Abstract
Recombination-based DNA construction methods, such as Gibson assembly, have made it possible to easily and simultaneously assemble multiple DNA parts and hold promise for the development and optimization of metabolic pathways and functional genetic circuits. Over time, however, these pathways and circuits have become more complex, and the increasing need for standardization and insulation of genetic parts has resulted in sequence redundancies — for example repeated terminator and insulator sequences — that complicate recombination-based assembly. We and others have recently developed DNA assembly methods that we refer to collectively as unique nucleotide sequence (UNS)-guided assembly, in which individual DNA parts are flanked with UNSs to facilitate the ordered, recombination-based assembly of repetitive sequences. Here we present a detailed protocol for UNS-guided assembly that enables researchers to convert multiple DNA parts into sequenced, correctly-assembled constructs, or into high-quality combinatorial libraries in only 2–3 days. If the DNA parts must be generated from scratch, an additional 2–5 days are necessary. This protocol requires no specialized equipment and can easily be implemented by a student with experience in basic cloning techniques.
Keywords: DNA assembly, synthetic biology, metabolic engineering, genetic circuits, insulation
INTRODUCTION
Synthetic biological circuits and metabolic pathways are employed in the production of commodity chemicals and biofuels 3–7, as well as in biosensing 8,9 and biomedical 10–15 applications. As circuits become more complex, however, the need for standardization and insulation of genetic parts 16–18 has resulted in substantial sequence redundancy. Circuits characterized by sequence redundancy can be assembled in a piecewise fashion 19 (e.g. via restriction cloning), but this approach is slow, especially when applied to experiments that require several design–build–test cycles 20. Gibson isothermal assembly 21, an in vitro homologous recombination-based approach, can assemble multiple DNA parts of up to several hundred kb in a one-step, one-pot reaction 22,23. However, with this approach recombination between redundant sequences can result in assembly errors.
Unique nucleotide sequence (UNS)-guided assembly was developed in our 1,2 and others’ 24,25 labs to enable the accurate isothermal assembly of multiple DNA parts with significant sequence similarity. In UNS-guided assembly, UNSs (also called linkers 26) are first attached to the DNA sequences of interest via cloning or PCR; the assemblies thus obtained provide the homology needed for isothermal assembly and buffer against inappropriate recombination between parts. Although this general approach had previously been demonstrated 26,27, only recently have sophisticated algorithm-based approaches to UNS construction been described that maximize assembly efficiency and minimize unexpected biological activity 1,24,25. Our group recently demonstrated the use of these algorithmically-designed UNSs to independently titrate the expression of multiple genes, and thereby improve the activity of an alkaloid biosynthetic pathway 1; we utilized this approach also to rapidly build and optimize a mammalian transcriptional logic gate integrated into the genome of embryonic stem cells 2. Others, in particular the Weiss and Ellis groups, have used UNS-guided assembly to construct large (11-part, 64-kb-long) transcriptional arrays in mammalian cells 24, and to generate gene expression libraries in yeast 25.
Here we present a detailed protocol for the application of UNS-guided assembly to construct multi-gene circuits and metabolic pathways. This protocol can be used to assemble specific multi-part constructs or combinatorial libraries. Although UNS-flanked parts can be generated in several different ways, we focus on their generation using a modular vector system developed in our lab that can reduce development time and improve the reusability of UNS-flanked parts. The design of UNSs has been described previously, and we encourage readers to consult Torella et al. 1 for a detailed description of this process. In brief, UNSs are generated by computationally generating a large pool of random 40-mers, then systematically removing those that contain barriers to assembly (e.g. stable hairpins, GC tracts, and sequences with homology to other UNSes) as well as biologically active sequences (e.g. those with known start codons, promoter-like sequences, or which have high BLAST scores against the host’s genome). Both the UNSs we use and the software needed to produce them are publicly available at http://openwetware.org/wiki/Silver_Lab.
Protocol overview
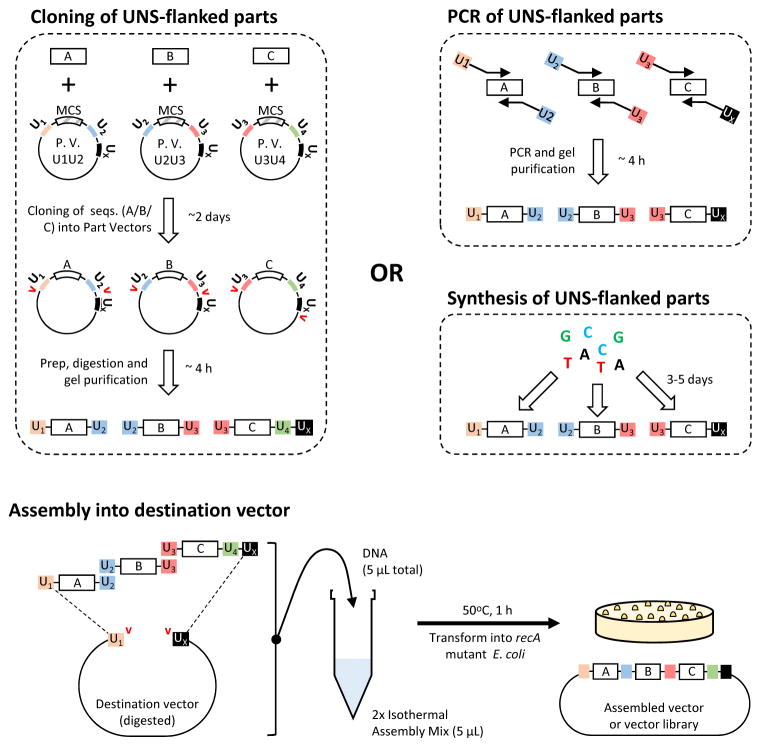
An overview of UNS-guided assembly is provided in Figure 1. UNSs are first appended to the DNA sequences to be assembled. This procedure may be carried out by cloning the sequences into standardized UNS-containing vectors, by PCR amplification with primers bearing UNSs at their 5′ ends or by total synthesis. The sequences of ten UNSs previously generated by our lab1 are provided in Table 1. These can equally be used for PCR-based and synthesis-based construction of UNS-flanked DNA sequences. In this protocol, we focus on the cloning-based approach.
Figure 1. Overview of UNS-guided assembly.
In UNS-guided assembly, DNA parts are first flanked with UNSs by cloning into standardized vectors, PCR or total synthesis. In the cloning approach, each part vector (“P.V.” in figure) contains a multiple cloning site (MCS), into which the sequences of interest are cloned, as well as a series of UNSs flanked by rare restriction sites (indicated by red carets). The vectors thus assembled can be digested to yield UNS-flanked parts for assembly. Once UNS-flanked dsDNA parts are generated, they are gel-purified, assembled via Gibson isothermal assembly and transformed into a recA mutant strains of E. coli. By including multiple versions of each UNS-flanked part in the assembly, combinatorial libraries can be generated.
Table 1.
List of UNSs.
| UNS# | Sequence (40 bp) |
|---|---|
| 1 | CATTACTCGCATCCATTCTCAGGCTGTCTCGTCTCGTCTC |
| 2 | GCTGGGAGTTCGTAGACGGAAACAAACGCAGAATCCAAGC |
| 3 | GCACTGAAGGTCCTCAATCGCACTGGAAACATCAAGGTCG |
| 4 | CTGACCTCCTGCCAGCAATAGTAAGACAACACGCAAAGTC |
| 5 | GAGCCAACTCCCTTTACAACCTCACTCAAGTCCGTTAGAG |
| 6 | CTCGTTCGCTGCCACCTAAGAATACTCTACGGTCACATAC |
| 7 | CAAGACGCTGGCTCTGACATTTCCGCTACTGAACTACTCG |
| 8 | CCTCGTCTCAACCAAAGCAATCAACCCATCAACCACCTGG |
| 9 | GTTCCTTATCATCTGGCGAATCGGACCCACAAGAGCACTG |
| X | CCAGGATACATAGATTACCACAACTCCGAGCCCTTCCACC |
In the cloning approach, standardized ‘part’ vectors are designed that possess a multiple cloning site (MCS) flanked by two distinct, part-specific UNSs (UN and UN+1) and a third UNS common to all part vectors (UX). 8-bp-long restriction sites, found in biological sequences only at low frequency, flank all UNSes; type IIS restriction sites overlap each of these restriction sites and act as a ‘backup’ in case one of the 8-bp sites cannot be used. Sequences of interest are cloned into the MCS, then excised by restriction digestion to generate UNS-flanked parts. All but the 3′-terminus part vector are digested around their UN and UN+1 sites, whereas the 3′-terminus part is digested around its UN and UX sites. This approach enables protocol users to perform isothermal assembly of all parts into a ‘destination’ vector bearing UNSes U1 and UX.
