This paper reports health-related quality of life (HRQoL) and quality-adjusted time without symptoms of disease or toxicity results from the ToGA (Trastuzumab for Gastric Cancer) phase III trial. Compared with chemotherapy alone, trastuzumab plus chemotherapy prolongs time to deterioration of HRQoL and increases quality-adjusted survival in patients with HER2-positive gastric or gastroesophageal junction cancer.
Keywords: Chemotherapy, Gastric cancer, HER2, Quality of life, Trastuzumab
Abstract
Background.
The Trastuzumab for Gastric Cancer phase III trial demonstrated that combining trastuzumab with chemotherapy significantly improved overall survival compared with chemotherapy alone in HER2-positive advanced gastric or gastroesophageal junction cancer. We report health-related quality of life (HRQoL) and quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) results from this trial.
Patients and Methods.
Patients were randomized to receive six cycles of chemotherapy given every 3 weeks (capecitabine or fluorouracil, plus cisplatin) either alone or combined with administration of trastuzumab every 3 weeks until disease progression. At each clinical visit, HRQoL was assessed using two European Organization for Research and Treatment of Cancer quality of life questionnaires, QLQ-C30 and QLQ-STO22. Q-TWiST methodology was applied retrospectively using the clinical data and utility coefficients.
Results.
Trastuzumab plus chemotherapy prolonged time to 10% definitive deterioration in all QLQ-C30 and QLQ-STO22 scores, including QLQ-C30 global health status versus chemotherapy alone, from 6.4 months to 10.2 months. In addition, trastuzumab plus chemotherapy extended Q-TWiST by 2.42 months compared with chemotherapy alone.
Conclusion.
Compared with chemotherapy alone, trastuzumab plus chemotherapy prolongs time to deterioration of HRQoL and increases quality-adjusted survival in patients with HER2-positive gastric or gastroesophageal junction cancer.
Abstract
摘要
背景。使用曲妥珠单抗治疗胃癌的 III 期研究表明,与单纯化疗相比,曲妥珠单抗与化疗药物的联合使用可显著提高 HER2 阳性晚期胃癌或食管胃结合部癌患者的总生存期。本文报告了这项研究的健康相关生存质量 (HRQoL) 及生存质量校正后的无疾病症状无毒性生存期 (Q-TWiST) 结果。
患者与方法。患者被随机分为两组,分别接受单纯化疗(卡培他滨或氟尿嘧啶,联合顺铂,每 3 周一个周期,共 6 个周期)或化疗联合曲妥珠单抗(每 3 周一次)治疗,直至出现疾病进展。在每次临床就诊时,我们采用欧洲肿瘤研治组织的两份生存质量调查问卷 QLQ-C30 和 QLQ-STO22 对受试者进行 HRQoL 评估。Q-TWiST 的评估则采用回顾性方式依据临床数据和效用系数来进行。
结果。与单纯化疗相比,曲妥珠单抗与化疗药物的联合使用将所有 QLQ-C30 和 QLQ-STO22 评估指标的“至 10% 确定性下降时间”从 6.4 个月推迟到了 10.2 个月,其中包括 QLQ-C30 总体健康状态这一指标。此外,与单纯化疗相比,曲妥珠单抗与化疗的联合使用也将 Q-TWiST 延长了 2.42 个月。
结论。与单纯化疗相比,曲妥珠单抗与化疗的联合使用推迟了 HRQoL 的至下降时间,提高了 HER2 阳性胃癌或食管胃结合部癌患者的生存质量校正后生存期。
The Oncologist 2014;19:712–719
Implications for Practice:
In the randomized phase III ToGA (Trastuzumab for Gastric Cancer) trial, adding trastuzumab to chemotherapy increased overall survival in patients with first-line HER2-positive advanced gastric cancer without increasing the overall incidence of adverse events. The results from the trial led to the approval of trastuzumab for this indication. Advanced gastric cancer is incurable, and most patients are symptomatic. Consequently, it is important that new treatments improve survival without compromising health-related quality of life (HRQoL). We show that adding trastuzumab to chemotherapy prolonged the time to deterioration of HRQoL and extended quality-adjusted survival.
