Abstract
Background
Descriptions of the sequelae of ABO incompatible (ABOi) kidney transplantation are limited to single-center reports which may lack power to detect important effects.
Methods
We examined United States Renal Data System registry data to study associations of ABOi live donor kidney transplantation with clinical complications in a national cohort. Among 14,041 Medicare-insured transplants in 2000–2007, 119 non-donor-A2 ABOi transplants were identified. A2 incompatible (A2i, n=35) transplants were categorized separately. Infection and hemorrhage events were identified by diagnosis codes on billing claims. Associations of ABOi with complications were assessed by multivariate Cox regression.
Results
Recipients of ABOi transplants had significantly (P<0.05) higher incidence of wound infections (12.7% vs 7.3%), pneumonia (7.6% vs 3.8%), and urinary tract infections (UTIs)/pyelonephritis (24.5% vs 15.3%) in the first 90 days compared with ABO compatible (ABOc) recipients. In adjusted models, ABOi was associated with twice the risk of pneumonia (aHR 2.10, 95%CI 1.08-4.09) and 55% higher risk of UTIs/pyelonephritis (aHR 1.55, 95% CI 1.05-2.29) in the first 90 post-transplant days, and 3.5-times the relative risk of wound infections in days 91-365 (aHR 3.55, 95% CI 1.92–6.57). The adjusted risk of hemorrhage was higher in ABOi recipients (aHR 1.85, 95%CI 1.12-3.05), 19% of whom underwent splenectomy before transplantation. A2i transplantation was associated only with early risk of UTIs/pyelonephritis.
Conclusions
ABOi transplantation offers patients with potential live donors an additional transplant option, but with higher risks of infectious and hemorrhagic complications.Awareness of these complications may help improve protocols for the management of ABOi transplantation.
Keywords: Blood group incompatibility, Hemorrhage, Infection, Kidney transplantation, Living donors, Medicare
INTRODUCTION
Blood group incompatibility (ABOi) remains a significant barrier to further expansion of live donor kidney transplantation. Estimates based upon blood group prevalence in the U.S. suggest that more than 35% of willing, healthy potential live donors are blood group incompatible with their intended recipients (1). While kidney paired donation (KPD) has emerged as a successful approach to address antibody incompatibilities for those who have a willing, but incompatible live donor, blood group O candidates continue to have much lower rates of success on KPD lists than their non-O counterparts, particularly in circumstances of broad HLA sensitization (2). To address this disparity, some U.S. transplant programs have successfully performed ABOi live donor kidney transplants (3, 4); and protocols based primarily on plasmapheresis without need for splenectomy seem successful (5). Following an early reduction in graft survival relative to blood type compatible (ABOc) live donor kidney transplant recipients (3), the average long-term graft survival in ABOi live donor transplant recipients is not inferior to, and often exceeds, that of ABOc deceased donor transplant recipients (3, 6).
Although post-transplant mortality and graft survival rates in ABOi recipients have been reported in national analyses, the impact of preconditioning treatments for ABOi transplantation on infectious and hemorrhagic complications, which may increase the cost and morbidity of this procedure, have not been well described. The preemptive treatment regimen for ABOi transplantation involves an escalation in pre- and post-transplant immunosuppression, resulting in suppressed cell-medicated immunity. Furthermore, many protocols use anti-CD20 antibody therapy as part of the induction strategy, resulting in suppression of humoral immunity and, potentially, increased risk of post-transplant infection. Apheresis, a common component of preemptive treatment regimens, induces a transient coagulopathy resulting from the apheresis-associated declines in plasma coagulation factors. While no longer commonly used as a routine component of the preconditioning regimen, splenectomy remains recommended in cases of uncontrolled acute humoral rejection among antibody incompatible recipients (7). These factors have the potential to increase the risk of early peri-operative, and potentially long-term post-operative, complications in recipients of ABOi transplants. However, these morbidity outcomes are not captured in current national registry data collected by the organ Procurement and Transplantation Network (OPTN).
To advance understanding of early clinical complications following ABOi transplantation, we identified a representative cohort of live donor kidney transplant recipients captured in the United States Renal Data System (USRDS) which links the OPTN registry and Medicare claims data. The objective of this study was to investigate infectious and hemorrhagic complications in the first year post-transplant among a national sample of U.S. Medicare-insured live donor transplant recipients by supplementing clinical registry data with diagnostic information from administrative billing claims. Using these integrated data, we sought to compare the frequencies of complications among ABOi recipients versus patients who received ABOc grafts without preconditioning therapy.
RESULTS
Demographic and Clinical Characteristics
Among 366 non-donor-A2 ABOi transplants performed nationally from 2000–2007, 32.5% (119 patients) had Medicare primary insurance and were included in this analysis (Table 1). During the study period, 35 Medicare-insured transplants were performed with A2 living donors (30 A2-to-O, 5 A2-to-B), 31.5% of who had Medicare primary insurance, and 13,887 Medicare-insured ABOc live donor kidney transplants were performed. By comparison, 26% of all live donor kidney transplant recipients in the study period had Medicare coverage. Among the study sample, ABOi recipients had higher frequencies of HLA sensitization including 13.4% with panel reactive antibodies ≥80% compared with only 5.1% of ABOc recipients. ABOi live donor transplantation was more common in recent years. A2i transplants involved more female recipients and more common use of induction immunosuppression, but otherwise had similar baseline characteristics as blood type compatible transplants. When induction was used, the regimen was dominantly rabbit antithymocyte globulin (thymoglobulin) among ABOi and A2i recipients (78% and 67% of cases treated with induction).
Table 1.
Baseline demographic and clinical characteristics of the study sample of Medicare-insured live donor kidney transplant recipients according to ABO compatibility.
