Abstract
There is limited information in the use of antitumor necrosis factor α, infliximab, in patients on hemodialysis. In Crohn’s disease (CD), only 3 cases are reported.
A 76-year-old man on hemodialysis for renal failure caused by immunoglobulin A nephropathy developed diarrhea and abdominal pains. A marked edema was observed in the pretibia and ankle. An increase of C-reactive protein (CRP) and erythrocyte sedimentation rate, hypoalbuminemia, hypocholesterolemia, and moderate anemia was found. Ultrasonography and computed tomography (CT) found wall thickness in the left colon. Sigmoidoscopy revealed multiple ulcers in the sigmoid colon and noncaseating epithelioid granuloma was found in the biopsy specimen. Barium enema study exhibited collar button signs and longitudinal ulcers in the left colon.
A severe form of CD was diagnosed. Metronidazole seemed to decrease CRP but was ineffective in ameliorating diarrhea. Infliximab rather than steroid hormone was chosen for the treatment. Standard induction therapy with infliximab was initiated. Symptoms rapidly improved then disappeared. CD activity index decreased from 747 to a remission level of 134 after 2 infusions of infliximab. Scheduled maintenance infliximab therapy was administered after the induction therapy. Ultrasonography and CT showed a disappearance of the wall thickness of the colon. Adverse reactions were not observed.
Infliximab was effective and safe in a patient with CD on hemodialysis. Our case has added additional literature in accordance with previous reports supporting infliximab as effective and safe in patients on hemodialysis.
INTRODUCTION
The efficacy of antitumor necrosis factor α (TNF-α), infliximab, has been shown to be effective in various disease treatment including Crohn’s disease (CD).1 However, there is limited information in the use of infliximab in patients on hemodialysis. Although the highest incidence in CD is among younger generations, 10–30 years old, older people are also affected.2 Here we report a case of an old man on hemodialysis who developed CD and was successfully treated with infliximab.
CASE REPORT
A 76-year-old man, 166 cm tall and weighing 47.1 kg, receiving hemodialysis for 6 years was referred to our Division of Gastroenterology, Akita City Hospital, Akita City, Japan, in the middle of November 2013, because of diarrhea, abdominal pain, and fever for 1 week. Hematochezia was absent. He was diagnosed with Wolff-Parkinson-White syndrome in 1973, paroxysmal atrial fibrillation in 1982, myocardial infarction in 1993, and immunoglobulin A (IgA) nephropathy in 2005. IgA nephropathy resulted in renal failure leading to hemodialysis thrice a week since 2007. Tenderness in the left lower abdomen and a marked edema in both sides of the pretibia and ankle were observed. Laboratory data were as follows: an increase of C-reactive protein (CRP), 10.73 mg/dL (normal range ≤0.19); an increase of erythrocyte sedimentation rate, 46 mm/h; hypoalbuminemia, 2.0 g/dL; hypocholesterolemia, 107 mg/dL; and moderate anemia, hemoglobin 9.0 g/dL (Figure 1). Ultrasonography and computed tomography (CT) found wall thickness in continuous form in the left colon. Sigmoidoscopy revealed multiple irregular-shaped ulcers in the sigmoid colon (Figure 2). Barium enema study exhibited collar button ulcers, longitudinal ulcers, and coarse mucosa in the left colon (Figure 3). Negative results were obtained in the following tests: cytomegalovirus antigenemia, stool culture, and Clostridium difficile toxin. Neither inclusion body nor immunohistologic cytomegalovirus positive cell was found in the biopsy specimen. Neither ghost tubules nor subepithelial collagen thickening were observed. But noncaseating epithelioid granuloma was found. Therefore, a severe form of CD was diagnosed.3 Metronidazole seemed to decrease CRP but was ineffective in ameliorating diarrhea. Rapid efficacy of infliximab in CD4 and reports of safety of infliximab in patients on hemodialysis5–12 encouraged us and our patient to use infliximab rather than steroid hormone that has an adverse osteoporotic effect. Latent tuberculosis was excluded by normal roentgenogram of the chest and negative Elispot assay for Mycobacterium tuberculosis.13 Hepatitis B infection was also excluded by negative test in surface antigen, surface antibody, and core antibody.13 Written informed consent on the use of infliximab and publishing the study was obtained. The standard induction therapy (3 infusions at week 0, 2, and 6) of infliximab (300 mg) was initiated.14 Infliximab was infused on alternative days to hemodialysis. Symptoms rapidly improved then disappeared. Crohn’s disease activity index (CDAI) decreased from 747 to a remission level (less than 150) of 1343 after 2 infusions of infliximab. CRP became normal and increase of albumin was ascertained before discharge (Figure 1). A third infusion of infliximab was administered as planned (Figure 1). Although albumin was further increased to 2.9 g/dL, fecal occult blood was more than 1000 ng/mL (Figure 1) indicating that there is still active inflammation in the intestine. Scheduled maintenance infliximab therapy15 was administered. An easy tendency to arrhythmia and tachycardia and renal failure hampered invasive morphological studies of the bowel after the standard induction therapy. Noninvasive studies with ultrasonography and CT found a disappearance of the wall thickness of the colon. Adverse reactions of infliximab were not observed during the course.
