Abstract
Background
Methylphenidate is a stimulant prescribed to treat Attention Deficit Hyperactivity Disorder. Its primary mechanism of action is in the dopamine system, alterations of which are associated with vulnerability to alcohol abuse. There are concerns that juvenile MPH treatment may influence adult drinking behavior. This study examined the interaction of MPH treatment and environmental rearing conditions, which are known to independently influence ethanol (EtOH) drinking behavior, on anxiety-like behavior and vulnerability to alcohol abuse in a juvenile rodent model.
Methods
Male Sprague Dawley rats were housed in enriched, standard, or isolated conditions for four weeks, starting at postnatal day 21. Rats were concurrently treated with 8 mg/kg/day MPH or saline, delivered via osmotic minipump. Anxiety-like behavior was determined at the end of the treatment session, and 5 weeks later. After MPH treatment, rats were exposed to a two-bottle choice EtOH drinking procedure that lasted three weeks.
Results
Early life chronic MPH treatment was associated with greater EtOH intake and greater EtOH preference, but only in socially isolated animals. Isolated animals had greater levels of anxiety-like behavior than standard-housed or enriched animals after 4 weeks of exposure to the housing conditions, a difference that persisted even after all animals had been individually housed for an additional 5 weeks and exposed to EtOH.
Conclusions
These results suggest that early life MPH treatment may increase vulnerability to EtOH drinking in adulthood in a subset of the population. Additionally, this study highlights the importance of early rearing condition for establishing long-lasting behavioral phenotypes. Environmental histories should be considered when prescribing MPH treatment to young children.
Keywords: Social Isolation, Ethanol Drinking, Methylphenidate, Rearing Environment
1. Introduction
Methylphenidate (MPH) is a stimulant medication that is prescribed to treat Attention Deficit Hyperactivity Disorder (ADHD), the most common psychological disorder of childhood. It has been shown to be extremely effective in acutely reducing the hallmark symptoms of ADHD including inattention, hyperactivity, and impulsivity (for review see Findling, 2008). As ADHD diagnoses have dramatically increased over the past several decades (Olfson et al., 2002, Zuvekas et al., 2006), so have prescriptions for stimulant drugs such as MPH.
MPH exerts its actions on the brain by blocking the dopamine transporter (DAT), a key regulator of dopamine transmission in brain areas that are associated with motivation and reward (Patrick et al., 1987, Volkow et al., 1995). Alterations of the dopamine system are regularly associated with vulnerability to substance abuse and the development of addiction. Thus, the potential for MPH treatment to have dopaminergic consequences, coupled with increased use, has led to concerns about the possibility for chronic MPH treatment to alter vulnerability to substance abuse.
Clinical studies have addressed this question and have suggested that early stimulant medication for ADHD may have a protective effect against the development of substance abuse disorders in adulthood (Wilens et al., 2003, Mannuzza et al., 2003, Volkow and Swanson, 2008, Biederman, 2003). However, varying treatment histories and differing diagnostic criteria in ADHD patients, among other factors, can produce bias and make interpretation of these studies difficult (Volkow and Insel, 2003). Furthermore, ADHD itself is associated with an increased risk for the development of a substance abuse disorder (Biederman et al., 1995, Milberger et al., 1997). In contrast, animal studies afford careful control over factors that introduce bias or variability into epidemiologic studies, allowing direct examination of the variable of interest, in this case exposure to methylphenidate.
There are very few studies in animal models investigating the effects of chronic MPH treatment on future intake of ethanol (EtOH), and of those that are available, results are conflicting (Vendruscolo et al., 2008, Soeters et al., 2008). This question is critical as alcohol is the most commonly abused drug in age groups that are likely to have a history of MPH prescription, such as adolescents and young adults (Johnston et al., 2013). There is also a clear association between ADHD diagnosis and alcohol dependence (Edwards and Kendler, 2012, Ameringer and Leventhal, 2013), which may or may not be related to stimulant treatment. One goal of the present study was to examine the effects of chronic MPH treatment on vulnerability to EtOH abuse in a rodent model.
Most studies of the effects of chronic MPH treatment on vulnerability to substance abuse have not taken into account the interacting effects of environmental conditions. However, it is known that environment can shape future behaviors, as well as vulnerability to substance abuse. For example, children who are exposed to early life stress have higher rates of depression, anxiety, and substance abuse as adults (Turner and Lloyd, 2004). Likewise, rodents who are exposed to early life stress in the form of social isolation have greater levels of anxiety-like behavior (McCool and Chappell, 2009, Chappell et al., 2013, Yorgason et al., 2013, Lodge and Lawrence, 2003, Lukkes et al., 2009) and depressive-like behavior (Brenes and Fornaguera, 2008, Brenes Saenz et al., 2006). Notably, socially isolated animals have been shown to have greater EtOH intakes than environmentally enriched or group housed animals (Chappell et al., 2013, McCool and Chappell, 2009, Deehan et al., 2007, Deehan et al., 2011, Lodge and Lawrence, 2003, Hall et al., 1998, Wolffgramm, 1990). Contrarily, rodents raised in enriched environments have been shown to be less sensitive to the rewarding effects of drugs of abuse (Bardo et al., 1995) and have lower drug intakes (Bardo et al., 2001, Alvers et al., 2012, Deehan et al., 2011).