Introduction
Gastric cancer is a major health concern and a leading cause of cancer-related death [1]. In most patients, the prognosis is poor, and surgical resection is often the only curative treatment modality. There is no established standard of care for advanced gastric cancer, but fluoropyrimidine-containing and platinum-containing regimens are widely used [2].
Human epidermal growth factor receptor 2 (HER2) overexpression or HER2 amplification occurs in 7%–34% of patients with gastric and gastroesophageal junction (GEJ) tumors [3–5]. Trastuzumab, a humanized monoclonal antibody specifically targeting HER2 [6], was investigated in combination with chemotherapy in patients with HER2-positive advanced gastric or GEJ cancer in the international, randomized, phase III ToGA (Trastuzumab for Gastric Cancer) trial [5]. Adding trastuzumab to chemotherapy (capecitabine or 5-fluorouracil and cisplatin) significantly prolonged overall survival (OS) in patients with HER2-positive advanced gastric or GEJ cancer (median: 13.8 vs. 11.1 months; hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.60–0.91; p = .0046) [6]. The safety profiles were similar between the two treatment arms [5].
Because advanced gastric or GEJ cancer is incurable and most patients are symptomatic, it is particularly important to identify treatments that may improve survival without compromising health-related quality of life (HRQoL). Consequently, current clinical decisions are often informed by HRQoL data, especially for advanced disease [7]. In this paper, we present the results of HRQoL analyses from the ToGA trial, including quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) analyses, which provide an assessment of quality-adjusted survival [8, 9].
Materials and Methods
Study Design and Conduct
The ToGA trial (ClinicalTrials.gov identifier: NCT01041404) was conducted in accordance with the Declaration of Helsinki. Trial design and baseline characteristics have been reported previously [5].
Randomization and Masking
Patients were block randomized (1:1) based on five stratification factors to receive trastuzumab (Herceptin; F. Hoffmann-La Roche Ltd., Basel, Switzerland, http://www.roche.com) plus chemotherapy (cisplatin plus capecitabine [Xeloda; F. Hoffmann-La Roche Ltd.] or fluorouracil, chosen at the investigator’s discretion) or chemotherapy alone [5]. Neither investigators nor patients were masked to treatment assignment.
Treatments
Chemotherapy was given every 3 weeks for six cycles. Capecitabine at 1,000 mg/m2 was given orally twice a day for 14 days followed by a 1-week rest, or fluorouracil at 800 mg/m2 per day was given by continuous intravenous infusion on days 1–5 of each cycle. Cisplatin at 80 mg/m2 on day 1 was given by intravenous infusion. Trastuzumab was given by intravenous infusion at a dose of 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3 weeks until disease progression (PD), unacceptable toxicity, or withdrawal of consent [5].
Quality-of-Life Assessments
HRQoL was assessed as a secondary objective of the ToGA trial by asking patients to complete validated local-language versions of two European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaires: QLQ-C30 (version 3.0) and QLQ-STO22. The self-administered QLQ-C30 questionnaire and the gastric-cancer-specific QLQ-STO22 module have been fully discussed previously [10–12]. In addition, pain intensity was assessed using a visual analog scale on a 100-mm horizontal line (0 mm = no pain, 100 mm = worst pain possible) [13]. Assessments were performed at baseline and then every 3 weeks (on day 1 of each cycle) until PD or withdrawal from the trial. All questionnaires were completed by the patients themselves at the study site before any other study-specific assessments (e.g., efficacy and safety assessments) and before intravenous infusions. In the case of missing or incomplete questionnaires, when at least half of the items from the scale were answered, values for missing items were imputed as the average of those items present for the respondent. Otherwise, the raw score of the scale was set to missing.
Statistical Analysis of Quality of Life
HRQoL was assessed in all randomized patients who received at least one dose of study medication (the full analysis set). The raw scores of each of the EORTC QLQ instruments were linearly transformed into scores ranging from 0 to 100 in accordance with the EORTC Scoring Manual [14, 15].
Mean and standard error of the mean were calculated for each treatment arm at each scheduled clinic visit up to 64 weeks after treatment initiation for both of the EORTC QLQ instruments.
As defined in the ToGA trial protocol, qualitative assessments of changes in each HRQoL measure during the study were made by preparing plots of mean values and their standard errors by treatment arm over time. Changes from baseline were depicted for each scale or item from the two HRQoL questionnaires up to 64 weeks after treatment initiation.
