Abstract
Objectives
Methylene blue, once discarded due to its unsettling yet mild side effects, has now found a renewed place in the pharmacopoeia of modern medicine. The continued spread of drug-resistant Plasmodium vivax and Plasmodium falciparum has also led to a recent re-examination of methylene blue's potent antimalarial properties. Here we examine the ex vivo susceptibility profile of Plasmodium spp. isolates to methylene blue; the isolates were from a region on the Thai–Myanmar border where there are increasing rates of failure when treating vivax malaria with chloroquine.
Methods
To do this we used a newly developed ex vivo susceptibility assay utilizing flow cytometry and a portable flow cytometer with a near-UV laser.
Results
P. vivax (median methylene blue IC50 3.1 nM, IQR 1.7–4.3 nM) and P. falciparum (median methylene blue IC50 1.8 nM, IQR 1.6–2.3 nM) are susceptible to methylene blue treatment at physiologically relevant levels. Unfortunately, the addition of chloroquine to combination treatments with methylene blue significantly reduces the ex vivo effectiveness of this molecule.
Conclusions
Our data support further efforts to employ methylene blue as a safe, low-cost antimalarial to treat drug-resistant malaria.
Keywords: Plasmodium falciparum, Plasmodium vivax, drug susceptibility assays, drug sensitivity assays
Introduction
Methylene blue was first synthesized in 1876 as a textile dye and was rather surprisingly adopted 15 years later as the world's first synthetic antimalarial.1 Other dyes were used later as antimalarial compounds after the discovery of methylene blue.2 Since then and despite its rather disconcerting side effects (green urine and blue sclera), methylene blue has been used in a bewildering array of therapeutic applications, including in the treatment of cancer, as an antidote for cyanide poisoning, to prevent methaemoglobinaemia and recently in the treatment of patients with Alzheimer's disease.3–6 The renewed interest in methylene blue is due in part to its relatively low toxicity (rat LD50 1250 mg/kg),7 its pharmacokinetics (oral absorption, 53%–97%; peak plasma concentration, 30–60 min; and plasma half-life, 5–6 h) and, most importantly, its low cost.8 Consequently, methylene blue has also recently been revived as an antimalarial.9 Methylene blue clearly appears to have good efficacy against chloroquine-resistant Plasmodium falciparum malaria.10–12 A recent study additionally showed that treatment with methylene blue alone or in combination with atorvastatin significantly reduced or prevented murine cerebral malaria.13 Unfortunately, little is known about the susceptibility of Plasmodium vivax to methylene blue, resulting from past failures to recognize that this parasite is the most important cause of malaria outside sub-Saharan Africa. The increasing number of regions in Asia and South America reporting the failure to treat vivax malaria with first-line drugs (chloroquine and antifolates) has provided an increased impetus to examine new therapies against P. vivax. As the development of novel molecules as antimalarials is time consuming and expensive, there is considerable advantage in adjusting the indications for pre-existing licensed therapeutics with modalities against malaria, such as methotrexate and now methylene blue.14 Consequently, the aim of this study was to assess the ex vivo susceptibility of P. vivax to methylene blue. As chloroquine is still often used as the first-line treatment of vivax malaria, we were also interested to examine whether methylene blue can be effectively used in combination with chloroquine to block the maturation of P. vivax.
Materials and methods
Sample collection
The 28 P. vivax and 18 P. falciparum isolates used in this study were obtained from Mae Sod District, Tak Province, Thailand. Samples were collected from patients attending the clinics of the Shoklo Malaria Research Unit (SMRU) under the ethical guidelines OxTREC 58-09 and 04-10. Isolates were collected in 5 mL lithium-heparinized tubes and sent to the laboratory within 5–6 h. After platelets and leucocytes had been removed using a cellulose filter column,15 the parasites were frozen down in Glycerolyte 57 (Baxter, Belgium).16
Parasite thawing
Prior to the antimalarial susceptibility assay, the isolates were thawed using a standard NaCl gradient methodology, which is described in detail in Kosaisavee et al.16 It should be noted that cryopreserved isolates have been successfully used in a number of ex vivo susceptibility studies involving P. vivax.16–19 Only 5 of the 28 P. vivax and 2 of the 18 P. falciparum isolates thawed failed to reach the required level of schizont development (≥50% healthy schizonts) in the drug-free control. Of the five P. vivax isolates that failed to reach this level, high gametocyte counts were the cause in three isolates. After thawing and centrifugation, the pellet was suspended in a blood medium mixture (BMM), consisting of McCoy's 5A medium supplemented with 2.4 g/L d-glucose and 20% heat-inactivated human AB serum, and the BMM was then incubated in a gas mix incubator (5% CO2, 5% O2 and 90% N2) at 37.5°C for ∼5 h before being used for the drug susceptibility test (this pre-incubation step helps the parasite to equilibrate to normal cellular function post-thawing before being exposed to antimalarials).
