Abstract
Purpose
The aim of our meta-analysis was to compare the efficacy and safety of radiofrequency ablation (RFA) versus surgical resection for patients with small hepatocellular carcinoma (SHCC).
Methods
Randomized controlled trials (RCTs) or retrospective studies comparing the RFA with surgical resection for patients with SHCC published from 2004 to 2014 were selected from database of PubMed, EMBASE and Cochrane library. The outcomes including overall survival rate, recurrence-free survival rate, recurrence rate and complications (mortality rate and morbidity rate) were abstracted. Individual and pooled odds ratio with 95 % confidence interval of each outcome was analyzed.
Results
Three RCTs and twenty retrospective studies were included with a total of 15,482 patients. The 1-, 3- and 5-year overall survival rate and recurrence-free survival rate of surgical resection were significantly higher than RFA. The 2- and 3-year but not 1-year recurrence rate of RFA was significantly higher than surgical resection. The morbidity rate of complication in surgical resection group was higher than it in RFA group, but the mortality was not different between the two groups.
Conclusion
Surgical resection led to a higher overall survival and recurrence-free survival rate in treating SHCC. However, RFA led to a lower morbidity rate of complication than surgical resection.
Keywords: Radiofrequency ablation, Surgical resection, Small hepatocellular carcinoma, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide, which leads to a 500,000 deaths per year. At the same time, 660,000 new cases were identified in the world every year with an increasing incidence (Llovet et al. 2003; Marrero 2013; Venook et al. 2010). Liver transplantation might be the best cure for patients with HCC, yet it is limited by the high cost and donor shortage. Surgical resection has been widely accepted for patients who are unwilling or unable to receive liver transplantations (Duffy et al. 2008). Recently, radiofrequency ablation (RFA) has attracted great attention to become a first-line treatment because of its safety, cost-effectiveness and minimal invasiveness (Rhim et al. 2010). A few of meta-analyses have been conducted to compare the efficacy and safety of the surgical resection with RFA in the treatment of patients with small hepatocellular carcinoma (SHCC) (Chen et al. 2006; Cho et al. 2011; Feng et al. 2012; Huang et al. 2010; Li et al. 2012; Xu et al. 2012; Zhou et al. 2010). But the results are still controversial. Interestingly, these studies have not mentioned the effect of characteristic of tumor on the treatment outcomes.
Several RCTs and retrospective cohort studies were newly conducted regarding the curative effect of RFA and surgical resection in recent years. Herein, we performed this meta-analysis to compare RFA with surgical resection in the treatment of SHCC using these recently reported studies. Moreover, we also conducted two subgroup analysis based on characteristics of tumors to evaluate the effect of single tumor size (≤3 or >3 cm) and number of tumor nodules (single or multiple) on the treatment outcomes.
Methods
Study selection
Two reviewers independently carried out a comprehensive search of PubMed, EMBASE and Cochrane library for relevant articles published January 1, 2004 to February 1, 2014. The key words in this strategy with MeSH heading: “radiofrequency ablation,” “surgical resection,” “hepatocellular carcinoma.” Only articles written in English were included in. The searches were limited to human subjects, and the research design type was RCTs or retrospective studies.
Criteria for inclusion and exclusion
For inclusion in the meta-analysis, a study had to fulfill the following criteria: (1) Patients involved in these studies had not treated with RFA or surgical resection before; (2) First RFA treatment was conducted by percutaneous; (3) All patients were suitable candidates for surgical resection and/or RFA; (4) For similar studies reported by the same institution and/or authors, only the most recent study with high quality was included in this analysis; and (5) Included studies must report on at least one of the following outcomes: the overall and recurrence-free survival rate of 1-, 3- and 5-year, the recurrence rate of 1-, 2- and 3-year and complications (including mortality and morbidity).
Briefly, according to Yao et al. (2001), SHCC was defined as a single HCC nodule of up to 6.5 cm, or with up to 3 lesions, the largest of which is no larger than 4.5 cm.
Data abstraction
Two reviewers independently abstracted data in duplicate. The extracted data included first author, publication year, type of study, period of patient enrolment, cases of patients, gender, age, number of nodules, size of tumors; and result data include overall and recurrence-free survival rate of 1-, 3- and 5-year, recurrence rate of 1-, 2- and 3-year, mortality and morbidity rate. Any disagreements were resolved by discussion.