Kaplan-Meier methodology was used to measure time to deterioration of global health status (GHS), functioning, and symptom scores. Time was calculated from randomization to the first occurrence of a 10% deterioration (or death). A 10% deterioration was defined as a deterioration from baseline of at least 10 points on the 0−100 scale that continued until the patient withdrew from the study. A deterioration was not counted as an event if a subsequent improvement returned the overall deterioration from baseline to less than 10 points. If a patient did not have an event (death or 10% deterioration), they were censored at their last clinic visit at which HRQoL was measured. The two-sided log-rank test was used to compare time to deterioration between the treatment arms. All analyses were conducted using SAS version 8.2 (SAS Institute Inc., Cary, NC, http://www.sas.com). The same analysis methodology was applied to time to first occurrence of a 5% deterioration (or death). All HRQoL analyses from the ToGA trial were planned to be descriptive only; therefore, statistical testing is of an exploratory nature.
Although the trial protocol did not specify comparisons of quantitative thresholds, the proportion of patients whose GHS improved or worsened by ≥10% from baseline at weeks 7, 16, 25, 34, 43, 52, and 61 has been calculated for each treatment arm.
Q-TWiST Analysis
The Q-TWiST analysis consisted of three steps [8].
Step 1: Partitioning of OS Into Nonoverlapping Health States
The area under the OS curves was partitioned into three nonoverlapping health states: (a) duration of toxicity (TOX), the average time spent with grade 3–4 adverse events after randomization and prior to PD for patients who received at least one dose of study medication; (b) time without disease symptoms or treatment toxicity (TWiST), calculated as progression-free survival (PFS) minus TOX; (c) disease relapse (REL), calculated as time from progression until end of follow-up or death (OS minus PFS).
Step 2: Estimation of Health-State Durations
The mean duration of TOX, REL, and TWiST was estimated from the clinical trial data as the area under the partitioned Kaplan-Meier curves. The chemotherapy-alone arm had a shorter follow-up time. Consequently, to remove any potential bias favoring trastuzumab plus chemotherapy, data were truncated to 31.31 months, the longest follow-up in the shortest progression-free curve of the chemotherapy-alone arm.
Step 3: Estimation of Q-TWiST
A quality-adjusted survival model was developed that included utility coefficients uTOX, uREL and uTWiST [8]. Utility coefficients incorporate the patient’s perception of his or her health and enable quality adjustment of the time spent in each health state. Values range from 0 (representing death) to 1 (representing full health). Quality-adjusted OS (Q-TWiST) is defined as the weighted sum of the time spent in each health state and is calculated as follows:
This Q-TWiST analysis assumed a TWiST utility of 1.0. A utility weight of 0.58 was selected for REL based on published literature [15, 16] and is associated with best supportive care in gastric cancer. This was considered appropriate because few patients with advanced gastric cancer receive second or later lines of chemotherapy. Because treatment duration varied between treatment arms and no treatment-related differences in HRQoL or duration of treatment toxicity were detected, it was considered reasonable to allocate the same utility weight to TOX in both treatment arms. In the absence of an established utility standard for TOX in this setting, the utility of TOX was conservatively assumed to be equivalent to that of REL (0.58). Thus, 1.72 days in REL or TOX was considered equivalent to 1.0 day of TWiST.
Q-TWiST values were compared between treatment arms, and 95% CIs for mean differences in each health state were estimated from the 2.5 and 97.5 percentiles of the bootstrapped values (5,000 iterations). The p values were based on the standard z-statistic. The increase in Q-TWiST and associated 95% CIs were estimated for a wide range of TOX and REL utility value combinations (0.1–0.9) while keeping the TWiST utility at 1.0.
Results
Patient Disposition and Characteristics
The full analysis-set population comprised 584 patients: 294 in the trastuzumab-plus-chemotherapy arm and 290 in the chemotherapy-alone arm. The first patient was randomized in September 2005 and the last patient in December 2008; the clinical cutoff date was January 2009. The demographics and baseline characteristics of these patients have been described previously [5]. Patient flow during the trial is shown in supplemental online Figure 1.