Part vectors have the advantage of enabling the user to test individual elements of a circuit for sequence and activity prior to assembly. Moreover, they facilitate modification and reuse of parts and thereby speed up the implementation of design–build–test cycles. Because parts can be assembled into any appropriate destination vector, destination vectors provide a convenient way to change the final construct’s origin of replication, antibiotic resistance, copy number or capabilities (e.g. transposition, integration or viral packaging functions). Typically we use bacterial artificial chromosome (BAC)-based destination vectors that can stably accommodate large (150 kb+) inserts. BACs also have the advantage of low copy numbers and therefore low leaky expression in bacterial hosts. As we use BACs that can be induced to produce high copy numbers by arabinose, their purification is straightforward.
Part and destination vectors are described in Tables 2 and 3. We have also set up a webpage on which these vectors and UNSs are described and can be requested, and which includes software for generating new UNSs, part vectors, and destination vectors (http://openwetware.org/wiki/Silver_Lab).
Table 2.
List of part vectors.
| Name | Features | Resistance | Copy Number | Size (kb) | Enz 1 A/B | Enz 2 A/B | Enz 3 A/B |
|---|---|---|---|---|---|---|---|
| pFL_U1U2 | U1-MCS-U2-UX | Amp | ~500 | 2.4 | AscI/BspMI | MauBI/BbsI | MreI/BsaI |
| pFL_U2U3 | U2-MCS-U3-UX | Amp | ~500 | 2.4 | AscI/BspMI | MauBI/BbsI | MreI/BsaI |
| pFL_U3U4 | U3-MCS-U4-UX | Amp | ~500 | 2.4 | AscI/BspMI | MauBI/BbsI | MreI/BsaI |
| pFL_U4U5 | U4-MCS-U5-UX | Amp | ~500 | 2.4 | AscI/BspMI | MauBI/BbsI | MreI/BsaI |
| pJT170 | U1-T7 Promoter-MCS-T7 Terminator-U2-UX. MCS contains BioBrick and BglBrck cloning sites allowing optional His-Tag fusion. | Amp | ~40 | 4.9 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT172 | U2-T7 Promoter-MCS-T7 Terminator-U3-UX. MCS contains BioBrick and BglBrck cloning sites allowing optional His-Tag fusion. | Amp | ~40 | 4.9 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT174 | U3-T7 Promoter-MCS-T7 Terminator-U4-UX. MCS contains BioBrick and BglBrck cloning sites allowing optional His-Tag fusion. | Amp | ~40 | 4.9 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT176 | U4-T7 Promoter-MCS-T7 Terminator-U5-UX. MCS contains BioBrick and BglBrck cloning sites allowing optional His-Tag fusion. | Amp | ~40 | 4.9 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT260 | U1-Trc Promoter-mCherry-Triple Terminator-U2-UX | Amp | ~40 | 5.7 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT288 | U2-Trc Promoter-mCherry-Triple Terminator-U3-UX | Amp | ~40 | 5.7 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT290 | U3-Trc Promoter-mCherry-Triple Terminator-U4-UX | Amp | ~40 | 5.7 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
| pJT292 | U4-Trc Promoter-mCherry-Triple Terminator-U5-UX | Amp | ~40 | 5.7 | AflII/SapI | MauBI/BbsI | MreI/BsaI |
Table 3.
List of destination vectors.
| Plasmid | Features | Resistance | Copy Number | Size (kb) | Enz 4 A/B | Enz 5 A/B |
|---|---|---|---|---|---|---|
| pDestET | Created from pETDuet-1 (Novagen) by replacing its promoter, MCS, T7Term and F1 Ori with U1 and UX. | 100 μg/mL Amp | ~40 | 4.4 | MauBI/BbsI | AvrII/BpmI |
| pDestBAC | Created from pETcoco-1 (Novagen) by removing inserting a stop codon at its NheI site and inserting U1 and UX. | 10 μg/mL Cam | ~1 (Amplifiable to 40 with arabinose) | 12.5 | MauBI/BbsI | AvrII/BpmI |
| pDestRmceBAC | Created from pDestBAC by inserting a cassette for recombinase-mediated cassette exchange (RMCE). Allows single-copy integration into a specific site in mammalian genomes. | 10 μg/mL Cam | ~1 (Amplifiable to 40 with arabinose) | 10.8 | MauBI/BbsI | AvrII/BpmI |
| pDestPBBAC | Created from pDestBAC by inserting a cassette for PiggyBAC transposition. Allows low-copy random integration into TTAA sites in mammalian genomes. | 10 μg/mL Cam | ~1 (Amplifiable to 40 with arabinose) | 16.4 | MauBI/BbsI | AvrII/BpmI |
| pDestBBR | Broad-host-range destination vector derived from pBBR1MCS (Kovach et al. 1994) and containing an arabinose-inducible promoter. Capable of replication in E. coli, P. putida, R. eutropha, M. extorquens, C. crescentus, V. cholera, P. denitrificans, Bordetella spp., and other microorganisms. | 50 μg/mL Kan | Depends on host | 6.3 | MauBI/BbsI | AvrII/BpmI |
Following PCR or part digestion, each UNS-flanked dsDNA part is gel-purified. The destination vector (which may be either PCR-purified or gel-purified) is mixed with equimolar amounts of each purified part in a solution 5 μl in volume; different versions of each part may be included in this mixture to generate combinatorial libraries. 5 μl of 2x isothermal assembly enzyme mixture is then added to the 5-μl solution of DNA in a 50-μl PCR tube, and this mixture is immediately moved to a thermal cycler with a 50-°C bed and 105-°C lid, the latter of which prevents evaporative reduction in sample volume. After 1 h, assembly mixtures are transformed into chemically competent Escherichia coli. We note that E. coli with mutations in recA should be used (e.g. DH5α, TOP10 or Mach1), as using strains with wild-type recA (e.g. NEB TURBO) considerably increases the proportion of incorrectly-assembled constructs.
The successful assembly of the desired products can typically be validated by culturing 2–3 colonies, mini-prepping their DNA, and performing analytical restriction digestions to confirm that the isolated plasmids are of the correct size. Comprehensive sequencing should then be performed to ensure no unexpected errors have occurred during assembly, though in our experience such errors are rare.
If an estimate of assembly efficiency is desired, for instance following library construction, 24–48 colonies can be grown to saturation in a 96-well plate, pooled, and the mixture mini-prepped and the digested for analysis. Densitometry can then be performed on gel bands of the correct (fully-assembled) size to estimate assembly efficiency. If assembly efficiency appears sufficient for the desired application, a subset of individual wells should be mini-prepped, analytically digested and comprehensively sequenced to ensure there are no common assembly errors before screening the library for the desired function.
Strengths and limitations
The present protocol has several advantages over conventional isothermal assembly as well as other methods of DNA assembly:
Assembly accuracy is high, enabling the simultaneous assembly of multiple parts in a one-pot reaction, including when the objective is to produce high-quality combinatorial libraries. For instance, we have generated 4- and 5-part libraries of metabolic pathways and genetic circuits 1,2.
UNSs decrease the dependence of assembly accuracy on the sequence of the DNA parts, enabling the construction of genetic circuits that include repeated sequences, such as the standard promoters and insulators that are common to synthetic circuits. We previously demonstrated assembly of four identical DNA parts (where only the UNSs varied) to demonstrate the assembly of extremely redundant sequences 1.
Standardized vectors for UNS-guided assembly (i.e. those that are identical except for the UNSes they contain, allowing facile assembly of any sequences cloned into them) enable straightforward modification and reuse of existing parts to speed up the design–build–test cycle, or to repurpose parts for new applications. The standardized vectors we describe also contain BioBrick 28 and BglBrick 29 sites for backwards compatibility with existing synthetic biology parts.
Cloning sequences of interest into these standardized vectors prior to UNS-guided assembly may also permit the assembly of complex sequences that are difficult to generate by PCR or synthesis (e.g. highly repetitive sequences).
This approach also has some limitations:
UNS-guided assembly is not scarless (i.e. UNSs remain in the final, fully assembled construct).
