Abstract
The generation and conduction of neuronal action potentials (APs) were the subjects of a cell physiology exercise for first-year medical students. In this activity, students demonstrated the all-or-none nature of AP generation, measured conduction velocity, and examined the dependence of the threshold stimulus amplitude on stimulus duration. For this purpose, they used the median giant nerve fiber (MGF) in the ventral nerve cord of the common earthworm (Lumbricus terrestris). Here, we introduce a specialized stimulation and recording chamber that the nonanesthetized earthworm enters completely unforced. The worm resides in a narrow round duct with silver electrodes on the bottom such that individual APs of the MGF can be elicited and recorded superficially. Our experimental setup combines several advantages: it allows noninvasive single fiber AP measurements taken from a nonanesthetized animal that is yet restrained. Students performed the experiments with a high success rate. According to the data acquired by the students, the mean conduction velocity of the MGF was 30.2 m/s. From the amplitude-duration relationship for threshold stimulation, rheobase and chronaxie were graphically determined by the students according to Lapicque's method. The mean rheobase was 1.01 V, and the mean chronaxie was 0.06 ms. The acquired data and analysis results are of high quality, as deduced from critical examination based on the law of Weiss. In addition, we provide video material, which was also used in the practical course.
Keywords: action potential, extracellular recording, conduction velocity, rheobase, chronaxie, Lapicque, Weiss
signaling in excitable tissue relies on the generation of action potentials (APs). The AP is a biophysical all-or-none response of the nerve or muscle cell to a suprathreshold stimulation. Typically, in a nerve cell, the AP is 1 ms long. However, this short signal can be actively propagated, sometimes over large distances, along nerve fibers in a regenerative manner, since the AP itself automatically excites neighboring membrane areas. In this activity, students elicited and recorded single fiber APs from an intact, nonanesthetized, but yet restrained earthworm using a specially designed stimulation and recording chamber. Students become familiar with extracellularly recorded AP signals, demonstrate the all-or-none nature of AP generation, measure conduction velocity, and examine the amplitude-duration relationship of electrical threshold stimulation.
Background
Comprehensive knowledge of the basic principles of cellular excitability (1, 20, 27) is fundamental to the education of medical students, because the measurement of neuronal excitability and signal conduction represents an essential part of electrodiagnostic testing in clinical practice (6). In addition, it is important to understand the principles of electrical excitability in nerve and muscle cells for the appropriate use of stimulation devices like deep brain electrodes, cardiac pacemakers, and defibrillators (16, 22, 23).
Excitability of nerve and muscle cells is defined by a threshold membrane potential that needs to be reached to fire an AP. The membrane potential can be experimentally brought to threshold by electrical stimulation. Once the threshold potential is reached, the AP is generated in an all-or-none fashion (1, 20, 27). Two aspects of neuronal excitation are of physiological and pathophysiological relevance: 1) how strong the electrical stimulation must be in order for the nerve cell membrane to reach AP firing threshold and 2) how fast the conduction of the AP occurs once the signal has been generated. As an intrinsic feature of nerve cell excitation, the stimulus amplitude necessary to reach the AP firing threshold depends on the duration of the stimulus. With very long stimulus durations (one to several milliseconds), a specific minimal stimulus amplitude is needed. When decreasing stimulus duration (10- to 100-fold), higher stimulus amplitudes are necessary to elicit an AP, which results in a tissue- or cell type-specific amplitude-duration curve for the AP firing threshold. Figure 1A shows how the threshold stimulus amplitude depends on stimulus duration. As defined by Lapicque at the turn of the 20th century (30), the minimal stimulus amplitude to reach threshold with very long stimulus durations is called “rheobase,” and the stimulus duration obtained from the amplitude-duration curve at two times rheobase is called “chronaxie” (Fig. 1A) (25). Rheobase and chronaxie are important measures of excitability, and, remarkably, the chronaxie defines conditions where the electrical energy of stimulation is minimal (Fig. 1C) (14) (see also the results). From a historical viewpoint, the definitions given by Lapicque are based on the law of Weiss, who discovered earlier that stimulus quantity (stimulus amplitude × stimulus duration) shows a linear dependence on stimulus duration (Fig. 1B) (24, 43). Since it was difficult to apply rectangular pulses with the stimulation devices available at that time (slow rise time, slow decay time, and unstable plateau), it was useful to define the dependence of quantity rather than amplitude on stimulus duration. Although no reference was made to the terms “rheobase” and “chronaxie,” these parameters were already part of the law of Weiss (Fig. 1) (see the appendix). For educational purposes, Lapicque's approach is preferable, first, because it illustrates the inverse dependence of the threshold stimulus amplitude on stimulus duration and the existence of a minimal amplitude at long durations and, second, because rheobase and chronaxie can be determined graphically without the need of any calculations (Fig. 1A) (see also materials and methods).
