Abstract
Culturing cells and tissues in vitro has provided valuable insights into the molecular mechanisms regulating redox signaling in cells with implications for medicine. However, standard culture techniques maintain mammalian cells in vitro under an artificial physicochemical environment such as ambient air and 5% CO2. Oxidative stress is caused by the rapid oxidation of cysteine to cystine in culture media catalyzed by transition metals, leading to diminished intracellular cysteine and glutathione (GSH) pools. Some cells, such as fibroblasts and macrophages, express cystine transport activity, designated as system , which enables cells to maintain these pools to counteract oxidative stress. Additionally, many cells have the ability to activate the redox sensitive transcription factor Nrf2, a master regulator of cellular defenses against oxidative stress, and to upregulate xCT, the subunit of the transport system leading to increases in cellular GSH. In contrast, some cells, including lymphoid cells, embryonic stem cells and iPS cells, express relatively low levels of xCT and cannot maintain cellular cysteine and GSH pools. Thus, fibroblasts have been used as feeder cells for the latter cell types based on their ability to supply cysteine. Other key Nrf2 regulated gene products include heme oxygenase 1, peroxiredoxin 1 and sequestosome1. In macrophages, oxidized LDL activates Nrf2 and upregulates the scavenger receptor CD36 forming a positive feedback loop to facilitate removal of the oxidant from the vascular microenvironment. This review describes cell type specific responses to oxygen derived stress, and the key roles that activation of Nrf2 and membrane transport of cystine and cysteine play in the maintenance and proliferation of mammalian cells in culture.
Keywords: Oxygen, Glutathione, Cystine transporter, xCT, Nrf2, Feeder cells, 2-Mercaptoethanol, CD36, Lymphocytes, Embryonic stem cells, iPS cells
Abbreviations: BCS, bathocuproine sulfonate; ES cells, embryonic stem cells; HO-1, heme oxygenase 1; Prx1, peroxiredoxin 1; SQSTM1, sequestosome1; 4HNE, 4-hydroxynonenal; iPS cells, induced pluripotent stem cells; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; MRPs, multidrug resistance-associated proteins; oxLDL, oxidized low density lipoprotein
Highlights
-
•
Rapid air-oxidation of cysteine to cystine occurs in culture media.
-
•
Cystine transporter activity maintains cellular GSH and cysteine pools.
-
•
Nrf2 and ATF4 upregulate expression of the cystine transporter xCT.
-
•
Many lymphoid and stem cells lack cystine transporter activity.
-
•
Fibroblast feeder cells can supply cysteine to cystine transporter deficient cells.
Graphical abstract
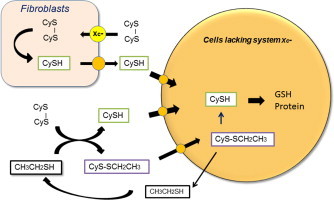
Introduction
Culturing mammalian cells in vitro is a basic technology supporting modern biology and clinical applications. However, freshly isolated cells from mammalian tissues are exposed to artificial physicochemical environments, quite different from those encountered in vivo, and only cells with the ability to adapt to such conditions can survive and proliferate.
In the 1970s, feeder layer cells or small thiol compounds were introduced to support survival, proliferation and differentiation of various cell types unable to adapt to culture media (Table 1). Unlike fibroblasts, non-adherent mouse spleen lymphocytes exhibit limited viability during culture in conventional media [1]. Chen and Hirsch were the first to demonstrate that co-culture with peritoneal macrophages enhanced the viability and antibody production by spleen lymphocytes [1]. Moreover, replacement of macrophages by supplementation of 2-mercaptoethanol (2ME, 10–100 µM) to culture media restored the viability and antibody forming capacity of lymphocytes [1]. Thymus feeder cell layers have also been used to sustain proliferation and differentiation of T lymphocytes [2], while human skin fibroblasts and epithelial cells can serve as feeder cells for human malignant lymphoma cell lines [3]. Broome and Jeng reported that low concentrations of selected thiol compounds, such as a-thioglycerol and 2ME, promote growth of mouse lymphoma L1210 cells in vitro [4]. Oshima established that feeder layer dependent embryonic carcinoma cells can proliferate in the absence of feeder cells when 2ME is supplemented [5]. Similarly, fibroblasts feeder cells can enhance proliferation of pluripotent hemopoietic stem cells in culture [6]. These studies establish the validity of using feeder cells or 2ME in the maintenance of various lymphoid and stem cells, although the mechanisms by which feeder cells and/or 2ME sustained cell viability were unknown at that time. Recent reviews [7–11] describe the application of these methods for the culture of human embryonic stem (hES) cells and induced pluripotent stem (iPS) cells (see Table 1).
Table 1.
Mammalian cells used as feeder cells (A) and 2-mercaptoethanol dependent cells (B).
| A. Feeder cells used to maintain mammalian cells | Feeder-dependent cells | References |
|---|---|---|
| Mouse macrophages | Mouse spleen lymphocytes | [1] |
| Mouse thymus stromal cells | Mouse T lymphocytes | [2] |
| Human skin fibroblasts | Human malignant lymphoma cells | [3] |
| Human epithelial cells | Human malignant lymphoma cells | [3] |
| Mouse embryonic Fibroblasts | Mouse embryonal carcinoma cells | [5] |
| Mouse fibroblasts | Mouse pluripotent haemopoietic stem cells | [6] |
| Human mesenchymal stromal cells | Human haematopoietic stem cells | [7] |
| Mouse embryonic fibroblasts | Human embryonic stem cells, iPS cells | Cited in reviews [8–11] |
| Human fibroblasts | Human embryonic stem cells | Cited in reviews [8–11] |
| B. 2-mercaptoethanol-dependent cells | References | |
| Mouse spleen lymphocytes | [1] | |
| Mouse lymphoma L1210 | [4] | |
| Human embryonic stem cells, iPS cells | Cited in reviews [8–11] | |
An important environmental stress in culture medium is oxygen dependent transition metal-catalyzed rapid oxidation of cysteine to cystine, resulting in depletion of intracellular cysteine and glutathione (GSH) pools critical for protein synthesis and detoxification, respectively [12,13]. In the 1980s, pioneering research led by Shiro Bannai established the importance of membrane cystine transport activity in maintaining intracellular cysteine and GSH pools to counteract oxidative stress [14,15]. Many cells, such as fibroblasts, macrophages, hepatocytes and vascular smooth muscle cells, express cystine transport activity and thus can survive and proliferate in defined culture media. Such cells are able to change air-oxidized media to reduced media by generating cysteine and protein thiols as a result of cystine uptake and diffusion of cysteine from cells [16].
