The authors conducted a single-center, prospective observational study of treatment with 5-fluorouracil and oxaliplatin (FOLFOX-4 regimen) in patients with unresectable or recurrent peritoneal pseudomyxoma of appendiceal origin. Results showed that FOLFOX-4 chemotherapy is tolerable and active in patients with peritoneal pseudomyxoma when disease is deemed unresectable or relapsed after peritonectomy and hyperthermic intraperitoneal chemotherapy.
Keywords: Peritoneal pseudomyxoma, Appendiceal cancer, FOLFOX-4, Chemotherapy
Abstract
Purpose.
The standard treatment of peritoneal pseudomyxoma is based on cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC). The establishment of newer systemic treatments is an unmet clinical need for unresectable or relapsed peritoneal pseudomyxoma. The aim of our study was to assess the activity of chemotherapy with 5-fluorouracil and oxaliplatin (FOLFOX-4 regimen) in terms of response rate in this subset of patients.
Materials and Methods.
Patients were included in a single-center, observational study and treated with FOLFOX-4 administered every 2 weeks for up to 12 cycles or until progressive disease or unacceptable toxicity.
Results.
Twenty consecutive patients were reviewed from July 2011 to September 2013. Only partial responses were observed, with an objective response rate of 20%. Median progression-free survival and overall survival were 8 months and 26 months, respectively. Two patients were able to undergo laparotomy with complete cytoreduction and HIPEC in one case. Safety data for FOLFOX-4 were consistent with the literature. By means of a mutant enriched polymerase chain reaction, KRAS mutation was found in 16 of 19 cases (84%), and MGMT promoter methylation was found in 8 (42%, all KRAS mutant).
Conclusion.
FOLFOX-4 chemotherapy is tolerable and active in patients with peritoneal pseudomyxoma when disease is deemed unresectable or relapsed after peritonectomy and HIPEC. The identification of predictive biomarkers, such as KRAS for resistance to anti-epidermal growth factor receptor monoclonal antibodies and MGMT for response to temozolomide, is a priority for the development of evidence-based treatment strategies for peritoneal pseudomyxoma.
Implications for Practice:
Little evidence is available about the role of modern chemotherapy for patients with peritoneal pseudomyxoma when the disease relapses after curative surgery or is deemed unresectable at tertiary cancer centers. Our study proved that FOLFOX-4 regimen is tolerable and active in this patients population, establishing a new therapeutic option in this orphan disease.
Introduction
Peritoneal pseudomyxoma (PPM) is an extremely rare clinical condition characterized by progressive accumulation of mucinous ascites and tumor implants throughout the peritoneum, almost always caused by appendiceal mucinous neoplasm [1]. The classic histology of PPM is constituted by sparse neoplastic cells within a background of abundant mucinous deposits, whereas hematogenous and lymph node metastases are unusual. In the fourth edition of the WHO Classification of Tumours of the Digestive System [2], PPM is classified as low or high grade based on histological criteria previously proposed by Bradley et al. [3].
The standard treatment of PPM is based on cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) to extirpate gross and microscopic disease, respectively. As confirmed by an expert consensus conference, CRS and HIPEC confer a significant overall survival (OS) benefit compared with historical data for debulking surgery, with a 20-year OS rate greater than 70% [4, 5]. However, even after adequate treatment, about one-third of patients with PPM will develop a recurrence and eventually will die of the disease and its complications [6]. Outcome following curative surgery is influenced by validated prognostic factors, such as completeness of surgical cytoreduction, peritoneal carcinomatosis index, and histological grade, as well as the number of prior operations and the values of circulating tumor markers [6, 7].
Chemotherapy plays only a marginal role in the treatment of PPM, initially due to the difficulty in establishing tumor cell lines and relevant in vivo models. Above all, PPM was traditionally considered chemoresistant because of its low or borderline malignant potential and low proliferation index. However, modern chemotherapy regimens currently used in gastrointestinal malignancies may improve outcomes of patients with unresectable or recurrent PPM. Consequently, we conducted a single-center, prospective observational study of treatment with 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX-4 regimen) in patients with unresectable or recurrent PPM of appendiceal origin.