Compliance With Questionnaire Completion
Patient compliance for completion of the QLQ-C30 and QLC-STO22 questionnaires was high in both treatment arms throughout the study (Table 1). Compliance with both questionnaires was greater than 92% in both treatment arms throughout all visits.
Table 1.
Patient compliance with EORTC QLQ-C30 and EORTC QLQ-STO22 questionnaires

Change From Baseline
Mean changes from baseline GHS scores revealed no worsening in the trastuzumab-plus-chemotherapy arm compared with the chemotherapy-alone arm during treatment with chemotherapy, at the end of chemotherapy, and during weeks 16–28 (Fig. 1A). Beyond week 34, there was no decrease from baseline GHS scores in the trastuzumab-plus-chemotherapy arm compared with the chemotherapy-alone arm; however, caution is needed in interpreting the data beyond week 34 due to the low patient numbers. A follow-up analysis, with a clinical cutoff date of January 2010, revealed consistent findings (Fig. 1B).
Figure 1.
Change from baseline global health status scores. (A): Initial analysis. (B): Follow-up analysis. Data are means and standard errors of the mean. Assessments were performed at baseline and then every 3 weeks until progression of disease or withdrawal. Data beyond 64 weeks are not presented due to the low number of patients whose disease progressed after this time point.
Abbreviations: BL, baseline; C, chemotherapy; T, trastuzumab.
Time to Definitive Deterioration
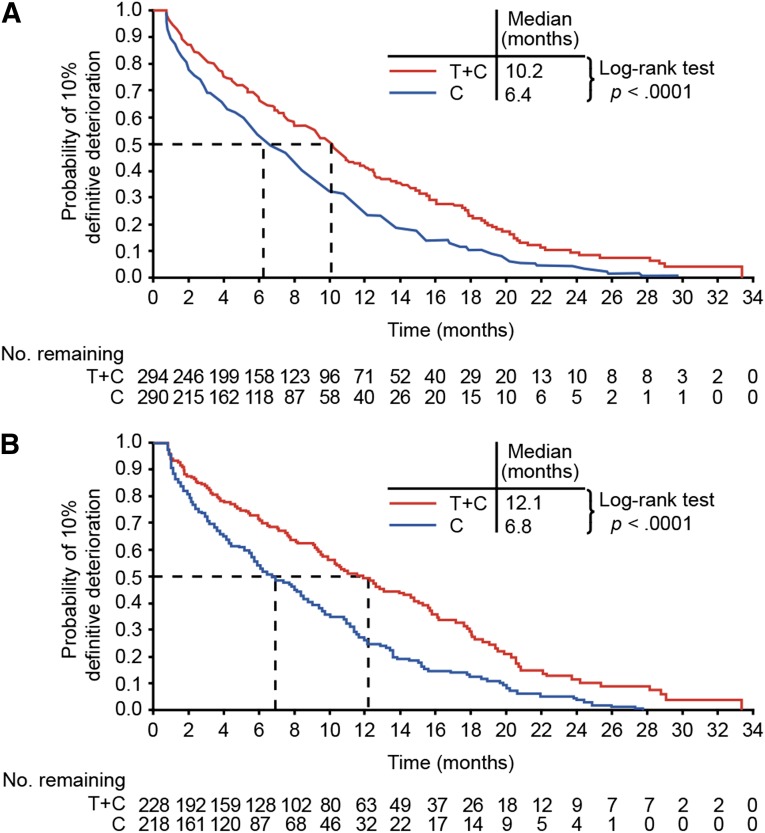
The median time to a 10% definitive deterioration in the GHS score of the QLQ-C30 questionnaire was 6.4 months and 10.2 months in the chemotherapy-alone and trastuzumab-plus-chemotherapy arms, respectively (p < .0001 by two-sided log-rank test) (Fig. 2A). In preliminary analyses of patients with high-level HER2 expression (immunohistochemistry [IHC] 2+/fluorescence in situ hybridization-positive [FISH-positive] or IHC 3+; n = 446), median times to 10% definitive deterioration in GHS score were 6.8 months and 12.1 months, respectively (p < .0001 by two-sided log-rank test), suggesting a greater effect of trastuzumab in this subgroup (Fig. 2B).
Figure 2.
