Abstract
The pro-inflammation factor high-mobility group box protein 1 (HMGB1) has been implicated in the pathogenesis of asthma. In this study, we used a murine model of chronic asthma to evaluate the effects of HMGB1 on airway remodeling. Female BALB/c mice were randomly divided into four groups: control, ovalbumin (OVA) asthmatic, OVA+isotype antibody and OVA+anti-HMGB1 antibody. Anti-HMGB1 antibody therapy was started on day 21 and was administered three times per week for 6 weeks before intranasal challenge with OVA. In this mouse model, HMGB1 expression is significantly elevated. The anti-HMGB1 antibody group exhibited decreased levels of immunoglobulin E (IgE) and inflammatory mediators and reduced inflammatory cell accumulation, airway hyperresponsiveness (AHR), mucus synthesis, smooth muscle thickness and lung collagen content compared with the OVA groups. Treatment with HMGB1 increased proliferation, migration, collagen secretion and α-smooth muscle actin (SMA) expression in MRC-5 cells. Treatment with the HMGB1/IL-1β complex significantly increased the expression and secretion of transforming growth factor (TGF-β1), matrix metalloproteinase (MMP)-9 and vascular endothelial growth factor (VEGF). Altogether, these results suggest that blocking HMGB1 activity may reverse airway remodeling by suppressing airway inflammation and modulating lung fibroblast phenotype and activation.
Keywords: airway remodeling, asthma, high-mobility group box protein 1 (HMGB1), murine mouse model
Introduction
High-mobility group box protein 1 (HMGB1), a highly conserved DNA-binding protein, acts as a potent endogenous ‘danger signal' for the initiation of innate immunity.1 Many clinical studies and animal trials have shown that HMGB1 acts as a pro-inflammatory mediator when it is localized in the extracellular space.1 In fact, elevated levels of HMGB1 have been detected in several clinical inflammatory diseases, including sepsis and systemic lupus erythematosus.2,3 We recently reported that HMGB1 levels are increased in patients with asthma and COPD and are significantly negatively correlated with the pulmonary function index and positively associated with neutrophil counts.4 We also demonstrated that HMGB1 levels are increased in the lungs as well as the bronchial alveolar lavage fluid (BALF) of ovalbumin (OVA)-induced acute asthmatic mice.5 Shim et al.6 showed that HMGB1 plays a central role in eosinophilic airway inflammation in asthma. More interestingly, HMGB1 has been shown to play a pivotal role in pulmonary fibrosis, partly through reduction of fibroblast proliferation and downregulation of interleukin (IL)-1β levels.7 Furthermore, some researchers have found that HMGB1 promotes secretion of vascular endothelial growth factor (VEGF)8 and that treatment with anti-HMGB1 antibodies suppresses the activity of matrix metalloproteinase (MMP)-9,9 both of which are involved in airway remodeling.
Based on this evidence, we hypothesized that HMGB1 released into the airway may be involved in the pathogenesis of airway remodeling. To test this, we measured the levels of HMGB1 in a mouse model of chronic asthma and then investigated the effects of anti-HMGB1 antibody treatment by examining respiratory mechanics, OVA-specific immunoglobulin (Ig)E, collagen deposition, smooth muscle thickness, epithelial mucus and inflammatory mediators. In addition, we investigated whether HMGB1 had direct effects on human fetal lung fibroblast proliferation, migration and collagen synthesis in vitro to elucidate the mechanisms involved in these processes. Finally, we identified the cell types that produce transforming growth factor (TGF)-β1, VEGF and MMP-9 in the asthmatic response to treatment with HMGB1 or the HMGB1/IL-1β complex.
Materials and methods
Murine model of chronic asthma
Thirty-two female BALB/c mice (aged 6–8 weeks) were purchased from the Guangxi Medical University Animal Center and maintained in the same center. The mice were housed under specific pathogen-free conditions. Eight mice were used per group. All experimental animal protocols were approved by the Animal Care and Use Committee of the Guangxi Medical University. The mice were randomly divided into four groups: phosphate-buffered saline (PBS) control, OVA, OVA+isotype antibody and OVA+anti-HMGB1 antibody. The mice were immunized by i.p. injection on days 0, 7, and 14 with 20 µg (grade V; Sigma-Aldrich; St. Louis, MO) plus 0.5 mg aluminum hydroxide (Thermo Scientific) and then challenged from day 21 with OVA (40 µg per mouse) i.n. three times a week for 6 weeks. An anti-HMGB1 antibody (Abcam, Cambridge; MA; 50 µg/mg body weight) or an (Abcam, Cambridge; MA) was injected i.p. 30 min before the challenge. The mice in the PBS group were treated with PBS instead of OVA.
Assessment of airway hyperresponsiveness
Airway hyperresponsiveness (AHR) was induced with methacholine (Sigma-Aldrich; St. Louis, MO) 24 h after the final i.n. challenge and assessed using whole-body plethysmography (Buxco Electronics, Troy, NY). Each mouse was exposed to aerosolized PBS (baseline) for 3 min followed by the administration of increasing concentrations of methacholine solutions. Airway resistance (enhanced pause (Penh)) values were evaluated for 5 min. The results are expressed as the percentage of baseline Penh value for each concentration of methacholine. To confirm the findings from the noninvasive body plethysmography experiments, we determined the respiratory mechanics during mechanical ventilation using an invasive method. Briefly, the mice were anesthetized with a pentobarbital sodium (70 mg/kg body weight), and the trachea was cannulated with a needle. The mice were transferred into a whole-body chamber (Buxco Electronics) and then mechanically ventilated. The baseline lung resistance was recorded for 3 min. After challenge with increasing concentrations of aerosolized methacholine (from 3.12–50 mg/ml), the lung resistance was recorded from 10 s to 2 min. Maximum RL values were selected to demonstrate the changes in the airway function of the mice (for a detailed description, see Supplementary Information).
Mouse sample collection
BALF and lung tissue were collected 48 h after the final allergen challenge. The total and differential cell counts from the BALF were determined by staining with hematoxylin and eosin (H&E), and the BALF supernatants were stored at −70 °C for further evaluation. The right lung was stored in liquid nitrogen for later determination of collagen content (upper lobe) and for use in an enzyme-linked immunosorbent assay (ELISA) and western blotting (lower lobe). The left lung was fixed with 4% formaldehyde and paraffin-embedded, followed by immunohistochemistry and staining with H&E, Masson's trichrome and periodic acid-Schiff.
Measurement of lung collagen content
The collagen assay was performed using a Sirius Red Collagen Detection Kit (Chondrex, redmond, USA) according to the manufacturer's instructions. Briefly, mouse lung tissues were homogenized and then mixed with 0.5 ml of sirius red solution for 20 min. The collagen–dye complex was collected by centrifugation at 10 000 r.p.m. for 3 min and then resuspended with 0.25 ml of extraction buffer. The solution was evaluated at 540 nm using a microtiter plate reader. The data are expressed as µg of collagen per g lung.
Immunohistochemistry
The left lung was fixed in 4% formaldehyde and stained with hematoxylin and eosin (JianCheng, Nanjing, China) to evaluate inflammation. The tissue sections were subjected to antigen retrieval by heating, and the endogenous peroxidase activity was quenched with 3% H2O2 in PBS. The sections were blocked with goat serum from a Histo-mouse-SP bulk kit (Zymed Laboratories, ZhongShang; Beijing, China). The slides were then incubated overnight with an anti-α-smooth muscle actin (SMA) antibody (1∶300; Immunoway, newark, USA) or an anti-HMGB1 antibody (1∶300; Immunoway).The sections were exposed to anti-mouse HRP secondary antibodies, and diaminobenzidine (Zymed Laboratories) was used as a substrate for the immunoperoxidase reaction. Finally, the slides were counter-stained with hematoxylin (JianCheng) for 2 minutes. Images were captured with a DP72 camera (BX53; ×10, ×200 or ×400 magnification; Tokyo, Japan, Olympus), and the images were analyzed using the freeware ImageJ.