Patients and Methods
Patients
Patients with unresectable or recurrent PPM of appendiceal origin were enrolled in this study. Eligibility criteria included age ≥18 years; pathologically confirmed appendiceal borderline mucinous tumor or well differentiated appendiceal adenocarcinoma associated with PPM; histologically and/or radiologically confirmed disease relapse following standard CRS and HIPEC, or, alternatively, histologically confirmed PPM judged unresectable by our multidisciplinary team assessment; Eastern Cooperative Oncology Group performance status 0–2; life expectancy of >3 months; and adequate bone marrow, hepatic, and renal functions. Key exclusion criteria were prior oxaliplatin-based chemotherapy, although one prior treatment line with fluoropyrimidine-based chemotherapy was allowed; surgery within 28 days of starting treatment or surgery anticipated during the study; patients with uncontrolled hypertension; clinically significant cardiovascular disease, hemorrhagic diathesis, or coagulopathy; patients with history of malignancy in the previous 5 years; women of childbearing potential (men must agree to use adequate contraception from enrollment until at least 3 months after the last study drug administration). The study was conducted according to Good Clinical Practices and was approved by the local ethics committee (study INT 14/14). All subjects provided written informed consent prior to study procedures. No formal definition of “unresectability” was provided in the study protocol because the exclusion criterion of anticipation of surgery based on multidisciplinary team assessment was deemed to exclude potentially resectable patients. All patients were screened for malnutrition using the Malnutrition Universal Screening Tool. In patients at high risk for malnutrition, a nutrition plan was developed and patients were supported with long-term artificial nutrition, according to the American Society for Parenteral and Enteral Nutrition guidelines [8].
Treatment Regimen
Eligible patients received a FOLFOX-4 regimen composed of a 3-hour infusion of oxaliplatin (85 mg/m2) on day 1 and a 2-hour infusion of L-folinic acid (100 mg/m2) followed by bolus 5-FU (400 mg/mq) and a 22-hour continuous infusion of 5-FU (600 mg/m2) for 2 consecutive days. Treatment was repeated every 2 weeks for a maximum of 12 cycles or until disease progression, consent withdrawal, or unacceptable toxicity. In case of adverse events, dose modification and treatment delays were performed according to the protocol (supplemental online Table 1). Dose modification of oxaliplatin alone was planned if the patient experienced grade 2 sensory neuropathy; oxaliplatin was permanently discontinued in case of grade 3 neurotoxicity, and 5-FU could be continued until the 12th cycle.
Study Endpoints and Assessments
The primary study endpoint was response rate according to Response Evaluation Criteria In Solid Tumors version 1.1 to define complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [9]. Secondary endpoints were progression-free survival (PFS), defined as the time from enrollment to investigator-assessed progression or death, whichever occurred first; OS, defined as time from enrollment to death; response duration, defined as time from first documented response to PD or death; circulating tumor markers response; and safety. A response rate of at least 20% was considered to be acceptable in this study, and at least four responses had to be observed with FOLFOX-4 because no patient was expected to show tumor response without active treatment.
Baseline evaluations included medical history and physical examination; complete blood count and biochemical profile; electrocardiogram; chest x-ray; computed tomography (CT) scan of the chest, abdomen, and pelvis, with documentation of tumor measurements; and assessment of circulating tumor markers including carcinoembryonic antigen, cancer antigen (CA) CA 19-9, CA 125, and CA 15-3. During treatment, complete blood cell counts and biochemical profiles, physical examinations, and assessment of toxicities were done prior to each treatment cycle. CT scans and tumor marker assessment were repeated every 12 weeks during the treatment phase and thereafter. At the discretion of the investigators, CT scans could be performed earlier than required by protocol if appropriate. Treatment toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0.
Biomarker Evaluation
Formalin-fixed paraffin-embedded tissues of peritoneal deposits were reviewed for tumor content. Macrodissection of 7 μm methylene blue-stained sections allowed the separation of neoplastic and normal cells. Genomic DNA was extracted using the QIAamp FFPE DNA kit (Qiagen, Chatsworth, CA, http://www.qiagen.com), following the manufacturer’s instructions. Mutational analysis of KRAS (exons 2, 3, and 4) was performed, as described previously [10, 11]. KRAS exon 2 status was further confirmed through a specific mutant-enriched polymerase chain reaction (PCR) known to be a more sensitive approach [12].
