Abstract
Adhesion molecule signaling is critical to human pluripotent stem cell (hPSC) survival, self-renewal, and differentiation. Thus, hPSCs are grown as clumps of cells on feeder cell layers or poorly defined extracellular matrices such as Matrigel. We sought to define a small molecule that would initiate adhesion-based signaling to serve as a basis for a defined substrate for hPSC culture. Soluble angiopoeitin-1 (Ang-1)-derived peptide QHREDGS added to defined serum-free media increased hPSC colony cell number and size during long- and short-term culture when grown on feeder cell layers or Matrigel, i.e. on standard substrates, without affecting hPSC morphology, growth rate or the ability to differentiate into multiple lineages both in vitro and in vivo. Importantly, QHREDGS treatment decreased hPSC apoptosis during routine passaging and single-cell dissociation. Mechanistically, the interaction of QHREDGS with β1-integrins increased expression of integrin-linked kinase (ILK), increased expression and activation of extracellular signal-regulated kinases 1/2 (ERK1/2), and decreased caspase-3/7 activity. QHREDGS immobilization to polyethylene glycol hydrogels significantly increased cell adhesion in a dose-dependent manner. We propose QHREDGS as a small molecule inhibitor of hPSC apoptosis and the basis of an affordable defined substrate for hPSC maintenance.
Introduction
Human pluripotent stem cells (hPSCs) are maintained in a pluripotent state through a series of interconnected mechanisms that balance survival, inhibition of differentiation, and the promotion of unlimited proliferation. The ability of hPSCs to self-renew and differentiate into multiple lineages offers the possibility of providing an unlimited number of cells for tissue engineering and regenerative medicine applications. However, the procurement of billions of functional cells for regenerative therapies remains a limiting factor due in part to the loss in cell numbers at each stage of manipulation [1] but also to the prohibitive expense of culturing the cells. As such, numerous studies have focused on finding molecular pathways responsible for the dissociation-induced cell death in hPSCs [1–3] and on identifying small molecules that can prevent or protect hPSCs from cell death [4]. The most successful among these molecules is Y-27632, a selective inhibitor of p160-Rho-associated coiled-coil kinase (ROCK), which is now routinely used in hPSC culture [1].
Recently, a growing number of reports have shown that adhesion molecules mediating either cell-to-cell or cell-to-extracellular matrix (ECM) contact contribute to the survival, self-renewal and differentiation potential of hPSCs [5]. E-Cadherin, for example, is a Ca2+-dependent cell surface glycoprotein that mediates cell-cell adhesion [5]. RNAi knockdown of E-Cadherin induces hyperactivation of actomyosin and apoptosis in hESCs [2,3], suggesting that cell-cell adhesion may prevent apoptosis through the regulation of actin-myosin contraction. Similarly, ROCK inhibition has been reported to suppress actomyosin hyperactivation downstream of Rho [3], which may explain the anti-apoptotic effects observed in hPSCs treated with Y-27632 [1]. Cell-ECM interactions have also been shown to be critical for hPSC survival [4]. Integrins are a class of heterodimeric transmembrane receptors that mediate the interaction of cells with the ECM [6]. Upon activation, integrins associate with adaptor and signaling proteins at their cytoplasmic tails [6,7], which includes the protein Integrin-linked kinase (ILK), a specific binding partner of β1- and β3-integrin subunits [8]. ILK additionally binds to Parvin and PINCH to form the IPP complex, which functions both as a scaffold linking integrin receptors to the actin cytoskeleton and as a hub for intracellular signaling molecules [9,10]. Integrin receptors may therefore affect cell-cell interactions indirectly through regulation of the IPP complex, in addition to the direct interaction they have with the ECM. Thus, integrin receptors sit at the crossroads of both cell-cell and cell-ECM interactions and have the potential to be potent regulators of hPSC survival.
Due to the importance of hPSCs to both the fields of tissue engineering and regenerative medicine, significant efforts have been made to determine ideal standardized culture conditions for the expansion and maintenance of hPSCs. While the ultimate objective is to standardize the culture procedure with a defined substrate in combination with a defined serum-free media [11,12], the complexity of the role of the ECM coupled with our poor understanding of all the requirements for hPSC maintenance and differentiation, has necessitated our continued reliance on poorly defined matrices, such as feeder cell layers and Matrigel substrate for hPSCs culture. Matrigel is a purified matrix derived from Engelbreth-Holm-Swarm sarcoma cells that contains various factors and has been shown to support the growth and maintenance of hESCs in feeder-free conditions [13] but is unsuitable for directed differentiation of hPSCs for autologous cell therapy [11] because of lot-to-lot variability, as well as possible viral contaminants [14]. Efforts are underway to produce synthetic wholly-defined surfaces that display proteins, such as laminin [15,16], vitronectin [17] and/or E-cadherin [12,18], or proprietary cell adhesion peptides (Corning Synthemax™ plates) to support self-renewal and expansion of hESCs and iPSCs in serum-free defined media [19–21] but as yet, feeder cell layers and Matrigel remain the most widely used substrates. Additionally, a substrate that promotes cell adhesion as well as cell survival would be beneficial.
Angiopoeitin-1 (Ang-1) is a secreted glycoprotein that acts as a ligand for the membrane receptor Tie2 on endothelial cells through which it regulates vascular maturation during development and promotes endothelial cell survival and adhesion [22]. However, in cell types that do not express Tie2, Ang-1 binds to integrins [23] and has been demonstrated to promote survival more effectively than other traditional integrin-binding proteins, including ECM proteins containing the RGD-motif [24]. While Ang-1 lacks a common integrin-binding motif, the highly conserved sequence QHREDGS was identified [24] and this peptide fragment was found to be capable of inducing cell survival and adhesion in non-endothelial cell types [25,26], as well as in endothelial cells wherein the effects of QHREDGS were inhibited by antiintegrin antibodies [27]. Given the aforementioned central role of integrins in adhesion and that hPSCs rely heavily on adhesion—both cell-cell and cell-ECM—to promote survival, self-renewal, and differentiation [5], we postulated that treatment of hPSCs with the Ang-1-derived integrin-binding fragment QHREDGS may enhance hPSC adhesion and thereby promote hPSC survival during both clump and single-cell passaging of hPSCs. Moreover, we also hypothesize that the peptide QHREDGS could be used to produce an affordable defined culture surface for hPSCs.
Specifically, we sought to determine if the small Ang-1 derived peptide QHREDGS could act as a soluble small molecule inhibitor of hPSC apoptosis to improve cell survival in existing culture conditions, and if QHREDGS could be used in the design of a defined culture surface with significantly improved hPSCs adhesion. We cultured hPSCs both long- and short-term in the presence of QHREDGS to determine the effect of QHREDGS treatment on hPSC morphology, proliferation or pluripotency. We also conjugated QHREDGS to a defined surface—a non-adhesive polyethylene glycol (PEG) hydrogel—to investigate the effect on hPSC adhesion. Furthermore, we investigated the mechanism by which QHREDGS affects hPSCs survival and adhesion by determining the interaction of QHREDGS with β1-type integrin receptors on the hPSC surface and the downstream effectors ILK, ERK1/2, AKT and caspase activity.
Materials and Methods
Antibodies
A complete list of antibodies is provided in the Supplementary Data.
Stem Cell Culture on Feeder Cell Layers
Normal human induced pluripotent stem cell (hiPSC) lines—BJ1D, 0901B, IMR90 (3)—and the human embryonic cell line, H9, were generated using standard retroviral or lentiviral mediated reprogramming and validated as previously described [28–30]. Different cell lines were used to validate results and the specific lines used in a given experiment are designated in the figure legends. Cells were cultured on a feeder layer of mouse embryonic fibroblast (MEF) cells (Mount Sinai Hospital, Toronto, ON) inactivated with 10µg/ml mitomycin C and seeded at 1.8 × 105 per 3.5cm dish. For single-cell passaging experiments, hiPSC colonies were dissociated in 0.25% Trypsin-EDTA for 5min, followed by trituration with a pipette. To ensure a single-cell suspension, the dissociated cells were passed through a 40µm cell strainer and seeded onto MEFs at a density of 100 cells/10-cm plate. Cells were allowed to grow for 7 days and the total number of colonies was counted. In the treated groups, QHREDGS (Biomatik, Cambridge, ON) or DGQESHR (Biomatik) dissolved in PBS (Lonza, Allendale, NJ) was added at the reported concentrations to culture media at the onset of the first treatment passage and replenished daily with each media change thereafter. The peptides or equivolume PBS (0.4% of volume) were included during clump or single-cell passaging. Cells were analyzed after 5 passages in continuous treatment with 50µM QHREDGS, 50µM DGQESHR or equivolume PBS (0µM QHREDGS), unless specified otherwise.
Live/Dead Staining and Immunohistochemistry
Human iPSCs were treated for 5 passages and stained for CFDA-Live/PI-Dead as described [31]. Live cells were counted using the cell counter plug-in of the ImageJ software (NIH, Bathesda, MD). Immunohistochemistry was performed as described [31]. Cells were fixed in 4% paraformaldehyde and the following primary antibodies were used: Oct4 (1:500), SSEA-4 (1:300), Ki67 (1:300), SMA (1:200), Gata6 (1:300), and β-III Tubulin (1:100), anti-BrdU (1:300). All secondary antibodies were used at 1:200 dilutions. DAPI counterstain was used at 300nM.
