Abstract
Chemokine-directed leukocyte migration is crucial for effective immune and inflammatory responses. Conventional chemokine receptors (cCKRs) directly control cell movement, atypical chemokine receptors (ACKRs) regulate co-expressed cCKRs, and both cCKRs and ACKRs internalize chemokines to limit their abundance in vivo, a process referred to as scavenging. A leukocyte’s migratory and chemokine scavenging potential is determined by which cCKRs and ACKRs it expresses, and by the ligand specificity, signaling properties, and chemokine internalization capacity of these receptors. Most chemokines can bind at least one cCKR and one ACKR. CCL2 can bind to CCR2 (a cCKR) and two ACKRs (ACKR1 and ACKR2). Here, by using fluorescent CCL2 uptake to label cells bearing functional CCL2 receptors, we have defined the expression profile, scavenging activity, and ligand specificity of CCL2 receptors on mouse leukocytes. We show that qualitative and quantitative differences in the expression of CCR2 and ACKR2 endow individual leukocyte subsets with distinctive CCL2 receptor profiles and CCL2 scavenging capacities. We reveal that some cells, including plasmacytoid dendritic cells, can express both CCR2 and ACKR2; that Ly6Chi monocytes have particularly strong CCL2 scavenging potential in vitro and in vivo; and that CCR2 is a much more effective CCL2 scavenger than ACKR2. We confirm the unique, overlapping, ligand specificities of CCR2 and ACKR2, and, unexpectedly, find that cell context influences the interaction of CCL7 and CCL12 with CCR2. Fluorescent chemokine uptake assays were instrumental in providing these novel insights into CCL2 receptor biology, and the sensitivity, specificity and versatility of these assays is discussed.
Introduction
Precise temporospatial leukocyte positioning is crucial for physiological and pathological immune and inflammatory responses. The chemokine family of secreted chemoattractants plays a central role in orchestrating this process by controlling leukocyte navigation into, within, and between tissues (1, 2). There are over forty chemokines in mammals which, based on the organization of cysteine residues in the mature protein, are subdivided into four subfamilies (CC, CXC, C and CX3C). They signal through heptahelical G-protein coupled receptors that decorate leukocyte surfaces, and 18 receptors have been identified that can induce cell migration after binding their cognate chemokine ligand. These ‘conventional’ chemokine receptors (cCKRs) are usually specific for a single chemokine subfamily and there are 10 CC chemokine receptors, 6 CXC chemokine receptors, and one receptor each for C and CX3C chemokines. Four ‘atypical’ chemokine receptors (ACKRs) also exist that were previously called DARC, D6, CXCR7 and CCRL1, and which have recently been renamed ACKR1, ACKR2, ACKR3 and ACKR4, respectively (2, 3). ACKRs structurally resemble cCKRs, but cannot directly initiate migratory responses. Instead they scavenge, sequester or transport chemokines to control cCKR-driven responses, and can also, in some contexts, regulate co-expressed cCKRs (3). Chemokine scavenging is not restricted to ACKRs. cCKR activation is accompanied by internalization of chemokine/cCKR complexes, and, interestingly, migrating cells can use cCKRs to actively scavenge the chemokines that are driving their migration (4). Moreover, like ACKRs, cCKRs have been shown to modulate chemokine abundance in vivo through ligand uptake (5-7).
Interactions between chemokines and their receptors are complex. Many chemokines bind multiple receptors, and some cCKRs and ACKRs show remarkable ligand promiscuity (2). This is prominent amongst chemokines and receptors that regulate leukocyte trafficking during inflammation. A leukocyte’s response to a specific inflammatory chemokine will depend on which cCKRs and ACKRs it carries; the level of expression and specificity of these receptors; and their ability to translate chemokine binding into biological responses. In addition, the extent of chemokine scavenging mediated by its cCKRs and ACKRs will determine how effectively it modifies chemokine abundance. Subsets of leukocytes are likely to show qualitative and quantitative differences in these parameters that will dictate how they respond to, and regulate, chemokines. Here we have examined these issues by exploring how leukocytes interact with the chemokine CCL2.
CCL2 is a key pro-inflammatory chemokine that can direct the migration of a variety of leukocytes, including subsets of monocytes, dendritic cells, NK cells, and T cells (2, 8-14). Responses to CCL2 are mediated by the cCKR CCR2, but CCL2 can also bind to ACKR1 and ACKR2. CCR2 is activated by other chemokines (e.g. CCL7 and CCL12 in mice) and ACKR1 and ACKR2 show broad specificity for inflammatory chemokines (3). ACKR1 is not expressed by leukocytes: it is found on red blood cells, where it acts as a chemokine buffer (3, 15), and blood vessel endothelial cells, where it participates in chemokine transcytosis (3, 16, 17). Lymphatic endothelial cells are a prominent source of ACKR2 (18, 19), but it is also expressed by mouse innate-like B cells (marginal zone (MZ) and B1 B cells) and can suppress the migration of these cells (20). It is unclear whether other mouse leukocytes express ACKR2, but this could contribute to the many indispensable in vivo functions that have been defined for ACKR2 (3). CCR2 plays a particularly prominent role in the biology of inflammatory Ly6Chi monocytes. It mediates their recruitment into inflamed tissues, but is also important for their mobilization from the bone marrow (BM) under steady state conditions (10, 13, 14). Interestingly, ACKR2 has also been implicated in regulating homeostatic monocyte release from mouse BM and circulating monocyte count in humans (21, 22).
Theoretically, immunostaining could be used to profile expression of CCR2 and ACKR2 on mouse leukocytes. However, effective anti-mouse ACKR2 Abs are not available, and Abs provide no insight into receptor specificity or ‘activity’ i.e. whether the detected receptors can bind chemokine, transduce signals, and mediate scavenging. Moreover, alternative splicing, post-translational modification or heterodimerization could mask Ab epitopes on receptors that are competent for chemokine binding. The use of fluorescently labeled chemokines overcomes these restrictions and limitations. We used AlexaFluor®-647 tagged CCL2 (CCL2AF647) to reveal ACKR2 expression by innate-like B cells (20). Binding of CCL2AF647 at 4°C was insufficiently sensitive to detect ACKR2, and cells had to be allowed to internalize CCL2AF647 by incubation at 37°C. Significantly, this showed that ACKR2 was functional with respect to the binding and internalization of CCL2 (20). This is critical for chemokine scavenging, and driven by constitutive ACKR2 trafficking to and from the cell surface (23). Some CCR2-dependent CCL2AF647 uptake was also observed in our previous work (20). The labeled cells carry CCR2 molecules that bind and internalize CCL2AF647, so, since internalization of CCR2 requires chemokine-induced signaling (24), these CCR2 molecules must presumably be capable of initiating intracellular signals upon CCL2 binding. Therefore, unlike Ab staining, CCL2AF647 uptake assays specifically identify cells carrying ‘functionally competent’ cCKRs and ACKRs for CCL2. Moreover, the extent of uptake reflects a cell’s chemokine scavenging potential, and the inclusion of unlabeled competitor chemokines allows receptor specificity to be defined.
In this paper, we have systematically determined which mouse leukocytes express functionally competent CCL2 receptors. We have compared the ex vivo and in vivo CCL2 scavenging potential of different leukocyte subsets, and revealed the contribution of CCR2 and ACKR2 to CCL2 receptor activity. We have also examined if the ligand specificity of CCR2 and its sensitivity to chemokine exposure are influenced by the cellular context in which the receptor is expressed. These studies have provided novel insights into the expression, regulation, ligand specificity, and scavenging potential of CCL2 receptors.
Materials and Methods
Animals and in vivo procedures
WT and Ccr2−/− C57Bl/6 mice were bred and maintained under specific pathogen-free conditions at the Central Research Facility, University of Glasgow. Ccr2−/− mice were originally from Jackson Labs (stock number: 004999) (25). In all experiments, 8–12 wk old male mice were used. For in vivo expansion of pDCs, ~2×106 Flt3L-producing B16FL cells (26) (provided by Oliver Pabst, Hannover Medical School, Germany) were injected subcutaneously into WT mice and tumor growth monitored for 10-14 days until sacrifice. For in vivo fluorescent chemokine uptake, WT mice were injected via the tail vein with 1μg of CCL2AF647 in 100μl of PBS, or with 100μl of PBS alone, and sacrificed 2h later. All procedures had received approval from Glasgow University’s ethical review boards, and were performed under license in accordance with the UK Home Office regulations.
