Abstract
Background
Proteins pathogenic in Alzheimer’s disease (AD) were extracted from neurally-derived blood exosomes and quantified to develop biomarkers for staging of sporadic AD.
Methods
Blood exosomes obtained at one time-point from patients with AD (n=57) or frontotemporal dementia (FTD) (n=16), and at two time-points from others (n=24) when cognitively normal and one-ten years later when diagnosed with AD were enriched for neural sources by immunoabsorption. AD-pathogenic exosomal proteins were extracted and quantified by ELISAs.
Results
Mean exosomal levels of total Tau, P-T181-tau, P-S396-tau and Aβ1-42 for AD and levels of P-T181-tau and Aβ1-42 for FTD were significantly higher than for case-controls. Stepwise discriminant modeling incorporated P-T181-tau, P-S396-tau and Aβ1-42 in AD, but only P-T181-tau in FTD. Classification of 96.4% of AD patients and 87.5% of FTD patients was correct. In 24 AD patients, exosomal levels of P-S396-tau, P-T181-tau and Aβ1-42 were significantly higher than for controls both one to ten years before and when diagnosed with AD.
Conclusions
Levels of P-S396-tau, P-T181-tau and Aβ1-42 in extracts of neurally-derived blood exosomes predict development of AD up to 10 years prior to clinical onset.
Keywords: preclinical AD, neural exosomes, P-tau, Aβ1-42, biomarkers
1. Introduction
Roles in the pathogenesis of Alzheimer’s disease (AD) have been attributed to altered proteins accumulating inside and on the surface of neurons [1, 2]. Increases in brain tissue oligomeric amyloid β (Aβ) peptides and phosphorylated tau (P-tau) detected by CNS imaging and in cerebrospinal fluid (CSF) levels of soluble amyloid Aβ1-42 and P-tau have been documented years before signs of AD [3–6]. Times for progression from preclinical stages to clinically apparent AD with threshold detectable amyloid deposition and abnormal elevation of CSF P-tau proteins are estimated to be up to 17 years [3, 5]. The potential prognostic sensitivity of protein biomarkers is supported by the timing of induction of AD-like disease in rodent models after transgenic over-expression of putatively neuropathogenic proteins [7–9].
In recent studies, low CSF levels of Aβ1-42 and high CSF levels of P-tau, as well as positive CNS images of amyloid deposits accurately predicted development of mild cognitive impairment (MCI) and probable AD [10, 11]. However, there was substantial overlap in these biomarkers between patients who subsequently developed AD and those who later manifested other forms of dementia or no signs of dementia, even when concentrations of these CSF proteins were considered together or as ratios. The overlap was even greater when plasma levels of these proteins were used for diagnosis or prediction [12–15]. This high level of prognostic uncertainty combined with the morbidity and expense of repeated CSF sampling and of neuroimaging procedures emphasizes the importance of developing accurate blood-based tests that predict high risk for AD and distinguish AD from other forms of dementia.
Exosomes are one class of endosome-derived membrane vesicles shed by neural cells, that contain proteins and other constituents of their cellular origin [16]. Exosomes accept amyloid precursor protein (APP) from early endosomes, after its cleavage by β-secretase, and the Aβ peptide fragments subsequently generated by γ-secretase are secreted in exosomes [17]. Although this exosome pathway accounts for only a small portion of the total Aβ peptides in neural plaques, it constitutes a prionoid-like mechanism for CNS spread of proteinopathies [18]. The detection of exosome signature proteins in neural amyloid plaques supports the possibility of their role in generation of AD-associated lesions [17]. Here we use a combination of chemical and immunochemical methods to harvest and enrich neurally-derived exosomes from small volumes of plasma or serum in quantities that provide readily detectable amounts of proteins implicated in the pathogenesis of AD.