Previous studies have shown that MPH, when given acutely, interacts with rearing condition to improve some behavioral deficits, such as impulsivity, associated with social isolation (Perry et al., 2008). Additionally, MPH, when administered during a drinking procedure, has been shown to decrease EtOH intake in rodents (Griffin et al., 2010). However, the effects of prior chronic MPH treatment on future EtOH drinking remain unclear. The purpose of the present study was to examine the interaction of chronic MPH and postweaning rearing environment on anxiety-like behavior and future EtOH drinking in young rats. To study chronic treatment, osmotic minipumps (Alzet©; Durect Corporation, Cupertino,CA) were used to administer MPH continuously, keeping blood levels of drug relatively stable. We have used this drug administration method previously as a way to model some of the pharmacokinetic aspects of the commonly prescribed extended-release formulations of methylphenidate, namely the generation of blood drug levels that persist for up to 12 hours (Swanson 2004). In a previous study, 8 mg/kg/day was found to generate average blood plasma levels of MPH of 15.4 ± 5.27 ng/ml (Gill et al., 2013), which is on the high level, but still very close to the therapeutic range of 10–15 ng/ml as indicated for treating children (Volkow and Swanson 2003). As adults, animals were tested for anxiety-like behavior and EtOH drinking to determine if MPH differentially influenced these behaviors as a function of environment.
2. Materials and Methods
Subjects and Housing Conditions
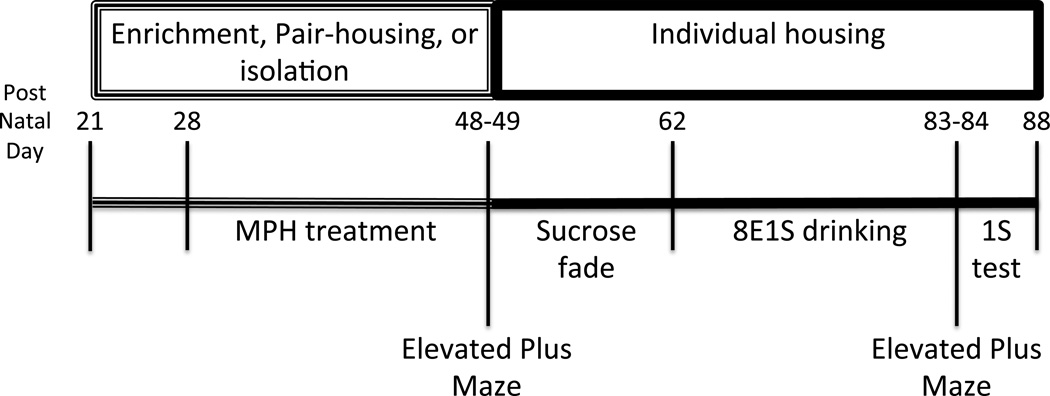
Forty-eight male Sprague Dawley rats were acquired at postnatal day (PND) 21 from Harlan Industries and placed into one of three housing conditions (16 rats per condition): environmentally enriched, pair-housed, or socially/environmentally isolated. Enriched animals were housed 4 animals per cage in large (280 square inches floor, 8 inches high) clear plastic cages with toys and climbing structures. Toys were rotated twice weekly and all animals were handled daily. Paired animals were housed 2 animals per cage in a standard size (142 square inches floor, 8 inches high) clear plastic cage without toys and with only limited handling. Isolated animals were housed singly in standard (142 square inches floor, 8 inches high) size opaque cages, without toys and were not handled except for weighing immediately prior to surgeries. All animals had 24-hour access to food and water and lights were maintained on a 12-hour on/off schedule with lights on at 8:00 am. At PND 50, all animals were individually housed in standard-size cages for the remainder of the study (see Figure 1 for study timeline). All studies were carried out in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, and experimental procedures were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Figure 1. Post-natal days and study timeline.
Drugs and Surgeries
Methylphenidate hydrochloride was obtained from Mallinckrodt (Covidien Pharmaceuticals; Hazelwood, MO). After one week of habituation to the housing conditions, animals were assigned to one of two conditions: 0 or 8 mg/kg/day MPH (8 animals per housing/drug dose). Animals that were housed together in the same cage were assigned to the same drug condition. On the morning of the scheduled surgery, each animal was weighed and its dose for the week was calculated. As young rats grow rapidly in this stage, end of the week body weights were estimated using normative growth charts for Sprague Dawley rats published by Harlan Industries (2008). The estimated end of the week weight was, on average, within 5% of the actual measured weight at the end of the week. The “dose weight” was the average of the current body weight and the estimated end of the week weight. After dose weights were calculated, the correct amount of MPH for each animal was mixed into a sterile saline solution (0.9%) and loaded into an osmotic minipump.
Osmotic minipumps were implanted subcutaneously to deliver a steady dose of MPH over 21 days. Animals were anesthetized with 3–4% isoflurane and given a dose of ketoprofen (5 mg/kg; s.c.) for pain relief. The skin on the animal’s back was shaved and washed in three stages with betadine surgical scrub, 70% isopropyl alcohol, and betadine surgical solution. A small incision was made in the skin between the scapulae. Using a hemostat, a small pocket was formed by spreading the subcutaneous connective tissue apart. The pump was inserted into the pocket and the skin was closed with Gluture® (Abbott Animal Health; Abbott Park, IL) tissue adhesive. The minipumps lasted for 7 days, at which point, old pumps were removed and replaced with new pumps. Drug treatment lasted for 21 days, and the final pumps were removed at the end of the third week of treatment. Each animal had a total of four surgeries (three implants, and a final removal), each lasting under 5 minutes.