Fig. 1.
Parameters of electrical threshold stimulation. A: hyperbolic dependence of stimulus amplitude (a) on stimulus duration (t). According to Lapicque, a at a very long stimulus duration is called “rheobase” (r) and t at two times r (2r) is called “chronaxie” (c) (25, 30). The parameters r (and 2r) and c are indicated by dotted lines and arrows. B: linear dependence of stimulus quantity [q; integral of a over t (see inset)] on t according to the law of Weiss (24, 43). The linear graph intersects the y-axis at r × c and the x-axis at (−)c; thus, its slope is r. The formulas in A and B are explained in the appendix. C: the energy (e) of an electrical stimulus has a minimum under chronaxie conditions (14). The graph combines the hyperbolic curve for a with the linear dependence of q to illustrate the derivation of e, which is the product of a and q (14).
Different experimental setups are used for the conception of the amplitude-duration relationship and AP conduction velocity in practical courses. Human subjects (e.g., course participants) are often used in neurophysiology courses for the induction of reflexes by electrical surface stimulation and to noninvasively determine nerve conduction velocity (27). However, although practicable and clinically useful, such experiments do not allow recording from a single nerve fiber to demonstrate the all-or-none nature of AP generation as such. For this purpose, classical preparations like the neuronal ganglia of the leech (18) are often used. A disadvantage of such tissue preparations is that they are time consuming, and the outcome critically depends on the preparational skills of the students. Moreover, special recording solutions need to be prepared to work with the isolated material. For these reasons, such experiments may not be fitted into the curricular timetable, their success rate may be low, or they may not be practicable at all due to staff shortage.
The common earthworm (Lumbricus terrestris) represents a model organism perfectly suited for students to noninvasively elicit and record APs in a single axon. Noninvasive recordings from the giant nerve fibers of the earthworm were first conducted >60 yr ago with different restraining methods (5, 39). Later, Drewes and coworkers (8) described a method to record from the giant nerve fibers of freely moving earthworms. A simple method suitable for classroom experiments based on the electrical stimulation of and recording from the giant nerve fibers of restrained anesthetized earthworms has been suggested by Heinzel and coworkers (19, 29). Here, we present a further development of the earthworm experiment: we used a specially designed stimulation and recording chamber, which the nonanesthetized earthworm enters freely. This earthworm chamber allows superficial electrical stimulation and recording with a high success rate. In this article, we first document the execution and analysis of the experiments and present the results based on a total of 77 trials conducted by a cohort of first-year medical students. We also outline the issues that are important for teaching the basic principles of neuronal excitation and for the discussion of the clinical relevance of the experiments at different student levels. Finally, we point out the merits of our experimental setup and evaluate the quality of the data acquired by the students by putting their results in relation to the law of Weiss.
Learning Objectives
After completing this activity, students should be able to:
1. Explain the all-or-none principle of AP generation
2. Describe the main parameters that determine AP conduction velocity
3. Describe the experimental setup for measuring AP conduction velocity
4. Interpret AP signals obtained with extracellular differential recordings
5. Explain the parameters rheobase and chronaxie as well as the experimental procedure to obtain these values
6. Describe the scientific and practical significance of a threshold stimulus amplitude-duration curve
Activity Level
This activity was performed by first-year medical students, but it is also suitable for higher levels of medical education. It can also be used in university animal physiology, biophysics, and biotechnology courses or in the education of physiotherapists. Furthermore, it may serve as a demonstration experiment in biology courses at school.