Materials and methods
2.1. Study design, subject characterization, and blood collection
Fifty-seven patients with amnestic MCI (aMCI) or dementia attributable to AD, who had donated blood at one time-point, were identified retrospectively at the Clinical Research Unit of the National Institute on Aging (CRU-NIA) in Harbor Hospital, Baltimore, MD, at the Jewish Home of San Francisco (JHSF), San Francisco, CA and in the neurology clinical services of the University of Rochester, Rochester, NY (UR), the University of California, Irvine, CA (UCI) and Georgetown University Medical Center, Washington, DC (GUMC) (Table 1). Twenty-four additional patients with AD had provided blood at two time-points in studies at the Mayo Clinic (MC) and the University of Kentucky (UK), first when cognitively intact and later when diagnosed with AD. For both groups, the diagnosis of AD had been established according to the revised NINDS-ADRDA criteria [19]. The patients classified as having aMCI had a Clinical Dementia Rating (CDR) global score of 0.5 [20]. Those with AD and mild to moderate dementia had a CDR global score of 1.0. Twenty-eight of the 57 single-time sample AD patients were taking an acetyl-cholinesterase inhibitor and/or memantine, and 12 were on anti-depressant medications; blood was drawn at least eight hours after their last medication.
Table 1.
Characteristics of Patients and Control Subjects
| Diagnosis
|
Total
|
MCI
|
Dementia
|
||||
|---|---|---|---|---|---|---|---|
| Number | Male/Female | Ages Mean±S.D (range) |
number | MMSE Scores Mean±S.E.M |
number | MMSE Scores Mean±S.E.M |
|
| AD | 57 | 30/27 | 79.5±6.05 (64–90) | 29 | 27.6±0.30 | 28 | 22.9±1.02** |
| AC | 57 | 30/27 | 79.6±6.03 (64–90) | 0 | 0 | ||
| Mild Dementia
|
Moderate Dementia
|
||||||
| FTD | 16 | 12/4 | 63.1±8.79 (48–79) | 9 | 26.7±0.73 | 7 | 15.0±3.65* |
| FTC | 16 | 12/4 | 63.7±7.43 (48–79) | 0 | 0 | ||
The significance of differences in values between the MCI/Mild Dementia and Dementia/Moderate Dementia groups were calculated by an unpaired t test;
p<0.01 and
p<0.001.
MMSE = Mini-Mental State Examination.
Sixteen patients with behavioral variant FTD (bv-FTD) had been evaluated and selected for study at the Memory and Aging Center of the Department of Neurology of the University of California, San Francisco (MAC) (Table 1). Their diagnosis and assignment to mild dementia or moderate dementia groups (Table 1) was based on standard clinical, mental status and psychiatric criteria, including discriminant analyses of neuropsychiatric elements, phonological performance and object understanding that distinguish FTD from AD [21, 22]. Seven of the FTD patients were receiving an anti-depressant, two were taking an acetyl-cholinesterase inhibitor and one was on memantine. Ninety-two cognitively normal subjects were recruited at the JHSF, CRU-NIA and GUMC to be age- and gender-matching controls for the several groups of AD and FTD patients (five served as controls for two clinical groups). Each subject studied and some patient-designates signed a consent form approved with the study protocol at each institution. All plasma and serum was stored at −80°C.
2.2. Isolation of exosomes from plasma or serum for ELISA quantification of exosome proteins
One-half ml of plasma was incubated with 0.15 ml of thromboplastin-D (Fisher Scientific, Inc., Hanover Park, IL) at room temperature for 60 min, followed by addition of 0.35 ml of calcium- and magnesium-free Dulbecco’s balanced salt solution (DBS−2) with protease inhibitor cocktail (Roche Applied Sciences, Inc., Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce Halt, Thermo Scientific, Inc., Rockford, IL). For serum, 0.5 ml was mixed with 0.5 ml of DBS−2 containing the inhibitor cocktails. After centrifugation at 1,500 x g for 20 min, supernates were mixed with 252 μl of ExoQuick exosome precipitation solution (EXOQ; System Biosciences, Inc., Mountainview, CA), and incubated for 1 hr at 4°C. Resultant exosome suspensions were centrifuged at 1,500 x g for 30 min at 4°C and each pellet was re-suspended in 150 μl of DBS−2 with inhibitor cocktails before immunochemical enrichment of exosomes from a neural source, as described for immune cell exosomes [23].