Data analysis
Binary end points (for example, survival and recurrence rate) were analyzed by calculating odds ratios (ORs) with 95 % confidence intervals (CIs). The heterogeneity of included studies was assessed using Chi square (χ 2) and I 2 statistics. Significant statistical heterogeneity between studies was identified when P < 0.1 when random effects model was used; otherwise, the fixed effects model was used. Sensitive analysis was conducted by eliminating each study in the analysis in each turn. Potential publication bias was assessed by visually inspecting of the Begg funnel plots in which the log ORs were plotted against their SEs. The presence of publication bias was also evaluated by using the Begg and Egger tests which were applied by Stata 11.0 software. To investigate the relationship among tumor number or size and overall or recurrence-free survival rate, we performed subgroup meta-analyses including single or multiple nodules with the single nodule ≤3 or >3 cm. All calculation was conducted using Revman 5.1 software if there is no special mention.
Results
Selection of studies
In accordance with the search, 38 potentially relevant publications were identified after the first round of selection. Among these publications, six were reported for one identical group of patients, eleven were comments or reviews. Two additional NRCT were identified by hand-searched. Finally, a total of 23 studies (3 RCTS and 20 retrospective studies) (Abu-Hilal et al. 2008; Chen et al. 2006; Desiderio et al. 2013; Feng et al. 2012; Guglielmi et al. 2008; Guo et al. 2013; Hasegawa et al. 2013; Hiraoka et al. 2008; Huang et al. 2010; Hung et al. 2011; Ikeda et al. 2011; Imai et al. 2012; Kong et al. 2011; Lupo et al. 2007; Nishikawa et al. 2011; Peng et al. 2012; Pompili et al. 2013; Sung et al. 2005; Tohme et al. 2013; Ueno et al. 2009; Wang et al. 2012; Wong et al. 2012; Yun et al. 2011) were selected, which involving 7,958 patients treated with RFA and 7,524 patients treated with surgical resection (Fig. 1). The details of these studies were listed in Table 1.
Fig. 1.
Flow diagram for identification of relevant clinical designs examining effect of RFA or surgical resection on the treatment of SHCC
Table 1.
Baseline patient and tumor characteristics presented in the studies for SHCC, treated with radiofrequency ablation or surgical resection
| Author | Year | Design | Study period | Treatment | Cases | Sex (M/F) | Age (years) | Tumor number (single/multiple) | Mean tumor size (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Sung N. H. | 2005 | NRCT | 1999–2001 | RFA | 55 | 41/14 | 59.1 ± 9.6 | NA | 2.4 ± 0.6 |
| Surgical resection | 93 | 69/24 | 49.2 ± 9.9 | NA | 2.5 ± 0.8 | ||||
| Chen M. S. | 2006 | RCT | 1999–2004 | RFA | 71 | 56/15 | 51.9 ± 11.2 | NA | ≤3/>3; 37/34 |
| Surgical resection | 90 | 75/15 | 49.4 ± 10.9 | NA | ≤3/>3; 42/48 | ||||