Quantification of airway remodeling
Both large and small airways were evaluated for the airway remodeling analysis. The lung sections were stained with periodic acid-Schiff (PAS) reagent to quantify the mucus-producing goblet cells. The number of PAS-positive cells in airways was counted by light microscopy (×200 magnification), and the results are expressed as the percentage of PAS+ epithelial cells out of the total number of epithelial cells in the airway. The peribronchial area that stained with Masson's trichrome was outlined and quantified to assess the extent of subepithelial fibrosis. The thickness of the airway smooth muscle layer was evaluated by immunostaining for α-SMA. The peribronchial smooth muscle layer was outlined and quantified using ImageJ. The results are expressed as the area of α-SMA-positive staining (square micrometers) per millimeter of length of the bronchiole basement membrane.
Impact on HMGB1 on human fetal lung fibroblasts (MRC-5)
The human fetal lung fibroblast cell line MRC-5 was purchased from American Type Culture Collection. The cells were maintained in DMEM supplemented with 10% fetal calf serum (Hiclone, Utah, USA).
Fibroblast migration
A ‘scratch-wound' assay was used to assess fibroblast migration fibroblast.10 Briefly, 2×105 fibroblasts/well were seeded into 6-well plates and grown to confluence (37 °C, 5% CO2). A pipette tip was used to scratch the monolayer to create a cell-free wound area. Untreated cells or cells treated with HMGB1 were allowed to migrate into this cell-free wound for 12 h. The recovery of the wound area was quantified and expressed as the ratio of the recovered wound area to the initial wound area.
Fibroblast proliferation
Lung fibroblasts (5×104 cells/well) were seeded into 12-well plates in DMEM medium (Hiclone, Utah, USA) with HMGB1 or without HMGB1 on day 0. On days 1, 2 and 3, the cells were trypsinized and stained with trypan blue, and the number of viable cells was counted.11
Detection of the expression of α-SMA
Fibroblasts (2×105 cells/well) were seeded into six-well plates and incubated for 48 h with or without recombinant HMGB1, or with TGF-β1 as a positive control (R&D Systems, MN, USA). The cells were then homogenized for detection of α-SMA expression by real-time polymerase chain reaction (PCR) and western blotting.
Collagen synthesis by lung fibroblasts
Fibroblasts (2×105 cells/well) were seeded into six-well plates and incubated for 24 h in the presence or absence of recombinant HMGB1. The cell supernatants were assessed for collagen secretion using the Sirius Red Collagen Detection Kit (Chondrex, redmond, USA).
Primary culture of human bronchial epithelial cells (PBECs)
Human bronchial epithelial cells were derived from bronchi removed from three patients with lung cancer undergoing lobectomy at the First Affiliated Hospital of Guangxi Medical University. The study protocol was approved by the Ethics Committee of Guangxi Medical University. The specimens were measured, and the bronchial tissue was added to 24-well tissue culture plates (Corning, NY, USA) coated with human placental collagen (Sigma). The tissues were cultured for 10–12 days in Airway Epithelial Cell Growth Medium (PromoCell, PromoCell, Heidelberg, Germany) in a humidified incubator at 37 °C with a 5% CO2 atmosphere12 (a detailed description is included in Supplementary Information). The epithelial cells were then trypsinized and seeded into 12-well plates at 2×105/well before being challenged with HMGB1, IL-1β or HMGB1/IL-1β complexes for 24 h.
Preparation of HMGB1-IL-1β complexes
Recombinant HMGB1 protein (Sigma) dissolved in 1×PBS was incubated with different concentrations of IL-1β (Sigma) to achieve the indicated final concentrations in cell cultures. After 16-h incubation at 4 °C, the solutions were added to the cell cultures.13
Cell lines, culture conditions and differentiation
A549, 16-HBE, THP-1 and U937 cells were purchased from the American Type Culture Collection and grown in RPMI-1640 medium (Hyclone) containing 10% fetal calf serum (Hyclone) and 100 U/ml penicillin/streptomycin. To differentiate the U937 and THP-1 cells to a monocyte/macrophage-like phenotype, the cells were incubated with 20 ng/ml phorbol-12-myristate-13-acetate (Sigma-Aldrich, Santa clara, USA) for 48 h (THP-1) or 72 h (U937) at a density of 1×106 cells/ml. For each experiment, 2×105 epithelial cells or 1×106 U937 or THP-1 cells were seeded into the 12-well culture plates and then allowed to attach for 24 h. The cells were then exposed to the indicated concentrations of HMGB1, IL-1β or HMGB1/IL-1β complexes. After stimulation for 24 h, the cell culture supernatants, cell lysates, and total RNA were collected for further analyses.
Isolation of cells from the peripheral blood of asthma patients
Blood samples were obtained by venipuncture from eight healthy nonsmoking donors (four women and four men, 26–45 years old). The records for all donors participating in this trial are held at the First Affiliated Hospital of Guangxi Medical University, and informed consent was obtained according to the protocol approved by the Ethics Committee of Guangxi Medical University.
Isolation and culture of eosinophils
The blood eosinophils were isolated using a negative immunomagnetic procedure.14 The purity of the isolated eosinophils was at least 95%, as assessed by H&E staining. After isolation, the eosinophils were cultured in RPMI-1640 medium containing 50 µg/ml gentamicin (Hiclone, Utah, USA). A total of 5×105 cells/ml were seeded into 24-well culture plates and were exposed to HMGB1 or HMGB1/IL-1β complexes for 24 h.
Neutrophil isolation and treatment
Neutrophils were isolated from the blood of healthy donors using a standard Percoll (GE Healthcare, Pittsburgh, USA) density gradient centrifugation protocol15 (a detailed description is included in Supplementary Information). The isolated neutrophils were resuspended in 4 ml FBS-free RPMI-1640 medium (106 cells/ml) and then seeded into 24-well culture plates. After incubation at 37 °C for 1 h, the neutrophils were treated with HMGB1 or HMGB1/IL-1β complexes for 30 min. Untreated cells were used as controls.
Quantitative PCR
Total RNA from A549, 16-HBE, PBECs, U937, THP-1, MRC-5, AM, EOS and NEU cells was extracted using Trizol (Invitrogen, Carlsbad, USA). cDNA was synthesized using an oligo dT primer in a total volume of 20 µl according to the manufacturer's instructions (Format; Waltham, Thermo Scientific). Real-time PCR was performed using an ABI 7900 Sequence Detection System and SYBR Premix EX Taq (Takara, Tokyo, Japan). The primer pairs used were as follows: MMP-9: 5′-GTA ACC CTG GTC ACC GGA CTT-3′ and 5′-ATA CGT TCC CGG CTG ATC AG-3′ β-actin: 5′-AAG AGA GGC ATC CTC ACC CT-3′ and 5′-TAC ATG GCT GGG GTG TTG AA-3′ VEGF: 5′-GCC TTG CCT TGC TGC TCT AC-3′ and 5′-TGA TTC TGC CCT CCT CCT TCT G-3′ TGF-β1: 5′-TGC GCC TGC AGA GAT TCA AG-3′ and 5′-AGG TAA CGC CAG GAA TTG TTG CTA-3′ α-SMA: 5′-AGC CAG TCG CCA TCA GGA AC-3′ and 5′-CCGGAGCCATTGTCACACAC-3′ HMGB1: 5′-GAT GGG CAA AGG AGA TCC TA-3′ and 5′-CTT GGT CTC CCT TTG GGG-3′. The mRNA levels were normalized to β-actin. The fold-change for each gene was calculated by comparing the cycle threshold (Ct) value of the gene with the Ct value of the control.