BRAF (exons 15) and NRAS (exons 2 and 3) mutational analysis was performed by means of PCR using specific primers described previously [11]. The PCR products were subjected to direct sequencing using an ABI Prism 3500 DX Genetic Analyzer (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) and then evaluated by means of the ChromasPro software.
MGMT promoter methylation was assessed by methylation-specific PCR. One microgram of DNA was bisulfate treated using the Methylation KIT (EZ DNA Methylation kit, Orange, CA, http://www.zymoresearch.com). The bisulfate-modified template was amplified by using primers specific for methylated and unmethylated templates, as described previously [13, 14]. Two templates provided by the Methylation KIT were used as positive controls for methylated and unmethylated reactions. The products of PCR-specific amplifications were separated by means of 2% agarose gel electrophoresis and visualized using ethidium bromide staining.
Results
Patients and Disease Characteristics
From July 2011 to September 2013, 20 consecutive patients were reviewed at Fondazione IRCCS Istituto Nazionale dei Tumori of Milan, Italy, and all received at least three cycles of chemotherapy. Patient demographics and disease characteristics are shown in Table 1.
Table 1.
Patients and disease characteristics

Fourteen patients (70%) underwent CRS, which included peritonectomy procedures and multivisceral resections aiming at removing the macroscopic disease, according to the technique described by Sugarbaker et al. [15]. Closed-abdomen HIPEC was performed using cisplatin and mitomycin C, as reported previously [7]. Six patients (30%) were deemed unresectable by multidisciplinary assessment. Five patients received one prior treatment line with capecitabine, in association with mytomycin C in two cases.
Patients and Disease Outcome
All patients had serial measurements adequate to determine their response. As expected, no CR occurred; four patients (20%) showed PR, nine (45%) had SD, and seven (35%) had PD as best response. The overall objective response rate was 20%, and a decrease in the amount of mucus was invariably observed. The median duration of response was 14.2 months (range: 4.7–16.2 months). The disease control rate (CR plus PR plus SD ≥3 months) was 65%. Two patients initially deemed unresectable were able to undergo laparotomy, reaching complete cytoreduction (CC-0) in one case. Seventeen (85%) patients had at least one elevated circulating tumor marker. All patients with PR and four of nine patients with SD (44%) showed significant decreases in tumor marker levels.
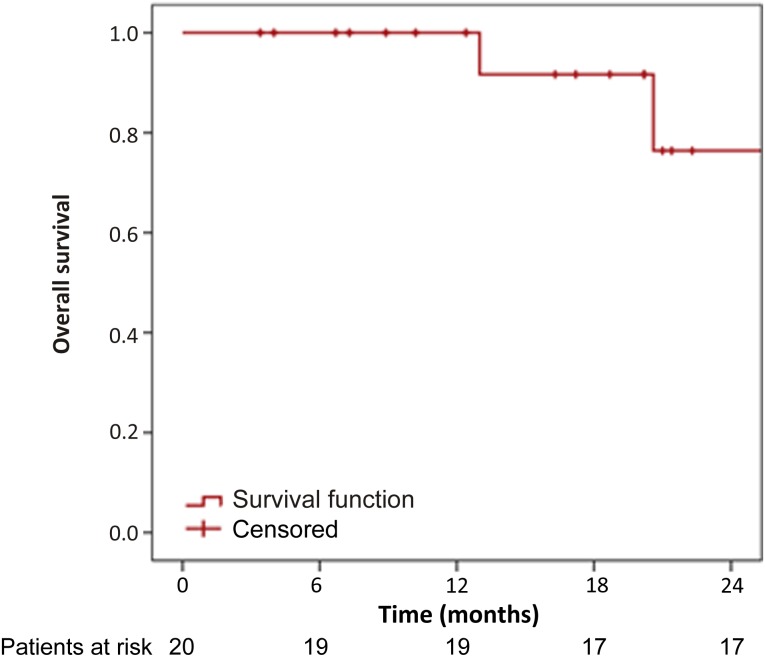
At a median follow-up time of 18 months, 15 patients (75%) experienced PD and only 4 (20%) died. Kaplan-Meier curves for PFS and OS of the study population are shown in Figures 1 and 2, respectively. The median PFS was 8 months (95% confidence interval [CI]: 0–16.8 months), and the median OS was 26.2 months (95% CI: 18.1–34.3 months). Six-month, 12-month, and 18-month PFS rates were 50%, 40%, and 25%, respectively. The median PFS was significantly improved for patients achieving clinical benefit compared with patients with PD (16.6 vs. 2.6 months; p = .0007); however, the median PFS was not significantly different between the group of patients with PR and those with SD (p = .95).