In vitro drug susceptibility assay
The antimalarial susceptibility of P. falciparum and P. vivax isolates was measured using a protocol modified from the WHO microtest, as previously described.17,20,21 Briefly, 200 μL of BMM was added to each well of the pre-dosed drug plates. Drug plates containing the BMM were incubated in a gas mix incubator at 37°C. To assess the stage specificity of antimalarials on the cryopreserved P. vivax, we used a method described by Sharrock et al.17 Briefly, the thawed and pre-incubated isolates were split into two groups. Half of the sample was added immediately to the pre-dosed drug plates (ring stage exposure). The other half of the thawed isolate was cultured in a flask for another 20 h. The predominantly trophozoite isolate (matured in the flask) was then added to the pre-dosed plates (late trophozoite stage exposure); importantly, the drug was washed out of the ring stage treatment to ensure a length of drug exposure equal to that of the trophozoite stage treatment. Both samples were harvested when ≥50% of parasites had matured to schizonts (∼40–44 h).
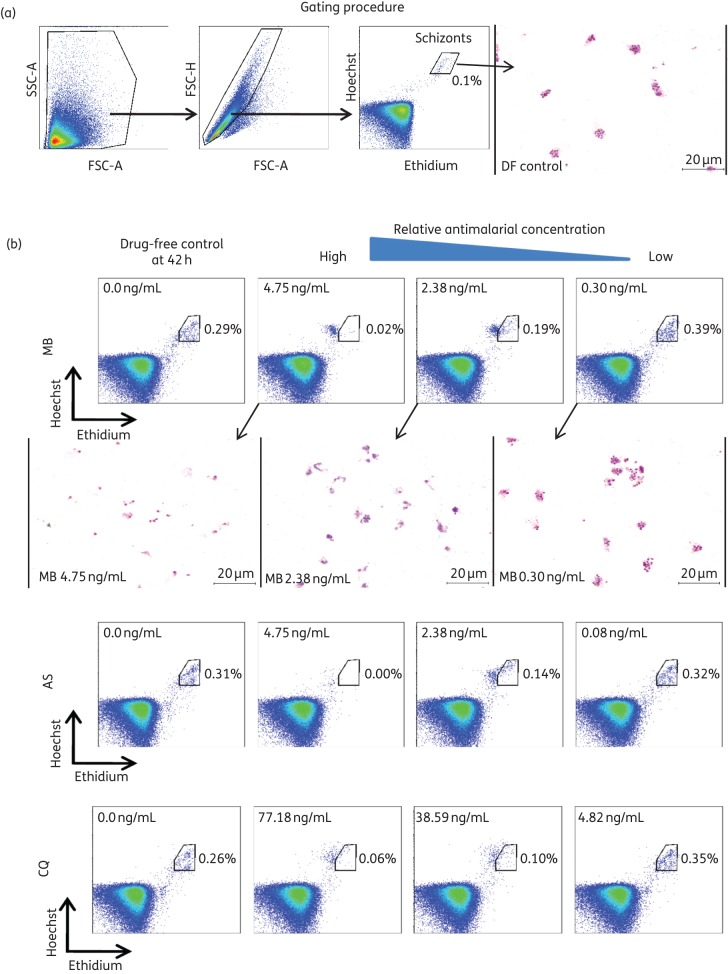
In addition to microscopy, schizont maturation was determined using a new flow cytometry method [Accuri™ C6 (Special Order), near-UV (375 nm) and blue (488 nm) lasers; Becton, Dickinson] at the same timepoint as for harvesting for the microscopic method as previously described, with some modifications.21,22 After ∼42 h post-culture, the 200 μL of BMM in each well was mixed by pipette, and 20 μL from each well was dispensed into a small curved-bottom tube (Micronic) before being treated with 0.5 μL of 1 mg/mL dihydroethidium (Sigma) and 1 μL of 800 μM Hoechst 33342 (Sigma), made up to 100 μL with PBS. The fluorescent staining reaction was stopped after 20 min at room temperature with the addition of 400 μL of PBS, and the reaction products were stored on ice. During the staining time, thick films (3 μL of packed red blood cells) were made from each of the wells for Giemsa staining. The initial gating strategy used for flow cytometry on Accuri™ C6 (Special Order) is described in Figure 1(a). Post-flow cytometry analysis was conducted using FlowJo version 7.6.5 (Tree Star Inc.). For parasitaemias of 0.05%–0.1%, we used 100 000 events (Figure 1a). However, at higher parasitaemias, 60 000 events can be used to reduce the count time per well to ∼20 s. In preliminary trials using the newly modified ‘near-UV’ Accuri C6, we found that the use of the Hoechst stain rather than SYBR green limited the background (generally detected events corresponding to uninfected reticulocytes), resulting in much clearer gating clusters for P. vivax.21 Figure 1(b) shows the side-by-side comparison of endpoints from the microscopy assays (thick films) and flow cytometry (final gates) for a methylene blue susceptibility assay. We limited the number of thick films shown so as to provide a better magnification of the parasites, which are generally difficult to see in smaller more numerous panels.
