Abstract
Background Mangroves are a group of highly salt-tolerant woody plants. The high water use efficiency of mangroves under saline conditions suggests that regulation of water transport is a crucial component of their salinity tolerance.
Scope This review focuses on the processes that contribute to the ability of mangroves to maintain water uptake and limit water loss to the soil and the atmosphere under saline conditions, from micro to macro scales. These processes include: (1) efficient filtering of the incoming water to exclude salt; (2) maintenance of internal osmotic potentials lower than that of the rhizosphere; (3) water-saving properties; and (4) efficient exploitation of less-saline water sources when these become available.
Conclusions Mangroves are inherently plastic and can change their structure at the root, leaf and stand levels in response to salinity in order to exclude salt from the xylem stream, maintain leaf hydraulic conductance, avoid cavitation and regulate water loss (e.g. suberization of roots and alterations of leaf size, succulence and angle, hydraulic anatomy and biomass partitioning). However, much is still unknown about the regulation of water uptake in mangroves, such as how they sense and respond to heterogeneity in root zone salinity, the extent to which they utilize non-stomatally derived CO2 as a water-saving measure and whether they can exploit atmospheric water sources.
Keywords: Mangrove, salinity tolerance, water uptake, hydraulic anatomy, salt secretion, aquaporins, halophyte, water use efficiency, WUE, Avicennia, Rhizophora, vapour pressure deficit
INTRODUCTION
Mangroves are a diverse group of ∼70 tree species that grow in saline, tidal wetlands on tropical and subtropical coastlines (Table 1). Mangroves tolerate a wide range of soil salinity (Lugo and Snedaker, 1974; Odum et al., 1982; Hutchings and Saenger, 1987). While salinity has long been recognized as an important factor that limits mangrove growth and productivity (Clough and Sim, 1989; Lin and Sternberg, 1992; Ball, 2002) mangroves are nonetheless highly adapted to salt concentrations in soils that exceed concentrations tolerated by most other plant species (Ball, 1988). Saline habitats represent a physiological challenge for plants because of the highly negative water potentials of the soil pore water, making water acquisition more energetically unfavourable than in non-saline soils. The ability to maintain water uptake in saline conditions is key to salt tolerance. Another physiological challenge is ion toxicity, as high concentrations of salt are potentially cytotoxic to all plants, including mangroves. The high water use efficiency of mangroves under saline conditions suggests that regulation of water transport, in conjunction with managing ions, is a crucial component of their salinity tolerance.
Table 1.
Occurrence of salt glands, leaf pubescence and relative salinity tolerance of mangrove tree species. Salt glands occur in four genera. Leaf pubescence has been noted in seven genera. Where leaf pubescence has been assessed in multiple species within a genus (e.g. Avicennia and Bruguiera), species within the genus appear to have similar leaf pubescence. High levels of salinity tolerance occur in species with and without salt glands and in species with and without leaf pubescence. The global distribution is from Duke (1992). High salinity tolerance indicates growth is observed in soil salinities that exceed those of seawater. Salinity tolerance data are based on Johnstone and Frodin (1982), Jimenez (1984), Jimenez and Soto (1985), Clough (1992), Allen et al. (2003) and Duke (2010). Pubescence is based on Stace (1965), Tomlinson (1986), Das (2002) and Wilson (2011). Taxonomy is based on the APGII system of flowering plant classification (APG, 1998)
| Order | Family | Genus | Species | World distribution | Salt glands | Leaf pubescence | Salinity tolerance |
|---|---|---|---|---|---|---|---|
| Arecaceae | Palmae | Nypa | fruicans | WP* | – | – | Low |
| Phoenix | paludosa | IM | – | – | Mid | ||
| Caryophyllales | Plumbaginaceae | Aegialitis | annulata | WP | + | + | High |
| rotundifolia | IM | + | + | High | |||
| Ericales | Myrsinaceae | Aegiceras | corniculatum | WP | + | + | Mid |
| floridum | IM | + | NA | NA | |||
| Pellicieraceae | Pelliciera | rhizophorae | Americas | – | – | Low | |
| Lamiales | Acanthaceae | Acanthus | ebracteatus | WP | + | NA | NA |
| ilicifolius | WP | + | + | Low | |||
| Avicenniaceae | Avicennia | alba | WP | + | + | Mid | |
| bicolor | W. America | + | + | High | |||
| germinans | AEP | + | + | High | |||
| integra | Australasia | + | + | Mid | |||
| marina | IWP | + | + | High | |||
| officinalis | WP | + | + | High | |||
| rumphiana | WP | + | + | Mid | |||
| schaueriana | E. America | + | + | NA | |||
| Malpighiales | Euphorbiaceae | Excoecaria | agallocha | IWP | – | – | Low |
| indica | IM | – | NA | NA | |||
| Rhizophoraceae | Bruguiera | cylindrica | WP | – | – | Low | |
| exaristata | Australasia | – | – | High | |||
| gymnorrhiza | IWP | – | – | Mid | |||
| hainesii | WP | – | – | NA | |||
| parviflora | WP | – | – | Mid | |||
| sexangula | WP | – | – | Low | |||
| Ceriops | australis | Australasia | – | – | High | ||
| decandra | WP | – | – | Low | |||
| tagal | IWP | – | – | Mid | |||
| Kandelia | candel | IM | – | – | Mid | ||
| Rhizophora | apiculata | WP | – | – | Mid | ||
| mangle | AEP | – | – | High | |||
| mucronata | IWP | – | – | Low | |||
| racemosa | AEP | – | – | Low | |||
| samoensis | W. America | – | – | Mid | |||
| stylosa | WP | – | – | High | |||
| Malvaceae | Sterculiaceae | Heritiera | fomes | IM | – | + | Low |
| globosa | IM | – | NA | NA | |||
| littoralis | IWP | – | + | Mid | |||
| Myrtales | Combretaceae | Conocarpus | erectus | AEP | – | + | Mid |
| Laguncularia | racemosa | AEP | – | + | Mid | ||
| Lumnitzera | littorea | WP | – | + | High | ||
| racemosa | IWP | – | + | High | |||
| Lythraceae | Sonneratia | alba | IWP | – | – | Mid | |
| apetala | IM | – | – | Low | |||
| caseolaris | WP | – | – | Low | |||
| griffithii | IM | – | – | NA | |||
| lanceolata | WP | – | – | Low | |||
| ovata | WP | – | – | NA | |||
| Myrtaceae | Osbornia | octodonta | WP | – | – | High | |
| Sapindales | Meliaceae | Xylocarpus | granatum | IWP | – | – | Low |
| mekongensis | WP | – | – | Mid |
IWP, Indo-West Pacific; WP, Western Pacific; IM, Indomalesia; AEP, Atlantic East Pacific; NA, data were not available for the species.
*Naturalized at other locations throughout the tropics.
SALT EXCLUSION MEASURES
Mangroves exert tight control over salt concentrations in their tissue by decoupling water uptake from ion uptake. Xylem sap in all mangrove species tested has relatively low salt concentrations (Table 2), with up to 99 % of the salts in the soil solution prevented from entering the xylem stream (Waisel et al., 1986; Werner and Stelzer, 1990; Melcher et al., 2001; Stuart et al., 2007) regardless of soil salinity (Table 2). Such efficient filtration at the root is achieved by the prevention of non-selective apoplastic water uptake (Krishnamurthy et al., 2014).
Table 2.
