SUMMARY
RIG-I activates interferon signaling pathways by promoting filament formation of the adaptor molecule, MAVS. Assembly of the MAVS filament is mediated by its CARD domain (CARDMAVS) and requires its interaction with the tandem CARDs of RIG-I (2CARDRIG-I). However, the precise nature of the interaction between 2CARDRIG-I and CARDMAVS, and how this interaction leads to CARDMAVS filament assembly has been unclear. Here we report a 3.6 Å electron microscopy structure of the CARDMAVS filament and a 3.4 Å crystal structure of the 2CARDRIG-I:CARDMAVS complex, representing 2CARDRIG-I “caught in the act” of nucleating the CARDMAVS filament. These structures, together with functional analyses, show that 2CARDRIG-I acts as a template for the CARDMAVS filament assembly, by forming a helical tetrameric structure and recruiting CARDMAVS along its helical trajectory. Our work thus reveals that signal activation by RIG-I occurs by imprinting its helical assembly architecture on MAVS, a previously uncharacterized mechanism of signal transmission.
INTRODUCTION
RIG-I and MDA5 represent a conserved family of the vertebrate innate immune receptors that detect viral RNAs at the early stage of infection and elicit the antiviral immune response by transcriptional up-regulation of type I interferons (IFNs) (Pichlmair and Reis e Sousa, 2007; Takeuchi and Akira, 2009). RIG-I and MDA5 are expressed by a wide range of tissues and are functional in the cytoplasm. They also share the same domain architecture, consisting of an N-terminal tandem caspase activation recruitment domain (2CARD) that is responsible for signal activation, and a central DExD/H motif helicase domain and C-terminal domain (CTD), which together function as a RNA recognition unit (Yoneyama and Fujita, 2008). Despite the shared domain architecture and the common downstream signaling pathways, RIG-I and MDA5 play non-redundant roles by recognizing largely distinct groups of viruses through distinct RNA specificity (Kato et al., 2011; Wilkins and Gale, 2010).
Structural and biochemical analysis of RIG-I and MDA5 from our laboratory and others have revealed how these receptors utilize the common domain architecture to recognize distinct groups of viral RNAs (Jiang et al., 2011; Kowalinski et al., 2011; Luo et al., 2011; Wu et al., 2013). We have shown that while RIG-I binds to dsRNA ends as a monomer in the absence of ATP, it forms short filamentous oligomers near a dsRNA end during ATP hydrolysis (Peisley et al., 2013). This oligomer assembly allows clustering of 2CARD for its own oligomerization and, at the same time, enables recognition of viral signatures at dsRNA ends (such as 5′-triphosphate) as well as the overall dsRNA length (Patel et al., 2013; Peisley et al., 2013). By contrast, MDA5 binds to dsRNA in a highly cooperative manner, forming long filaments on dsRNA in the absence of ATP (Berke et al., 2012; Peisley et al., 2011; Wu et al., 2013). ATP hydrolysis however triggers rapid filament end-disassembly, which enables MDA5 to regulate its filament stability and the signaling activity according to the length of dsRNA over a wide range (Kato et al., 2008; Peisley et al., 2012).
In stark contrast to the detailed understanding of how RIG-I and MDA5 recognize viral RNAs, the molecular mechanisms by which these receptors activate antiviral signaling pathway and how they interact with the downstream adaptor molecule, MAVS, have been poorly understood. In the absence of viral infection, 2CARD of RIG-I (and possibly MDA5) is in the auto-suppressed state by forming an intramolecular interaction with the helicase domain (Kowalinski et al., 2011). This interaction is presumably released upon RNA binding as 2CARD competes with RNA for the same binding site in the helicase domain (Kowalinski et al., 2011). The released 2CARD of RIG-I (2CARDRIG-I) then forms a homo-tetramer, upon binding to K63-linked polyubiquitin chains (K63-Ubn) (Jiang et al., 2012) and/or when brought into proximity upon RIG-I filament formation (Peisley et al., 2013). Our recent crystal structure of 2CARDRIG-I in complex with K63-Ub2 revealed a core tetrameric structure of 2CARDRIG-I encircled and stabilized by K63-Ub2, explaining how K63-Ubn promotes 2CARD tetramerization (Peisley et al., 2014). The 2CARDRIG-I tetramer then interacts with the single CARD of MAVS (CARDMAVS), and stimulates formation of self-perpetuating filaments of CARDMAVS on mitochondria (Hou et al., 2011). MAVS filament in turn activates the IFNα/β signaling pathway by recruiting further downstream signaling molecules, such as TRAF2, 5 and 6 (Liu et al., 2013). The importance of MAVS filament formation in its cellular function has been further demonstrated by a strong correlation between filament formation in vitro and signaling activity in cells (Peisley et al., 2014; Peisley et al., 2013), and the sufficiency of CARDMAVS filament seed to induce filament formation by full-length MAVS and subsequent activation of downstream signaling pathway (Hou et al., 2011).
MAVS filament nucleation by RIG-I and MDA5 thus represents the first inter-molecular signal propagation event in the signaling pathway of these receptors. A few models have been proposed to explain how RIG-I and MDA5 trigger MAVS filament nucleation (Berke et al., 2013; Peisley et al., 2014; Rawling and Pyle, 2014; Xu et al., 2014), but the challenge commonly associated with structural investigation of the nucleator:filament interaction has thus far prevented mechanistic understanding of this key signal transduction event. We here report our 3.6 Å EM structure of the CARDMAVS filament and 3.4 Å crystal structure of the 2CARDRIG-I:CARDMAVS complex obtained through a novel protein-engineering approach. Our CARDMAVS filament structure is vastly different from recently published 9.6 Å model of the CARDMAVS filament (Xu et al., 2014). Our structures, together with functional analyses, reveal previously uncharacterized signal activation mechanism of RIG-I and MAVS.