BrdU Proliferation Assay
After 5 passages in treatment, hiPSCs were passaged as described above in the presence of 50µM QHREDGS, 50µM DGQESHR or equivolume PBS (0µM QHREDGS) onto MEF plates and grown for 3 days. Cultures were pulsed for 1h with 10µM BrdU (Sigma Aldrich) and immediately stained and analyzed for immunohistochemistry (see below). Ki67 (1:300) co-staining was used to differentiate MEFs from hiPSCs. All samples were counterstained with DAPI (300nM, Sigma Aldrich).
FACS Analysis
Analytical flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences) as described [32] with an additional permeabilization step with 0.25% Triton-X (Sigma Aldrich) for 10 min at 4°C. All antibodies were used at 1:100.
Human iPSC Differentiation and Teratoma Assay
Following 5 passages in continuous treatment, hiPSCs were collected and differentiated into embryoid bodies (EBs) containing cells of all three germ layers as described [33]. For further differentiation and analysis, EBs were plated onto plates coated with 0.2% gelatin for another 10 days then fixed in 4% paraformaldehyde for immunohistochemistry. In vivo differentiation was assessed by teratoma assays performed following 10 passages of BJ1D hiPSCs in continuous treatment with QHREDGS. Specifically for this assay, cells were treated for 2h with 10µM Y-27632 ROCK inhibitor (Sigma Aldrich) prior to collection to ensure that a sufficient number of cells survived through to implantation. Cells were harvested following 15min incubation in 1mg/ml collagenase (STEMCELL Technologies, Vancouver, BC) and resuspended in 1 volume Teratoma mix (2:1:2 ratio of: Knockout DMEM, Life Technologies; hESC-qualified Matrigel, BD Biosciences; Collagen, STEMCELL Technologies) and injected into NOD.CB17-Prkdcscid/J (NOD-SCID) mice. In addition to the treated hiPSCs, H9 hESCs were used as a positive control and mouse fibroblasts as a negative control. Teratomas were extracted 3 months after injection and analyzed by H&E staining. Experiments were conducted in accordance with procedures approved by the Institutional Committee on Animal Care.
Quantitative RT-PCR
RT-PCR was performed as previously described [34], using previously published primer sets [35].
Stem Cell Culture on Matrigel
Human iPSCs were grown on 6-well plates coated with hESC-qualified Matrigel in mTeSR™1 medium (STEMCELL Technologies) and were passaged with Versene (Life Technologies) for 5mins at 37°C, as previously described [36]. For single-cell passaging experiments, hiPSCs was washed with PBS, incubated with 0.25% trypsin-EDTA for 5mins at 37°C, pelleted by centrifugation for 5mins at 200g, resuspended in mTeSR™1 and pelleted again. The cell pellet was resuspended, strained through a pre-wet cell strainer, counted and 100 cells were seeded into a well of a 24-well plate coated with hESC-qualified Matrigel. To each well, QHREDGS peptide or equivolume PBS (0µM QHREDGS) was added to a final concentration of 5µM, 50µM, or 500µM (0h). Three scenarios were tested: (i) cells remained undisturbed for 48h (1×48h No Media Change), (ii) at 24h, a second dose of PBS/QHREDGS was added to the wells without changing the medium (2×24h No Media Change) or (iii) at 24h, the medium was changed and a second dose of PBS/QHREDGS was added to the wells (2×24h Media Change). In all cases, the medium was changed to fresh mTeSR™1 without PBS/QHREDGS at 48h and subsequently changed daily. On day 7, the cells were fixed with 4% paraformaldehyde for 15mins at room temperature, washed ×3, blocked for 1h in Blocking Buffer (5% FBS, 0.3% TritonX-100 in PBS), and incubated with α-Oct4 (1:500 in Blocking Buffer) overnight at 4°C. The wells were washed ×3, incubated with secondary antibody (1:200 in Blocking Buffer) for 2h at room temperature, washed ×3 with PBS and then counterstained with DAPI (1µg/mL in PBS) for 15mins at room temperature. The wells were washed ×3 and imaged in PBS with an Olympus IX-81 fluorescent microscope with CellSens Dimension Imaging software (Olympus America Inc., Center Valley, PA). Each individual colony was imaged, counted and its area determined.
Hydrogel Preparation
The QHREDGS peptide was conjugated to polyethylene glycol (PEG) by incubating 4.0mg of acrylate-PEG-NHS (MW 3500; Jenkem Technologies, Allen, TX) in a reaction solution of 0mM, 7.2mM (6mg/mL), 14.5mM (12mg/mL) or 29.0mM (24mg/mL) QHREDGS in 50mM Tris pH 8.5 with a final volume of 100µL for 3h at 700rpm. After 3h, the reaction solutions were dialyzed (MWCO 3500) extensively against ddH2O; lyophilized and resuspended in ddH2O, to which 40mg polyethylene glycol diacrylate (PEGDA Mn 700; Sigma Aldrich) and 0.4mg 2-Hydroxy-2-methyl-propiophenone (Sigma Aldrich) was added in a final volume of 80µL; 25µL of this solution was added to each 22×22mm cut square of GelBond PAG film (Lonza) and a vinyl coverslip of equal size was gently placed on top. The hydrogel was placed under UV light for 10min to polymerize, washed for 10min then incubated at 4°C for 1h. After 1h the coverslip was gently peeled off the hydrogel and the hydrogel was placed in 70% EtOH under UV for 1h to sterilize. Sterilized hydrogels were incubated in mTeSR™1 medium overnight prior to seeding with cells. To determine the conjugation efficiency, FITC-aminohexanoic acid-QHREDGS-CONH2 (FITC-QHREDGS; Institute for Biomolecular Design, Edmonton, AB) peptide was conjugated to acrylate-PEG-NHS as above, except that the lyophilized powder was resuspended in 100µL ddH2O. The amount of FITC-QHREDGS contained in the reconstituted solution was determined from a standard curve by fluorescence using a SpectraMax GeminiXS fluorescent plate reader and SoftMax Pro 3.2.1 software (Molecular Devices, Sunnyvale, CA).
EDTA/Integrin Adhesion Assay
Human iPSCs were washed and incubated with equal volumes of 10mM EDTA, 50µg/mL anti-β1-integrin (α-CD29) antibody (in PBS) or PBS alone at 37°C for 20mins. The cells were harvested by using a cell scraper, pelleted by centrifugation (200g for 5 min), resuspended, seeded onto PEG-hydrogels in a 20µL volume and incubated at 37°C for 40mins. After 40mins, medium was added to each well and returned to 37°C. After the 6h of incubation, cells were fixed with 4% paraformaldehyde, blocked overnight in Blocking Buffer at 4°C and incubated with DAPI (1µg/mL in PBS) for 15mins at room temperature. Washed hydrogels were imaged using an Olympus IX-81 fluorescent microscope with CellSens Dimension Imaging software. The surface of each hydrogel was imaged in its entirety and the total number of cells was determined using the maxima plug-in filter of ImageJ software and summing the values determined from each individual image for a given hydrogel. The total number of adherent cells was normalized by dividing by the total number of cells that adhered to the unconjugated PEG hydrogels for a given experiment.
Time Course and Western Blot
Human iPSC colonies were washed, incubated with 0.25% trypsin-EDTA for 5min at 37°C and the cell suspension was pelleted (200g for 5 min). The cell pellet was resuspended, and 250,000 cells were seeded into a well of a 24-well plate coated with hESC-qualified Matrigel. To each well, QHREDGS (final concentration 50µM) or equivolume PBS (0µM QHREDGS) was added. At each time point, the cells were harvested by using a cell scraper, pelleted by centrifugation (200g for 5 min) and resuspended in a minimal volume of Lysis Buffer (10X Cell Lysis Buffer, Cell Signaling Technology; cOmplete Mini, EDTA-free protease inhibitor cocktail tablet, Roche; in ddH2O). DNase (1mg/mL stock in ddH2O) was added to the lysates. Samples were run on Novex Tris-Glycine 1.5mm, 10-well, 10% Gels (Life Technologies) for 1.5h at 125V then transferred to nitrocellulose membrane by wet transfer at 125V for 2.5h. Membranes were probed with: α-phospho-AKT (1/1000 in 5% BSA w/v in PBS with 0.1% Tween20), α-AKT (1/1000), α-phospho-ERK1/2 (1/1000), α-ERK (1/1000), α-ILK (1/1000); secondary antibodies (1/2000 in 1% skim milk powder w/v in PBS with 0.1% Tween20); developed using ECL reagents and exposed to film. The films were scanned and densitometry was performed using ImageJ software.
Statistical Analyses
All statistical analyses were performed using the Student’s t-test. P<0.05 denotes a statistical significance.