| ABOi N =119 | A2i N =35 | ABOc N = 13,887 | |
|---|---|---|---|
| Characteristics | % or mean +/- SD | % or mean +/- SD | % or mean +/- SD |
| Recipient Variables | |||
| Recipient age , mean (SD), yr | 48.2 (15.8) | 40.1 (16.6) | 45.7 (16.3) |
| Female, % | 37.8 | 57.1 | 40.6 |
| Race,% | |||
| White | 73.1 | 68.6 | 70.4 |
| African-American | 18.5 | 22.9 | 19.4 |
| Other | 8.4 | 8.6 | 10.2 |
| Body mass index, mean (SD), kg/m2 | 26.1 (5.5) | 26.1 (6.4) | 26.4 (5.6) |
| Missing | 4.2 | 2.9 | 6.9 |
| Cause of ESRD, % | |||
| Diabetes | 25.2 | 14.3 | 22.3 |
| Glomerulonephritis | 15.1 | 34.3 | 19.5 |
| Hypertension | 12.6 | 17.1 | 18.4 |
| Polycystic kidney disease | 7.6 | 0 | 5.1 |
| Other | 39.5 | 34.3 | 34.7 |
| Diabetes, % | 27.7 | 20.0 | 26.2 |
| Previous transplant | 34.5‡ | 20.0 | 15.7 |
| Pretransplant dialysis | |||
| None (Preemptive), % | 7.6 | 0 | 4.6 |
| Years pre-transplant of dialysis, mean (SD) | 4.4 (5.0) | 4.1 (4.6) | 3.4 (4.2) |
| Peak panel reactive antibody level, % | ‡ | ||
| < 10 | 53.8 | 62.9 | 74.8 |
| 10-79 | 21.6 | 22.8 | 16.4 |
| >=80 | 13.4 | 8.6 | 5.1 |
| Missing | 10.9 | 5.71 | 3.7 |
| HLA mismatches, % | |||
| Zero A, B, and DR | 11.8 | 5.7 | 8.8 |
| Zero DR | 21.0 | 8.6 | 18.2 |
| Serum albumin at listing, mean (SD), g/dl | 3.2 (1.6) | 2.6 (1.9) | 2.6 (1.9) |
| Missing | 17.6† | 34.3 | 31.9 |
| Donor Variables | |||
| Age, mean (SD), yr | 41.3 (12.1) | 36.8 (11.0) | 39.2 (11.1) |
| Weight, mean (SD), kg | 83.6 (25.1)* | 74.5 (16.1) | 77.4 (16.6) |
| Missing, % | 57.1 | 57.1 | 69.3 |
| Hypertension, % | 0.8 | 0 | 0.7 |
| Cytomegalovirus positive, % | 44.5 | 62.9 | 53.0 |
| Race, % | |||
| White | 76.5 | 80.0 | 71.3 |
| African-American | 14.3 | 14.3 | 17.5 |
| Other | 9.2 | 5.7 | 11.2 |
| Year of Transplant, % | ‡ | ||
| 2000-2002 | 10.9 | 40.0 | 35.4 |
| 2003-2005 | 45.4 | 25.7 | 42.2 |
| 2006-2007 | 43.7 | 34.3 | 22.4 |
| Induction | 57.1 | 85.7* | 60.9 |
| Maintenance immunosuppression at discharge, % | |||
| Steroids | 81.5 | 80.0 | 78.1 |
| Tacrolimus and MMF | 80.7 | 77.1 | 55.5 |
| Tacrolimus and AZA | 0.8 | 0 | 0.9 |
| CSA and MMF | 0.8 | 2.9 | 0.9 |
| CSA and AZA | 0 | 0 | 0.3 |
| Rapamycin-based | 5.0 | 2.9 | 11.9 |
| Other | 12.6 | 17.1 | 30.7 |
ABOi, ABO incompatible; A2i, A2 incompatible; ABOc, ABO compatible P-values vs ABOc
0.001 to <0.05
0.0001 to <0.001
p<0.0001
Frequencies of Post-transplant Complications according to Blood Type Compatibility
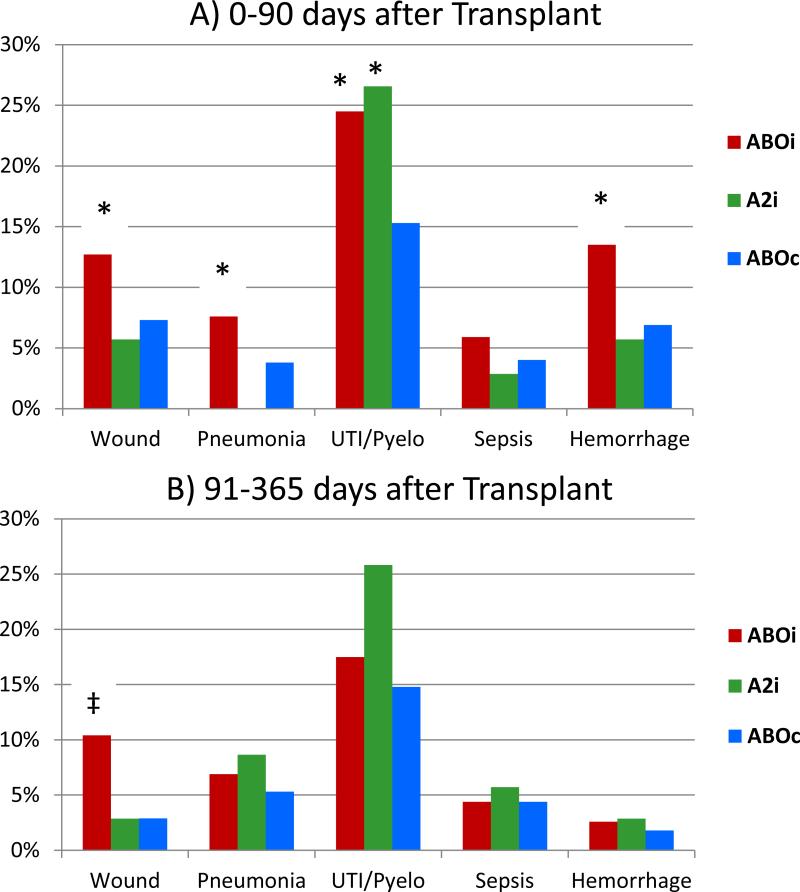
Kaplan-Meier estimates of the frequencies of infectious complications and hemorrhage during the periods 0-90 days and 91-365 days after transplantation are displayed in Figure 1. Recipients of ABOi transplants had significantly (P<0.05) higher incidences of wound infections (12.7% vs. 7.3%), pneumonia (7.6% vs. 3.8%),urinary tract infections (UTIs)/pyelonephritis (24.5% vs. 15.3%), and hemorrhage (13.5 vs. 6.9%) compared with ABOc recipients in the first 90 days. In the 91 to 365 day period, the unadjusted frequency of wound infections was significantly higher among ABOi versus compatible recipients (10.4% vs. 2.9%). The unadjusted frequency of sepsis did not differ by blood type compatibility in either study time window. Compared with ABOc transplant recipients, recipients of A2i transplants had significantly higher frequencies of UTI/pyelonephritis in the first 90 days (28.6% vs. 15.55). The distributions of subcategories of each type of infection and bleeding event according to individual ICD9 codes during days 0 to 90 (SDC1, Table) and 91 to 365 (SDC2, Table) are provided in the supplement.
Figure 1.
Kaplan-Meier estimates of infectious complications and hemorrhage frequencies over periods of 0 to 90 days and 91 to 365 days, according to blood type compatibility.
ABOi, ABO incompatible; A2i, A2 incompatible; ABOc, ABO compatible.
P-values vs ABOc: * 0.0001 to <0.05; ‡<0.0001
Adjusted Associations of Blood Type Compatibility with Post-transplant Complications
In multivariable regression including adjustment for baseline recipient, donor and transplant factors listed in Table 1, ABOi status was associated with more than twice the risk of pneumonia (aHR 2.10, 95% CI 1.08–4.09) and 55% higher risk of UTIs/pyelonephritis (aHR 1.55, 95% CI 1.05–2.29) in the first 90 post-transplant days compared with blood type compatible transplantation (Table 2). Compared with ABOc recipients ABOi recipients also had 85% higher relative risk of early hemorrhage (aHR 1.85, 95% CI 1.12–3.05). A2i transplantation was associated with twice the adjusted risk of UTI/pyelonephritis (aHR 2.12, 95% CI 1.14–3.95) as ABOc status in the early post-transplant period.
Table 2.