Published concentrations of sodium ions measured in the xylem sap of mangroves grown at a range of salinities, and means and range of sodium ions measured in the xylem sap of mangroves, other halophytes and glycophyte species grown at a range of salinities. Exclusion percentages were calculated from the ratio between the sodium concentrations in the xylem and that in the soil. The complete table can be accessed in Supplementary Data Table S1
| Vegetation type | [Na+]soil (mm) | [Na+]xylem sap (mm) | Exclusion (%) | References |
|---|---|---|---|---|
| Mangroves | ||||
| Aegiceras corniculatum | 75 | 5·2 | 92·9 | Popp et al., 1993 |
| Aegiceras corniculatum | 360 | 6·9 | 98·1 | Popp et al., 1993 |
| Avicennia alba | 140 | 4·4 | 96·9 | Paliyavuth et al., 2004 |
| Avicennia alba | 285 | 17·8 | 93·8 | Paliyavuth et al., 2004 |
| Avicennia alba | 430 | 42 | 90·2 | Paliyavuth et al., 2004 |
| Avicennia alba | 560 | 115 | 79·5 | Paliyavuth et al., 2004 |
| Avicennia marina | 110 | 6·7 (6·4) | 93·9 | Moon et al., 1986 |
| Avicennia marina | 900 | 130 (60) | 85·6 | Waisel et al., 1986 |
| Bruguiera gymnorrhiza | 140 | 1 | 99·3 | Paliyavuth et al., 2004 |
| Bruguiera gymnorrhiza | 285 | 1 | 99·6 | Paliyavuth et al., 2004 |
| Bruguiera gymnorrhiza | 430 | 3 | 99·3 | Paliyavuth et al., 2004 |
| Bruguiera gymnorrhiza | 560 | 5 | 99·1 | Paliyavuth et al., 2004 |
| Conocarpus erectus | 75 | 0·9 | 98·8 | Popp et al., 1993 |
| Conocarpus erectus | 360 | 7·5 | 97·9 | Popp et al., 1993 |
| Laguncularia racemosa | 0 | 2 (0·4) | - | Sobrado, 2004 |
| Laguncularia racemosa | 215 | 4·3 (0·7) | 98 | Sobrado, 2004 |
| Laguncularia racemosa | 240 | 8·7 (0·3) | 96·4 | Sobrado, 2004 |
| Laguncularia racemosa | 400 | 11·6 (0·9) | 97·1 | Sobrado, 2004 |
| Laguncularia racemosa | 430 | 8·9 (0·7) | 98 | Sobrado, 2004 |
| Rhizophora mangle | 0 | 11·1 (5·4) | – | Werner and Stelzer, 1990 |
| Rhizophora mangle | 200 | 20·1 (8·7) | 90 | Werner and Stelzer, 1990 |
| Range | 0–900 | 0·9–130 | 90–99·6 | |
| Mean ± s.d. | 20 ± 35 | 95 ± 6·6 | ||
| Other halophytes | ||||
| Range | 0–530 | 0·5–46·6 | 76·7–98·6 | Flowers, 1985; Arndt et al., 2004; Kant et al., 2006 |
| Mean ± s.d. | 13 ± 12 | 93 ± 7·5 | ||
| Glycophytes | ||||
| Range | 0–430 | 0–31·5 | 71–97·6 | Munns, 1985, 1988; Gouia et al., 1994; Seel and Jeschke, 1999; Chen et al., 2001; Sunarpi et al., 2005; Kant et al., 2006 |
| Mean ± s.d. | 7 ± 0·7 | 90·4 ± 6·6 | ||
Mangrove root epidermal cells are highly suberized (Krishnamurthy et al., 2014). Suberin is a hydrophobic polyphenol-based compound that is deposited between the cell wall and the plasma membrane, providing an efficient barrier to water movement into the cell (Kolattukudy, 1984). Additionally, the endodermal layer of mangroves has a highly developed Casparian strip, starting very close to the root cap (Lawton et al., 1981). In Avicennia marina this suberization significantly limits passive ion and water transport into the stele and blocks almost all apoplastic water (Moon et al., 1986; Krishnamurthy et al., 2014). Therefore, water must enter the stele through membranes (Steudle and Peterson, 1998). This transcellular transport relies on the high permeability of these membranes to water.
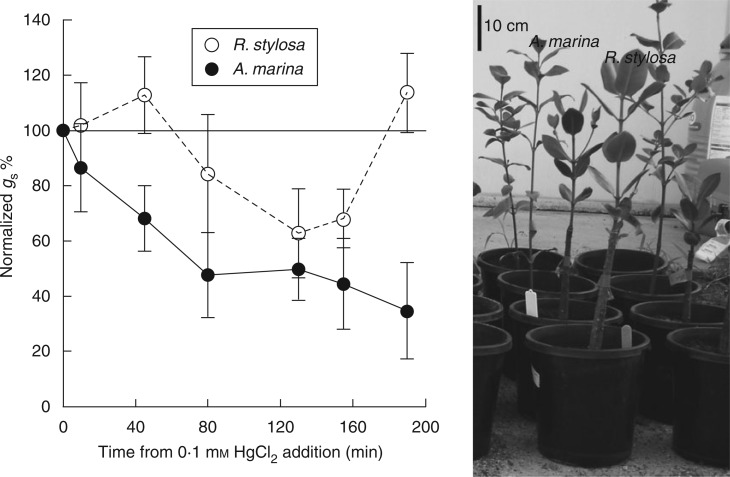
Most of the hydraulic conductivity of plant cell membranes, and subsequently the cell-to-cell pathway for water uptake, is attributed to the presence of water channel proteins known as aquaporins (Chrispeels and Maurel, 1994; Maurel, 1997; Tyerman et al., 1999; Maurel et al., 2008). Aquaporins are 27-kDa proteins belonging to the major intrinsic protein (MIP) family. The presence of aquaporins in membranes increases the water permeability along the cell-to-cell pathway. Using the aquaporin blocker 0·1 mm HgCl2, applied to the roots of mangrove seedlings, we established a significant role for aquaporins in water uptake in two mangrove species, A. marina and Rhizophora stylosa, as evidenced by significant reductions in stomatal conductance and an increase in xylem absisic acid (ABA) concentrations (Figs 1 and 2) following HgCl2 application. The HgCl2 was applied in low concentration and did not lead to damage to the photosynthetic apparatus based on fluorescence measurements [PSII maximum efficiency (Fv/Fm) was not lower in seedlings that received HgCl2 treatment]. Addition of the aquaporin blocker to the soil resulted in a significant reduction in stomatal conductance in both species within 60 min (Fig. 1). Avicennia marina showed a 65 % reduction in stomatal conductance within 200 min (3·3 h) and R. stylosa stomatal conductance was lower by 40 % in seedlings receiving HgCl2 than in seedlings that did not, although full recovery was recorded within 3·5 h. This is within the highest range of sensitivity measured for plants (Wan and Zwiazek, 1999; Martre et al., 2002; Martinez-Ballesta et al., 2006). We also detected a significant increase in ABA concentration in xylem sap in HgCl2-treated plants in both species (Fig. 2).
Fig. 1.
Temporal changes in mean stomatal conductance (gs) of 0·1 mm HgCl2-treated plants normalized against the mean stomatal conductance of control plants at each time point for Avicennia marina and Rhizophora stylosa seedlings (as indicated in the key). Repeated measures ANOVA: F(5,131) = 17·08, P < 0·001 for A. marina and F(1,131) = 16·197, P = 0·0008 for R. stylosa. Error bars are s.e.m. (n = 5). Representative seedlings of both species are shown to indicate size and developmental stage.
Fig. 2.
Mean ± s.e.m. (n = 5) ABA concentration (pmol ml–1 xylem) in xylem sap of Avicennia marina and Rhizophora stylosa in HgCl2-treated seedlings and control seedlings (see key) 200 min (3·5 h) following HgCl2 addition. Xyem sap was collected by low-speed centrifugation of stem segments and ABA concentrations were determined by competitive ELISA (Phytodetek PDK 09347/0096 Agdia Inc. Elkhart, IN, USA). Differences in ABA concentration were significant for the HgCl2 treatment. Factorial ANOVA, F(1,15) = 5·35, P = 0·0391.
The rapid and strong reduction in stomatal conductance and the increase in xylem sap ABA concentrations indicates that when the cell-to-cell water transport pathway was inhibited, a drought response developed in the mangrove seedlings, which indicates that very little apoplastic transport occurs in these species. This provides further support for previous work that showed that in order to maintain a relatively salt-free xylem, mangroves avoid apoplastic water transport through suberization and highly developed Casparian strips.