FIGURE 1.

Clinical course. CRP (normal range ≤0.19 mg/dL); ESR (≤10 mm/h); fecal occult blood (<100 ng/mL); T chol (≥120 to ≤220 mg/dL). CDAI = Crohn’s disease activity index; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; IFX = infliximab; T chol = total cholesterol.
FIGURE 2.

Endoscopic pictures of sigmoidoscopy. Inflamed mucosa with (A) multiple irregular-shaped ulcers (arrow) and (B) punched-out ulcer (arrow) was observed in the sigmoid colon.
FIGURE 3.
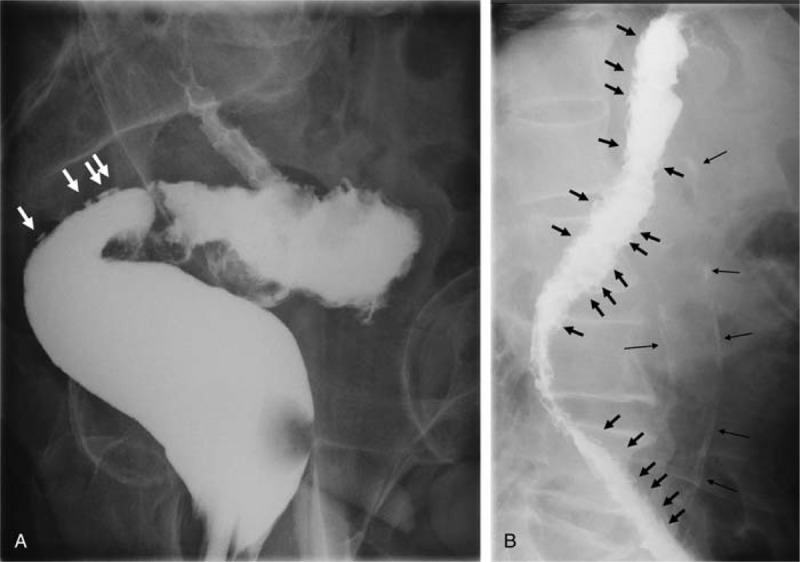
Radiograph of barium enema study. Clear collar button signs were observed in the (A) sigmoid colon (arrows) and the (B) descending colon (short arrows). Long fine arrows in (B) show calcification of the aorta.
DISCUSSION
Collar button signs and longitudinal ulcers are observed in both CD and ulcerative colitis.16–18 In the present case, an absent hematochezia and a positive noncaseating epithelioid granuloma led to a diagnosis of CD.
