.002, 0.010) |
| BTP | ||||
| Δc-statistic | 0.020 (−0.001, 0.041) | 0.024 (0.005, 0.043) | 0.006 (−0.000, 0.012) | 0.009 (0.002, 0.017) |
| Categorical NRI | 0.109 (0.029, 0.186) | 0.063 (−0.020, 0.140) | 0.043 (0.018, 0.066) | 0.063 (0.034, 0.094) |
| Continuous NRI | 0.214 (0.079, 0.352) | 0.093 (−0.052, 0.227) | 0.188 (0.124, 0.250) | 0.183 (0.118, 0.250) |
| IDI | 0.028 (0.016, 0.041) | 0.020 (0.008, 0.032) | 0.010 (0.007, 0.012) | 0.015 (0.012, 0.019) |
cTnT: cardiac troponin T, NT-proBNP: N-terminal pro–B-type natriuretic peptide, B2M: β2 microglobulin, BTP: β-trace protein, NRI: net reclassification improvement. IDI: integrated discrimination improvement.
CKD was defined as eGFRcre <60 ml/min/1.73m2 or ACR ≥ 30 mg/g.
Positive values indicate better prediction with a model with cardiac marker.
The superiority of cardiac markers to kidney markers for CVD prediction was largely consistent in those without CKD, although the prediction statistics for cardiac markers were more prominent in CKD than in non-CKD (Tables 3 and 4). In non-CKD, the prediction improvement for B2M was greater than that for the other two kidney markers and similar to that for the two cardiac markers.
When we analyzed CHD, stroke, and HF, separately, although there were some variations, the best value for each statistic was generally observed for a model with either cTnT or NT-proBNP regardless of CKD status (Tables 3 and 4). In both CKD and non-CKD, the improvement in prediction by these cardiac and kidney markers was most evident for HF.
Overall, conventional kidney measures of eGFR and ACR did not outperform cTnT and NT-proBNP for predicting CVD outcomes in both CKD and non-CKD groups (Tables VII and VIII). Finally, we repeated the analyses using eGFR based on serum creatinine and cystatin C11 for defining CKD population and observed similar results (data not shown).
Discussion
This study compared the additional value of several cardiac and kidney markers for CVD risk prediction among individuals with and without CKD. In line with previous reports not necessarily specific to CKD populations,9, 12–16 every marker was associated with incident CVD, independently of conventional risk factors, and contributed to improvement in CVD prediction in both CKD and non-CKD. Of note, the associations of cTnT and NT-proBNP with CVD were stronger than those of three non-traditional kidney markers, cystatin C, B2M, and BTP or well-studied non-traditional marker, hsCRP. Hence, the improvement in CVD prediction was most evident for these cardiac markers, particularly in persons with CKD.
There has been concern about cTnT and NT-proBNP as predictors of CVD in CKD populations, since their levels can increase as a consequence of kidney dysfunction (i.e., volume overload and/or reduced kidney excretion) and may not necessarily reflect cardiac abnormalities.10 However, several recent studies report the associations between these cardiac markers and poor prognosis, even among this specific population.10, 17, 18 Of importance, the associations of cTnT and NT-proBNP with CVD were independent of kidney function and actually stronger in those with CKD than in those without in our study. The present study expands current knowledge in several other aspects. First, the rigorous assessment of risk prediction statistics is clinically important, since a significant association does not necessarily ensure improvement in risk prediction.19 The prediction improvement for CVD by cTnT and NT-proBNP in our study was considerably larger than what was recently reported for the combination of total cholesterol, high-density lipoprotein cholesterol, and hsCRP for CHD prediction in a meta-analysis.20 Second, cTnT and NT-proBNP improved risk prediction independently of each other and their combination may gain more than each separately, probably since they reflect different pathological conditions of the heart.16, 21 Third, their contributions to improved CVD prediction were largest for HF, followed by CHD and stroke. Fourth, we confirmed prediction values of these cardiac markers in persons without history of CVD (i.e., the primary prevention setting).10, 22 Finally, these cardiac markers outperformed non-traditional kidney markers including cystatin C for CVD prediction. This is a clinically important aspect when we consider parsimonious prediction models given the cost to measure non-traditional predictors.