Western blotting analysis
The lung tissue and cells were homogenized in a protein extraction buffer (Biyuntian; Shanghai, China), and the total protein concentration was estimated using a BCA Protein Assay Kit (Biyuntian, Shanghai, China). The lung and cells lysates were boiled at 95 °C for 5 min in 1×SDS sample buffer. The lung lysates (30 mg protein per lane) and cell lysates (50 mg protein per lane) were separated on 10% SDS-Tricine gels. The proteins were then transferred to nitrocellulose membranes (PALL, NY, USA) and blocked in 5% nonfat milk in TBS with 0.1% Tween 20 for 60 min at room temperature. The membranes were exposed to rabbit anti-VEGF (Boster, Wuhan, China), rabbit anti-β-actin (Cell Signaling Biotechnology; Boster, USA), rabbit anti-β-tubulin (Cell Signaling Biotechnology; Boster, USA), mouse anti-MMP-9 (Abcam, Cambridge, UK), mouse anti-TGF-β1 (Abcam), or mouse anti-HMGB1 (Immunoway, newark, USA), followed by HRP-conjugated secondary antibodies (Sigma, Santa clara, USA). β-actin expression and β-Tubulin expression (for MRC-5 only) were monitored in lung tissues and cells as an internal control. The bands were detected using ECL substrate (Pierce, Thermo Scientific, Waltham, USA) and visualized on X-ray films. The images were subsequently analyzed using Quantity One to quantify the protein expression (BioRad, California, USA).
Determination of MMP-9 Activity
Metalloproteinase-9 activity was assessed in lung lysates and cell supernatants by gelatin zymography (GENMED, Boster, USA). Briefly, equal amounts of total protein were mixed with loading buffer and then separated on a 10% SDS-tricine gel with 1 g/l of gelatin for 120 min. After incubation in zymography buffer for 30 min, the gels were transferred to developing buffer and incubated for 20 h (neutrophils) or 40 h (all other cells) at 25 °C. The gels were stained with Coomassie brilliant blue for 60 min, and the gel was then washed in wash buffer for 30 min. The bands were scanned to determine the gelatinolytic activity.
ELISA
The levels of interferon-γ, tumor-necrosis factor (TNF)-α, VEGF, IL-1β, IL-4, IL-5, IL-6, total MMP-9, IL-13 and active TGF-β1 in BALF, cell culture supernatants or lung lysates were quantified using commercial ELISA kits. These ELISA kits were purchased from BOSTER Technology (Wuhan, China) with the exception of the HMGB1 ELISA, which was purchased from the SHINO-TEST Corporation (Tokyo, Japan). The absorbance of each sample at 450 nm was measured using a microplate reader (Thermo Labsystems, Waltham, USA). The serum levels of total and OVA-specific IgE were determined using an Anti-Ovalbumin IgE (mouse) EIA Kit (Cayman Chemical, Michigan, USA).
Statistical analysis
The results are expressed as the mean±s.e. Significance was determined by either one-way or two-way ANOVA (SPSS 16.0). P values <0.05 were considered statistically significant.
Results
HMGB1 expression is elevated in a mouse model of chronic asthma
HMGB1 expression was significantly higher in the lungs of OVA-induced asthmatic mice (n=8) than in those of PBS-treated mice (Figure 1a and b). HMGB1 levels were also higher in the BALF of OVA-induced mice than those in PBS-treated mice, and anti-HMGB1 antibody treatment reduced HMGB1 levels in the BALF (Figure 1c).
Figure 1.
HMGB1 levels in lung tissue and BALF, HMGB1 RNA expression in lung tissue, and immunohistochemistry for HMGB1 in a murine model of chronic asthma. (a) Western blot analysis of HMGB1 levels in lung tissue (PBS: PBS control group; OVA: chronic asthma group). (b) Real-time PCR analysis of HMGB1 expression in lung tissues (PBS: PBS control group; OVA: chronic asthma group). (c) ELISA analysis of HMGB1 levels in BALF (PBS: PBS control group; OVA: chronic asthma group; OVA+Control antibody: OVA+isotype antibody group; OVA+anti-HMGB1 antibody: OVA+anti-HMGB1 antibody group). (d) Immunohistochemical staining of lung tissue sections (bronchial area) from the PBS control group (PBS) and the chronic asthma group (OVA) (HMGB1 (dark brown); ×100, ×200, ×400). (e) Immunohistochemical staining of lung tissue sections (perivascular area) from the PBS control group (PBS) and the chronic asthma group (OVA) (HMGB1 (dark brown); ×100, ×200, ×400). #P<0.01 versus the PBS control group. The data are presented as the mean±s.e.m. N=8/group. BALF, bronchial alveolar lavage fluid; HMGB1, high-mobility group box protein 1; OVA, ovalbumin; PBS, phosphate-buffered saline; PCR, polymerase chain reaction.
Immunohistochemistry analysis showed that HMGB1 was expressed in the bronchiolar epithelial cells and alveolar macrophages, while weak expression of HMGB1 was also detected in vascular smooth muscle in mice from the PBS control group (Figure 1d and e). The number of HMGB1-positive cells was markedly higher in chronic asthmatic mice than in the control mice. These HMGB1-positive cells were predominantly found in the peribronchial and perivascular areas of the lung tissue (Figure 1d and e). However, the number of HMGB1-stained bronchiolar epithelial cells was lower, suggesting that HMGB1 was secreted from these epithelial cells in this model.
Effects of anti-HMGB1 treatment on OVA-induced chronic airway remodeling
Effects on airway inflammation
The total number of inflammatory cells, including eosinophils, lymphocytes and neutrophils, was increased in the BALF from mice in the OVA-induced asthmatic group as compared to PBS control mice (Figure 2a). Moreover, mice in the anti-HMGB1 antibody group exhibited fewer total inflammatory cells, eosinophils and neutrophils as compared to mice from the OVA-induced asthmatic group and the OVA+isotype antibody group (Figure 2a). The lung sections from anti-HMGB1 antibody-treated mice stained with H&E also showed a reduction in inflammatory infiltrates compared with mice from the OVA-induced asthmatic group and the OVA+isotype antibody group (Figure 2c). As expected, OVA-induced asthmatic mice exhibited a significant increase in AHR, However, administration of an anti-HMGB1 antibody to OVA-induced mice led to a decrease in AHR, as detected by both noninvasive and invasive methods (Figure 2d and e).
Figure 2.