Prerequisite Student Knowledge and Skills
Ideally, students participating in this activity should have a basic understanding of the following:
1. Changes in ion conductances underlying a neuronal AP
2. Continuous and saltatory AP propagation and the neuroanatomic correlates
3. The difference between stimulation electrodes (terms and definition of “anode” and “cathode”) and recording electrodes
4. The difference between intracellular and extracellular recordings and electrical stimulation
5. The terms “electrotonus” and “threshold stimulus”
6. The anatomy of an earthworm with its ventral cord harboring giant nerve fibers
Time Required and Course Organization
The activity described here is designed as a single session. The session was repeated multiple times with different groups of students, and the whole practical course ran for 2 wk. Including the graphical analysis of the experimental data by the students and a concluding discussion, one session requires ∼150 min. The experiments can also be performed as a demonstration in ∼30 min. The activity can be modified by skipping or adding experiments. Additional experiments are outlined below in Possible Extensions and Modifications.
Inquiry Application
The activity, as described here, is on the “Methods” level. The questions to be answered and tasks to be performed were defined by the teacher. However, depending on the time available, the activity may be modified to fit higher levels of inquiry. One may, for instance, only provide the individual components of the experimental setup and let students connect them with cables. One may also provide appropriate literature, from which students have to extract experimental approaches and the relevant parameters to be measured.
MATERIALS AND METHODS
Animals
For an expected number of ∼100 experiments to be performed during the whole practical course, we purchased a stock of 40 earthworms (L. terrestris) from a local sports fishing dealer. Animals were held in plastic boxes filled with moistened soil (4 boxes each containing 10 earthworms) in the laboratory refrigerator at 8°C (see also the Supplemental Material).1 As an annelid, Lumbricus has a ventral nerve cord, which harbors two lateral giant nerve fibers (LGFs) and one median giant nerve fiber (MGF; Fig. 2). The diameter of these giant nerve fibers is much larger (LGF: ∼0.05 mm and MGF: ∼0.08 mm) (5, 42) compared with all other nerve cells in Lumbricus, and, moreover, the giant nerve fibers possess a myelin-like glial cell wrapping (38) with node-like fenestrations, particularly found in the MGF (17). Accordingly, the conduction velocities found in the giant nerve fibers of Lumbricus are very high and comparable to those in fast vertebrate myelinated axons measured at room temperature (5, 11). When the earthworm is touched at its anterior or posterior end, the giant nerve fibers and downstream motor neurons trigger a muscle twitch as part of an escape reflex. The MGF conducts from the front to the rear (when the worm is touched at its front end), and the two LGFs, which are combined to one functional unit by numerous anastomoses (5), conduct from the rear to the front (when the worm is touched at its rear end). Experimentally, the giant nerve fibers are easily accessible by ventral superficial electrical stimulation and recording (39). In this activity, we were interested in threshold stimulation of a single nerve fiber. This was easily accomplished, since, compared with LGFs, the AP firing threshold of the MGF is considerably lower at all stimulus durations (5, 29). Right before the experiment, the earthworm was briefly rinsed with tap water and dried on a piece of paper towel. The animal was not anesthetized but immediately used for the experiment. No injury occurred during the experiment, and animals were reused in different sessions and finally set free in an appropriate environment after the practical course was finished.
Fig. 2.
The common earthworm (Lumbricus terrestris). A schematic drawing of a cross section of Lumbricus is shown. The worm is surrounded by the body wall, which mainly consists of muscle tissue penetrated by two pairs of lateral bristles and two pairs of ventral bristles (setae). Almost all segments contain a pair of nephridia; the gut and three main blood vessels as well as the ventral nerve cord traverse the coelom at almost full length. The median giant nerve fiber and two lateral giant nerve fibers run within the ventral nerve cord.
Experimental Setup
Each experimental setup was used by a group of three to four students. The experimental setup consisted of a stimulator, a computer-controlled data-acquisition unit, and a specialized earthworm chamber (Fig. 3 and Supplemental Material). The stimulator created rectangular voltage pulses that could be modified in duration and amplitude (Fig. 3A). It sent a trigger signal to the data-acquisition unit to start the recording and storage of data (stimulus delay: 0.5 ms; see Fig. 4, A and B, and Supplemental Material). The data-acquisition unit was a BIOPAC system (BIOPAC Systems, Goleta, CA), which offers four input channels: channels 1 and 2 received the signals from the differential recording sites 1 and 2, respectively, channel 3 was the stimulus signal, and channel 4 was the trigger signal from the stimulator (Fig. 3A). Other commercially available digitizing systems for AP recording from earthworm giant fibers are, for instance, offered by AD Instruments (Colorado Springs, CO) or iWorx Systems (Dover, NH). The experiment can also be executed without a digitization system, just by using an appropriate stimulator (either constant current or constant voltage generator, see below), amplifier, filter (0.1–3 kHz), and oscilloscope (time base: 1 ms/division, gain: 20 μV/division without preamplification, negative slope external alternating current trigger).