| Lupo L. | 2007 | NRCT | 1999–2006 | RFA | 60 | 47/13 | 68 (42–85) | NA | 3.65 (3.0–5.0) |
| Surgical resection | 42 | 33/9 | 67 (28–80) | NA | 4.0 (3.0–5.0) | ||||
| Abu-Hilal M. | 2008 | NRCT | 1991–2003 | RFA | 34 | 27/7 | 65 | NA | 3.0 (2.0–5.0) |
| Surgical resection | 34 | 26/8 | 67 | NA | 3.8 (1.3–5.0) | ||||
| Hiraoka A. | 2008 | NRCT | 2000–2007 | RFA | 105 | 76/29 | 69.4 ± 9.1 | NA | 1.98 ± 0.52 |
| Surgical resection | 59 | 44/15 | 62.4 ± 10.6 | NA | 2.27 ± 0.55 | ||||
| Guglielmi A. | 2008 | NRCT | 1996–2006 | RFA | 32 | NA | NA | NA | NA |
| Surgical resection | 31 | NA | NA | NA | NA | ||||
| Ueno S. | 2009 | NRCT | 2000–2005 | RFA | 155 | 100/55 | 66 (40–79) | 101/54 (65/35 %) | 2.0 ± 0.1 |
| Surgical resection | 123 | 82/41 | 67 (28–85) | 110/13 (89/11 %) | 2.7 ± 0.1 | ||||
| Huang J. | 2010 | RCT | 2003–2005 | RFA | 115 | 79/36 | 56.57 ± 14.30 | 84/31 (73/27 %) | NA |
| Surgical resection | 115 | 85/30 | 55.91 ± 12.68 | 89/26 (77 %/23 %) | NA | ||||
| Yun W. K. | 2011 | NRCT | 2000–2007 | RFA | 255 | 197/58 | 57.0 ± 9.9 | NA | 2.1 ± 0.5 |
| Surgical resection | 215 | 171/44 | 51.7 ± 9.7 | NA | 2.1 ± 0.5 | ||||
| Hung H. H. | 2011 | NRCT | 2002–2007 | RFA | 190 | 121/69 | 67.42 ± 11.45 | 152/38 (80/20 %) | 2.37 ± 0.92 |
| Surgical resection | 229 | 184/45 | 60.07 ± 12.56 | 181/48 (79/21 %) | 2.88 ± 1.06 | ||||
| Nishikawa H. | 2011 | NRCT | 2004–2010 | RFA | 162 | 95/67 | 68.4 ± 8.7 | NA | 1.99 ± 0.62 |
| Surgical resection | 69 | 50/19 | 67.4 ± 9.7 | NA | 2.68 ± 0.49 | ||||
| Ikeda K. | 2011 | NRCT | 1999–2006 | RFA | 236 | 145/91 | 67 (38–87) | NA | 1.8 (0.8–3.0) |
| Surgical resection | 138 | 101/37 | 62.5 (29–80) | NA | 2.0 (0.5–3.0) | ||||
| Kong W. | 2011 | NRCT | 2002–2009 | RFA | 47 | 37/10 | 57 ± 14 | 40/7 (85/15 %) | ≤2/2–5; 27/73 % |
| Surgical resection | 40 | 35/5 | 53 ± 13 | 38/2 (95/5 %) | ≤2/2–5; 21/79 % | ||||
| Feng K. | 2012 | RCT | 2005–2008 | RFA | 84 | 79/5 | 51 (24–83) | 48/36 (57/43 %) | 2.4 ± 0.6 |
| Surgical resection | 84 | 75/9 | 47(18–76) | 52/32 (62/38 %) | 2.6 ± 0.8 | ||||
| Wong K. M. | 2012 | NRCT | 2004–2009 | RFA | 36 | 18/18 | 63.5 ± 13 | NA | 1.9 ± 0.6 |
| Surgical resection | 46 | 30/16 | 55.1 ± 12 | NA | 2.1 ± 0.6 | ||||
| Wang J. H. | 2012 | NRCT | 2002–2009 | RFA | 52 | 35/17 | ≤60/>60; 29/23 | NA | NA |
| Surgical resection | 52 | 38/14 | ≤60/>60; 35/17 | NA | NA | ||||
| RFA | 254 | 161/93 | ≤60/>60; 85/169 | 173/81 (68 %/32 %) | NA | ||||
| Surgical resection | 208 | 168/40 | ≤60/>60; 113/95 | 189/19 (91 %/9 %) | NA | ||||
| Peng Z. W. | 2012 | NRCT | 2003–2008 | RFA | 71 | 63/8 | 53.1 ± 12.1 | NA | NA |
| Surgical resection | 74 | 65/9 | 51.5 ± 12.1 | NA | NA | ||||
| Imai K. | 2012 | NRCT | 2000–2011 | RFA | 82 | 46/36 | 67.6 ± 8.5 | NA | 1.87 ± 0.50 |
| Surgical resection | 101 | 75/26 | 63.3 ± 9.7 | NA | 2.14 ± 0.55 | ||||
| Tohme S. | 2013 | NRCT | 2001–2011 | RFA | 60 | 38/22 | 65.6 ± 12 | 47/13 (78.3/21.7 %) | 2.36 ± 0.94 |