Cell isolation
Single cell suspensions of mouse spleen, lymph node and BM were prepared as previously described (20). RBC were lysed in spleen and BM samples by incubating cells in Red Blood Cell Lysis Buffer (Sigma Aldrich) for 1min at room temperature. Mouse peripheral blood was harvested by terminal cardiac puncture using a 1ml syringe with a 25-gauge needle that had previously been flushed with 0.5M EDTA (pH7.5). RBC were lysed by adding 9 volumes of Ammonium Chloride Solution (StemCell Technologies) to the blood and incubating on ice for 10min. Cells were then washed twice by centrifugation at 400xg with complete RPMI (RPMI 1640 containing 10 U/ml penicillin/streptomycin, 0.2mM L-glutamine, and 10% FCS [all Invitrogen]) for 5min at 4°C. Cells were re-suspended in complete RPMI and viable cells counted on a hemocytometer using Trypan blue exclusion.
Chemokines
CCL2AF647 (Almac Sciences) is a chemically synthesized form of human CCL2 that carries AlexaFluor®-647 on its extreme C-terminus. It has equivalent bioactivity to unlabelled recombinant human CCL2 in in vitro chemotaxis assays (20). Unlabelled chemokines were from Peprotech or R&D Systems.
Fluorescent chemokine uptake
1-2×106 cells were incubated in the dark for 1h at 37°C or 4°C in 50μl of binding buffer (complete RPMI with 20mM HEPES (pH7.2)) containing 25nM CCL2AF647 (Almac Sciences), with or without unlabelled chemokine competitor (20). Cells were then washed in binding buffer and stained with fluorescently labeled Abs as described below. In some experiments, cells were pre-incubated with unlabelled chemokine at 37°C for 30min, washed three times at 4°C in binding buffer, and then incubated with fluorescent chemokine as above.
Antibodies and flow cytometry
Cells were incubated in ice-cold FACS buffer (PBS containing 1% FCS, 0.02% NaNH3 and 5mM EDTA) with Fc block (BD biosciences) at 4°C for 15min. Cells were then stained with Abs for 15min at 4°C, and washed twice with FACS buffer. Where necessary, cells were incubated in secondary detection reagents for 15min at 4°C, before being washed twice with FACS buffer. To allow dead cell exclusion, the cell viability dye Viaprobe (BD Biosciences) was added after antibody labeling or cells were labeled with fixable viability dyes (eBiosciences) before antibody staining, each according to the manufacturers’ instructions. The following Abs, labeled with various fluorophores, were used (clone names are in parentheses): Anti-mouse CCR2 (475301) was from R&D Systems; antibodies against mouse Ly6C (AL-21), CD21 (7G6), and CD11b (M1/70) were from BD Biosciences; and antibodies against mouse Gr1 (RB6-8C), CD317/PDCA1 (129c), CD49b/DX5 (DX5), IA/IE (MHCII) (M5/114.15.2), CD11c (N418), CD8 (53-6.7), F4/80 (BM8), γδ TCR (GL3), CD19 (1D3), CD4 (GK1.5), CD3 (17A2), and SiglecH (440c) were from eBioscience. Unstained cells, cells stained with only one fluorescent Ab, and ‘Fluorescent minus one’ controls were used in all experiments to allow appropriate acquisition parameters to be established, and to aid gating during data analysis. ‘Fluorescent minus one’ controls contained all Abs except one, which was replaced with an equivalent quantity of an isotype-matched Ab control. Data were acquired on a Miltenyi MACSQuant or Becton Dickinson LSRII and analyzed using FlowJo software (Treestar Inc.). Dead cells and cell doublets/clusters were excluded from all analyses.
pDC purification and chemotaxis
Plasmacytoid dendritic cells (pDCs) were purified using a FACS Aria (Becton Dickinson) as live singlet B220+CD11c+Ly6C+CD11b−SiglecH+ cells to >95% purity. pDCs were re-suspended to 106/ml in chemotaxis buffer (RPMI plus 0.5% BSA and 25mM HEPES (pH7.2)) and 100μl added to inserts of a 24-well Transwell chemotaxis plate (5μm pores) sitting above 600μl of chemotaxis buffer containing 0-50nM CCL2. Plates were incubated for 3h at 37°C. Migrated cells were retrieved from the lower chamber, washed, resuspended in 200μl of FACS buffer, and stained with Abs as above. Cells were counted on a Miltenyi MACSQuant set to analyze a defined sample volume. Data were analyzed using FlowJo software.
Statistical Analysis
All statistical analyses were performed using GraphPad PRISM software. The statistical tests used are indicated in the Figure Legends. p values of less than 0.05 were considered to demonstrate statistically significant differences between groups.
Results
CCL2AF647 uptake identifies mouse leukocytes expressing functionally competent CCL2 receptors
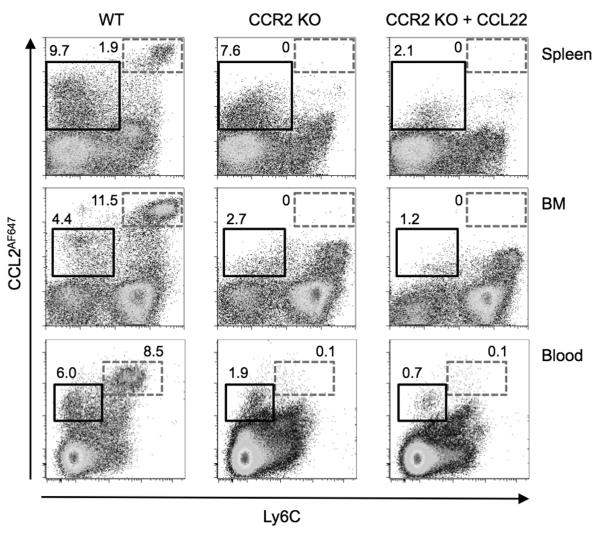
First we sought to identify leukocytes bearing functionally competent CCL2 receptors in the spleen, BM and blood of WT mice at steady state. Single cell suspensions were prepared and incubated with CCL2AF647. To determine if CCR2 was responsible for mediating CCL2AF647 uptake, cells from Ccr2−/− mice were also used. Ccr2−/− samples were also included that contained excess unlabelled mouse CCL22 in addition to CCL2AF647. ACKR2, unlike CCR2, binds CCL2 and CCL22, so any ACKR2-mediated CCL2AF647 uptake by Ccr2−/− cells will be blocked by CCL22 (20). After CCL2AF647 uptake, cells were stained with anti-Ly6C antibodies because we anticipated that Ly6Chi monocytes would demonstrate CCR2-dependent CCL2AF647 uptake. Indeed, as shown in Figure 1, most Ly6Chi cells in spleen, BM and blood internalized substantial quantities of CCL2AF647 in a CCR2-dependent fashion, and clearly had higher CCL2 scavenging potential than any other cells in the samples. Lower levels of CCL2AF647 uptake were achieved by a subset of WT Ly6C−/int cells. This was partially CCR2-dependent, but unlabelled CCL22 reduced CCL2AF647 uptake by Ccr2−/− Ly6C−/int cells, demonstrating the involvement of ACKR2. This was particularly evident amongst Ly6C− splenocytes presumably due, at least in part, to the expression of functionally competent ACKR2 receptors by MZ B cells (20). As in previous studies (20), CCL2AF647 labeling was dependent on active ligand internalization because cells did not accumulate any CCL2AF647 if they were incubated at 4°C rather than 37°C (Supplementary Figure 1). Even in the presence of CCL22, some Ccr2−/− cells, most notably Ly6Chi BM cells, could be weakly labeled with CCL2AF647 (Figure 1). This was not seen when incubations were performed at 4°C (Supplementary Figure 1), implying that at 37°C CCL2AF647 was being actively internalized by these cells rather than just binding to the cell surface. However, like CCL22, many other unlabelled chemokines (including mouse CCL2, CCL3, CCL4, CCL11, CCL17 and CXCL12) or, significantly, an unlabelled version of the fluorescent chemokine (human CCL2), were unable to block CCL2AF647 uptake by Ccr2−/− Ly6Chi BM cells (Supplementary Figure 1 and data not shown), demonstrating that it was due to non-specific uptake mechanisms, such as pinocytosis.
Figure 1. CCL2AF647 uptake enables specific and sensitive detection of CCL2 receptors on mouse leukocytes.
Cells from the spleen, BM and blood WT or Ccr2−/− (CCR2 KO) mice were incubated with CCL2AF647 (+/− 10-fold molar excess of unlabelled CCL22), stained with fluorescently labeled anti-Ly6C Ab, and examined by flow cytometry. Dead cells and cell doublets were excluded by pre-gating. The boxes indicate populations of cells discussed in the Results text, and the adjacent numbers represent the percentage of live cells found in the box, rounded to one decimal place. Data are representative of three of more repeat experiments each containing three or more individual mice per genotype.