Results
QHREDGS-mediated effect on attachment, colony number and size in feeder-dependent culture
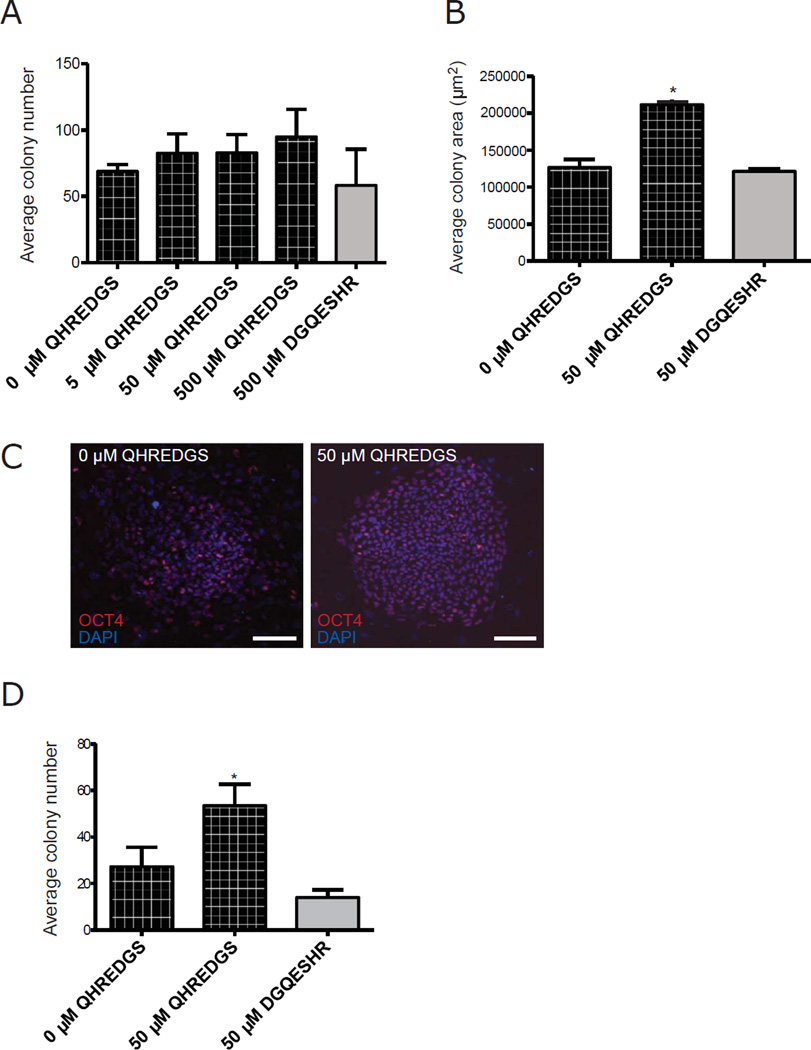
Given that hPSC expansion on MEF feeder layers in serum-free media remains one of the most common hPSC culturing protocols, we aimed to establish the effect of treating hiPSCs with soluble QHREDGS peptide under these standard culture conditions. In order to determine the optimal concentration of QHREDGS for hiPSC expansion, we performed a dose-response experiment and found that long-term (5 passage) pre-treatment with the intermediate concentration of 50µM soluble QHREDGS—added to culture medium with each media change—most effectively increased both the number of colonies (Figure 1A) and the average colony diameter (P = 0.01, n = 3, Figure 1B) compared to 0µM QHREDGS during routine passaging. Having selected an effective concentration, we investigated the effect of longterm pre-treatment with 50µM QHREDGS on the colony-forming efficiency of single-cellsby dissociating the hiPSCs to single cells, plating them on MEFs at a low density and culturing the cells for 7 days in medium containing 50µM QHREDGS, 0µM QHREDGS or 50µM DGQESHR (scrambled) peptide. After one week, QHREDGS treatment of hiPSCs resulted in larger colonies (Figure 1C) and in significantly more colonies than the 0µM QHREDGS control, in three different iPSC lines and one hESC line (BJ1D, P = 0.003, n = 3, Figure 1D; 0901B, P = 0.02, n = 3; IM90(3), P = 0.01, n = 3; H9, P > 0.05, n = 3, Supplementary Figure 1A). Thus, QHREDGS treatment improved hiPSC expansion during routine passaging and enhanced the colony-forming efficiency of single-cell dissociated hiPSCs under standard feeder layer culture conditions.
Figure 1. QHREDGS increases hiPSC colony number and size during clump and single-cell passaging in serum-free, feeder layer culture conditions.
[A–B] BJ1Ds hiPSCs pre-treated for 5 passages with PBS alone (0µM QHREDGS), an increasing concentration of QHREDGS peptide or the scrambled peptide DGQESHR (grey bar) were passaged in clumps in the presence of the treatments for 7 days, then colony number [A] and colony size [B] were determined. [C–D] Human iPSCs were pre-treated for 5 passages with PBS alone (0µM QHREDGS), 50µM QHREDGS or 50µM DGQESHR, dissociated to single-cell and plated at a low density, then cultured for 7 days in the presence of the treatments. [C] Representative Oct4- and DAPI staining images of BJ1D hiPSC colonies pre-treated with PBS (0µM QHREDGS) or 50µM QHREDGS, 7 days after single-cell dissociation (scale bar = 100µm). [D] Colony number determined for BJ1D hiPSCs 7 days after single-cell dissociation. Data presented are the mean ± SEM. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3).
QHREDGS-mediated effect on caspase-dependent apoptosis
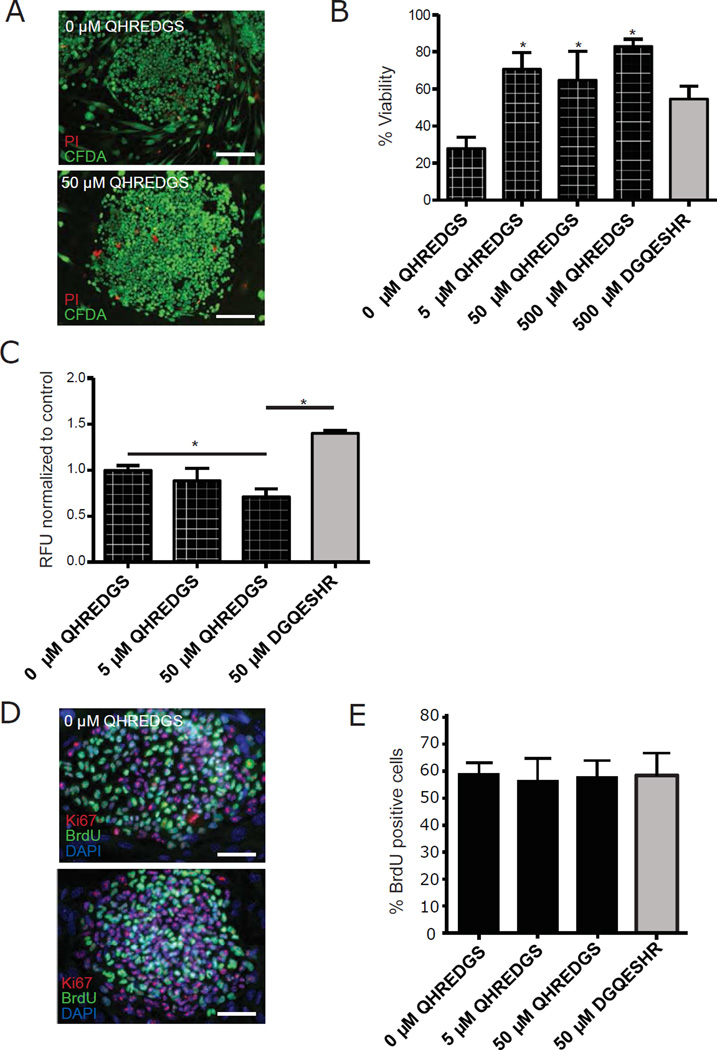
We then sought to understand the mechanism by which QHREDGS promoted increased hiPSC colony number and size. The effect of long-term pre-treatment with 50µM QHREDGS on hiPSC viability was determined by live/dead staining at the end of culture, and it was observed that QHREDGS significantly increased the percent viability of hiPSCs relative to the 0µM QHREDGS control (5µM QHREDGS: P = 0.009; 50µM: P = 0.05; 500µM: P < 0.001; n = 3; Figure 2A–B). However, there was not a significant difference in percent viability among the different concentrations of QHREDGS tested (P > 0.05; n = 3; Figure 2B). To further deconstruct the mechanism, we selected the intermediate 50µM QHREDGS concentration and investigated the effect of QHREDGS treatment on the processes of apoptosis and proliferation: the two possible processes resulting in increased cell numbers. We found that long-term pre-treatment with 50µM QHREDGS significantly decreased caspase-3/7 activity in two different hiPSC lines relative to either the 0µM QHREDGS control or DGQESHR (scrambled) treatment (BJ1D, 50µM QHREDGS: P = 0.04, 50µM DGQESHR: P = 0.002, n = 3, Figure 2C; 0901B, 50µM QHREDGS: P = 0.002, 50µM DGQESHR: P = 0.002, n = 3, Supplementary Figure 1B). To assess the effect of QHREDGS treatment on cell proliferation, hiPSCs were pulsed with BrdU and assayed for incorporation by immunohistochemistry. We found that all groups contained similar percentages of BrdU-positive cells (Figure 2D–E). Therefore, QHREDGS treatment improved cell viability due to increased cell survival resulting from decreased caspase-dependent apoptosis rather than increased proliferation.
Figure 2. QHREDGS promotes hiPSC survival by inhibiting caspase-dependent apoptosis but does not affect proliferation.