Figure 1.
Kaplan-Meier curve for progression-free survival of the study population.
Figure 2.
Kaplan-Meier curve for overall survival of the study population.
Safety
Overall, 169 chemotherapy cycles were administered, with a median number of 8 cycles per patient (range: 3–12 cycles). The toxicity profile of the FOLFOX-4 regimen was manageable, and no toxic death occurred. Any-grade adverse events were reported in 14 patients (70%). Treatment-related side effects are detailed in Table 2. The most common toxicities were hematological, with grade 3–4 neutropenia occurring in 15% of cases and no patient experiencing febrile neutropenia. Grade 1–2 anemia, thrombocytopenia, and neutropenia occurred in 50%, 25%, and 50% of patients, respectively. Among nonhematological toxicities, fatigue was frequently reported (40%), but it was of grade 3 only in 1 patient (5%). Mild to moderate neurosensory toxicity was observed in 35% of patients (grade 1 in four patients and grade 2 in three patients) but was graded as severe in one patient (5%), who permanently discontinue oxaliplatin. Among patients with disease control, early treatment discontinuation occurred in one patient due to severe fluoropyrimidine-associated toxicity, whereas dose reductions of FOLFOX-4 were needed in four patients (20%).
Table 2.
Treatment-related toxicity

Biomarker Assessment
One patient was not evaluable for molecular biomarkers. Of the 19 assessable PPM cases, 16 (84%) displayed exon 2 mutations of KRAS when mutant-enriched PCR was added to Sanger sequencing. As shown in Table 3, in the tumors with mutated KRAS, the normal DNA sequence GGT (glycine) at codon 12 was altered in 14 cases (88%)—to GAT (aspartic acid) in 10 cases (63%), to GTT (valine) in 3 cases (19%), and to TGT (cysteine) in a single case (6%). KRAS codon 13 mutations were present in two tumors (12%) and were always constituted by the replacement of the glycine amino acid with aspartate (G13D). In three cases (19%), the KRAS gene was considered to be wild type based on Sanger sequencing, whereas a mutation was detected through the mutant-enriched PCR. No BRAF and NRAS mutation was identified, particularly in those patients with KRAS wild-type PPM. MGMT promoter hypermethylation was found in 8 of 19 patients (42%). Interestingly, none of the 3 KRAS wild-type samples showed MGMT methylation, compared with 8 of 16 KRAS-mutant cases.
Table 3.
KRAS mutational status and MGMT promoter hypermethylation status in 19 assessable patients

Discussion
In this study of patients with unresectable or relapsed PPM, FOLFOX-4 chemotherapy obtained a 20% response rate and a 65% disease control rate, with a median PFS of 8 months. The median OS of 26.2 months was consistent with the low malignant potential of the disease. It has been clearly demonstrated that a combined approach of CRS and HIPEC is the standard treatment of patients with PPM because it is able to provide a survival advantage compared with historical controls. Treatment failure may be predicted by several prognostic factors, which were validated in large series [6, 16–18]. Several retrospective analyses documented that previous use of systemic chemotherapy was an independent poor prognostic factor for patients undergoing curative CRS and HIPEC for appendiceal neoplasms associated with PPM [19–21]. However, such studies may be biased by the fact that systemic chemotherapy was offered to patients who were considered suboptimal candidates for curative surgery or was administered in peripheral hospitals without experience in treating PPM, thus delaying the curative approach.