Leukocyte-specific CCL2AF647 uptake profiles
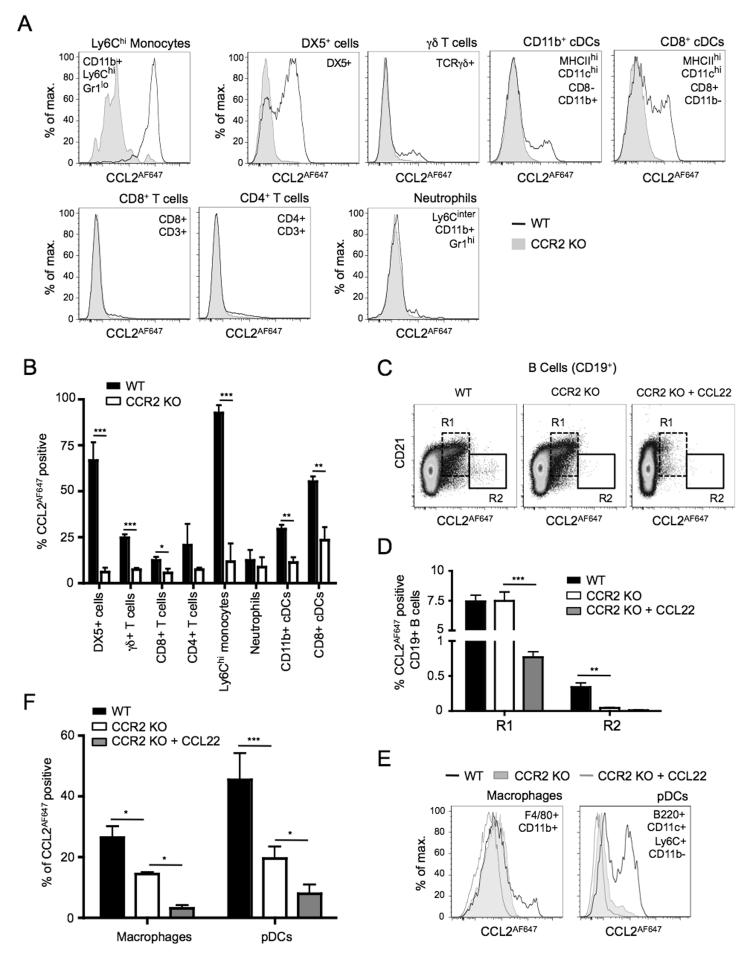
Next we sought to identify the cell types capable of CCR2- and/or ACKR2-dependent CCL2AF647 uptake, and compare the extent of uptake between different leukocyte populations. When specific leukocyte populations in the spleen were examined (identified as shown in Supplementary Figure 2), five distinct patterns of CCL2AF647 labeling were seen (Figure 2). First, virtually all Ly6Chi monocytes showed very strong CCR2-dependent CCL2AF647 uptake. Second, most cells expressing the NK cell marker DX5, along with subsets of γδ T cells, CD11b+ conventional dendritic cells (cDCs), and CD8+ cDCs, were capable of CCR2-dependent CCL2AF647 uptake (Figure 2A-B), although the amount of CCL2AF647 internalized per cell was substantially less than that achieved by Ly6Chi monocytes (Figure 2A). A few WT CD4+ and CD8+ T cells also internalized CCL2AF647 and this was reduced in Ccr2−/− cells (Figure 2A-B). Third, some cell types, including neutrophils, showed no CCR2-dependent CCL2AF647 uptake (Figure 2A-B and data not shown). A fourth pattern of CCL2AF647 uptake was seen amongst B cells. None of the splenic cell types discussed above showed any evidence of ACKR2 activity in Ccr2−/− or WT mice, as there was no significant reduction of CCL2AF647 uptake in the presence of CCL22 (data not shown), but, consistent with our previous work (20), nearly all CCL2AF647 uptake by CD21hi MZ B cells was ACKR2-dependent (Figure 2C-D) . In addition, a small population of CD21-/lo B cells showed strong CCR2-dependent CCL2AF647 uptake that resembled that achieved by Ly6Chi monocytes (Figure 2C-D). Ccr2 transcripts are found in immature T1 B cells and plasmablasts (27, 28), but to our knowledge expression of CCR2 protein by mouse B cells has not been reported. Finally, analysis of pDCs and macrophages provided evidence of a fifth CCL2AF647 uptake profile. CCL2AF647 internalization by these cells was reduced by Ccr2 deletion, but could be lowered further by inclusion of CCL22 (Figure 2E and F). Thus, pDCs and macrophages can express CCR2 and ACKR2.
Figure 2. CCL2AF647 uptake identifies splenic leukocyte subsets expressing CCR2 and/or ACKR2.
WT and Ccr2−/− (CCR2 KO) splenocytes were incubated with CCL2AF647 (+/− a 10-fold molar excess of unlabelled CCL22), stained with fluorescently labeled Abs, and examined by flow cytometry. Dead cells and cell doublets have been excluded from all data. (A & E) Overlaid CCL2AF647 uptake profiles of WT and CCR2 KO splenic leukocyte subsets identified by the surface immunophenotype indicated. (B) Mean percentage (+SD) of CCL2AF647 positive cells in splenic leukocyte subsets (n=3). CCL2AF647 positive WT cells were defined based on arbitrary gates set using equivalent populations of CCR2 KO splenocytes. The percentage of CCL2AF647 positive CCR2 KO cells remaining in this gate is shown in the white columns. *p<0.05, **p<0.01, ***p<0.001 using Student’s t test. (C) Dot-plots of live splenic B cells (CD19+) showing CCL2AF647 uptake against CD21 expression. R1 and R2 identify cells with specific CCL2AF647 uptake properties that are discussed in the Results text. (D) Mean percentage (+SD) of CCL2AF647 positive cells in R1 and R2 (n=3). (F) Mean percentage (+SD) of CCL2AF647 positive WT and CCR2 KO splenic macrophages and pDCs (n=3). CCL2AF647 positive WT and CCR2 KO cells were defined based on arbitrary gates set using equivalent populations of CCR2 KO splenocytes that had been incubated with CCL2AF647 and an excess of unlabelled CCL22. The percentage of CCL2AF647 positive cells in this gate is shown in the white columns. In D and F, data were analyzed using one-way ANOVA with Tukey post-test, *p<0.05, **p<0.01, ***p<0.001. Data are representative of four of more repeat experiments each containing three or more individual mice per genotype.
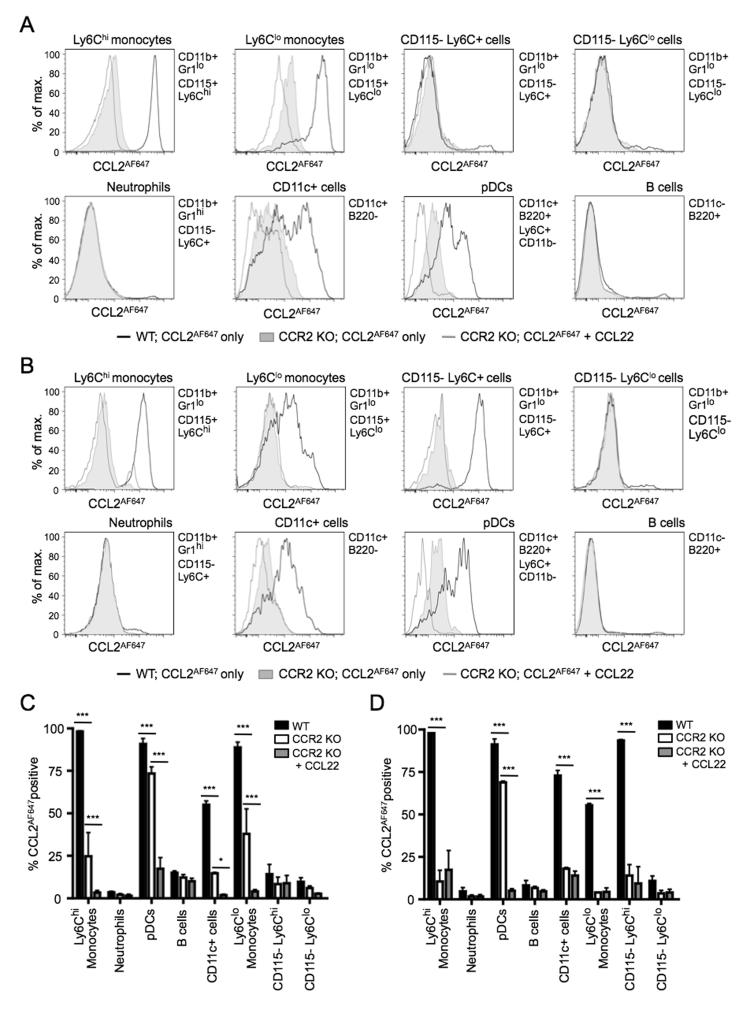
Similar analyses were undertaken on cells from BM, blood, and skin-draining lymph nodes (Figure 3 and data not shown). CCL2AF647 uptake profiles of leukocyte subsets in lymph nodes were broadly similar to those seen in the spleen (data not shown). In BM, CCL2AF647 uptake was restricted to CD11b+ and CD11c+ cells (Figure 3A and 3C; populations identified as shown in Supplementary Figure 3). Amongst CD11b+ BM cells, CCR2-dependent CCL2AF647 uptake was an exclusive feature of CD115+Gr1lo monocytes: nearly all cells in this population (Ly6Chi or Ly6Clo) showed strong CCR2 activity. Virtually all CD115−Gr1lo cells and neutrophils lacked functional CCL2 receptors. As in the spleen, pDCs and CD11c+B220− cells in BM showed biphasic CCL2AF647 uptake, with readily identifiable subsets with differing CCL2 receptor activity. Interestingly, all BM cells expressing active CCR2 in WT mice, particularly Ly6Clo monocytes and pDCs, showed evidence of ACKR2 activity as revealed by the ability of CCL22 to inhibit CCL2AF647 uptake by Ccr2−/− cells (Figure 3A and 3C). Analysis of Ccr2−/− cells provided a reliable reflection of ACKR2 expression by WT cells because unlabelled CCL22 reduced CCL2AF647 uptake by a similar amount when Ccr2−/− and WT BM cells were compared (data not shown), although, as described below, Ccr2 deficiency was associated with a reduction in the ACKR2 activity of pDCs.