However, some cells including mouse lymphocytes and lymphoid cells lack cystine transport activity and depend exclusively on supply of extracellular cysteine for their survival and proliferation [17–19]. These cells cannot adapt to conventional culture conditions using incubators gassed with ambient oxygen and 5% CO2. However, there are some culture methods to surmount this difficulty. One of the widely used methods is to co-culture with feeder cells, which express cystine transport activity and are thus able to change an oxidized culture media to a reduced one, thereby providing cysteine for the cells deficient in cystine transport activity. We here review the development of methods used for supporting the survival and proliferation of cells deficient in cystine transport activity.
After the discovery of the cystine/glutamate specific anionic amino acid transporter designated system , further research established the importance of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) as a regulator of the cystine transporter (xCT, a component of system ) and stress-induced antioxidant proteins involved in the protection of cells against oxidative stress [20,21]. As an example, we describe here the major Nrf2-regulated gene products expressed in activated macrophages and their involvement in protection against oxidative stress. Macrophages play important roles in effectively removing oxidized low density lipoproteins (oxLDL) and lipid oxidative products such as 4-hydroxynonenal (4HNE) following the activation of Nrf2.
Importance of cystine transport in the regulation of cellular redox balance
The disulfide amino acid cystine is one of the essential amino acids in defined culture media used for many mammalian cell types including human diploid fibroblasts. Bannai et al. found that cystine deficiency in culture media specifically causes rapid cell death due to depletion of cellular GSH [12]. He subsequently characterized a novel membrane transport activity mediating exchange of extracellular cystine and intracellular glutamate, and designated this amino acid transporter as system [14 ,15,22,23] (Fig. 1). This transport system plays a key role in regulating cystine uptake, leading to maintenance of intracellular cysteine and GSH pools [15,22].
Fig. 1.
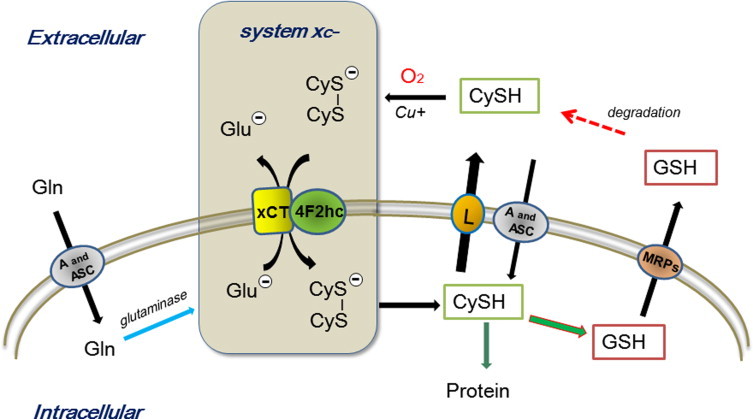
Role of cystine transport in the maintenance of intracellular cysteine and GSH levels. Cysteine (CySH) is rapidly air-oxidized to cystine (CyS–SCy) in culture media catalyzed by reactions with transition metals. The specific cystine/glutamate Na+-independent transport system transports one cystine into cells in exchange for export of one glutamate molecule [14,23]. The cystine transporter is composed of a heterodimer, 4F2hc and xCT [28]. Induction of xCT expression by oxidative stress is regulated by Nrf2 while 4Fhc is constitutively expressed. Cystine is reduced to cysteine in cells, but effluxes from cells via neutral amino acid transporters including system L. A high concentration of glutamine in culture media contributes to the maintenance of high concentrations of intracellular glutamate, which facilitate efficient import of cystine via system . GSH has a short half-life, since it is rapidly exported through transporters such as multidrug resistance-associated proteins (MRPs) [29]. GSH is cleaved into cysteinylglycine and amino acids by plasma membrane-bound enzymes [29].
Amino acid membrane transport is mediated by transporters recognizing both chemical structure and ionic charge of amino acids [24–26]. They are classified into acidic, neutral and basic amino acid transporters depending on their net charge. The transport of neutral amino acids with short, polar, or linear side chains is mediated by Na+-dependent, highly concentrative system A and ASC, whereas branched chain and aromatic amino acids are mainly transported by a Na+-independent system L [24–27]. Notably, cysteine is mainly taken up by cells via system A and ASC, and effluxes from cells through system L [24–27]. The cystine/glutamate transport system is a Na+-independent exchange transporter preferring the acidic/anionic form of cystine and glutamate having a net negative charge [23]. Due to a high intracellular glutamate content and presence of cystine only in the medium, system catalyzes export of one glutamate and import of one cystine in an exchange reaction (Fig.1) [15,23]. Cystine is readily reduced to cysteine in cells. Extracellular glutamate and homocysteate competitively inhibit import of cystine [14]. Many of the amino acid transporters have been cloned [25,26] and system is comprised of two components xCT and 4F2hc linked by disulfide [25,28]. The former component is stress-inducible and the latter is constitutively expressed [28]. Notably, the heavy subunit 4F2hc associates with other amino acid exchanger systems such as system L, y+L and asc [25].
The rate-limiting step for GSH synthesis is the pool size of cellular cysteine in cultured cells, as it is relatively small compared to those of glycine and glutamate. Cystine transport activity and cysteine efflux from cells determines the size of the cellular cysteine pool. Notably, the reduced form of GSH can also efflux from cells via transporters such as multidrug resistance proteins (MRPs) and is rapidly degraded to dipeptides and amino acids at the extracellular surface [29]. The oxidation of cysteine is catalyzed by heavy metals such as Cu+[1] in the presence of oxygen [13]. Most culture media contain a 5–20-fold greater concentration of glutamine to support cell growth. Glutamine is taken up via system A and ASC [25,26] and metabolized to glutamate by cellular glutaminase, and hence the cellular glutamate pool depends largely on levels of extracellular glutamine [30]. The high intracellular glutamate thus formed is used for uptake of cystine via the exchange transport system [23]. It has been estimated that one-third to one-half of the total consumption of glutamine is used for the uptake of cystine during culture of human diploid fibroblasts [30].