Effect of anti-HMGB1 antibody treatment on OVA-induced chronic airway inflammation and OVA-specific IgE. (a) Inflammatory cells in the BALF. (b) The level of OVA-specific IgE in serum. (c) H&E-stained lung tissue (×100). (d) The effect of anti-HMGB1 antibody treatment on OVA-induced AHR as assessed by a non-invasive method. (e) The effect of anti-HMGB1 antibody treatment on OVA-induced AHR as assessed by an invasive method. PBS: control group; OVA: chronic asthma group; OVA+isotype antibody: OVA+isotype antibody group; OVA+anti-HMGB1: OVA+anti-HMGB1 antibody group. #P<0.01 versus the PBS control group. The data are presented as the mean±s.e.m. N=8/group. AHR, airway hyperresponsiveness; BALF, bronchial alveolar lavage fluid; HMGB1, high-mobility group box protein 1; OVA, ovalbumin; PBS, phosphate-buffered saline; PCR, polymerase chain reaction.
Effects on OVA-specific IgE
We determined the effects of an anti-HMGB1 neutralizing mAb on OVA-specific IgE by measuring the levels of OVA-specific IgE in the serum. As shown in Figure 2b, OVA-specific IgE was increased in chronic asthmatic mice, and administration of anti-HMGB1 antibodies resulted in a significant reduction in the levels of OVA-specific IgE.
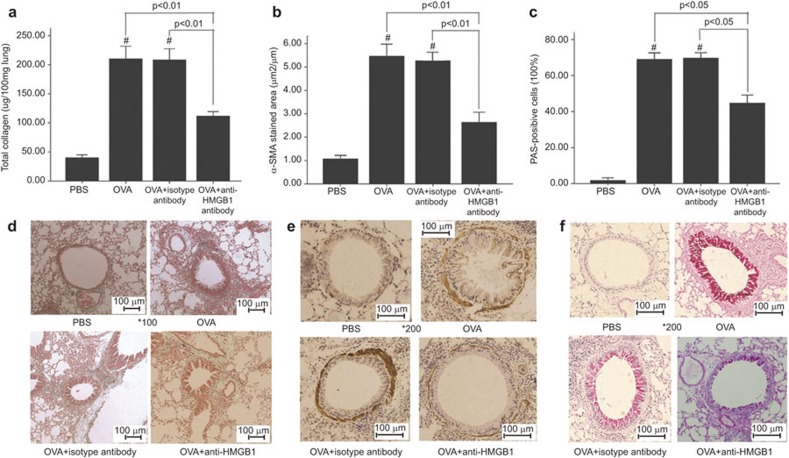
Effect of anti-HMGB1 antibody administration on airway remodeling
Two parameters (the area of peribronchial trichrome staining and lung collagen content) were used to evaluate peribronchial fibrosis. As shown in Figure 3a, the area of peribronchial trichrome staining in chronic asthmatic mice was significantly greater than in PBS-treated control mice. Treating asthmatic mice with an anti-HMGB1 antibody led to decreased collagen deposition in the bronchovascular regions compared with the OVA-induced asthmatic mice. Similarly, mice treated with an anti-HMGB1 antibody and repeatedly challenged with OVA exhibited reduced levels of lung collagen compared with the chronic asthmatic mice (Figure 3d). The peribronchial smooth muscle layer was thicker, and the percentage of airway epithelium cells that stained positively with PAS was significantly increased in chronic asthmatic mice compared with the PBS-treated mice. However, treatment with an anti-HMGB1 antibody administration led to a significant decrease in the percentage of PAS-positive cells in the airway epithelium (Figure 3b and e) and α-SMA-stained cells in the peribronchial area (Figure 3c and f) as compared with the chronic asthmatic mice.
Figure 3.
Reduced airway remodeling in mice treated with an anti-HMGB1 antibody. (a) Reduced lung collagen content. (d) Reduced peribronchial collagen deposition. (b, e) Reduced area of peribronchial α-SMA staining. (c, f) Reduced production of mucus in the airway compared with the OVA chronic asthma group. PBS: control group; OVA: chronic asthma group; OVA+isotype antibody: OVA+isotype antibody group; OVA+anti-HMGB1: OVA+anti-HMGB1 antibody group. #P<0.01 versus the PBS control group. The data are presented as the mean±s.e.m. N=8/group. HMGB1, high-mobility group box protein 1; OVA, ovalbumin; PBS, phosphate-buffered saline.
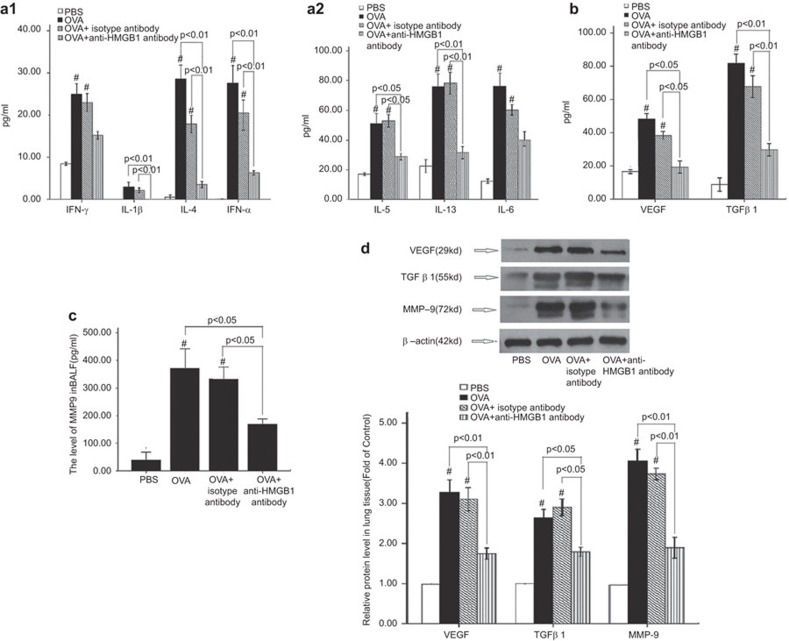
Effects on cytokine expression
The levels of IL-5, IL-4, IL-13, IL-1β, TNF-α (Figure 4a1 and a2), VEGF, active-TGF-β1 (Figure 4b) and total MMP-9 (Figure 4c) were significantly reduced in the BALF from mice in the anti-HMGB1 antibody group compared with the mice in the OVA+isotype antibody group. No differences were observed in the levels of interferon-γ (Figure 4a1) or IL-6 (Fig. 4a2). We further confirmed the changes in VEGF, total TGF-β1 and MMP-9 expression levels by western blot analysis of lung tissue lysates (Figure 4d). In addition, the high MMP-9 activity detected by gelatin zymography of BALF from the chronic asthmatic mice decreased following administration of an anti-HMGB1 antibody (Supplementary Information)
Figure 4.
Cytokine levels in mouse BALF and cytokine expression in mouse lung tissue. (a1, a2) Levels of IFN-γ, IL-1β, IL-4, IL-5, IL-6, IL-13 and TNF-α expression in BALF, as detected by ELISA (n=8). (b, c) VEGF, total MMP-9 and total TGF-β1 expression in BALF, as detected by ELISA (n=8). (d) VEGF, total TGF-β1 and MMP-9 expression in the lung tissue, as detected by western blot (n=6). PBS: control group; OVA: chronic asthma group; OVA+isotype antibody: OVA+isotype antibody group; OVA+anti-HMGB1: OVA+anti-HMGB1 antibody group. #P<0.01 versus the PBS control group. The data are presented as the mean±s.e.m. BALF, bronchial alveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; HMGB1, high-mobility group box protein 1; IFN, interferon; MMP, matrix metalloproteinase; OVA, ovalbumin; PBS, phosphate-buffered saline; TGF, transforming growth factor; TNF, tumor-necrosis factor; VEGF, vascular endothelial growth factor.