It may not be surprising that both cTnT and NT-proBNP performed better than kidney markers for CVD prediction since these cardiac markers reflect subclinical alteration of cardiac structure or function, which would progress to clinical HF or CHD. Nevertheless, our results may have important clinical implications, since identifying high risk individuals is an important element for prevention strategy. Future studies are warranted to assess whether cTnT and NT-proBNP would help guide prevention strategies, e.g., statin therapy,22 in persons with CKD. Also, given that individuals with CKD are prone to vascular calcification,23–26 comparison of cardiac markers with markers of calcification such as coronary artery calcium and serum calcium or phosphate would be important.
Our observations for kidney markers might be conservative, since, by stratifying our study population according to CKD status, we restricted the variance of kidney markers, in each of CKD and non-CKD. Nevertheless, these kidney measures contributed to better CVD prediction more evidently in CKD stage 3–5 than in stage 1–2. Also, cystatin C and B2M performed better than hsCRP for CVD prediction in the entire CKD population. Also, our analytic frame is in line with a recognized approach to use novel kidney filtration markers in addition to eGFRcre and ACR to identify the high-risk CKD population (e.g., a “triple-marker approach” using serum creatinine, cystatin C, and ACR27, 28). Although additional kidney filtration markers may be useful to more precisely estimate kidney function or predict kidney outcomes,9 our results suggest that overall cardiac-specific markers would be more useful to predict CVD outcomes in CKD population.
Among kidney markers, B2M predicted CVD similarly to cystatin C in CKD and was the best in non-CKD. B2M is a polypeptide forming a small subunit of the major histocompatibility class I molecule on the surface of nucleated human cells and thrombocytes and is eliminated by the kidney.9, 29 A few studies have reported a significant prospective association between B2M and CVD,29, 30 but the mechanisms by which B2M confers CVD risk is not well understood. B2M may merely be a good marker for kidney dysfunction,31 but, some investigators have suggested non-kidney pathways. B2M is known as a marker of inflammation31 and may also directly damage vessels through its involvement in amyloid formation.29 Nevertheless, B2M warrants further investigations in the context of CVD.
There are several limitations in our study. Few participants had CKD. However, it is still one of the largest CKD datasets ever tested for both cTnT and NT-proBNP as well as non-traditional kidney and inflammatory markers in terms of CVD prediction.10 Moreover, most participants with CKD were at stages 1–3. However, the vast majority of people with CKD are at these stages.32 Also, we did not have information on causes of CKD. However, as this is a community-based cohort, it is highly likely that two most prevalent causes of CKD, hypertension and diabetes,33 play a major role in most cases and Table 1 is consistent with this perspective. The mean age of our study population at baseline was 62 years old, and thus a majority of women in our study were likely to be postmenopausal. Thus, our results may not be generalizable to premenopausal women. The frequency of statin prescription has substantially increased since the baseline of this study (1996–1998)34 and its impact on our estimates is unclear. We had only a single measurement of cardiac and kidney markers, and thus there might be a degree of misclassification due to their short-term variability, leading to attenuated/conservative estimates. Identification of HF cases predominantly relied on ICD codes in hospital records and thus might not capture mild cases managed in outpatient setting.
In conclusion, cardiac and kidney dysfunction/damage markers improved CVD prediction in those with and without CKD. The improvement was more evident for cTnT and NT-proBNP compared to kidney markers, particularly in CKD. Our results suggest that these cardiac markers are useful to better classify CVD risk in CKD population.
Supplementary Material
Significance.
This is the first article, to our knowledge, comparing five non-traditional cardiac and kidney markers (high-sensitivity cardiac troponin T [cTnT], N-terminal pro-B-type natriuretic peptide [NT-proBNP], cystatin C, β2-microglobulin, and β-trace protein) as well as a representative inflammatory marker, high-sensitivity C-reactive protein (hsCRP), regarding their ability to predict cardiovascular disease (CVD) in individuals with and without chronic kidney disease (CKD). Despite the concern about their predictability performance in persons with CKD, the present study shows that cTnT and NT-proBNP improved CVD prediction more evidently than all the kidney markers and hsCRP, indeed more evidently in those with CKD than in those without. Their contributions to improved cardiovascular prediction were largest for heart failure, followed by coronary heart disease and stroke. Given that novel kidney markers attract attention for better CVD risk classification in CKD population, our results about superiority of cardiac markers for CVD prediction have important clinical implications.