Culture media are usually air-oxidized during storage. Cysteine, if added, is oxidized to cystine over short time periods and reactive cysteine residues in serum albumin are masked mainly by cysteine through disulfide formation. When pre-confluent human diploid fibroblasts are incubated in standard culture medium containing 10% (v/v) fetal calf serum, sulfhydryls are produced in culture medium, achieving maximal levels within 6 h (Fig. 2) [16]. Cysteine is rapidly produced in the medium upon incubation with cells which is dependent on cellular cystine uptake and the efflux of cysteine from cells. Levels of cysteine after 6 h or later can reach around 20 µM in the culture medium, a level almost equivalent to that measured in blood [15]. Masked sulfhydryl residues in albumin are gradually reduced by the sulfhydryl-disulfide exchange reaction with cysteine and reach a similar level to cysteine within 48 h (Fig. 2) [16]. Thus, fibroblasts are able to produce cysteine from cystine and sulfhydryl residues in albumin in the culture medium [16]. Fibroblasts are often used as feeder layer cells to ensure survival and growth of cells deficient in cystine transporter activity (Table 1). Cells expressing cystine transport activity like fibroblasts can release cysteine into the culture medium, which in turn supports the proliferation of other cells which lack or have low cystine transport activity (Fig. 2).
Fig. 2.
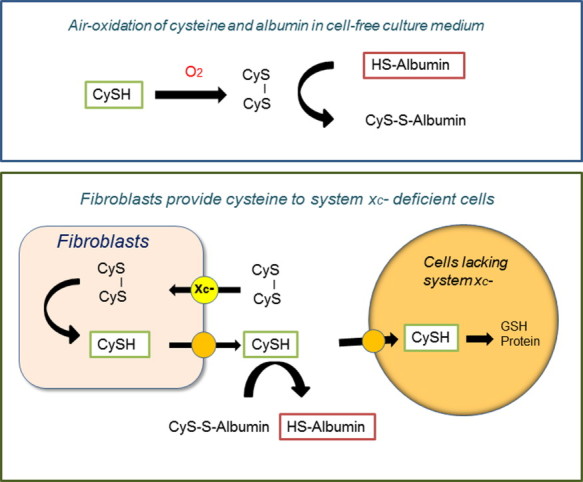
Fibroblasts serve as feeder cells by providing cysteine to co-cultured cells deficient in cystine transport activity. When human diploid fibroblasts are cultured in fresh medium containing 10% (v/v) fetal bovine serum, which has been oxidized during storage, they rapidly produce sulfhydryls in the medium [16]. These cells take up cystine via system and reduce it to cysteine intracellularly. Cellular cysteine in turn can efflux from cells and accumulate in the medium. Cysteine gradually unmasks cysteine residues in albumin, producing protein sulfhydryls via an SH/S-S exchange reaction. As fibroblasts release cysteine into the culture medium, these cells can be used as feeder cells to provide cysteine for other cells lacking cystine transporter activity.
A recent review by Lewrenz et al. summarizes expression of system in vivo [31]. The expression of xCT was detected in brain including meninges and some circumventricular organs [32] and spinal cord [33], but not in lung, heart, liver and kidney [31–33]. Mice lacking xCT are healthy in appearance and fertile, although cystine and GSH contents in plasma are lower in knockout compared to wild type mice [34]. As expected, embryonic fibroblasts derived from xCT deficient mice require 2ME for their survival and proliferation in in vitro [34].
Effects of cysteine stabilization and 2-mercaptoethanol on proliferation of lymphocytes
Although cystine transport activity is important for growth and survival of cells in culture, some cells such as murine lymphocytes and lymphoid cells are deficient in cystine transport activity [13,18]. These cells cannot survive and proliferate in standard defined culture media, as they depend on the supply of cysteine which is unstable in culture medium. Culture media contain trace amounts of heavy metals that catalyze oxidation of cysteine to cystine in the presence of oxygen. Copper ions are one of the metal ions catalyzing oxidation of cysteine. Supplementing a nontoxic copper-specific chelating agent, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline disulfonic acid (bathocuproine sulfonate, BCS) to culture medium (e.g. RPMI 1640) inhibits oxidation of cysteine and enhances proliferation of murine lymphoid L1210 cells, which lack cystine transport activity [13]. Notably, following addition of cysteine at a concentration of 50 µM to fresh culture medium in the absence of cells, roughly 90% of cysteine is oxidized within 2 h. However, in the presence of 10 µM BCS, only about 20%, 50%, and 75% cysteine is oxidized after 2, 8 and 24 h, respectively (Fig. 3). When 50 µM cysteine is supplemented at 24 h intervals in the presence of 10 µM BCS, L1210 cells continuously proliferate at maximal rates (Fig. 3) [13].
Fig. 3.
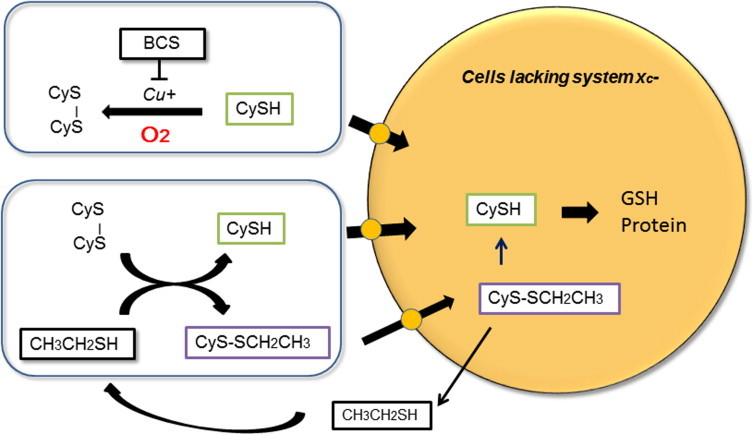
Cells deficient in cystine transport activity depend on cysteine for their survival and proliferation. Some cells such as murine lymphocytes and lymphoma L1210 cells do not express cystine transporters and largely depend on supply of extracellular cysteine for their survival. Supplemented cysteine is rapidly auto-oxidized in culture media in cell free conditions, but the presence of the copper chelator bathocuproine sulfonate (BCS) significantly prolongs the half-life of cysteine from about 30 min to 8 h. Addition of 50 µM cysteine to culture media every 24 h in the presence of 10 µM BCS supports continuous growth of L1210 murine lymphoma cells in RPMI1640 medium containing 10% (v/v) fetal calf serum [13]. Another method is to supplement a small thiol compound 2-mercaptoethanol (2ME) to the culture medium, which catalytically facilitates cysteine transport [19]. The mixed disulfide of cysteine and 2ME formed in the medium is stable and can be taken up by the cells via neutral amino acid transporters. It is reduced within the cells to cysteine and 2ME, with 2ME released back into medium. Due to its catalytic action, 2ME is routinely used at 20–50 µM concentrations for prolonged support of cell survival and growth of murine lymphocytes.