Figure 1.
(a) Flow cytometry gating strategy for determination of schizont parasitaemia in a 42 h ex vivo culture of P. vivax in the drug-free (DF) control. (b) A series of scatter plots from the Accuri C6 (with near-UV and blue lasers) comparing the effect of three antimalarials [methylene blue (MB), artesunate (AS) and chloroquine (CQ)] at various concentrations (the first panel being the DF control) on the maturation of P. vivax schizonts (shown in the gate). The y-axis represents the Hoechst signal and the x-axis represents the ethidium signal. Below the MB panels are a series of Giemsa-stained thick films from the same wells measured by flow cytometry. The thick films clearly show complete schizont inhibition at the highest concentration and a sequential increase in the maturity of stages as the concentration of MB decreases (the lowest concentration predominated by late schizont forms).
Statistical analysis
The growth responses either the number of schizonts/200 infected red blood cells (microscopy) or the total number of schizonts (per 100 000 events) in the designated gate (flow cytometry) (Figure 1a) for each of the treatments and assays were converted to a percentage of the drug-free positive control at 40 h. The background 0 h was subtracted from each of the data points. IC50 data were calculated using the online ICEstimator (http://www.antimalarial-icestimator.net/MethodIntro.htm) and the IC50 was calculated by non-linear regression analysis.23 Non-parametric analysis of data shown in Figures 2 and 3 (Friedman's and Dunn's post hoc tests) was performed using GraphPad Prism 6.
Figure 2.
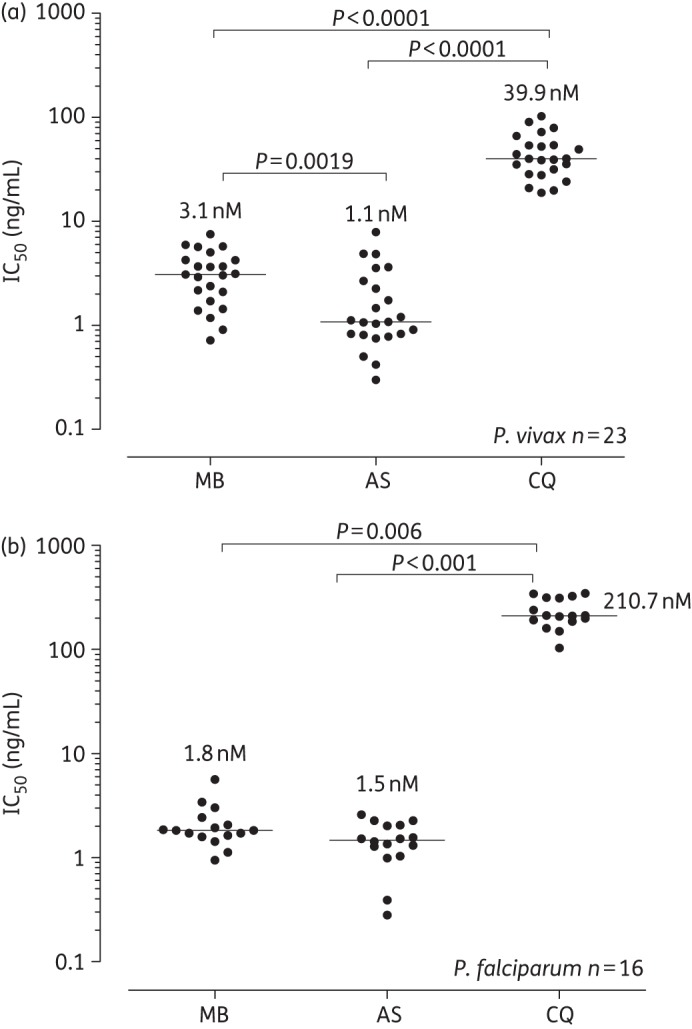
Comparison of the antimalarial effect of methylene blue (MB), artesunate (AS) and chloroquine (CQ) on (a) P. vivax (n = 25) and (b) P. falciparum (n = 16) isolates from the Thai–Myanmar border. The lines represent the median IC50 values (nM) (also indicated by the numerical values on the graph). The repeated measure medians were compared using Friedman's and Dunn's post hoc tests.