UNSs are designed to have minimal secondary structure and could promote cleavage of mRNAs 30, potentially making UNSs more appropriate for the design of multi-promoter arrays than operons.
Standardized UNS vectors need to undergo enzymatic restriction digestion to generate linear UNS-flanked parts prior to assembly, which imposes sequence constraints on the parts to be assembled. However, most restriction sites in the standardized UNS vectors are 8-bp long and occur very rarely in biological sequences. In principle these sites could be converted to meganuclease sites 24 in order to further relax sequence constraints.
Although we recommend using standardized UNS vectors to maximize part reuse and the fidelity of assembled constructs, this approach is also slower than PCR, which may be preferable when constructing high-complexity libraries, when part order must be varied, or when maximizing speed is a priority (e.g. see Casini et al. 25). In either case, the Procedure detailed below should serve as a useful starting point for UNS-guided assembly.
Comparison with other methods
Beside conventional isothermal assembly, whose differences with UNS-guided assembly have already been discussed, two additional techniques that accomplish similar goals with different advantages and disadvantages should be considered: golden gate cloning 31,32 and PCR-based methods, such as circular polymerase extension cloning 33,34 (CPEC) and overlap-extension PCR 35,36 (OE-PCR).
In golden gate cloning, type IIS restriction enzymes are used to generate sticky-ended dsDNAs that can be assembled by conventional cloning 31, including in a one-pot, multi-part format 32. Because type IIS enzymes cleave outside their recognition region, the final construct may be scarless (i.e. not contain the original restriction sites), unlike UNS-guided assembly which is not scarless (because UNSes remain in the final construct). Golden gate cloning is similarly fast and convenient to UNS-guided assembly but type IIS restriction sites are extremely common in biological sequences, imposing significant design constraints on the assembly strategy.
CPEC and OE-PCR are PCR-based methods of multi-part assembly, in which homology between the ends of DNA parts is used to anneal them such that they can be extended by PCR in order to create fused final products. These methods are attractive due to their rapidity; indeed, these methods will generally require less hands-on time than UNS-guided assembly. However, PCR can introduce point mutations in the final product (especially in large constructs), and annealing of unintended sequences can easily result in incorrectly assembled products. As a result, it is our experience that UNS-guided assembly produces the desired product substantially more frequently than CPEC and related methods.
Finally, we note that a recent publication described the use of a ligase cycling reaction, in which repeated cycles of melting, annealing and splinted ligation were used to rapidly assemble up to 20 independent DNA parts in a single reaction 37. This approach is promising but, like Gibson isothermal assembly, requires unique terminal sequences on each part to facilitate assembly. UNSs may therefore be useful in facilitating ligase cycling reaction–based assembly of synthetic gene circuits.
Applications
The ability to construct high-quality combinatorial libraries using repeated, standardized DNA parts makes it feasible to construct sophisticated metabolic pathways and genetic circuits with substantially less effort than that afforded by some alternative assembly methodologies. Applications may include:
Optimization of complex multi-gene and multi-operon metabolic pathways. Yield can be altered by changing the expression and translation strengths of multiple genes in parallel, for instance by varying promoters, ribosome binding sites, terminators, riboswitches, and activator and repressor binding sites.
Construction of metabolic pathways with novel products. This can be achieved by permuting existing pathways (e.g. by varying subunit composition in polyketide synthases) or by constructing entirely novel pathways using genes culled from enzyme databases.
Refactoring of existing but cryptic metabolic pathways from genomic or metagenomic data (‘synthetic metagenomics’ 38).
Construction of genetic circuits with unprecedented size and function. Circuit size and complexity is limited by the speed of the design–build–test cycle time and the ability to adequately insulate functional parts from one another. UNS-guided assembly can be used to assemble multiple highly redundant parts (e.g. those bearing repeated terminators or insulator sequences), thereby speeding the design–build–test cycle for well-insulated circuits.
By designing UNSs for insertion into coding sequences, large multi-domain proteins could be varied in composition in a combinatorial fashion to generate novel functions. Examples include polyketide synthases, nonribosomal peptide synthases, type I fatty acid synthases, and mammalian DNA-binding proteins. Although the UNSs described in this Protocol (Table 1) generally contain stop codons and are 40-bp-long (and would therefore introduce a frame shift), UNSs could easily be designed to be translated in-frame into flexible peptide linkers for this application.
EXPERIMENTAL DESIGN
Cloning into standardized part vectors
Although PCR or synthesis may be used to generate DNA parts, we recommend the use of standard UNS-bearing vectors for part generation. A list of standard part vectors is provided in Table 2; however, in most cases it will be necessary to clone sequences of interest into these vectors in order to generate parts with the desired function. Cloning can be performed using restriction–ligation (including BioBrick and BglBrick formats for our standard vectors) of sequences originally included in other plasmids or generated by PCR amplification. Isothermal assembly or other approaches to cloning part vectors can also be employed. Construction, sequence verification and preservation as glycerol stocks of clones of each desired part should be performed before initiating the Procedure.
Positive controls
It is helpful to have a positive control for successful UNS-guided assembly, and we recommend implementing the full Procedure on empty part vectors to first verify that the method is performing as expected, before using it to assemble parts of interest. For this purpose, digest empty part vectors pJT170 and pJT172 (Table 2) with AflII and MauBI and pJT174 (Table 2) with AflII and MreI to generate parts, and digest pDestET (Table 3) with MauBI and AvrII to generate linearized destination vector. Following assembly and transformation into an appropriate recA strain of E. coli, the resulting plasmids should be purified from 8–10 individual colonies and analytically digested with AflII and MreI. A 1,288-bp-long band should indicate successful assembly of these parts.
Ensuring proper sequence overlap between parts
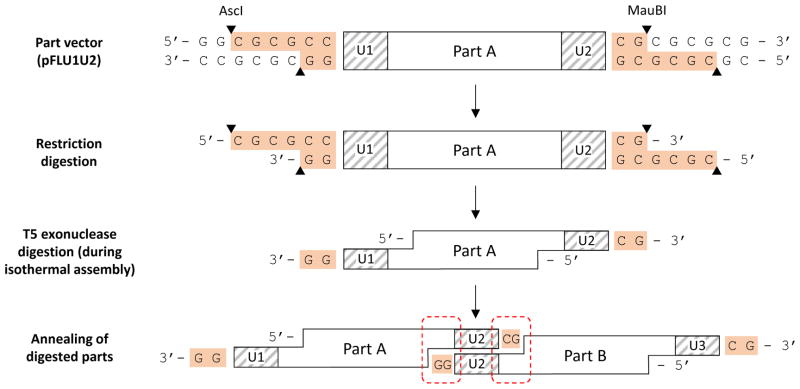
UNS-flanked parts may be generated by PCR, synthesis, or restriction digestion of part vectors. If any of the parts in a given assembly include those generated by restriction digestion, additional precautions may be necessary to ensure sequence compatibility between the parts, and between the parts and destination vector (Fig. 2). Specifically, if restriction sites are external to the UNS-flanked parts, digestion may leave additional nucleotides at the termini. Although the 5′->3′ exonuclease in the 2x isothermal assembly mixture will remove those nucleotides at the 5′ termini, those at the 3′ termini will still be present, and care must be taken to ensure that restriction-digested parts do not produce mismatches with one another or with the destination vector.
Figure 2. Sequence considerations when using standard part vectors.
When using currently available part vectors (Table 2), restriction sites lying just outside of the UNSs are digested to generate linear parts. Shown is an example in which pFLU1U2 (Table 2) containing “Part A” is digested with AscI and MauBI. This digestion leaves behind terminal nucleotides from the restriction sites (orange). The 5′ nucleotides at the termini are digested by T5 exonuclease during isothermal assembly, but those at the 3′ ends are left behind. When Part A anneals to another part, “Part B” (bottom), the terminal nucleotides in Part A anneal with nucleotides internal to the UNSs in Part B and vice versa. Caution must be taken to ensure these terminal nucleotides do not create mismatches that might interfere with assembly.