In contrast, little evidence is available about the role of modern chemotherapy for patients with PPM when the disease relapses after curative surgery or is deemed unresectable at tertiary cancer centers. Although currently used regimens may improve patient and disease outcomes compared with data derived from the 5-fluorouracil era, PPM was traditionally considered chemoresistant. In fact, it is reasonable that slow growth rates of appendiceal mucinous neoplasms may be partially responsible for the resistance to cell-cycle phase-specific chemotherapy. This is known as “cytokinetic drug resistance,” published more than 40 years ago by Skipper et al. [22] We hypothesized that appendiceal mucinous tumors might be more sensitive to chemotherapy agents that are cytotoxic to cells that have a prolonged G0-phase cell cycle. Consequently, continuous exposure to an antimetabolites, such as continuous infusion 5-FU or capecitabine, might be active in patients with PPM. A previous retrospective single-institution study was carried out in patients with peritoneal metastases from appendiceal cancer, with or without PPM, and who were poor candidates for CRS and/or HIPEC [23]. Despite the limitation of the heterogeneity of the chemotherapy regimens used, the authors observed a disease control rate of 55.6% and PFS of 7.6 months.
At present, there is no standard systemic chemotherapy regimen for PPM relapsing or progressing after CRS and HIPEC, although drugs and combinations commonly used for colorectal cancer may be generally offered to patients. The establishment of newer systemic treatments as well as the identification of predictive biomarkers helping to treat patients who are more likely to obtain a benefit and avoid potential toxicity in case of resistance are still an unmet clinical need for patients with PPM.
A previous phase II study was carried out in 40 patients with advanced unresectable PPM, with the aim of assessing the efficacy of the combination of mitomycin C and capecitabine [24]. Of the 39 assessable patients, 15 (38%) had a clinical benefit in terms of reduction in mucus (with or without a solid component) or development of stability when known to be progressing prior to treatment. One-year and 2-year overall survival was 84% and 61%, respectively, whereas PFS was not reported. A previous case series assessed the role of neoadjuvant oxaliplatin-based chemotherapy in the management of peritoneal metastases in high-grade mucinous carcinoma of the appendix [25]. This retrospective study included 30 patients treated with the FOLFOX regimen and 4 patients treated with capecitabine and oxaliplatin (XELOX regimen); 21 of the 34 subjects received bevacizumab as a part of their neoadjuvant treatments at the discretion of the managing medical oncologist. In this study, the most accurate assessment of the clinical status of these patients was achieved by extensive surgical exploration compared with radiological assessment. At laparotomy, a clinical response was observed in 8 patients, SD was observed in 9, and PD was observed in 17. Two case reports described a good outcome when bevacizumab was added to fluoropyrimidine-based chemotherapy [26, 27]. The authors speculated that modulation of the tumor microenvironment and angiogenesis by bevacizumab may be beneficial in borderline tumors such as PPM. Given the absence of evidence-based data, at present, the addition of biologics to chemotherapy in the treatment of PPM cannot be recommended outside of clinical trials.
The molecular profiling of cancers may provide new targets for therapeutic intervention [28], and the identification of predictive biomarkers is an unmet clinical need in patients with appendiceal neoplasm associated with PPM. The role of biologics commonly used in advanced colorectal cancer is not known at present in patients with PPM. It is well known that KRAS mutations are predictive biomarkers of resistance to anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab in patients with advanced colorectal cancer [29]. It is well described that KRAS mutations are involved in the development and progression of mucinous cancers arising from several locations, including appendiceal cancers [30–32]. Even if our sample size was small, we reported dramatically high incidence of KRAS mutations in appendiceal mucinous neoplasms using the extremely sensitive mutant-enriched PCR. This technique may detect a mutant allele among as many as 103 to 104 copies of wild-type alleles [12] and may be particularly suitable for molecular profiling of PPM, which is histologically characterized by sparse and rare neoplastic cells within a large amount of mucinous deposits. We emphasize that the role of anti-EGFR monoclonal antibodies should be assessed mainly in the subset of PPM patients with wild-type KRAS status. Moreover, this study is the first to describe the presence of MGMT promoter hypermethylation in patients with PPM. Similar to colorectal cancer, we found that MGMT methylation is associated with KRAS mutation; it may be involved in the early steps of carcinogenesis through a defective DNA repair mechanism and an increase in the mutational rate of target genes such as KRAS itself. Moreover, MGMT methylation is associated with benefit from alkylating agents such as temozolomide in several cancers, including glioblastoma and colorectal cancer [14, 33]. Recently, the combination of capecitabine and temozolomide was shown to be highly active in patients with well- to moderately differentiated neuroendocrine carcinomas [34], which are characterized by slow growing rates, similar to PPM.