Adjusted associations of ABO compatibility and other baseline recipient, donor and transplant factors with the risk of infections and hemorrhage 0 to 90 days after live donor kidney transplantation.
| Baseline Factor | Wound Infection | Pneumonia | UTI/Pyelonephritis | Sepsis | Hemorrhage |
|---|---|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| ABOi | 1.64 (0.96-2.79) | 2.22 (1.14-4.33)* | 1.56 (1.05-2.30)* | 1.49 (0.70-3.16) | 1.96 (1.19-3.24)* |
| A2i | 0.86 (0.21-3.44) | N/A | 2.14 (1.15-3.99)* | 0.76 (0.11-5.42) | 0.91 (0.23-3.65) |
| ABOc | Reference | Reference | Reference | Reference | Reference |
| Candidate/Recipient Variables | |||||
| Recipient age (years/10) | 1.11 (1.05-1.17)† | 1.24 (1.15-1.33)‡ | 1.04 (1.00-1.07)* | 1.10 (1.02-1.17)* | 1.05 (1.00-1.11)* |
| Female | 1.38 (1.21-1.58)‡ | 1.02 (0.84-1.22) | 1.79 (1.63-1.96))‡ | 1.04 (0.87-1.25) | 1.01 (0.88-1.16) |
| Previous transplant | 0.94 (0.76-1.16) | 1.10 (0.83-1.47) | 0.86 (0.74-1.00)* | 0.90 (0.69-1.19) | 0.96 (0.78-1.19) |
| Pretransplant dialysis | |||||
| Preemptive transplant | 1.32 (0.93-1.88) | 1.19 (0.75-1.89) | 1.47 (1.17-1.85)† | 1.62 (1.04-2.54)* | 1.57 (1.12-2.21)* |
| Natural log of (yr of dialysis+1) | 1.24 (1.12-1.38)‡ | 1.13 (0.98-1.31) | 1.17 (1.09-1.26)‡ | 1.35 (1.18-1.56)‡ | 1.24 (1.12-1.38)* |
| Body mass index (kg/m2) | 1.07 (1.06-1.08)‡ | 1.01 (0.99-1.03) | 1.01 (1.00-1.02) | 1.01 (0.99-1.03) | 1.00 (0.99-1.01) |
| >20 | 0.71 (0.54-0.94)* | 0.70 (0.50-0.98)* | 0.95 (0.80-1.13) | 0.70 (0.50-0.96)* | 0.88 (0.69-1.12) |
| Cause of ESRD | |||||
| Diabetes/ Glomerulonephritis | Reference | Reference | Reference | Reference | Reference |
| Hypertension | 0.91 (0.75-1.12) | 1.01 (0.76-1.33) | 1.06 (0.92-1.22) | 1.19 (0.91-1.56) | 0.97 (0.80-1.19) |
| Polycystic kidney disease | 1.00 (0.73-1.38) | 0.98 (0.63-1.51) | 1.20 (0.98-1.48) | 1.36 (0.91-2.04) | 0.68 (0.47-0.99)* |
| Other | 1.06 (0.90-1.26) | 1.10 (0.87-1.40) | 1.33 (1.18-1.49)‡ | 1.35 (1.07-1.70)* | 1.08 (0.91-1.28) |
| Diabetes | 1.80 (1.06-3.06)* | 2.16 (0.99-4.70) | 1.07 (0.71-1.60) | 1.00 (0.46-2.17) | 0.85 (0.45-1.59) |
| Diabetes × Age (years/10) | 0.96 (0.87-1.06) | 0.91 (0.79-1.05) | 1.01 (0.94-1.09) | 1.07 (0.93-1.23) | 1.01 (0.90-1.14) |
| Peak panel reactive antibody level | |||||
| < 10 | Reference | Reference | Reference | Reference | Reference |
| 10-79 | 1.10 (0.93-1.30) | 1.24 (0.98-1.58) | 0.94 (0.83-1.06) | 1.15 (0.91-1.45) | 1.12 (0.94-1.34) |
| >=80 | 1.18 (0.89-1.55) | 1.79 (1.25-2.55)* | 0.96 (0.79-1.17) | 1.09 (0.74-1.60) | 1.59 (1.23-2.05)† |
| Missing | 1.59 (1.19-2.11)* | 1.24 (0.79-1.96) | 1.01 (0.81-1.26) | 1.59 (1.08-2.34)* | 1.14 (0.82-1.60) |
| HLA mismatches | |||||
| Zero A, B, and DR | 1.31 (1.05-1.62)* | 0.79 (0.55-1.12) | 0.86 (0.73-1.02) | 0.96 (0.70-1.31) | 0.79 (0.61-1.02) |
| Zero DR | 1.08 (0.92-1.28) | 1.01 (0.80-1.26) | 0.97 (0.86-1.08) | 0.89 (0.70-1.12) | 1.03 (0.88-1.22) |
| Serum albumin at listing (g/dl) | 0.82 (0.69-0.99)* | 0.87 (0.68-1.12) | 0.92 (0.82-1.05) | 0.81 (0.63-1.05) | 0.97 (0.81-1.15) |
| >3.5 | 1.03 (0.82-1.31) | 0.97 (0.69-1.35) | 1.01 (0.86-1.20) | 0.83 (0.6-1.16) | 0.90 (0.71-1.15) |
| Missing | 0.42 (0.23-0.76)* | 0.56 (0.25-1.27) | 0.76 (0.50-1.15) | 0.40 (0.17-0.90)* | 0.79 (0.44-1.40) |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| African-American | 1.22 (0.86-1.72) | 1.21 (0.74-2.00) | 0.95 (0.74-1.23) | 1.15 (0.71-1.85) | 1.30 (0.92-1.84) |
| Other | 0.90 (0.64-1.25) | 0.93 (0.59-1.45) | 0.97 (0.79-1.20) | 0.82 (0.52-1.28) | 0.82 (0.60-1.13) |
| Year of transplant | 1.01 (0.97-1.06) | 0.98 (0.93-1.03) | 1.04 (1.01-1.07)* | 1.00 (0.95-1.06) | 1.02 (0.98-1.06) |
| Donor Variables | |||||
| Age (in yrs) | 1.00 (0.99-1.00) | 1.01 (1.00-1.01) | 1.01 (1.00-1.01)* | 1.01 (1.00-1.01) | 1.00 (1.00-1.01) |
| >18 | 0.46 (0.25-0.84)* | 0.72 (0.23-2.29) | 0.82 (0.47-1.42) | 0.47 (0.19-1.15) | 0.60 (0.29-1.22) |
| Hypertension | 0.92 (0.41-2.07) | 1.02 (0.33-3.20) | 1.05 (0.64-1.72) | 1.43 (0.59-3.49) | 1.13 (0.53-2.38) |
| Cytomegalovirus positive | 1.02 (0.90-1.16) | 1.17 (0.97-1.40) | 1.04 (0.95-1.14) | 1.14 (0.96-1.36) | 1.02 (0.89-1.16) |
| Natural log of weight (kg) | 0.93 (0.59-1.46) | 0.77 (0.40-1.48) | 0.84 (0.62-1.14) | 0.95 (0.49-1.84) | 0.96 (0.60-1.53) |
| Missing | 0.74 (0.10-5.37) | 0.44 (0.03-7.50) | 0.44 (0.12-1.63) | 0.83 (0.05-14.59) | 0.79 (0.10-5.92) |
| Race | |||||
| White | Reference | Reference | Reference | Reference | Reference |
| African-American | 0.95 (0.67-1.36) | 0.80 (0.47-1.35) | 0.99 (0.76-1.28) | 0.93 (0.57-1.54) | 1.13 (0.79-1.62) |
| Other | 0.84 (0.62-1.16) | 1.12 (0.74-1.71) | 0.94 (0.76-1.15) | 0.96 (0.63-1.45) | 1.22 (0.91-1.63) |
| Induction | 0.97 (0.85-1.11) | 0.94 (0.79-1.13) | 1.05( 0.95-1.15) | 1.02 (0.85-1.22) | 1.02 (0.89-1.17) |
| Maintenance at discharge | |||||
| Steroids | 1.03 (0.88-1.20) | 1.11 (0.88-1.40) | 1.07 (0.96-1.20) | 1.16 (0.93-1.46) | 0.91 (0.77-1.06) |
| Tacrolimus and MMF | Reference | Reference | Reference | Reference | Reference |