BJ1D hiPSCs were pre-treated for 5 passages with PBS alone (0µM QHREDGS), an increasing concentration of QHREDGS peptide or the scrambled peptide DGQESHR, dissociated to single cells and plated at a low density, then cultured for 7 days in the presence of the treatments. [A–B] The cells were stained with Propidium Iodide (PI, dead cells) and Carboxyfluorescein Diacetate Succinimidyl Ester (CFDA, live cells) (scale bar = 150µm). [A] Representative PI/CFPA staining images of BJ1D hiPSC colonies pre-treated with PBS (0µM QHREDGS) or 50µM QHREDGS, 7 days after single-cell dissociation. [B] Quantification of percent viability (live/dead cells). Grey bar denotes scrambled DGQESHR peptide treatment. [C] The cells were assayed for caspase-3/7 in BJ1D hiPSCs. The relative fluorescence units (RFU) per cell is indicative of caspase-3/7 activity. [D–E] The cells were assayed for BrdU incorporation, a measure of proliferation. [D] Representative BrdU, Ki67 and DAPI staining images of BJ1D hiPSC colonies pre-treated with PBS (0µM QHREDGS) or 50µM QHREDGS, 7 days after single-cell dissociation. [E] Quantification of percent BrdU-positive cells. Data presented are the mean ± SEM. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3).
Long-term QHREDGS treatment of hiPSCs
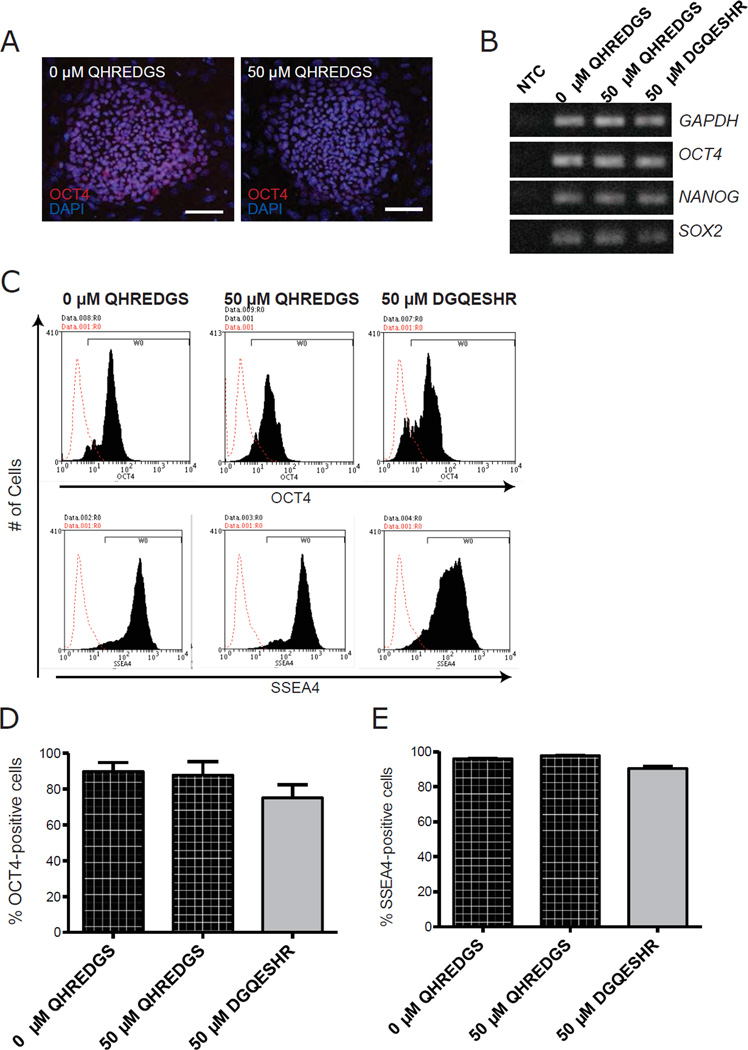
A critical parameter of successful hiPSC culture is the retention of pluripotency, we therefore sought to determine whether long-term (5 passage) pre-treatment with 50µM QHREDGS would affect the pluripotency of the hiPSCs in vitro or in vivo. By immunohistochemistry, the QHREDGS-treated BJ1D hiPSCs continued to express the pluripotency markers Oct4 (Figure 3A) and SSEA4 (data not shown); by RT-PCR, OCT4, NANOG, and SOX2 gene expression was equivalent among the treatment groups (Figure 3B); and by flow cytometry analysis, the percentage of Oct4+ and SSEA4+ cells in both BJ1D and 0901B hiPSCs were comparable among all treatment groups (BJ1D, Figure 3C–E; 0901B, Supplementary Figure 2). Furthermore, long-term pre-treatment with 50µM QHREDGS of hiPSCs did not affect their ability to differentiate into neural cells, mesodermal cells and endodermal cells in vitro (Figure 4A). The layer-specific differentiated cells had equivalent gene expression of markers for all three germ layers, i.e. PAX6 (ectoderm), AFP (endoderm), BRACHYURY and CDX2 (mesoderm) irrespective of treatment (Figure 4B). To determine the in vivo pluripotency capacity of the QHREDGS-treated cells, hiPSCs were treated for 10 passages with 50µM QHREDGS and it was determined that the cells retained the ability to form teratomas displaying tissues of all three germ layers in immunodeficient NOD-SCID mice (Figure 4C–D). We, therefore, determined that prolonged exposure to the QHREDGS peptide did not adversely alter pluripotency of hiPSCs either in vitro or in vivo.
Figure 3. QHREDGS treatment does not affect the pluripotency of hiPSCs in vitro.
BJ1D hiPSCs pretreated for 5 passages with PBS alone (0µM QHREDGS), 50µM QHREDGS or 50µM DGQESHR were assessed for their ability to express pluripotency markers. [A] Representative Oct4 and DAPI staining images of BJ1D hiPSC colonies pre-treated with PBS (0µM QHREDGS) or 50 µM QHREDGS, 7 days after single-cell dissociation. [B] Agarose gels showing RT-PCR products generated by primers for the pluripotency markers OCT4, NANOG and SOX2 and the housekeeping gene, GAPDH. No Template Control is denoted by NTC. [C–E] Flow cytometric analysis of PBS (0µM QHREDGS), 50µM QHREDGS and 50µM DGQESHR treated BJ1D hiPSCs. [C] Representative Oct4 (top panels) and SSEA4 histograms (bottom panels). Red denotes secondary antibody only control and black denotes cell population positive for the designated antibody. [D–E] Quantification of Oct4+ [D] and SSEA4+ [E] cells. Data presented are the mean ± SEM. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3).
Figure 4. Long-term QHREDGS treatment does not affect the ability of hiPSCs to differentiate into cells of all three germ layers in vitro and in vivo.
[A–B] BJ1D hiPSCs pre-treated for 5 passages with PBS (0µM QHREDGS), 50µM QHREDGS or 50µM DGQESHR were subjected to an embryoid body-based differentiation protocol. [A] Representative images of differentiated cells pre-treated with PBS alone (0µM QHREDGS; top row) or 50µM QHREDGS (bottom row) and stained for SMA (mesoderm), GATA 6 (endoderm) or βIII-tubulin (ectoderm) (n=3). [B] Agarose gels showing RT-PCR products generated by primers for the germ layer markers PAX6 (ectoderm), AFP (endoderm), BRACHYURY (mesoderm), and CDX2 (mesoderm) (n=3). [C–D] BJ1D hiPSCs treated with PBS alone (0µM QHREDGS), 50µM QHREDGS or 50µM DGQESHR for 10 passages were subjected to a teratoma formation assay (n=3). [C] Representative images of teratomas formed from cells pre-treated with PBS alone (0µM QHREDGS) or 50µM QHREDGS. [D] Representative images of teratomas formed from cells pre-treated with PBS alone (0µM QHREDGS), 50µM QHREDGS or 50µM DGQESHR sectioned and stained with H&E. Arrows, ectodermal; asterisks, mesodermal; and arrowheads, endodermal structures.