Kaplan-Meier plots of time to a 10% definitive deterioration in European Organization for Research and Treatment of Cancer QLQ-C30 global health status for the full analysis set population (A) and for patients with high-level HER2 expression (immunohistochemistry [IHC] 2+/fluorescence in situ hybridization-positive or IHC 3+) (B).
Abbreviations: C, chemotherapy; T, trastuzumab.
Median times to 10% definitive deterioration in all QLQ-C30 and QLQ-STO22 scores and visual analog scale of pain intensity are summarized in Table 2. The results suggested benefits in favor of trastuzumab plus chemotherapy for all QLQ-C30 and QLQ-STO22 scores and the visual analog scale. Median times to 5% definitive deterioration similarly showed increases with trastuzumab plus chemotherapy for all scores assessed (supplemental online Table 1).
Table 2.
Summary of median times to 10% definitive deteriorations of scale items for EORTC QLQ-C30, EORTC QLQ-STO22, and the visual analog scale

Proportion of Patients Improving or Worsening by ≥10%
The proportion of patients with QLQ-C30 GHS scores improving by ≥10% in the trastuzumab-plus-chemotherapy arm was no worse than in the chemotherapy-alone arm over time (Fig. 3).
Figure 3.
Proportions of patients with European Organization for Research and Treatment of Cancer QLQ-C30 global health status scores improving by at least 10%, worsening by at least 10%, and with no change (improvement or worsening by <10%).
Abbreviations: C, chemotherapy; T, trastuzumab.
Q-TWiST Analysis
The median follow-up time for patients in the analysis population was 17.18 months. During the truncated follow-up period (31.31 months), patients receiving trastuzumab plus chemotherapy gained a mean 2.52 months of unadjusted OS (95% CI: 1.12–4.02; p < .001) and a mean 2.29 months of progression-free survival (95% CI: 1.31–3.83; p < .001) compared with chemotherapy alone (Table 3). There was no significant difference between the two arms in the duration of TOX (2.54 vs. 2.52 months, p = .31) or REL (6.18 vs. 5.85 months, p = .48) (Table 3). Patients receiving trastuzumab plus chemotherapy gained a mean 2.27 months of TWiST compared with chemotherapy alone (95% CI: 1.15–3.80; p < .001) (Table 3, Fig. 4). Furthermore, when the utility coefficients were applied to the three health states, trastuzumab plus chemotherapy increased the mean duration of Q-TWiST by 2.42 months compared with chemotherapy alone (95% CI: 1.24–3.86; p < .001).
Table 3.
Summary of mean differences in health states and Q-TWiST

Figure 4.
Time spent (months) in quality-adjusted TWiST (Q-TWiST) health states. (A): Unadjusted analysis: a = comparison of OS; b = comparison of PFS; c = comparison of TWiST. (B): Utility-adjusted analysis: c = comparison of TWiST; d = comparison of Q-TWiST. ∗p < .001.
Abbreviations: C, chemotherapy; REL, disease relapse; T, trastuzumab; TOX, duration of toxicity; TWiST, time without disease symptoms or treatment toxicity.
Discussion
The ToGA trial demonstrated that the addition of trastuzumab to cisplatin-based chemotherapy in patients with HER2-positive advanced gastric or GEJ cancer significantly prolonged median OS by 2.7 months (4.2 months in patients with high HER2 expression) and led to the approval of trastuzumab for this indication [5]. In this paper, we show that in patients receiving trastuzumab plus chemotherapy, quality-adjusted survival was extended without altering the overall treatment toxicity burden, and HRQoL was preserved for longer than in patients receiving chemotherapy alone, as shown by the longer median times to definitive deterioration in all aspects of HRQoL analyzed. According to published research, changes in QLQ-C30 scores of 5%–10% and 10%–20% correspond to small and moderate changes in HRQoL, respectively [17].
Although potentially influenced by the number of patients at risk or alive, particularly in the final part of the assessment period, Figure 1 may suggest that the HRQoL of ToGA patients progressed through three distinct phases. During the slight rise in GHS score in both arms in the first part of the curve, we hypothesize that the toxicity of chemotherapy may have partially offset any improvement in HRQoL. In addition, the majority of ToGA patients (90%) had an Eastern Cooperative Oncology Group performance status of 0 or 1 at baseline [5], and this could also explain why HRQoL did not significantly improve in either arm. In the postulated second phase, HRQoL did not worsen in the trastuzumab-plus-chemotherapy arm compared with chemotherapy alone. Finally, the preservation in GHS scores in the trastuzumab-plus-chemotherapy arm exceeded that with chemotherapy alone, and this may reflect sustained effects of trastuzumab continuation after chemotherapy. Similar findings were also evident in the subgroup of patients with high HER2 expression [18]. Consistent results were reported with trastuzumab plus chemotherapy in metastatic breast cancer [19].