In addition to tight control over water and ion uptake, 14 of the 51 species in Table 1, from the families Plumbaginaceae, Myrsinaceae, Acanthaceae and Avicenniaceae, have salt glands that excrete salt on the leaf surface. While six of these species are known to have a high level of salinity tolerance, the other eight species have moderate and even low (Acanthus) salinity tolerance. Additionally, high levels of salinity tolerance can be achieved in species without salt glands (Table 1). Therefore, salt excretion (the salt gland trait) is not sufficient or necessary to confer high levels of salinity tolerance. The presence of salt glands (Table 1) is also not linked to levels of salt exclusion (Table 2). Although salt glands are important for excreting some of the salt that enters the plant in the species in which they occur, below we suggest that a benefit of the presence of salt on the leaf surface may be a reduction in leaf vapour pressure deficit.
Mangroves are not only exposed to variation in salinity at the root zone but also to variation in the level of root zone anoxia because of fluctuating water levels during tidal inundation, as well as variability in sediment characteristics and the capacity of root systems to transport air (McKee, 1996). Aquaporins are sensitive to levels of anoxia (Tournaire-Roux et al., 2003), which may indicate that anoxic conditions in soils and variation in inundation tolerance among species may interact with salinity to influence water uptake in mangroves (Ye et al., 2010).
MAINTAINING LOW WATER POTENTIALS
In order to take up water through the root filtration system, mangroves need to maintain water in their tissues against a strong osmotic gradient. Furthermore, if the water potential of the soil is lower than that of the plant, water could potentially be lost from the plant to the soil. Dephosphorylation of root aquaporins can significantly reduce root hydraulic conductivity and minimize water loss under hyperosmotic conditions (Horie et al., 2011), and the strong dependence of water uptake in mangroves on cell-to-cell pathways can provide strong regulation of water loss to the environment during periods of hyperosmotic conditions. However, in order to maintain water uptake, mangroves need to maintain water potentials that are lower than those of the soil. The leaf water potential of Avicennia germinans in Belize and Florida was between 0·1 and 1 MPa lower than the water potential of the soil pore water, and that for Rhizophora mangle was 0·6–0·8 MPa lower (Melcher et al., 2001; Lovelock et al., 2006a). In order to exceed the osmotic pressure of seawater, mangroves need to maintain a water potential (the combination of osmotic and hydrostatic xylem sap pressure) of at least –3·5 MPa. Beginning with the pioneering work of Scholander et al. (1962), numerous publications have reported particularly negative water potential values for mangrove sap. In a high-resolution diel analysis of water potential in A. marina, hydrostatic potentials as low as –5 MPa were recorded at midday and –3 MPa at night, when the plant was not transpiring (Waisel et al., 1986). Similar maximal daytime values were measured for A. germinans (Lovelock et al., 2006a). Vandegehuchte et al. (2014) measured values between –2·5 and –4 MPa for A. marina and between –2 and –3·5 MPa for R. stylosa during the night and day respectively. The two species from the same location differed in their water potential, indicating interspecific differences in gradients driving water uptake, which may also relate to differences among species in salinity tolerance.
Organic and inorganic osmotica
Mangroves, like many other halophytes, use ions as solutes to decrease osmotic potential in cells (Scholander et al., 1962). As ambient salinity increases, so do ion concentrations in all mangrove tissue (roots, leaves and stems) (e.g. R. mangle; Werner and Stelzer, 1990). To a certain level, leaf sap osmolality, determined mainly by the concentration of Na+ and Cl– ions in the cells, is positively correlated with soil salinity (Medina and Francisco, 1997). However, the capacity to sequester ions within the vacuoles is not limitless. Summaries of the few studies that have measured cellular ionic concentrations can be found in Supplementary Data Tables S1 and S2 and illustrate the difference between external and internal sodium concentrations over a range of salinities. In mangroves growing at high salinities, the predicted osmotic potential of the shoot, calculated from cellular ion concentrations, is higher than the observed osmotic potential and is not low enough to counter the low water potential of the soil. Thus, above a salinity threshold other osmotica are necessary to reduce water potential; these are organic solutes such as mannitol, proline, glycinebetaine and triterpenoids (Popp et al., 1985). These compatible solutes are also used to adjust the osmotic potential in the cytoplasm, which must maintain much lower Na+ and Cl– concentrations than the vacuole to allow enzyme function. Since the cytoplasm makes up <10 % of the cell volume (Mallery and Teas, 1984), readily available inorganic ions are the main osmotica used by mangroves to decrease leaf water potentials.
Cavitation-resistant hydraulic anatomy
Operating at low xylem water potentials puts mangroves at a high risk of cavitation (the formation of gas bubbles), because the pressurized water may vaporize in vulnerable areas such as xylem pit membranes (Tyree et al., 1994; Melcher et al., 2001; Hacke et al., 2006). These gas bubbles can then block the ascent of water through the embolized xylem vessel and thus reduce overall hydraulic conductivity. Cavitation events can be repaired by refilling, although the exact mechanism by which this process occurs is still debated (Woodruff et al., 2007; Zwieniecki and Holbrook, 2009; Wheeler et al., 2013). However, evidence suggests that vessels can only be repaired a limited number of times before cavitation becomes permanent (cavitation fatigue). Cavitation fatigue varies among tree species, with some species able to withstand repeated cavitation and repair cycles better than others (Hacke et al., 2001). Cavitation fatigue has not been studied in mangroves, so it is unknown whether they are better adapted than other species to withstand repeated cavitation. However, mangrove species are better than their rainforest family counterparts at avoiding cavitation (Sperry et al., 1988). Mangroves (especially at high salinities) use less water than similar sized freshwater wetland trees and thus have lower rates of sap flow (Krauss et al., 2007), which in turn lowers the xylem tension and the associated risk of cavitation. By adopting a cavitation-resilient anatomy, mangroves can safely operate at higher xylem tensions and thus colonize areas with higher soil salinities.
Mangroves have a range of anatomical adaptations to maintain hydraulic conductivity under the very low xylem water potentials needed to extract water under saline conditions (Sobrado, 2000, 2001; Melcher et al., 2001; Ewers et al., 2004). One such adaptation is a high degree of plasticity in vessel size and density. Xylem vessels become narrower and form at higher densities as salinity increases (Melcher et al., 2001; Yáñez-Espinosa et al., 2004; Lovelock et al., 2006b; Schmitz et al., 2006; Hao et al., 2009), thus reducing the risk of cavitation [possibly by reducing the pit area (Hacke et al., 2006)] and providing alternative routes for water transport when bubbles do form (Schmitz et al., 2006). However, smaller vessels, while safer in terms of embolism development under low water potentials, significantly reduce hydraulic conductivity (Hacke et al., 2006; Lovelock et al. 2006c) and may impose constraints on photosynthetic carbon assimilation rates and growth (Lovelock et al., 2006c). A high degree of plasticity in the anatomical response to the salinity environment is advantageous, allowing plants to modify their vulnerability to embolisms as well as their photosynthetic carbon gain over time and space. Differences in the ability to avoid embolisms contribute to interspecific differences in mangrove salinity tolerance (Ewers et al., 2004; Robert et al., 2009). Under high salinity, xylem vessels also become more circular (Robert et al., 2009). The advantage for the change in eccentricity is unclear; however, we hypothesize that it could provide an advantage as the area over which two vessels touch becomes smaller, thereby reducing the risk of air aspiration into functional vessels from embolized ones.