In the design of the pJT part vectors (Table 2) and pDestET, pDestBAC and pDestBBR destination vectors (Table 3) for instance, all UNSs have been flanked (on the top strand) by 5′ G and 3′ CG sequences to accommodate the extra terminal nucleotides left behind by restriction digestion with AflII, MauBI and MreI. In the design of the pFL part vectors (Table 2) and pDestRmceBAC and pDestPBBAC destination vectors (Table 3), all UNSs are flanked on the top strand by 5′ CC as AscI is used instead of AflII, and leaves behind a different set of terminal nucleotides (Fig. 2, Table 4). Assembly of a mixture of parts from the pJT and pFL series would therefore cause single-bp mismatches during assembly that may decrease assembly accuracy. Likewise, if PCR- or synthesis-derived parts are assembled with digestion-derived parts, care must be taken to design primers or synthetic sequences that accommodate these additional nucleotides. Although this is true for currently available part vectors (Table 2), future UNSs could be designed that contain internal restriction sites, thereby obviating the need for additional sequence considerations. In Table 4, we provide a list of all currently available part and destination vectors and the terminal nucleotides surrounding their UNSs, in order to judge compatibility.
Table 4.
List of terminal nucleotides (top strand) surrounding UNSs in each part and destination vector.
| Part or Destination Vectors | UN* 5′ end | UN* 3′ end | UX 5′ end | UX 3′ end |
|---|---|---|---|---|
| pFL part vectors | 5′-CC | CG-3′ | 5′-G | CG-3′ |
| pDestRmceBAC pDestPBBAC | 5′-CC | CG-3′ | 5′-G | CG-3′ |
| pJT part vectors | 5′-G | CG-3′ | 5′-G | CG-3′ |
| pDestET pDestBAC pDestBBR | 5′-G | CG-3′ | 5′-G | CG-3′ |
UN refers to all numbered UNSs in the given part or destination vector(s)
MATERIALS
REAGENTS
A suitable destination vector (see Table 3) as a glycerol stock. Destination vectors can be obtained at http://openwetware.org/wiki/Silver_Lab. Their propagation and purification is described in Reagent Setup.
Part vectors containing the sequences to be assembled (see Table 2 for a list of empty part vectors) as glycerol stocks. Basic part vectors may be obtained at http://openwetware.org/wiki/Silver_Lab. Propagation and purification of part vectors is described in Reagent Setup. CRITICAL Part vectors are not required if parts will be generated by PCR or synthesis. For PCR only the appropriate template DNA template solution is required.
Distilled, deionized, sterile H2O (ddH2O)
Magnesium chloride (MgCl2, anhydrous; Sigma-Aldrich, cat. no. M8266-100G)
DL-Dithiothreitol (DTT; Sigma-Aldrich, cat. no. D0632-10G)
dNTP mix (10 mM each; New England Biolabs, cat. no. N0447S)
Poly(ethylene glycol) (PEG, avg. mol. wt. 8,000; Sigma-Aldrich, cat. no. P2139-500G)
Nicotinamide adenine dinucleotide (NAD+; Applichem, cat. no. A1124,0005)
T5 Exonuclease (EPICENTRE, cat. no. T5E4111K)
Phusion™ high-fidelity DNA polymerase (NEB, cat. no. F-530S). CRITICAL Do not use HotStart polymerases in the relevant step of the Procedure. The isothermal assembly reaction begins at room temperature (20 °C–25 °C) and never goes above 50 °C; as a result, HotStart polymerases may not function properly during isothermal assembly.
Taq DNA Ligase (NEB, cat. no. M0208L)
Fermentas FastDigest enzymes indicated in Tables 2 and 3 (as needed), including 10x FD Green Buffer (e.g. AscI/MauBI/MreI; Thermo Scientific, cat. nos. FD1894, FD2084, FD2024)
Zymoclean™ Gel DNA Recovery Kit (Zymo Research, cat. no. D4001)
Tris-EDTA buffer solution pH 7.4 (TE buffer; Sigma-Aldrich, cat. no. 93302-100ML)
SeaKem LE Agarose (Lonza, cat. no. 50004)
Ethidium bromide (Sigma-Aldrich, cat. no. E8751) CAUTION Ethidium bromide is a mutagen and potential carcinogen. Gloves should be worn and care taken to avoid contact with skin.
Tris Acetate-EDTA buffer (10× TAE buffer; Sigma-Aldrich, cat. no. T9650)
Tris HCl buffer (1 M, pH 7.5; Sigma-Aldrich, cat. no. T2319)
Nalgene sterile disposable bottle top filters (0.20 μm filter; Thermo Scientific, cat. no. 09-740-22K)
LB broth powder (Sigma-Aldrich, cat. no. L3022)
LB broth with agar (Sigma-Aldrich, cat. no. L3147)
SOC medium (Sigma-Aldrich, cat. no. S1797)
Ampicillin sodium salt (Sigma-Aldrich, cat. no. A9518)
Chloramphenicol (Sigma-Aldrich, cat. no. C0378)
Poly(ethylene glycol) (PEG, avg. mol. wt. 4,000; Sigma-Aldrich, cat. no. 81240-1KG)
Dimethyl sulfoxide (DMSO, Sigma-Aldrich, cat. no. D2650) CAUTION Although non-toxic, DMSO can carry contaminants through skin and into the bloodstream. Gloves should be worn and care taken to avoid contact with skin.
L-arabinose (Sigma-Aldrich, cat. no. A3256)
QIAprep Spin Miniprep Kit (QIAGEN, cat. no. 27104)
QIAGEN Plasmid Plus Midi Kit (QIAGEN, cat. no. 12943)
One Shot Mach1 or TOP10 chemically competent E. coli (Life Technologies, cat. nos. C8620-03 and C4040-03
Nunc 96-well deep-well plates (VWR, cat. no. 73520-474)
Ready-Load 1 kb Plus Ladder (Life Technologies, cat. no. 10381-010)
EQUIPMENT
Nanodrop 1000 (Fisher Scientific, cat. no. NC9904842)
Veriti® thermal cycler (Life technologies, cat. no. 4375305) CRITICAL Whatever thermal cycler is used, it must be capable of holding the lid at 105°C to prevent sample volume reduction
Rotary evaporator (rotavap)
PowerPac basic power supply for electrophoresis (Bio-Rad, cat. no. 164-5050)
Sub-Cell GT system for agarose gel electrophoresis (Bio-Rad, cat. no. 170-4401)
FluorChem SP UV transilluminator and imaging system (Alpha Innotech)
Heidolph Titramax vibrating microtiterplate shaker (VWR, cat. no. 82004-940)
REAGENT SETUP
5x isothermal assembly buffer
Combine 3 ml of 1 M Tris-HCl pH 7.5, 300 μl of 1 M MgCl2, 600 μl of 10 mM dNTP mix, 300 μl of 1 M DTT, 1.5 g of PEG (mol. wt. 8,000) and 20 mg of NAD+. Raise the final volume to 6 ml with ddH2O and mix thoroughly. Prepare 20x 300 μl aliquots of this mixture and store at −20 °C. This buffer should be stable at −20 °C for at least six months.
2x isothermal assembly mixture
Thaw one aliquot of 5x isothermal assembly buffer on ice and add the following to it: 1.2 μl of T5 exonuclease, 20 μl of Phusion polymerase, 160 μl of Taq DNA ligase, and 270 μL of ddH2O. Mix thoroughly but gently to avoid introducing bubbles and causing the enzymes to misfold. Transfer 20-μl aliquots of this mixture into 50-μl PCR tubes, seal them tightly, and store them at −20 °C. Each tube will be sufficient for 3–4 isothermal assembly reactions and should retain its activity for several months. CRITICAL we recommend storing 2x isothermal assembly aliquots in volumes no smaller than 20 μl. In some freezers, small but meaningful loss of water content from the aliquots may occur over time, which can be problematic for aliquots under 20 μl in volume during extended storage.
TSS broth
Combine 100 ml of LB broth (prepared as recommended from powder manufacturer’s package), 2 ml of 1 M MgCl2, and 10 g of PEG (mol. wt. 4,000). Microwave for 30 s to dissolve fully, then filter-sterilize and add 5 ml of DMSO. Mix thoroughly and store at 4 °C. When stored at 4 °C, TSS broth should be stable for at least six months.
TSS competent cell preparation
Grow desired E. coli strain (typically Mach1 or TOP10 chemically competent E. coli) overnight (16–20 h) in 5 ml of LB broth at 37 °C, 250 rpm. Dilute 1 ml of the overnight culture into 100 ml of LB broth in a 500-mL flask, and shake at 37 °C, 250 rpm until an OD600 of 0.3–0.4 is reached. Transfer the culture into two 50-ml Falcon tubes and submerge the falcon tubes in an ice–water bath for 15 min. Centrifuge the 50-ml Falcon tubes at 3,000 g, 4 °C, for 20 min. Decant the supernatant and resuspend both pellets completely in a total of 10 ml of ice-cold TSS broth. Prepare 100 μl aliquots of the cell mixtures and flash-freeze them in a dry ice–ethanol bath before storing them at −80 °C. TSS competent cells should be stable for at least six months.