Figure 3. Cells expressing CCL2 receptors in mouse BM and blood.
Cells from WT or Ccr2−/− (CCR2 KO) BM (A and C) or blood (B and D) cells were incubated with CCL2AF647 (+/− a 10-fold molar excess of unlabelled CCL22), stained with fluorescently labeled Abs, and examined by flow cytometry. Dead cells and cell doublets have been excluded from all data. Leukocyte subsets were identified using the surface immunophenotype indicated to the right of each histogram overlay and using the gating strategy shown in Supplementary Figure 3. (A-B) Representative overlaid histograms of CCL2AF647 uptake profiles for each of the populations indicated. A, BM; B, blood. (C-D) Mean percentage (+SD) of CCL2AF647 positive cells in each leukocyte subset (n=3). CCL2AF647 positive WT cells were defined based on arbitrary gates set using equivalent populations of CCR2 KO BM cells that had been incubated with CCL2AF647 and CCL22: the percentage of cells in this gate is shown (grey columns). Data were analyzed using one-way ANOVA with Tukey post-test, *p<0.05, ***p<0.001. Three of more repeat experiments generated similar datasets.
Circulating peripheral blood cells had a CCL2AF647 uptake profile that was similar to BM (Figure 3B and 3D), although there were several notable differences. First, CCR2 activity was lower on most Ly6Clo monocytes. Second, in contrast to CD11b+Gr1loCD115−Ly6C+ cells in the BM, cells with this surface phenotype in the blood had a CCR2-dependent CCL2AF647 uptake profile comparable to Ly6Chi monocytes. And third, active ACKR2 was only reproducibly detectable on pDCs.
Collectively, these data reveal marked qualitative differences in CCL2 receptor usage between leukocyte subsets. In addition, by using the extent of CCL2AF647 uptake to gauge scavenging potential, it is clear that CCR2 is capable of mediating much more CCL2 scavenging than ACKR2, and that Ly6Chi monocytes have a greater capacity for CCL2 scavenging than any other leukocyte subset examined.
CCL2 receptors in mouse pDCs
We were interested in the biphasic CCL2AF647 uptake profiles of pDC, and the evidence that these cells can express CCR2 and ACKR2. Previous work using the anti-mouse CCR2 antibody MC-21 (29) has reported that CCR2 can only be detected on 15-25% of mouse BM pDCs (30, 31), while a slightly higher proportion of pDCs in the spleen and lymph node carry surface CCR2 (31). However, our data indicate that virtually all WT pDCs carry functionally competent CCL2 receptors, and that pDCs can be subdivided into two subsets based on CCL2AF647 uptake (CCL2hi and CCL2lo). We were satisfied with our definition of pDCs as CD11c+B220+Ly6C+ CD11b− cells, because they expressed SiglecH and PDCA1 (CD317/BST2), two surface proteins highly restricted to pDCs (Supplementary Figure 4A). These pDC markers were absent from most CD11c+B220+ cells lacking Ly6C or expressing CD11b (Supplementary Figure 4A). To further examine CCL2 receptors on pDCs, we undertook CCL2AF647 uptake experiments in the presence or absence of excess unlabelled CCL22, using cells from WT and Ccr2−/− BM, spleen, blood, inguinal lymph node and mesenteric lymph node (Figure 4). Biphasic CCL2AF647 uptake profiles were seen for WT pDC populations from all anatomical locations. In WT lymph nodes and blood, CCL22 only significantly reduced CCL2AF647 uptake by CCL2lo pDCs. This was seen with WT splenic and BM pDCs as well, although the size of the CCL2hi pDC population was also reduced when CCL22 was present. These data indicated that CCL2AF647 uptake by WT CCL2lo pDCs was mediated primarily by ACKR2, and that, consistent with the observations of others (30, 31), only a subset of WT pDC express CCR2. However, it was notable that the CCL2lo pDC population was also affected by Ccr2 deletion. In all tissues examined, Ccr2−/− pDCs displayed levels of CCL2AF647 uptake that were markedly lower than the majority of WT pDCs, including most of the CCL2lo pDC. Thus, WT pDCs can express functionally competent CCR2 and/or ACKR2, and genetic deletion of CCR2 reduces ACKR2-mediated CCL2AF647 internalization by these cells.
Figure 4. pDCs express CCR2 and ACKR2.
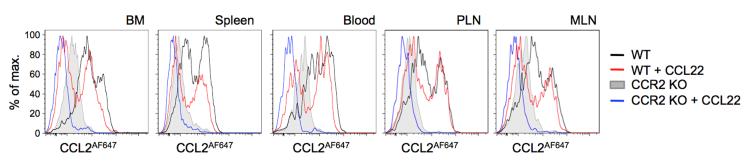
Cells from blood and the tissues indicated of WT and Ccr2−/− (CCR2 KO) mice were incubated with CCL2AF647 +/− a 10-fold molar excess of CCL22. pDC were identified as shown in Supplementary Figures 2-4. Overlaid histograms are shown of CCL2AF647 uptake by pDCs and are representative of data from three or more repeat experiments, each containing at least three mice per genotype. MLN, mesenteric lymph node; PLN, skin-draining peripheral lymph node.
Ly6Chi monocytes efficiently scavenge CCL2AF647 in live mice
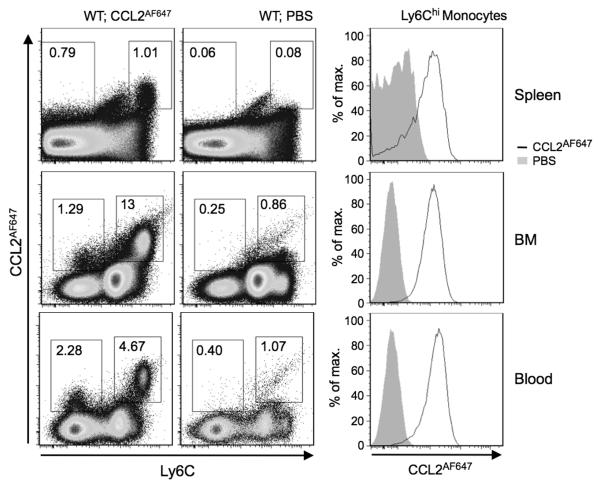
We next considered whether the scavenging potential of leukocytes revealed by ex vivo CCL2AF647 uptake assays accurately reflected their scavenging potential in vivo. To do this, CCL2AF647 was injected i.v. into live WT mice, and leukocytes in the spleen, BM and blood were assessed by flow cytometry. Control mice received carrier (PBS) alone. As in the ex vivo CCL2AF647 uptake assays, more CCL2AF647 was internalized by Ly6Chi cells than by Ly6C−/int cells (dot-plots; Figure 5), and the overall profile of CCL2AF647-labelled cells was similar to that seen after ex vivo labeling (compared Figure 5 to Figure 2). The Ly6Chi CCL2AF647-positive cells in spleen, blood and BM were Ly6Chi monocytes, as expected (histogram plots; Figure 5). All Ly6Chi monocytes in BM and blood were CCL2AF647-positive. However, some splenic Ly6Chi monocytes were poorly labeled perhaps because the injected CCL2AF647 did not access the subcapsular red pulp where these cells are known to reside (32).