Conclusion
Our study may open a new therapeutic window for patients with PPM because the FOLFOX-4 regimen was proven to be active and promising. Moreover, KRAS mutation and MGMT methylation are not only involved in the cancer-development process but may be used as predictive biomarkers for molecular enrichment for future trials with targeted agents or cytotoxics.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This investigator-driven study was supported by NORD (National Organization for Rare Disorders) Grant 2011. This work was also supported in part by funds obtained through Italian Law 12 November 2011 n. 183, which allows taxpayers to allocate 0.5% share of their income tax contribution to a research institution of their choice.
Author Contributions
Conception/Design: Filippo Pietrantonio, Maria Di Bartolomeo, Marcello Deraco, Federica Perrone, Filippo de Braud
Provision of study material or patients: Filippo Pietrantonio, Claudia Maggi, Giuseppe Fanetti, Roberto Iacovelli, Maria Di Bartolomeo, Francesca Ricchini, Marcello Deraco, Federica Perrone, Dario Baratti, Shigeki Kusamura, Elena Tamborini, Alessandra Castano, Paola Valentina Consonni, Ilaria Bossi, Cecilia Gavazzi, Massimo Milione, Giuseppe Pelosi, Filippo de Braud
Collection and/or assembly of data: Filippo Pietrantonio, Claudia Maggi, Giuseppe Fanetti, Roberto Iacovelli, Maria Di Bartolomeo, Francesca Ricchini, Marcello Deraco, Federica Perrone, Dario Baratti, Shigeki Kusamura, Elena Tamborini, Alessandra Castano, Paola Valentina Consonni, Ilaria Bossi, Massimo Milione, Filippo de Braud
Data analysis and interpretation: Filippo Pietrantonio, Claudia Maggi, Giuseppe Fanetti, Roberto Iacovelli, Maria Di Bartolomeo, Francesca Ricchini, Marcello Deraco, Federica Perrone, Dario Baratti, Shigeki Kusamura, Elena Tamborini, Alessandra Castano, Paola Valentina Consonni, Ilaria Bossi, Filippo de Braud
Manuscript writing: Filippo Pietrantonio, Marcello Deraco, Federica Perrone, Cecilia Gavazzi, Massimo Milione, Giuseppe Pelosi, Filippo de Braud
Final approval of manuscript: Filippo Pietrantonio, Claudia Maggi, Giuseppe Fanetti, Roberto Iacovelli, Maria Di Bartolomeo, Francesca Ricchini, Marcello Deraco, Federica Perrone, Dario Baratti, Shigeki Kusamura, Elena Tamborini, Alessandra Castano, Paola Valentina Consonni, Ilaria Bossi, Cecilia Gavazzi, Massimo Milione, Giuseppe Pelosi, Filippo de Braud
Disclosures
The authors indicated no financial relationships.