Ethanol Drinking
Animals were individually housed for the EtOH self-administration portion of the study, starting at PND 50. Animals were weighed daily between 8:00 and 9:00am and weights were recorded. At 9:00am regular water bottles were removed and EtOH/sucrose and water sipper tubes were hung on the cage side by side and were available for one hour. EtOH/sucrose and water tube sides were changed daily to eliminate side preference. At 10:00am, bottles were removed and measured and regular water bottles were replaced on cages.
A modified Samson sucrose-fade technique was used to eliminate inherent problems of taste aversion to EtOH in Sprague-Dawley rats (Samson, 1986). Each day animals were given identical bottles containing two different solutions, one mixture of EtOH/sucrose and one plain water bottle. On day 1, animals were given a bottle of 10% sucrose (10S) mixed into water. On following days the solution was adjusted to decrease the sucrose concentration and increase the EtOH concentration. Concentrations were as follows: 2% EtOH/7% sucrose (2E7S), 2E5S, 4E5S, 4E3S, 4E3S, 5E2S, 5E1S, 6E2S, 6E1S, 7E2S, 8E1S. Removing sucrose from the solution entirely and testing only a concentration of 8% EtOH drastically reduced drinking behavior, thus the final concentration used for the remainder of the study was 8E1S. Animals had access to the 8E1S solution one hour per day for 21 consecutive days. Each day, mLs consumed (mLs pre drinking session – mLs post drinking session), g/kg EtOH intake, and % EtOH preference were calculated. G/kg EtOH intake was calculated using the following equation: g/kg EtOH = 1000/body weight(g)*mLs ethanol consumed*0.789*ethanol concentration/100. Percent EtOH preference was calculated as the mLs EtOH consumed over the total liquid consumed, thus preference scores greater than 50% reflected a preference for the EtOH/sucrose solution.
Anxiety-like behavior
On PND 48 or 49 (Pre-EtOH), rats were tested for anxiety-like behavior on the elevated plus maze (Med Associates; St. Albans, VT). The elevated plus maze consisted of two opposite open arms and two opposite closed arms that were elevated 40 cm above the floor. Photosensors recorded the entries and time spent in each individual arm. At the beginning of the assay, animals were placed in the center of the plus maze facing an open arm. After 5 minutes, animals were immediately returned to their home cages. Animals were re-tested on the elevated plus maze near the conclusion of the study, at PND 84–85 (Post-EtOH), after approximately five weeks of daily EtOH exposure (sucrose fade and then three weeks of two bottle choice at 8E1S). Importantly, this assay can be used to reliably re-assess anxiety-like behavior, provided the tests are separated by at least 28 days (Schneider et al, 2011). For the Post-EtOH run, animals had a normal EtOH drinking session in the morning, and sufficient time was allowed to pass for EtOH clearance before testing on the plus maze.
Blood EtOH concentrations
On two different occasions during the 8E1S two-bottle choice study, animals’ blood ethanol concentrations (BECs) were tested at the end of the drinking session. A tail-snip procedure was used to collect approximately 15 µL of blood from each rat. The tail tip was cleaned, sterilized, and treated with analgesic cream. Using a sharp pair of sterile surgical scissors, the very tip of the tail below the tail vertebrae was lacerated and blood was collected. Blood EtOH concentrations were determined using a commercially available alcohol dehydrogenase/NADH enzymatic assay kit (Diagnostic Chemicals, Oxford, CT).
Sucrose Preference
In order to assess the impact of the addition of sucrose to the EtOH solution, a 1% sucrose solution, without EtOH, was presented with a water bottle for three consecutive days following the three-week EtOH/sucrose exposure. Procedures were identical to those of the EtOH/sucrose drinking.
Statistical Analysis
Mixed-model three-way analyses of variance (ANOVAs) were used to examine the effects of day, MPH, and housing on the following measures: daily g/kg EtOH intake over three weeks of 8E1S drinking, and mg/kg sucrose consumption during the sucrose preference test. Post-hoc (Bonferroni) tests were used where appropriate. Where data violated assumptions of sphericity, Greenhouse-Geisser corrections were used. Two-way ANOVAs (drug × housing) were used to compare average intakes and average preferences over the three-week drinking procedure. T-tests with a Bonferroni correction were used for planned comparisons between housing and drug groups where appropriate. In all cases, p <0.05 was considered statistically significant.
Two-way ANOVAs (drug condition × housing condition) were run on the following measures: Time spent in the open arms, and number of closed arm entries on Pre-EtOH and Post-EtOH runs of the elevated plus maze. Student’s t-tests were used for planned comparisons between housing groups where there were significant main effects. In all cases, p <0.05 was considered statistically significant.
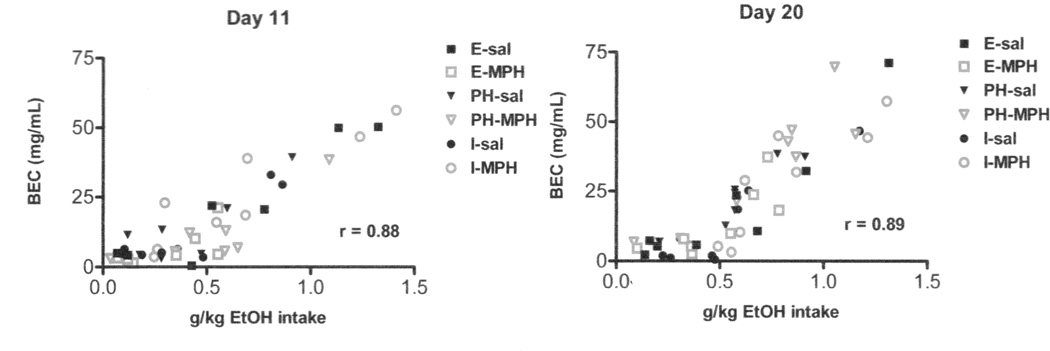
On days 11 and 20 of the drinking procedure, BECs were measured and Pearson’s correlations were used to compare g/kg EtOH intake with the BEC across all animals, regardless of drug or housing condition. Again, p <0.05 was considered statistically significant.