Each sample received 100 μL of 3% BSA (1:3.33 dilution of Blocker BSA 10% solution in DBS−2 [Thermo Scientific, Inc.]) and was incubated for 1 hr at 4°C each with 2 μg of mouse anti-human NCAM antibody (ERIC 1, sc-106, Santa Cruz Biotechnology, Santa Cruz, CA), that had been biotinylated with the EZ-Link sulfo-NHS-biotin system (Thermo Scientific, Inc.), or for some preparations with 1 μg of mouse anti-human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) and then 25 μl of streptavidin-agarose resin (Thermo Scientific, Inc.) plus 50 μL of 3% BSA. After centrifugation at 200 xg for 10 min at 4°C and removal of the supernate, each pellet was suspended in 50 μL of 0.05 M glycine-HCl (pH 3.0) by vortexing for 10 sec. Each suspension then received 0.45 ml of DBS−2 with 2 g/100 ml of BSA, 0.10 % Tween 20 and the inhibitor cocktails followed by incubation for 10 min at 37°C with vortex-mixing and was storage at −80°C prior to ELISAs. Relative yields of exosomes from plasma and serum at this stage were compared using both sources from six patients with AD. The respective mean levels of P-T181-tau and CD81 extracted from serum-derived exosomes were 58% and 56% of that from plasma-derived exosomes. Although the yield from serum was lower, it was correctible by normalization for the exosome marker CD81 as shown [23]. To recover exosomes for counting, immunoprecipitated pellets were re-suspended in 0.25 ml of 0.05 M glycine-HCl (pH=3.0) at 4°C, centrifuged at 200 x g for 15 min and supernate pH adjusted to 7.0 with 1 M Tris-HCl (pH 8.6). Exosome suspensions were diluted 1:200 to permit counting in the range of 3–15 × 108/ml, with an NS500 nanoparticle tracking system (NanoSight, Amesbury, UK), as described [23].
Exosome proteins were quantified by ELISA kits for human Aβ1-42, human total tau and human P-S396-tau (Life Technologies/Invitrogen, Camarillo, CA), human P-T181-tau (Innogenetics Division of Fujirebio US, Inc., Alpharetta, GA) and human CD81 (Hölzel Diagnostika-Cusabio, Cologne, Germany) with verification of the CD81 antigen standard curve using human purified recombinant CD81 antigen (Origene Technologies, Inc., Rockville, MD), according to suppliers’ directions. The mean value for all determinations of CD81 in each assay group was set at 1.00 and the relative values for each sample used to normalize their recovery. The minor constituent of secreted neural exosomes P-S396-tau, as well as the usually examined major neural exosome component P-T181-tau were both quantified to provide more complete information about the possible relationship between neurally secreted and plasma neurally-derived exosome constituents in AD and FTD [24].
2.3. Statistical analyses
The statistical significance of differences between group means for patients with AD or FTD and their respective normal controls was determined with an unpaired t test including a Bonferroni correction in the interpretation (GraphPad Prism 6, La Jolla, CA). The significance of differences between serial values for AD patients taken before and after onset of aMCI or dementia was calculated with a paired t test (GraphPad). Separate discriminant classifier analyses were conducted to define the best simple linear models for comparing AD with AC and FTD with FTC. Two discriminant analyses considered all variables and were performed step-wise. Final models retained only variables with a minimum partial F of 3.84 to enter and 2.71 to remove. Prior probabilities were considered equal for all groups. Fisher Function Coefficients and within group covariances were computed. Receiver operating characteristics (ROC) analyses were conducted under the non-parametric distribution assumption for Standard Error of Area to determine the performance of the models for discriminating AD from AC and FTD from FTC. Discriminant and ROC analyses were conducted with SPSS v21.0 (IBM).
3. Results
3.1. Patient characteristics
The 57 patients with AD consisted nearly equally of those with aMCI or dementia, with the latter group having significantly lower MMSE scores (P< .001) (Table 1). The 16 patients with FTD had nearly equal numbers with mild or moderate dementia, with greater severity for the latter group documented by the significantly lower MMSE scores (P< .01). As cognitively normal control subjects were matched individually with patients, group male/female ratios and mean (±SD) ages were expectedly nearly equal.