Effects of HMGB1 on the migration, proliferation, differentiation and collagen synthesis of human fetal lung fibroblasts (MRC-5 cells)
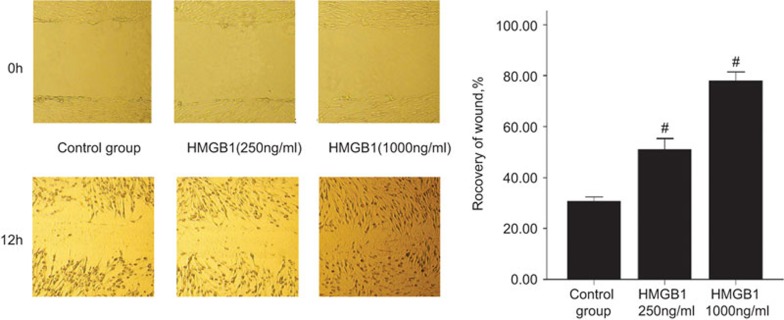
The migration of MRC-5 cells was evaluated by a scratch wound assay.19 Treatment with HMGB1 (250 and 1000 ng/ml) increased the migration capacity of MRC-5 cells in a concentration-dependent manner compared with the untreated control (Figure 5).
Figure 5.
The effect of HMGB1 treatment on the migration of MRC-5 cells. The results are expressed as the percentage of recovery in the wound area. Representative images (×100) of MRC-5 cells treated with HMGB1 or untreated, at time 0 and after 12 h of incubation, are shown. #P<0.01 versus the control group. The mean±s.e.m. from six experiments is shown. HMGB1, high-mobility group box protein 1.
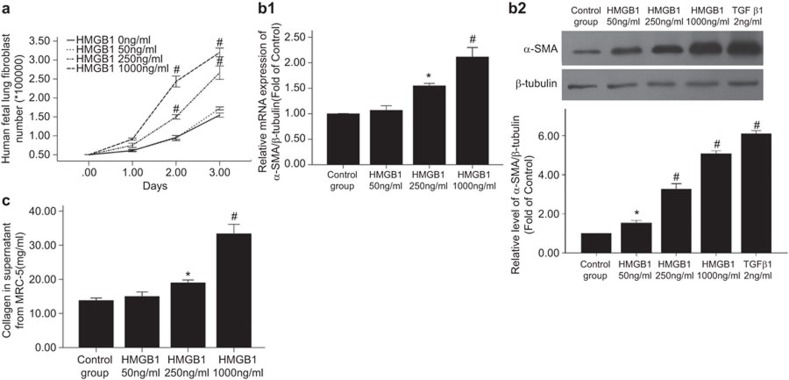
Treatment with 250 or 1000 ng/ml HMGB1 also significantly increased the number of MRC-5 cells as compared to the untreated control (Figure 6a). A time-course experiment using 1000 ng/ml HMGB1 showed that the maximal proliferation response occurred at day 2. The greatest difference in cell numbers between cells treated with 250 ng/ml HMGB1 and untreated cells was observed at day 3.
Figure 6.
The effect of HMGB1 treatment on human fetal lung fibroblast (MRC-5) proliferation, differentiation and collagen synthesis. (a) Proliferation. The cells were treated with 0, 50, 250 or 1000 ng/ml HMGB1 0–3 days, then resuspended and counted (n=4/group). (b) Differentiation. MRC-5 cells were treated with 0, 50, 250 or 1000 ng/ml HMGB1 for 48 h. (b1) The effect of HMGB1 treatment on α-SMA mRNA expression in MRC-5 cells, as determined by real-time PCR (n=4). (b2) The effect of HMGB1 treatment on α-SMA protein expression in MRC-5 cells, as determined by western blotting (n=4). The control cells were treated with TGF-β1 2 ng/ml). The upper panel shows a representative immunoblot, and the lower panel shows the pooled relative densitometric scanning values. (c) The effect of HMGB1 on collagen synthesis by MRC-5 cells. MRC-5 cells (2×105 cells/well in six-well plates) were treated with 0, 50, 250 or 1000 ng/ml HMGB1 for 48 h (n=4). The supernatants were collected and assayed using a sirius red collagen detection kit. *P<0.05 versus the control group, #P<0.01 versus the control group. The data are presented as the mean±s.e.m. HMGB1, high-mobility group box protein 1; PCR, polymerase chain reaction; TGF, transforming growth factor.
The upregulation of α-SMA expression is a characteristic marker for differentiation of lung fibroblasts to myofibroblasts. Using real-time PCR and western blotting, we detected the expression of α-SMA in MRC-5 cells after 48 h of treatment with HMGB1 (250 and 1000 ng/ml) and found that it significantly increased α-SMA mRNA expression (Figure 6b1) and protein expression in a concentration-dependent manner compared with the untreated control cells (Figure 6b2).
Treatment with HMGB1 (250 ng/ml) only slightly increased the level of MRC-5 collagen production compared with untreated control cells, whereas treatment with 1000 ng/ml HMGB1 led to 2–3 times as much collagen production compared with untreated control cells (Figure 6c).
Treatment with HMGB1 slightly increases the level of TGF-β1 secreted by eosinophils
TGF-β1 is produced by many types of cells during the airway remodeling process and plays a very important role in regulating the cellular processes that lead to airway remodeling and.16 Therefore, we investigated which types of cells expressed and secreted increased levels of TGF-β1 in response to HMGB1 treatment using ELISAs. The types of cells we tested included human peripheral blood eosinophils, neutrophils, lung macrophages, U937 cells, THP-1 monocytes, human umbilical vascular endothelial cells, human fetal lung fibroblasts (MRC-5 cells), human primary bronchial epithelial cells and two epithelial cell lines (16-HBE and A549). We found that that HMGB1 alone, even at the highest concentration used in this study (1000–2000 ng/ml), did not induce cytokine production. HMGB1 has been shown to increase the cytokine response induced by other cytokines such as IL-1β through the formation of complexes,13 and elevated levels of HMGB1/IL-1β complex have been found in serum from patients with asthma.4,6 Therefore, we investigated whether the HMGB1/IL-1β complex affected the synthesis and secretion of cytokines. The cell types listed above were incubated with varying concentrations of HMGB1, IL-1β or HMGB1/IL-1β complexes. Interestingly, stimulation with the HMGB1/IL-1β complex, but not HMGB1 or IL-1β alone, for 24 h led to a slight increase in TGF-β1 levels in the supernatant from eosinophils only (Supplementary Information). We did not detect this increase in the supernatants from other cells (data not shown). Treatment with HMGB1 alone or with the HMGB1/IL-1β complex did not induce an increase in the levels of TGF-β1 mRNA or protein expression in eosinophils compared with the untreated control cells (data not shown).