Some mangrove species also have a high sugar and water storage capacity in the stem and leaves, which may allow the rapid repair of embolisms when they occur. Some of the most salt-tolerant species and drought-tolerant species have successive cambia, where phloem and parenchyma cells are in close proximity to xylem, arranged in concentric bands (Carlquist, 2007; Robert et al., 2011). Over gradients of increasing salinity, the number of parenchyma cells and fibres in the mangrove genus Avicennia, which has successive cambia, increased (Schmitz et al., 2008; Santini et al., 2012). In Laguncularia racemosa, higher salinities resulted in a more confluent parenchyma (Yáñez-Espinosa et al., 2004). Confluent parenchyma, like successive cambia, may allow the storage of water and sugars near the xylem vessels, which could facilitate the rapid repair of embolisms (Carlquist, 2007).
WATER-SAVING ADAPTATIONS
Since water acquisition is more energetically costly in saline than in non-saline soils, mangroves have evolved a range of adaptations that facilitate efficient water use during photosynthetic carbon gain during the day and reduce losses of water to saline soils at night. Mangroves have a number of properties, from the scale of the arrangement of leaves in the canopy to microscopic structures within leaves, that contribute to high photosynthetic water use efficiencies.
Leaf temperature regulation
In most mangrove species, stomata are present only on the abaxial leaf surface and are usually located in crypts (slightly sunken within the epidermis) (Tomlinson, 1986). Both of these anatomical characteristics function to reduce transpiration, while not affecting CO2 uptake, by significantly increasing the humidity (reducing the leaf-to-air vapour pressure deficit) around the stomatal pore (Roth-Nebelsick, 2007). Mangrove leaves are held at almost vertical orientations when exposed to full sunlight (up to 75° from horizontal), which corresponds to a projected area that can be as low as 10 % of that of horizontal leaves (Ball et al., 1988; Lovelock and Clough, 1992). The leaf angle of sun-exposed leaves differs among mangrove species (Lovelock and Clough, 1992), which, in addition to leaf anatomical traits, could be a contributory factor influencing the distribution of species over salinity gradients. A lower leaf display area results in a reduction in direct radiation, allowing the leaf to remain at temperatures that are favourable for photosynthesis whilst requiring minimal evaporative cooling (Ball et al., 1988).
Another water-saving adaptation is reduction in leaf size; mangroves growing in high salinity conditions develop smaller leaves. Smaller leaves result in higher conductance across the leaf boundary layer, allowing the leaf temperature to equilibrate with the atmosphere more effectively. This too minimizes the need for evaporative cooling while enabling photosynthesis rates to remain high (Ball et al., 1988). Correspondingly, a negative correlation between leaf size and leaf sap osmolality was found in many mangrove species (Medina and Francisco, 1997). Mangrove leaves have a high water content per unit area (salt succulence), which increases with salinity (Camilleri and Ribi, 1983). High water content increases leaf heat capacity, thus reducing the need for evaporative cooling.
Uptake of non-stomatally derived CO2
Another water-saving feature is the use of CO2 for photosynthesis acquired through non-leaf tissues, and thus acquired at lower water costs. Fixation of CO2 can occur through stem photosynthesis, whereby respired, non-stomatally derived CO2 is fixed by chloroplasts within the bark and pith of mangrove trees (Teskey et al., 2008). While this phenomenon is not unique to mangroves, it occurs in a number of mangrove species to a significant extent, contributing up to 5 % of the CO2 fixed by the plant (Schmitz et al., 2012). Uptake of CO2 by roots, while not yet studied in mangroves, has been shown in other submerged and wetland plants (Raven et al., 1988; Brix, 1990; Rich et al., 2008). Many wetland plants, including mangroves, have continuous gas spaces extending from the stomata to the roots. This aerenchyma, which is not limited to roots but is formed in most parts of the plant (Raven, 1996), can hold significant amounts of CO2 (Constable et al., 1992). Utilization of this aerenchyma CO2 for photosynthesis has been shown in a number of wetland plants, such as Typha latifolia (Constable and Longstreth, 1994) and Phragmites australis (Brix, 1990). Mangroves have highly developed aerenchyma, an adaptation to their anoxic environment (Pi et al., 2009), and occupy soils with high concentrations of organic carbon (Donato et al., 2011). In non-flooded terrestrial plants, where there is little or no aerenchyma, such soil-derived CO2 can contribute 1–3 % of total C fixed by the plant (Ford et al., 2007); it may make a significantly higher contribution in mangroves, although this is yet to be established.
USE OF NON-SALINE WATER SOURCES AND INCREASING HUMIDITY
Uptake of less saline water sources within the rhizosphere
High-salinity soils are often characterized by high levels of spatial and temporal heterogeneity in salinity (Silvestri et al., 2005; Hao et al., 2009). The ability of some halophytes to tolerate high salinity has been linked to an ability to utilize those less saline patches in the rhizosphere (Semeniuk, 1983; Yakir and Yechielie, 1995). Mangroves can be relatively large trees that have expansive root systems (Comley and McGuinness, 2005). With such a large below-ground biomass, roots are exposed to heterogeneous salinities throughout the rhizosphere. Moreover, due to their location in the intertidal zone, mangroves may be exposed to both terrestrial and marine water sources, which vary greatly in salinity, from 0 to >160 p.p.t., where evaporation is high (Ball, 1998). Thus, while salinity might be high in part of the rhizosphere, some roots are often in contact with less saline water sources [e.g. groundwater, rainwater or riverine water (Ewe et al., 2007)].
When less-saline water sources are available, they can become the main source of water in the xylem of mangroves, as has been documented for some species (Sternberg and Swart, 1987; Lin and Sternberg, 1992; Ewe et al., 2007; Lambs et al., 2008; Wei et al., 2013). Successful utilization of less-saline water sources, when available, can increase halophyte survivorship, growth and productivity under conditions in which salinity limits growth rates (Yakir and Yechielie, 1995). In other halophytes, long-term responses to heterogeneity in salinity that is spatially or temporally stable leads to a proliferation of root growth into areas of lower salinity, and this can contribute to the preferential uptake of freshwater in some cases (Mensforth and Walker, 1996; Bazihizina et al., 2012). For mangroves, this has not yet been tested.
Uptake of atmospheric water and decreasing leaf-to-air vapour pressure deficit
Using atmospheric water sources is another way of reducing the utilization of saline water. A number of plant species can take up water that condenses on their leaves (Slayter, 1956; Munné-Bosch and Alegre, 1999; Martin and von Willert, 2000), most likely through their stomatal pores (Eichert et al., 2008). This is especially common in cloud forests (Johnson and Smith, 2008; Eller et al., 2013) and foggy coastal areas (Burgess and Dawson, 2004). Fog uptake by leaf surfaces of the cloud forest tree Drimys brasiliensis can contribute more than 40 % of the leaf's water content, leading to the reverse flow of sap from the leaf down to the roots. Some evidence for reverse flow has been reported in mangroves at night (Rada et al., 1989; Hao et al., 2009) and during rainfall events (K. Steppe, University of Ghent, unpublished results) and could potentially be associated with the uptake of atmospheric water sources (top-down rehydration). However, the contribution of atmospheric water sources to leaf water is yet to be determined for mangroves.
Salt secretion in mangroves could be important for improving the leaf water balance. The capacity to absorb water from the air may be assisted by salt crystals on the surface of leaves, which both attract liquid to the leaf surface by lowering the dew point and enable liquid penetration into the stomata, by making the surface of the leaf less hydrophobic and reducing the water surface tension (Burkhardt et al., 2012). Salt secretion exists only in some mangrove species (Table 1) but salt also accumulates on the leaves of non-salt-secreting species due to salts transported in air. The direct uptake of water from the leaf surface, with its high salt concentrations, is unlikely because of the low water potential of water on the surface of mangrove leaves. Furthermore, uptake of salty water through the stomata would introduce high concentrations of salt into the leaf. However, the generation of a humid environment surrounding the leaf surface would significantly reduce loss of water through the stomata during carbon acquisition by significantly decreasing the leaf-to-air vapour pressure deficit (VPD).