Results
Drinking
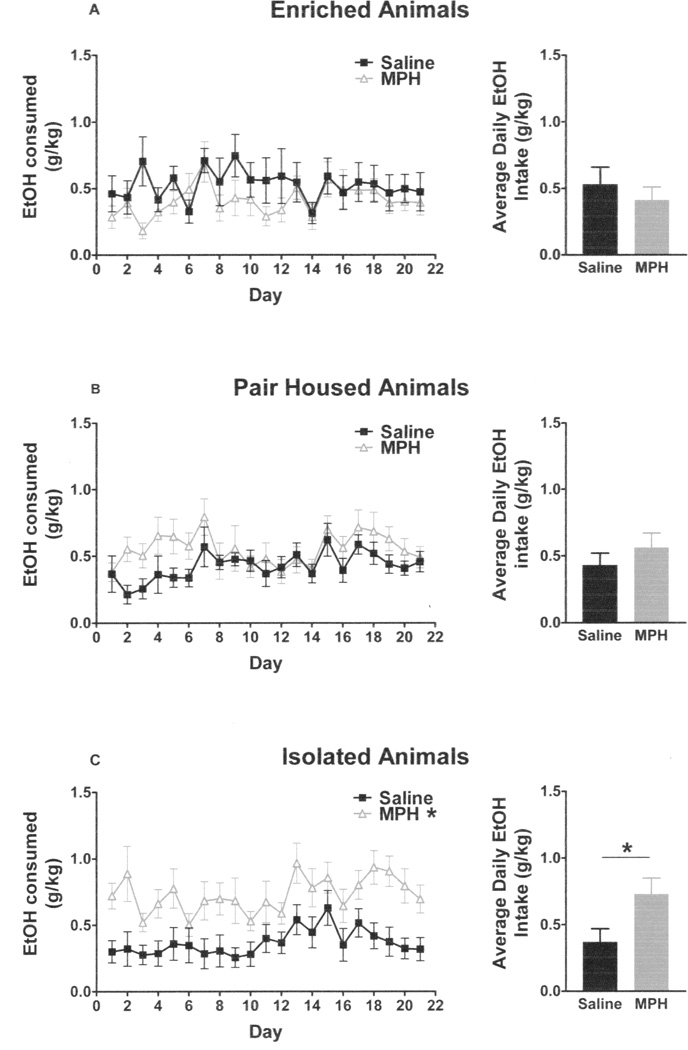
The final concentration of EtOH/sucrose was 8% EtOH and 1% sucrose. A mixed model three-way ANOVA (day × drug × housing) violated assumptions of sphericity, so a Greenhouse-Geisser Correction factor was used on the following data. There was a main effect of day (F10,413 = 4.924; p < 0.001), but no interactions between day and any of the other variables. There were no main effects of drug (F1,42 = 3.554) or housing (F2,42 = 0.523), but there was a significant interaction between drug and housing (F2,42 = 4.457, p < 0.02) (Figure 2 A–C). Post-hoc testing showed that isolated, MPH-treated rats drank more than isolated, saline-treated rats (F1,14 = 7.344; p < 0.02). There were no differences between paired and enriched animals, regardless of drug treatment. Likewise, MPH-treated, socially isolated animals had greater average EtOH intakes than untreated socially isolated animals (t14 = 2.71, p < 0.02), but there were no differences between the drug treatment groups within enriched (t14 = 1.03) and paired housing groups (t14 = 1.44).
Figure 2. Daily g/kg EtOH intakes.
MPH- and saline-treated animals within the enriched (A) and pair-housed (B) groups did not differ in their daily intakes of an EtOH/sucrose solution over a three-week drinking period. MPH-treated isolated animals had significantly greater average EtOH/sucrose intakes than saline-treated isolated animals (C).
There was a main effect of housing on water consumption (F2,42 = 7.036, p < 0.01), and a main effect of day (F9.5,398.5 = 4.659, p < 0.001), but no effect of methylphenidate (F1,42 = 1.595). Enriched animals had the greatest average water intakes over the course of the study at 7.95 ± 1.37 g/kg (mean ± SEM), followed by pair-housed animals that drank an average of 5.13 ± 0.74 g/kg (mean ± SEM), and finally isolated animals had average water intakes of 3.03 ± 0.39 g/kg (mean ± SEM).