RESULTS
EM reconstruction of the CARDMAVS filament
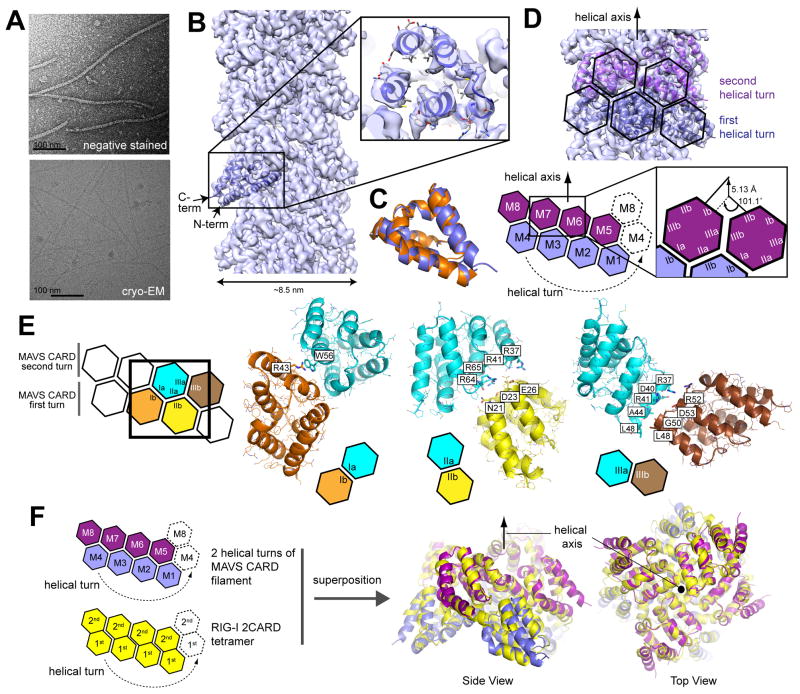
CARDMAVS is both necessary and sufficient to form the MAVS filament (Hou et al., 2011). To understand the structural organization of the CARDMAVS filament, we first generated filaments using isolated CARDMAVS with 19 additional amino acids (aa) at the N-terminus and the P2A mutation (Figures 1A & S1A). This modification improved solubility of the CARDMAVS filament without affecting the cellular signaling activity in full-length MAVS (Figure S1B). The average power spectrum of the 31.7 nm long overlapping filament segments indicated that the CARDMAVS filament exhibits a helical symmetry of a twist angle of 101.1 and a axial rise of 5.13 Å (Figure S1D). Using these parameters and a helical geometrically constrained reconstruction approach (Supplemental Method), we reconstructed the electron density map of the CARDMAVS filament (Figure 1B). Final map has an overall resolution of 3.6 Å based on the Fourier shell correlation (FSC) = 0.5 criterion (Figure S1E).
Figure 1. MAVS CARD forms a left-handed single-stranded filament using a common helical symmetry shared with the RIG-I 2CARD tetramer.
A. Representative electron micrographs of negatively stained and cryo CARDMAVS filaments.
B. Reconstructed EM density map of the CARDMAVS filament and monomeric CARDMAVS (PDB: 2VGQ) docked into the map (using the program Chimera). The N- and C-termini (residues 1 and 97) face the periphery of the filament and are indicated by arrows. The density for the 19 aa extension at the N-terminus was unresolved, suggesting its flexibility.
C. Superposition of the structures of CARDMAVS before refinement (PDB: 2VGQ, orange) and after refinement (slate).
D. A model of the CARDMAVS filament, showing two helical turns. Shown below is the 2D representation of the helical assembly. The CARDMAVS filament displays a left-handed single-stranded structure with a twist angle of 101.1 and a rise of 5.13 Å. Each CARDMAVS interacts with six nearest neighbors: two neighbors along the helical trajectory through the IIIa:IIIb interaction (intra-strand interaction), and two neighbors each through the Ia:Ib and IIa:IIb interactions between adjacent helical turns (inter-strand interaction). See also Figure S1F.
E. Details of the Ia:Ib, IIa:IIb and IIIa:IIIb interactions. Residues at the interface are shown in stick models. Color code was chosen to simplify the comparison with Figure 5A.
F. Superposition of the two helical turns of the CARDMAVS filament from (D) and the helical tetramer of 2CARDRIG-I (PDB: 4NQK).
We first performed rigid-body docking of monomeric CARDMAVS (Potter et al., 2008) to the reconstructed EM map, followed by structural refinement of individual side chains using a crystallographic refinement program (Table 1). High resolution of the density map not only enabled unambiguous identification of the orientation of individual CARDs within the filament, but also correctly predicted many of the bulky side chains (Figure 1B). The final model after refinement showed a nearly identical conformation as the monomeric CARDMAVS (Figure 1C), suggesting that the filament is formed through rigid-body assembly. Cross-beta structure commonly observed in amyloid fibers was absent, consistent with the previous report that the MAVS filament is not stained with Congo Red (Hou et al., 2011).
Table 1.
Refinement statistics of the CARDMAVS filament
| Statistics for CARDMAVS filament model and pseudo-crystallographic refinement | |
|---|---|
| Cell dimensionsa | |
| a, b, c (Å) | 105.6, 105.6, 88.0 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Resolution (Å) | 99–3.64 |
| No. reflections | 33916 (2900) |
| Rworkb | 23.8% |
| Rfreec | 27.6% |
| No. of protein atoms | 6344 |
| Average B-factors | 136.08 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 1.257 |
| Dihedral angle (°) | 14.668 |
| Ramachandran Plot | |
| Outliers | 1.2% |
| Allowed | 98.8% |
| Favored | 95.0% |
Arbitrary cell dimensions were chosen to cover the two helical turns of the CARDMAVS filament
R=Σh||F(h)obs|−|F(h)calc||/Σh|F(h)|
Rfree was calculated for 5.1% of reflections randomly excluded from the refinement
Each CARDMAVS interacts with six nearest neighbors using six distinct surface areas (Ia/b, IIa/b and IIIa/b) forming three types of intermolecular interactions (Ia:Ib, IIa:IIb and IIIa:IIIb) (Figure 1D). These interactions resemble those previously identified from the 2CARDRIG-I tetramer (Peisley et al., 2014) and oligomers of other Death Domains (DDs) (Ferrao and Wu, 2012). Each CARDMAVS interacts with the neighboring units in the identical manner regardless of its position within the filament. Thus, the CARDMAVS filament is best described as a single-stranded left-handed helix (Figure 1D), in which IIIa:IIIb constitutes the intra-strand interaction whereas Ia:Ib and IIa:IIb form the inter-strand interactions (Figures S1F & 1E). Although detailed nature of some of these interactions could not be clearly defined due to limited resolution of the map, survey of residues at the interface indicates that inter-CARD interactions are mediated by a combination of electrostatic and hydrophobic interactions (Figure 1E).
Interestingly, our previous crystal structure of the 2CARDRIG-I tetramer also displayed a single-stranded left-handed helical arrangement with similar twist and rise values (Peisley et al., 2014). In this structure, the 1st and 2nd CARDs of 2CARDRIG-Iare tightly joined together through the IIa:IIb interaction, and the resultant 2CARD are stacked together in a way that the first helical turn of the 1st CARD is extended by the second helical turn of the 2ndCARD (Figure 1F). To compare the helical assembly of the CARDMAVS filament and the 2CARDRIG-I tetramer, we superposed the two helical turns of the CARDMAVS filament onto the 2CARDRIG-I tetramer structure (Figure 1F). The two structures aligned with remarkable agreement, suggesting that the two assembly structures share the same helical geometry. This observation prompted us to hypothesize that that 2CARDRIG-I tetramer triggers the CARDMAVS filament formation by serving as a helical template (to be discussed further in Figure 3).
Figure 3. Protein engineering strategies for crystallization of the 2CARDRIG-I:CARDMAVS complex.
A. 2CARDRIG-I with E52A (filled circle) was fused to CARDMAVS with the triple mutation, E23K/E26K/E80K (KKK, open circles). Surface locations of the mutations were indicated in the 2D representation. See Figure S3 for detailed characterization of these mutants.