Short-term QHREDGS treatment of hiPSCs in feeder-free culture
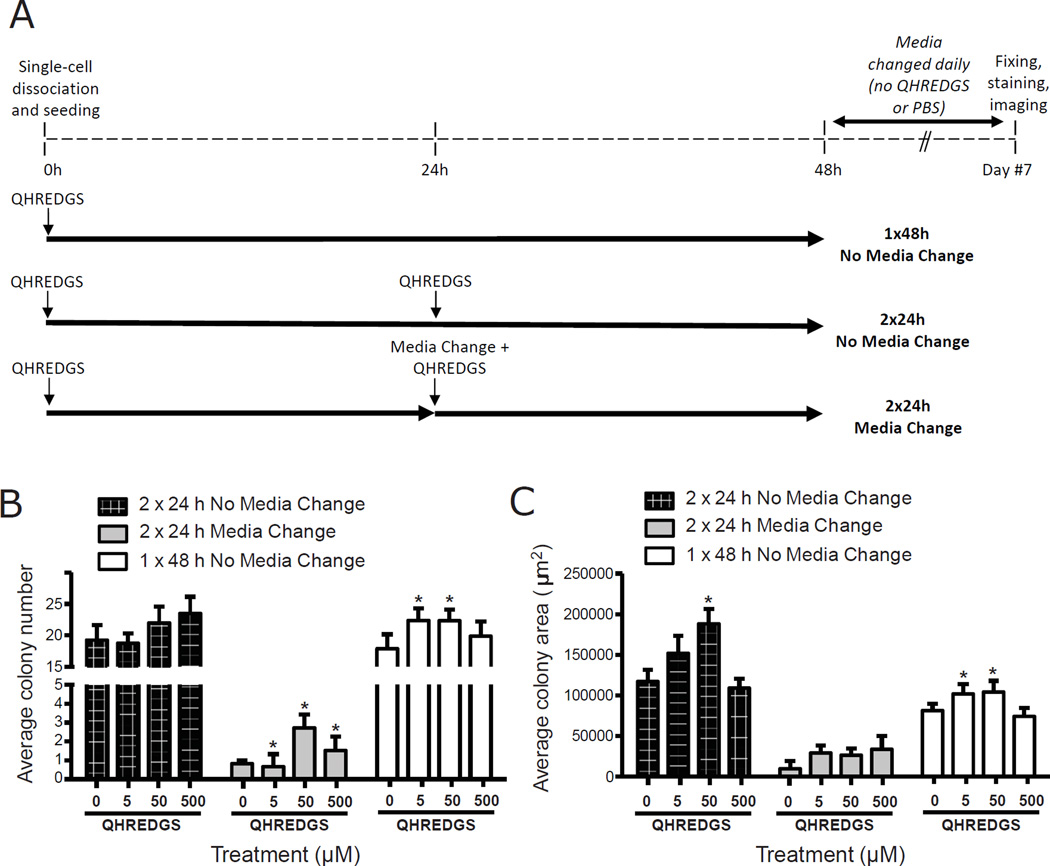
To determine whether the effect of QHREDGS on hiPSCs was dependent on specific culture conditions, on a prolonged treatment time and/or on a specific concentration of QHREDGS, we cultured hiPSCs in a more defined culture on Matrigel in serum-free media and investigated the survival of hiPSCs under short-term (48h) QHREDGS treatment over a 100-fold range of concentrations. To determine the minimum practical application time and the minimal practical application dose of QHREDGS required to promote hPSC viability, we compared three concentrations of QHREDGS (5, 50 and 500µM) and three treatment regimens: (i) incubation of the cells in culture media with QHREDGS for 48h without changing the media, (ii) incubation of the cells in culture media with QHREDGS for 24h, change of media and additional QHREDGS added at the same concentration for 24h, or (iii) incubation of the cells in culture media with QHREDGS for 24h, no change in media but an additional dose of QHREDGS added at the same concentration for 24h (Figure 5A). In general, short-term QHREDGS treatment of hiPSCs grown on Matrigel in serum-free media resulted in increased colony number and size of the colonies formed (Figure 5B–C). Relative to the 0µM QHREDGS control, treatment of hiPSCs for 48h with 5 or 50µM QHREDGS resulted in a significant 25% increase in colony number on average (5µM QHREDGS: P = 0.05; 50µM: P = 0.05; n = 3; Figure 5B 1×48h No Media Change) and a significant 40% and 42% increase, respectively, in colony size on average (5µM QHREDGS: P = 0.004; 50µM: P = 0.001; n = 3; Figure 5C 1×48h No Media Change). Treatment with 500µM QHREDGS for 48h yielded an 11% increase in colony number on average and had no effect on colony size (P > 0.05; n=3; Figure 5B–C 1×48h No Media Change). Whereas incubation of hiPSCs with 5, 50 or 500µM QHREDGS for 2×24h (without media change) resulted in a 0%, 14% and 22% increase in colony number, respectively (P > 0.05; n = 3; Figure 5B 2×24h No Media Change); and a 22%, 55% and 0% increase in colony size, relative to the control (50µM: P = 0.01; n = 3; Figure 5C 2×24h No Media Change). Finally, incubation of hiPSCs with 5, 50 or 500µM QHREDGS for 2×24h (with media change) resulted in a massive significant increase in colony number of 300%, 600% and 450%, respectively (5µM QHREDGS: P = 0.05; 50µM: P = 0.01; 500µM: P = 0.03; n = 3; Figure 5B 2×24h Media Change); but despite this increase the number of colonies that could be obtained under this condition was substantially less than in the other two treatment regimens (Figure 5B). Similarly, the average colony area for this regimen was substantially smaller than for the other two regimens even considering the QHREDGS-induced increase in cell area of 0%, 13% and 34%, respectively (P > 0.05; n = 3; Figure 5C 2×24h Media Change). Taken together, we determined that the effect of the QHREDGS peptide on hiPSC survival was not dependent on a particular culture condition and did not require long-term treatment, as 48h was sufficient to significantly improve hiPSC colony number and size. Additionally, the most effective regimen—when both colony number and colony size were considered—was a single dose of QHREDGS incubated with the cells for 48h undisturbed, at a concentration of 5µM QHREDGS, the minimum concentration tested.
Figure 5. Single dose, low concentration QHREDGS treatment improves colony number and size of single-cell dissociated hiPSCs in serum-free, feeder-free culture conditions.
[A] Short-term QHREDGS treatment protocol. At 0h, BJ1D hiPSCs were single-cell dissociated and seeded onto Matrigel in the presence of PBS alone (0µM QHREDGS) or QHREDGS. Three different treatment schemes were investigated: (1) cells remained undisturbed for 48h at 37°C (1×48h No Media Change); (2) at 24h, an additional dose of PBS or QHREDGS was added to the media and the cells returned to the incubator (2×24h No Media Change); or (3) at 24h, the media was aspirated and replaced, and an additional dose of PBS or QHREDGS was added to the media and the cells returned to the incubator (2×24h Media Change). At 48h, the media was replaced with PBS/QHREDGS-free medium for all groups. The cells were fed daily with QHREDGS-free medium for the following 5 days. On day 7, the cells were fixed and stained for Oct4 and counterstained with DAPI. [B–C] BJ1D hiPSCs were single-cell dissociated and seeded onto Matrigel in the presence of PBS alone (0µM QHREDGS) or QHREDGS (5, 50 or 500µM), as described in [A]. After 7 days, the cells were fixed and stained for Oct4 and the number of Oct4-positive colonies in each well was counted [B] and the area of the colonies was measured [C]. Data presented are the average ± SEM. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3–7).
ILK expression and ERK1/2 activation in QHREDGS treated cells
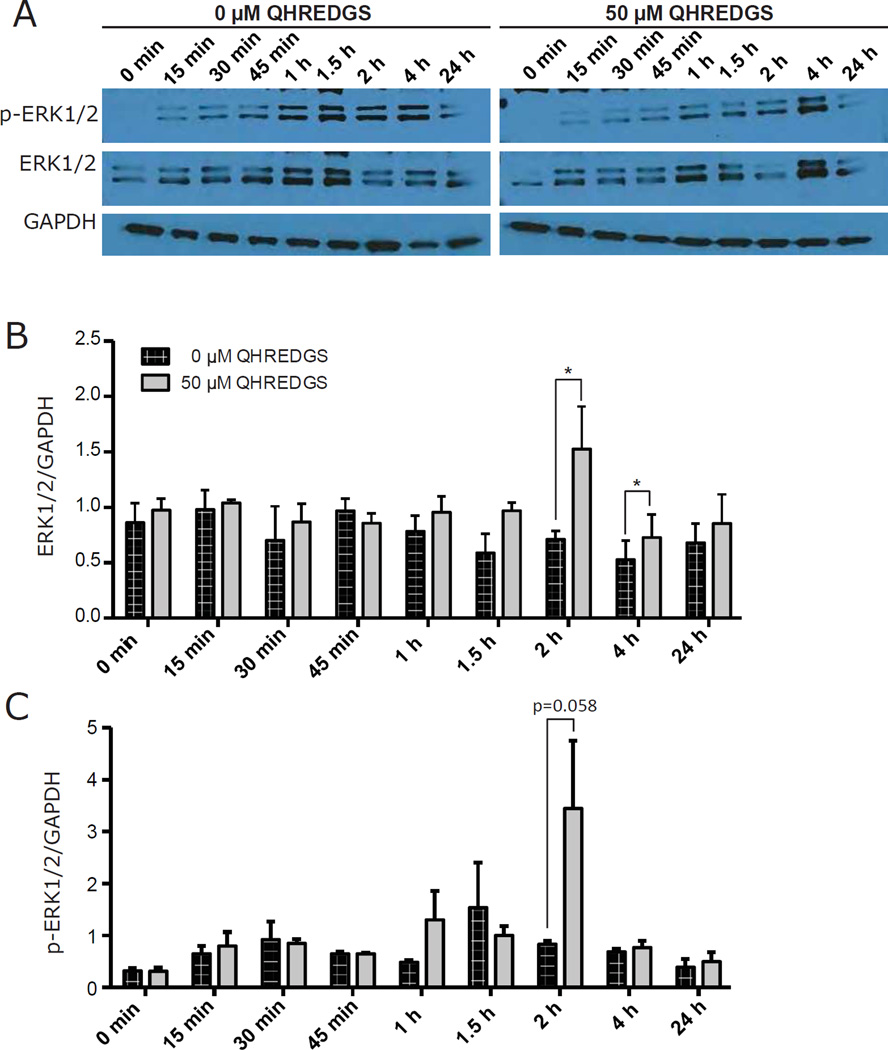
Having identified a pro-survival effect of QHREDGS on hPSC cultures, we sought to understand the mechanism. To identify intracellular signaling effectors that were activated by QHREDGS in hPSCs, we first focused on ILK because it is a known binding partner of β1- and β3-integrins, the receptors we identified for QHREDGS in endothelial cells [27], and ILK has been implicated in cell survival pathways [10]. Single-cell hiPSCs were seeded onto Matrigel-coated plates in the presence of 50µM QHREDGS and lysed at various time points over 24h. ILK protein expression, as determined by Western blot analysis, increased following QHREDGS treatment at all time points relative to the 0µM QHREDGS control, with peak expression at 1.5–2h (0min: P = 0.04; 30min: P = 0.02; 1.5h: P = 0.02; 2h: P = 0.05; 4h: P = 0.002; 24h: P = 0.03; n = 3; Figure 6A–B).