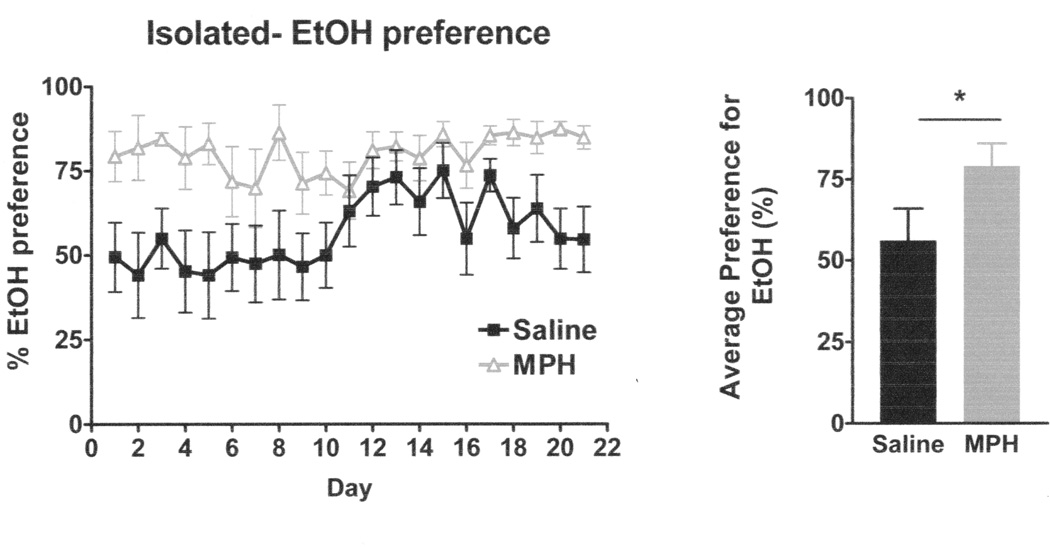
As EtOH intakes were only different in the isolated group, further analysis for EtOH/sucrose preference over water was conducted only within the isolated group. A mixed-model two-way ANOVA (day × drug) showed a significant main effect of day (F4,58 = 3.464, p <0.02; Greenhouse-Geisser correction) and a significant main effect of drug (F1,14 = 4.772, p <0.05), but no interaction on EtOH preference (Figure 3). MPH-treated, isolated animals showed greater average preference (t14 = 2.17, p < 0.05) for EtOH/sucrose versus water than saline-treated, isolated animals.
Figure 3. Ethanol Preference in Isolated Animals.
MPH-treated isolated animals had significantly greater EtOH preference over water than saline-treated isolated animals.
Anxiety-like behavior
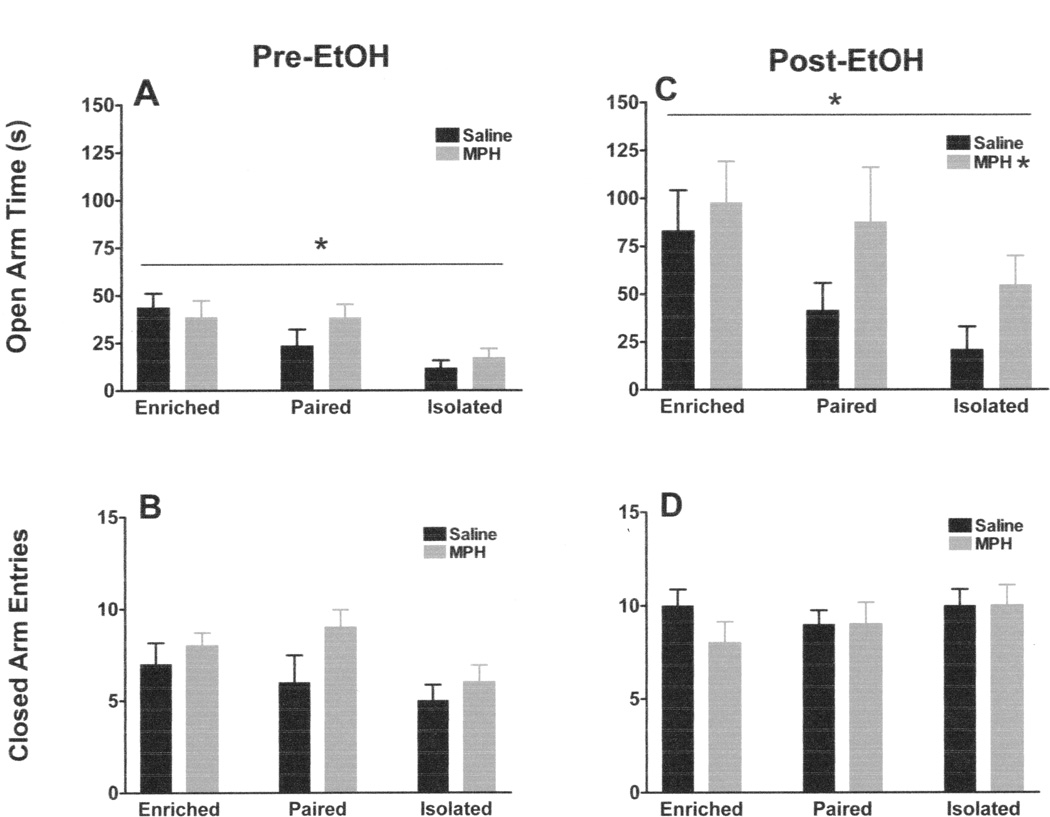
On the pre-EtOH run on the elevated plus maze, there was a main effect of housing condition on time spent in the open arms (F2,42 = 8.961; p <0.002), but no effect of MPH (F1,42 = 0.943) and no interaction (F2,42 = 1.215) (Figure 4A). Post-hoc t-tests indicated that isolated animals spent significantly less time on the open arms of the plus maze than paired (t30 = 2.549; p <0.05) or enriched animals (t30 = 4.959; p <0.01). The difference between paired and enriched animals was not statistically significant (t30 = 1.427). There were no differences between groups on closed arm entries (Housing effect: F2,42 = 2.410; MPH effect: F1,42 = 2.920; Interaction: F2,42 = 0.717) (Figure 4B), a measure of general locomotor activity.