Because most patients with advanced gastric or GEJ cancer are symptomatic and incurable when diagnosed, maintaining HRQoL for longer is a critical component of their anticancer therapy. Several previous trials have prospectively evaluated HRQoL with different chemotherapy regimens in this setting; however, ToGA is the first large trial conducted exclusively in HER2-positive disease. The V-325 study in 445 randomized patients with advanced gastric or GEJ cancer showed that during protocol chemotherapy and follow-up, docetaxel plus cisplatin and fluorouracil therapy was associated with a significantly longer time to 5% definitive deterioration in QLQ-C30 GHS than cisplatin and fluorouracil alone (6.5 vs. 4.2 months; HR: 1.45; 95% CI: 1.08–1.93; log-rank test p = .01) [20]. Other chemotherapy regimens have also demonstrated at least trends toward improvement in HRQoL versus comparators [21–23]; however, all these trials were restricted by early dropout and lack of compliance.
Consistent with observations from a systematic Medline search of clinical studies in advanced GEJ cancer [24] and the V-325 study [20], our results suggest time to definitive deterioration is the most appropriate method to evaluate the impact of therapy on HRQoL, and they support the recommendation to establish a standardized approach to such analyses [24]. In contrast with the V-325 study, we did not evaluate postprogression HRQoL. Nevertheless, the decreased compliance over time noted in the V-325 study [20] does not apply to ToGA; therefore, we were able to determine time to deterioration. Curran and colleagues found that 58% of their patients had censored time to events when analyzing GHS quality of life [23], whereas in ToGA, approximately 30% of patients were censored, showing a robust number of events. HRQoL scores were generally decreased at withdrawal, which seems crucial for determining time to deterioration because it allowed the capture of definitive deterioration without affecting compliance and avoided the potential bias introduced by missing events. Curran and colleagues observed a similar “deterioration prior to dropout” [23] but did not use this in further analysis.
Although investigation of the effect of adding trastuzumab to chemotherapy on HRQoL was an important objective of the ToGA trial, these were secondary endpoints for which no a priori hypotheses were formulated. Because several HRQoL outcomes were assessed without correction for multiple comparisons, our findings should be interpreted with caution.
As in most oncology trials, HRQoL assessments in the ToGA trial were discontinued at progression or withdrawal. This is a potential limitation because significant quality-of-life changes might have resulted from symptomatic changes following PD; however, differences in use of postprogression therapies would complicate interpretation of subsequent HRQoL assessments.
The outstanding high compliance with questionnaire completion in the ToGA trial may relate to conduct of assessments at the study site prior to clinical evaluations at every visit until progression. No HRQoL assessments were made following PD, when compliance is typically poor. If a definitive deterioration was observed after a missing value, it was assumed that the deterioration occurred at the time of the missing value. Moreover, it was mandatory for data management to follow up missing HRQoL data, further enhancing compliance.
The numerical increase in some adverse events in the trastuzumab-plus-chemotherapy arm of ToGA [5] was not reflected in the HRQoL data and may be explained by the different approach adopted in safety analyses and HRQoL assessments. Whereas safety outcomes record the occurrence of events at least once in a patient during the study and record the highest severity, HRQoL data show patient perception of this event or symptom at each visit from baseline until progression. Furthermore, in accordance with other published data, HRQoL in oncology does not seem to be related only to toxicity of treatment [24, 25] but rather may reflect differences in efficacy [24].