B. Analysis of the oligomeric state of the fusion construct in (A) by Multi-Angle Light Scattering (MALS) coupled to Size Exclusion Chromatography (SEC) using a Superdex200 column. MALS data indicate that the peaks eluted at ~12 ml and ~14.5 ml correspond to the tetramer and monomer, respectively. Shown on the bottom right corner is the SDS-PAGE analysis (Coomassie stained) of the fusion constructs.
C. SEC trace of the fusion constructs (16 aa linker) with E137A/E138A (green circle, mut1) on 2CARDRIG-I and R37A/R41A (brown circle, mut2) on CARDMAVS, using a Superdex200 column. Both mut1 and mut2 fusion proteins elute at ~14.5 ml, suggesting that these mutants exist as monomers. Inset: SDS-PAGE analysis of the mut1 and mut2 fusion proteins.
D. MAVS stimulatory activity of the fusion constructs. Monomer-to-filament transition of fluorescently labeled CARDMAVS-S was monitored by native gel assay. CARDMAVS-S was incubated with wild-type or E52A 2CARDRIG-I or fusion constructs in the presence or absence of K63-Ubn.
Charge-complementary mutations support the CARDMAVS filament structure
One way to test the functionality of the MAVS filament model is to examine the effect of mutations in the interface on filament formation in vitro and signaling activity in cells. Surface residues involved in each of the interactions revealed in our structure closely matched those previously identified to be important in our screens and others (Peisley et al., 2014; Peisley et al., 2013; Xu et al., 2014) (Table S1). However, the large surface areas involved in the interface makes such a loss-of-function mutation analysis insufficient to validate the structure (Egelman, 2010). In fact, the same loss-of-function mutation data in Table S1 is consistent with both our filament structure and the one recently proposed (Xu et al., 2014). Note that the latter differs significantly from our structure in both overall architecture (left-handed, 3-stranded) as well as local inter-CARD interactions (Figure S2B). Considering the similarity between the power spectra of our filaments and Xu et al.’s, this large discrepancy in the final models most likely arose from the use of incorrect helical symmetry in their reconstruction, rather than the intrinsic difference in the filament samples. The validity of our helical symmetry can be seen at higher resolution by the visualization of the protein α-helical secondary structure with correct handedness, which is absent in Xu et al.’s reconstruction.
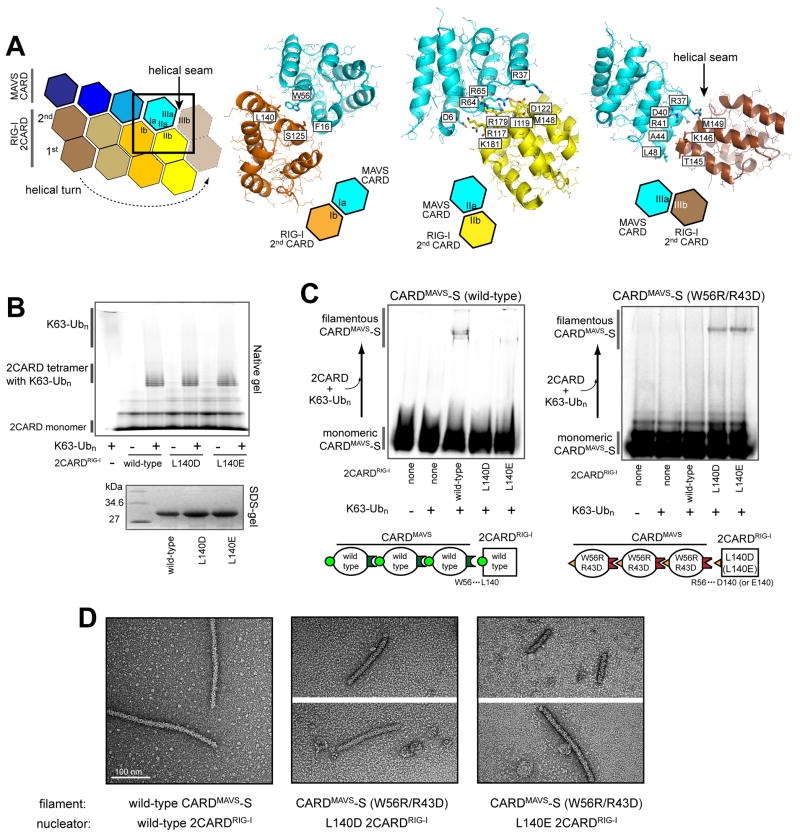
For more rigorous validation of our structural model, we employed a charge-complementary mutagenesis approach, where filament formation was monitored upon mutation of either one or both of the interacting residues into complementary amino acids. We chose to investigate W56 and R43 in the Ia/Ib interface as these residues were identified to be important from both our functional assays and Xu et al.’s (Table S1), and the Ia/Ib interface is simpler than IIa/IIb and IIIa/IIIb with a clear density map supporting a direct interaction between W56 and R43 (Figure 2A). Finally, unlike our structure, W56 and R43 are far apart in Xu et al.’s (Figure S2B), further providing an opportunity to distinguish between our model and Xu et al’s.
Figure 2. Charge-complementary mutagenesis supports the CARDMAVS filament reconstruction.
A. The CARDMAVS filament model and the EM map, showing the inter-CARDMAVS interface. See Figure 1E for the color code. Shown on the right is the magnified view of the type I interface, unambiguously showing that W56 and R43 form a direct contact, likely involving a pi:cation interaction.
B. Effect of charge-complementary mutations on filament formation of CARDMAVS, as measured by native gel analysis. CARDMAVS was fused to a fluorescent tag, SNAP, (CARDMAVS-S), which was conjugated with Alexa647 for gel imaging. Shown on the right are pictorial diagrams that illustrate the effect of charge-complementary mutations. See also Figures S2C–D.
C. EM images of the samples in (B). Wild-type CARDMAVS filaments are shown at 52K and 21K magnifications for clear visualization of the filamentous structure.
D. Filament seeding assays to test the cross-reactivity between wild-type and the double mutants W56R/R43D and W56R/R43E. Monomeric CARDMAVS-S was prepared by either refolding of the filament (for wild-type) or fractionation by Size Exclusion Chromatography (SEC). Purified monomer was fluorescently labeled using Alexa647, and mixed with unlabeled seed filaments (sample from (B)) at a monomer to seed mass ratio of 20:1.
In our previous mutagenesis analyses of MAVS (Peisley et al., 2014; Peisley et al., 2013), the intrinsic ability of CARDMAVS to form filaments in vitro and E. coli showed a strong correlation with signaling activity in human 293T cells (Table S1). In these experiments, we have used CARDMAVS fused to a fluorescent tag, SNAP (CARDMAVS-S), which enables us to monitor its filament formation and extension by native gels as well as EM. As expected, single point mutations, W56R, R43D and R43E, completely abrogated filament formation by CARDMAVS-S (Figures 2B–C). However, the double mutations W56R/R43D and W56R/R43E restored the filament formation activity of CARDMAVS-S, albeit at lower efficiency than wild-type (Figure 2B). While the W56R/R43D and W56R/R43E filaments showed a similar thickness as the wild-type filament, they were significantly longer, which likely reflects less efficient nucleation of the mutant filaments, consistent with the fewer number of filaments. While filaments of wild-type and the double mutant CARDMAVS-S can be rapidly extended by the monomer of the same type, they could not cross-seed each other (Figure 2D), consistent with the notion that the charge complementarity between residues 56 and 43 are required for filament formation. Together, these results suggest that W56 and R43 form a direct interaction within the MAVS filament, which is consistent with our MAVS filament model, but not Xu et al.’s.