Fig. 3.
Experimental setup. A: the experimental setup consisted of a stimulator, a data-acquisition unit (connected to a personal computer), and the earthworm chamber. The schematic drawing shows the worm in the chamber with its anterior end on the left where stimulation takes place (stimulus electrodes and ground). Two pairs of recording electrodes in the center of the chamber are connected to the data-acquisition unit, and the alternative places for stimulus or recording electrodes are not in use. B–E: photographs of the earthworm chamber. B: open chamber (lid removed) with a metric plastic ruler to measure distances between electrodes. The rubber strip on the ruler fits between the rubber strips delineating the central “halfpipe” with the electrodes. C: the worm has entered the chamber and is restrained by plugs inserted in the rubber tubings on both sides. D: closed chamber with plugs (syringe needle covers). The chamber is covered with a black lid gently tightened with wing nuts (to avoid escape of the worm and to darken the chamber). E: view from the bottom. The closed chamber has been turned upside down to check whether the worm has contact with all connected stimulation and recording electrodes (see also Supplemental Material for the details of the earthworm chamber).
Fig. 4.
Experimental action potential (AP) generation and signal conduction. A and B: the preset user surface of the data acquisition and analysis software with three horizontal acquisition segments. Channels 1 and 2 show differential recordings from two recording sites, and channel 3 shows the stimulus signal. The time point of stimulation is indicated by the vertical dotted lines (S). A: subthreshold stimulation with a 0.1-ms pulse; stimulus amplitude = 0.9 V. Stimulus artifacts but no AP can be seen in channels 1 and 2. B: suprathreshold stimulation with a 0.1-ms pulse and stimulus amplitude = 1.2 V. The first biphasic AP signals in channels 1 and 2 are caused by the passing through of the median giant nerve fiber AP. C: frequency distribution of stimulus amplitudes determined at stimulus duration = 0.1 ms (bin width: 0.3 V). D: frequency distribution of the conduction velocities (bin width: 3.75 m/s). Conduction velocity was calculated based on the latency difference between the first negative peaks in channels 1 and 2, respectively (time between dotted lines 1 and 2) and the measured distance between the recording electrodes (see Fig. 3B).
The earthworm chamber designed for this activity has four different pairs of silver electrodes (and two electrodes for grounding; Fig. 3A); two central pairs of recording electrodes are routinely used in the experiments. In Fig. 3A, the anterior end of the worm is on the left, and the electrode pair on the left is connected to the stimulator together with a grounding electrode. An AP elicited in the MGF at this site will travel from the front to the rear, as it happens normally in the worm (from left to right in Fig. 3A). With a long enough worm, an additional pair of electrodes may be used for recording (not connected in Fig. 3A). Alternatively, stimulation may occur at this additional site such that the elicited AP would travel from right to left (Fig. 3A). Figure 3, B–E, shows our earthworm chamber (see also Supplemental Material): the chamber is a transparent box, with an elongated rounded depression (roughly 8 cm long and 0.5 cm wide) on its upper surface (Fig. 3B, lid removed). This “halfpipe” is bounded by two elongated rubber strips and harbors on its bottom the silver wire electrodes (diameter: 0.5 mm) connected to easily accessible pin jacks on the outside of the chamber (Fig. 3B). With a metric plastic ruler, the distance between electrodes in the chamber can be measured (Fig. 3B) to calculate the conduction velocity. The ruler is equipped with an elongated rubber strip that fits between the rubber strips delineating the central halfpipe. A construction plan of the earthworm chamber can be found in the Supplemental Material.