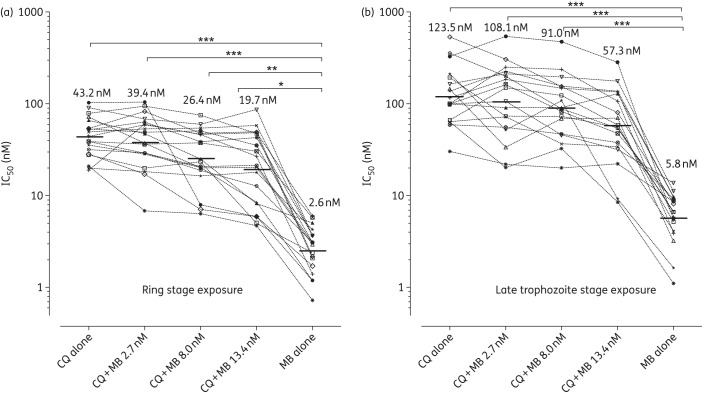
Figure 3.
Repeated-measures comparison of methylene blue (MB) and chloroquine (CQ) treatments, alone and in combination, against the ex vivo maturation of P. vivax schizonts (n = 17). (a) The effect on IC50 values (nM) if P. vivax is exposed to methylene blue for 20 h from the early ring stage (1–6 h post-invasion) and (b) shows the effect of methylene blue on late trophozoites (∼21–26 h post-invasion). Each line connects the same P. vivax isolate. Isolates are listed in decreasing order of susceptibility to chloroquine. *, <0.05; **, <0.01; ***, <0.001.
Results and discussion
Here we tested the ex vivo effectiveness of methylene blue against P. vivax from a region where there is an increased incidence of chloroquine-resistant vivax malaria.24,25 We found that these P. vivax isolates were susceptible to methylene blue in the nM range (Figure 2a). Methylene blue was described as an oxidative agent acting on cytosolic dehydrogenation in the glucose 6-phosphate pathway in rabbit reticulocytes and could explain the susceptibility of P. vivax to methylene blue.26 Notably, the highly chloroquine-resistant isolates of P. falciparum from this region were susceptible to methylene blue (median methylene blue IC50 1.8 nM, IQR 1.6–2.3 nM), as were the P. vivax isolates (median IC50 3.1 nM, IQR 1.7–4.3 nM) (Figure 2a). Methylene blue was certainly as effective as artesunate in its ability to inhibit schizont maturation in P. vivax and P. falciparum (Figure 2b). While we believe this latter observation to be true, the precise IC50 values for P. vivax and P. falciparum artesunate should be interpreted with caution as the methodology used a standard extended ex vivo exposure of (∼40 h) whereas in vivo this exposure level to artesunate is <4 h. We used the traditional standard exposure method so that our results would be directly comparable to those with methylene blue and other in vitro studies conducted on P. falciparum.
While most of the P. vivax isolates tested in this study were relatively susceptible to chloroquine, three isolates (Figure 3) had chloroquine IC50 values >80 nM [2-fold higher than the P. vivax population median chloroquine IC50 of 39.9 nM (Figure 2a)], similar to the chloroquine IC50 values seen in West Papua, where a high proportion of patients fail chloroquine treatment.19,20 Importantly, these relatively chloroquine-non-susceptible isolates were effectively killed by methylene blue (methylene blue IC50 values of 1.2–3.6 nM).