The maintenance of GSH in murine spleen lymphocytes also largely dependent on cysteine [18]. Stimulation of lymphocytes either with lipopolysaccharide (LPS) or Concanavalin A markedly increases cysteine transport activity. The uptake of cysteine is mainly mediated through system A and ASC. In contrast, both LPS-stimulated and fresh resting lymphocytes have extremely low cystine transport activities, and thus depend on extracellular cysteine for maintenance of intracellular GSH levels [18]. In the absence of cysteine in culture medium, intracellular GSH decreases by roughly one-third within 8 h. However, addition of 50 µM cysteine every 8 h in the presence of 20 µM BCS maintains cellular GSH levels in the activated lymphocytes [18].
A small thiol compound 2ME has routinely been supplemented in culture medium for murine lymphocytes and lymphoid cells (Table 1), which lack cystine transport activity [18]. When 2ME is supplemented to culture medium at 10–50 µM, these cells survive and proliferate in a standard cystine containing medium, noting that RPMI1640 medium contains 0.23 mM cystine. The major role of 2ME is to maintain intracellular cysteine and GSH levels by facilitating cystine and cysteine transport [35]. A molecule of 2ME reacts with a cystine producing cysteine and a mixed-disulfide of 2ME via an SH and S-S exchange reaction in culture medium (Fig. 3). Both cysteine and the mixed disulfide can be transported into cells via membrane transporters for neutral amino acids. The mixed disulfide is then reduced in cells generating cysteine and 2ME, with the latter effluxing from cells and reacting again with cystine. Due to this catalytic action of 2ME, cells can maintain cellular cysteine and GSH levels during culture in the absence of cystine transport activity [35] (Fig. 3).
Although expression of system is low in murine lymphocytes, it can be expressed in splenic T lymphocytes isolated from mice 10 days after thermal injury [36]. Thermal injury induces oxidative stress, decreases GSH and protein levels, leading to enhanced expression of xCT. The acquired cystine transport activity is sufficient to support cell proliferation without 2ME in culture medium [36].
Upregulation of cystine transport activity by Nrf2 and ATF4
Notably, cystine transport activity is upregulated under oxidative stress [37–40]. Fig. 4 shows the effect of electrophilic agents such as diethylmaleate on the upregulation of cellular cystine transport activity and GSH levels [37]. Diethylmaleate is known as a GSH depleting agent, and addition of this agent at 1 mM to culture media of human fibroblasts causes a rapid decline in cellular GSH to 20% within 3–4 h and to almost negligible levels within 12 h leading to cell death [38]. However, diethylmaleate at 0.1 mM induces more than a 2-fold increase in cellular GSH over 15 h, noting however that there is a transient decrease in GSH over the first 3 h [38]. The increase in GSH is accompanied by a marked increase in cystine transport activity, resulting from the activation of Nrf2 and upregulation of xCT expression [28] (Fig. 4). Thus, a low dose of diethylmaleate can induce cellular responses that enable cells to become more resistant to electrophilic agents and oxidative stress.
Fig. 4.
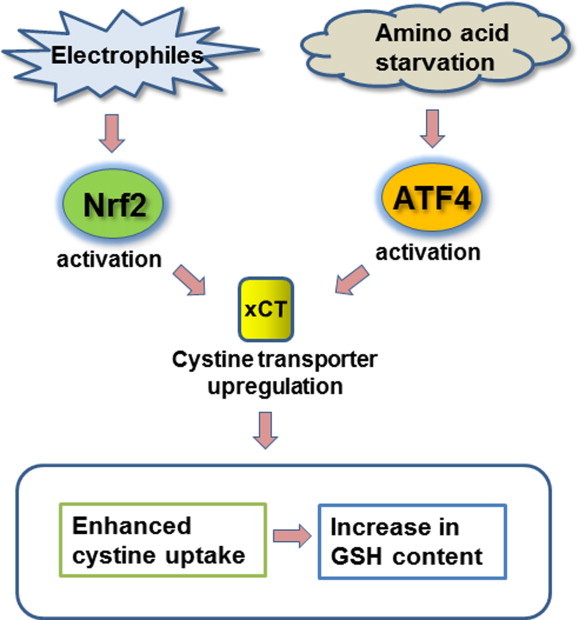
Electrophilic agents and amino acid starvation up regulate xCT, resulting in increases in intracellular GSH. Diethylmaleate (DEM) is an electrophilic agent reactive with GSH and depletes GSH in human fibroblasts when added at 1 mM to culture media. However, when supplemented at 0.1 mM, it increases cellular GSH more than 2 fold within 15 h, preceded by an initial transient decrease over the first 3 h. DEM activates Nrf2 and upregulates expression of xCT, resulting in an increase in cystine uptake and cellular GSH [20,28,38]. Amino acid starvation activates transcription factor ATF4 leading upregulation of xCT expression [41].
Notably xCT expression is also upregulated in NIH3T3 cells following removal of amino acids including cystine in culture media [41]. In addition to Nrf2, the transcription factor ATF4 activates xCT gene expression through a cis-element termed amino acid response element [41]. An oxidation product of ?-tocopherol, ?-tocopheryl quinone, reacts with nucleophiles inducing toxicity and activates ATF4 leading upregulation of xCT expression [42].
Nrf2-mediated regulation of redox balance in cultured cells
The transcription factor Nrf2 regulates expression of many genes via its interaction with ARE/EpRE elements in their regulatory region [43]. Nrf2 target genes include a group of phase II detoxification enzymes including glutathione-S-transferase [43]. In addition to xCT, Nrf2 also regulates expression of other key proteins important for defense against oxidative stress [20]. Heme oxygenase 1 (HO-1), peroxiredoxin 1 (Prx1) and sequestosome1/p62 (Fig. 5) are major proteins induced following Nrf2 activation in murine peritoneal macrophages [20] and aortic smooth muscle cells [21]. HO-1 and Prx1 are well known endogenous antioxidants, and enhanced expression of these proteins and increases GSH via xCT up regulation ensure protection of cells against oxidative damage. Sequestosome1/p62 is a modulator of cell signaling with multifunctional activities including anti-inflammatory functions [44,45].
Fig. 5.