0.8% (wt/vol) agarose-TAE gel with ethidium bromide
Combine 0.8 g of SeaKem LE agarose (or similar) and 100 mL 1xTAE buffer and microwave until fully dissolved. Cool to 50 °C then add 100 μl of a 500-μg/ml stock solution of ethidium bromide. Mix well and pour into an appropriate gel-casting tray with comb. Leave at room temperature for half an hour or until the gel is nearly opaque.
Propagation and purification of multi-copy plasmids (part and destination vectors)
Streak glycerol stocks of each desired multi-copy part and destination vector (see Tables 2 and 3) on an LB-agar plate containing appropriate antibiotics and incubate at 37 °C overnight (16–20 h). The antibiotics, typically ampicillin or chloramphenicol, depend on the vector used, and LB-agar plates containing these antibiotics should be prepared according to powder manufacturer’s instructions. For each plasmid, inoculate one colony into 10 mL of LB containing appropriate antibiotics (depending on the vector used) and shake overnight (16–20 h) at 250 rpm and 37 °C. To purify the vector, use the QIAprep Spin Miniprep Kit according to the manufacturer’s instructions and elute into 30 μL of 0.2x TE buffer. Implementing this procedure should provide ~5 μg of vector. Purified vector should be stored at −20 °C, and is stable for several years at this temperature.
Propagation and purification of amplifiable, single-copy destination vectors
For destination vectors derived from amplifiable copy-number bacterial artificial chromosomes (BACs; see Table 3), first streak out and start liquid cultures for each desired destination vector as described for multi-copy plasmids. Following overnight growth, transfer 1.5 ml of culture to 80 ml of LB broth containing 1.0% (wt/vol) of L-arabinose with appropriate antibiotics (depending on vector used) in a 500-ml flask. Shake at 250 rpm and 30 °C for 24 h. Purify the BACs with the QIAGEN Plasmid Plus Midi Kit. Follow the manufacturer’s high-yield protocol and elute in 100 μl of 0.2x TE. Implementing this procedure should provide ~100 μg of vector, which may be stored at −20 °C for several years.
Primer design for PCR-based part generation
If PCR is to be used to generate UNS-flanked parts (Step 1Bi), first design forward and reverse primers to amplify the sequences of interest. We recommend using Phusion polymerase, and therefore recommend following the Phusion manufacturer’s instructions for primer design. Once primers are designed for amplification, append two sequential UNSs from Table 1 to the 5′ ends of the upstream and downstream primers (the latter as a reverse complement). The resulting oligos can be used to generate UNS-flanked parts by PCR (Step 1Bii). CRITICAL Oligos designed in this way are typically > 60 bp, which can decrease synthesis quality or increase cost (e.g. this is the cutoff for the most inexpensive oligos available from IDT with acceptable quality). If shorter oligos (< 60 bp) are required, UNSs can be shortened by up to 10 nucleotides at the 5′ ends of the primers.
PROCEDURE
Preparation of linear, UNS-flanked parts TIMING 2–5 d
-
1
Generate linear UNS-flanked parts by synthesis (Option A), PCR (Option B) or digestion of part vectors (Option C):
-
Generation of linear UNS-flanked parts by synthesis
-
Select two sequential UNSs from Table 1 and design a DNA sequence in which the chosen UNSs are fused to the ends of your desired sequence. Submit the sequence thus designed for synthesis to IDT’s gBlock service (or similar). This approach provides 200 ng of purified, linear UNS-flanked part, enough for ~10–20 five-part reactions.
CRITICAL STEP In some assembly efforts, the user may wish to combine synthesized parts with parts generated by other methods. If any of the parts included in the assembly reaction are to be generated by part vector digestion, then for those parts generated by synthesis, additional sequence considerations may be necessary. See Experimental Design section and Table 4 for details.
-
-
Generation of linear UNS-flanked parts by PCR
Design primers for PCR-based generation of UNS-flanked parts (see Reagent Setup).
-
While PCR practices vary substantially between laboratories, an example of a protocol used commonly in our laboratory is as follows:
-
Prepare a 20 μl PCR reaction in a 50 μl PCR tube as follows:
Component Amount (μl) Final concentration 2x Phusion HF Master Mix 10.0 1x ddH2O 7.0 – Template DNA (10 ng/μL) 1.0 0.5 ng/μL Forward primer (10 μM) 1.0 0.5 μM Reverse primer (10 μM) 1.0 0.5 μM -
Run the following PCR program with the lid heated to 105 °C. For the Phusion enzyme, the annealing temperature should be 3 °C higher than the lower-Tm primer (as calculated according to the manufacturer’s instructions):
Cycle number Denature Anneal Extend 1 98 °C, 30 s 1x 2–36 98 °C, 15 s (Tm + 3) °C, 30 s 72°C, 20 s/kb 37 72 °C, 5 min -
PCR products may be stored at −20 °C for at least six months.
CRITICAL STEP In some assembly efforts, the user may wish to combine synthesized parts with parts generated by other methods. If any of the parts included in the assembly reaction are to be generated by part vector digestion, then for those parts generated by synthesis, additional sequence considerations may be necessary. See Experimental Design section and Table 4 for details.
CRITICAL STEP We recommend the use of Phusion polymerase or a polymerase with similarly high fidelity to ensure that parts have the correct sequence, especially for larger parts or final assembled constructs.
TROUBLESHOOTING
-
-
Generation of linear UNS-flanked parts by digestion of part vectors
-
Propagation and purification of part vectors is described in Reagent Setup, and is the same for both empty part vectors (Table 2) and part vectors into which sequences of interest have been cloned (as described below). Generate UNS-flanked parts from part vectors by setting up, for each part, the following restriction digestion, and incubating it at 37 °C for 1 h:
Component Amount (μl) Final concentration 2.0 μg of part vector X 100 ng/μl ddH2O 16.0 – X – 10x FD Green Buffer 2.0 1x FastDigest Enz1A or Enz1B 1.0 0.05 FDU/μl FastDigest Enz2A or Enz2B (except for the 3′-terminus part) 1.0 0.05 FDU/μl FastDigest Enz3A or Enz3B (except for the 3′-terminus part) 1.0 0.05 FDU/μl The enzyme designations refer to specific sites on the vector. Enz1 cuts the DNA at a restriction site near the UN sequence, Enz2 at one near the UN+1 sequence, and Enz3 at one near the UX sequence (Figure 3). A and B refer to the two restriction enzymes that can be used to cut each site. In each case, enzyme A recognizes the rare, 8-bp restriction site, whereas enzyme B recognizes a IIS restriction site that overlaps the A restriction site and can serve as a backup if A cannot be used (e.g. because the A restriction site is found in the part itself). The A and B restriction sites for each part vector are provided in Table 2.
-
-
-
2
Perform a restriction digestion of the destination vector by preparing the following digestion mixture and incubating it at 37 °C for 1 h:
Component Amount (μl) Final concentration 2.0 μg of destination vector X 100 ng/μl ddH2O 16.0 – X – 10x FD Green Buffer 2.0 1x FastDigest Enz4A or Enz4B 1.0 0.05 FDU/μl FastDigest Enz5A or Enz5B 1.0 0.05 FDU/μl Enz4 and Enz5 cut DNA at restriction sites nearest the U1 and UX sites, respectively, in a given destination vector. The identity of these enzymes is provided in Table 3. Like part vectors, destination vectors have both primary and backup (A and B) restriction enzymes for each restriction site (Figure 3).
-
3
Load the entirety of the destination vector restriction reaction mixture (from Step 2), part vector restriction reaction mixtures (from Step 1C), PCR-generated parts (from Step1B), and 10 μl of Ready-Load 1 kb Plus Ladder, into the lanes of a 0.8% (wt/vol) agarose-TAE gel containing 0.5 μg/mL ethidium bromide, then run electrophoresis at 150 V for 20 min.
CRITICAL STEP Loading dye does not need to be added to these reactions prior to running the gel; 1x FD Green Buffer acts as a loading dye on its own.
-
4
Using a FluorChem UV transilluminator and camera (or similar), image the gel with 280-nm UV light and determine whether the digestion yielded the expected products.
TROUBLESHOOTING
-
5
Assuming the expected size products were identified, gel-purify each UNS-flanked part and destination vector using the Zymoclean Gel DNA Recovery Kit. Follow the manufacturer’s instructions and elute each product into 7 μl of DNA Elution Buffer (provided with kit).