Biologic agents revolutionized treatment in medicine. TNF-α is a key molecule of the inflammatory response and is involved in the pathogenesis of various autoimmune and noninfectious inflammatory conditions. TNF-α antagonists have been shown to be effective in CD, ulcerative colitis, rheumatoid arthritis, Behcet’s disease, ankylosing spondylitis, uveitis, psoriasis, pyoderma gangrenosum, and hidradenitis suppurativa.1 In CD, TNF-α antagonists are effective in inducing and maintaining remission,15 systemic complications such as pyoderma gangrenosum and arthritis,19,20 and secondary amyloidosis.21 Infliximab was the first biologic agent approved in Japan for CD in 2002 and for rheumatoid arthritis in 2003. There are very few reports on the use of infliximab in patients on dialysis: rheumatoid arthritis,6,7 ankylosing spondylitis,9 sarcoidosis,5 and psoriatic arthritis.8 In CD, 3 case reports are available and all are from Japan.10–12 In these reports, CD was first diagnosed followed by renal failure resulting in hemodialysis. Infliximab was used for enterocutaneous fistula,10 relapse of CD,11 and uncontrolled activity with conventional treatment.12 Conventional treatment such as 5-aminosalicylate and steroid hormone was also ineffective in the former 2 cases.10,11 Therefore, in all the 3 cases of CD, infliximab was used after conventional therapy. Infliximab was effective in these patients including CD.5–12 Adverse effects were described in 3 cases: postinfusion transient itching,7 deep vein thrombosis,5 and pneumocystis jivoreci pneumonia.11 The latter 2 hampered the succession of infliximab treatment. Except for these 2 cases, scheduled infliximab maintenance therapy was administered in these diseases.6–10,12 Needless to say, attention to be paid to a variety of adverse events associated with infliximab.22–25 Pharmacokinetics of TNF-α antagonists in patients on dialysis is not known. However, Kume et al,12 in CD, measured the serum concentration of infliximab before and after hemodialysis and showed that the serum level of infliximab was essentially unchanged by hemodialysis. In our case, CD appeared in a patient on hemodialysis. No adverse event of infliximab was encountered.
The number of patients on regular dialysis treatment in Japan is steadily increasing: 103,296 in 1990, 206,134 in 2000, and 309,946 in 2012.26 The primary disease for dialysis is diabetic nephropathy holding the first place since 1998 accounting for 44.1% followed by chronic glomerulonephritis in the second place (19.4%). The mean age for initiation of hemodialysis is 68.4 years.26 The number of patients with CD is also steadily increasing in Japan: 6609 in 1990, 19,651 in 2000, and 36,418 in 2012.27 Both diabetes mellitus and CD are popular in wealthy nations and they are not restricted to western countries anymore but distributed worldwide and known as global diseases.28,29 It is anticipated that the number of CD patient on dialysis will increase in the future not only in Japan but also worldwide.
Our case has contributed additional literature in accordance with previous reports supporting infliximab as effective and safe in patients on hemodialysis.
Footnotes
Abbreviation CD = Crohn’s disease, CDAI = Crohn’s disease activity index, CRP = C-reactive protein, CT = computed tomography, ESR = erythrocyte sedimentation rate, IFX = infliximab, T chol = total cholesterol, TNF-α = TNF-αtumor necrosis factor α
The authors have no funding and conflicts of interest to disclose.
References
- 1.Karampetsou MP, Liossis SN, Sfikakis PP. TNF-α antagonists beyond approved indications: stories of success and prospects for future. QJM. 2010;103:917–928. [DOI] [PubMed] [Google Scholar]
- 2.Tokayer AZ, Brandt LJ. Idiopathic inflammatory bowel diseases in the elderly. In: Inflammatory Bowel Disease. Philadelphia, PA: W.B. Saunders; 2000:335–341. [Google Scholar]
- 3.Lichtenstein GR, Hanauer SB, Sandborn WJ. The Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. [DOI] [PubMed] [Google Scholar]
- 4.Chiba M, Sugawara T, Tsuda H, et al. Esophageal ulcer of Crohn’s disease: disappearance in 1 week with infliximab. Inflamm Bowel Dis. 2009;15:1121–1122. [DOI] [PubMed] [Google Scholar]