| Surgical resection | 50 | 31/19 | 66.3 ± 1 | 39/11 (78/22 %) | 3.07 ± 1.17 | ||||
| Pompili M. | 2013 | NRCT | 1999–2010 | RFA | 116 | 92/24 | 69 (38–85) | NA | 2.3 (1.3–3.0) |
| Surgical resection | 116 | 87/29 | 67 (41–83) | NA | 2.3 (0.8–3.0) | ||||
| Guo W. X. | 2013 | NRCT | 2002–2007 | RFA | 94 | 78/16 | 56 (19–75) | 63/31 (67/33 %) | ≤3/>3; 62/32 |
| Surgical resection | 102 | 94/8 | 52 (18–75) | 75/27 (74/26 %) | ≤3/>3; 75/27 | ||||
| Desiderio J. | 2013 | NRCT | 2004–2012 | RFA | 44 | 35/9 | 64.4 ± 6.5 | 19/25 (43/57 %) | NA |
| Surgical resection | 52 | 37/15 | 65.6 ± 4.8 | 22/30 (42/58 %) | NA | ||||
| Hasegawa K. | 2013 | NRCT | 2000–2005 | RFA | 5,548 | 3,569/1,979 | 69 (52–80) | 4,068/1,480 (73/27 %) | 2.0 (1.0–3.0) |
| Surgical resection | 5,361 | 3,967/1,394 | 66 (48–77) | 4,458/903 (83/17 %) | 2.3 (1.2–3.0) |
RFA Radiofrequency ablation, M/F male/female, NA not available, RCT randomized controlled trial, NRCT non-randomized controlled trial
Overall survival
Most studies reported the overall survival rate, twenty-one, twenty-two and eighteen articles reported 1-, 3- and 5-year survival rate, respectively. The patients of surgical resection group had higher overall survival rates than RFA group in 1-year (OR 0.71, 95 % CI 0.52–0.96) (Fig. 2), 3-year (OR 0.62, 95 % CI 0.49–0.78) and 5-year (OR 0.55, 95 % CI 0.47–0.66). Significant heterogeneity was found in the analysis of the 3- and 5-year overall survival rate but not 1-year overall survival rate (Table 2).
Fig. 2.
The results of meta-analysis about 1-year overall survival rate. M–H Mantel–Haenszel test, RFA radiofrequency ablation
Table 2.
Relevant results indicators of RFA versus surgical resection on the treatment of SHCC
| T/P | Results (%) | OR (95 % CI) | P value | P–He* | Model | ||
|---|---|---|---|---|---|---|---|
| RFA | Surgical resection | ||||||
| Overall survival | |||||||
| 1-Year | 21/4,199 | 95.0 | 96.4 | 0.71 (0.52, 0.96) | 0.03 | 0.100 | Fixed effects |
| 3-Year | 22/15,414 | 80.4 | 85.4 | 0.62 (0.49, 0.78) | <0.001 | <0.001 | Random effects |
| 5-Year | 18/14,686 | 61.7 | 72.2 | 0.55 (0.47, 0.66) | <0.001 | 0.02 | Random effects |
| Recurrence-free survival | |||||||
| 1-Year | 19/3,544 | 72.7 | 82.4 | 0.58 (0.45, 0.76) | <0.001 | 0.004 | Random effects |
| 3-Year | 18/3,389 | 40.1 | 58.9 | 0.52 (0.40, 0.68) | <0.001 | <0.001 | Random effects |
| 5-Year | 15/2,984 | 23.5 | 44.3 | 0.50 (0.34, 0.76) | 0.001 | 0.000 | Random effects |
| Recurrence | |||||||
| 1-Year | 4/1,004 | 15.1 | 14.1 | 1.11 (0.78, 1.59) | 0.547 | 0.491 | Fixed effects |
| 2-Year | 3/772 | 38.2 | 25.5 | 1.75 (1.28, 2.39) | 0.001 | 0.717 | Fixed effects |
| 3-Year | 4/1,004 | 54.1 | 41.5 | 1.68 (1.30, 2.17) | 0.000 | 0.947 | Fixed effects |
| Complication | |||||||
| Mortality | 7/1,217 | 0.6 | 0.9 | 0.80 (0.30, 2.15) | 0.65 | 0.62 | Fixed effects |
| Morbidity | 13/1,937 | 9.4 | 23.5 | 0.37 (0.24, 0.58) | <0.001 | 0.01 | Random effects |