| Tacrolimus and AZA | 0.87 (0.39-1.95) | 0.50 (0.12-2.02) | 2.17 (1.55-3.06)‡ | 2.64 (1.39-5.02)* | 0.49 (0.16-1.52) |
| CSA and MMF | 0.98 (0.46-2.10) | 2.40 (1.23-4.69)* | 1.24 (0.76-2.02) | 3.50 (1.91-6.39)‡ | 0.69 (0.26-1.88) |
| CSA and AZA | 1.06 (0.26-4.34) | 1.62 (0.48-5.42) | 0.31 (0.08-1.28) | 0.32 (0.04-2.47) | 0.59 (0.08-4.27) |
| Sirolimus-based | 1.68 (1.40-2.01)‡ | 1.84 (1.43-2.36)‡ | 1.09 (0.94-1.26) | 1.91 (1.49-2.44)‡ | 1.67 (1.39-2.02)‡ |
| Other | 1.13 (0.97-1.31) | 1.38 (1.13-1.70)* | 1.08 (0.97-1.20) | 1.34 (1.10-1.65)* | 1.34 (1.15-1.56)† |
P-values
0.001 to <0.05
0.0001 to <0.001
p<0.0001
Other significant correlates of early post-transplant complications 0 to 90 days post-transplant included older recipient age, which was associated with increased risk of all the infectious outcomes. Women experienced higher adjusted risks of wound infections and UTIs/pyelonephritis compared with men. Each 1 unit increase in BMI was associated with 7% increase in the relative risk of wound infections (aHR 1.07, 95% CI 1.06–1.08), although there was a concomitant risk reduction among patients with BMI >20 kg/m2 compared with underweight patients. Diabetic patients had an 81% increase in the relative risk of wound infections (aHR 1.81, 95% CI 1.06–3.08) and more than twice the relative risk of early pneumonia (aHR 2.22, 95% CI 1.02–4.84), while sensitized recipients with PRA ≥80% had 60% higher risk of early hemorrhage (aHR 1.60, 95% CI 1.24–2.06) and 78% higher risk of pneumonia (aHR 1.78, 95% CI 1.25–2.53) as those with PRA <10%. Compared with use of tacrolimus and mycophenolate mofetil (MMF)-based maintenance immunosuppression at transplant discharge, sirolimus-based immunosuppression was associated with approximately 70% to 90% increases in the relative risks of early hemorrhage (aHR 1.67, 95% CI 1.39–2.02), wound infection (aHR 1.68, 95% CI 1.40–2.01), pneumonia (aHR 1.84, 95% CI 1.43–2.36) and sepsis (aHR 1.91, 95% CI 1.49–2.44).
Compared with compatible living donor kidney transplant recipients, ABOi transplant recipients experienced 3.5-times the adjusted relative risk of wound infections in the period 91 to 365 days post-transplant (aHR 3.55, 95% CI 1.92–6.57). Other correlates of wound infections during this period included older recipient age, female sex, white race, and higher BMI but again with a risk reduction for BMI >20 kg/m2 versus underweight status (SCD3, Table). Diabetic patients had more than 3-times the relative risk of wound infections in this period as non-diabetic patients (aHR 3.45, 95% CI 1.55–7.68), and patients who received sirolimus-based immunosuppression at discharge had more than twice the risk as those discharged with tacrolimus and MMF (aHR 2.35, 95% CI 1.78–3.09). The risks of the other study complications in this period did not vary significantly by ABO compatibility.
Outcomes of ABOi Transplantation according to Splenectomy Status
Nineteen percent (n=24) of ABOi transplants were performed with splenectomy, with recorded timing of splenectomy ranging from 6 months before transplant to 5 days post-transplant (within the initial transplant hospitalization). An additional splenectomy event at 33 days post-transplant was considered as a baseline factor in the models of complications beyond 3 months post-transplant. Splenectomy was a more common component of the conditioning regimen for ABOi transplantation in early years of the study (32.6% of ABOi transplants in 2000–2004 versus 13.2% in 2005–2007), but splenectomy was performed throughout the study period. Stratifying ABOi transplantation according to the presence of splenectomy demonstrated that the unadjusted frequency of hemorrhage was more common among ABOi recipients managed with versus without splenectomy in both the 0 to 90 (25.9% vs 9.9%, P=0.02) and 91 to 365 day periods (11.1% vs 0%, P=0.001). The frequency of hemorrhage among ABOi recipients treated with splenectomy also substantially exceeded that of ABOc patients in the 0 to 90 day (25.9% vs 6.9%, P<0.0001) and 91 to 365 day periods (11.1% vs 1.8%, P=0.0002). While the point estimate of hemorrhage within 0 to 90 days was higher among ABOi recipients managed without splenectomy compared to ABOc recipients, this difference was not statistically significant (9.9% vs 6.9%, P=0.27). The unadjusted frequencies of other complications among ABOi recipients did not differ significantly according to splenectomy status (Table 3). Importantly, however, there were no statistically significant interactions of ABOi transplantation and splenectomy after multivariable adjustment upon the risk of any study outcome including hemorrhage. Thus, while ABOi overall was associated with higher risks of some infections and hemorrhage compared with ABOc transplantation in adjusted models as described above, we did not detect statistically significant heterogeneity in the complications of ABOi transplantation according to splenectomy status after covariate adjustment.
Table 3.