Figure 5. Ly6Chi monocytes scavenge CCL2AF647 in live mice.
1μg of CCL2AF647 in PBS, or PBS alone, was injected i.v. into WT mice. Cells were isolated from spleen, BM and blood 2h later, labeled with fluorescently labeled Abs, and analyzed by flow cytometry. In the dot-plots, CCL2AF647 uptake is plotted against Ly6C expression. The percentage of cells in each gate, as a proportion of live cells, is shown. The histograms show CCL2AF647 uptake by Ly6Chi monocytes, identified as in Supplementary Figures 2 and 3. Plots are representative of data from two independent experiments, each containing at least three mice per treatment.
Thus, Ly6Chi monocytes have the greatest capacity for CCL2 scavenging in vivo, while CCR2- or ACKR2-mediated CCL2 uptake by Ly6C−/int cells only makes a minor contribution to the total CCL2 scavenging achieved by leukocytes.
Cell-specific interactions between CCR2 and its ligands
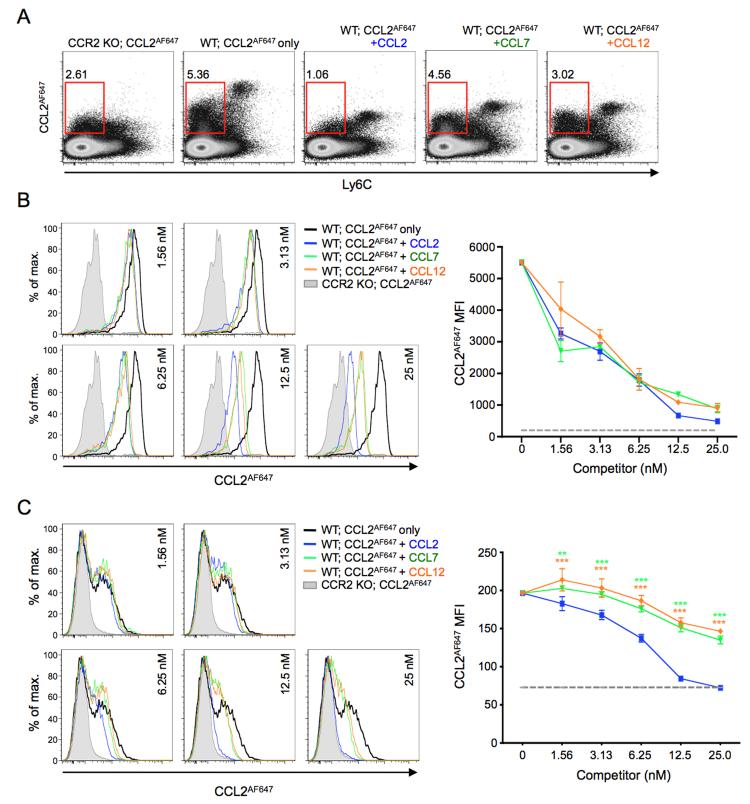
Next, we explored the nature of the interaction of CCL7 and CCL12 with CCR2 and ACKR2 on mouse leukocytes. Fluorescently labeled versions of these chemokines were not available, so we examined this indirectly by assessing the ability of unlabelled versions of these chemokines to interfere with CCL2AF647 uptake. We initially assessed CCL2AF647 uptake by WT splenocytes co-incubated with or without 25nM of unlabelled CCL2, CCL7 or CCL12 (Figure 6A). Ccr2−/− splenocytes incubated with CCL2AF647 alone were included as a control. Interestingly, each competitor chemokine left a unique profile of residual CCL2AF647 uptake. All three chemokines substantially reduced uptake by Ly6Chi cells, although CCL7 and CCL12 were somewhat less effective than CCL2. As expected, CCL2 had more impact than CCR2 deletion on CCL2AF647 uptake by Ly6C− cells because of its ability to interfere with ACKR2-mediated uptake (20). However, CCL12 and CCL7 were much less effective than CCL2 at blocking CCL2AF647 uptake by Ly6C− cells. The ligand specificity of ACKR2 contributes to these differences: 25nM CCL2 and CCL12 completely inhibited CCL2AF647 uptake by MZ B cells, while CCL7 had no effect (data not shown). However, the data indicated that CCL7 and CCL12 differ from CCL2 in their ability to interact with CCR2.
Figure 6. Mouse CCR2 ligands show unique, cell type-specific interactions with CCR2.
(A) Representative flow cytometry profiles showing CCL2AF647 uptake by WT and Ccr2−/− (CCR2 KO) splenocytes in the presence or absence of 25nM unlabelled CCL2, CCL7 or CCL12, as indicated. Cells were separated according to Ly6C expression. Red boxes gate CCL2AF647-positive Ly6C− cells, and the percentage of cells in this gate, as a proportion of live cells, is shown. (B-C) Left panels: Representative overlaid histogram plots showing CCL2AF647 uptake by live (B) Ly6Chi monocytes or (C) CD11b+Ly6C− cells from WT and Ccr2−/− (CCR2 KO) spleens in the presence or absence of a range of concentrations of unlabelled CCL2, CCL7 or CCL12, as indicated. The right panels show the average mean fluorescent intensity (MFI) (+/−SD) of CCL2AF647 uptake by (B) Ly6Chi monocytes or (C) CD11b+ Ly6C− cells (n=3 WT mice). CCL2AF647 uptake by CCR2 KO cells is indicated by the grey dotted line. Data were analyzed by two-way ANOVA with Bonferroni post test, **p<0.01, ***p<0.001 (green, CCL2 vs. CCL7; orange, CCL2 vs. CCL12). Dead cells and cell doublets have been excluded from all data shown. Two or more repeat experiments yielded similar results.
To explore this in more detail, we compared the impact of a range of concentrations of unlabelled chemokine on the high CCR2-dependent CCL2AF647 uptake of splenic Ly6Chi monocytes, and, in the same samples, on the lower levels of CCL2AF647 uptake achieved by CD11b+Ly6C− cells (Figure 6B-C). There are no ACKR2-expressing splenocytes in the CD11b+Ly6C− population, and their CCL2AF647 uptake is mediated by CCR2 expressed primarily by NK cells (33) and cDCs (Supplementary Figure 2). When Ly6Chi monocytes were examined, even the lowest concentration (~1.5nM) of all chemokines inhibited some CCL2AF647 uptake, and 25nM reduced uptake to levels only slightly higher than Ccr2−/− cells (Figure 6B). CCL2 was marginally more effective than CCL7 or CCL12 at higher concentrations. With CD11b+Ly6C− cells, CCL2 showed a dose response curve for inhibition of CCL2AF647 uptake that was similar to that seen with Ly6Chi monocytes. However, CCL7 and CCL12 were much less effective competitors (Figure 6C). Even when they were present at 25nM, these two chemokines only partially inhibited CCL2AF647 uptake by these cells. Thus, the way that CCL7 and CCL12 interact with CCR2 depends on which cell type is expressing the receptor.