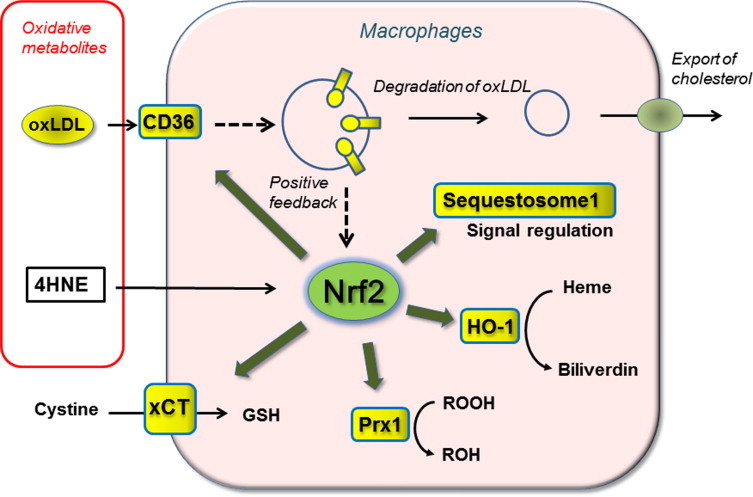
Activation of transcription factor Nrf2 by oxidized LDL and 4-hydroxynonenal in peritoneal macrophages. Both oxLDL and membrane permeable lipid metabolite 4HNE function as alarm signals for oxidative stress and activate Nrf2 leading to the upregulation of different endogenous antioxidants and CD36 expression in macrophages [21]. CD36 is a scavenger receptor which mediates roughly half of oxLDL uptake by macrophages, thereby contributing to the removal of toxic oxLDL from the micro-environment [21]. As oxLDL upregulates its receptor CD36 mainly via Nrf2 activation, the reaction provides a positive feedback loop. Exposure of murine peritoneal macrophages to elevated oxLDL concentrations leads to an upregulation of CD36 and accumulation of oil droplets in the cytoplasm [21]. In this context, Nrf2 facilitates foam cell formation under enhanced oxLDL loading [55,56]. Nrf2 activation in macrophages also upregulates antioxidant enzymes such as heme oxygenase-1 (HO-1) and peroxiredoxin 1 (Prx1) and the subunit of the transport system xCT to protect the cells from oxidative damage. Sequestosome1/p62 is known to modulate receptor mediated signaling and suppresses inflammation, however its antioxidant function remains to be defined [45,54].
Various electrophilic agents such as diethylmaleate activate Nrf2 through direct interaction with the reactive sulfhydryls in the cytosolic sensor protein Keap1 [46]. Oxidized lipid metabolites, continuously produced in cells and tissues, contain intrinsic activators of Nrf2 such as 4-hydroxynonenal (4HNE), and thus function as an alarm signal for oxidative stress [21,47]. Circulating oxidized LDL (oxLDL) is associated with clinical manifestations of atherosclerosis. Continual oxidation of LDL occurs in vivo, and the products are removed by cells expressing scavenger receptors for oxLDL [48,49]. Exposure of oxLDL induces cellular responses such as upregulation of cystine transport activity and GSH synthesis in mouse peritoneal macrophages [50] and human arterial smooth muscle cells [51]. Upregulation of GSH by oxLDL is accompanied by an increase in glutamate-cysteine ligase in bovine aortic endothelial cells [52]. GSH upregulation by oxLDL is mediated via AREs/EpREs [53], and the transcription factor Nrf2 plays key role in oxLDL induced cellular responses [21]. We examined effects of 4HNE, lipid hydroperoxides, oxysterols and other aldehydes contained in oxidized LDL on Nrf2 activation, and found that 4HNE was the most effective activator of Nrf2 [21]. The estimated amount of 4HNE in oxLDL was sufficient to cause Nrf2 activation [21].
A scavenger receptor CD36, which is expressed in macrophages and platelets, is a major receptor for oxLDL. Notably, oxLDL upregulates CD36 expression in macrophages forming a positive feedback to facilitate removal of toxic oxLDL from the microenvironment. Induction of CD36 expression by oxidative stress largely depends on Nrf2 [21,54]. However, it should be noted that feeding a high fat diet in the absence of ApoE, which facilitates HDL-mediated cholesterol efflux from macrophages, results in enhanced atherosclerotic lesion formation in wild type compared to Nrf2 deficient mice [55,56]. These results highlight that the Nrf2-CD36 pathway could exacerbate atherosclerosis under conditions of elevated oxLDL levels.
A small lipid oxidation metabolite 4HNE is a membrane permeable activator of Nrf2 and upregulates expression of CD36 and other gene products in murine macrophages [21]. 4HNE also activates Nrf2 in vascular smooth muscle cells but CD36 is not expressed in murine smooth muscle cells. However, there are studies reporting CD36 expression in human vascular smooth muscle cells [57]. As oxLDL does not efficiently activate Nrf2 in murine vascular smooth muscle cells, incorporation of oxLDL into cells may necessary for Nrf2 activation [21]. In this context, upregulation of GSH is important, as 4HNE is conjugated with GSH by glutathione-S-transferase and subsequently reduced by aldo-keto reductases [58].
Importance of redox balance in stem cell culture
Culturing stem cells commonly requires feeder cells to maintain an undifferentiated state without losing pluripotency in culture [7–11]. Fibroblasts are usually used as feeder cells for ES cells and iPS cells [7–11] (Table1). Mesenchymal stromal cells are also used for maintenance of hematopoietic stem cells in vitro [7,59]. A key function of feeder cells is to stably provide cysteine for stem cell survival (Fig. 2) and, in addition to other secreted factors, extracellular matrix and cellular contacts for proliferating the stem cells. The requirement of feeder cells or 2ME dependency of mammalian stem cells and human iPS cells [7–11] suggests they express low cystine transport activity similar to mouse lymphocytes and lymphoid cells.