Frequencies of infectious complications and hemorrhage over periods of 0 to 90 days and 91 to 365 days among ABOi live donor kidney transplant recipients according to splenectomy status
| Evaluation Period: 0 to 90 days post-transplant | |||||
|---|---|---|---|---|---|
| Wound Infection | Pneumonia | UTI/Pyelonephritis | Sepsis | Hemorrhage | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| ABOi with Splenectomy (n=24) | 14.8 (8.8-20.8) | 3.7 (0-8.4) | 33.3 (25.6-41.1) | 7.4 (3.1-11.7) | 25.9 (19.8-32.1)* |
| ABOi without Splenectomy (n=95) | 12.0 (4.3-19.7) | 8.7 (8.7-8.7) | 21.9 (7.0-36.9) | 5.5 (0-11.1) | 9.9 (2.2-17.5) |
| Evaluation Period: 91 to 365 days post-transplant | |||||
|---|---|---|---|---|---|
| Wound Infection | Pneumonia | UTI | Sepsis | Hemorrhage | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| ABOi with Splenectomy (n=24) | 11.1 (5.1-17.1) | 3.7 (-1.0-8.4) | 15.3 (7.5-23.0) | 4.7 (0.4-8.9) | 11.1 (5.0-17.3)* |
| ABOi without Splenectomy (n=95) | 10.1 (2.5-17.8) | 8.0 (8.0-8.0) | 18.1 (3.1-33.1) | 2.9 (0-8.4) | 0 |
P-value <0.05 comparing ABOi recipients managed with versus without splenectomy
Literature Review
Frequencies of bleeding and infectious complications reported in previously published single center experiences with ABOi transplantation (8-16) are summarized in Table 4 to provide context for the complication rates observed in our study. Although there were trends towards higher frequencies of these complications in many (but not all) of the reported studies, sample sizes were 40 ABOi recipients or fewer in each study, and statistical significance was not reached in any reported comparison.
Table 4.
Summary of published reports of frequencies of infectious and hemorrhagic complications after ABOi kidney transplantation
| Reference | Sample | Splenectomy or Rituximab | Pretransplant Oral ISx | Apheresis Method | Induction + Maintenance ISx | Complications |
|---|---|---|---|---|---|---|
| Schaefer et al., 2013 (14) | 3 ABOi pediatric recipients, Heidelberg, Germany, 2009-2012 | Rituximab (375mg/m2), 1-44 d pretransplant | Steroids + TAC + MMF, started at first IA | IA (1.5 PV) to achieve isoagglutinin titers ≤ 1:8 pretransplant | Basiliximab + IVIG (0.5g/kg) + Steroids + TAC + MMF |
Hemorrhage • 67% (2/3), both requiring transfusions and 1 requiring surgery |
| Morath et al., 2012 (12) | 17 ABOi adult recipients, Heidelberg, Germany , 2005-2010 | Rituximab (375 mg/m2), median 31d pretransplant | Steroids + TAC + MPA, started at first IA | • Antigen-specific (n=5) or non-antigen specific (n=12) IA • Plasmapheresis subsequently used in 6 cases with isoagglutinin titer ≥ 1:16 despite IA |
Basiliximab in 12 recipients, otherwise not noted IVIG (0.5g/kg) in 11 recipients |
Bacterial infections • UTI: 24% (4/17) • Pneumonia: 6% (1/17) • Bacteremia: 6% (1/17) Hemorrhage • Antigen-specific IA group: 20% (1/5) • Non-antigen specific IA group: 17% (2/12) |
| Hwang et al., 2011 (11) | 12 ABOi and 50 ABOc adult recipients, Seoul, Korea, 2009-2010 | • ABOi: Rituximab (100-375 mg/m2), 1 mo pretransplant • ABOc with +CDC crossmatch (n=2): Rituximab (375 mg/m2), 1 week pretransplant |
ABOi: Steroids + TAC + MMF/MPA, started 7 d pretransplant | • PE (1 PV with 5% albumin) every other day to achieve isoagglutinin titer ≤ 1:32 • Final pretransplant PE and all posttransplant PE performed with FFP replacement fluid |
Basiliximab + Steroids + TAC + MMF/MPA ABOi and ABOc with +CXM: IVIG 100mg/kg |
Bacterial infections ABOi: 0% (0/12), *P=0.19 ABOc: 18% (9/50) Hemorrhage • ABOi: 25% (3/12), *P=0.08 • ABOc: 6% (3/50) |
| Habicht et al., 2011 (10) | 21 ABOi and 47 ABOc adult recipients, Hannover, Germany , 2007-2009 | Rituximab (375 mg/m2), 1 mo pretransplant | Steroids + TAC + MMF, started 1 mo pretransplant | IA daily to achieve isoagglutinin titer ≤ 1:8 | Basiliximab + Steroids + TAC + MMF IVIG (30g) -1 to -2d pretransplant |
Pneumonia • ABOi: 5% (1/21), * P NR • ABOc:4% (2/47) Urosepsis • ABOi: 10% (2/21), *P NR • ABOc: 6% (3/47) Hemorrhage • ABOi: 10% (2/21), *P NR • ABOc: 2% (1/47) • Cases required intervention, aspiration or transfusion |
| Flint et al., 2011 (8) | 37 ABOi and 52 ABOc adult recipients, Melbourne, Australia, 2005-2008 | none | MMF (500-1000 mg bid), 10-14 d pretransplant | PE to achieve isoagglutinin titer <1:32, with 5% albumin or FFP (for final pretransplant and posttransplant PE) | Basiliximab + Steroids + TAC + MMF IVIG (0.5g/kg) immediately pretransplant prior to 2008 |
UTI within first 6 wks • ABOi: 19% (7/37), *P NS • ABOc: 15% (8/52) Septicemia within first 6 wks • ABOi: 14% (5/37), *P NS • ABOc: 2% (1/52) |
| Wilpert et al., 2010 (16) | 40 ABOi and 43 ABOc adult recipients, Freiburg, Germany, 2004-2009 | Rituximab (375 mg/m2), 1 mo pretransplant | Prednisone (30mg/d) + TAC+ MMF (2g/day), started 7d pretransplant | Antigen-specific IA (2.5-3 PV) every other day to achieve isoagglutinin titer ≤ 1:4 | Basiliximab + Steroids + TAC + MPA IVIG (0.5g/kg) -1 to -5d pretransplant |
Hospitalized Infections • ABOi: 23% (9/40), *P=0.62 • ABOc: 30% (13/43) Recurrent UTI • ABOi: 8% (3/40), *P=1.00 • ABOc: 9% (4/43) Sepsis • ABOi: 3% (1/40), *P=1.00 • ABOc: 2% (1/43) Hemorrhage requiring surgery • ABOi: 25% (10/40), *P=0.16 • ABOc: 12% (5/43) |
| Renner et al., 2010 (13) | 14 ABOi adult recipients, Giessen, Germany, 2007-2010 | Rituximab (375 mg/m2), 1 mo pretransplant | Steroids + TAC + MMF, started 1 mo pretransplant | IA to achieve isoagglutinin titer ≤ 1:4 | Basiliximab (1 case with high PRA received TMG)+ Steroids + TAC + MMF/MPA |
Hemorrhage • 29% (4/14), including 3 cases requiring surgery |
| Genberg et al, 2008 (9) | 15 ABOi and 30 ABOc adult recipients, Stockholm, Sweden, 2001-2005 | Rituximab (375 mg/m2), 1 mo pretransplant | Steroids + TAC + MMF, started 10d pretransplant | IA to achieve titer < 1:8 | Steroids + TAC + MMF IVIG 0.5g/kg, 1d pretransplant |
Wound infections • sABOi 13% (2/15), *P NS ABOc 10% (3/30) UTI • ABOi: 13% (2/15), *P NS • ABOc 37% (11/30) Sepsis • ABOi 7% (1/15), *P NS • ABOc 20% (6/30) |
| Schwartz et al., 2006 (15) | 40 ABOi and 77 ABOc adult transplant recipients, Rochester, MN, 1999-2003 | • Splenectomy until 2003 • Rituximab (375 mg/m2), 1 wk pretransplant starting in 2003 |
None | • PE (1 PV) every 1-2 d pretransplant to achieve isoagglutinin titer < 1:8 • Replacement fluid was FFP in first 4, and 5% albumin in next 36 who also received IVIG (10g) after each PE |
TMG (1.5mg/kg/d for 10 d) + Steroids + TAC + MMF |
Infectious complications • ABOi: 25% (10/40), *P=0.33 • ABOc: 17% (13/77) Hemorrhage (p=0.228) • ABOi: 10% (4/40), *P=0.23 • ABOc: 4% (3/77) |
CDC, complement-dependent cytotoxicity; d, day; FFP, fresh frozen plasma; IA, immunoadsorption; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; mo, month; MPA, mycophenolic acid; NR, not recorded; NS, not significant; PE, plasma exchange; PV, plasma volume; TAC, tacrolimus; TMG, thymoglobulin; UTI, urinary tract infection; wk, week
P value for comparison of event frequencies among ABOi vs ABOc recipients
DISCUSSION