Attention has recently been drawn to the fact that P. vivax trophozoites are intrinsically non-susceptible to chloroquine.17,20 Our data are in agreement with these past studies, clearly showing that exposing the ring stages to chloroquine produces significantly lower IC50 values (geometric mean chloroquine IC50 43.2 nM, 95% CI 33.9–55.1 nM) compared with the IC50 values that result from exposing trophozoites to chloroquine (geometric mean chloroquine IC50 123.5 nM, 95% CI 86.3–176.8 nM) (P = 0.0005) (Figure 3). Interestingly, this stage-specific action of chloroquine on P. vivax was also observed for the methylene blue treatments, with ring stage exposure (geometric mean methylene blue IC50 2.6 nM 95% CI 2.0–3.4 nM) producing significantly lower methylene blue IC50s than trophozoite stage exposure (geometric mean methylene blue IC50 5.8 nM, 95% CI 4.2–8.0 nM) (P = 0.0007) (Figure 3). New data on the importance of the late stages (trophozoites and schizonts) to the pathobiology of P. vivax, especially with regard to cytoadhesion and rosetting, indicate that molecules such as mefloquine and artesunate, which kill P. vivax trophozoites, would be a valuable attribute to vivax malaria therapeutics.27–30 While it is a little disappointing that methylene blue does not share this characteristic with mefloquine and artesunate, the methylene blue IC50s against trophozoites were all <11.2 nM and well within the useful therapeutic range of methylene blue treatment (∼130 nM/h in humans after a 500 mg oral dose).31 It is interesting to note that the presence of 2.7–13.4 nM methylene blue failed to significantly increase the susceptibility of P. vivax trophozoites to chloroquine treatment (Figure 3).
Although coadministration with chloroquine significantly increases the plasma concentration of methylene blue, it was found that methylene blue was antagonistic to the chloroquine treatment of P. falciparum.10,31 Initial attempts to combine methylene blue with chloroquine were clearly not effective in the treatment of patients with malaria in Africa.11 Unfortunately, our ex vivo data suggest this may be the case with P. vivax, as the addition of relatively high fixed doses of methylene blue to the chloroquine susceptibility assays resulted in disappointingly high IC50 values. For example, the addition of a fixed dose of 8.0 nM methylene blue (a dose ∼3-fold higher than the methylene blue IC50 against the ring stages) to the chloroquine ring stage susceptibility assay resulted in a susceptibility value that was 10-fold higher than that for treatment with methylene blue alone (Figure 3). There was certainly no significant decrease in the mean chloroquine IC50 values despite the addition of up to 13.4 nM of methylene blue (Figure 3a). Our findings that the combination of chloroquine and methylene blue is incompatible in terms of its pharmacodynamics against P. vivax has important consequences for the treatment and control of vivax malaria in many regions (such as India and the Middle East) where chloroquine is still favoured as the first-line treatment for vivax malaria due to its relative safety, cheapness and long half-life (∼30 days), protecting patients from early relapses. Although our study clearly demonstrates the potential of methylene blue as an effective therapeutic agent against P. vivax, it would only be of utility in areas no longer using chloroquine as a first-line treatment for vivax malaria. Methylene blue should certainly be considered as a useful alternative to artesunate for the rapid clearance of P. vivax. The challenge is to find a useful partner drug for methylene blue with an extended half-life similar to that of piperaquine to protect patients from early relapses.
Funding
This study received funding from SIgN and from the Horizontal Programme on Infectious Diseases under the Agency for Science, Technology and Research (A*STAR, Singapore). SMRU is sponsored by The Wellcome Trust of Great Britain, as part of the Oxford Tropical Medicine Research Programme of Wellcome Trust-Mahidol University. The Vivax Malaria Laboratory, Department of Microbiology at the National University of Singapore, received funding from a Singapore Ministry of Education (MOE) Tier 2 Grant (#MOE2013-T2-1-145).
Transparency declarations
None to declare.
Acknowledgements
We thank the patients and staff of the SMRU. We particularly wish to emphasize our gratefulness to the SMRU obstetrics doctors and nurses (led by Professor Rose McGready); without whom none of our studies would be possible. We also wish to highlight the role of the expert microscopy staff at SMRU (trained by Stephane Proux).