References
- 1.Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur J Surg Oncol. 2008;34:196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 2.et al. In: WHO Classification of Tumours of the Digestive System. 4th ed. Bosman FT, Carneiro F, Hruban RH, editors; Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 3.Bradley RF, Stewart JH, IV, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 4.Austin F, Mavanur A, Sathaiah M, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–1393. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 6.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 7.Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: Biological features are the dominant prognostic determinants after complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2009;249:243–249. doi: 10.1097/SLA.0b013e31818eec64. [DOI] [PubMed] [Google Scholar]
- 8.August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: Nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472–500. doi: 10.1177/0148607109341804. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Perrone F, Lampis A, Orsenigo M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 11.Di Bartolomeo M, Pietrantonio F, Perrone F, et al. Lack of KRAS, NRAS, BRAF and TP53 mutations improves outcome of elderly metastatic colorectal cancer patients treated with cetuximab, oxaliplatin and UFT. Target Oncol. 2014;9:155–162. doi: 10.1007/s11523-013-0283-8. [DOI] [PubMed] [Google Scholar]
- 12.Molinari F, Felicioni L, Buscarino M, et al. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res. 2011;17:4901–4914. doi: 10.1158/1078-0432.CCR-10-3137. [DOI] [PubMed] [Google Scholar]
- 13.Herman JG, Graff JR, Myöhänen S, et al. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietrantonio F, Perrone F, de Braud F, et al. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Ann Oncol. 2014;25:404–408. doi: 10.1093/annonc/mdt547. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 1996;82:235–253. doi: 10.1007/978-1-4613-1247-5_15. [DOI] [PubMed] [Google Scholar]
- 16.Smeenk RM, Verwaal VJ, Antonini N, et al. Progression of pseudomyxoma peritonei after combined modality treatment: Management and outcome. Ann Surg Oncol. 2007;14:493–499. doi: 10.1245/s10434-006-9174-x. [DOI] [PubMed] [Google Scholar]
- 17.Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007;14:2289–2299. doi: 10.1245/s10434-007-9462-0. [DOI] [PubMed] [Google Scholar]
- 18.Baratti D, Kusamura S, Martinetti A, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14:2300–2308. doi: 10.1245/s10434-007-9393-9. [DOI] [PubMed] [Google Scholar]
- 19.Smith JW, Kemeny N, Caldwell C, et al. Pseudomyxoma peritonei of appendiceal origin. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1992;70:396–401. doi: 10.1002/1097-0142(19920715)70:2<396::aid-cncr2820700205>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: Clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–534. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 22.Skipper HE, Schabel FM, Jr, Mellett LB, et al. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970;54:431–450. [PubMed] [Google Scholar]
- 23.Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: A single-institution experience. Cancer. 2010;116:316–322. doi: 10.1002/cncr.24715. [DOI] [PubMed] [Google Scholar]
- 24.Farquharson AL, Pranesh N, Witham G, et al. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer. 2008;99:591–596. doi: 10.1038/sj.bjc.6604522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugarbaker PH, Bijelic L, Chang D, et al. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol. 2010;102:576–581. doi: 10.1002/jso.21679. [DOI] [PubMed] [Google Scholar]
- 26.Sun WL, Hutarew G, Gradl J, et al. Successful antiangiogenic combination therapy for pseudomyxoma peritonei with bevacizumab and capecitabine. Cancer Biol Ther. 2009;8:1459–1462. doi: 10.4161/cbt.8.15.8943. [DOI] [PubMed] [Google Scholar]
- 27.Powell ED, Macdonald DB, Elkeilani AM, et al. A case of appendiceal adenocarcinoma with clinical benefit from FOLFOX and bevacizumab. Case Rep Oncol. 2009;2:111–115. doi: 10.1159/000229245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Hoff DD, Stephenson JJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 29.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 30.Ichikawa Y, Nishida M, Suzuki H, et al. Mutation of K-ras protooncogene is associated with histological subtypes in human mucinous ovarian tumors. Cancer Res. 1994;54:33–35. [PubMed] [Google Scholar]
- 31.Raghav KP, Shetty AV, Kazmi SM, et al. Impact of molecular alterations and targeted therapy in appendiceal adenocarcinomas. The Oncologist. 2013;18:1270–1277. doi: 10.1634/theoncologist.2013-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabbani W, Houlihan PS, Luthra R, et al. Mucinous and nonmucinous appendiceal adenocarcinomas: Different clinicopathological features but similar genetic alterations. Mod Pathol. 2002;15:599–605. doi: 10.1038/modpathol.3880572. [DOI] [PubMed] [Google Scholar]
- 33.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 34.Fine RE, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol. 2014;32(suppl 3):179a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.