T/P No. of trials/no. of patients, OR odds ratio, P–He* P value of heterogeneity
Recurrence-free survival
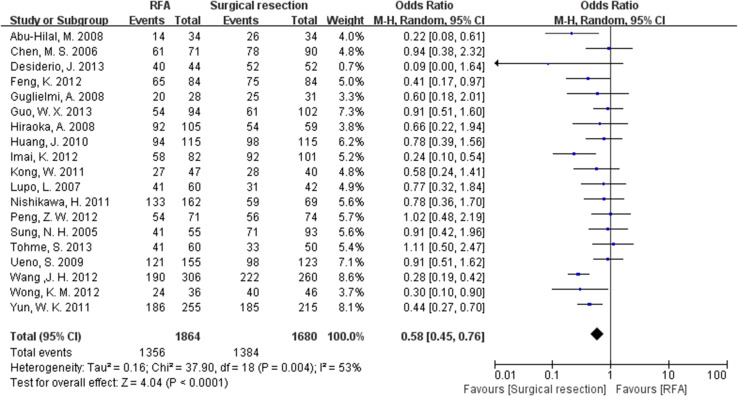
Nineteen, eighteen and fifteen articles, respectively, reported the 1-, 3- and 5-year recurrence-free survival rate. The surgical resection group had a relatively higher recurrence-free survival rate than RFA group (OR 0.58, 95 % CI 0.45–0.76 for 1-year; OR 0.52, 95 % CI 0.40–0.68 for 3-year; OR 0.50, 95 % CI 0.34–0.76 for 5-year) (Fig. 3). Significant heterogeneity was found in the analysis of recurrence-free survival rate (Table 2).
Fig. 3.
The results of meta-analysis about 1-year recurrence-free survival rate
Recurrence
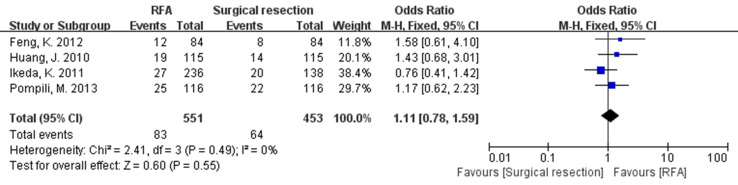
Four studies reported the 1- and 3-year recurrence rate while only three studies reported the 2-year recurrence rate. No significant difference was observed between the two groups (OR 1.12, 95 % CI 0.78–1.59 for 1-year; OR 1.75, 95 % CI 1.28–2.39 for 2-year; OR 1.68, 95 % CI 1.30–2.17 for 3-year) (Fig. 4). No significant heterogeneity was found in the analysis of recurrence rate (Table 2).
Fig. 4.
The results of meta-analysis about 1-year recurrence rate
Complication
Seven studies reported the mortality rate, and thirteen articles reported the morbidity rate. The mortality rate was of no difference between the two groups (OR 0.80, 95 % CI 0.30–2.15). However, the morbidity rate in RFA group was significantly lower than in surgical resection group (OR 0.37, 95 % CI 0.24–0.58) (Fig. 5). Significant heterogeneity was found in the meta-analysis of mortality rate but not morbidity rate (Table 2).
Fig. 5.
The results of meta-analysis about morbidity rate
Subgroup analysis
A total of seven studies were included in the subgroup analysis of Child–Pugh class. The results suggested that RFA or surgical resection has the equally effect on the overall survival rate (1-, 3-, 5-year) and recurrence-free survival rate (1-, 5-year) when the patients have a liver function Child–Pugh class A (Table 3). Eight studies were included in the subgroup analysis of single nodule. Generally, the results suggested that surgical resection led to a better survival rate than RFA (Table 3). In brief, for single nodule ≤3 cm, significant difference was observed in the 5-year but not 1-year, 3-year overall survival rate between RFA and surgical resection groups. However, significant difference was also observed in 1- and 3-year but not 5-year recurrence-free survival rate; for single nodule >3 cm, significant difference was found in 1- and 3-year but not 5-year overall survival rate, nor in recurrence-free rate. In addition, for single tumor nodule, significant difference was observed in 3- and 5-year but not in 1-year overall survival rate as well as in 1-, 3- and 5-year recurrence-free rate; however, for multiple nodules, significant difference was only found in 5-year overall survival rate (Table 3).
Table 3.