Feeder-free stem cell culture has been developed in order to avoid unfavorable effects such as contamination of pathogens from feeder cells [8–11]. Interestingly, feeder-free media generally contain 2ME at a concentration similar to that used for murine lymphocyte in culture [8–11,60]. As described previously, 2ME can improve the redox balance by enhancing cysteine uptake to maintain cellular GSH levels (Fig. 3). Another method to maintain stem cells without feeder cells is to use fibroblast conditioned medium. In an open protocol provided by NIH [60], fresh medium was first applied to a monolayer of fibroblasts for one day, and stem cells were then incubated with this conditioned medium and seeded on Matrigel, which provides matrix for cell attachment. The conditioned medium is replaced daily to support continuous growth of stem cell colonies [60]. It has been reported that air-oxidized medium can be reduced to produce cysteine and albumin sulfhydryls following incubation with fibroblasts (Fig. 2) [16]. Interestingly, cysteine either present in the medium or supplemented to the medium is relatively stable, which may partly be due to stability of protein thiols and reduced free transition metal ions after incubation with the fibroblasts. In this context, supplementing conditioned media daily mimics a culture condition with repeated cysteine supply in the presence of a copper ion chelator [13] (Fig. 3).
Basic fibroblast growth factor (FGF-2) is commonly included as a supplement in culture media for various stem cells and iPS cells [8–11]. FGF-2 activates cellular signaling through FGF receptors in cooperation with heparin to induce its pleiotropic effects including angiogenic and neurogenic effects [61–63]. Notably, FGF-2 activates the stress related transcription factor ATF4 in vascular smooth muscle cells [64] and osteoblasts [65]. FGF-2 upregulated GSH synthesis in glial [66] and Sertoli cells [67] and cystine/glutamate transport (system ) in astrocytes [68]. These results suggest that cystine transport activity may be upregulated in the presence of FGF-2 in stem cells or differentiating cells via FGF-2/ATF4 signaling, leading to improved survival and growth, however, to our knowledge this hypothesis remains to be investigated.
Summary
Understanding the mechanisms regulating redox balance in cells cultured in vitro is important for the design of cell type specific culture conditions. Maintenance of cellular GSH levels during culture is a key redox indicator for protection of DNA against oxidative damage. Most mammalian cells, excluding hepatocytes, which can synthesize cysteine from methionine, depend largely on supply of cysteine or cystine for GSH synthesis, as glycine and glutamate are usually maintained at higher levels in cells cultured in conventional culture media.
Improving the culture conditions for various stem cells and iPS will be particularly important for clinical applications. Further research is required to establish feeder-free and serum-free defined culture conditions for the maintenance of stem and iPS cells in healthy conditions and for inducing their differentiation into desired cell types. Stem cell biology will be significantly improved by designing redox balance based culture conditions which will allow different stem cells to maintain proliferation without losing pluripotency, to enhance their differentiation and to select desired differentiated cells from others.
Acknowledgements
This work was supported in part by the British Heart Foundation (FS/10/59/28533; PG/13/1/29801), Wellcome Trust (Collaborative Award 059752), and Great Britain-Sasakawa Foundation(Collaborative Award B41) .
References
- 1.Chen C., Hirsch J.G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. Journal of Experimental Medicine. 1972;136:604–617. doi: 10.1084/jem.136.3.604. 4559193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorczynski R.M., Macrae S. Differentiation of functionally active mouse T lymphocytes from functionally inactive bone marrow precursors. III. Induction of T-cell activities by growth of bone marrow on feeder layers prepared from mouse thymocytes. Immunology. 1979;38:1–12. 315913 [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein A.L., Kaplan H.S. Feeder layer and nutritional requirements for the establishment and cloning of human malignant lymphoma cell lines. Cancer Research. 1979;39:1748–1759. 371794 [PubMed] [Google Scholar]
- 4.Broome J.D., Jeng M.W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. Journal of Experimental Medicine. 1973;138:574–592. doi: 10.1084/jem.138.3.574. 4727914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshima R. Stimulation of the clonal growth and differentiation of feeder layer dependent mouse embryonal carcinoma cells by ß-mercaptoethanol. Differentiation: Research in Biological Diversity. 1978;11:149–155. doi: 10.1111/j.1432-0436.1978.tb00978.x. 720785 [DOI] [PubMed] [Google Scholar]
- 6.Löwenberg B., Dicke K.A. Induction of proliferation of haemopoietic stem cells in culture. Experimental Hematology. 1977;5:319–331. 891671 [PubMed] [Google Scholar]
- 7.Andrade P.Z., Santos F.D., Cabral J.M., da Silva C.L. Stem cell bioengineering strategies to widen the therapeutic applications of haematopoietic stem/progenitor cells from umbilical cord blood. Journal of Tissue Engineering and Regenerative Medicine. 2013 doi: 10.1002/term.1741. 23564692 [DOI] [PubMed] [Google Scholar]
- 8.Unger C., Skottman H., Blomberg P., Dilber M.S., Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Human Molecular Genetics. 2008;17:R48–R53. doi: 10.1093/hmg/ddn079. 18632697 [DOI] [PubMed] [Google Scholar]
- 9.Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E., Wagner R., Lee G.O., Antosiewicz-Bourget J., Teng J.M.C., Thomson J.A. Chemically defined conditions for human iPSC derivation and culture. Nature Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. 21478862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crocco M.C., Fratnz N., Bos-Mikich A. Substrates and supplements for hESCs:a critical review. Journal of Assisted Reproduction and Genetics. 2013;30:315–323. doi: 10.1007/s10815-012-9914-8. 23288664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamasaki S., Taguchi Y., Shimamoto A., Mukasa H., Tahara H., Okamoto T. Generation of human induced pluripotent stem (iPS) cells in serum- and feeder-free defined culture and TGF-ß1 regulation of pluripotency. PLoS One. 2014;9:e87151. doi: 10.1371/journal.pone.0087151. 24489856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannai S., Tsukeda H., Okumura H. Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochemical and Biophysical Research Communications. 1977;74:1582–1588. doi: 10.1016/0006-291x(77)90623-4. 843380 [DOI] [PubMed] [Google Scholar]