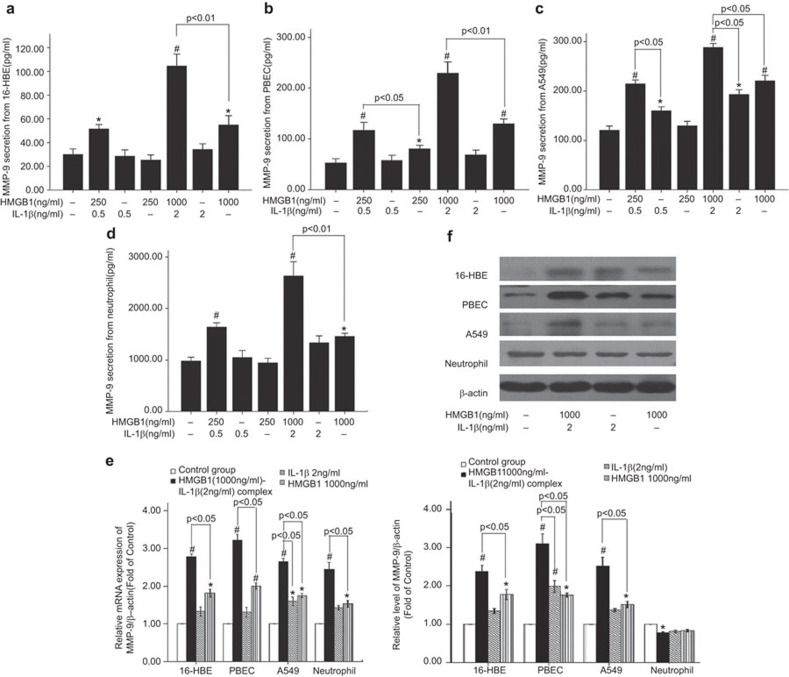
Treatment with HMGB1 increased MMP-9 expression and activity
MMP-9 is a significant pathogenic factor in asthmatic airway remodeling. The primary cellular sources of MMP-9 in airway remodeling are eosinophils, epithelial cells, alveolar macrophages and neutrophils.17 Thus, we evaluated the MMP-9 expression and activity in human peripheral blood eosinophils, neutrophils, alveolar macrophages, U937 cells, THP-1 monocytes, human primary bronchial epithelial cells and two epithelial cell lines (A549 and 16-HBE). Treatment with HMGB1 alone or the HMGB1/IL-1β complex did not result in increased MMP-9 secretion or expression by eosinophils, alveolar macrophages, U937 cells or THP-1 monocytes compared with the untreated control cells. Additionally, stimulation with 250 or 1000 ng/ml HMGB1 or 0.5 or 2 ng/ml IL-1β induced little or no production of MMP-9 by 16-HBE cells, PBECs, A549 cells and neutrophils. In contrast, significant levels of MMP-9 production were observed in 16-HBE cells, PBECs, A549 cells and neutrophils pre-incubated with 0.5 or 2 ng/ml IL-1β followed by treatment with 250 or 1000 ng/ml HMGB1, as compared to cells treated with HMGB1 or IL-1β alone (Figure 7a–d). Similarly, treatment with the HMGB1/IL-1β complex led to an increase in MMP-9 secretion, as detected by gelatin zymography analysis (Supplementary Information). Treatment with 1000 ng/ml HMGB1 or 2 ng/ml IL-1β induced little (one- to twofold) to no significant changes in MMP-9 mRNA or protein expression in 16-HBE cells, PBECs, or A549 cells as compared with the untreated control cells. However, treatment with the HMGB1/IL-1β complex resulted in a 2.5- to 4-fold upregulation in MMP-9 expression (Figure 7e and f). Treatment with the HMGB1/IL-1β complex decreased MMP-9 content in neutrophil lysates compared with untreated control cells (Figure 7f). We observed similar results by ELISA and gelatin zymography. These data suggest that the MMP-9 secretion induced by treatment with the HMGB1/IL-1β complex resulted in the downregulation of MMP-9 expression in the neutrophil lysates.
Figure 7.
Treatment with HMGB1 in complex with IL-1β stimulates 16-HBE cells, PBECs, A549 cells and neutrophils to increase MMP-9 production. 16-HBE, PBECs and A549 cells (2×105 cells/well) and neutrophils (1×106 cells/ml) were seeded into 24-well plates and then treated with HMGB 1(250 ng/ml or 1000 ng/ml), IL-1β (0.5 ng/ml or 2 ng/ml) or the HMGB1/IL-1β complex (HMGB1 250 ng/ml+IL-1β 0.5 ng/ml or HMGB1 1000 ng/ml+IL-1β 2 ng/ml) for 24 h (for epithelial cells) or 30 min (for the neutrophils). Untreated cells were included as controls. The cell culture supernatants, cell lysates and total RNA were collected for further analysis. (a) The level of MMP-9 in 16-HBE supernatants, as detected by ELISA. (b) The level of MMP-9 in PBEC supernatant, as detected by ELISA. (c) The level of MMP-9 in A549 supernatant, as detected by ELISA. (d) The level of MMP-9 in the neutrophil supernatant, as detected by ELISA. (e) The expression of MMP-9 mRNA relative to β-actin in 16-HBE, PBEC and A549 cells and neutrophils, as detected by real-time PCR. (f) The expression of MMP-9 protein relative to β-actin in 16-HBE, PBEC and A549 cells and neutrophils, as detected by western blotting. *P<0.05 vs. the control group, #P<0.05 vs. the control group. The data are presented as the mean±s.e.m. N=4. HMGB1, high-mobility group box protein 1; ELISA, enzyme-linked immunosorbent assay; MMP, matrix metalloproteinase; PBEC, primary human bronchial epithelial cell; PCR, polymerase chain reaction.
HMGB1 increased the expression and secretion of VEGF
Studies in a transgenic mouse model that expresses VEGF in the lungs have suggested that VEGF is an important mediator of vascular inflammation and airway remodeling.18 In this study, we found that treatment with HMGB1 or the HMGB1/IL-1β complex did not increase VEGF secretion from eosinophils, human mast cells or differentiated U937 monocyte/macrophage-like cells compared with the untreated control cells. Treatment with HMGB1 or IL-1β induced little (one- to twofold) or no significant change in VEGF secretion from 16-HBE cells, PBECs, A549 cells, undifferentiated U937 cells, undifferentiated and differentiated THP-1 cells, or alveolar macrophages compared with the untreated control cells. However, treatment with the HMGB1/IL-1β complex resulted in a 2.5- to 4-fold upregulation in VEGF secretion (Figure 8a–e). The effect of this complex was strongest in 16-HBE cells. HMGB1 in complex with IL-1β also resulted in increased VEGF mRNA (Figure 8f–h) and protein expression (Figure 9) in the cell lines listed above. The effects of the complexes were the most significant in undifferentiated and differentiated THP-1 cells. These data suggest that the reduced VEGF expression in mice treated with an anti-mouse HMGB1 antibody was due to inhibition of VEGF expression and secretion in lung epithelial cells and macrophages.
Figure 8.
HMGB1 in complex with IL-1β stimulates 16-HBE cells, PBECs, A549 cells, U937 cells, THP-1 monocyte/macrophages and alveolar macrophages to increase VEGF production. 16-HBE, PBECs and A549 cells, alveolar macrophages and differentiated THP-1 cells (2×105 cells/well) and undifferentiated U937 cells and THP-1 cells (1×106 cells/ml) were seeded into six-well plates and then treated with HMGB1 (250 ng/ml or 1000 ng/ml), IL-1β (0.5 ng/ml, 2 ng/ml or 5 ng/ml) or the HMGB1/IL-1β complex (HMGB1 250 ng/ml+IL-1β 0.5 ng/ml, HMGB1 1000 ng/ml+IL-1β 2 ng/ml or 1000 ng/ml+IL-1β 5 ng/ml) for 24 h. Untreated cells were included as controls. The cell culture supernatants, cell lysate and total RNA were collected for further analysis. VEGF secretion from 16-HBE cells (a), PBEC (b), A549 cells (c), U937 cells (d), undifferentiated and differentiated THP-1 cells, and alveolar macrophages (e), as detected by ELISA. Expression of VEGF mRNA relative to β-actin in 16-HBE cells and PBEC (f), A549 cells (g), U937 cells, undifferentiated and differentiated THP-1 cells and alveolar macrophages (b), as detected by real-time PCR. *P<0.05 vs. the control group, #P<0.05 vs. the control group. The data are presented as the mean±s.e.m. N=4. HMGB1, high-mobility group box protein 1; ELISA, enzyme-linked immunosorbent assay; PBEC, primary human bronchial epithelial cell; PCR, polymerase chain reaction; VEGF, vascular endothelial growth factor.