Figure 4. Elevated Plus Maze Behavior.
In the Pre-EtOH run, there was a main effect of housing condition, but no effect of MPH and no interaction between MPH and housing on open arm time (A), and there were no effects of housing, MPH and no interactions on closed arm entries (B). In the Post-EtOH run, there was still a main effect of housing condition, as well as an effect of MPH, but no interaction (C). Again, there were no effects of housing, MPH, and no interactions on closed arm entries (D).
On the post-EtOH run of the elevated plus maze, there was again an effect of housing on time spent in the open arms (F2,42 = 3.724; p < 0.05), as well as an effect of MPH (F1,42 = 6.827; p <0.02), but no interaction (F2,42 = 0.440) (Figure 4C). Post-hoc t-tests confirmed that enriched animals still spent significantly more time on the open arms than isolated animals (t30 = 2.984; p < 0.05) after EtOH exposure. There was no significant difference between isolated and paired animals (t30 = 1.722), or paired and enriched animals (t30 = 0.726). Although there was a main effect of MPH on the time spent in the open arms on the Post-EtOH run, post-hoc t-tests between the individual MPH- and saline- treated housing groups did not reach significance. Post-EtOH, again, there were no differences on closed arm entries (Housing effect: F2,42 = 1.042; MPH effect: F1,42 = 0.167; Interaction: F2,42 = 1.042) (Figure 4D). The Post-EtOH run on the elevated plus maze was completed at least six hours after the end of that days drinking session. Based on calculated g/kg EtOH intakes for that day, this was ample time to allow for EtOH clearance, without disrupting behavior by omitting the drinking session entirely on plus maze testing day.
Blood Ethanol Concentrations
BECs were determined twice during the study, on days 11 and 20 of the drinking procedure. On both days, BECs correlated with EtOH intake for that day (day 11: r = 0.88, p < 0.0001; day 20: r = 0.89, p <0.0001). This test verified that the animals were ingesting measurable quantities of ethanol and that their BECs reflected that intake (Figure 5).
Figure 5. Blood Ethanol Concentrations.
BECs taken on two separate drinking days correlated with g/kg EtOH intake on that day. Animals were consuming measurable quantities of EtOH.
Sucrose Drinking
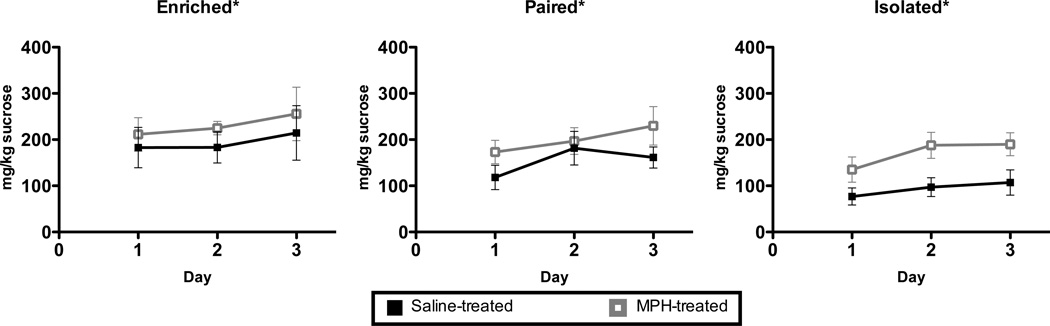
In order to control for the presence of sucrose in the EtOH solution, the animals were given 1-hour per day access to a 1% sucrose solution and water for three days. A three way mixed model ANOVA showed a main effect of day (F2,84 = 5.466, p <0.01), a main effect of housing (F2,42 = 4.011, p <0.03), and a main effect of drug (F1,42 = 5.412, p <0.03) (Figure 6) on overall sucrose consumption (mg/kg). There were no significant interactions. Collapsed across housing and drug conditions, animals increased their intake of the 1% sucrose solution (mg/kg) over the three-day period, to the point that sucrose intake on Day 3 of the procedure was significantly greater than on Day 1 (t47 = 3.255; p < 0.01). Post-hoc (Bonferroni) testing on the main effect of housing condition confirmed that sucrose intake of enriched animals (mean ± SEM: 197.0 ± 27.7 mg/kg) was significantly greater than the sucrose intake of isolated animals (mean ±SEM: 106.0 ± 17.6 mg/kg; p < 0.027), while pair-housed animals (mean ± SEM: 145.6 ± 19.0) were not significantly different from the other groups. Additionally, the main effect of drug supported greater overall sucrose consumption in MPH-treated animals.
Figure 6. Sucrose drinking.
There was a main effect of day, a main effect of housing condition and a main effect of MPH treatment on sucrose intake over a three-day sucrose preference test (A–C). Enriched animals drank significantly more sucrose than isolated animals. MPH-treated animals consumed more sucrose than saline-treated animals. Animals drank significantly greater amounts of sucrose on Day 3 than on Day 1. There was no interaction between housing and MPH treatment. * p < 0.05.
Discussion
Here we report that three weeks of chronic MPH treatment during pre-adolescence/adolescence was associated with greater EtOH/sucrose intake and preference, but only in socially isolated animals, suggesting that MPH treatment may increase vulnerability to alcohol abuse in adulthood in a subset of the population. Additionally, we show a persistent effect of early environmental enrichment on anxiety-like behavior, when compared to social isolation. Early enriched animals continued to spend more time on the open arms of the elevated plus maze than early isolated animals even after 5 weeks of individual housing. This suggests that housing conditions, specifically during the pre-adolescent/adolescent ages of PND 21–50, can have long enduring effects on adult behaviors, regardless of adult environment.