The earthworm enters the chamber through one of two lateral rubber tubings (inner diameter: 5–6 mm) and then reaches the central halfpipe, which should be covered by the plastic ruler to guide the worm. Finally, the worm enters the tubing on the other side of the chamber (Fig. 3C). Now, plugs (e.g., syringe needle covers) are introduced into the rubber tubings on both sides of the chamber to restrain the worm, and the black lid is gently screwed upon the chamber (Fig. 3D). This blocks the worm's escape and also darkens the chamber to mimic the worm's natural environment. Once the worm has fully entered the chamber and the plugs and lid have been added, the chamber can easily be turned upside down to check for the correct position of the worm. This is important because the worm has to be in contact with the stimulation and recording electrodes (Fig. 3E).
All students initially performed a “dummy experiment” with a piece of moistened gauze bandage rolled up to adopt an elongated worm-like shape. This familiarized students with the experimental setup. Furthermore, students got an impression of what a null response (stimulus artifacts not followed by an AP) would look like with subthreshold stimulation of the worm (see results). The handling of the earthworm chamber, including the transferral of the worm into the chamber, is shown in several videos available in the Supplemental Material.
Experiments
Each student had a printed tutorial containing a detailed description of the experiments, analysis instructions, and a coordinate system to plot the results (see below). Initially, students had to find the threshold stimulus amplitude at a preset stimulus duration of 0.1 ms. This first experiment demonstrated the all-or-none nature of AP generation. Care had to be taken that proper AP signals appeared in both recording channels, because the latency difference between the signals was later used to determine conduction velocity (see below). If the worm was long enough, two different recording site distances were used (see Fig. 3A). Next, students were asked to switch the cable connections at one of the recording electrode pairs and repeat the stimulation. The effect of this maneuver demonstrates a basic principle of extracellular AP recording, to be addressed in a concluding discussion (see below). Finally, students determined the threshold stimulus amplitude for different stimulus durations. This experiment started with the longest stimulus duration of 1 ms, where the threshold stimulus amplitude is expected to be low. Stimulus duration was then decreased stepwise down to 0.01 ms, where the threshold stimulus amplitude is much higher. The stimulus durations between these two extremes were 0.5, 0.2, 0.1, 0.08, 0.06, 0.04, and 0.02 ms. Starting with the longest one and decreasing instead of increasing stimulus duration allows swift execution of the experiment, since it is safe to assume that stimulus amplitude either remains unchanged from the previous run or has to be increased. All experiments were conducted at room temperature.
Data Analysis
The threshold amplitude at a stimulus duration of 0.1 ms was determined twice, and the results were averaged. Next, the difference between the latency periods of two corresponding signals recorded via channel 1 and 2, respectively, was measured (see Fig. 4B). Conduction velocity (in m/s) was calculated from the recording site distance (Δs; in mm) and the latency difference (Δt; in ms). Two values were determined corresponding to two different recording site distances, if available (depending on the worm's length), and averaged. To create the amplitude-duration curve, the different threshold stimulus amplitudes were plotted against the corresponding stimulus duration. Students were asked to draw a smooth line through the data points. The semilogarithmic coordinate system in the handout tutorial (Fig. 5A) flattened the hyperbolic shape of the amplitude-duration curve (see Fig. 1A), which facilitated visual inspection and graphic analysis of the data. The asymptote of the hand-drawn curve was determined by eye with the help of a ruler. The obtained value is the rheobase (in V). According to Lapicque's method, the chronaxie value (in ms) was then obtained by determining, again with the help of a ruler, the stimulus duration at two times rheobase (see Fig. 5A).
Fig. 5.
Rheobase and chronaxie values determined by the students. A: amplitude-duration diagram for threshold stimulation from a typical experiment. The stimulus amplitudes necessary to induce AP firing are plotted against the corresponding stimulus duration on a semilog scale. Due to the logarithmic scaling of the x-axis, the hyperbolic shape of the curve (see Fig. 1A) appears flattened, which facilitates graphic analysis. The dashed line through the data points is a smooth fit that represents the drawing by eye, performed by the students. The dashed leftward pointing arrow represents the asymptote of the curve, which yields the rheobase value (1.2 V in the experiment shown); the dashed downward pointing arrow originates from the point on the curve corresponding to two times rheobase and intersects the x-axis at the chronaxie value (0.054 ms in the experiment shown). B: frequency distribution of rheobase values obtained by the students (bin width: 0.3 V). C: frequency distribution of the corresponding chronaxie values (bin width: 0.015 ms).