3.2. Exosomal protein levels
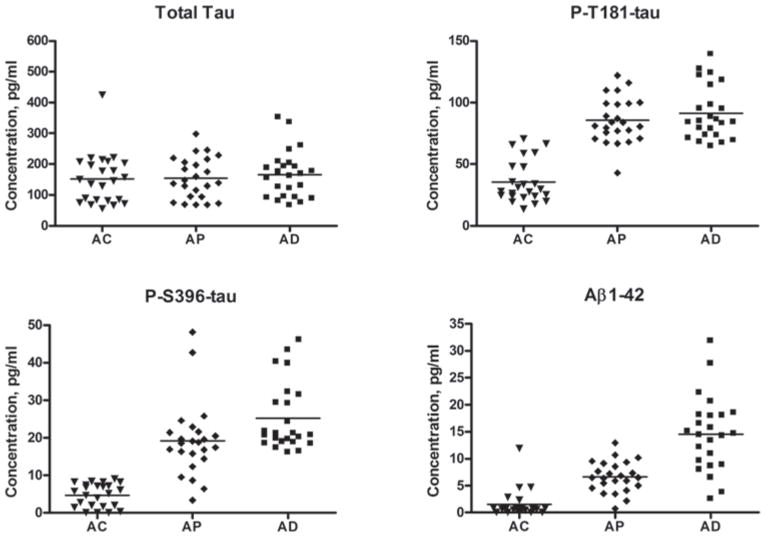
Cross-sectional comparisons of results of one-time studies of 57 AD patients and 57 matched case-controls (AC) revealed that AD exosomal concentrations of total tau (191±12.3 pg/ml, mean ± SEM, P= .0005), P-T181-tau (106±6.10 pg/ml, P< .0001), P-S396-tau (25.4±2.25 pg/ml, P< .0001) and Aβ1-42 (18.5±2.97 pg/ml, P< .0001) were significantly higher than for AC (130±11.9 pg/ml, 16.9±1.89 pg/ml, 3.88±0.26 pg/ml and 0.83±0.13 pg/ml, respectively) (Fig. 1). P-S396-tau levels showed the least overlap with only five AD values in the AC range, of which two had P-T181-tau levels, two others had Aβ1-42 levels and one had both P-T181-tau and Aβ1-42 levels above the AC range. Thus the AD profile of these three exosomal proteins together was completely distinct from that of AC. Step-wise discriminant analyses resulted in a model progressively incorporating P-T181-tau, P-S396-tau and Aβ1-42, but not total tau, which produced a Wilk’s Lambda of 0.229 and an exact F of 119 (P< .001). The final model correctly classified 96.4% of MCI/AD patients contrasted with AC subjects (93% of MCI/AD and 100% of AC). The Area Under the Curve (AUC) for the final model from the ROC analysis was 0.999 and individual AUC values for the individual proteins were 0.991, 0.988, 0.987 and 0.731, respectively, for P-T181-tau, P-S396-tau, Aβ1-42 and total tau (Fig. 1S).
Fig. 1.
Levels of proteins in blood exosomes of patients with AD, FTD and cognitively normal matched case-controls (AC, FTC). The horizontal line in each cluster here and in Fig. 2 depicts the mean for that set.
Cross-sectional comparisons of the results of one-time studies of 16 FTD patients and 16 matched case-controls (FTC), showed that FTD exosomal concentrations of P-T181-tau (82.6±9.20 pg/ml, mean ± SEM) and Aβ1-42 (7.54±1.01 pg/ml) were significantly higher than for the FTC group (9.32±2.86 pg/ml and 0.76±0.35 pg/ml, respectively)(both P< .0001), whereas those of total tau and P-S396-tau did not differ significantly between the FTD (135±15.8 pg/ml and 2.13±0.33 pg/ml, respectively) and FTC (148±30.1 pg/ml and 3.13±0.46 pg/ml) groups (Fig. 1). Fourteen of the 16 levels of P-T181-tau for FTD patients were higher than the upper end of the range for FTC subjects and the Aβ1-42 levels for the two FTD patients with P-T181-tau levels in the FTC range both were above the FTC Aβ1-42 range. In a step-wise discriminant analysis, only P-T181-tau entered the model, and attained a Wilk’s Lambda value of 0.324 and an exact F of 62.5 (P< .001). In the final model, exosomal P-T181-tau correctly classified 87.5% of FTD patients contrasted with FTC subjects (75% of FTD and 100% of FTC). For the final model from the ROC analysis, AUC for P-T181-tau was 0.992 and for Aβ1-42 was 0.969. Most remarkably and in contrast to the AD group, none of the concentrations of P-S396-tau for the FTD group was higher than the upper end of the range for the FTC subjects.