Subgroup analyses based on characteristics of tumors (RFA vs surgical resection)
| Variables | Duration (years) | Overall survival | Recurrence-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S/P | OR (95 % CI) | Model | P value | S/P | OR (95 % CI) | Model | P value | ||
| Child–Pugh class | |||||||||
| Child A | 1 | 7/825 | 1.07 (0.50, 2.26) | Fixed effects | 0.87 | 5/549 | 0.70 (0.44, 1.12) | Fixed effects | 0.14 |
| 3 | 7/825 | 0.62 (0.34, 1.14) | Random effects | 0.12 | 5/549 | 0.57 (0.34, 0.96) | Random effects | 0.04 | |
| 5 | 3/245 | 0.61 (0.36, 1.06) | Fixed effects | 0.08 | 3/245 | 0.44 (0.14, 1.36) | Random effects | 0.15 | |
| Single nodule | |||||||||
| ≤3cm | 1 | 8/1,081 | 0.55 (0.28, 1.08) | Fixed effects | 0.083 | 6/743 | 0.49 (0.26, 0.94) | Random effects | 0.031 |
| 3 | 8/1,081 | 0.51 (0.25, 1.04) | Random effects | 0.063 | 6/743 | 0.53 (0.34, 0.82) | Random effects | 0.005 | |
| 5 | 7/849 | 0.35 (0.17, 0.74) | Random effects | 0.006 | 6/743 | 0.53 (0.23, 1.24) | Random effects | 0.143 | |
| >3cm | 1 | 2/112 | 0.08 (0.01, 0.78) | Fixed effects | 0.029 | 1/41 | 0.46 (0.09, 2.39) | NA | 0.357 |
| 3 | 2/112 | 0.15 (0.05, 0.50) | Fixed effects | 0.002 | 1/41 | 0.24 (0.03, 2.16) | NA | 0.202 | |
| 5 | 2/112 | 0.56 (0.24, 1.30) | Fixed effects | 0.177 | 1/41 | 0.37 (0.04, 3.48) | NA | 0.388 | |
| Nodules | |||||||||
| Single | 1 | 15/2,215 | 0.82 (0.54, 1.27) | Fixed effects | 0.377 | 13/1,699 | 0.59 (0.41, 0.84) | Random effects | 0.003 |
| 3 | 15/2,178 | 0.64 (0.41, 0.98) | Random effects | 0.043 | 12/1,631 | 0.60 (0.49, 0.73) | Fixed effects | 0.000 | |
| 5 | 12/1,567 | 0.53 (0.36, 0.79) | Random effects | 0.001 | 11/1,390 | 0.59 (0.35, 0.99) | Random effects | 0.047 | |
| Multiple | 1 | 4/237 | 0.95 (0.18, 5.05) | Random effects | 0.952 | 2/122 | 0.34 (0.10, 1.13) | Fixed effects | 0.078 |
| 3 | 4/237 | 0.53 (0.10, 2.66) | Random effects | 0.437 | 2/122 | 0.53 (0.11, 2.57) | Random effects | 0.426 | |
| 5 | 2/112 | 0.40 (0.17, 0.92) | Fixed effects | 0.032 | 1/55 | 0.57 (0.10, 3.38) | NA | 0.532 | |
OR Odds ratio, CI confidence interval, NA not available, S sample size of trials, P patients
Sensitivity analysis and publication bias
We investigated the influence of single study on the overall pooled estimate by eliminating one study in each turn. No significant influence was observed for the results of meta-analysis except the 1-year overall survival rate. Publication bias was assessed using the Begg and Egger test. No significant publication bias was found for the overall survival rate, the recurrence-free survival rate and the morbidity rate. The funnel plot of 1-year overall survival rate was almost visually symmetrical (Fig. 6). The publication bias was not assessed for the recurrence rate and mortality rate, because only a small number of studies reported those outcomes.
Fig. 6.