- 13.Ishii T., Bannai S. The synergistic action of the copper chelator bathocuproine sulphonate and cysteine in enhancing growth of L1210 cells in vitro. Journal of Cellular Physiology. 1985;125:151–155. doi: 10.1002/jcp.1041250119. 4044667 [DOI] [PubMed] [Google Scholar]
- 14.Bannai S., Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. Journal of Biological Chemistry. 1980;255:2372–2376. 7358676 [PubMed] [Google Scholar]
- 15.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochimica et Biophysica Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. 6383474 [DOI] [PubMed] [Google Scholar]
- 16.Bannai S., Ishii T. Formation of sulfhydryl groups in the culture medium by human diploid fibroblasts. Journal of Cellular Physiology. 1980;104:215–223. doi: 10.1002/jcp.1041040211. 7410490 [DOI] [PubMed] [Google Scholar]
- 17.Hishinuma I., Ishii T., Watanabe H., Bannai S. Mouse lymphoma L1210 cells acquire a new cystine transport activity upon adaptation in vitro. In Vitro Cellular and Developmental Biology: Journal of the Tissue Culture Association. 1986;22:127–134. doi: 10.1007/BF02623499. 2869021 [DOI] [PubMed] [Google Scholar]
- 18.Ishii T., Sugita Y., Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. Journal of Cellular Physiology. 1987;133:330–336. doi: 10.1002/jcp.1041330217. 3680392 [DOI] [PubMed] [Google Scholar]
- 19.Ishii T., Hishinuma I., Bannai S., Sugita Y. Mechanism of growth promotion of mouse lymphoma L1210 cells in vitro by feeder layer or 2-mercaptoethanol. Journal of Cellular Physiology. 1981;107:283–293. doi: 10.1002/jcp.1041070215. [DOI] [PubMed] [Google Scholar]
- 20.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Journal of Biological Chemistry. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. 10821856 [DOI] [PubMed] [Google Scholar]
- 21.Ishii T., Itoh K., Ruiz E., Leake D.S., Unoki H., Yamamoto M., Mann G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circulation Research. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. 14752028 [DOI] [PubMed] [Google Scholar]
- 22.Bannai S., Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. Journal of Membrane Biology. 1986;89:1–8. doi: 10.1007/BF01870891. 2870192 [DOI] [PubMed] [Google Scholar]
- 23.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. Journal of Biological Chemistry. 1986;261:2256–2263. 2868011 [PubMed] [Google Scholar]
- 24.Christensen H.N. Distinguishing amino acid transport systems of a given cell or tissue. Methods in Enzymology. 1989;173:576–616. doi: 10.1016/s0076-6879(89)73040-8. 2674620 [DOI] [PubMed] [Google Scholar]
- 25.Fotiadis D., Kanai Y., Palacin M. The SLC3 and SLC7 families of amino acid transporters. Molecular Aspects of Medicine. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. 23506863 [DOI] [PubMed] [Google Scholar]
- 26.Hundal H.S., Taylor P.M. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. American Journal of Physiology: Endocrinology and Metabolism. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. 19158318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato H., Watanabe H., Ishii T., Bannai S. Neutral amino acid transport in mouse peritoneal macrophages. Journal of Biological Chemistry. 1987;262:13015–13019. 3115975 [PubMed] [Google Scholar]
- 28.Sato H., Tamba M., Ishii T., Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. Journal of Biological Chemistry. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. 10206947 [DOI] [PubMed] [Google Scholar]
- 29.Ballatori N., Krance S.M., Marchan R., Hammond C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Molecular Aspects of Medicine. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. 18786560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannai S., Ishii T. A novel function of glutamine in cell culture: utilization of glutamine for the uptake of cystine in human fibroblasts. Journal of Cellular Physiology. 1988;137:360–366. doi: 10.1002/jcp.1041370221. 2903864 [DOI] [PubMed] [Google Scholar]
- 31.Lewerenz J., Hewett S.J., Huang Y., Lambros M., Gout P.W., Kalivas P.W., Massie A., Smolders I., Methner A., Pergande M., Smith S.B., Ganapathy V., Maher P. The cystine/glutamate antiporter system xc- in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxidants and Redox Signaling. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato H., Tamba M., Okuno S., Sato K., Keino-Masu K., Masu M., Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. 12223556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.Y., Kanai Y., Chairoungdua A., Cha S.H., Matsuo H., Kim D.K., Inatomi J., Sawa H., Ida Y., Endou H. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochimica et Biophysica Acta. 2001;1512:335–344. doi: 10.1016/s0005-2736(01)00338-8. 11406111 [DOI] [PubMed] [Google Scholar]
- 34.Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., Takahashi S., Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. Journal of Biological Chemistry. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. 16144837 [DOI] [PubMed] [Google Scholar]
- 35.Ishii T., Bannai S., Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. Journal of Biological Chemistry. 1981;256:12387–12392. 7298664 [PubMed] [Google Scholar]
- 36.D’Elia M., Patenaude J., Dupras C., Bernier J. Burn injury induces the expression of cystine/glutamate transporter xc- in mouse T cells. Immunology Letters. 2009;125:137–144. doi: 10.1016/j.imlet.2009.06.011. 19576933 [DOI] [PubMed] [Google Scholar]
- 37.Bannai S., Kitamura E. Adaptive enhancement of cystine and glutamate uptake in human diploid fibroblasts in culture. Biochimica et Biophysica Acta. 1982;721:1–10. doi: 10.1016/0167-4889(82)90017-9. 6127114 [DOI] [PubMed] [Google Scholar]
- 38.Bannai S. Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. Journal of Biological Chemistry. 1984;259:2435–2440. 6142042 [PubMed] [Google Scholar]
- 39.Watanabe H., Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages. Journal of Experimental Medicine. 1987;165:628–640. doi: 10.1084/jem.165.3.628. 2880923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannai S., Sato H., Ishii T., Sugita Y. Induction of cystine transport activity in human fibroblasts by oxygen. Journal of Biological Chemistry. 1989;264:18480–18484. 2808385 [PubMed] [Google Scholar]
- 41.Sato H., Nomura S., Maebara K., Sato K., Tamba M., Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochemical and Biophysical Research Communications. 2004;325:109–116. doi: 10.1016/j.bbrc.2004.10.009. 15522208 [DOI] [PubMed] [Google Scholar]
- 42.Ogawa Y., Saito Y., Nishio K., Yoshida Y., Ashida H., Niki E. ?-Tocopheryl quinone, not a-tocopheryl quinone, induces adaptive response through up-regulation of cellular glutathione and cysteine availability via activation of ATF4. Free Radical Research. 2008;42:674–687. doi: 10.1080/10715760802277396. 18654882 [DOI] [PubMed] [Google Scholar]