Analysis of CARDMAVS-S from duck showed that they also form filaments (Figure S2E), suggesting that filament formation is a feature of MAVS that is conserved across species. Sequence alignment showed, however, that residues at the inter-CARD interface, in particular W56 and R43, in human are poorly conserved in duck (Figure S2A). Despite the lack of conservation, mutation of Y56 or H43 in duck MAVS, which correspond to W56 and R43 in human MAVS, abrogated its filament formation in vitro and IFNβ signaling in chicken fibroblast cell line, DF-1 (Figures S2E–F). The charge-complementary mutation Y56R and H43E partially restored its ability to form filaments (Figure S2E). Although the double mutant filaments appeared more curvy than the wild-type, likely reflecting less optimal surface compatibility of the mutant, the complete abrogation of filament formation by either of the single mutations and rescue of filament formation by the double mutation supports the notion that residues 56 and 43 interact with each other in duck MAVS filament, as well as in human MAVS filament.
Protein engineering strategies for crystallization of the 2CARDRIG-I:CARDMAVS complex
To understand precisely how the 2CARDRIG-I tetramer initiates the CARDMAVS filament formation, we developed strategies to crystallize the 2CARDRIG-I and CARDMAVS complex. As crystallization of polymerizing proteins, such as CARDMAVS, is challenging, we employed a series of protein engineering approaches, which led to identification of the fusion construct (Figure 3A) that formed a homogeneous tetramer (Figure 3B) and produced well-diffracting crystals.
From our initial screens of multiple surface mutants of both 2CARDRIG-I and CARDMAVS, we identified two candidate mutants, E52A in 2CARDRIG-I and D23K/E26K/E80K (KKK) in CARDMAVS. E52A resides on the bottom surface of the 2CARD tetramer (surface IIa of the 1stCARD, Figure 3A) and improved solubility and the tetramer stability without interfering with IFN signaling in cells (Figures S3A–B). The KKK residues, on the other hand, reside on the top surface of the filament (surface IIb, Figure 3A) and are involved only in the vertical inter-strand interaction, not in formation of the single helical turn. Accordingly, the KKK mutant of CARDMAVS did not form long filaments, but formed oligomers of heterogeneous size (Figure S3C). MAVS with the KKK mutation retained a moderate level of signaling activity in cells (Figure S3A), suggesting that the small oligomeric structure formed by the mutant likely recapitulates the functional form of the CARDMAVS filament.
We hypothesized that fusion of CARDMAVS to 2CARDRIG-I would restrict the stoichiometry of the 2CARD:CARD interaction to a 1:1 molar ratio, and would prevent oligomerization of CARDMAVS beyond a tetramer. The covalent fusion of the two would also stabilize the 2CARD:CARD interaction, whose transient nature has thus far prohibited its detailed biochemical analysis. Consistent with our prediction, the 2CARDRIG-I(E52A)-CARDMAVS(KKK) fusion protein formed a tetramer but not larger oligomers, and shortening of the linker between 2CARDRIG-I and CARDMAVS from 42 to 16 amino acids (aa) further promoted its tetramerization (Figure 3B). Unlike isolated 2CARDRIG-I (Figure S3B), the tetramer formation of the fusion protein did not require K63-Ubn. Instead, it depended on the intact surface III of CARDMAVS as well as 2CARDRIG-I (Figure 3C), suggesting that homo-oligomerization of both CARDMAVS and 2CARDRIG-I cooperate to drive tetramer formation of the fusion construct.
Note that the 42 aa linker between 2CARDRIG-I and CARDMAVS is long enough (~13 nm, in comparison to 5 nm long 2CARD) so that it alone would not impose geometric restraints to the conjugated proteins. Thus, the impact of the 2CARDRIG-I(E52A) fusion on blocking CARDMAVS(KKK) from forming oligomers beyond a tetramer suggests that 2CARDRIG-I(E52A) forms a specific interaction with CARDMAVS(KKK). Consistent with this interpretation, the 2CARDRIG-I(E52A)-CARDMAVS(KKK) fusion protein did not stimulate filament formation of wild-type CARDMAVS-S regardless of the linker length or K63-Ubn (Figure 3D). This is unlike isolated 2CARDRIG-I(E52A), which robustly stimulates the monomer-to-filament transition of CARDMAVS-S in the presence of K63-Ubn, and suggests that 2CARDRIG-I(E52A) in the fusion construct interacts with CARDMAVS(KKK) using the surface that is otherwise involved in stimulating wild-type CARDMAVS-S.
Our initial effort to crystallize the 2CARDRIG-I(E52A)-CARDMAVS(KKK) tetramer with the 16 aa linker produced small crystals. The crystal quality was dramatically improved upon addition of mono-Ub, which did not bind the 2CARD-CARD tetramer in solution, but acted as a crystallographic packing reagent (Figure S4F). The structure was determined by molecular replacement, and refined to 3.4 Å at Rwork and Rfree of 19.1% and 23.9%, respectively (Table 2).
Table 2.
Data collection and refinement statistics of the 2CARDRIG-I:CARDMAVS complex structure
| Crystal 1 | |
|---|---|
| Data collection | |
| Space group | P21 21 21 |
| Cell dimensions | |
| a, b, c (Å) | 111.8, 117.3, 257.5 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Resolution (Å) | 46.8–3.4 (3.64–3.40)* |
| Rsym or Rmerge | 0.16 (1.32) |
| I/σI | 8.28 (1.26) |
| Completeness (%) | 99.1 (97.8) |
| Redundancy | 3.3 (3.2) |
| Refinement | |
| Resolution (Å) | 46.8–3.40 (3.44–3.40) |
| No. reflections | 89450 (3005) |
| Rwork/Rfree | 0.19 (0.32)/0.24 (0.33) |
| No. atoms | |
| Protein | 43375 |
| Ligand/ion | |
| Water | |
| B-factors | |
| Protein | 104.09 |
| Ligand/ion | |
| Water | |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.011 |
| Bond angles (°) | 1.268 |
| Ramachandran Plot | |
| Outliers | 0.5% |
| Allowed | 99.5% |
| Favored | 96.3% |
Values in parentheses are for highest-resolution shell.