The funnel plot of 1-year overall survival rate. SE Standard error
Discussion
Although meta-analysis has been commonly applied for evaluations of controversy trials especially of randomized controlled trials (RCTs), it is also available for non-randomized controlled trials (NRCTs), in which either the number or the sample size is insufficient for RCT (Mathurin et al. 2003). Our study suggested that surgical resection was a better treatment for patients with SHCC than RFA. Surgical resection had a higher overall survival and recurrence-free rate of 1-, 3- and 5-year. These conclusions were consistent with Li et al. (2012) and Zhou et al. (2010), which should be attributed to the increased understanding and improvement of surgical techniques (Kudo 2010). Moreover, it seemed that patients treated with surgical resection had a lower recurrence rate than those treated with RFA. Feng et al. (2012) also recommended surgery treatment as a better choice for the SHCC located at specific sites of liver. Compared to surgical resection, RFA was an emerging technology which developed fast. However, RFA technology in hepatic ablation was limited by following factors: (1) limitation of ablation volume, (2) technically infeasible in some tumors due to conspicuity and dangerous location, and (3) the heat sink effect (Rhim and Lim 2010).
The subgroup analysis indicated that RFA should be the first choice for the patients with SHCC and Child–Pugh class A, because RFA obtained equivalent overall survival rate and caused minor damage to the patients compared with surgical resection. Surgical resection led to a higher 5-year overall survival rate than RFA when the single nodule is ≤3 cm, while RFA led to a higher 5-year overall survival rate for larger single nodule. In addition, when the single nodule is >3 cm, surgical resection led to a higher 1-, 3-year overall survival rate than RFA, which was consistent with the meta-analysis conducted by Li et al. and Zhou et al. They also suggested that surgical resection was better than RFA in treating SHCC particularly when tumor size was >3 cm (Li et al. 2012; Zhou et al. 2010). Actually, when treating large tumors (>3 cm) using RFA, additional difficulty including vascular invasion was often arisen. However, our result of subgroup analysis regarding multiple nodules should be treated with caution, as only two trials concerning 112 patients were included. Our results indicated that surgical resection is better for patients with single nodules but not multiple nodules, which may be due to multiple nodules arise the difficulty of surgery.
In addition, we should also keep eyes on the characteristics of population data and tumor nodules. Pompili et al. (2013) reported the older age and the higher alpha-fetoprotein level as independent predictors of a poor overall survival rate. In multivariate analyses, Abu-Hilal et al. (2008) reported that the overall survival rate was related to tumor diameter, while Nishikawa et al. (2011) reported the serum albumin level as the sole significant factor contributing to overall survival. Recently, more and more studies are exploring the combined outcome of RFA. Kagawa et al. (2010) reported RFA combined with TACE is an efficient and safe treatment that provides overall survival rates similar to those achieved with surgical resection. If the tumor size is over than 5 cm, we can combine TACE with RFA to achieve complete local tumor control (Rhim and Lim 2010).
The results of this meta-analysis should be interpreted with caution for several reasons. Firstly, as the number of qualified RCTs was limited, most data included in the present study were abstracted from NRCTs. The unpredictable confounding factors may affect our analysis of data, even the well-analyzed cohort studies. Even if we had some subgroup analysis, but the heterogeneity was still existing. We expect that more RCTs will be designed to provide guidance for clinical treatment. Secondly, heterogeneity existed in some subgroups, which could be due to the bias in patient demographics and tumor characteristics generated during the process of patient selection. Thirdly, RFA can be performed by percutaneous, laparoscopic or open approaches, the pros and cons of different approaches should be further noticed. In addition, the long-term of survival should be paid more attention in further trial.
Conflict of interest
None.