Figure 6. QHREDGS increases ILK expression.
[A–B] BJ1D hiPSCs were single-cell dissociated and seeded onto Matrigel in the presence of PBS (0µM QHREDGS) or 50µM QHREDGS. At various time points, cells were scraped and lysed. Cell lysates were analyzed by Western blot analysis using the denoted antibodies. The resultant bands were quantified by densitometry. [A] Representative images of Western blot membranes probed with denoted antibodies. [B] Quantification of Western blot analysis. Data presented are the average normalized ILK concentration determined from the ILK:GAPDH ratio ± SEM. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3–5).
Integrin signaling has been reported to promote hPSC viability through pathways in which AKT and ERK1/2 are activated [37]. Also, AKT- and ERK1/2-dependent cell survival pathways can be regulated by the ILK-PINCH-Parvin (IPP) complex [9,10,38]. Finally, AKT and ERK1/2 were identified as effectors of the interaction between Ang-1, the QHREDGS parent protein, and integrins [24]. Thus, we investigated the possibility that AKT and ERK1/2 might be activated downstream of ILK upregulation. While we did not observe an increase in AKT phosphorylation (at Ser473) following QHREDGS treatment (Supplementary Figure 3), we did find significant upregulation of ERK1/2 expression following QHREDGS treatment at 2h and 4h (P = 0.05, P = 0.04, respectively; n = 3; Figure 7A–B), and an increase in ERK1/2 phosphorylation (at Thr202/Tyr204) at 2h (P = 0.05; n = 3; Figure 7C). Therefore, our results demonstrate that treatment of hiPSCs with QHREDGS increased ILK expression, increased expression and activation of ERK1/2 but did not affect AKT expression and activation.
Figure 7. QHREDGS increases activation of ERK1/2.
[A–C] BJ1D hiPSCs were single-cell dissociated and seeded onto Matrigel in the presence of PBS (0µM QHREDGS) or 50µM soluble QHREDGS. At various time points, cells were scraped and lysed. Cell lysates were analyzed by Western blot analysis using the denoted antibodies and the resultant bands were quantified by densitometry. [A] Representative images of Western blot membranes probed with denoted antibodies. [B–C] Quantification of Western blot analysis. Data presented are the average normalized ERK1/2 concentration determined from the ERK1/2:GAPDH ratio ± SEM [B] and the average normalized phospho-ERK1/2 (p-ERK1/2) concentration determined from the p-ERK1/2:GAPDH ratio ± SEM [C]. P values are derived from Student’s t-test, P < 0.05 considered significant (n=3).
QHREDGS-β1-integrin interaction
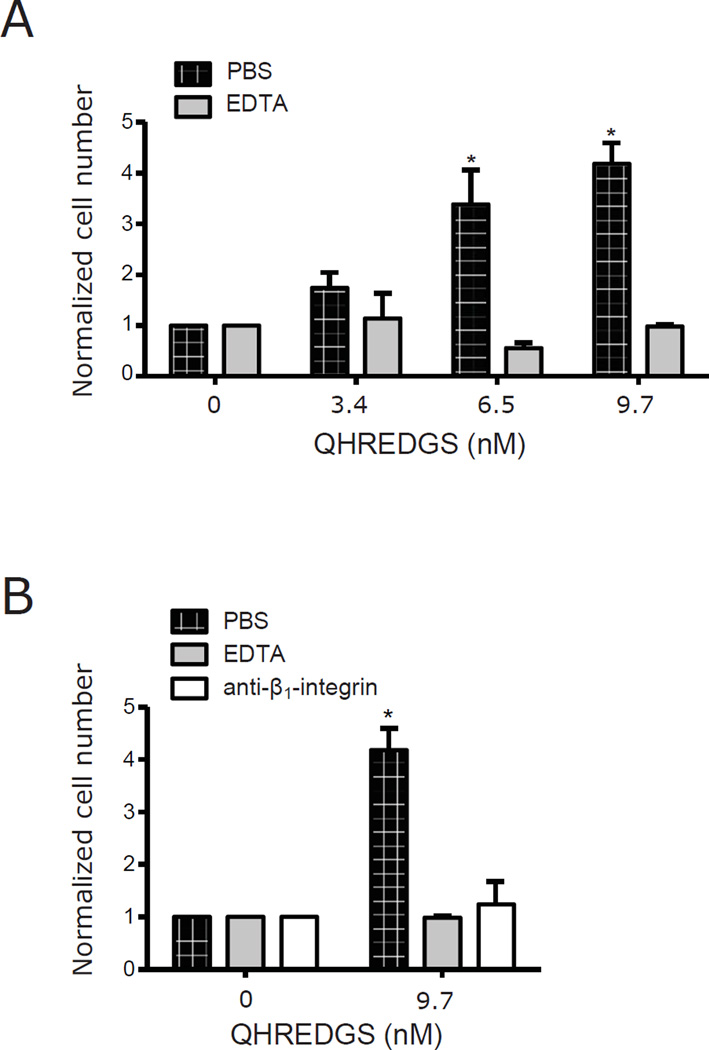
The QHREDGS peptide was first suggested as a possible motif that would allow Ang-1 to bind to integrin receptors in cell types that did not express the Tie2 receptor [24]. Integrins are an important component of the hiPSC-ECM interaction and can enhance survival during dissociation stress [4]. Recently, we demonstrated that QHREDGS-mediated effects on tube formation, metabolism and survival in endothelial cells could be inhibited by using antibodies against the α5β1- and αvβ3-integrin receptors [27]. In order to investigate the interaction of integrin receptors on hiPSCs with the QHREDGS peptide, we generated PEG hydrogels onto which we covalently attached QHREDGS peptide. PEG hydrogels are non-adhesive, non-fouling surfaces during day- to week-long periods of culture [39] and are therefore not ideal substrates for the propagation of hiPSCs, which have specific adhesion requirements [4]. However, PEG hydrogels are useful tools for studies aimed at isolating the effects of specific adhesion molecules on cells because PEG surfaces are resistant to nonspecific protein adsorption [39], a problem that plagues standard plastic tissue culture surfaces and which can yield skewed or misleading results due to the inability to disentangle the contribution of proteins adsorbed from the media vs surface immobilized peptide. In order to determine whether QHREDGS interacts with integrin receptors on hiPSCs, we generated PEG-hydrogels conjugated with increasing amounts of QHREDGS. Using a fluorescently-labeled QHREDGS (FITC-QHREDGS), we quantified the amount of peptide that was conjugated to the PEG variant, acrylate-PEG-NHS, in reaction solutions with increasing amounts of peptide and the respective conjugation efficiencies (Table 1). We observed a significant, dose-dependent increase in the number of adherent hiPSCs to the PEG-QHREDGS hydrogels compared to the unconjugated controls following single-cell dissociations (50µM: P = 0.04; 500µM: P = 0.008; n = 3; Figure 8A). By pre-incubating hiPSCs with EDTA to chelate divalent ions necessary for integrin activation [40], we found that adhesion to the QHREDGS-hydrogels was completely inhibited (Figure 8B). To identify the specific integrin receptor type involved, we performed the same experiment using an anti-β1-integrin antibody in lieu of EDTA and found the same result of complete loss of adhesion (Figure 8B). Thus, we determined that the interaction of QHREDGS with hiPSCs is mediated by β1-integrins. Moreover, we found that QHREDGS conjugation significantly enhanced the adherence of hiPSCs to PEG hydrogels. The PEG-QHREDGS hydrogel, therefore, constitutes the basis for an entirely defined surface for hPSC culture.
Table 1.
Quantification of conjugation of FITC-QHREDGS to acrylate-PEG-NHS.
| Mass of acrylate-PEG- NHS in reaction solution (mg) |
Mass FITC- QHREDGS in reaction solution (mg) |
Concentration FITC-QHREDGS reaction solution (mg/mL) |
Mass peptide conjugated (mg) |
Moles of peptide in hydrogel (nM) |
Conjugation efficiency |
|---|---|---|---|---|---|
| 4.0 | 0.6 | 6.0 | 0.33±0.12 | 3.4±1.3 | 54±20% |
| 4.0 | 1.2 | 12.0 | 0.63±0.14 | 6.5±1.4 | 52±11% |
| 4.0 | 2.4 | 24.0 | 0.94±0.36 | 9.7±3.7 | 39±15% |
Measured values reported as mean ± SEM, n=3.
Figure 8. QHREDGS binding to β1-integrin receptors is sufficient to promote adhesion of single-cell dissociated hiPSCs to non-fouling PEG hydrogels.