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Assays of B2M, BTP, and Cystatin C were funded by R01DK076770 from the National Institute of Diabetes and Digestive and Kidney Diseases. Roche Diagnostics provided reagents and loan of an instrument to conduct the cTnT and NT-proBNP assays. Siemens Healthcare Diagnostics provided reagents and loan of an instrument to conduct the B2M and BTP assays. Roche and Siemens had no role in design, analysis, or manuscript preparation.
Footnotes
Disclosures: None.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet. 2012;380:807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, Yusuf S, Mann JF. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: A cohort study. Ann Intern Med. 2011;154:310–318. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Pacold IV, Durazo-Arvizu R, Liu K, Shilipak MG, Goff DC, Jr, Tracy RP, Kramer H. The effect of including cystatin c or creatinine in a cardiovascular risk model for asymptomatic individuals: The multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174:949–957. doi: 10.1093/aje/kwr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European guidelines on cardiovascular disease prevention in clinical practice (version 2012): The fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts)developed with the special contribution of the european association for cardiovascular prevention & rehabilitation (eacpr) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013;2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of esrd, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59:653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, Bakker SJL, Gansevoort RT group ftPs. High-sensitive troponin t and n-terminal pro-b type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;33:2272–2281. doi: 10.1093/eurheartj/ehs163. [DOI] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin c. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kragelund C, Grønning B, Køber L, Hildebrandt P, Steffensen R. N-terminal pro–b-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Medicine. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 14.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 15.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: The framingham heart study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dierkes J, Domröse U, Westphal S, Ambrosch A, Bosselmann H-P, Neumann KH, Luley C. Cardiac troponin t predicts mortality in patients with end-stage renal disease. Circulation. 2000;102:1964–1969. doi: 10.1161/01.cir.102.16.1964. [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Yi S, Hiremath L, et al. N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: The african american study of kidney disease and hypertension (aask) Circulation. 2008;117:1685–1692. doi: 10.1161/CIRCULATIONAHA.107.724187. [DOI] [PubMed] [Google Scholar]
- 19.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 20.Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadu RT, Nambi V, Ballantyne CM. Developing and assessing cardiovascular biomarkers. Transl Res. 2012;159:265–276. doi: 10.1016/j.trsl.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 24.Campean V, Neureiter D, Varga I, Runk F, Reiman A, Garlichs C, Achenbach S, Nonnast-Daniel B, Amann K. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28:280–289. doi: 10.1159/000090182. [DOI] [PubMed] [Google Scholar]
- 25.Amann K, Tyralla K, Gross ML, Eifert T, Adamczak M, Ritz E. Special characteristics of atherosclerosis in chronic renal failure. Clinical Nephrology. 2003;60 (Suppl 1):S13–21. [PubMed] [Google Scholar]
- 26.Tyralla K, Amann K. Morphology of the heart and arteries in renal failure. Kidney Int Suppl. 2003:S80–83. doi: 10.1046/j.1523-1755.63.s84.1.x. [DOI] [PubMed] [Google Scholar]
- 27.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin c, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waheed S. Combined association of creatinine, albuminuria and cystatin c with all-cause mortality cardiovascular and kidney outcomes: The atherosclerosis risk in communities (aric) study. Clin J Am Soc Nephrol. 2013;8:434–442. doi: 10.2215/CJN.04960512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke H-D, Vanholder R, Diouf M, Choukroun G, Massy ZA. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297–1303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 30.Amighi J, Hoke M, Mlekusch W, Schlager O, Exner M, Haumer M, Pernicka E, Koppensteiner R, Minar E, Rumpold H, Schillinger M, Wagner O. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke. 2011;42:1826–1833. doi: 10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 31.Shinkai S, Chaves P, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, Suzuki T. B2-microglobulin for risk stratification of total mortality in the elderly population: Comparison with cystatin c and c-reactive protein. Arch Intern Med. 2008;168:200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 34.Molokhia M, McKeigue P, Curcin V, Majeed A. Statin induced myopathy and myalgia: Time trend analysis and comparison of risk associated with statin class from 1991–2006. PLoS One. 2008;3:e2522. doi: 10.1371/journal.pone.0002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.