To date, descriptions of post-operative complications in ABOi transplant recipients aside from graft failure or death have been limited to single-center reports as these complications are not captured in clinical registry data. However, results obtained in highly skilled centers of excellence may not accurately reflect national experience. Furthermore, given the limited use of ABOi transplantation, no single center study to date has demonstrated sufficient statistical power to identify potentially important differences in the frequencies of post-transplant infectious and bleeding complications (8-16). We examined United States transplant registry and billing claims data to study associations of ABOi live donor kidney transplantation with clinical complications in a national cohort of Medicare-insured recipients to address these concerns. When compared with ABOc patients, the ABOi transplant recipients in this cohort were significantly more likely to suffer from wound infections, pneumonia, urinary tract infections (UTIs)/pyelonephritis and hemorrhage in the first 90 days after transplant, as well as more common wound infections in days 91 to 365 post-transplant. These patterns were significant after multivariate adjustment for other baseline recipient, donor and transplant factors collected in the transplant registry. A2i transplantation was associated only with increased risk of early UTIs/pyelonephritis.
Specifically, ABOi recipients in the current analysis had approximately 50% to 100% increases in the risks of early wound infections (aHR 1.58), pneumonia (aHR 2.10) and urinary tract infections (aHR 1.55) compared with blood type compatible recipients. The higher rates of infections are consistent with trends from previous single center reports, although statistical significance was not reached in any prior report due to insufficient sample sizes (8-11, 15, 16). Increased risk of post-transplant infections may reflect the use of rituximab and/or splenectomy, duration and type of pre-transplant immunosuppression, administration of cell depleting antibody therapy and more intensive post-transplant maintenance immunosuppression regimens. Although the nature of our data precludes estimation of risks attributable to specific components of the conditioning regimen, the core treatment modality, apheresis, has been a component of all successful ABOi programs and likely contributes significantly to the risk of complications. There were no significant differences in infection risk according to the presence versus absence of splenectomy among ABOi recipients in our current study, suggesting that the shift away from splenectomy may not alleviate infectious risk.
Preconditioning for ABOi transplantation may contribute to the higher rates of infection and wound complications observed in the current study. Apheresis may increase infection risk both as a result of the use of transcutaneous vascular access lines and by inducing hypogammaglobulinemia and hypocomplementemia. A single plasma volume exchange can reduce serum immunoglobulin levels by 60% (17), while a single double-filtration plasmapheresis session depletes serum IgG by 40% (18). Multiple plasma exchange sessions result in hypogammaglobulinemia that may persist for several weeks. Apheresis also results in hypocomplementemia (19). Transient hypogammaglobulinemia and hypocomplementemia may help to explain our finding of increased rates of urinary tract infections and pneumonia in the first three months after transplant, but not beyond.
The finding that ABOi transplantation increases the rate of peri-operative hemorrhage in this analysis is consistent with trends suggested in prior single center studies comparing bleeding in ABOi to ABOc transplants, although unlike our current national study, patterns in prior single center studies did not reach statistical significance (10, 11, 15, 16). Several factors specific to ABOi transplants may result in an increased risk of bleeding including splenectomy, coagulopathy induced by apheresis, and the administration of anticoagulation medications during apheresis or post-operatively. Our analysis suggests that ABOi recipients undergoing splenectomy had particularly increased risk of hemorrhage, with nearly four times the crude bleeding rate as observed among ABOc recipients and twice the bleeding rate among ABOi recipients without splenectomy. However, we did not detect statistical evidence of variation in the bleeding risk associated with ABOi transplantation according to splenectomy status after multivariate adjustment. High rates of hemorrhage continue to be suggested in single center ABOi cohorts despite the reduction in the use of routine splenectomy in recent years (10-14, 16).
The observed increased risk of bleeding among ABOi recipients may also reflect coagulopathy and thrombocytopenia resulting from necessary pre-transplant apheresis treatments. Each of the methods of apheresis used in ABOi transplantation (plasma exchange, double-filtration plasmapheresis, and immunoadsorption) requires either heparin or citrate anticoagulation to avoid clotting within the circuit (20). Plasma exchange, the only apheresis method presently available in the United States, depletes coagulation factors non-selectively and also removes complement and immunoglobulins (21). A single plasma exchange reduces coagulation factors including fibrinogen by approximately 60% (17). Depletion of coagulation factors also develops after double-filtration plasmapheresis and immunoadsorption (22, 23). Thrombocytopenia results from loss of platelets in the discarded plasma, thrombosis within the plasma filter, and dilutional effects of replacement fluid (17). Unfortunately, assessment and management of apheresis-induced coagulopathy is difficult in the context of ABOi transplantation, as relationships between absolute levels of coagulation factors and bleeding risk have not been well established.
Limitations of the current study include the necessary restriction of the analytic cohort to patients with Medicare claims. The use of this analytic platform allowed us to assess the outcomes of nearly one third of all patients undergoing ABOi live donor transplantation in the U.S. in a robust, multiyear analysis. While Medicare claims are surrogate measures for diagnoses and coding errors are possible, the use of claims data provides the sole option for long-term, nationally representative data collection given the inherent limitations of registry data. In addition, kidney transplant recipients who have Medicare as their primary insurer may differ systematically from those who use other reimbursement systems. However, an interaction between insurance coverage and ABOi transplantation in contributing to complications (such that ABOi transplantation affects privately-insured patients differently than Medicare-insured patients in terms of causing complications) is unlikely. Therefore, while the absolute incidence of complications may be affected by population characteristics on the basis of primary payer, the relative differences between blood type incompatible and compatible groups are likely robust estimates of the true impact of the conditioning regimen for ABOi transplantation. Furthermore, Medicare claims are particularly relevant to research among kidney transplant recipients because, unlike the eligibility requirements of age >65 or disability in the general population, renal allograft recipients are offered disease-specific Medicare entitlement and Medicare is the largest single insurer in this population. As a result, Medicare billing claims have been used to study a variety of complications after kidney transplantation (24-27). Study of a Medicare-insured sample appears particularly relevant for issues in incompatible transplantation as Medicare insurance is more common among ABOi live donor recipients than among recipients of live donor transplants overall.