Crystal structure of the 2CARDRIG-I:CARDMAVS complex
The crystal structure revealed that CARDMAVS is stacked on top of the 2ndCARD RIG-I through IIa:IIb interactions, and that the four copies of the 2CARD-CARD fusion protein form a helical tetramer (Figures 4A–B). The electron density map for the linker between 2CARDRIG-I and CARDMAVS was poorly defined, suggesting flexibility in the linker. The identical tetrameric architecture of 2CARD-CARD was observed in two independent copies in the asymmetric unit (Figures S4A–B), which supports that the crystal structure represents their native assembly architecture in solution. The two copies of the tetramer in the crystal lattice are stacked bottom-to-bottom, using the Ia, IIa and IIIa surface areas of the 1stCARD RIG-I (Figure S4A). The functional implication of this stacking is unclear as the tetramer formation of 2CARDRIG-I is sufficient to stimulate MAVS (Peisley et al., 2014), and mutations of the bottom (IIa) of the 1stCARD RIG-I have little impact on the signaling activity of RIG-I (Peisley et al., 2014). As expected from the requirement for mono-Ub in crystallization, two or three Ub’s occupy previously identified Ub-binding sites (Peisley et al., 2014) on each of the 2CARDRIG-I tetramers, and mediate crystallographic contacts with adjacent complexes (Figures S4E–F). We here limit our discussion to the inter-CARDMAVS or RIG-I interactions, as 2CARDRIG-I:Ub interactions were previously discussed (Peisley et al., 2014).
Figure 4. Crystal structure of the 2CARDRIG-I:CARDMAVS complex reveals how the 2CARDRIG-I tetramer nucleates the CARDMAVS filament.
A. 2D representation of the helical architecture of the 2CARDRIG-I:CARDMAVS complex. The mutations E52A and E23K/E26K/E80K are represented by filled and open circles, respectively. On the right is the schematic model of the helical architecture of the complex. The same color code is used throughout Figure 4. See also Figure S4.
B. Structure of the 2CARDRIG-I:CARDMAVS complex shown in ribbon (left) and surface (right) representations. The N- and C-termini of 2CARDRIG-I (residues 1 and 188) and CARDMAVS (residues 1 and 97) face the periphery of the helical assembly architecture and are indicated by arrows.
C. Three helical turns in the 2CARDRIG-I:CARDMAVS complex, looking down the helical axis. The first helical turn formed by the 1stCARD RIG-I (bottom) is extended by the second helical turn formed by the 2nd CARDRIG-I (middle) and the third helical turn formed by the CARDMAVS (top). The arrows indicate the angular relationship (twist) between the adjacent CARDs.
D. A model of the CARDMAVS filament nucleated by the 2CARDRIG-I tetramer. The EM structure of the CARDMAVS filament (slate) was stacked on top of the crystal structure of the 2CARDRIG-I:CARDMAVS complex, showing that the helical trajectory of the 2CARDRIG-I:CARDMAVS complex can be further extended to support the CARDMAVS filament propagation. Shown on the right are the 2D (top) and 3D (bottom) schematic models.
Comparison of the structure of the 2CARDRIG-I tetramer within the 2CARDRIG-I:CARDMAVS complex and that formed in complex with K63-Ub2 without CARDMAVS (Peisley et al., 2014) showed that their structures are identical (Figure S5A, bottom panel). Similarly, the CARDMAVS tetramer in the 2CARDRIG-I:CARDMAVS complex also showed little difference from the single helical turn of CARDMAVS in the CARDMAVS filament (Figure S5A, top panel). These observations suggest that the covalent fusion of 2CARDRIG-I and CARDMAVS had little impact on their ability to homo-oligomerize. Interestingly, the two helical axes of the 2CARDRIG-I tetramer and the CARDMAVS tetramer coincide such that the first helical turn of the 1st CARDRIG-I, which is extended by the second helical turn of the 2ndCARD RIG-I, is further extended by the third helical turn of CARDMAVS without any noticeable discontinuity (Figure 4C). In further support of our notion that a single helical symmetry runs from the bottom most 1stCARD RIG-I (chain A in Figure 4A) all the way to the top most CARDMAVS (chain H in Figure 4A), superposition of the 2CARDRIG-I:CARDMAVS complex onto the 3-helical turns of our CARDMAVS filament model showed a remarkable match between the two structures (Figure S5B). Accordingly, the CARDMAVS filament structure can be stacked on top of the 2CARDRIG-I:CARDMAVS complex without introducing any discontinuity in the helical symmetry (Figure 4D). Altogether, these observations are consistent with the model that the 2CARDRIG-I tetramer nucleates the CARDMAVS filament by serving as a helical template that recruits individual CARDMAVS along the extended helical trajectory pre-defined by 2CARDRIG-I (Figure 4D).
Charge-complementary mutations support the 2CARDRIG-I:CARDMAVS complex structure
Examination of the 2CARDRIG-I:CARDMAVS interface shows that surfaces Ia, IIa and IIIa of CARDMAVS interact with Ib, IIb and IIIb of the 2ndCARD RIG-I, respectively, using a combination of hydrophobic and electrostatic residues (Figure 5A). This interaction mode between 2CARDRIG-I and CARDMAVS is consistent with our previous data (Peisley et al., 2014), showing that D122 and E178/R179 on the surface IIb of the 2ndCARD RIG-I are crucial for MAVS stimulation, but not for its homo-tetramerization. To further test whether our structure of the 2CARDRIG-I:CARDMAVS complex indeed recapitulates the functional state of 2CARDRIG-I nucleating the CARDMAVS filament, we have extended the charge complementary mutation analysis of MAVS in Figure 2 to RIG-I.
Figure 5. CARDMAVS interacts with 2CARDRIG-I as it does with CARDMAVS in the filament.
A. Details of the 2CARDRIG-I: CARDMAVS interactions. Residues at the interface are shown in stick models. See Figure 1E to compare with the CARDMAVS: CARDMAVS interaction.
B. Tetramerization of wild-type, L140D and L140E 2CARDRIG-I in the presence of K63-Ubn, as measured by a native gel analysis. The gel was Krypton-stained.
C. MAVS stimulatory activity of wild-type and charge-complementary mutant 2CARDRIG-I. Monomer-to-filament transition of wild-type CARDMAVS-S was stimulated by wild-type 2CARDRIG-I, but not by L140D or L140E. Conversely, monomer-to-filament transition of W56R/R43D CARDMAVS-S was stimulated by L140D and L140E 2CARDRIG-I, but not by wild-type 2CARDRIG-I. Shown below are pictorial diagrams that illustrate the effect of charge-complementary mutations.
D. Representative electron micrographs of the wild-type or W56R/R43D CARDMAVS-S filaments stimulated by wild-type, L140D or L140E 2CARDRIG-I.