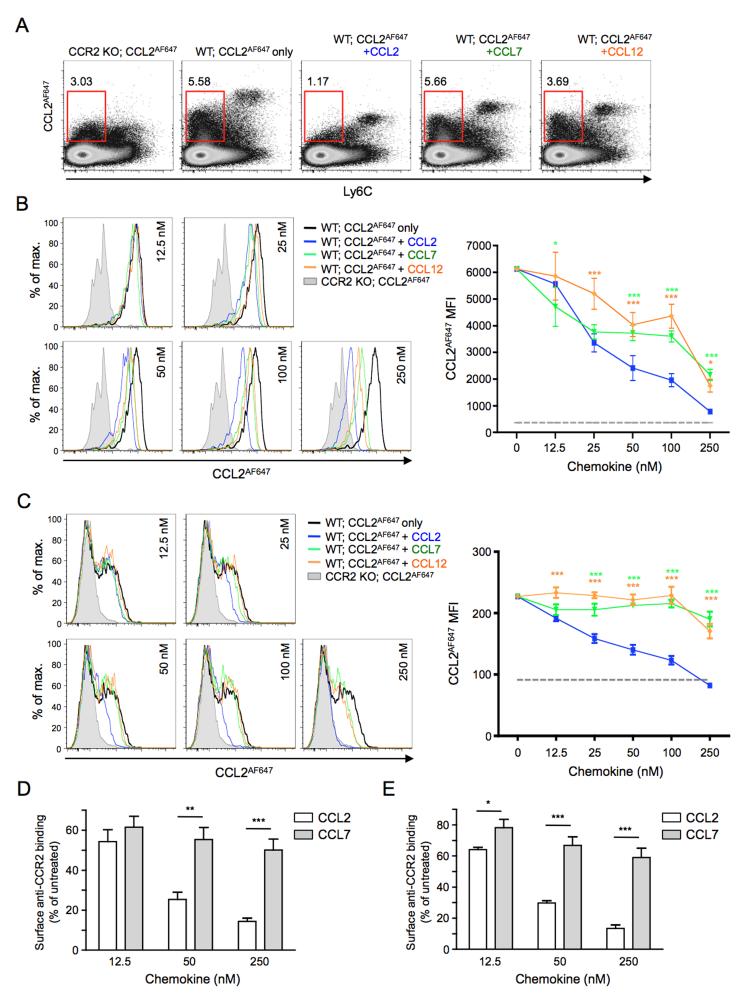
Further evidence of differences in CCR2 between cell types emerged when the ability of pre-incubation with CCL7 and CCL12 to prevent CCR2-mediated CCL2AF647 uptake was examined (Figure 7). Splenocytes were exposed to unlabelled chemokine for 30mins at 37°C, washed thoroughly at 4°C, and then their CCL2AF647 uptake properties were assessed. Compared to co-incubation (Figure 6), much higher concentrations of chemokine were required to inhibit CCL2AF647 uptake using this pre-incubation approach. For example, pre-exposure to 12.5nM CCL2 had barely any impact on subsequent CCL2AF647 uptake (Figure 7B-C), but nearly completely blocked CCL2AF647 internalization when it was included during the uptake period (Figure 6B-C). Moreover, only pre-incubation with 250nM CCL2 inhibited uptake to a level approaching that seen by Ccr2−/− cells (Figure 7). However, as in the co-incubation experiments, pre-incubation with each chemokine left a distinct CCL2AF647 uptake profile (Figure 7A). CCL2 was more effective than CCL7 or CCL12 at reducing uptake by Ly6Chi monocytes (Figure 7B), and this difference between chemokines was even more striking when CD11b+Ly6C− cells were examined. With these cells, exposure to 100nM CCL7 or CCL12 was unable to prevent any subsequent uptake of CCL2AF647, and only minimal inhibition was achieved at 250nM, while equivalent concentrations of CCL2 resulted in strong suppression of CCL2AF647 uptake (Figure 7C).
Figure 7. Ligand- and cell type-specific modification of CCR2 behavior.
(A) Representative flow cytometry profiles showing CCL2AF647 uptake by WT and Ccr2−/− (CCR2 KO) splenocytes that had been pre-incubated for 30 minutes with or without 250nM unlabelled CCL2, CCL7 or CCL12, as indicated. Cells were separated according to Ly6C expression. Red boxes gate CCL2AF647-positive Ly6C− cells, and the percentage of cells in this gate, as a proportion of live cells, is shown. (B-C) Left panels: Representative overlaid histogram profiles showing CCL2AF647 uptake by (B) Ly6Chi monocytes or (C) CD11b+Ly6C− cells from WT and Ccr2−/− (CCR2 KO) spleens pre-incubated for 30 minutes with or without a range of concentrations of unlabelled CCL2, CCL7 or CCL12, as indicated. The right panels show the average mean fluorescent intensity (MFI) (+/−SD) of CCL2AF647 uptake by (B) Ly6Chi monocytes or (C) CD11b+ Ly6C− cells (n=3 WT mice). CCL2AF647 uptake by CCR2 KO cells is shown by the grey dotted line. Data were analyzed by two-way ANOVA with Bonferroni post test, *p<0.05, ***p<0.001 (green, CCL2 vs. CCL7; orange, CCL2 vs. CCL12). (D) WT and Ccr2−/− splenocytes were incubated for 30 minutes with or without unlabelled CCL2 or CCL7 (12.5nM, 50nM or 250nM), and Ly6Chi monocytes then examined for their ability to bind anti-CCR2 antibody. (E) Cells were treated as in D, except that, before labeling with antibodies, cells that had been exposed to unlabeled chemokines were washed thoroughly and allowed to internalize CCL2AF647 for 1h at 37°C. In D and E, the average mean fluorescence intensity (MFI) of anti-CCR2-stained Ccr2−/− cells was subtracted from the MFI of anti-CCR2-stained WT cells. Anti-CCR2 binding to chemokine-treated cells was then calculated as a percentage of that seen with WT cells that had not been exposed to any chemokine (mean (+SEM) n=3). Data were analyzed using a Student’s t test: *p<0.05, **p<0.01, ***p<0.001. Dead cells and cell doublets have been excluded from all data shown. Two or more repeat experiments gave comparable results.
We also wished to explore the impact of CCL2 and CCL7 on the surface anti-CCR2 immunoreactivity of Ly6Chi monocytes and CD11b+Ly6C− cells from WT spleens. Only one of four commercially available anti-mouse CCR2 antibodies tested showed specificity for CCR2 (determined by comparing the flow cytometry profiles of untreated WT and Ccr2−/− cells (data not shown)), and while this antibody (monoclonal 475301) provided robust detection of CCR2 on Ly6Chi monocytes it was limited in its ability to detect CCR2 on untreated CD11b+Ly6C− cells. Nonetheless, it allowed us to explore if exposure to chemokine resulted in changes in anti-CCR2 antibody binding to WT Ly6Chi monocytes, and if CCL2 and CCL7 differed in their ability to modulate this binding (Figure 7D-E). When used at 12.5nM, CCL2 and CCL7 both reduced subsequent anti-CCR2 antibody binding to Ly6Chi monocytes by 40-50% (Figure 7D). However, at higher concentrations (50 or 250nM), CCL2 was able to further reduce anti-CCR2 antibody binding to these cells, but CCL7 was far less effective (Figure 7D). Similar results were obtained if Ly6Chi monocytes pre-exposed to CCL2 or CCL7 were allowed to internalize CCL2AF647 before binding of the anti-CCR2 antibody was examined (Figure 7E). Thus, CCL2 and CCL7 clearly differ in their ability to modify CCR2 on the surface of Ly6Chi monocytes, and this could contribute to the differences in CCL2AF647 uptake seen after pre-treatment with these chemokines.
Collectively, these data reveal that CCL2, CCL7 and CCL12 have unique properties that are determined, at least in part, by the ligand binding capacity of ACKR2, and the cell type-specific ligand recognition properties and ligand-specific responsiveness of CCR2.
Discussion
Fluorescent chemokines complement existing tools, such anti-chemokine receptor Abs or reporter gene ‘knock-in’ mice, that are typically used to identify cells expressing chemokine receptors (34-36). However, our study shows that fluorescent chemokine uptake assays have a number of added benefits. First, because they exploit the inherent specificity of chemokines for their receptors, we find that they are easier to control than Ab-mediated detection methods. In our hands, CCL2AF647 uptake is more sensitive, reliable and reproducible than immunostaining with commercial anti-mouse CCR2 Abs as a way of detecting mouse cells expressing CCR2. Moreover, cells can be identified that express ACKR2, for which there is no Ab available for use in mice. Second, the only cells labeled are those carrying functionally competent chemokine receptors i.e. receptors able to bind and internalize the labeled chemokine. ACKR2 internalizes chemokine without chemokine-induced signaling, but activation of CCR2 is required for the internalization of CCL2/CCR2 complexes. Thus, cells showing CCR2-dependent CCL2AF647 uptake carry CCR2 molecules capable of initiating intracellular signaling upon CCL2 binding. Third, all cells carrying functionally competent cCKRs and/or ACKRs for the labeled chemokine are labeled in these assays. As a result, they provide a comprehensive picture of how that chemokine is sensed and regulated by complex mixtures of cells. Fourth, they reveal the chemokine scavenging potential of different leukocyte subsets. Ly6Chi monocytes are the most effective CCL2 scavengers and CCR2 mediates much more scavenging than ACKR2. Finally, fluorescent chemokines can be used to define the ligand specificity of cCKRs and ACKRs on primary cells, and compare how chemokines interact with these receptors when expressed by different cell types in the same sample. Our data indicate that CCL2, CCL7 and CCL12 will have distinct properties in vivo because of the unique ways in which they interact with ACKR2 and CCR2. For all these reasons, we consider fluorescent chemokine uptake assays to be a particularly versatile method that has broad applicability in the detection and analysis of chemokine receptors on primary cells. Indeed, we have successfully used them to detect and characterize CCR1, CCR5, CCR7, CXCR2, CXCR3 or ACKR4 on primary cells, and found that single fluorescent chemokines work across a variety of mammalian species (unpublished observations).