The current study is also limited by the inclusion of patients with a variety of preconditioning regimens. Based upon the high risk of humoral rejection among preconditioned ABOi recipients that was initially observed by Alexandre and colleagues in the 1980's (28, 29), pre-transplant splenectomy remained in common use through the early 2000's. Following a series of reports in the early to mid-2000's suggesting that ABOi kidney transplantation could be safely and successfully performed with the use of anti-CD20 therapy in place of splenectomy (30-32), or in the absence of both therapies (5), many transplant centers eliminated the practice of preoperative splenectomy in their ABOi recipients. Our study spanned this period of change in the preconditioning regimen. To address this issue, we performed sub-analyses considering interactions according to splenectomy status. The unadjusted frequency of hemorrhage was more common among ABOi recipients managed with versus without splenectomy, although statistical interaction of ABOi transplantation and splenectomy was not significant after multivariate adjustment, perhaps as a result of sample size or the inherent risk of hemorrhage among all ABOi patients. Due to limitations of the OPTN data, we also were unable to characterize the use of treatments such as rituximab, plasmapheresis, and IVIG. While lack of this information limits analysis of regimen-specific estimates of the frequency of early complications after ABOi transplantation, the overall impact of the conditioning regimen on the observed complications reflects a national experience and supports the need for continued assessment of complication rates.
Despite its limitations, this study is strengthened by the use of a nationally representative sample which exceeds the size and scope of prior single center reports of ABOi transplantation, by relatively complete follow-up of Medicare beneficiaries, and by focus on understudied outcomes which may contribute to patient morbidity and costs of care. This study offers important insights into the actual practice of ABOi transplantation in the U.S. that complements information from small series and cohorts, independent of center-reporting and publication bias. It appears that performing ABOi transplant is a viable, yet clinically complicated endeavor designed to serve patients whose other option is long-term dialysis. The observed complications should be considered within the context of the morbidity and mortality associated with chronic dialysis. Comparison of ABOi to ABOc transplantation offers important insights into the clinical impact of incompatibility on center practice and complication rates. However, from a patient perspective, ABOi transplantation is used only when a compatible donor is not available. Thus, patients need to be counseled about the relative risk of morbidity and mortality associated with long-term dialysis therapy compared with ABOi transplant, as well as options to seek alternative pathways to an ABOc transplant such as KPD programs. In conclusion, this national study of Medicare-insured live donor transplant recipients demonstrates that ABOi transplantation offers patients with potential live donors an additional transplant option, but with higher risks of post-transplant infections and hemorrhage. Awareness of these complications may help improve protocols for the management of ABOi transplantation to reduce the incidence and severity of post-operative complications.
MATERIALS AND METHODS
Data Sources and Study Samples
Study data were drawn from records of the USRDS which integrate OPTN records with Medicare billing claims. The primary study sample comprised recipients of live donor kidney transplants in the U.S. from 2000 to 2007 with Medicare as the primary payer at time of transplantation (33). The similarities and differences of patients in the USRDS with and without Medicare as their primary payer have been described previously (26). This study was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996; all standards regarding the security and privacy of an individual's health information were maintained.
Definitions of Blood Type Compatibility and other Baseline Factors
Blood type compatibility was ascertained using donor and recipient ABO blood types as reported to the national registry. A-to-(O or B), B-to-(O or A), and AB to (O, A, or B) were considered ABOi. A2-to-(O or B) transplants were categorized as an additional comparison group, due to increasing data that A2 incompatible (A2i) transplantation may be safe without preconditioning (34, 35). Recipients of ABOc live donor transplants were considered as the reference group. Use of splenectomy was ascertained by submission of a procedure (International Classification of Diseases Code 9th Edition Clinical Modification (ICD9-CM) procedure or Common Procedure Terminology (CPT)) code any time before transplant through the end of transplant hospitalization. Information on other baseline recipient clinical and demographic traits, donor characteristics and transplant factors were drawn from the OPTN Transplant Candidate Registration and Transplant Recipient Registration forms incorporated in the USRDS, as summarized in Table 1.
Clinical Outcomes Definitions
Diagnoses of post-transplant complications were defined by identification of billing claims with corresponding ICD9-CM diagnosis codes for wound infections, pneumonia, UTIs/pyelonephritis, sepsis, and hemorrhage (SDC 4, Appendix). For pneumonia, sepsis and UTIs/pyelonephritis, we required one inpatient claim or two other claims on separate dates to define serious infections, as performed in previous studies of claims data to identify infections in the kidney transplant population (25).
Statistical Analyses
Data management and analysis were performed with SAS for Windows software, version 9.2 (SAS Institute Inc., Cary, NC). Continuous data were summarized as means and standard deviations, and categorical data were summarized as proportions. Distributions of baseline traits among ABOi and A2i recipients versus recipients of blood type compatible transplants were compared with Chi-square for proportions and t-tests for continuous variables.
Time-to-event analyses were considered in periods of 0 to 90 days and 91 to 365 days after transplantation, and were censored at death not concomitant with a study end-point, end of the risk period of interest (day 90 or day 365), end of Medicare benefits, or end of the study (December 31, 2007). We estimated the unadjusted frequencies of each clinical event by the Kaplan-Meier method, and applied the Log Rank test to compare frequencies among ABOi, A2i and ABOc recipients. Multivariable Cox's regression was used to estimate the relative risk of each clinical event associated with ABOi transplantation, including adjustment for recipient, donor and transplant factors as per our recent studies of post-transplant outcomes (36-39). As a secondary analysis, we tested the interaction between ABOi transplantation and use of splenectomy to assess for heterogeneity of observed associations related to splenectomy.
Literature Review
To frame our results in the context of bleeding and infectious rates previously published single center experiences with ABOi transplantation, we queried the MEDLINE electronic database for reports published through July 1, 2013 using the medical subject headings (MeSH) terms “blood group incompatibility”, “kidney transplantation”, “infection”, “pneumonia”, “surgical wound infection”, “sepsis”, “urinary tract infections”, and “hemorrhage.” Searches were limited to articles involving human subjects and published in English language. Manual review of the reference list of identified articles was also performed.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government. An abstract describing a portion of this work was presented at the 2013 American Transplant Congress in Seattle, WA, June 2013.
The roles of the authors in this work are as follows:
K.L.L., D.A. and M.A.S. participated in study design, data acquisition, data analysis, and writing of the paper. H.X. participated in study design, data analysis and writing of the paper. C.K., C.S., J.T.N., V.R.D., D.C.B., and D.L.S. participated in study design, interpretation, and writing of the paper. All authors agreed to publish the paper.
Forms of Support: Dr. Segev received support from a grant from the National Institutes of Health (NIH), R01-DK098431.
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose.