Figure 9.
HMGB1 in complex with IL-1β stimulates 16-HBE cells, PBECs, A549 cells, U937 cells, THP-1 monocyte/macrophages and alveolar macrophages to increase VEGF protein expression, as detected by western blot. 16-HBE cells, PBECs, A549 cells, alveolar macrophages and differentiated THP-1 cells (2×105 cells/well) and undifferentiated U937 and THP-1 cells (1×106cells/ml) were seeded into six-well plates and then treated with HMGB1 (250 ng/ml or 1000 ng/ml), IL-1β (0.5 ng/ml, 2 ng/ml or 5 ng/ml) and the HMGB1/IL-1β complex (HMGB1 250 ng/ml+IL-1β 0.5 ng/ml,HMGB1 1000 ng/ml+IL-1β 2 ng/ml or 1000 ng/ml+IL-1β 5 ng/ml) for 24 h. Untreated cells were included as controls. The cell lysates were collected and subjected to western blotting, as described in the section on ‘Materials and methods'. (a1, a2) Expression of MMP-9 protein relative to β-actin in 16-HBE, PBECs and A549 cells. (b1, b2) Expression of MMP-9 protein relative to β-actin in U937 cells, THP-1 monocyte/macrophages and alveolar macrophages. *P<0.05 vs. the control group, #P<0.05 vs. the control group. The data are presented as the mean±s.e.m. N=4.
Discussion
In the present study, we demonstrated that blocking HMGB1 activity inhibits airway remodeling in an OVA-induced mouse model of chronic asthma. First, we showed that HMGB1 expression was significantly higher in chronic asthmatic mice. Moreover, in comparison with OVA-induced asthmatic mice, mice treated with an anti-HMGB1 antibody exhibited reduced airway inflammation, AHR and airway remodeling. We also showed that treatment with HMGB1 induced lung fibroblast activation. Finally, HMGB1 alone and particularly in complex with IL-1β directly affects the expression and secretion of TGF-β1, MMP-9 and VEGF, which are important mediators of airway remodeling. We previously reported increased levels of HMGB1 in asthma patients and asthmatic mice.7,8 In this study, we present additional evidence that HMGB1 may be involved in the pathogenesis of asthma, including airway remodeling.
Our finding that anti-HMGB1 treatment inhibits airway remodeling in a mouse model of chronic asthma is consistent with a recent report by Lee et al.19 However, our study identified a different mechanism. Lee et al.19 showed that HMGB1 had a regulatory effect on immune helper T cells and that elevated TLR-2 and TLR-4 expression decreased in response to treatment with an anti-HMGB1 antibody. In contrast, we found that treatment with HMGB1 directly affects specific structural cells, including lung fibroblasts and airway smooth muscle, and induces the expression and release of some important remodeling mediators.
In this study, we found that the HMGB1-positive cells were primarily infiltrating inflammatory cells, and that the number of these cells was higher in the chronic asthmatic mice than in the PBS-treated control mice. These findings suggest that infiltrating inflammatory cells may contribute to the enhanced expression of HMGB1 in the lungs of asthmatic mice. However, the source of soluble HMGB1 in the BALF is difficult to determine. We previously demonstrated that hydrogen peroxide can induce human bronchial epithelial cells to actively release HMGB1.20 Interestingly, both the number of HMGB1-positive bronchiolar epithelial cells and the intensity of HMGB1 staining in the cytoplasm of bronchiolar epithelial cells were lower in chronic asthmatic mice than in the control mice. These findings suggest that airway epithelial cells are a potentially important source of HMGB1 secretion during airway remodeling. In addition, alveolar macrophages and mononuclear cells are another important source of HMGB1 in the lungs.6,21
Consistent with the findings by Shim et al.,6 administration of an anti-HMGB1 antibody resulted in decreased lung inflammation overall, resulting in reduced numbers of inflammatory cells and decreased cytokine levels. Moreover, anti-HMGB1 administration also led to a marked decrease in the number of neutrophils in the BALF. We previously showed4 that HMGB1 levels in asthma patients are positively correlated with the number of neutrophils in the sputum. HMGB1-induced neutrophil recruitment may explain this correlation.22 In addition, it is likely that chronic AHR correlates with chronic airway inflammation in asthma and may be associated with the persistent structural alterations (so-called airway remodeling) observed in chronic asthma.23 Thus, the reduction in AHR that we observed after treatment with an anti-HMGB1 antibody may be related to the reduced production of Th2 cytokines such as IL-13 as well as structural changes in the airway.
Sub-epithelial fibrosis is considered a hallmark of chronic airway remodeling in asthma.24 In this study, we observed that anti-HMGB1 treatment led to reduced peribronchial collagen deposition as well as total lung collagen content. Our results are consistent with other observational studies, which showed that administration of an anti-HMGB1 antibody reduced the levels of collagen in the lungs in a bleomycin-induced mouse model of pulmonary fibrosis7 and in an allergen-induced mouse model of chronic asthma.19 TGF-β1 and MMP-9 have been shown to play important roles in the modulation of lung collagen homeostasis.24 Our results suggest that blocking HMGB1 activity may affect lung fibrosis in chronic asthma in a TGF-β1- and MMP-9-dependent manner. In addition, Lappalainen and colleagues25 previously demonstrated that IL-1β causes lung inflammation, mucus metaplasia and airway fibrosis in adult transgenic mice. TNF-α has also been shown to play an important role in chronic airway remodeling, as it leads to increased levels of sub-epithelial fibrosis, peribronchial smooth muscle and deposition of extracellular matrix.26 Furthermore, this study shows that HMGB1 treatment increases α-SMA expression in human fetal lung fibroblasts. Myofibroblasts are highly active cells that can secrete extracellular matrix proteins.24 An increase in airway smooth muscle mass is another feature of airway remodeling. In this study, we demonstrated that blocking HMGB1 activity results in a reduction in peribronchial smooth muscle, implying that HMGB1 is an important mediator of OVA-induced airway smooth muscle cell hyperplasia and hypertrophy. Palumbo and colleagues27 showed that HMGB1 and its receptor RAGE may be involved in muscle tissue regeneration through inducing the migration and proliferation of vessel-associated stem cells. Indeed, we found that HMGB1 induces the proliferation of airway smooth muscle cells (data not shown). We are currently investigating the mechanism by which HMGB1 stimulates airway smooth muscle cell hyperplasia and hypertrophy and to determine whether HMGB1 has an effect on the migration and secretion of airway smooth muscle cells. Lung tissue from IL-13-transgenic mice exhibits mucus overproduction and goblet cell hyperplasia.28 In this study, we found that treatment with an anti-HMGB1 antibody led to a reduction in the number of PAS-stained goblet cells in the airway. Thus, we suggest that HMGB1 may induce goblet cell hyperplasia and mucus overproduction indirectly through IL-13 and other cytokines.