TROUBLESHOOTING
-
6
Measure the concentration of each gel-purified sample by applying 1 μl of DNA solution to a Nanodrop spectrophotometer (or similar).
Pause point Following gel purification and DNA quantification, the purified parts can be stored at −20 °C for at least three months for future assembly reactions.
Figure 3. Restriction site locations in part and destination vectors.
(A) Each part vector contains restriction sites at which it can be cleaved to produce UN-UN+1 or UN-UX-flanked parts. We label the enzymes used to cleave at near UN as Enz1, near UN+1 as Enz2 and near UX as Enz3. Each restriction site contains both a primary, rare, 8-bp restriction site (site ‘A’) and a secondary, overlapping type IIS restriction site (‘B’) to be used as a backup. (B) Destination vectors contain restriction sites (recognized by Enz4 and Enz5) that must be digested to remove the DNA segment between U1 and UX. As in the part vectors, each of these DNA sequences has both a primary and secondary restriction site. In (A) and (B) restriction sites are indicated by red carets.
Isothermal assembly TIMING ~1.5 h
-
7
In a 50-μl PCR tube, mix 50–100 ng of destination vector with equimolar amounts of each purified part and use ddH2O to raise the volume to 5 μl. If you are performing combinatorial assembly to generate a library, then in place of a given part supply X versions of that part, each at 1/X the normal molar amount.
CRITICAL STEP If the volume obtained after mixing together the DNA solutions is greater than 5 μl, leave the lid of the PCR tube open and use a rotavap set at 37 °C to reduce the volume to 5 μl. If a rotavap is not available, the volume may be reduced by incubating at 60 °C with the cap ajar to concentrate the sample. Alternatively, the total amount of DNA can be reduced while keeping the molar ratios of the parts equal (though this approach will result in a decrease of assembly yield).
-
8
Combine the 5-μl DNA mixture from step 7 with 5 μl of 2x isothermal assembly mixture in a 50-μl PCR tube. In the end, this mixture will have the components detailed in the in-text table reported below.
CRITICAL STEP Pipette thoroughly to mix the DNA with the viscous 2x isothermal assembly mixture, but avoid introducing bubbles, as they may disrupt enzyme activity. Once this is done, immediately move to the next step.
Component Amount (μl) Final concentration 5–20 ng of destination vector per kb X 0.5–2.0 ng/kb μl Equimolar amount of each part Y 0.5–2.0 ng/kb μl ddH2O 5.0 – X – Y – 2x isothermal assembly mixture 5.0 1x -
9
Place the reaction mixture from step 8 in a thermal cycler. Set the bed to 50 °C and the lid to 105 °C for 1 h to complete the assembly reaction.
CRITICAL STEP We strongly recommend using a thermal cycler with a hot-lid for this step. The 10-μl reaction volume keeps reagent costs low, but incubation in a 50-°C water bath would result in evaporative concentration that decreases assembly efficiency. If a thermal cycler with hot-lid is not available, double the volume of the reaction mixture (i.e., 10 μl DNA + 10 μl 2x isothermal assembly mixture) and incubate the reaction mixture in a 50-°C water bath for 1 h.
-
10
Place the assembly reaction mixture on ice.
Pause point If not used immediately, the assembly reaction mixture can be frozen at −20 °C and stored at this temperature for at least a month.
Transformation of the assembly reaction TIMING 1 d
CRITICAL Steps 11–16 detail the implementation of One Shot chemically competent cell manufacturer’s instructions, with minor modifications, to perform transformations of two different types of competent cells with the assembled reaction mixture from step 10.
-
11
Thaw a tube of competent E. coli cells on ice for each assembly reaction performed. When assembling a single, defined product, and only one successful clone is required, use a 100-μl aliquot of low-efficiency (~107 cfu/μg) TOP10 or Mach1 chemically competent cells prepared according to the TSS competent cell method reported in the Reagent Setup. Alternatively, when constructing a combinatorial library, use a 50-μl aliquot of commercially supplied, high-efficiency (>109 cfu/μg) One-Shot TOP10 or Mach1 chemically competent cells
CRITICAL STEP Be sure only to use E. coli strains with mutations in recA, such as TOP10, DH5α or Mach1. In our experience, cloning strains with functional recA (e.g. NEB TURBO) yield a lower proportion of correct constructs when transformed with isothermal assembly mixtures.
-
12
Add either up to 5 μl (for TSS competent cells) or up to 2 μl (for One Shot competent cells) of the assembly reaction from step 9 to the thawed competent cells and stir gently with the pipette tip.
-
13
Incubate the resulting mixture on ice for 30 min.
-
14
Heat shock the competent cells for 30 s at 42 °C then place them back on ice for 2 min.
-
15
Add 250 μl of SOC medium to the tube of competent cells and incubate at 37 °C for 1 h, horizontally, with shaking at 250 rpm.
-
16
Plate each transformation mixture on LB-agar plates containing appropriate antibiotics (depending on the destination vector used) and incubate overnight (16–20 h) at 37 °C. Place any leftover transformation mixture in a 4-°C refrigerator so that it can be plated the next day, if necessary. We do not recommend keeping the transformation mixture for longer than 24 h.
CRITICAL STEP We recommend plating two different volumes of the transformation mixture, typically 10 μl and 100 μl, to ensure single colonies can be picked from at least one of these plates.
TROUBLESHOOTING
Testing assembly accuracy TIMING ~2 d
-
17
Pick 2–3 colonies from the transformation plate, inoculate each into 10 ml of LB broth containing 1.0% (wt/vol) glucose + an appropriate antibiotic (depending on destination vector used), and shake overnight (16–20 h) at 37 °C and 250 rpm. The plate can be stored at 4 °C for up to one week if more colonies are needed.
-
18
Extract and purify the plasmid from each culture prepared in step 17 using the QIAprep Spin Miniprep Kit according to the manufacturers’ instructions.
-
19
Digest the purified plasmid by preparing a digestion mixture as described in the in-text table below, and incubating it at 37 °C for 30 min. Restriction enzymes can be chosen by considering the expected sequence of the correctly assembled vector, and identifying restriction sites for which the size of the excised band is likely to appear only in a correctly-assembled product. For example, we typically choose restriction sites bracketing the insert that is assembled into the destination vector. If the insert excised from the plasmid turns out to be of the expected size, it suggests that all parts have successfully been assembled, whereas smaller bands would suggest an incompletely assembled product.
Component Amount (μl) Final concentration 500 ng of vector X 50 ng/μl ddH2O 8.0 – X – 10x FD Green Buffer 1.0 1x FastDigest Enzyme 1 0.5 FastDigest Enzyme 2 0.5 0.05 FDU/μL Incubate at 37°C for 30 min -
20
Load each reaction mixture, as well as 10 μl of Ready-Load 1 kb Plus Ladder, onto a 0.8% (wt/vol) agarose-TAE gel containing 0.5 μg/ml of ethidium bromide and perform electrophoresis at 150 V for 20 min.
-
21
Using a FluorChem UV transilluminator and camera (or similar), image the gel with 280-nm UV light and determine whether the digestion yielded the expected products. TROUBLESHOOTING
-
22
Using appropriate primers, submit each plasmid with the correct digestion pattern for Sanger sequencing using your preferred sequencing service.
CRITICAL STEP It is ideal to comprehensively sequence the full insert assembled into the destination vector to ensure no rearrangements occurred during assembly.
TROUBLESHOOTING
Testing assembly efficiency for libraries TIMING ~2 d
CRITICAL Please note that this sub-section of the Procedure is optional. It should be implemented if the experimenter is attempting to assemble a high-quality combinatorial library and would like to estimate assembly efficiency before proceeding to perform functional assays.
-
23
Pick 24–48 colonies from the plates prepared in step 16 and transfer them into individual wells of a Nunc 96-well deep-well (2.0 ml) plate containing 1.0 ml of LB broth containing 1.0% (wt/vol) glucose + an appropriate antibiotic (depending on destination vector used). Incubate the plate on a vibrating microtiter plate shaker overnight (16–20 h) at 30 °C and 1,200 rpm.
-
24
Pool 200 μl of culture per well into a single tube and extract and purify the plasmid mixture using the QIAprep Spin Miniprep Kit according to the manufacturers’ instructions.
-
25
Digest the plasmid mixture with appropriate restriction enzymes, load the mixture on an agarose gel, run electrophoresis, and image as described in Steps 19–21.