Our structures indicate that W56 in CARDMAVS interacts with R43 in CARDMAVS within the filament (Figure 1E) and L140 in 2CARDRIG-I within the 2CARDRIG-I:CARDMAVS complex (Figure 5A). We asked whether W56R in CARDMAVS can interact with a charge-complementary mutant L140D (or L140E) in 2CARDRIG-I, as it did with R43D (or R43E) in CARDMAVS (Figure 2). If so, L140D (or L140E) 2CARDRIG-I would stimulate filament formation of CARDMAVS with the W56R/R43D mutation, but not wild-type CARDMAVS. Purified 2CARDRIG-I with L140D and L140E formed the tetramer as well as wild-type 2CARDRIG-I in the presence of K63-Ubn (Figure 5B). While L140D and L140E significantly reduced the ability of 2CARDRIG-I to stimulate wild-type CARDMAVS (Figure 5C), they conferred a new ability to stimulate filament formation of W56R/R43D CARDMAVS (Figure 5D). A residual stimulatory activity observed with L140E against wild-type CARDMAVS may reflect more hydrophobic nature of Glu than Asp due to its longer aliphatic side chain. This result suggests that W56 in CARDMAVS directly interacts with L140 in 2CARDRIG-I, and further supports our structure of the 2CARDRIG-I:CARDMAVS complex.
Plasticity of the 2CARDRIG-I:CARDMAVSinterface
Comparison of the 2CARDRIG-I:CARDMAVS and CARDMAVS:CARDMAVS interactions shows that the same residues in surfaces Ia, IIa and IIIa of CARDMAVS interact with Ib, IIb and IIIb of CARDMAVS within the MAVS filament (Figure 1E) and those of the 2nd CARDRIG-I in the 2CARD:CARD complex (Figure 5A). Sequence comparison between 2CARDRIG-I and CARDMAVS shows that, while CARDMAVS is more homologous to the 2nd CARDRIG-I than to the 1stCARD RIG-I, residues in Ib-IIIb of 2nd CARDRIG-I and CARDMAVS are dissimilar (with the exception of D122 in RIG-I, which corresponds to E26 in MAVS) (Figure 6A). These results suggest the plasticity of the CARDMAVS surface, which allows its interaction with at least two distinct combinations of surface residues.
Figure 6. Versatility in molecular interface between CARDMAVS and 2CARDRIG-I.
A. Structure-based sequence alignment between 2CARDRIG-I and CARDMAVS. The surface residues interacting with Ia-IIIa of CARDMAVS were highlighted yellow, and the surface types were indicated below (blue).
B. Superposition of duck 2CARDRIG-I (green, PDB:4A2W) onto human 2CARDRIG-I in the 2CARDRIG-I: CARDMAVS complex. E115 and T175/T176 (black spheres) are located in two loops (colored black on the right) in the 2CARDRIG-I: CARDMAVS interface, which show distinct conformations in human and duck 2CARDRIG-I. See also Figure S6A for sequence comparison.
C. IFNβ reporter assay of human and duck 2CARDRIG-I in 293T cells (mean ± SD, n=3). All 2CARDs are N-terminally fused to GST. See also Figure S6B.
D. Native gel analysis of tetramerization of wild-type and T175K/T176E 2CARDRIG-I from duck in the presence and absence of K63-Ubn (Krypton stain). SDS-PAGE analysis (Coomassie stain) are shown on the left.
E. Human MAVS stimulatory activity of wild-type and T175K/T176E duck 2CARDRIG-I. Monomer-to-filament transition of fluorescently labeled human CARDMAVS-S was monitored in the presence of duck 2CARDRIG-I and increasing concentrations of K63-Ubn. Shown on the right is a representative electron micrograph of the human CARDMAVS-S filaments stimulated by duck 2CARDRIG-I with T175K/T176E.
Sequence comparison among RIG-I orthologs shows the lack of strict conservation in the 2CARDRIG-I:CARDMAVS interface (Figure S6A). The sequence divergence is such that none of the residues on the Ib-IIIb surface of human 2CARDRIG-I are preserved in duck. Duck 2CARDRIG-I is inefficient in activating IFNβ signaling pathway in the human cell 293T (Figure 6C), and cannot stimulate filament formation of human MAVS in vitro (Figure 6E). Comparison of the structures of duck and human 2CARDRIG-I suggests that while the overall conformation of 2CARD is well conserved (Figure 6B, Cα RMSD of 0.8 Å), two loops in IIb slightly differ in their backbone geometry (Figure 6B). In our effort to understand how human MAVS discriminates between human and duck 2CARDRIG-I, we have mutated E115 and T175/T176 in these loops in duck 2CARDRIG-I to mimic human 2CARDRIG-I. A replacement of E115 by T and insertion of R (E115TR) as in human RIG-I did not increase the signaling activity of duck 2CARDRIG-I in 293T cells. However, replacement of T175/T176 by K175/E176 (as in human RIG-I) increased the signaling activity of duck 2CARDRIG-I in 293T cells, to the level comparable to human 2CARDRIG-I (Figure 6C). Consistently, our in vitro assay showed that duck 2CARDRIG-I with T175K/T176E, but not the wild-type, induced filament formation of human CARDMAVS (Figure 6E), while both T175K/T176E and wild-type can form tetramers in the presence of K63-Ubn (Figure 6D). Interestingly, T175K/T176E in duck RIG-I did not result in a compensatory loss of its signaling activity in chicken cells, DF-1 (Figure S6B), suggesting that the mutation leads to selective increase in its stimulatory activity against human MAVS without affecting its activity against chicken MAVS. Considering that the composition of the residues in the 2CARD:CARD interface remain significantly different between human and duck 2CARDRIG-I even after the T175K/T176E mutation, the newly acquired compatibility between duck 2CARDRIG-I and human CARDMAVS further highlights the remarkable plasticity of the 2CARD:CARD interface.
DISCUSSION
Mechanistic studies of filament nucleation processes often confront multiple challenges, including the difficulty of crystallizing a polymerizing protein and the intrinsic limitation of EM helical reconstruction in analyzing non-repeating elements, such as the nucleator. In the case of RIG-I and MAVS, the apparently transient interaction between 2CARDRIG-I and CARDMAVS has further defied previous studies to understand how 2CARDRIG-I, in particular its tetrameric form, triggers the CARDMAVS filament formation, the committed step in the IFNβ signaling pathway of RIG-I. The structures of the CARDMAVS filament and the 2CARDRIG-I:CARDMAVS complex reported in this manuscript thus reveal a long-sought-after mechanism of MAVS filament nucleation by RIG-I. We found that the helical tetrameric architecture of 2CARDRIG-I serves as a template that recruits individual CARDMAVS along the extended helical trajectory pre-defined by the 2CARDRIG-I tetramer (Figure 4D). As a result, the left-handed single-stranded helical architecture of the 2CARDRIG-I tetramer (with a helical pitch of a single CARD) is precisely preserved in the CARDMAVS filament (Figure 1F). As CARDMAVS is recruited to the composite surface on the 2CARDRIG-I tetramer formed by adjacent 2CARDRIG-I subunits, our findings thus explain the requirement for 2CARD tetramerization in MAVS activation and argue against a scenario where individual 2CARD:CARD interactions can stimulate MAVS filament formation. While only the 2nd CARDRIG-I forms a direct contact with MAVS, we have previously shown that the tight intramolecular interaction between the 1st and 2ndCARDs is required for stable tetramerization of 2CARDRIG-I (Peisley et al., 2014), which explains the observed requirement for both the 1st and 2ndCARDs for RIG-I to activate downstream signaling pathways (Gack et al., 2008).