Leaf cuticles can provide an important hydrophobic barrier to water loss and the composition of epicuticular lipids could also affect leaf wettability (Ahmad and Wainwright, 1976). In the mangrove A. marina, cuticle thickness increases with salinity (Naidoo et al., 2011), but data on the relationship between salinity and mangrove leaf cuticle composition are not available. Leaf pubescence could be another water-conserving feature found in some mangrove plants, mainly from the genus Avicennia but also from Acanthus, Heritiera, Excoecaria and Conocarpus (Table 1). Leaf hairs at lower densities can introduce turbulence into the flow boundary layer of the leaf surface and thus enhance heat and water transfer from the leaf to the atmosphere (Benz and Martin, 2006); at higher densities they can increase surface roughness, create a thicker boundary layer and thus reduce gas diffusion and consequently water loss (Schuepp, 1993). However, increases and decreases in the boundary layer both have negligible effects on diffusion rates when conditions are even slightly windy (Roth-Nebelsick, 2001), and thus the contribution of leaf hairs to water conservation through changes to the boundary layer are likely to be very small under field conditions. A positive effect of pubescence on water conservation is more likely to be due to increased capacity to take up water through leaf surfaces, possibly by retaining rain or dew water on the leaf surface for longer (Grammatikopoulos and Manetas, 1994) or through direct uptake by the epidermal cells forming the hair.
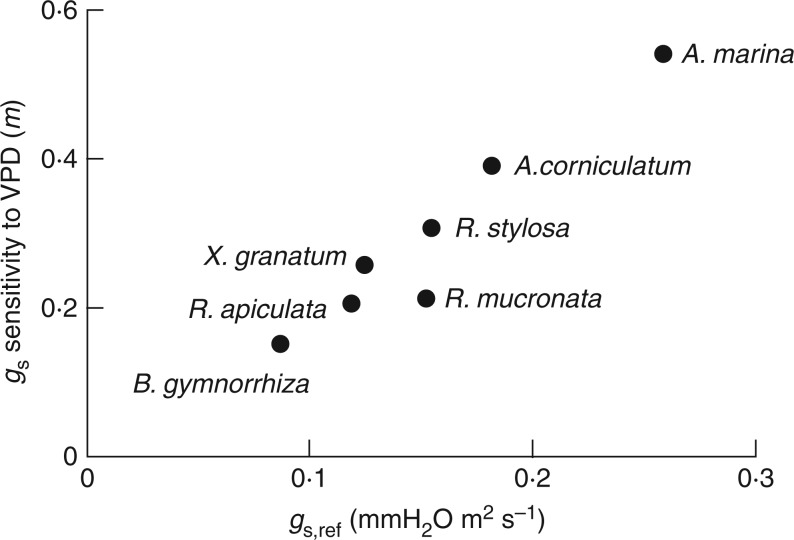
Many mangrove adaptations attributed to salinity tolerance contribute to decreased VPD around their leaves. Such adaptations include small leaf size, leaf hairs, salt crystals and sunken stomata as well as adaptations for lowering leaf temperature, such as steep leaf angles and succulence. The VPD is perhaps the strongest environmental determinant of water loss through transpiration (Oren et al., 1999) and is an important factor in plant growth and development (Leuschner, 2002). High VPD leads to stomatal closure, lower photosynthetic rates, higher rates of xylem cavitation and reduction in leaf area, among other effects. In mangroves, in which water use efficiency is central to their ability to survive in saline environments, a lower VPD environment can reduce the cost of carbon gain. A number of studies have indicated that mangroves are highly responsive to changes in VPD (Clough and Sim, 1989; Ball et al., 1997). Plant species show large differences in the sensitivity of stomata to changes in VPD, but a general positive relationship exists between the sensitivity to increasing VPD and the stomatal conductance at low VPD (Oren et al., 1999; Leuschner, 2002). A reanalysis of the data in Clough and Sim (1989) reveals a similar relationship among mangrove species (Fig. 3). It also reveals that the more salt-tolerant species (e.g. A. marina) are more sensitive to changes in VPD. The high sensitivity of salt-tolerant mangrove stomata to VPD corroborates previous findings that suggest that lower VPD can significantly improve mangrove salinity tolerance (Ball and Farquhar, 1984). Leaf-to-air VPD is thus likely to be an important component in how mangroves have adapted to tolerate saline environments and could be one of the drivers of regional and global mangrove species distributions and patterns in primary productivity.
Fig. 3.
Relationship between the sensitivity of stomatal conductance to VPD (sensu ‘m’; Oren et al., 1999, calculated as the slope of the regression line describing the relationship between gs and VPD) and stomatal conductance rates at a low reference VPD (interpolated form the relationship between gs and VPD, at VPD = 1) for a number of Australian mangrove species (data from Clough and Sim, 1989). Species plotted are Avicennia marina, Aegiceras corniculatum, Rhizophora stylosa, Rhizophora mucronata, Rhizophora apiculata, Xylocarpus granatum and Bruguiera gymnorrhiza.
CONCLUSIONS
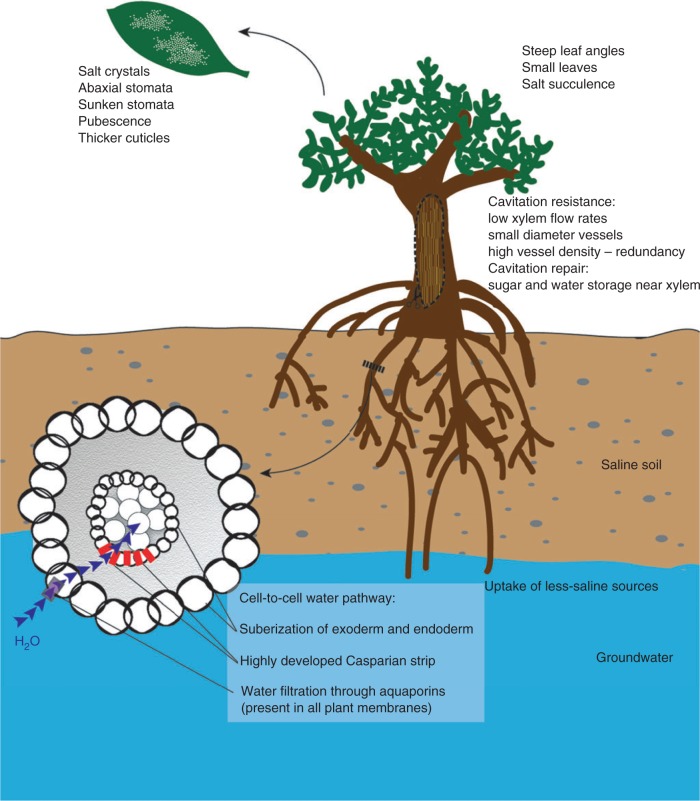
In mangrove species, adaptations that influence water uptake, transport and loss while maintaining photosynthetic carbon gain are important for salinity tolerance (Fig. 4). The complexity of the suite of characteristics required for salinity tolerance may be linked to the wide variation in salinity tolerance evolved among different mangrove taxa. The variation in adaptations in water conducting pathways among mangrove taxa likely contributes to the maintenance of high levels of productivity in heterogeneous saline environments that occur in mangrove habitats.
Fig. 4.
Schematic representation of the different strategies employed by mangroves to regulate water balance in saline environments.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals and consist of the following. Table S1: Published concentrations of sodium ions in the xylem sap of mangrove, other halophytes and glycophyte species growing at a variety of salinities. Exclusion percentages were calculated from the ratio between the sodium concentrations in the xylem and in the soil. Table S2: Published concentrations of sodium ions in the leaf tissues of mangroves grown at a range of salinities and the sodium concentrations in the growing medium.
ACKNOWLEDGEMENTS
This work was supported by an Australian Research Council Discovery Early Career Researcher Award (grant DE120101706) to R.R. and Discovery Awards (grants DP1096749 and DP0774491) to C.E.L. Support was also obtained from the Australian Research Council Special Research Initiatives, Co-funded Centre for Groundwater Research and Training to C.E.L.