The results of the ToGA Q-TWiST analysis further support the HRQoL data because they demonstrate that first-line trastuzumab plus chemotherapy extends quality-adjusted survival without altering the overall treatment toxicity burden. The Q-TWiST methodology provides an assessment of quality-adjusted survival that can be applied to clinical trial data to compare different treatments [8, 9]. Q-TWiST is more sensitive than general quality-of-life methodologies in detecting clinically important differences between treatments [26], and unlike classical clinical assessments of safety, efficacy, or HRQoL, Q-TWiST evaluates both the quantity and quality of survival. It should be noted, however, that the Q-TWiST analysis has limitations in that it applied a utility coefficient to the REL health state derived from the literature and a utility coefficient to the TOX health state that was assumed to be equivalent to that of REL. In practice, a patient may assign different weights to the two health states because the utility coefficients incorporate the patient’s perception of his or her health.
In a preplanned post hoc subgroup analysis of the 446 ToGA patients with high-level HER2 expression (IHC 2+/FISH-positive or IHC 3+), there was a 4.2-month increase in median OS with trastuzumab plus chemotherapy versus chemotherapy alone, and this is greater than the 2.7-month increase observed in the overall study population [5]. The impact of trastuzumab on HRQoL also appears greatest in this subgroup. An exploratory analysis was also performed in patients without PD before week 37 (beyond the median time to progression) to investigate the effect of PD on the perception of HRQoL. This analysis suggested that the HRQoL in this subpopulation was generally consistent with those in the overall population but might have been more pronounced, particularly in the trastuzumab-containing arm [27].
Conclusion
For patients with HER2-positive advanced gastric or GEJ cancer enrolled in the ToGA trial, the addition of trastuzumab to chemotherapy compared with chemotherapy alone improved OS without apparently compromising HRQoL. Both the QLQ-C30 and QLQ-STO22 questionnaires indicate that the time to deterioration in patients’ HRQoL with the addition of trastuzumab to chemotherapy was prolonged. Furthermore, quality-adjusted survival (Q-TWiST) was extended by the addition of trastuzumab to chemotherapy.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank the patients, their families, the nurses, and the investigators who participated in this study. We also thank Rick Aultman for performing statistical analysis and Alicyn Campbell and Vivian Ng for critical review of the manuscript. The study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland, and support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Preliminary analyses of quality of life and Q-TWiST data from the ToGA (Trastuzumab for Gastric cancer) trial have been presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, Orlando, Florida, January 22–24, 2010 (abstract 7); the 12th World Congress on Gastrointestinal Cancer, Barcelona, Spain, June 30 to July 3, 2010 (abstract O-0011); Digestive Disease Week, Chicago, Illinois, May 7–10, 2011 (abstract Sa1016); and the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, June 4–8, 2010 (abstract 4048).
Author Contributions
Conception/Design: Taroh Satoh, Yung-Jue Bang, Eric Van Cutsem, Nedal Al-Sakaff, Harald A. Weber
Provision of study material or patients: Taroh Satoh, Yung-Jue Bang, Evgeny A. Gotovkin, Yasuo Hamamoto, Yoon-Koo Kang, Vladimir M. Moiseyenko, Atsushi Ohtsu, Eric Van Cutsem, Hyun-Cheol Chung
Collection and/or assembly of data: Yung-Jue Bang, Eric Van Cutsem, Hyun-Cheol Chung
Data analysis and interpretation: Taroh Satoh, Yung-Jue Bang, Yoon-Koo Kang, Atsushi Ohtsu, Eric Van Cutsem, Nedal Al-Sakaff, Alexa Urspruch, Julie Hill, Harald A. Weber, Hyun-Cheol Chung
Manuscript writing: Taroh Satoh, Yung-Jue Bang, Evgeny A. Gotovkin, Yasuo Hamamoto, Yoon-Koo Kang, Vladimir M. Moiseyenko, Atsushi Ohtsu, Eric Van Cutsem, Nedal Al-Sakaff, Alexa Urspruch, Julie Hill, Harald A. Weber, Hyun-Cheol Chung
Final approval of manuscript: Taroh Satoh, Yung-Jue Bang, Evgeny A. Gotovkin, Yasuo Hamamoto, Yoon-Koo Kang, Vladimir M. Moiseyenko, Atsushi Ohtsu, Eric Van Cutsem, Nedal Al-Sakaff, Alexa Urspruch, Julie Hill, Harald A. Weber, Hyun-Cheol Chung
Disclosures
Taroh Satoh: Chugai Pharmaceutical (RF); Yung-Jue Bang: F. Hoffmann-La Roche Ltd. (C/A, H, RF); Eric Van Cutsem: Bayer (RF); Roche (RF); Yoon-Koo Kang: F. Hoffmann-La Roche Ltd. (C/A, RF); Nedal Al-Sakaff: F. Hoffmann-La Roche Ltd. (E, OI); Alexa Urspruch: F. Hoffmann-La Roche Ltd. (E); Julie Hill: F. Hoffmann-La Roche Ltd. (C/A); Harald A. Weber: F. Hoffmann-La Roche Ltd. (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.GLOBOCAN stomach cancer fact sheet 2012. Available at http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp Accessed May 23, 2014.