Resuspended initial precipitates from control subjects (n=3) and AD patients with dementia (n=3), respectively, contained 3.49±0.90 × 109 exosomes/ml of plasma (mean±SEM) and 2.78±0.26 × 109 exosomes/ml of plasma as determined by the Nanosight system. Suspensions of immunoabsorbed exosomes from the same initial suspensions contained 0.417±0.023 × 109 exosomes/ml of plasma and 0.472±0.090 × 109 exosomes/ml of plasma. The range of yields of immunoabsorbed neurally-derived exosomes for both AD and AC groups was 12–17% of the initial precipitates. Diameters of total plasma exosomes and immunoabsorbed putatively-neural plasma exosomes ranged from 78 nm to 126 nm, which encompasses the expected exosomal size. No difference between exosomes of AD patients and control subjects was statistically significant, so that differences in the levels of pathogenic proteins are not due to divergent yields of exosomes and have been corrected by normalization with the exosomal marker CD81.
To support the capacity of neural adhesive protein immunoabsorption to enrich plasma exosomes for a neural source, immunoabsorption was carried out in parallel both with anti-NCAM-1 antibody and anti-L1CAM antibody for six plasmas of AD patients and six plasmas of matched controls (Table S1). Unlike the anti-NCAM-1 antibody, the anti-L1CAM antibody does not bind to NK and NKT cells of the immune system and is differently distributed in the nervous system. Extracted exosomal levels of CD81, P-T181-tau, P-S396-tau, total tau and Aβ1-42 were statistically indistinguishable whether enriched with anti-NCAM-1 antibody or anti-L1CAM antibody.
3.3. Relationship of exosomal protein levels to severity and stage of AD
Comparing the 29 AD patients with aMCI to the 28 AD patients with dementia showed no differences in the exosomal levels of P-S396-tau, P-T181-tau, total tau or Aβ1-42 (Table 2). This suggested that increased exosomal levels of these pathogenic proteins might be detectable early in the preclinical course. Blood exosomal proteins therefore were measured for an additional group of 24 AD patients at two time-points, the first at one to ten years before their diagnosis and the second at the time of initial diagnosis of AD. This group consisted of 12 men and 12 women with a mean age (±S.D.) of 71.8±7.30 years at the time of the first blood sample. The later diagnosis was aMCI for 13 and dementia for 11. Intervals between the two blood samples (number of patients) were: one year (one), two years (one), three years (four), four years (two), five years (two), six years (two), seven years (three), eight years (three), nine years (three) and ten years (three).
Table 2.
Levels of Serum Exosome Proteins in Relation to Severity of Dementia in AD
| Patient Group
|
P-S396-tau
|
P-T181-tau
|
Total tau
|
Aβ1-42
|
|---|---|---|---|---|
| AD, MCI | 23.8±3.27 | 114±10.6 | 201±20.9 | 23.0±4.57 |
| AD, dementia | 27.0±3.12 | 102±7.08 | 181±12.8 | 12.8±1.60 |
All values are mean±S.E.M., pg/ml; none of the differences between values for the MCI and dementia groups were significant.
As for the single time-point values (Fig. 1), the mean levels (±SEM) of P-S396-tau (25.2±1.85 pg/ml), P-T181-tau (91.1±4.42 pg/ml) and Aβ1-42 (14.5±1.41 pg/ml) at the time of diagnosis of AD (AD) were significantly higher than those of their case-controls (AC) (4.72±0.64 pg/ml, 35.6±3.49 pg/ml and 1.51±0.52 pg/ml, respectively)(all P< .0001) (Fig. 2). The mean level of total tau for the AD patients (165±15.8 pg/ml) was no different from that of their AC group (148±116.5 pg/ml). Further, the mean preclinical (AP) level of total tau (154±13.6 pg/ml) also was no different from that of AC. For P-S396-tau and P-T181-tau, the AP (19.2±2.00 pg/ml and 85.7±3.75 pg/ml, respectively) as well as AD values were significantly higher than those of the corresponding AC sets (both P< .0001). The mean P-S396-tau and P-T181-tau values of the AD group were no different from those of the corresponding AP group. Elevated exosomal levels of P-S396-tau and P-T181-tau thus were clearly detectable in a high-risk but cognitively normal AP group and had attained a plateau as early as 10 years before clinical diagnosis of AD. For Aβ1-42, the mean levels for the AD and AP (6.64±0.58 pg/ml) sets both were significantly higher than that of the AC set, but the mean AD level also was significantly higher than that of AP. Therefore Aβ1-42 may represent a biomarker for progression as well as early detection. A comparison of all AP and AD exosomal protein levels of patients converting to aMCI with those converting to AD did not show any significant differences. Further, a comparison of all AP and AD exosomal protein levels of patients converting to aMCI or AD after 1 to 5 years with those converting after 6 to 10 years also did not show any significant differences.