LITERATURE CITED
- Ahmad I, Wainwright SI. Ecotype differences in leaf surface properties of Agrostis stolonifera from salt marsh, spray zone and inland habitats. New Phytologist. 1976;76:361–366. [Google Scholar]
- Allen JA, Krauss KW, Hauff RD. Factors limiting the intertidal distribution of the mangrove species Xylocarpus granatum. Oecologia. 2003;135:110–121. doi: 10.1007/s00442-002-1167-2. [DOI] [PubMed] [Google Scholar]
- APG An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- Arndt SK, Arampatsis C, Foetzki A, Li X, Zeng F, Zhang X. Contrasting patterns of leaf solute accumulation and salt adaptation in four phreatophytic desert plants in a hyperarid desert with saline groundwater. Journal of Arid Environments. 2004;59:259–270. [Google Scholar]
- Ball MC. Ecophysiology of mangroves. Trees – Structure and Function. 1988;2:129–142. [Google Scholar]
- Ball MC. Mangrove species richness in relation to salinity and waterlogging: a case study along the Adelaide River floodplain, northern Australia. Global Ecology & Biogeography Letters. 1998;7:71–82. [Google Scholar]
- Ball MC. Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees – Structure and Function. 2002;16:126–139. [Google Scholar]
- Ball MC, Farquhar GD. Photosynthetic and stomatal responses of two mangrove species, Aegiceras corniculatum and Avicennia marina, to long term salinity and humidity conditions. Plant Physiology. 1984;74:1–6. doi: 10.1104/pp.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MC, Cowan IR, Farquhar GD. Maintenance of leaf temperature and the optimisation of carbon gain in relation to water loss in a tropical mangrove forest. Australian Journal of Plant Physiology. 1988;15:263–276. [Google Scholar]
- Ball MC, Cochrane MJ, Rawson HM. Growth and water use of the mangroves Rhizophora apiculata and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant, Cell & Environment. 1997;20:1158–1166. [Google Scholar]
- Bazihizina N, Barrett-Lennard EG, Colmer TD. Plant responses to heterogeneous salinity: growth of the halophyte Atriplex nummularia is determined by the root-weighted mean salinity of the root zone. Journal of Experimental Botany. 2012;63:6347–6358. doi: 10.1093/jxb/ers302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz BW, Martin CE. Foliar trichomes, boundary layers, and gas exchange in 12 species of epiphytic Tillandsia (Bromeliaceae) Journal of Plant Physiology. 2006;163:648–656. doi: 10.1016/j.jplph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Brix H. Uptake and photosynthetic utilization of sediment-derived carbon by Phragmites australis (Cav.) Trin. ex Steudel. Aquatic Botany. 1990;38:377–389. [Google Scholar]
- Burgess SSO, Dawson TE. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant, Cell & Environment. 2004;27:1023–1034. [Google Scholar]
- Burkhardt J, Basi S, Pariyar S, Hunsche M. Stomatal penetration by aqueous solutions – an update involving leaf surface particles. New Phytologist. 2012;196:774–787. doi: 10.1111/j.1469-8137.2012.04307.x. [DOI] [PubMed] [Google Scholar]
- Camilleri JC, Ribi G. Leaf thickness of mangroves (Rhizophora mangle) growing in different salinities. Biotropica. 1983;15:139–141. [Google Scholar]
- Carlquist S. Successive cambia revisited: ontogeny, histology, diversity, and functional significance. Journal of the Torrey Botanical Society. 2007;134:301–332. [Google Scholar]
- Chrispeels MJ, Maurel C. Aquaporins: the molecular basis of facilitated water movement through living plant cells. Plant Physiology. 1994;105:9–15. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li J, Wang S, Hüttermann A, Altman A. Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to -increasing soil NaCl. Trees. 2001;15:186–194. [Google Scholar]
- Clough BF. Primary productivity and growth of mangrove forests. In: Robertson AI, Alongi DM, editors. Tropical mangrove ecosystems. Washington, DC: American Geophysical Union; 1992. doi:10.1029/CE041p0225. [Google Scholar]
- Clough BF, Sim RG. Changes in gas exchange characteristics and water use efficiency of mangroves in response to salinity and vapour pressure deficit. Oecologia. 1989;79:38–44. doi: 10.1007/BF00378237. [DOI] [PubMed] [Google Scholar]
- Comley B, McGuinness KA. Above- and below-ground biomass, and allometry, of four common northern Australian mangroves. Australian Journal of Botany. 2005;53:431–436. [Google Scholar]
- Constable JVH, Longstreth DJ. Aerenchyma carbon dioxide can be assimilated in Typha latifolia L. leaves. Plant Physiology. 1994;106:1065–1072. doi: 10.1104/pp.106.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable JVH, Grace JB, Longstreth DJ. High carbon dioxide concentrations in aerenchyma of Typha latifolia. American Journal of Botany. 1992;79:415–418. [Google Scholar]
- Das S. On the ontogeny of stomata and glandular hairs in some Indian mangroves. Acta Botanica Croatica. 2002;61:199–205. [Google Scholar]
- Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M. Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience. 2011;4:293–297. [Google Scholar]
- Duke NC. Mangrove floristics and biogeography. Coastal and Estuarine Studies. 1992;41:63–100. [Google Scholar]
- Duke NC. Overlap of eastern and western mangroves in the South-western Pacific: hybridization of all three Rhizophora (Rhizophoraceae) combinations in New Caledonia. Blumea. 2010;55:171–188. [Google Scholar]
- Eichert T, Kurtz A, Steiner U, Goldbach HE. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Eller CB, Lima AL, Oliveira RS. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae) New Phytologist. 2013;199:151–162. doi: 10.1111/nph.12248. [DOI] [PubMed] [Google Scholar]
- Ewe SML, Sternberg LdSL, Childers DL. Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia. 2007;152:607–616. doi: 10.1007/s00442-007-0699-x. [DOI] [PubMed] [Google Scholar]
- Ewers FW, Lopez-Portillo J, Angeles G, Fisher JB. Hydraulic conductivity and embolism in the mangrove tree Laguncularia racemosa. Tree Physiology. 2004;24:1057–1062. doi: 10.1093/treephys/24.9.1057. [DOI] [PubMed] [Google Scholar]
- Flowers TJ. Physiology of halophytes. Plant and Soil. 1985;89:41–56. [Google Scholar]
- Ford CR, Wurzburger N, Hendrick RL, Teskey RO. Soil DIC uptake and fixation in Pinus taeda seedlings and its C contribution to plant tissues and ectomycorrhizal fungi. Tree Physiology. 2007;27:375–383. doi: 10.1093/treephys/27.3.375. [DOI] [PubMed] [Google Scholar]
- Gouia H, Ghorbal MH, Touraine B. Effects of NaCl on flows of N and mineral ions and on NO3– reduction rate within whole plants of salt-sensitive bean and salt-tolerant cotton. Plant Physiology. 1994;105:1409–1418. doi: 10.1104/pp.105.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikopoulos G, Manetas Y. Direct absorption of water by hairy leaves of Phlomis fruticosa and its contribution to drought avoidance. Canadian Journal of Botany. 1994;72:1805–1811. [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiology. 2001;125:779–786. doi: 10.1104/pp.125.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Hao G-Y, Jones TJ, Luton C, et al. Hydraulic redistribution in dwarf Rhizophora mangle trees driven by interstitial soil water salinity gradients: impacts on hydraulic architecture and gas exchange. Tree Physiology. 2009;29:697–705. doi: 10.1093/treephys/tpp005. [DOI] [PubMed] [Google Scholar]
- Horie T, Kaneko T, Sugimoto G, et al. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant & Cell Physiology. 2011;52:663–675. doi: 10.1093/pcp/pcr027. [DOI] [PubMed] [Google Scholar]
- Hutchings P, Saenger P. Ecology of mangroves. St Lucia, Australia: University of Queensland Press; 1987. [Google Scholar]