-
26
If a quantitative estimate of assembly efficiency is desired, densitometry of individual bands can be performed with ImageJ, freely available from http://rsbweb.nih.gov/ij/.
-
27
Assuming assembly efficiency appears high enough for your desired application, miniprep a subset of wells (~10) from the 96-well plate individually, then digest and sequence them as described in Steps 19–21 to confirm proper assembly.
TIMING
Steps 1–6, preparation and gel purification of linear, UNS-flanked parts: 2–5 d if part vectors must be generated, if primers must be ordered, or if parts are to be generated by synthesis. If part vectors are already available for digestion-based part assembly, or templates and primers are already available for PCR-based assembly, this step takes only ~2–4 h.
Steps 7–10, isothermal assembly: ~ 1.5 h.
Steps 11–16, transformation of the assembly reaction: 1 d.
Steps 17–22, testing assembly accuracy for specific constructs: 2 d.
Steps 23–27, testing average assembly accuracy of a library (optional): 2 d.
TROUBLESHOOTING
Table 5.
Troubleshooting table
| Step | problem | possible reason | solution |
|---|---|---|---|
| 1bi | No PCR product | Incorrectly designed primers or amplification protocol | Check for primer hairpin or primer dimer formation (we recommend using the Oligo Analyzer at http://www.idtdna.com for this purpose) Ensure that the melting temperature is appropriate If primers are GC-rich, repeat PCR in the presence of between 3.0% and 7.0% (vol/vol) DMSO. Re-design primers if necessary |
| Interference of UNS sequence with priming due to inappropriate annealing, primer hairpin formation or primer dimer formation | Check for homology between UNSs and primer and/or template sequences. Shorten UNSs in primers by up to 10 nt or use different UNSs not homologous to primers and/or template | ||
| 4 | Digestion of part vector yields multiple bands that are smaller than expected | Restriction sites recognized by the enzymes are present in the part | Check the part sequence for restriction sites used to digest the part vector. If present, see if an alternative restriction site may be used. For instance if an AscI recognition site (Enz1 ‘A’ site in pFL vectors used to cut the first UNS) is present in the part, see if a BspMI recognition site (Enz1 ‘B’ site that also cuts the first UNS in pFL vectors) is present in the part. If both are present, the part must be modified, cloned into a part vector with different restriction sites, or PCR-amplified to avoid the use of restriction enzymes |
| 5 | Low concentration of gel-purified parts | Insufficient starting material | Repeat digestion with 2–3x the original amount of plasmid before gel purifying |
| Poor gel extraction | Consult manufacturer’s troubleshooting instructions for Zymoclean Gel DNA RecoveryKit. | ||
| 16 | Few or no colonies produced following transformation | Competent cells have low transformation efficiency | For commercial competent cells, measure cfu/μg according to manufacturers’ instructions. For prepared competent cells, measure cfu/μg by transforming 0.1 ng of pUC19 plasmid DNA into competent cells and multiplying the number of resulting colonies by 10^4. If cfu/μg is < 5*106 for prepared competent cells, re-prepare. If cfu/μg is < 5*108 for commercial competent cells, re-order them |
| Assembly inefficient due to old or mis-prepared isothermal assembly mixture | Repeat previously successful assembly as positive control. If necessary, NEB provides a positive control with their Gibson Assembly Master Mix (cat. no. E2611S). If the positive control fails, prepare a new batch of 2x isothermal assembly mixture | ||
| Assembly inefficient due to failure of parts to anneal | Ensure part assembly strategy is properly designed. In particular, if any parts generated by restriction digest are included in the assembly, be sure all UNSs are flanked by appropriate 5′GG or and 3′ CG nucleotides to accommodate the extra nucleotides generated by restriction digestion | ||
| 21, 22 | Colonies contain re-ligated destination vector | Inefficient digestion of original destination vector | Check activity of restriction enzymes used to digest the destination vectors and re-order if necessary. Digestion time can also be increased 2–3-fold |
| Colonies contain incorrectly-sized products, or sequencing reveals DNA deletions and/or rearrangements | Incorrect annealing, extension or ligation of original parts | Ensure part assembly strategy is properly designed. In particular, if any parts generated by restriction digest are included in the assembly, be sure all UNSs are flanked by appropriate 5′GG or and 3′ CG nucleotides to accommodate the extra nucleotides generated by restriction digest | |
| Inaccurate assembly can result from old or partially inactivated isothermal assembly mixture. Perform a positive control assembly (see above) and re-prepare 2x isothermal assembly mixture if necessary | |||
| If UNSs are frequently recombining with a specific sequence within one of your parts, either modify the part or swap the offending UNS for another before repeating the assembly | |||
| Transformation was performed into an E. coli strain that has wild-type recA | Cloning strains with wild-type recA can produce higher yields of incorrect assembly products. Switch to a recA mutant strain (e.g. TOP10 or Mach1) and repeat the transformation |
ANTICIPATED RESULTS
Starting from part vectors (or equivalent PCR products or synthesized dsDNAs), UNS-guided assembly of complex genetic circuits and circuit libraries takes only ~5–6 h to complete, with an additional 2–3 days required to transform and sequence-verify these constructs. As new part vectors may be generated in as little as 2 days (depending on the approach taken), it is possible to generate, assemble and verify a multi-part library in less than a week.
Assuming all parts are designed correctly, the 2x isothermal assembly mixture has been properly prepared and stored, and the E. coli used for transformation have a mutation in recA, we find that assembly is typically highly accurate (85–100% of all clones isolated contain the expected sequence), even when the parts to be assembled have substantial sequence redundancy. We observed 98% and 95% assembly efficiencies for 3- and 4-part (including destination vector) combinatorial libraries respectively 1, with substantial sequence redundancy (repeating terminator sequences) and sizes up to 15 kb. The 4-part assembly was performed to generate a combinatorial expression library of three genes (vioB, vioA, vioE), which together convert cytosolic tryptophan into the insoluble green pigment deoxychromoviridans 39. An analytical restriction digest of 60 pooled clones from this library showed high assembly accuracy (Fig. 4A, i); however, visual inspection of individual colonies from the isothermal assembly reaction showed distinct differences in deoxychromoviridans production by different library members (Fig. 4A, ii and iii).
Figure 4. Constructing individual circuits and combinatorial libraries.
(A) Combinatorial assembly of a 3-part biosynthetic pathway for deoxychromoviridans (an insoluble green alkaloid pigment) by UNS-guided assembly. Each part contained one of six promoters, a triple terminator, and one of the three genes required for deoxychromoviridans biosynthesis (vioB, vioA, vioE). (i) Analytical restriction digestion of a pool of 60 clones obtained by this method. The pool was digested to isolate the destination vector backbone (black arrow) from the inserts assembled into it. Indicated are the frequent, correctly-sized (green arrow) and rare, incorrectly-sized (red arrows) inserts. (ii) Transformation of empty pDestET into TOP10 competent cells yields a lawn of unpigmented TOP 10 E. coli. (iii) Transformation of the isothermal assembly reaction (containing the vioBAE library, indicated by “Vio Lib” in the figure) into TOP10 E. coli yields colonies with variable levels of green pigmentation. (B) Construction of individual, four-part circuits for and logical computation in mammalian cells. (i) Parts were assembled into the pDestRmceBAC destination vector, which is capable of single-copy integration into appropriate mammalian cell lines. The first three parts (A, B, C1) were mixed with either the D1 or D2 part. Each mixture was assembled on its own, transformed into E. coli, and five clones (indicated by the numbers 1–5 in Figure 4bii) selected from each assembly reaction for further analysis. (ii) Analytical restriction digestion of these ten clones revealed the correct pattern for 9 out of 10. This panel is reprinted from 1.
In the same work, we also performed a 5-part assembly, in which four of the parts shared 80% sequence identity. Assembly accuracy was ~85% despite the extreme level of redundancy.
In a separate project to build mammalian AND-logic transcriptional circuits 2, four additional five-part assemblies of up to 27 kb in size were attempted. These attempts achieved a cumulative accuracy of 90% (18/20 correctly assembled), despite each part carrying repeated HS4 insulator sequences. Data regarding two of these four attempts are reported in Figure 4B.