References
- Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N (2008) Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg 12:1521–1526 [DOI] [PubMed] [Google Scholar]
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY (2006) A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 243:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Rhim H, Noh S (2011) Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol 26:1354–1360 [DOI] [PubMed] [Google Scholar]
- Desiderio J, Trastulli S, Pasquale R, Cavaliere D, Cirocchi R, Boselli C, Noya G, Parisi A (2013) Could radiofrequency ablation replace liver resection for small hepatocellular carcinoma in patients with compensated cirrhosis? A 5-year follow-up. Langenbeck’s Arch Surg/Deutsche Gesellschaft fur Chirurgie 398:55–62 [DOI] [PubMed] [Google Scholar]
- Duffy JP, Hiatt JR, Busuttil RW (2008) Surgical resection of hepatocellular carcinoma. Cancer J 14:100–110 [DOI] [PubMed] [Google Scholar]
- Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57:794–802 [DOI] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C (2008) Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg 12:192–198 [DOI] [PubMed] [Google Scholar]
- Guo WX, Sun JX, Cheng YQ, Shi J, Li N, Xue J, Wu MC, Chen Y, Cheng SQ (2013) Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg 37:602–607 [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O et al (2013) Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 58:724–729 [DOI] [PubMed] [Google Scholar]
- Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y et al (2008) Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology 55:2171–2174 [PubMed] [Google Scholar]
- Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y (2010) A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 252:903–912 [DOI] [PubMed] [Google Scholar]
- Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, Kao WY, Huo TI, Huang YH, Su YH et al (2011) Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol 9:79–86 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi M, Kawamura Y, Imai N, Seko Y, Hirakawa M, Hosaka T, Sezaki H, Akuta N, Saitoh S et al (2011) Stage progression of small hepatocellular carcinoma after radical therapy: comparisons of radiofrequency ablation and surgery using the Markov model. Liver Intern 31:692–699 [DOI] [PubMed] [Google Scholar]
- Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H (2012) Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res 43:853–864 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, Watanabe T, Mine T, Tokai RFASG (2010) Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer 116:3638–3644 [DOI] [PubMed] [Google Scholar]
- Kong WT, Zhang WW, Qiu YD, Zhou T, Bin H (2011) Prospective comparative study of cool-tip radiofrequency ablation and surgical resection for hepatocellular carcinoma. Chin-Ger J Clin Oncol 10(7):399–405 [Google Scholar]
- Kudo M (2010) Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology 78(Suppl 1):113–124 [DOI] [PubMed] [Google Scholar]
- Li L, Zhang J, Liu X, Li X, Jiao B, Kang T (2012) Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol 27:51–58 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917 [DOI] [PubMed] [Google Scholar]
- Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V (2007) Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB 9:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JA (2013) Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis 33(Suppl 1):S3–S10 [DOI] [PubMed] [Google Scholar]
- Mathurin P, Raynard B, Dharancy S, Kirzin S, Fallik D, Pruvot FR, Roumilhac D, Canva V, Paris JC, Chaput JC et al (2003) Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther 17:1247–1261 [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, et al (2011) Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol 11:143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS (2012) Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 262:1022–1033 [DOI] [PubMed] [Google Scholar]
- Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, et al (2013) Long term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma 3 cm. Results of a Multicenter Italian Survey. J Hepatol 59:89–97 [DOI] [PubMed] [Google Scholar]
- Rhim H, Lim HK (2010) Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver 4(Suppl 1):S113–S118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim H, Lim HK, Choi D (2010) Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg 2:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung NH, Lee SY, Moon SC, Joon HL, Kwang CK, Seung WP, Byung CY, Jong CR, Choi D, Hyo KL et al (2005) Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 39:247–252 [DOI] [PubMed] [Google Scholar]
- Tohme S, Geller DA, Cardinal JS, Chen HW, Packiam V, Reddy S, Steel J, Marsh JW, Tsung A (2013) Radiofrequency ablation compared to resection in early-stage hepatocellular carcinoma. HPB 15:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H, Kagoshima Liver Cancer Study Group (2009) Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg 16:359–366 [DOI] [PubMed] [Google Scholar]
- Venook AP, Papandreou C, Furuse J, de Guevara LL (2010) The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 15(Suppl 4):5–13 [DOI] [PubMed] [Google Scholar]
- Wang JH, Wang CC, Hung CH, Chen CL, Lu SN (2012) Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 56:412–418 [DOI] [PubMed] [Google Scholar]
- Wong KM, Yeh ML, Chuang SC, Wang LY, Lin ZY, Chen SC, Tsai JF, Wang SN, Kuo KK, Dai CY, et al (2012) Survival comparison between surgical resection and percutaneous radiofrequency ablation for patients in Barcelona Clinic Liver Cancer early stage hepatocellular carcinoma. Indian J Gastroenterol 32:253–257 [DOI] [PubMed] [Google Scholar]
- Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y, Miao Y (2012) Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol 10:163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33:1394–1403 [DOI] [PubMed] [Google Scholar]
- Yun WK, Choi MS, Choi D, Rhim HC, Joh JW, Kim KH, Jang TH, Lee JH, Koh KC, Paik SW et al (2011) Superior long-term outcomes after surgery in child–pugh class a patients with single small hepatocellular carcinoma compared to radiofrequency ablation. Hepatol Int 5:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J (2010) Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 10:78 [DOI] [PMC free article] [PubMed] [Google Scholar]