While there was no main effect of housing condition or MPH treatment on EtOH intake, there was an interaction between the two variables. MPH-treated isolated animals had greater EtOH intake and greater EtOH/sucrose preference than saline-treated isolated animals. Greater levels of EtOH drinking after MPH treatment are consistent with a previous report which documented greater EtOH intakes among adult rodents that had been treated with MPH during adolescence, even though those animals were group housed at the time (Vendruscolo et al., 2008). Notably this effect was in female Spontaneously Hypertensive Rats (SHRs), a strain that is a presumed model of ADHD (Sagvolden, 2000), and shares several behavioral characteristics with socially isolated animals including hyperactivity and impulsivity (Sagvolden et al., 1993). Yet, another study using the SHR strain documented no increases in EtOH consumption after chronic MPH treatment (Soeters et al., 2008), though the sex of the animals and the housing conditions were not specified. While doses of MPH (2 mg/kg/day) were the same and the duration of dosing was similar in the two studies, one study administered MPH orally (Soeters et al., 2008), and the other study administered drug via intraperitoneal injection (Vendruscolo et al., 2008). Additionally, the EtOH was presented differently between the two studies, with one study using ascending concentrations of EtOH over several days to a maximum of 6% EtOH solution (Soeters et al., 2008), and the other using a 10% solution throughout the study (Vendruscolo et al., 2008). Thus, there are several variables that may explain the discrepant results between the two studies. While the present study utilized a different strain of animal, these results are in line with those of Vendruscolo and colleagues (2008), indicating that chronic MPH treatment during early life is associated with greater EtOH drinking in adulthood. However, it should be noted that MPH treatment was associated with greater EtOH intakes only in isolated animals. There was no effect of drug treatment on the drinking behavior of paired or enriched animals.
The rearing environments significantly influenced the expression of anxiety-like behavior, without altering locomotor behavior, on the elevated plus maze. These results demonstrate that the environmental conditions in the present study were sufficient to produce the expected behavioral phenotype, despite the lack of a main effect of housing on drinking behavior. Additionally, this study extends previous work by re-examining anxiety-like behavior after changing housing conditions in adulthood. At PND 50 all enriched and paired animals were separated into individual housing, identical to the isolated condition, for the remainder of the study. All animals were retested on the elevated plus maze on PND 84–85 after 5 weeks of individual housing, daily handling, and daily exposure to EtOH. Despite the changing environmental conditions, the significant difference between isolated and enriched animals persisted. Animals that were previously enriched still spent significantly greater time on the open arms of the elevated plus maze than consistently isolated animals. It is notable that the pair-housed animals were not significantly different from the other two groups on the post-EtOH test, suggesting that only behavioral phenotypes resulting from the extreme conditions of postweaning environmental enrichment and social isolation may be associated with differential behavior in the long-term. These data are in accordance with a recent publication by Yorgason and colleagues (2013), which reported enduring lower levels of anxiety-like behavior in Long Evans rats that had been group-housed postweaning for 7 weeks and were subsequently isolated for an additional 4 months, when compared to animals that had been constantly isolated. The results of that work and this study highlight the importance of the postweaning environment, which can have a profound impact on adult behavior, regardless of the adult environment.
In addition to the effect of housing on the second run of the elevated plus maze, there was an effect of early MPH treatment that was not present in the first exposure to the plus maze, despite cessation of drug infusion 5 weeks earlier. Previous reports of the effects of chronic MPH treatment and subsequent drug washout on anxiety-like behavior are mixed, with some studies showing increases in anxiety-like behavior (Bolanos et al., 2003, Bolanos et al., 2008), and others showing decreases (Gray et al., 2007). It is also possible that EtOH exposure influenced the behavior on the elevated plus maze on the Post-EtOH run as all animals had been exposed to EtOH for 5 weeks by that time.
Most studies of EtOH drinking in rodents do not use a sweetened solution, and it is possible that the sucrose addition affected the drinking patterns in these animals. In order to control for the presence of sucrose, a three-day sucrose preference test was performed at the conclusion of the drinking period. When exposed to an EtOH-free 1% sucrose solution, enriched animals had significantly greater intakes than isolated animals. Additionally, MPH treatment was associated with greater intake of sucrose when compared to saline treatment. However, when sucrose was combined with the pharmacological effects of EtOH, only the isolated animals were affected by prior MPH treatment. It is possible that pair-housed and enriched, MPH-treated animals were averse to the EtOH addition to the solution, while it was reinforcing to the isolated, MPH-treated animals.
Notably, in the present study, isolation alone was not sufficient to elevate EtOH intakes of isolated rats over those of pair-housed and enriched animals. This is in contrast to much of the current literature that documents greater EtOH consumption in isolated animals when compared to group-housed or environmentally enriched animals (Chappell et al., 2013, McCool and Chappell, 2009, Deehan et al., 2007, Deehan et al., 2011, Lodge and Lawrence, 2003, Hall et al., 1998, Wolffgramm, 1990). One potential reason for the discrepancy between the results presented here and previous work is the rat strain. None of the above cited studies used outbred Sprague Dawley rats in their drinking experiments. Another study that did use Sprague Dawley rats also found that animals that were isolated for 30 days postweaning did not have greater EtOH intake than their group-housed counterparts, in concert with the present results (Pisu et al., 2011). There are also data that suggest that extended enrichment rearing (over 90 days) in Sprague Dawley rats will actually enhance voluntary ethanol intake when compared to extended isolation rearing (Rockman et al., 1989). Thus, it remains possible that the results of the present study would have been different if another strain of rat had been tested.