As expected, functionally competent CCR2 is expressed by all Ly6Chi monocytes in BM, blood and spleen. Ly6Clo monocytes in mouse blood show heterogeneous CCR2 activity. These cells develop from circulating Ly6Chi monocytes, during which they down-regulate CCR2 and Ly6C, and acquire more CX3CR1 (37, 38). It is likely therefore that the continuum of CCR2 activity amongst Ly6Clo monocytes is a reflection of their differentiation status. In line with previous reports (10, 14), we found that Ccr2 deficiency was associated with a reduction in the number of Ly6Chi monocytes in spleen (by ~75%) and blood (by ~90%), and a ~3-fold increase in their abundance in BM (data not shown). Interestingly, blood CD11b+Gr1loLy6C+ cells lacking CD115, which have a CCL2AF647 uptake profile that is indistinguishable from Ly6Chi monocytes, were also reduced by ~90% in the blood of Ccr2−/− mice (p<0.01; 5 mice per group), although the number of cells in the BM with this surface phenotype was unaffected by CCR2 loss (data not shown). The function and identity of these cells is uncertain. Expression of CD115, the MCSF/IL24 receptor, is used as a defining feature of monocytes and related macrophage/DC precursors (39), so it is perhaps not appropriate to refer to them as monocytes. However, their similarities with Ly6Chi monocytes suggest they are CD115-negative versions of these cells. It will be important to determine the origins of these cells, dissect their differentiation potential, and define their contribution to immune defects in Ccr2−/− mice.
Some populations of leukocytes, such as neutrophils, lack CCL2 receptors but several others, such as γδ T cells, cDCs and DX5+ (NK) cells, contain subsets carrying functionally competent CCR2. These CCR2+ cells have the potential to migrate in response to CCL2, and CCR2 on γδ T cells, cDCs and NK cells is known to be indispensible in certain contexts (8, 9, 11). Innate-like B cells express ACKR2, but not CCR2, but both these CCL2 receptors are clearly expressed by some populations of cells, including pDCs and BM Ly6CloCD115+ monocytes.
pDCs can be separated into CCL2lo and CCL2hi subsets. In general, in WT mice, CCL2lo pDCs express ACKR2 while CCL2hi pDC carry CCR2, but some splenic and BM pDCs appear to co-express these receptors. pDCs migrate towards CCL2 in vitro ((31) and Supplementary Figure 4B-D), and CCL2 recruits pDCs into the skin after imiquimod treatment to contribute to tumor cell killing (12). We have not been able to determine if CCL2hi and CCL2lo pDCs show differential chemotactic activity, because the exposure to CCL2AF647 required for their fractionation prevents subsequent migratory responses. We could not separate these pDC subsets in other ways either, because differential CCL2AF647 uptake activity does not co-segregate with other markers that can divide the pDC population into functionally distinct subsets (e.g. CD4, CD8, CD9, CCR9, Ly49Q) (data not shown), although it was notable that nearly all the rare CCR9− pDCs are in the CCL2hi subset (Supplementary Figure 4E). CCR9− pDCs can act as DC precursors and give rise to cDCs and pDCs (40, 41), while CCR9+ pDCs are reportedly enriched for tolerogenic activity (40, 42, 43) and can home to the small intestine (30). CCR2 is responsible for CCL2-induced cell migration, but the role of ACKR2 on pDCs is unclear. It may act solely as a scavenger, but it was only weakly active in this regard in the CCL2AF647 uptake assays. Other functions are perhaps more likely, and it is interesting that ACKRs, including ACKR2, have been shown to modulate co-expressed cCKRs (3). For example, CXCR4 and ACKR3 (the cCKR and ACKR respectively for CXCL12) are co-expressed by migrating interneurons during brain development and deletion of Ackr3 disrupts CXCR4-mediated signaling (44). Conversely, when Ackr2 is deleted, B1 B cells become more responsive to CXCL13 and can, unlike WT B1 B cells, respond weakly to some pro-inflammatory chemokines (20). Thus, ACKR2 on pDCs may control co-expressed cCKRs, including CCR2 and the closely-related receptor CCR5, which is expressed by all pDCs, shares ligands with ACKR2 (e.g. CCL3 and CCL4), and controls pDC release from the BM (31). Experiments are underway to explore these ideas and to examine if the CCL2lo and CCL2hi subsets represent functionally distinct populations of pDCs.
Our data reveal the CCL2 scavenging potential of individual leukocyte subsets. On a cell-by-cell basis, and after labeling ex vivo or in vivo, Ly6Chi monocytes internalize much more CCL2AF647 than any other leukocyte population. These cells are abundant in blood and lymphoid tissues, and use CCR2 to navigate into inflamed tissues in large numbers. Since their CCL2AF647 uptake can only be effectively blocked by relatively high concentrations of CCR2 ligand (Figure 7), they are likely to be able to scavenge chemokines through CCR2 during, and after, their recruitment into tissues. Indeed, elegant experiments performed by Volpe and colleagues demonstrated that migrating monocytes internalize substantial quantities of fluorescent CCL2, while retaining their ability to respond to gradients of this chemokine (4). Collectively, these data indicate that chemokine scavenging by CCR2 in vivo, which has been shown to regulate chemokine abundance at steady state and during inflammation induced by intratracheal LPS challenge or the implantation of allogeneic tissue (5-7), is most likely driven primarily by Ly6Chi monocytes. In contrast, ACKR2, which is considered to be a ‘professional’ chemokine scavenger (3), has only very low CCL2 scavenging potential on leukocytes. It seems unlikely that it will have a major impact on chemokine abundance in a tissue, and may instead operate only at specific microanatomical niches, such as the splenic MZ. Indeed, anatomically restricted ACKR2-mediated scavenging serves a key role in the skin (3, 45). Lymphatic endothelial cells in this tissue express ACKR2 to prevent them from becoming coated in inflammatory chemokines. This is important because it stops leukocytes accumulating around these vessels and interfering with the flow of tissue fluid and mature dendritic cells from the skin (45).
CCL2, CCL7 and CCL12 clearly differ in the way they interact with leukocytes. CCL2 and CCL12, but not CCL7, are ligands for ACKR2 (20), and the interaction of CCL7 and CCL12 with CCR2 is influenced by cell background. The data suggest that CCR2 exists in two forms. DX5+ (NK) cells and cDCs in the splenic CD11b+Ly6C− population express a version of CCR2 that is more readily activated by CCL2 than by CCL7 or CCL12. In contrast, since low concentrations of CCL2, CCL7 or CCL12 (up to 12.5nM) co-incubated with CCL2AF647 show very similar abilities to block fluorescent chemokine uptake by Ly6Chi monocytes, it appears that the dominant form of CCR2 on these cells does not readily discriminate between these three chemokines. However, at higher concentrations (50nM or above) CCL2 is more effective than CCL7 at reducing anti-CCR2 antibody binding to these cells, suggesting that it drives more extensive CCR2 internalization under these conditions. The molecular bases and biological implications of these observations are under investigation. In humans, alternative splicing generates two isoforms of CCR2 (termed CCR2A and CCR2B) (46), but there is no evidence that equivalent diversity exists in mice. However, differences in post-translational processing or heterodimerization could conceivably result in cell-specific differences in CCR2 behaviour. Human CCR2 can heterodimerise and hetero-oligomerise with human CCR5 and CXCR4, and negative binding cooperativity exists between these receptors in transfected cell lines and primary human cells (47-50). According to microarray data available through Immgen (www.immgen.org), the CCR2+ cell types examined in our study express CXCR4 but differ in their level of CCR5 expression. Thus, the precise nature of the dimers and higher order structures involving CCR2 could vary between Ly6Chi monocytes and CD11b+Ly6C− cells to modulate the behavior of CCR2. Moreover, the ability of CCL7 and CCL12 to interact with a broader array of chemokine receptors than CCL2 may also be relevant.
We expect that the high expression and enhanced ligand binding properties of CCR2 on Ly6Chi monocytes will make these cells more responsive than other CCR2+ cells to low concentrations of CCL7 and CCL12. Interestingly, under resting conditions, this form of CCR2 is only expressed by cells whose steady state trafficking is affected by deficiency in Ccr2 (i.e. Ly6Chi monocytes, CD115+ cells in the BM, and the circulating CD115−Ly6C+ monocyte-like cells discussed above) (data not shown) (10, 13, 14). It is possible that this form of CCR2 is specifically required for monocyte navigation out of the BM, a process dependent on both CCL2 and CCL7 (13, 14). It might also endow Ly6Chi monocytes with greater sensitivity than other CCR2+ cells when it comes to CCR2-dependent recruitment into inflamed tissues, and it will be of interest to see if other leukocytes switch to a Ly6Chi monocyte-like form of CCR2 to help facilitate their migration during inflammation or infection. Studies are also underway to examine if the ligand recognition properties of other chemokine receptors are, like CCR2, subject to cell-specific modulation.