A corollary of this nucleation mechanism is that CARDMAVS interacts with 2CARDRIG-I in the same manner as it does with other CARDsMAVS in the filament. The divergence between 2CARDRIG-I and CARDMAVS sequences, especially in residues interacting with a common surface on CARDsMAVS, indicates a remarkable plasticity in the CARD-CARD interactions utilized in the RIG-I-MAVS (and presumably MDA5-MAVS, Figures S6C–D) signaling pathway. It is possible that the apparently broad specificity reflects the sufficiency of transient (thus weak) interaction between 2CARDRIG-I and CARDMAVS for nucleating the MAVS filament. A transient interaction between 2CARDRIG-I and CARDMAVS may allow a small number of RIG-I molecules to nucleate multiple MAVS filaments, thus amplifying the antiviral signal, while a stable interaction could be restricted to nucleating a single MAVS filament. This raises an intriguing question of whether MAVS stimulation is limited to RIG-I and MDA5, or there could be other CARD-containing proteins that also stimulate MAVS filament formation. Understanding the precise nature of the CARD-CARD specificity for MAVS activation would require future investigation.
How do our findings on isolated CARDMAVS and 2CARDRIG-I translate to the signaling mechanism of full-length RIG-I/MDA5 and MAVS? We have previously shown that the RNA binding domains (helicase and CTD) of RIG-I and MDA5 form filamentous oligomers along dsRNA and utilize proximity-induced oligomerization mechanism (as well as ubiquitin-mediated mechanism for RIG-I) to assemble the 2CARD oligomers alongside the filament (Peisley et al., 2013; Wu et al., 2013) (Figure 7). Consistent with this notion, 2CARDRIG-I in its homo-tetrameric structure exposes its C-terminus to the periphery of the oligomer (Figure 4B), so that the linker (~50 aa) between 2CARD and the helicase domain could tether the 2CARD tetramer to the RIG-I core filament (Figure 7). The 2CARDRIG-I tetramer would then recruit individual MAVS CARDs by extending the helical trajectory of the 2ndCARD RIG-I, nucleating the self-perpetuating filament of CARDMAVS (Figure 7). As with 2CARDRIG-I, the C-terminus of CARDMAVS face outwards (Figure 1B), allowing formation of the CARDMAVS filament while being anchored to the mitochondrial outer-membrane via the ~400 aa long linker (Figure 7). Only upon CARDMAVS filament formation, MAVS can recruit TRAF molecules and activate the downstream signaling pathway (Hou et al., 2011), consistent with the known preference of TRAFs for oligomerized target proteins (Wu, 2004).
Figure 7. A model of signal activation by full-length RIG-I and MAVS.
Upon encountering viral dsRNA with 5′ ppp and blunt end, the RNA binding domain (helicase-CTD) of RIG-I forms filamentous oligomers along the length of dsRNA in an ATP-dependent manner (Peisley et al., 2013). This filament formation brings together 2CARDs into proximity, and induces tetramerization of 2CARD while being tethered to the core filament via a 50 aa flexible linker (Peisley et al., 2013). Tetramerization of 2CARD may also be induced by K63-Ubn, which stabilizes the 2CARD tetramer by bridging between adjacent 2CARDs and wrapping around the tetramer (Peisley et al., 2014). The 2CARD tetramer resembles the lock-washer (helical ring) structure, which serves as a helical template that nucleates the MAVS CARD filament. MAVS CARD is anchored to the mitochondrial outer membrane via a ~400 aa long linker, which contains the TRAF binding sites. Filament formation of MAVS CARD would bring together multiple TRAF binding sites into proximity, which appears to be important for efficient recruitment of TRAF molecules and activation of further downstream signaling pathway (Hou et al., 2011; Takamatsu et al., 2013).
Our findings not only provide unique insights into the signal activation mechanism of RIG-I and MAVS, but also implicate a novel type of signaling mechanism that could be potentially applicable to a broad range of innate immune receptors. These include AIM2 and NLRP3 in inflammasomes, which together with RIG-I are emerging as a new class of receptors that activate downstream immune response via filament nucleation of their respective adaptor molecules (Cai et al., 2014; Lu et al., 2014). Furthermore, the crystallization strategies developed in this work offers a new generalizable methodology to investigate filament nucleation mechanisms of other receptors.
EXPERIMENTAL PROCEDURES
Detailed experimental procedures are provided in Supplemental Information.
Cryo-Electron Microscopy and Helical Reconstruction of the MAVS CARD filament
Human CARDMAVS with an N-terminal 19 aa extension and P2A was purified in buffer A (20 mM Hepes pH 7.5, 150 mM NaCl) as described in Figure S1C. For cryo-EM, 3.5 μl of the CARDMAVS filament sample (0.2 mg/ml) was applied to glow-discharged Quantifoil R1.2/1.3 holy carbon grids (Quantifoil, Germany) and plunge-frozen using a Vitrobot Mark I (FEI). Data collection was performed on a Tecnai F20 electron microscope (FEI) operated at an acceleration voltage of 200 kV using a CT3500 cryo-specimen holder (Gatan) and the UCSF Image4 software (Li et al., 2013).
The SPARX software suite (Hohn et al., 2007) was used for all steps of image processing. Initially, we windowed segments 256 × 256 pixels with 75 pixels translation between them, yielding 12,817 segments. The average power spectrum showed an unambiguous layer lines pattern with a meridional reflection at 1/5.13 Å−1. Thus, the axial rise per asymmetric unit in the filament was 5.13 Å (Figure S1D). Indexing of the layer lines yielded an azimuthal rotation per subunit of 101.1° (Figure S1D). The helical reconstruction was performed using Helicon in SPARX. Using the established helical symmetry parameters the segments were automatically windowed from filaments using segment size of 256 × 256 pixels and 5 pixel gap between each segment. First, we performed five iterations of the ab initio structure determination, which converged rapidly yielding a filament structure, of which individual subunits unmistakably resembled the structure of the monomeric MAVS CARD (Potter et al., 2008). Therefore, we proceeded with twenty iterations of local refinement, during which progressively relaxed geometrical constrained due to helical symmetry and allowed out-of-plane tilts of segments (range 80 to 100 degrees). The resolution (3.6 Å) was estimated using the FSC = 0.5 criterion.
To create a model of the two helical turns of the CARDMAVS filament, eight copies of CARDMAVS were docked into the density map using the program Coot (Emsley and Cowtan, 2004). The density map was cropped to just cover the modeled portion of the filament (within 3 Å) using the phenix.cut_out_density command in the program Phenix (Adams et al., 2010). For refinement, phenix.refine was used with an arbitrary space group and unit cell dimensions. A summary of structure refinement statistics is provided in Table 1.