[A] BJ1D hiPSCs were single-cell dissociated and incubated with PBS or 10mM EDTA prior to seeding onto PEG hydrogels conjugated with different amounts of QHREDGS peptide. After 6 hours, cells were fixed, stained with DAPI, imaged and the cells adherent to the hydrogel were counted. Data presented are the average normalized values ± SEM (n = 3–5); values were normalized by dividing by the number of cells that adhered to the PEG hydrogel in a given experiment. P values were determined from paired Student’s t-tests, P < 0.05 considered significant. [B] BJ1D hiPSCs were single-cell dissociated and incubated with PBS, 10mM EDTA or 50µg/mL anti-β1-integrin antibody prior to seeding onto PEG hydrogels with or without QHREDGS conjugated. Data presented are the average normalized values ± SEM; values were normalized by dividing by the number of cells that adhered to the PEG hydrogel in a given experiment. P values were determined from paired Student’s t-tests, P < 0.05 considered significant (n = 3–5).
Discussion
In the hPSC field, there has been a great deal of research focused on defining the molecular pathways responsible for dissociation-induced cell death in hPSCs [1–3] and on identifying small molecules that can inhibit or modulate these pathways [4]. Parallel efforts have focused on determining the ideal standardized culture conditions for hPSC propagation and differentiation through the fabrication of synthetic culture substrates with defined characteristics and determining the specific formulation requirements for a compatible defined serum-free medium [11,12], with the objective of greater reproducibility of cultures and the elimination of xenogenic compounds. Additionally, a standardized reproducible culture system would provide a platform on which to study cell activity without the confounding of undefined and extraneous signaling. In recent years, some synthetic surfaces that display specific proteins, such as laminin [15,16], vitronectin [17] and/or E-cadherin [12,18], have been developed to be used in conjunction with serum-free media and have been demonstrated to support self-renewal and expansion of hESCs and hPSCs [19–21], but due to relative costs have failed to be widely implemented. In part, this is because the production of protein-based synthetic substrates is constrained by the fact that the simplest, most cost-effective methods for covalent protein immobilization can result in a heterogenous surface, with proteins immobilized in non-productive conformations or with blocked active sites [27]. Also, proteins are subject to denaturation and thus have a limited shelf-life [41]. An alternative is to generate substrates using peptide fragments derived from growth factors or ECM proteins that possess equivalent activity to the full-length protein and have the advantages of cost-effective production, fewer, if any, conformational requirements and increased stability [27].
In consideration of these facts, we directed our attention to QHREDGS, a peptide fragment derived from the growth factor Ang-1 that we previously reported can promote the survival of neonatal rat cardiomyocytes [31] and endothelial cells [27]. Moreover, we identified that QHREDGS interacts with integrin receptors on the endothelial cell surface [27]. As mentioned above, integrins are receptors that play an important role in both cell-cell and cell-ECM interactions, upon which hPSCs heavily rely for survival, self-renewal, and differentiation [5]. Herein, we demonstrated for the first time that QHREDGS could promote hPSC survival by regulating integrin-based intracellular signaling that includes the upregulation of ILK, the upregulation and activation of ERK1/2 and the downregulation of caspase-3/7 activity (Figure 9). Additionally, we demonstrated that a single, low concentration dose of QHREDGS added to the culture medium was sufficient to increase hPSC colony number and size. Finally, we identified the basis for a defined hPSC culture substrate by demonstrating that immobilized QHREDGS significantly increased the adhesion of hPSCs to a PEG substrate in a dose-dependent manner. Given it is a synthetic peptide, a QHREDGS-based substrate with a relatively long shelf-life could be produced in a cost-effective manner and optimized as a defined substrate for hPSC culture for wide-scale implementation.
Figure 9. QHREDGS-dependent pro-survival mechanism in hPSCs.
Model depicting the postulated mechanism for increased QHREDGS-dependent hPSC survival, wherein the binding of QHREDGS to a β1-integrin receptor on the hPSC surface increases ILK, stimulating the upregulation of the pro-survival ERK1/2 signaling pathway and the eventual downregulation of caspase-dependent apoptotic pathways.
In recent years, peptides containing the integrin-binding motif RGD have been used to make defined substrates for hPSCs, primarily with the intention of promoting adhesion to a non-adhesive substrates [42]. There have been a few reports of RGD-substrates supporting hPSC cultures but of them long-term hPSC culturing has not been demonstrated and questions have been raised as to the contribution of media components due to protein-fouling to the base hydrogel material [42]. Additionally, while RGD peptides have been reported to promote attachment and even survival in various terminally differentiated cell types [43,44], even in these cell types it does not downregulate caspase activity [45]. Conversely, we have demonstrated that the QHREDGS does support hPSC adhesion when bound to a non-fouling PEG hydrogel [39] and therefore forms the basis of a defined substrate for hPSC culture. Furthermore, we have demonstrated that QHREDGS acts as a small molecule inhibitor of apoptosis when added to hPSC cultures in its soluble form while maintaining hPSC pluripotency, proliferation and morphology. These distinctions between the RGD and QHREDGS peptides indicate that while both these peptides engage integrin receptors, the intracellular effects they induce are not equivalent.
We therefore investigated the particular intracellular signaling events initiated by the QHREDGS-β1-integrin interaction and demonstrated that the QHREDGS-mediated cell survival mechanism results in the inhibition of caspase-dependent hPSC death and involves increased ILK expression, and increased expression and activation ERK1/2 (Figure 9). Similarly, in other cell types, QHREDGS/Ang-1 has been reported to mediate cell survival by engaging β1- and β3-integrin receptors [24,27]; and while these previous studies did not directly investigate ILK signaling, ILK is a reported binding partner of both β1- and β3-integrin receptors [8], suggesting that ILK may be a participant in inducing cell survival in these milieus as well. Both AKT and ERK1/2 signaling pathways have been implicated in promoting integrin-mediated proliferation and maintenance of hPSCs [37]. Moreover, both ERK1/2 and AKT activation were identified as effectors of integrin-dependent cell survival mediated by Ang-1 [24]. While we did not observe an increase in AKT activation with QHREDGS treatment, this difference once again highlights that QHREDGS has its own unique attributes and that its derivation from Ang-1 does not guarantee they share all the same biological activities.
As mentioned above, integrins can affect both cell-cell and cell-ECM interactions and thereby regulate hPSC expansion. A common denominator linking integrin signaling to both cell-cell and cell-ECM interactions is the GTPase, Rho [4]. It is therefore possible that the QHREDGS-mediated effect of increased hPSC survival may involve Rho signaling pathways, although this remains to be investigated. Alternatively, given the role identified for cell-cell interactions in preventing hPSC death [4], it is possible that QHREDGS treatment of hPSCs may also affect the expression of the cell-cell adhesion molecule E-cadherin by integrin-mediated effects on the cytoskeleton. A better understanding of the QHREDGS-mediated survival mechanism can be beneficial to designing a more complex but more effective defined hPSC culture protocol that utilizes a combination of small molecules added to the culture medium and/or immobilized onto a defined substrate to produce either an additive effect upon a single signaling pathway or else a synergistic effect upon parallel pathways.
It has been reported recently that synthetic PEG-hydrogels can be used to support growth, pluripotency and self-renewal of hPSCs in serum-free, feeder-free, 3D but not 2D cultures [46] and that prolonged culture on this substrate retains the normal hiPSC karyotype [46]. Importantly, herein we have demonstrated that QHREDGS improved the adherence of hiPSCs to PEG-hydrogels in feeder-free, 2D cultures, which Jang et al reported did not support hPSC cultures [46]. Therefore, the addition of QHREDGS to 3D synthetic PEG-hydrogel matrices may have the potential to generate a superior defined substrate for serum-free, feeder-free, 3D hPSC cultures.
Additionally, an integral part of integrin signaling is a pulling force [47]. Substrate stiffness can therefore influence integrin-mediated signaling [47]. PEG-hydrogels can be easily modified to attain different mechanical properties using different concentrations, molecular weight monomers and/or branched variants. In this way, the effect of QHREDGS-integrin signaling can be fine-tuned. Similarly, the differentiation of hPSCs to a particular cell lineage can be regulated by controlling the stiffness of the substrate seen by the tissue and also by conjugating additional pro-differentiation signaling molecules/peptides to the substrate [48]. The simplicity of both PEG and the NHS-based conjugation method permits the addition of complexity at the level of co-immobilized molecules and substrate physical properties, allowing for specificity of design for particular applications. Complex 3D PEG hydrogels can be produced wherein biodegradability can be controlled by inclusion of matrix metalloprotease (MMP)-sensitive oligopeptides and by photopatterning, spatial control over peptide density can be exerted [49,50]. Advances have also been made in the assessment of hydrogel design parameters for the production of degradable, biofunctionalized, injectable PEG hydrogels for site-specific cell delivery [51]. The specificity of the hydrogel can be further modified by appropriate selection of hydrogel polymerization reaction type and network structure, which can provide additional cell behaviour cues [52]; and by selection of optimal immobilization chemistry and/or effective distance between substrate and peptide. Over the years, more elegant immobilization chemistries have been developed that produce homogenous substrate surfaces without post-synthetic peptide modification, for example using thiol-ene ‘click’ chemistry, peptides can be immobilized to PEG through cysteine residues [53]. This is in contrast to EDC/NHS chemistry, which uses the more ubiquitous carboxylic groups and whereby the QHREDGS peptide can be immobilized via carboxylic groups in the C-terminus or the side chains of either glutamic acid (E) or aspartic acid (D) to produce a heterogeneous substrate surface [31]. It is therefore possible to refine the effect of QHREDGS by using a homogenous immobilization technique and selecting one conformation as optimal for a specific application. With respect to distance between substrate and peptide, it has been demonstrated that cells have only limited adherence to PEG hydrogel surfaces immobilized with RGD in the absence of a spacer arm (MW=3400) [54] and that a PEG spacer of 3500Da—an effective distance of approximately 3.5nm—was optimal for adhesion [55]. Herein, we similarly immobilized the QHREDGS peptide to a PEG spacer arm of 3500Da, however the optimal effective distance may vary by application and therefore could be used as another avenue to improve specificity. Finally, in terms of large-scale production and differentiation of hPSCs, microcarrier beads in a bioreactor have been employed [56]; and RGD-containing recombinant peptides have been used to enhance adhesion to microcarrier beads [57]. As mentioned, RGD-substrates have met with minimal success in sustaining hPSC cultures [42], which suggests that the QHREDGS peptide may be a viable substitute for RGD in the context of microcarrier beads or alternatively as a small molecule inhibitor of hPSC apoptosis added to the culture medium in the bioreactor.