- Jimenez JA. A hypothesis to explain the reduced distribution of the mangrove Pelliciera rhizophorae Tr. & Pl. Biotropica. 1984;16:304–308. [Google Scholar]
- Jimenez JA, Soto R. Patrones regionales en la estructura y composición florística de los manglares de la costa Pacífica de Costa Rica. Revista de Biologia Tropical. 1985;33:25–37. [Google Scholar]
- Johnson DM, Smith WK. Cloud immersion alters microclimate, photosynthesis and water relations in Rhododendron catawbiense and Abies fraseri seedlings in the southern Appalachian Mountains, USA. Tree Physiology. 2008;28:385–392. doi: 10.1093/treephys/28.3.385. [DOI] [PubMed] [Google Scholar]
- Johnstone IM, Frodin DG. Mangroves of the Papuan subregion. In: Gressitt JL, editor. Biogeography and ecology of New Guinea. The Hague: Dr W. Junk; 1982. [Google Scholar]
- Kant S, Kant P, Raveh E, Barak S. Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant, Cell & Environment. 2006;29:1220–1234. doi: 10.1111/j.1365-3040.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE. Biochemistry and function of cutin and suberin. Canadian Journal of Botany. 1984;62:2918–2933. [Google Scholar]
- Krauss KW, Young PJ, Chambers JL, Doyle TW, Twilley RR. Sap flow characteristics of neotropical mangroves in flooded and drained soils. Tree Physiology. 2007;27:775–783. doi: 10.1093/treephys/27.5.775. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Jyothi-Prakash PA, Qin L, et al. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant, Cell & Environment. 2014;37:1656–1671. doi: 10.1111/pce.12272. [DOI] [PubMed] [Google Scholar]
- Lambs L, Muller E, Fromard F. Mangrove trees growing in a very saline condition but not using seawater. Rapid Communications in Mass Spectrometry. 2008;22:2835–2843. doi: 10.1002/rcm.3676. [DOI] [PubMed] [Google Scholar]
- Lawton JR, Todd ANN, Naidoo DK. Preliminary investigations into the structure of the roots of the mangroves, Avicennia marina and Bruguiera gymnorrhiza, in relation to ion uptake. New Phytologist. 1981;88:713–722. [Google Scholar]
- Leuschner C. Air humidity as an ecological factor for woodland herbs: leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high VPD levels. Flora. 2002;197:264–274. [Google Scholar]
- Lin G, Sternberg L. Comparative study of water uptake and photosynthetic gas exchange between scrub and fringe red mangroves, Rhizophora mangle L. Oecologia. 1992;90:399–403. doi: 10.1007/BF00317697. [DOI] [PubMed] [Google Scholar]
- Lovelock C, Clough B. Influence of solar radiation and leaf angle on leaf xanthophyll concentrations in mangroves. Oecologia. 1992;91:518–525. doi: 10.1007/BF00650325. [DOI] [PubMed] [Google Scholar]
- Lovelock CE, Ball MC, Feller IC, Engelbrecht BMJ, Ewe ML. Variation in hydraulic conductivity of mangroves: influence of species, salinity, and nitrogen and phosphorus availability. Physiologia Plantarum. 2006a;127:457–464. [Google Scholar]
- Lovelock CE, Ball MC, Feller IC, Engelbrecht BMJ, Ewe ML. Differential effects of nitrogen and phosphorus on hydraulic conductance of mangroves along salinity gradients. Physiologia Plantarum. 2006b;127:457–464. [Google Scholar]
- Lovelock CE, Ball MC, Choat B, Engelbrecht BMJ, Holbrook NM, Feller IC. Linking physiological processes with mangrove forest structure: phosphorus deficiency limits canopy development, hydraulic conductivity and photosynthetic carbon gain in dwarf Rhizophora mangle. Plant, Cell & Environment. 2006c;29:793–802. doi: 10.1111/j.1365-3040.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- Lugo AE, Snedaker SC. The ecology of mangroves. Annual Review of Ecology and Systematics. 1974;5:39–64. [Google Scholar]
- Mallery CH, Teas HJ. The mineral ion relations of mangroves. I. root cell compartments in a salt excluder and a salt secreter species at low salinities. Plant & Cell Physiology. 1984;25:1123–1131. [Google Scholar]
- Martin CE, von Willert DJ. Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib Desert in southern Africa. Plant Biology. 2000;2:229–242. [Google Scholar]
- Martinez-Ballesta MC, Silva C, Lopez-Berenguer C, Cabanero FJ, Carvajal M. Plant aquaporins: new perspectives on water and nutrient uptake in saline environment. Plant Biology. 2006;8:535–546. doi: 10.1055/s-2006-924172. [DOI] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology. 2002;130:2101–2110. doi: 10.1104/pp.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. Aquaporins and water permeability of plant membranes. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu D-T, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- McKee KL. Growth and physiological responses of neotropical mangrove seedlings to root zone hypoxia. Tree Physiology. 1996;16:883–889. doi: 10.1093/treephys/16.11-12.883. [DOI] [PubMed] [Google Scholar]
- Medina E, Francisco M. Osmolality and δ13C of leaf tissues of mangrove species from environments of contrasting rainfall and salinity. Estuarine and Coastal Marine Science. 1997;45:337–344. [Google Scholar]
- Melcher PJ, Goldstein G, Meinzer FC, et al. Water relations of coastal and estuarine Rhizophora mangle: xylem pressure potential and dynamics of embolism formation and repair. Oecologia. 2001;126:182–192. doi: 10.1007/s004420000519. [DOI] [PubMed] [Google Scholar]
- Mensforth L, Walker G. Root dynamics of Melaleuca halmaturorum in response to fluctuating saline groundwater. Plant and Soil. 1996;184:75–84. [Google Scholar]
- Moon G, Clough B, Peterson C, Allaway W. Apoplastic and symplastic pathways in Avicennia marina (Forsk.) Vierh. roots revealed by fluorescent tracer dyes. Functional Plant Biology. 1986;13:637–648. [Google Scholar]
- Munné-Bosch S, Alegre L. Role of dew on the recovery of water-stressed Melissa officinalis L. plants. Journal of Plant Physiology. 1999;154:759–766. [Google Scholar]
- Munns R. Na+, K+ and Cl− in xylem sap flowing to shoots of NaCl-treated barley. Journal of Experimental Botany. 1985;36:1032–1042. [Google Scholar]
- Munns R. Effect of high external NaCl concentrations on ion transport within the shoot of Lupinus albus. I. Ions in xylem sap. Plant, Cell & Environment. 1988;11:283–289. [Google Scholar]
- Naidoo G, Hiralal O, Naidoo Y. Hypersalinity effects on leaf ultrastructure and physiology in the mangrove Avicennia marina. Flora. 2011;206:814–820. [Google Scholar]
- Odum WE, McIvor CC, Smith TJ. The ecology of the mangroves of south Florida: a community profile. Washington DC: United States Fish and Wildlife Service, Office of Biological Services; 1982. [Google Scholar]
- Oren R, Sperry JS, Katul GG, et al. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell & Environment. 1999;22:1515–1526. [Google Scholar]
- Paliyavuth C, Clough B, Patanaponpaiboon P. Salt uptake and shoot water relations in mangroves. Aquatic Botany. 2004;78:349–360. [Google Scholar]
- Pi N, Tam NFY, Wu Y, Wong MH. Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquatic Botany. 2009;90:222–230. [Google Scholar]
- Popp M, Larher F, Weigel P. Osmotic adaption in Australian mangroves. In: Beeftink WG, Rozema J, Huiskes AHL, editors. Ecology of coastal vegetation. Dordrecht: Springer; 1985. [Google Scholar]
- Popp M, Polania J, Weiper M. Physiological adaptations to different salinity levels in mangrove. In: Lieth H, Masoom A, editors. Towards the rational use of high salinity tolerant plants. Dordrecht: Springer; 1993. [Google Scholar]
- Rada F, Goldstein G, Orozco A, Montilla M, Zabala O, Azócar A. Osmotic and turgor relations of three mangrove ecosystem species. Functional Plant Biology. 1989;16:477–486. [Google Scholar]
- Raven JA. Into the voids: the distribution, function, development and maintenance of gas spaces in plants. Annals of Botany. 1996;78:137–142. [Google Scholar]