Fig. 2.
Sequential levels of proteins in blood exosomes of patients with AD measured first at a time of normal cognition (preclinical, AP) and later at the time of development of aMCI or dementia (AD).
4. Discussion
Levels of total tau, P-T181-tau, P-S396-tau and Aβ1-42 previously quantified in plasma, serum or CSF have represented those in the fluid-phases. In contrast, the levels we now report are for proteins extracted predominantly from cellular structures consisting of neurally-derived blood exosomes. When contrasted with fluid-phase levels, exosomal levels are nearly two orders of magnitude higher for total tau and P-T181-tau, and similar in magnitude for Aβ1-42 [15, 25–27], and all were significantly greater than those in case-control exosomes (Fig. 1). When blood exosomal levels of P-S396-tau, P-T181-tau and Aβ1-42 were considered together, the sensitivity of distinguishing AD from AC was 96% (Figs. 1, 2, S1). In contrast to levels in AD, no concentration of P-S396-tau for the FTD group was higher than the upper end of the range for the FTC controls. Thus blood exosomal P-S396-tau alone separates FTD from AD with a high specificity. Levels of P-S396-tau, P-T181-tau and Aβ1-42 together also distinguished patients in the AP preclinical set from AC subjects with a sensitivity of 96% (Fig. 2). Most importantly, significantly elevated exosomal levels of these proteins were detected in high-risk, but cognitively normal AP subjects up to 10 years prior to clinical diagnosis. Further, blood exosomal levels of Aβ1-42 continued to increase progressively from preclinical AP levels to significantly higher levels at the time of diagnosis of AD, implying value for exosomal Aβ1-42 as a progression biomarker (Fig. 2).
A recent fascinating report of higher or lower than normal plasma levels of multiple lipids and other cellular constituents during an average 2.1 year preclinical phase of AD provided a method with 90% accuracy in predicting progression to MCI or mild AD [28]. The broad spectrum of structures, organ distribution, functions, biosynthetic pathways and biodegradative mechanisms of these molecules suggest that the disturbed pattern observed reflects major systemic perturbations in the early stages of AD. The two sets of results differ in three major respects. First, here we are assessing neural cell exosomal proteins implicated in the pathogenesis of AD, whereas they quantified plasma fluid-phase levels of amino acids and fats that are not characteristically altered in neural lesions of AD. Second, our present approach identified preclinical AD up to 10 years prior to clinical onset, as contrasted with the detection of plasma abnormalities to date only up to three years before diagnosis of dementia clinically. Third, the accuracy of classification of preclinical AD with our protein assays exceeded 96% as contrasted with 90% for the analyses of plasma lipids and amino acids. In further collaborative studies, we quantified neural exosomal P-tau and Aβ1-42 proteins in pairs of plasma samples from the 28 AD converters studied earlier by Dr. Federoff’s group [28]. Our results showed significant elevations compared to cognitively normal matched controls in 100% of the preclinical samples, as contrasted with 89% preclinical identification by the profile of plasma tests of the Georgetown University Medical Center.
At this point in the evolving understanding of the clinical significance of our findings, it appears that detection in individuals of elevations of blood exosomal P-T181-tau and Aβ1-42 support identification of present or future susceptibility to proteinopathic neurodegenerative disease, including AD and at least one form of FTD. Concurrent or subsequent recognition of elevated exosomal P-S396-tau suggests the presence or future likelihood of development of AD. At least two more points of information would strengthen the clinical usefulness of this blood exosomal profile of neuropathogenic proteins. The first would be quantification of levels of other relevant neurally-derived exosomal proteins, such as TDP-43, FUS and additional isoforms of P-tau, in relation to the type and severity of neurodegenerative disease. The second would be results of prospective longitudinal studies designed to delineate the neurological course of cognitively normal subjects with an abnormal blood exosomal profile of neuropathogenic proteins as contrasted with the course of those having a normal profile of these proteins. With this knowledge, it may be possible to identify high-risk subjects early in their preclinical stage, define their point in the preclinical trajectory and guide early applications of novel treatments.