Conclusions
We have identified QHREDGS as a small molecule inhibitor of hPSC cell death that can be used as a soluble supplement to hPSC maintenance media without affecting phenotype or morphology. Similarly, by immobilizing QHREDGS onto a PEG-hydrogel we have demonstrated the utility of QHREDGS as the basis of a defined substrate for hPSCs in serum-free, feeder-free culture. Furthermore, we have defined an integrin-mediated anti-apoptotic pathway activated by QHREDGS resulting in cultures of increased numbers. Taken together, we have developed two methods of maintaining hPSC cultures using the Ang-1-derived peptide QHREDGS and provided a mechanistic understanding of the way in which QHREDGS achieves its beneficial effects. The flexibility of the QHREDGS peptide with respect to its biomaterial application makes it amenable to integration into pre-existing technologies, such as 3D cultures and microcarrier beads; and its simplicity and ease of use will permit it to be used in combination to generate new, more complex and more effective biomaterials for specialized applications. The potential of the Ang-1 derived peptide QHREDGS as a tool for hPSC culture is proven here for the first time.
Supplementary Material
Acknowledgements
This work was funded by grants from Connaught Innovation Award, Ontario Research Fund–Global Leadership Round 2 (ORF-GL2), Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-126027), NSERC-CIHR Collaborative Health Research, Grant (CHRPJ 385981-10), NSERC Discovery Grant (RGPIN 326982-10), Heart and Stoke Foundation GIA, and NSERC Discovery Accelerator Supplement (RGPAS 396125-10).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 2.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Bennett SA, Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh Migr. 2012;6:59–70. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda S, Shirotani-Ikejima H, Tadokoro S, Maeda Y, Kinoshita T, Tomiyama Y, et al. Integrin-linked kinase associated with integrin activation. Blood. 2009;113:5304–5313. doi: 10.1182/blood-2008-07-169136. [DOI] [PubMed] [Google Scholar]
- 9.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Tu Y, Zhang Y, Blair HC, Zhang L, Wu C. PINCH-1 regulates the ERK-Bim pathway and contributes to apoptosis resistance in cancer cells. J Biol Chem. 2008;283:2508–2517. doi: 10.1074/jbc.M707307200. [DOI] [PubMed] [Google Scholar]
- 11.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev Biol. 2010;10 doi: 10.1186/1471-213X-10-60. 60-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaoka M, Koshimizu U, Yuasa S, Hattori F, Chen H, Tanaka T, et al. E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One. 2006;1:e15. doi: 10.1371/journal.pone.0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 14.Carlson Scholz JA, Garg R, Compton SR, Allore HG, Zeiss CJ, Uchio EM. Poliomyelitis in MuLV-infected ICR-SCID mice after injection of basement membrane matrix contaminated with lactate dehydrogenase-elevating virus. Comp Med. 2011;61:404–411. [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 17.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 18.Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 19.Derda R, Musah S, Orner BP, Klim JR, Li L, Kiessling LL. High-throughput discovery of synthetic surfaces that support proliferation of pluripotent cells. J Am Chem Soc. 2010;132:1289–1295. doi: 10.1021/ja906089g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh H, Tahir TA, Alawo DO, Issa E, Brindle NP. Molecular control of angiopoietin signalling. Biochem Soc Trans. 2011;39:1592–1596. doi: 10.1042/BST20110699. [DOI] [PubMed] [Google Scholar]
- 23.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 24.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–e24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 25.Rask F, Mihic A, Reis L, Dallabrida S, Ismail N, Sider K, et al. Hydrogels modified with QHREDGS peptide support cardiomyocyte survivalin vitro and after sub-cutaneous implantation. Soft Matter. 2010;6:5089–5099. [Google Scholar]
- 26.Reis LA, Chiu LL, Liang Y, Hyunh K, Momen A, Radisic M. A peptide-modified chitosan-collagen hydrogel for cardiac cell culture and delivery. Acta Biomater. 2012;8:1022–1036. doi: 10.1016/j.actbio.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Miklas JW, Dallabrida SM, Reis LA, Ismail N, Rupnick M, Radisic M. QHREDGS enhances tube formation, metabolism and survival of endothelial cells in collagen-chitosan hydrogels. PLoS One. 2013;8:e72956. doi: 10.1371/journal.pone.0072956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotta A, Cheung AY, Farra N, Vijayaragavan K, Seguin CA, Draper JS, et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat Methods. 2009;6:370–376. doi: 10.1038/nmeth.1325. [DOI] [PubMed] [Google Scholar]
- 29.Hotta A, Cheung AY, Farra N, Garcha K, Chang WY, Pasceri P, et al. EOS lentiviral vector selection system for human induced pluripotent stem cells. Nat Protoc. 2009;4:1828–1844. doi: 10.1038/nprot.2009.201. [DOI] [PubMed] [Google Scholar]
- 30.Chang WY, Lavoie JR, Kwon SY, Chen Z, Manias JL, Behbahani J, et al. Feeder-independent derivation of induced-pluripotent stem cells from peripheral blood endothelial progenitor cells. Stem Cell Res. 2013;10:195–202. doi: 10.1016/j.scr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Rask F, Dallabrida SM, Ismail NS, Amoozgar Z, Yeo Y, Rupnick MA, et al. Photocrosslinkable chitosan modified with angiopoietin-1 peptide, QHREDGS, promotes survival of neonatal rat heart cells. J Biomed Mater Res A. 2010;95:105–117. doi: 10.1002/jbm.a.32808. [DOI] [PubMed] [Google Scholar]
- 32.Chiang CK, Chowdhury MF, Iyer RK, Stanford WL, Radisic M. Engineering surfaces for site-specific vascular differentiation of mouse embryonic stem cells. Acta Biomater. 2010;6:1904–1916. doi: 10.1016/j.actbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 36.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 38.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 39.Harris JM. Poly(ethylene glycol) chemistry: Biotechnical and biomedical applications. New York and London: Plenum Press; 1992. [Google Scholar]
- 40.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 41.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 42.Lambshead JW, Meagher L, O'Brien C, Laslett AL. Defining synthetic surfaces for human pluripotent stem cell culture. Cell Regeneration. 2013;2:7. doi: 10.1186/2045-9769-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288:C30–C38. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 44.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 45.Erhardt JA, Ohlstein EH, Toomey JR, Gabriel MA, Willette RN, Yue TL, et al. Activation of caspase-3/caspase-3-like activity in rat cardiomyocytes by an RGD peptide, but not the GPIIb/IIIa antagonist lotrafiban. Thromb Res. 2001;103:143–148. doi: 10.1016/s0049-3848(01)00287-0. [DOI] [PubMed] [Google Scholar]
- 46.Jang M, Lee ST, Kim JW, Yang JH, Yoon JK, Park JC, et al. A feeder-free, defined three-dimensional polyethylene glycol-based extracellular matrix niche for culture of human embryonic stem cells. Biomaterials. 2013;34:3571–3580. doi: 10.1016/j.biomaterials.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 47.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraehenbuehl TP, Langer R, Ferreira LS. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods. 2011;8:731–736. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- 49.Leight JL, Alge DL, Maier AJ, Anseth KS. Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials. 2013;34:7344–7352. doi: 10.1016/j.biomaterials.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokuda EY, Leight JL, Anseth KS. Modulation of matrix elasticity with PEG hydrogels to study melanoma drug responsiveness. Biomaterials. 2014;35:4310–4318. doi: 10.1016/j.biomaterials.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhote V, Skaalure S, Akalp U, Roberts J, Bryant SJ, Vernerey FJ. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J Mech Behav Biomed Mater. 2013;19:61–74. doi: 10.1016/j.jmbbm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts JJ, Bryant SJ. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials. 2013;34:9969–9979. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Advanced Materials. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 55.Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1:107–114. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 56.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodrigues CA, Diogo MM, da Silva CL, Cabral JM. Microcarrier expansion of mouse embryonic stem cell-derived neural stem cells in stirred bioreactors. Biotechnol Appl Biochem. 2011;58:231–242. doi: 10.1002/bab.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.