Crystallization and Structure determination of the 2CARDRIG-I:CARDMAVS complex
The 2CARDRIG-I-CARDMAVS fusion construct was expressed in BL21(DE3) at 20 °C for 16–20 hr following induction with 0.4 mM IPTG, and was purified by Ni-NTA affinity chromatography. The 6xHis tag was removed by HRV 3C protease, and 2CARD was further purified by additional Ni-NTA affinity chromatography and Size Exclusion Chromatography (SEC) in buffer A. The tetrameric species were isolated from SEC, mixed with mono-Ub at a molar ratio of 2:1 (fusion construct:Ub), and was concentrated to ~10 mg/ml in buffer A with 2 mM DTT. Crystals were obtained as small rods using the hanging-drop vapor-diffusion method from a 1:1 mixture of sample and reservoir buffer that contained 0.22 M Tri-Li Citrate, 18% PEG3350, 10 mM Cr(III)Cl3. The crystals were cryo-protected in the reservoir buffer containing 15%–20% ethylene-glycol for 5 minutes prior to flash freezing in liquid nitrogen. Diffraction data were collected at beamline 21ID-F (LS-CAT) at the Advanced Photon Source and processed using the program XDS (Kabsch, 2010). The structure was determined by molecular replacement using Phaser (McCoy et al., 2007) and refined using Phenix (Adams et al., 2010) (see Supplemental Information). A summary of data collection and structure refinement statistics is provided in Table 2.
Supplementary Material
HIGHLIGHTS.
The CARDMAVS filament is a left-handed, single-stranded helix.
The CARDMAVS:2CARDRIG-I complex also forms the same helical architecture.
The 2CARDRIG-I tetramer nucleates the CARDMAVS filament by helical extension.
CARDMAVS utilizes the same surface to interact with both 2CARDRIG-I and CARDMAVS.
Acknowledgments
The authors acknowledge the Texas Advanced Computing Center (TACC) at The University of Texas at Austin for providing High Performance Computing resources. W.B. acknowledges the Charles King postdoctoral fellowship. E.H.E. acknowledges EB001567. K.E.M. acknowledges CIHR MOP 125865. T.W. is an Investigator in the Howard Hughes Medical Institute. P.A.P. acknowledges GM U54 094598 and GM R01 60635. S.H. acknowledges the Pew Scholarship and BCH Career Development Award.
Footnotes
ACCESSION NUMBER
The cryo-EM densities of the MAVS CARD filament, and the atomic models were deposited with accession codes EMD-5922 and PDB-3J6J, respectively. The PDB accession code for coordinates and structure factors for the 2CARDRIG-I:CARDMAVS complex is 4P4H.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke IC, Li Y, Modis Y. Structural basis of innate immune recognition of viral RNA. Cellular Microbiology. 2013;15:386–394. doi: 10.1111/cmi.12061. [DOI] [PubMed] [Google Scholar]
- Berke IC, Yu X, Modis Y, Egelman EH. MDA5 assembles into a polar helical filament on dsRNA. Proc Natl Acad Sci U S A. 2012;109:18437–18441. doi: 10.1073/pnas.1212186109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, Chen ZJ. Prion-like Polymerization Underlies Signal Transduction in Antiviral Immune Defense and Inflammasome Activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman EH. Reconstruction of helical filaments and tubes. Methods Enzymol. 2010;482:167–183. doi: 10.1016/S0076-6879(10)82006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. 2004 doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Ferrao R, Wu H. Helical assembly in the death domain superfamily. Curr Opin Struct Bio. 2012;22:241–247. doi: 10.1016/j.sbi.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Kirchhofer A, Shin YC, Inn KS, Liang C, Cui S, Myong S, Ha T, Hopfner KP, Jung JU. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci U S A. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn M, Tang G, Goodyear G, Baldwin PR, Huang Z, Penczek PA, Yang C, Glaeser RM, Adams PD, Ludtke SJ. SPARX, a new environment for cryo-EM image processing. J Struct Biol. 2007;157:47–55. doi: 10.1016/j.jsb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:1–14. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Ramanathan A, Miller MT, Tang GQ, Gale M, Jr, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-Induced Oligomerization of the RNA Sensors RIG-I and MDA5 Activates Antiviral Innate Immune Response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Cryst. 2010;D66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takahasi K, Fujita T. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immuno Rev. 2011;243:91–98. doi: 10.1111/j.1600-065X.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth C, Braunfeld MB, Gubbens S, Agard DA, Cheng Y. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, Sun L, Chen ZJ. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell. 2014;156:1194–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Jain A, Chou YY, Baum A, Ha T, Garcia-Sastre A. ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 2013;14:780–787. doi: 10.1038/embor.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Jo M, Lin C, Wu B, Orme-Johnson M, Walz T, Hohng S, Hur S. Kinetic Mechanism for Viral dsRNA Length Discrimination by MDA5 Filament. Proc Natl Acad Sci U S A. 2012;109:E3340–3349. doi: 10.1073/pnas.1208618109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Lin C, Bin W, Orme-Johnson M, Liu M, Walz T, Hur S. Cooperative Assembly and Dynamic Disassembly of MDA5 Filaments for Viral dsRNA Recognition. Proc Natl Acad Sci U S A. 2011;108:21010–21015. doi: 10.1073/pnas.1113651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Wu B, Xu H, Chen ZJ, Hur S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature. 2014;509:110–114. doi: 10.1038/nature13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Wu B, Yao H, Walz T, Hur S. RIG-I Forms Signaling-Competent Filaments in an ATP-Dependent, Ubiquitin-Independent Manner. Mol Cell. 2013;51:573–583. doi: 10.1016/j.molcel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Reis e Sousa C. Innate Recognition of Viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Potter JA, Randall RE, Taylor GL. Crystal structure of human IPS-1/MAVS/VISA/Cardif caspase activation recruitment domain. BMC Struct Biol. 2008;8:1–10. doi: 10.1186/1472-6807-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling DC, Pyle AM. Parts, assembly and operation of the RIG-I family of motors. Curr Opin Struct Biol. 2014;25:25–33. doi: 10.1016/j.sbi.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu S, Onoguchi K, Onomoto K, Narita R, Takahasi K, Ishidate F, Fujiwara TK, Yoneyama M, Kato H, Fujita T. Functional Characterization of Domains of IPS-1 Using an Inducible Oligomerization System. PLos One. 2013;8:e53578. doi: 10.1371/journal.pone.0053578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immuno Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural Basis for dsRNA Recognition, Filament Formation, and Antiviral Signal Activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Wu H. ASSEMBLY OF POST-RECEPTOR SIGNALING COMPLEXES FOR THE TUMOR NECROSIS FACTOR RECEPTOR SUPERFAMILY. Adv Protein Chem. 2004;68:225–279. doi: 10.1016/S0065-3233(04)68007-7. [DOI] [PubMed] [Google Scholar]
- Xu H, He X, Zheng H, Huang LJ, Hou F, Yu Z, de la Cruz MJ, Borkowski B, Zhang X, Chen ZJ, et al. Structural basis for the prion-like MAVS filaments in antiviral innate immunity. eLife. 2014;3:e01489. doi: 10.7554/eLife.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.