Chemokine receptors are attractive therapeutic targets in many diseases. Some chemokine receptor antagonists have reached the clinic, but many others have failed and evidence of effective inhibition chemokine receptor function in vivo is often lacking (51). Future efforts in this area will benefit from work that builds a greater understanding of the expression and functional properties of cCKRs and ACKRs in humans and experimental animals. Fluorescent chemokines will be a valuable tool in this type of work. Moreover, since they detect functionally active chemokine receptors, fluorescent chemokines could be used to assess receptor activity in blood samples during pre-clinical and clinical trials. This would determine if the drug being used has true potency against its target in vivo. We think that this could be particularly useful in trials that fail to ameliorate disease, because robust evidence of in vivo activity will strengthen the conclusion that the receptor under investigation is not a good target in that disease, and inspire confidence in its use in subsequent trials in other pathologies.
Supplementary Material
Acknowledgements
RJBN acknowledges support services provided by Dr A Wilson.
Source of support: This work was supported by the Wellcome Trust (L.B.F., R.J.B.N.), the Biotechnology and Biological Sciences Research Council (C.A.H.H., R.J.B.N.), the Medical Research Council (C.A.H.H., G.J.G), the TIMER EU consortium grant (C.A.H.H., G.J.G), and Arthritis Research UK (V.K., S.W.F.M.). A Medical Research Council Program Grant funds work in the labs of G.J.G. and R.J.B.N..
Abbreviations used in this article
- ACKR
atypical chemokine receptor
- CCL2AF647
AlexaFluor®-647 tagged CCL2
- cCKR
conventional chemokine receptor
- cDCs
conventional dendritic cells
- MZ
marginal zone
- pDCs
plasmacytoid dendritic cells
Footnotes
The authors have no conflicts of interest.
References
- 1.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadière C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, Matsushima K, Murphy PM, Nibbs R, Nomiyama H, Power CA, Proudfoot AEI, Rosenkilde MM, Rot A, Sozzani S, Thelen M, Yoshie O, Zlotnik A. International Union of Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nibbs RJB, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 4.Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. CCR2 Acts as Scavenger for CCL2 during Monocyte Chemotaxis. PLoS ONE. 2012;7:e37208. doi: 10.1371/journal.pone.0037208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Bohle RM, Seeger W, Lohmeyer J. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol. 2005;288:L350–8. doi: 10.1152/ajplung.00061.2004. [DOI] [PubMed] [Google Scholar]
- 7.Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, Gladue RP. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine. 2002;18:184–190. doi: 10.1006/cyto.2002.1031. [DOI] [PubMed] [Google Scholar]
- 8.van Helden MJG, Zaiss DMW, Sijts AJAM. CCR2 defines a distinct population of NK cells and mediates their migration during influenza virus infection in mice. PLoS ONE. 2012;7:e52027. doi: 10.1371/journal.pone.0052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lança T, Costa MF, Gonçalves-Sousa N, Rei M, Grosso AR, Penido C, Silva-Santos B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J Immunology. 2013;190:6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by the chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 11.Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J Exp Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive Roles for MCP-1 and MCP-3 in CCR2-Mediated Recruitment of Inflammatory Monocytes during Listeria monocytogenes Infection. J Immunol. 2008;180:6846–4853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsou C-L, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayr FB, Spiel AO, Leitner JM, Firbas C, Schnee J, Hilbert J, Derendorf H, Jilma B. Influence of the Duffy antigen on pharmacokinetics and pharmacodynamics of recombinant monocyte chemoattractant protein (MCP-1, CCL-2) in vivo. Int J Immunopathol Pharmacol. 2009;22:615–625. doi: 10.1177/039463200902200307. [DOI] [PubMed] [Google Scholar]
- 16.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJB, Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol. 2003;170:5244–5251. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, Brenner MB, Carroll MC, Mooney DJ, Turley SJ, The Immunological Genome Project Consortium Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:511–518. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansell CAH, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, Goodyear CS, Nibbs RJB. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savino B, Castor MG, Caronni N, Sarukhan A, Anselmo A, Buracchi C, Benvenuti F, Pinho V, Teixeira MM, Mantovani A, Locati M, Bonecchi R. Control of murine Ly6Chigh monocyte traffic and immunosuppressive activities by atypical chemokine receptor D6. Blood. 2012;119:5250–5260. doi: 10.1182/blood-2011-10-388082. [DOI] [PubMed] [Google Scholar]
- 22.Crosslin DR, McDavid A, Weston N, Zheng X, Hart E, de Andrade M, Kullo IJ, McCarty CA, Doheny KF, Pugh E, Kho A, Hayes MG, Ritchie MD, Saip A, Crawford DC, Crane PK, Newton K, Carrell DS, Gallego CJ, Nalls MA, Li R, Mirel DB, Crenshaw A, Couper DJ, Tanaka T, van Rooij FJA, Chen M-H, Smith AV, Zakai NA, Yango Q, Garcia M, Liu Y, Lumley T, Folsom AR, Reiner AP, Felix JF, Dehghan A, Wilson JG, Bis JC, Fox CS, Glazer NL, Cupples LA, Coresh J, Eiriksdottir G, Gudnason V, Bandinelli S, Frayling TM, Chakravarti A, van Duijn CM, Melzer D, Levy D, Boerwinkle E, Singleton AB, Hernandez DG, Longo DL, Witteman JCM, Psaty BM, Ferrucci L, Harris TB, O’Donnell CJ, Ganesh SK, Group CHW, Larson EB, Carlson CS, Jarvik GP, The eMERGE Network Genetic variation associated with circulating monocyte count in the eMERGE Network. Hum Mol Genet. 2013;22:2119–2127. doi: 10.1093/hmg/ddt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber M, Blair E, Simpson CV, O’Hara M, Blackburn PE, Rot A, Graham GJ, Nibbs RJB. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol Biol Cell. 2004;15:2492–2508. doi: 10.1091/mbc.E03-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franci C, Gosling J, Tsou CL, Coughlin SR, Charo IF. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. Critical role of carboxyl-tail serines/threonines in receptor function. J Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- 25.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 27.Flaishon L, Becker-Herman S, Hart G, Levo Y, Kuziel WA, Shachar I. Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration. Blood. 2004;104:933–941. doi: 10.1182/blood-2003-11-4013. [DOI] [PubMed] [Google Scholar]
- 28.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachý J, Brühl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlöndorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 30.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Förster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawai CM, Sisirak V, Ghosh HS, Hou EZ, Ceribelli M, Staudt LM, Reizis B. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J Exp Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 34.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou C-L, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, Jia T, Méndez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sunderkötter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 38.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 40.Schlitzer A, Loschko J, Mair K, Vogelmann R, Henkel L, Einwächter H, Schiemann M, Niess JH, Reindl W, Krug A. Identification of CCR9- murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood. 2011;117:6562–6570. doi: 10.1182/blood-2010-12-326678. [DOI] [PubMed] [Google Scholar]
- 41.Schlitzer A, Heiseke AF, Einwächter H, Reindl W, Schiemann M, Manta C-P, See P, Niess JH, Suter T, Ginhoux F, Krug AB. Tissue-specific differentiation of a circulating CCR9- pDC-like common dendritic cell precursor. Blood. 2012;119:6063–6071. doi: 10.1182/blood-2012-03-418400. [DOI] [PubMed] [Google Scholar]
- 42.Björck P, Leong HX, Engleman EG. Plasmacytoid dendritic cell dichotomy: identification of IFN-α producing cells as a phenotypically and functionally distinct subset. J Immunol. 2011;186:1477–1485. doi: 10.4049/jimmunol.1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, López-Bendito G, Stumm R, Marín O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, Garside P, McDonald V, Jenkins C, Ransohoff R, Liu L, Milling S, Cerovic V, Graham GJ. D6 facilitates cellular migration, and fluid flow, to lymph nodes by suppressing lymphatic congestion. Blood. 2011;118:6220–6229. doi: 10.1182/blood-2011-03-344044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohy D, Parmentier M, Springael J-Y. Allosteric transinhibition by specific antagonists in CCR2/CXCR4 heterodimers. J Biol Chem. 2007;282:30062–30069. doi: 10.1074/jbc.M705302200. [DOI] [PubMed] [Google Scholar]
- 48.Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael J-Y. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–31279. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Asmar L, Springael J-Y, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol. 2005;67:460–469. doi: 10.1124/mol.104.003624. [DOI] [PubMed] [Google Scholar]
- 50.Springael J-Y, Le Minh PN, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric modulation of binding properties between units of chemokine receptor homo- and hetero-oligomers. Mol Pharmacol. 2006;69:1652–1661. doi: 10.1124/mol.105.019414. [DOI] [PubMed] [Google Scholar]
- 51.Schall TJ, Proudfoot AEI. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–363. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.