- 43.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and Biophysical Research Communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. 9240432 [DOI] [PubMed] [Google Scholar]
- 44.Ishii T., Warabi E., Siow R.C., Mann G.E. Sequestosome1/p62:a regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radical Biology and Medicine. 2013;65:102–116. doi: 10.1016/j.freeradbiomed.2013.06.019. 23792273 [DOI] [PubMed] [Google Scholar]
- 45.Moscat J., Diaz-Meco M.T., Wooten M.W. Signal integration and diversification through the p62 scaffold protein. Trends in Biochemical Sciences. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. 17174552 [DOI] [PubMed] [Google Scholar]
- 46.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. 12193649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapple S.J., Cheng X., Mann G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biology. 2013;1:319–331. doi: 10.1016/j.redox.2013.04.001. 24024167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li A.C., Glass C.K. The macrophage foam cell as a target for therapeutic intervention. Nature Medicine. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. 12411950 [DOI] [PubMed] [Google Scholar]
- 49.Glass C.K., Witztum J.L. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. 11239408 [DOI] [PubMed] [Google Scholar]
- 50.Sato H., Takenaka Y., Fujiwara K., Yamaguchi M., Abe K., Bannai S. Increase in cystine transport activity and glutathione level in mouse peritoneal macrophages exposed to oxidized low-density lipoprotein. Biochemical and Biophysical Research Communications. 1995;215:154–159. doi: 10.1006/bbrc.1995.2446. 7575584 [DOI] [PubMed] [Google Scholar]
- 51.Siow R.C., Sato H., Leake D.S., Pearson J.D., Bannai S., Mann G.E. Vitamin C protects human arterial smooth muscle cells against atherogenic lipoproteins: Effects of antioxidant vitamins C and E on oxidized LDL-induced adaptive increase in cystine transport and glutathione. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1662–1670. doi: 10.1161/01.atv.18.10.1662. [DOI] [PubMed] [Google Scholar]
- 52.Moellering D.R., Levonen A.L., Go Y.M., Patel R.P., Dickinson D.A., Forman H.J., Darley-Usmar V.M. Induction of glutathione synthesis by oxidized low-density lipoprotein and 1-palmitoyl-2-arachidonyl phosphatidylcholine: protection against quinone-mediated oxidative stress. Biochemical Journal. 2002;362:51–59. doi: 10.1042/0264-6021:3620051. 11829739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bea F., Hudson F.N., Chait A., Kavanagh T.J., Rosenfeld M.E. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circulation Research. 2003;92:386–393. doi: 10.1161/01.RES.0000059561.65545.16. 12600891 [DOI] [PubMed] [Google Scholar]
- 54.Maruyama A., Tsukamoto S., Nishikawa K., Yoshida A., Harada N., Motojima K., Ishii T., Nakane A., Yamamoto M., Itoh K. Nrf2 regulates the alternative first exons of CD36 in macrophages through specific antioxidant response elements. Archives of Biochemistry and Biophysics. 2008;477:139–145. doi: 10.1016/j.abb.2008.06.004. 18585365 [DOI] [PubMed] [Google Scholar]
- 55.Sussan T.E., Jun J., Thimmulappa R., Bedja D., Antero M., Gabrielson K.L., Polotsky V.Y., Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PloS One. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. 19023427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barajas B., Che N., Yin F., Rowshanrad A., Orozco L.D., Gong K.W., Wang X., Castellani L.W., Reue K., Lusis A.J., Araijo J.A. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. 20947826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto K., Hirano K., Nozaki S., Takamoto A., Nishida M., Nakagawa-Toyama Y., Janabi M.Y., Ohya T., Yamashita S., Matsuzawa Y. Expression of macrophage (Mphi) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-gamma, which regulates gain of Mphi-like phenotype in vitro, and its implication in atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1027–1032. doi: 10.1161/01.atv.20.4.1027. 10764668 [DOI] [PubMed] [Google Scholar]
- 58.Alary J., Gueraud F., Cravedi J.P. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Molecular Aspects of Medicine. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. 12892995 [DOI] [PubMed] [Google Scholar]
- 59.Mehrasa R., Vaziri H., Oodi A., Khorshidfar M., Golpour M., Amirizadeh N. Mesenchymal stem cells as a feeder layer can prevent apoptosis of expanded hematopoietic stem cells derived from cord blood. International Journal of Molecular and Cellular Medicine. 2014;3:1–10. 24551815 [PMC free article] [PubMed] [Google Scholar]
- 60.NIH home page stem cell information. http://stemcells.nih.gov/research/nihresearch/scunit/pages/feederfree.aspx.
- 61.Woodbury M.E., Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2014;9(2):92–101. doi: 10.1007/s11481-013-9501-5. 24057103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M.H., Tang Z.H., Li G.H., Qu S.L., Zhang Y., Ren Z., Liu L.S., Jiang Z.S. Janus-like role of fibroblast growth factor 2 in arteriosclerotic coronary artery disease: atherogenesis and angiogenesis. Atherosclerosis. 2013;229:10–17. doi: 10.1016/j.atherosclerosis.2013.03.013. 23578358 [DOI] [PubMed] [Google Scholar]
- 63.Haley E.M., Kim Y. The role of basic fibroblast growth factor in glioblastoma multiforme and glioblastoma stem cells and in their in vitro culture. Cancer Letters. 2014;346:1–5. doi: 10.1016/j.canlet.2013.12.003. 24333730 [DOI] [PubMed] [Google Scholar]
- 64.Malabanan K.P., Kanellakis P., Bobik A., Khachigian L.M. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circulation Research. 2008;103:378–387. doi: 10.1161/CIRCRESAHA.107.168682. 18617696 [DOI] [PubMed] [Google Scholar]
- 65.Fei Y., Xiao L., Hurley M.M. Fibroblast growth factor 2 positively regulates expression of activating transcription factor 4 in osteoblasts. Biochemical and Biophysical Research Communications. 2010;391:335–339. doi: 10.1016/j.bbrc.2009.11.059. 19913500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou J.G., Cohen G., Mytilineou C. Basic fibroblast growth factor stimulation of glial cells protects dopamine neurons from 6-hydroxydopamine toxicity: involvement of the glutathione system. Journal of Neurochemistry. 1997;69:76–83. doi: 10.1046/j.1471-4159.1997.69010076.x. 9202296 [DOI] [PubMed] [Google Scholar]
- 67.Gualtieri A.F., Mazzone G.L., Rey R.A., Schteingart H.F. FSH and bFGF stimulate the production of glutathione in cultured rat Sertoli cells. International Journal of Andrology. 2009;32:218–225. doi: 10.1111/j.1365-2605.2007.00836.x. 18042181 [DOI] [PubMed] [Google Scholar]
- 68.Liu X., Albano R., Lobner D. FGF-2 induces neuronal death through upregulation of system xc- Brain Research. 2014;1547:25–33. doi: 10.1016/j.brainres.2013.12.018. 24374066 [DOI] [PubMed] [Google Scholar]