- 2.Kang H, Kauh JS. Chemotherapy in the treatment of metastatic gastric cancer: Is there a global standard? Curr Treat Options Oncol. 2011;12:96–106. doi: 10.1007/s11864-010-0135-z. [DOI] [PubMed] [Google Scholar]
- 3.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: A practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 4.Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53–59. doi: 10.1097/PAP.0b013e3182026d72. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 7.Bezjak A, Ng P, Skeel R, et al. Oncologists’ use of quality of life information: Results of a survey of Eastern Cooperative Oncology Group physicians. Qual Life Res. 2001;10:1–13. doi: 10.1023/a:1016692804023. [DOI] [PubMed] [Google Scholar]
- 8.Gelber S, Gelber RD, Cole BF, et al. The Q-TWiST Method. Quality of Life Newsletter. 1996;16:3–4. [Google Scholar]
- 9.Revicki DA, Feeny D, Hunt TL, et al. Analyzing oncology clinical trial data using the Q-TWiST method: Clinical importance and sources for health state preference data. Qual Life Res. 2006;15:411–423. doi: 10.1007/s11136-005-1579-7. [DOI] [PubMed] [Google Scholar]
- 10.Vickery CW, Blazeby JM, Conroy T, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 11.Blazeby JM, Conroy T, Bottomley A, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 14.Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLC-C30 Scoring Manual (3rd Edition) Brussels: EORTC, 2001. [Google Scholar]
- 15.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Xie F, O’Reilly D, Ferrusi IL, et al. Illustrating economic evaluation of diagnostic technologies: Comparing Helicobacter pylori screening strategies in prevention of gastric cancer in Canada. J Am Coll Radiol. 2009;6:317–323. doi: 10.1016/j.jacr.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Shen L, Kang YK, Lichinitser M, et al. Health-related quality of life in patients with HER2-positive advanced gastric or gastroesophageal junction cancer with high HER2 expression levels – exploratory analysis of the phase III ToGA Study. Eur J Cancer. 2011;47:S231–S232. [Google Scholar]
- 19.Osoba D, Slamon DJ, Burchmore M, et al. Effects on quality of life of combined trastuzumab and chemotherapy in women with metastatic breast cancer. J Clin Oncol. 2002;20:3106–3113. doi: 10.1200/JCO.2002.03.090. [DOI] [PubMed] [Google Scholar]
- 20.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25:3210–3216. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 21.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 22.Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996–2004. doi: 10.1200/JCO.2002.08.105. [DOI] [PubMed] [Google Scholar]
- 23.Curran D, Pozzo C, Zaluski J, et al. Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: Results of a randomised phase III trial. Qual Life Res. 2009;18:853–861. doi: 10.1007/s11136-009-9493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer. 2010;116:2511–2518. doi: 10.1002/cncr.25064. [DOI] [PubMed] [Google Scholar]
- 25.Funaioli C, Longobardi C, Martoni AA. The impact of chemotherapy on overall survival and quality of life of patients with metastatic colorectal cancer: A review of phase III trials. J Chemother. 2008;20:14–27. doi: 10.1179/joc.2008.20.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Sherrill B, Di Leo A, Amonkar MM, et al. Quality-of-life and quality-adjusted survival (Q-TWiST) in patients receiving lapatinib in combination with paclitaxel as first-line treatment for metastatic breast cancer. Curr Med Res Opin. 2010;26:767–775. doi: 10.1185/03007991003590860. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsu A, Wang L, Lim H, et al. Quality of life results from a phase III study of trastuzumab plus chemotherapy as first-line therapy in patients with HER2-positive advanced gastric and gastro-esophageal junction cancer. Ann Oncol. 2010;21(suppl 6):O-0011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.