References
- 1.Guttmann P, Ehrlich P. Ueber die Wirkung des Methylenblau bei Malaria. Berlin Klin Wochenschr. 1891;28:953–6. [Google Scholar]
- 2.Vennerstrom JL, Makler MT, Angerhofer CK, et al. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob Agents Chemother. 1995;39:2671–7. doi: 10.1128/aac.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radic Biol Med. 2007;43:178–90. doi: 10.1016/j.freeradbiomed.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wainwright M, Crossley KB. Methylene blue–a therapeutic dye for all seasons? J Chemother. 2002;14:431–43. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 5.Boylston M, Beer D. Methemoglobinemia: a case study. Crit Care Nurse. 2002;22:50–5. [PubMed] [Google Scholar]
- 6.Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer's disease. Biochem Pharmacol. 2009;78:927–32. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Vutskits L, Briner A, Klauser P, et al. Adverse effects of methylene blue on the central nervous system. Anesthesiology. 2008;108:684–92. doi: 10.1097/ALN.0b013e3181684be4. [DOI] [PubMed] [Google Scholar]
- 8.Misclescu A, Wiklund L. Methylene blue, an old drug with new indications? Jurnalul Român de Anestezie Terapie intensivã. 2010;17:35–41. [Google Scholar]
- 9.Schirmer RH, Coulibaly B, Stich A, et al. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–5. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- 10.Akoachere M, Buchholz K, Fischer E, et al. In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob Agents Chemother. 2005;49:4592–7. doi: 10.1128/AAC.49.11.4592-4597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meissner PE, Mandi G, Coulibaly B, et al. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;5:84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dormoi J, Briolant S, Desgrouas C, et al. Efficacy of Proveblue (methylene blue) in an experimental cerebral malaria murine model. Antimicrob Agents Chemother. 2013;57:3412–4. doi: 10.1128/AAC.02381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dormoi J, Briolant S, Desgrouas C, et al. Impact of methylene blue and atorvastatin combination therapy on the apparition of cerebral malaria in a murine model. Malar J. 2013;12:127. doi: 10.1186/1475-2875-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imwong M, Russell B, Suwanarusk R, et al. Methotrexate is highly potent against pyrimethamine-resistant Plasmodium vivax. J Infect Dis. 2011;203:207–10. doi: 10.1093/infdis/jiq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriprawat K, Kaewpongsri S, Suwanarusk R, et al. Effective and cheap removal of leukocytes and platelets from Plasmodium vivax infected blood. Malar J. 2009;8:115. doi: 10.1186/1475-2875-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosaisavee V, Suwanarusk R, Nosten F, et al. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp Parasitol. 2006;114:34–9. doi: 10.1016/j.exppara.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Sharrock WW, Suwanarusk R, Lek-Uthai U, et al. Plasmodium vivax trophozoites insensitive to chloroquine. Malar J. 2008;7:94. doi: 10.1186/1475-2875-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwanarusk R, Chavchich M, Russell B, et al. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J Infect Dis. 2008;198:1558–64. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suwanarusk R, Russell B, Chavchich M, et al. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One. 2007;2:e1089. doi: 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell B, Chalfein F, Prasetyorini B, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52:1040–5. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell B, Malleret B, Suwanarusk R, et al. Field-based flow cytometry for ex vivo characterization of Plasmodium vivax and P. falciparum antimalarial sensitivity. Antimicrob Agents Chemother. 2013;57:5170–4. doi: 10.1128/AAC.00682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malleret B, Claser C, Ong AS, et al. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci Rep. 2011;1:118. doi: 10.1038/srep00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Nagard H, Vincent C, Mentre F, et al. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011;104:10–8. doi: 10.1016/j.cmpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Phyo AP, Lwin KM, Price RN, et al. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin Infect Dis. 2011;53:977–84. doi: 10.1093/cid/cir631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijken MJ, Boel ME, Russell B, et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J. 2011;10:113. doi: 10.1186/1475-2875-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapoport SM, Muller M. The influence of methylene blue on the respiratory metabolism of the reticulocyte. Eur J Biochem. 1974;46:335–40. doi: 10.1111/j.1432-1033.1974.tb03625.x. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho BO, Lopes SC, Nogueira PA, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–47. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 28.De Las Salas B, Segura C, Pabon A, et al. Adherence to human lung microvascular endothelial cells (HMVEC-L) of Plasmodium vivax isolates from Colombia. Malar J. 2013;12:347. doi: 10.1186/1475-2875-12-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WC, Malleret B, Lau YL, et al. Glycophorin C (CD236R) mediates vivax malaria parasite rosetting to normocytes. Blood. 2014;123:e100–9. doi: 10.1182/blood-2013-12-541698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes SC, Albrecht L, Carvalho BO, et al. Paucity of Plasmodium vivax mature schizonts in peripheral blood is associated with their increased cytoadhesive potential. J Infect Dis. 2014;209:1403–7. doi: 10.1093/infdis/jiu018. [DOI] [PubMed] [Google Scholar]
- 31.Walter-Sack I, Rengelshausen J, Oberwittler H, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol. 2009;65:179–89. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]