More recently we have used UNS-guided assembly to construct five-part succinic acid production plasmids with assembly accuracies of ~88%, and have expanded our destination vectors to include broad-host-range plasmids such that UNS-guided assembly and optimization of metabolic pathways can be performed in non-model organisms (Torella et al., unpublished data; pDestBBR in Table 3 is one of these vectors and may be requested from our lab). We are also using UNS-guided assembly to develop more sophisticated mammalian cell circuits for logical computation. Further information on these and other efforts in UNS-guided assembly will be made public in http://openwetware.org/wiki/Silver_Lab.
Acknowledgments
The authors wish to thank C.T. Walsh, T.A. Wencewicz, P. Malkus and J. Paulsson for plasmids and reagents, and T.J. Ford, D.C. MacKellar, A.H. Chen and H.M. Salis for helpful discussions relating to this work. This work was supported by the Advanced Research Projects Agency-Energy ‘Electrofuels’ Collaborative Agreement [grant number DE-AR0000079 to P.A.S.]; a National Science Foundation Graduate Research Fellowship and Herchel Smith Graduate Research Fellowship to [J.P.T.]; a European Molecular Biology Organization and Human Frontier Science Program Fellowship to [F.L.]; a German National Academic Foundation Scholarship to [C.R.B]; a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship to [J.H.C.]; and the Defense Advanced Research Projects Agency [grant number 4500000572 to P.A.S.]. This material is based upon work supported by the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
CONTRIBUTIONS
J.P.T. conceived the project.
J.P.T., C.R.B., F.L., J.H.C., P.A.S., and J.C.W. contributed key ideas to the development of the project.
P.A.S. and J.C.W. supervised the project.
J.P.T., C.R.B., F.L. and J.H.C. performed the experiments.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Torella JP, et al. Rapid construction of insulated genetic circuits via synthetic sequence-guided isothermal assembly. Nucleic acids research. 2014;42:681–689. doi: 10.1093/nar/gkt860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lienert F, et al. Two-and three-input TALE-based AND logic computation in embryonic stem cells. Nucleic acids research. 2013;41:9967–9975. doi: 10.1093/nar/gkt758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keasling JD. Synthetic biology for synthetic chemistry. Acs Chemical Biology. 2008;3:64–76. doi: 10.1021/cb7002434. [DOI] [PubMed] [Google Scholar]
- 4.Torella JP, et al. Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proceedings of the National Academy of Sciences. 2013;110:11290–11295. doi: 10.1073/pnas.1307129110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prather KLJ, Martin CH. De novo biosynthetic pathways: rational design of microbial chemical factories. Current Opinion in Biotechnology. 2008;19:468–474. doi: 10.1016/j.copbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Savage DF, Way J, Silver PA. Defossiling fuel: How synthetic biology can transform biofuel production. Acs Chemical Biology. 2008;3:13–16. doi: 10.1021/cb700259j. [DOI] [PubMed] [Google Scholar]
- 7.Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nature Reviews Genetics. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purnick PEM, Weiss R. The second wave of synthetic biology: from modules to systems. Nature Reviews Molecular Cell Biology. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- 9.Bowen TA, Zdunek JK, Medford JI. Cultivating plant synthetic biology from systems biology. New Phytologist. 2008;179:583–587. doi: 10.1111/j.1469-8137.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 10.Lienert F, Lohmueller JJ, Garg A, Silver PA. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nature reviews. Molecular cell biology. 2014;15:95–107. doi: 10.1038/nrm3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruder WC, Lu T, Collins JJ. Synthetic Biology Moving into the Clinic. Science. 2011;333:1248–1252. doi: 10.1126/science.1206843. [DOI] [PubMed] [Google Scholar]
- 12.Weber W, Fussenegger M. Emerging biomedical applications of synthetic biology. Nature Reviews Genetics. 2012;13:21–35. doi: 10.1038/nrg3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benenson Y. Biomolecular computing systems: principles, progress and potential. Nature reviews. Genetics. 2012;13:455–468. doi: 10.1038/nrg3197. [DOI] [PubMed] [Google Scholar]
- 14.Bugaj LJ, Schaffer DV. Bringing next-generation therapeutics to the clinic through synthetic biology. Curr Opin Chem Biol. 2012;16:355–361. doi: 10.1016/j.cbpa.2012.04.009. S1367-5931(12)00058-0 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Chen YY, Galloway KE, Smolke CD. Synthetic biology: advancing biological frontiers by building synthetic systems. Genome Biol. 2012;13:240. doi: 10.1186/gb-2012-13-2-240. gb-2012-13-2-240 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agapakis CM, et al. Insulation of a synthetic hydrogen metabolism circuit in bacteria. Journal of biological engineering. 2010;4:3. doi: 10.1186/1754-1611-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YJ, et al. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Methods. 2013;10:659–664. doi: 10.1038/nmeth.2515. nmeth.2515 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Litcofsky KD, Afeyan RB, Krom RJ, Khalil AS, Collins JJ. Iterative plug-and-play methodology for constructing and modifying synthetic gene networks. Nat Methods. 2012;9:1077–1080. doi: 10.1038/nmeth.2205. nmeth.2205 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelcbuch L, et al. Spanning high-dimensional expression space using ribosome-binding site combinatorics. Nucleic Acids Research. 2013;41 doi: 10.1093/nar/gkt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esvelt KM, Wang HH. Genome-scale engineering for systems and synthetic biology. Molecular Systems Biology. 2013;9 doi: 10.1038/msb.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6:343-U341. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 22.Gibson DG, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008;319:1215–1220. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- 23.Gibson DG, Smith HO, Hutchison CA, Venter JC, Merryman C. Chemical synthesis of the mouse mitochondrial genome. Nature Methods. 2010;7:901-U905. doi: 10.1038/nmeth.1515. [DOI] [PubMed] [Google Scholar]
- 24.Guye P, Li Y, Wroblewska L, Duportet X, Weiss R. Rapid, modular and reliable construction of complex mammalian gene circuits. Nucleic acids research. 2013;41:e156–e156. doi: 10.1093/nar/gkt605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casini A, et al. One-pot DNA construction for synthetic biology: the Modular Overlap-Directed Assembly with Linkers (MODAL) strategy. Nucleic acids research. 2014;42:e7–e7. doi: 10.1093/nar/gkt915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramon A, Smith HO. Single-step linker-based combinatorial assembly of promoter and gene cassettes for pathway engineering. Biotechnol Lett. 2011;33:549–555. doi: 10.1007/s10529-010-0455-x. [DOI] [PubMed] [Google Scholar]
- 27.Du J, Yuan Y, Si T, Lian J, Zhao H. Customized optimization of metabolic pathways by combinatorial transcriptional engineering. Nucleic Acids Res. 2012;40:e142. doi: 10.1093/nar/gks549. gks549 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight TF. Idempotent Vector Design for Standard Assembly of Biobricks. Tech Rep, MIT Synthetic Biology Working Group Technical Reports. 2003 [Google Scholar]
- 29.Anderson JC, et al. BglBricks: A flexible standard for biological part assembly. Journal of biological engineering. 2010;4:1. doi: 10.1186/1754-1611-4-1. 1754-1611-4-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borujeni AE, Channarasappa AS, Salis HM. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic acids research. 2013 doi: 10.1093/nar/gkt1139. gkt1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PloS one. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler C, Gruetzner R, Kandzia R, Marillonnet S. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PloS one. 2009;4:e5553. doi: 10.1371/journal.pone.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan J, Tian J. Circular polymerase extension cloning of complex gene libraries and pathways. PloS one. 2009;4:e6441. doi: 10.1371/journal.pone.0006441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan J, Tian J. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nature protocols. 2011;6:242–251. doi: 10.1038/nprot.2010.181. [DOI] [PubMed] [Google Scholar]
- 35.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 36.Xiong AS, et al. PCR-based accurate synthesis of long DNA sequences. Nature protocols. 2006;1:791–797. doi: 10.1038/nprot.2006.103. [DOI] [PubMed] [Google Scholar]
- 37.de Kok S, et al. Rapid and reliable DNA assembly via ligase cycling reaction (LCR) ACS Synthetic Biology. 2014 doi: 10.1021/sb4001992. [DOI] [PubMed] [Google Scholar]
- 38.Bayer TS, et al. Synthesis of methyl halides from biomass using engineered microbes. Journal of the American Chemical Society. 2009;131:6508–6515. doi: 10.1021/ja809461u. [DOI] [PubMed] [Google Scholar]
- 39.Balibar CJ, Walsh CT. In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum. Biochemistry. 2006;45:15444–15457. doi: 10.1021/bi061998z. [DOI] [PubMed] [Google Scholar]