- Raven JA, Handley LL, Macfarlane JJ, et al. The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review, with new data on Eriocaulon decangulare L. New Phytologist. 1988;108:125–148. doi: 10.1111/j.1469-8137.1988.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata) Plant, Cell & Environment. 2008;31:1007–1016. doi: 10.1111/j.1365-3040.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Robert EMR, Koedam N, Beeckman H, Schmitz N. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. Functional Ecology. 2009;23:649–657. [Google Scholar]
- Robert EMR, Schmitz N, Boeren I, et al. Successive cambia: a developmental oddity or an adaptive structure? PloS One. 2011;6(1):e16558. doi: 10.1371/journal.pone.0016558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Nebelsick A. Computer-based analysis of steady-state and transient heat transfer of small-sized leaves by free and mixed convection. Plant, Cell & Environment. 2001;24:631–640. [Google Scholar]
- Roth-Nebelsick A. Computer-based studies of diffusion through stomata of different architecture. Annals of Botany. 2007;100:23–32. doi: 10.1093/aob/mcm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini N, Schmitz N, Lovelock C. Variation in wood density and anatomy in a widespread mangrove species. Trees. 2012;26:1555–1563. [Google Scholar]
- Schmitz N, Verheyden A, Beeckman H, Kairo JG, Koedam N. Influence of a salinity gradient on the vessel characters of the mangrove species Rhizophora mucronata. Annals of Botany. 2006;98:1321–1330. doi: 10.1093/aob/mcl224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Robert EMR, Verheyden A, Kairo JG, Beeckman H, Koedam N. A patchy growth via successive and simultaneous cambia: key to success of the most widespread mangrove species Avicennia marina. Annals of Botany. 2008;101:49–58. doi: 10.1093/aob/mcm280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Egerton JJG, Lovelock CE, Ball MC. Light-dependent maintenance of hydraulic function in mangrove branches: do xylary chloroplasts play a role in embolism repair. New Phytologist. 2012;195:40–46. doi: 10.1111/j.1469-8137.2012.04187.x. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Hemmingsen E, Garey W. Salt balance in mangroves. Plant Physiology. 1962;37:722–729. doi: 10.1104/pp.37.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepp PH. Tansley Review No. 59. Leaf boundary layers. New Phytologist. 1993;125:477–507. doi: 10.1111/j.1469-8137.1993.tb03898.x. [DOI] [PubMed] [Google Scholar]
- Seel WE, Jeschke WD. Simultaneous collection of xylem sap from Rhinanthus minor and the hosts Hordeum and Trifolium: hydraulic properties, xylem sap composition and effects of attachment. New Phytologist. 1999;143:281–298. [Google Scholar]
- Semeniuk V. Mangrove distribution in northwestern Australia in relationship to regional and local freshwater seepage. Vegetatio. 1983;53:11–31. [Google Scholar]
- Silvestri S, Defina A, Marani M. Tidal regime, salinity and salt marsh plant zonation. Estuarine and Coastal Marine Science. 2005;62:119–130. [Google Scholar]
- Slayter RO. Absorption of water from atmospheres of different humidity and its transport through plants. Australian Journal of Biological Sciences. 1956;9:552–558. [Google Scholar]
- Sobrado MA. Relation of water transport to leaf gas exchange properties in three mangrove species. Trees. 2000;14:258–262. [Google Scholar]
- Sobrado MA. Hydraulic properties of a mangrove Avicennia germinans as affected by NaCl. Biologia Plantarum. 2001;44:435–438. [Google Scholar]
- Sobrado MA. Influence of external salinity on the osmolality of xylem sap, leaf tissue and leaf gland secretion of the mangrove Laguncularia racemosa (L.) Gaertn. Trees. 2004;18:422–427. [Google Scholar]
- Sperry JS, Tyree MT, Donnelly JR. Vulnerability of xylem to embolism in a mangrove vs an inland species of Rhizophoraceae. Physiologia Plantarum. 1988;74:276–283. [Google Scholar]
- Stace CA. The significance of the leaf epidermis in the taxonomy of the Combretaceae. Journal of the Linnean Society of London, Botany. 1965;59:229–252. [Google Scholar]
- Sternberg LdSL, Swart PK. Utilization of freshwater and ocean water by coastal plants of southern Florida. Ecology. 1987;68:1898–1905. doi: 10.2307/1939881. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson C. How does water get through roots. Journal of Experimental Botany. 1998;49:775–788. [Google Scholar]
- Stuart SA, Choat B, Martin KC, Holbrook NM, Ball MC. The role of freezing in setting the latitudinal limits of mangrove forests. New Phytologist. 2007;173:576–583. doi: 10.1111/j.1469-8137.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- Sunarpi Horie T, Motoda J, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant Journal. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- Teskey RO, Saveyn A, Steppe K, McGuire MA. Origin, fate and significance of CO2 in tree stems. New Phytologist. 2008;177:17–32. doi: 10.1111/j.1469-8137.2007.02286.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson PB. The botany of mangroves. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, et al. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Tyerman S, Bohnert H, Maurel C, Steudle E, Smith J. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany. 1999;50:1055–1071. [Google Scholar]
- Tyree MT, Davis SD, Cochard H. Biophysical perspectives of xylem evolution – is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction. IAWA Journal. 1994;15:335–360. [Google Scholar]
- Vandegehuchte MW, Guyot A, Hubau M, et al. Long-term versus daily stem diameter variation in co-occurring mangrove species: environmental versus ecophysiological drivers. Agricultural and Forest Meteorology. 2014;192–193:51–58. [Google Scholar]
- Waisel Y, Eshel A, Agami M. Salt balance of leaves of the mangrove Avicennia marina. Physiologia Plantarum. 1986;67:67–72. [Google Scholar]
- Wan X, Zwiazek JJ. Mercuric chloride effects on root water transport in aspen seedlings. Plant Physiology. 1999;121:939–946. doi: 10.1104/pp.121.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Lockington DA, Poh SC, Gasparon M, Lovelock CE. Water use patterns of estuarine vegetation in a tidal creek system. Oecologia. 2013;172:485–494. doi: 10.1007/s00442-012-2495-5. [DOI] [PubMed] [Google Scholar]
- Werner A, Stelzer R. Physiological responses of the mangrove Rhizophora mangle grown in the absence and presence of NaCl. Plant, Cell & Environment. 1990;13:243–255. [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant, Cell & Environment. 2013;36:1938–1949. doi: 10.1111/pce.12139. [DOI] [PubMed] [Google Scholar]
- Wilson P. Myrtaceae. In: Kubitzki K, editor. Flowering plants. Eudicots: Berlin: Springer; 2011. [Google Scholar]
- Woodruff DR, McCulloch KA, Warren JM, Meinzer FC, Lachenbruch B. Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas fir. Plant, Cell & Environment. 2007;30:559–569. doi: 10.1111/j.1365-3040.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- Yakir D, Yechielie Y. Plant invasion of newly exposed hypersaline Dead Sea shores. Nature. 1995;374:803–805. [Google Scholar]
- Yáñez-Espinosa L, Terrazas T, López-Mata L, Valdez-Hernández J. Wood variation in Laguncularia racemosa and its effect on fibre quality. Wood Science and Technology. 2004;38:217–226. [Google Scholar]
- Ye Y, Gu YT, Gao HY, Lu CY. Combined effects of simulated tidal sea-level rise and salinity on seedlings of a mangrove species, Kandelia candel (L.) Druce. Hydrobiologia. 2010;641:287–300. [Google Scholar]
- Zwieniecki MA, Holbrook NM. Confronting Maxwell's demon: biophysics of xylem embolism repair. Trends in Plant Science. 2009;14:530–534. doi: 10.1016/j.tplants.2009.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.