Furthermore, we evaluated whether HMGB1 could directly alter the functional phenotype of lung fibroblasts in vitro. First, we found that HMGB1 can directly induce human lung fibroblast proliferation, which is consistent with a previous report by Hamada et al.7 Additionally, we observed that HMGB1 increases human lung fibroblast migration and α-SMA expression. These results demonstrate that HMGB1 promotes the differentiation of fibroblasts into myofibroblasts, which contributes to ECM protein overproduction and deposition in the form of sub-epithelial fibrosis.29 Consistent with the findings from Hamada et al.,7 50 ng/ml HMGB1 did not affect collagen synthesis; higher concentrations of HMGB1 were required to induce the production of collagen. Moreover, in this study, we did not observe any effects of HMGB1 on the secretion of TGF-β1, MMP-9 or VEGF from MRC-5 cells, strongly suggesting that HMGB1 also exerts a direct profibrotic effect on lung fibroblasts in vitro. HMGB1 may also induce activation of the lung fibroblasts indirectly by increasing the expression of other profibrogenic factors like TGF-β1 and TNF-α.
TGF-β1 has been identified to as an important mediator associated with tissue remodeling in chronic asthma.16 Although Hamada et al.7 reported that treatment with an anti-HMGB1 antibody did not lead to a reduction in TGF-β1 levels in BALF from mice, we detected a significant decrease in TGF-β1 expression levels in mice treated with an anti-HMGB1 antibody treatment compared to the control mice. As HMGB1 promotes the survival of human eosinophils and can enhanced eosinophil chemotaxis,30 it is reasonable to assume that blocking HMGB1 activity can decrease the number of eosinophils infiltrating into the lung, as suggested by this study and another study.6 Additionally, eosinophils are the main source of TGF-β1 in asthmatic airways. Thus, it is possible that TGF-β1 levels decrease together with the reduction in eosinophil numbers. In this study, we show that treatment with the HMGB1/IL-1β complex increases TGF-β1 secretion from eosinophils. This outcome demonstrates another possible mechanism by which treatment with HMGB1 may lead to a decrease in TGF-β1 expression.
In this study, we showed that treatment with an anti-HMGB1 antibody resulted in decreased levels of cytokines, including IL-1β, TNF-α, TGF-β1, MMP-9 and VEGF. Therefore, we sought to clarify whether HMGB1 directly affects the secretion and expression of these cytokines from structural immune cells and inflammatory cells in the lung. HMGB1 can induce the secretion of TNF-α and IL-1β from human monocytes and neutrophils.31,32 However, we did not detect a any direct effect of HMGB1 on the secretion of TGF-β1 from a variety of related cell lines. Other cells that are potential sources of TGF-β1, such as vascular smooth muscle cells, were not assessed. To the best of our knowledge, this is the first study to report that HMGB1 alone and in complex with IL-1β can induce of MMP-9 expression, secretion and activity in 16-HBE cells, PBECs and neutrophils, which are the important sources of MMP-9 in asthma. In addition, consistent with a previous report by Liu et al.,33 our findings confirmed that MMP-9 secretion and expression increase in A549 cells in response to HMGB1. We are the first to report that treatment with HMGB1 or the HMGB1/IL-1β complex induces VEGF expression and secretion in 16-HBE cells, PBECs and A549 cells. Kawahara and colleagues34 showed that treatment with HMGB1 enhanced VEGF production in murine macrophage-like RAW264.7 cells. Our study confirms that treatment with HMGB1 alone increases VEGF release from U937 cells, THP-1 cells and human alveolar macrophages, and that treatment with the HMGB1/IL-1β complex enhances this process. Further research is needed to identify HMBG1 ligands and the signaling activation and transduction mechanisms that participate in this process.
In conclusion, this study demonstrated that HMGB1 plays an important role in the development of OVA-induced chronic airway remodeling in a mouse model. HMGB1 directly affected the expression and secretion of inflammatory mediators, including TGF-β1, MMP-9 and VEGF, and indirectly modulated allergic airway inflammation. Our study provides evidence for HMGB1 as a profibrotic factor that modulates the activation and phenotype of lung fibroblasts. Further studies in humans are needed to determine whether blocking HMGB1 activity is a potential therapeutic approach for allergic airway remodeling. Although mucosal immunotherapy can lead to severe side effects such as severe anaphylaxis, it still represents a potentially beneficial treatment for allergic diseases.35 We propose that combining mucosal immunotherapy with anti-HMGB1 administration could represent a novel strategy for treating allergic diseases.
Acknowledgments
This study was supported by the Scientific Research and Technological Development Program Project of Guangxi Province (10124001A-32), the Young Science Foundation of Guangxi Medical University (GXMUSF201206) and the Innovation Project of Guangxi Graduate Education (YCBZ2013014).
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Pettilä V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–981. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, et al. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17:807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CC, Zhao HJ, Cai SX, Liu LY, Shen XB, Mo GW. Expression of high mobility group box-1 in the lung tissue and BALF of asthmatic mice and the influence of dexamethasone. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:2051–2054. [PubMed] [Google Scholar]
- Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42:958–965. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, et al. The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:440–447. doi: 10.1165/rcmb.2007-0330OC. [DOI] [PubMed] [Google Scholar]
- Biscetti F, Straface G, de Cristofaro R, Lancellotti S, Rizzo P, Arena V, et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 2010;59:1496–1505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rat. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocols. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol. 2007;37:424–430. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt JI, Boreiko CJ, Mangum JB, Martin JT, Iglehart JD, Hesterberg TW. Development of a tracheal implant xenograft model to expose human bronchial epithelial cells to to Xic gases. Toxicol Pathol. 1989;17:465–473. doi: 10.1177/019262338901700301. [DOI] [PubMed] [Google Scholar]
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Conkright JJ, Rothenberg ME. CC chemokine receptor-3 undergoes prolongedligand-induced internalization. J Biol Chem. 1999;274:12611–12618. doi: 10.1074/jbc.274.18.12611. [DOI] [PubMed] [Google Scholar]
- Schleimer RP, Rutledge BK. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986;136:649–654. [PubMed] [Google Scholar]
- Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–33. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005;4:177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Lai YT, Chang HT, Liao JW, Shyu WC, Li CY, et al. Inhibition of high-mobility group box 1 in lung reduced airway inflammation and remodeling in a mouse model of chronic asthma. Biochem Pharmacol. 2013;86:940–949. doi: 10.1016/j.bcp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Hou C, Zhao H, Li W, Cai S. Hydrogen peroxide induces high mobility group box 1 release in human bronchial epithelial cells. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1131–1134. [PubMed] [Google Scholar]
- Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, et al. Expression of high-mobility group box 1 and of receptor for advanced glycatio end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118:551–559. doi: 10.1016/j.jaci.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- Cho JY, Pham A, Rosenthal P, Miller M, Doherty T, Broide DH. Chronic OVA allergen challenged TNF p55/p75 receptor deficient mice have reduced airway remodeling. Int Immunopharmacol. 2011;11:1038–1044. doi: 10.1016/j.intimp.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007;56:331–340. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- Liu PL, Tsai JR, Hwang JJ, Chou SH, Cheng YJ, Lin FY, et al. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am J Respir Cell Mol Biol. 2010;43:530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Hashiguchi T, Kikuchi K, Tancharoen S, Miura N, Ito T, et al. Induction of high mobility group box 1 release from serotonin-stimulated human umbilical vein endothelial cells. Int J Mol Med. 2008;22:639–644. [PubMed] [Google Scholar]
- Ye YL, Chuang YH, Chiang BL. Strategies of mucosal immunotherapy for allergic diseases. Cell Mol Immunol. 2011;8:453–461. doi: 10.1038/cmi.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.