- 5.Yee AM, Pochapin MB. Treatment of complicated sarcoidosis with infliximab anti-tumor necrosis factor-alpha therapy. Ann Intern Med. 2001;135:27–31. [DOI] [PubMed] [Google Scholar]
- 6.Singh R, Cuchacovich R, Huang W, et al. Infliximab treatment in a patient with rheumatoid arthritis on hemodialysis. J Rheumatol. 2002;29:636–637. [PubMed] [Google Scholar]
- 7.Hammoudeh M. Infliximab treatment in a patient with rheumatoid arthritis on haemodialysis. Rheumatology. 2006;45:357–359. [DOI] [PubMed] [Google Scholar]
- 8.Saougou I, Papagoras C, Markatseli TE, et al. A case report of a psoriatic arthritis patient on hemodialysis treated with tumor necrosis factor blocking agent and a literature review. Clin Rheumatol. 2010;29:1455–1459. [DOI] [PubMed] [Google Scholar]
- 9.Marocchi E, Spadaro A, Giannakakis K, et al. Infliximab in a patient with ankylosing spondylitis and secondary IgA nephropathy requiring haemodialysis. Clin Exp Rheumatol. 2010;28:440. [PubMed] [Google Scholar]
- 10.Morita H. Infliximab treatment of a patient on hemodialysis with fistulating Crohn’s disease: the Japanese experience. Shinyaku To Rinsho (in Japanese, abstract in English). J New Rem Clin. 2007;56:675–680. [Google Scholar]
- 11.Kanai H, Noguchi T, Koyanagi H, et al. Infliximab treatment in a hemodialysis patient with relapse of Crohn’s disease after a 40-year interval. Nihon Tohseki Igakukai Zashi (in Japanese, abstract in English). J Jpn Soc Dial Ther. 2009;42:905–911. [Google Scholar]
- 12.Kume K, Yamasaki M, Yoshikawa I, et al. Infliximab treatment in a patient with Crohn’s disease on haemodialysis. Colorectal Dis. 2011;13:341. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn BP, Doherty GA, Gautam S, et al. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012;18:1057–1063. [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn’s disease: a user’s guide for clinicians. Am J Gastroenterol. 2002;97:2962–2972. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 16.Margulis AR, Goldberg HI, Lawson TL, et al. The overlapping spectrum of ulcerative and granulomatous colitis: a roentgenographic-pathologic study. Am J Roent Rad Ther Nucl Med. 1971;113:325–334. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein JE, Madewell JE, Feigin DS. The Collar button ulcer A radiologic–pathologic correlation. Gastrointest Radiol. 1979;4:79–84. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa T, Takei S, Nozawa A, et al. A case of ulcerative colitis associated with longitudinal ulcers (in Japanese, abstract in English). Endosc Forum Dig Dis. 1996;12:248–252. [Google Scholar]
- 19.Brooklyn TN, Dunnill MGS, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int. 2004;25:406–410. [DOI] [PubMed] [Google Scholar]
- 21.Pukitis A, Zake T, Groma V, et al. Effect of infliximab induction therapy on secondary systemic amyloidosis associated with Crohn’s disease: case report and review of the literature. J Gastrointestin Liver Dis. 2013;22:333–336. [PubMed] [Google Scholar]
- 22.Kaur N, Mahl TC. Pneumocystis jivoreci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481–1484. [DOI] [PubMed] [Google Scholar]
- 23.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–1276. [DOI] [PubMed] [Google Scholar]
- 24.Kerbleski JF, Gottlieb AB. Dermatological complications and safety of anti-TNF treatments. Gut. 2009;58:1033–1039. [DOI] [PubMed] [Google Scholar]
- 25.Bessissow T, Renard M, Hoffman I, et al. Review article: non-malignant haematological complications of anti-tumor necrosis factor alpha therapy. Aliment Phamacol Ther. 2012;36:312–323. [DOI] [PubMed] [Google Scholar]
- 26. Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan as of Dec. 31, 2012. 2014. http://docs.jsdt.or.jp/overview/index.html Accessed February 6, 2014. [Google Scholar]
- 27.The Ministry of Health, Welfare, and Labor, Japan. Japan Intractable Diseases Information Center. 2014. http://www.nanbyou.or.jp/entry/1356 Accessed February 19, 2014. [Google Scholar]
- 28.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systemic review. Gastroenterology. 2012;142:46–54. [DOI] [PubMed] [Google Scholar]
- 29.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. [DOI] [PubMed] [Google Scholar]