Institution at which work was performed: Saint Louis University, St. Louis, MO
REFERENCES
- 1.Segev DL, Gentry SE, Melancon JK, Montgomery RA. Characterization of waiting times in a simulation of kidney paired donation. Am J Transplant. 2005;5(10):2448. doi: 10.1111/j.1600-6143.2005.01048.x. [DOI] [PubMed] [Google Scholar]
- 2.Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis. 2011;57(1):144. doi: 10.1053/j.ajkd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93(6):603. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garonzik Wang JM, Montgomery RA, Kucirka LM, Berger JC, Warren DS, Segev DL. Incompatible live-donor kidney transplantation in the United States: results of a national survey. Clin J Am Soc Nephrol. 2011;6(8):2041. doi: 10.2215/CJN.02940311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segev DL, Simpkins CE, Warren DS, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5(10):2570. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 6.OPTN (Organ Procurement and Transplantation Network)/UNOS (United Network for Organ Sharing) [September 10, 2013];National Data Reports, Graft Survival by Donor Type. Latest Data http://optn.transplant.hrsa.gov/latestData/rptStrat.asp.
- 7.Locke JE, Zachary AA, Haas M, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant. 2007;7(4):842. doi: 10.1111/j.1600-6143.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 8.Flint SM, Walker RG, Hogan C, et al. Successful ABO-incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011;11(5):1016. doi: 10.1111/j.1600-6143.2011.03464.x. [DOI] [PubMed] [Google Scholar]
- 9.Genberg H, Kumlien G, Wennberg L, Berg U, Tyden G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85(12):1745. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 10.Habicht A, Broker V, Blume C, et al. Increase of infectious complications in ABO- incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant. 2011;26(12):4124. doi: 10.1093/ndt/gfr215. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JK, Kim SI, Choi BS, et al. Short-term results of ABO-incompatible living donor kidney transplantation: comparison with ABO-compatible grafts. J Korean Surg Soc. 2011;81(1):10. doi: 10.4174/jkss.2011.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morath C, Becker LE, Leo A, et al. ABO-incompatible kidney transplantation enabled by non-antigen-specific immunoadsorption. Transplantation. 2012;93(8):827. doi: 10.1097/TP.0b013e31824836ae. [DOI] [PubMed] [Google Scholar]
- 13.Renner FC, Czekalinska B, Kemkes-Matthes B, et al. Postoperative bleeding after AB0- incompatible living donor kidney transplantation. Transplant Proc. 2010;42(10):4164. doi: 10.1016/j.transproceed.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer B, Tonshoff B, Schmidt J, et al. Bleeding complications in pediatric ABO- incompatible kidney transplantation. Pediatr Nephrol. 2013;28(2):327. doi: 10.1007/s00467-012-2302-x. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz J, Stegall MD, Kremers WK, Gloor J. Complications, resource utilization, and cost of ABO-incompatible living donor kidney transplantation. Transplantation. 2006;82(2):155. doi: 10.1097/01.tp.0000226152.13584.ae. [DOI] [PubMed] [Google Scholar]
- 16.Wilpert J, Fischer KG, Pisarski P, et al. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25(11):3778. doi: 10.1093/ndt/gfq229. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan A. Complications of apheresis. Semin Dial. 2012;25(2):152. doi: 10.1111/j.1525-139X.2011.01026.x. [DOI] [PubMed] [Google Scholar]
- 18.Moriya Y, Yamaji K, Kanai Y, Tsuda H. The effectiveness of intravenous human immunoglobulin treatment after plasmapheresis in restoring serum immunoglobulin levels: a preliminary study. Ther Apher. 2002;6(2):154. doi: 10.1046/j.1526-0968.2002.00297.x. [DOI] [PubMed] [Google Scholar]
- 19.Biglarnia AR, Nilsson B, Nilsson Ekdahl K, et al. Desensitization with antigen-specific immunoadsorption interferes with complement in ABO-incompatible kidney transplantation. Transplantation. 2012;93(1):87. doi: 10.1097/TP.0b013e31823bb689. [DOI] [PubMed] [Google Scholar]
- 20.Lee G, Arepally GM. Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J Clin Apher. 2012;27(3):117. doi: 10.1002/jca.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chirnside A, Urbaniak SJ, Prowse CV, Keller AJ. Coagulation abnormalities following intensive plasma exchange on the cell separator. II. Effects on factors I, II, V, VII, VIII, IX, X and antithrombin III. Br J Haematol. 1981;48(4):627. doi: 10.1111/j.1365-2141.1981.00627.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanafusa N, Hamasaki Y, Kawarasaki H, et al. The effect of different apheresis modalities on coagulation factor XIII level during antibody removal in ABO-blood type incompatible living related renal transplantation. Transfus Apher Sci. 2013 doi: 10.1016/j.transci.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Yeh JH, Chiu HC. Coagulation abnormalities in serial double-filtration plasmapheresis. J Clin Apher. 2001;16(3):139. doi: 10.1002/jca.1026. [DOI] [PubMed] [Google Scholar]
- 24.Lentine KL, Rocca Rey LA, Kolli S, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3(4):1090. doi: 10.2215/CJN.03080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant. 2006;6(1):129. doi: 10.1111/j.1600-6143.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- 26.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 27.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre GP, Squifflet JP, De Bruyere M, et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19(6):4538. [PubMed] [Google Scholar]
- 29.Alexandre GP, De Bruyere M, Squifflet JP, Moriau M, Latinne D, Pirson Y. Human ABO- incompatible living donor renal homografts. Neth J Med. 1985;28(6):231. [PubMed] [Google Scholar]
- 30.Tyden G, Kumlien G, Genberg H, Sandberg J, Lundgren T, Fehrman I. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5(1):145. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 31.Tyden G, Kumlien G, Fehrman I. Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76(4):730. doi: 10.1097/01.TP.0000078622.43689.D4. [DOI] [PubMed] [Google Scholar]
- 32.Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4(8):1315. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 33.Whiting JF, Woodward RS, Zavala EY, et al. Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments.[see comment]. Transplantation. 2000;70(5):755. doi: 10.1097/00007890-200009150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Redfield RR, Parsons RF, Rodriguez E, et al. Underutilization of A2 ABO incompatible kidney transplantation. Clin Transplant. 2012;26(3):489. doi: 10.1111/j.1399-0012.2011.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gloor JM, Stegall MD. ABO incompatible kidney transplantation. Curr Opin Nephrol Hypertens. 2007;16(6):529. doi: 10.1097/MNH.0b013e3282f02218. [DOI] [PubMed] [Google Scholar]
- 36.Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L'Italien G, Schnitzler MA. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94(4):369. doi: 10.1097/TP.0b013e318259407f. [DOI] [PubMed] [Google Scholar]
- 37.Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, L'Italien G, Lentine KL. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241. doi: 10.1097/TP.0b013e318255f839. [DOI] [PubMed] [Google Scholar]
- 38.Schnitzler MA, Gheorghian A, Axelrod D, L'Italien G, Lentine KL. The cost implications of first anniversary renal function after living, standard criteria deceased and expanded criteria deceased donor kidney transplantation. J Med Econ. 2013;16(1):75. doi: 10.3111/13696998.2012.722571. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler MA, Lentine KL, Gheorghian A, Axelrod D, Trivedi D, L'Italien G. Renal function following living, standard criteria deceased and expanded criteria deceased donor kidney transplantation: impact on graft failure and death. Transpl Int. 2012;25(2):179. doi: 10.1111/j.1432-2277.2011.01395.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.