In summary, chronic MPH treatment interacted with rearing environment to increase EtOH intake and preference in animals that were socially isolated in early life. This suggests that MPH treatment may increase vulnerability to future alcohol abuse in a subset of the population. In addition, here we show that postweaning rearing conditions of isolation and enrichment are associated with persistent effects on anxiety-like behavior, regardless of adult housing conditions. This highlights the importance of early life experience in rodents for establishing long-term behavioral phenotypes. Overall, the current findings emphasize the importance of the potential interaction between environmental history and medication history when considering factors that influence substance abuse vulnerability.
Acknowledgements
We would like to thank Hilary Smith, Amanda Gogolak, and Mack Miller for their help with these experiments. These studies were funded by grants from NIAAA, AA 21099 (JLW), AA 17531 (JLW), and T32-AA00756 (KEG) and grants from NIDA, DA20648 (LJP) and DA06634 (LJP and TJRB).
This research was funded by grants from NIAAA, AA 21099 (JLW), AA 17531 (JLW), and T32-AA00756 (KEG), and grants from NIDA, DA20648 (LJP) and DA06634 (LJP and TJRB
References
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behavioural pharmacology. 2012;23:650–657. doi: 10.1097/FBP.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer KJ, Leventhal AM. Associations between attention deficit hyperactivity disorder symptom domains and DSM-IV lifetime substance dependence. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2013;22:23–32. doi: 10.1111/j.1521-0391.2013.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. The Journal of clinical psychiatry. 2003;64(Suppl 11):3–8. [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, Henderson RP. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett. 2008;436:278–282. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Brenes Saenz JC, Villagra OR, Fornaguera Trias J. Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav Brain Res. 2006;169:57–65. doi: 10.1016/j.bbr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–E403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res. 2007;31:1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Palmatier MI, Cain ME, Kiefer SW. Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behavioral neuroscience. 2011;125:184–193. doi: 10.1037/a0022627. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Twin study of the relationship between adolescent attention-deficit/hyperactivity disorder and adult alcohol dependence. Journal of studies on alcohol and drugs. 2012;73:185–194. doi: 10.15288/jsad.2012.73.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL. Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review. Clinical therapeutics. 2008;30:942–957. doi: 10.1016/j.clinthera.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Gill KE, Beveridge TJ, Smith HR, Porrino LJ. The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Res. 2013;1527:67–78. doi: 10.1016/j.brainres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf WD, Nagel SK, Epstein LG, Miller G, Nass R, Larriviere D. Pediatric neuroenhancement: Ethical, legal, social, and neurodevelopmental implications. Neurology. 2013 doi: 10.1212/WNL.0b013e318289703b. [DOI] [PubMed] [Google Scholar]
- Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, Zupan B, Menzer D, Rice J, Drake CT, Romeo RD, Brake WG, Torres-Reveron A, Milner TA. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Novak AJ, Middaugh LD, Patrick KS. The interactive effects of methylphenidate and ethanol on ethanol consumption and locomotor activity in mice. Pharmacol Biochem Behav. 2010;95:267–272. doi: 10.1016/j.pbb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Harlan Industries. Sprague Dawley Growth Curve. [Accessed October 15, 2009];2008 Available at: http://www.harlan.com/products_and_services/research_models_and_services/research_models/sprague_dawley_outbred_rat.hl. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume II: College students and adults ages 19–50. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. Monitoring the Future national survey results on drug use, 1975–2012. Volume II: College students and adults ages 19–50, in Series Monitoring the Future national survey results on drug use, 1975–2012; p. 400. [Google Scholar]
- Lodge DJ, Lawrence AJ. The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats. Behav Brain Res. 2003;141:113–122. doi: 10.1016/s0166-4328(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Hormones and behavior. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL., 3rd Does stimulant treatment place children at risk for adult substance abuse? A controlled, prospective follow-up study. J Child Adolesc Psychopharmacol. 2003;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 1997;6:318–329. [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Caldwell RW, Ferris RM, Breese GR. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmacol Exp Ther. 1987;241:152–158. [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Mostallino MC, Dore R, Maciocco E, Secci PP, Serra M. Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats. Eur Neuropsychopharmacol. 2011;21:414–425. doi: 10.1016/j.euroneuro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Gibson JE, Benarroch A. Effects of environmental enrichment on voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1989;34:487–490. doi: 10.1016/0091-3057(89)90545-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Pettersen MB, Larsen MC. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav. 1993;54:1047–1055. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schneider P, Ho YJ, Spanagel R, Pawlak CR. A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front Behav Neurosci. 2011;5:43. doi: 10.3389/fnbeh.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeters HS, Howells FM, Russell VA. Methylphenidate does not increase ethanol consumption in a rat model for attention-deficit hyperactivity disorder-the spontaneously hypertensive rat. Metab Brain Dis. 2008;23:303–314. doi: 10.1007/s11011-008-9098-1. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, Kollins S, Nguyen AS, DeCory HH, Hirshe Dirksen SJ, Hatch SJ. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113:e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Izidio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behavioural pharmacology. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry. 2008;165:553–555. doi: 10.1176/appi.ajp.2008.08020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013 doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuvekas SH, Vitiello B, Norquist GS. Recent trends in stimulant medication use among U.S. children. Am J Psychiatry. 2006;163:579–585. doi: 10.1176/ajp.2006.163.4.579. [DOI] [PubMed] [Google Scholar]