Supplementary Material
Acknowledgments
The authors are grateful to Lynn Kane (JHSF), Anna Karydas (UCSF MAC), Dana Swenson-Dravis and Matthew Miller (Mayo Clinic), Sonya Anderson (U. of Kentucky), Melissa Swaby (NIA), Eileen Johnson and Pamela Bailie (U. Rochester), Claudia Kawas, Dana Greenia, Mukti Patel and Archana Balasubramanian (UC Irvine), and Robert Padilla, Jamie McCann, Danielle Phelps and Ishmeal Conteh (Georgetown U.) for organizing and distributing clinical materials and data. We wish to thank Judith H. Goetzl for expert preparation of graphic illustrations.
Funding: Intramural Research Program of the National Institute on Aging (DK, EE), NIA RO1AG030753 from the National Institutes of Health (HJF), UK ADC P30 AG028383 (ELA) and Nanosomix, Inc. (EJG).
Special abbreviations
- aMCI
amnestic MCI
- DBS−2
calcium- and magnesium-free Dulbecco’s balanced salt solution
- AUC
Area Under The Curve
- AC
AD case-controls
- FTC
FTD case-controls
- AD,AP
cases of preclinical
Footnotes
Author contributions: MSF- A,W; DK- P,A,W; MM- P,A,W; AB- P,A,W; EE- L,A; JBS- P,A,W; ELA- P,A,W; RCP- P,A,W; HJF- A,W; BLM- P,A,W; EJG- D,L,A,W.
P= evaluation of patients, D= development of analytical methodology, L= laboratory benchwork, A= analysis of data, W= writing and/or editing of manuscript.
Conflicts of interest: Only two authors report possible conflicts of interest. Dr. Boxer delares grants from NIH / NIA, grants from Tau Research Consortium, grants from Corticobasal Degeneration Solutions, grants, personal fees and non-financial support from Archer Biosciences, grants from Allon Therapeutics, personal fees from Acetylon, personal fees from Ipierian, grants from Genentech, grants from Bristol Myers Squibb, grants from TauRx, grants from Alzheimer’s Association, grants from Bluefield Project to Cure FTD, grants from Association for Frontotemporal Degeneration, grants from Alzheimer’s Drug Discovery Foundation, grants from EnVivo, grants from C2N Diagnostics, grants from Pfizer, grants from Eli Lilly, outside the submitted work. Dr. Goetzl has filed a provisional application with the U.S. Patent Office for the platform and methodologies described in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 2.Golde TE, Borchelt DR, Giasson BI, Lewis J. Thinking laterally about neurodegenerative proteinopathies. J Clin Invest. 2013;123:1847–55. doi: 10.1172/JCI66029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–65. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gama Sosa MA, De Gasperi R, Elder GA. Modeling human neurodegenerative diseases in transgenic systems. Hum Genet. 2012;131:535–63. doi: 10.1007/s00439-011-1119-1. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Hashimoto T, Roe AD, Hori Y, Spires-Jones TL, Hyman BT. Brain interstitial oligomeric amyloid beta increases with age and is resistant to clearance from brain in a mouse model of Alzheimer’s disease. Faseb J. 2013;27:3239–48. doi: 10.1096/fj.13-229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal R, Tripathi CB. Diagnostic Utility of CSF Tau and Abeta(42) in Dementia: A Meta-Analysis. Int J Alzheimers Dis. 2011;2011:503293. doi: 10.4061/2011/503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer’s disease - current concepts. Mol Neurodegener. 2013;8:20. doi: 10.1186/1750-1326-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–64. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 13.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–67. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–71. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruhbeis C, Frohlich D, Kramer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingtdeux V, Sergeant N, Buee L. Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front Physiol. 2012;3:229. doi: 10.3389/fphys.2012.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 20.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuhashi M, Taub DD, Kapogiannis D, Eitan E, Zukley L, Mattson MP, et al. Aging enhances release of exosomal cytokine mRNAs by Abeta1-42-stimulated macrophages. Faseb J. 2013;27:5141–50. doi: 10.1096/fj.13-238980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–9. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–62. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 26.Seppala TT, Herukka SK, Hanninen T, Tervo S, Hallikainen M, Soininen H, et al. Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010;81:1123–7. doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. Jama. 2011;305:261–6. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–8. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.