The present report includes the results of 77 trials performed by different groups of students in different sessions. Results obtained by students during the whole practical course were further processed with Kaleidagraph (Synergy Software, Reading, PA), and pooled data are given as means ± SE unless stated otherwise. The number of observations (n) refers to the number of trials and not to the number of worms. Statistical analyses were based on Student's paired t-test.
Possible Extensions and Modifications
The described experimental setup offers further options, which were not made use of in the activity described here: the period with no voltage deflection in which the AP runs between the first and second of a pair of recording electrodes may be visualized. For this purpose, all recording cables have to be first unplugged and then any two electrodes with a larger distance may be chosen as a new pair of recording electrodes and connected accordingly. Also, basic principles of electrical stimulation may be demonstrated: since with a charged technical device depolarization occurs at the cathode and, accordingly, hyperpolarization at the anode, the latter must not be located between cathode and recording site. If one does separate the cathode from the recording site by the anode, conduction and AP recording may no longer be possible (anodal block), especially when longer stimulus durations are used. With the built-in and easily accessible pin jacks of our recording chamber, maneuvers like these are expeditious.
The experimental setup described above was tailored for the teaching of basic principles of electrical excitability and signal conduction. However, the earthworm chamber may also be used to examine other physiologically relevant parameters, including the refractory period of AP generation, the temperature dependence of AP conduction velocity, or synaptic depression, facilitation, and habituation (29). It should be noted, however, that the experimental setup we present here is not designed for tactile stimulation to study AP generation and conduction relevant to the muscle twitch reflex of Lumbricus. This may be accomplished with the Heinzel method (19, 29) or with an experimental setup designed for freely moving worms, as previously described by Drewes and coworkers (8).
Finally, if this activity is used at higher levels of education in university biophysics or biotechnology courses, more focus may be put on the mathematical connections between the parameters of electrostimulation, briefly addressed in the appendix.
Troubleshooting
The worm does not want to enter the tube.
Darken the chamber, e.g., with a paper towel (as shown in the video sequence provided in the Supplemental Material). First use the ruler with the rubber strip upward. Turn the ruler with the rubber strip downward, when the worm has entered the second part of the tube and the exits have been locked. For very thick worms, leave the ruler with the rubber strip upward. Extremely small worms should not be used; restraining them is difficult, and they might turn around within the tube.
No AP signal is detectable.
Good contact between the worm and electrodes is essential: the position of the worm should be checked by inspection from beneath. This is easy since the “main body” of our earthworm chamber is transparent. Before using stimulus amplitudes well above 3 V, the setup should be examined by an experienced supervisor.
There is an insufficient signal-to-noise ratio.
For the laptop, try to choose the battery operation to reduce electromagnetic noise. The use of a Faraday cage might also be helpful. However, in our teaching laboratories and other rooms tested, no Faraday cage was necessary with our experimental setup. Check any filter settings.
Stimulus artifact overspreads the AP signal.
Check whether the ground electrode positioned between stimulation and recording electrode is properly connected. Check whether recording and stimulation cables are separated. Check the silver electrodes for unwanted electrical connections caused by excess fluid in the chamber. Dry the worm and chamber with a paper towel.
The Virtual Experiment
On each computer in the teaching laboratory, a PDF file named “Earthworm experiment” was available, which contained embedded video sequences demonstrating the handling of the earthworm chamber and the execution of the experiments. It could be used as a descriptive online tutorial in addition to the printed handout. Moreover, the file also contained analysis instructions and links to data files that were acquired by us before the course under the same conditions as described for this activity. Thus, the Earthworm experiment file may also be used by students who refuse to perform animal experiments for ethical or esthetical reasons. Performing the “virtual experiment” based on the provided material (videos and previously acquired data) guaranteed a learning profit for these students (see below). Both the Earthworm experiment file and the example data files are provided with the Supplemental Material. The data files can be processed with free BIOPAC software (http://www.biopac.com/BSL-Analysis.asp).
RESULTS
Data Acquisition and Analysis
The user interface of the acquisition and analysis software preset for the practical course (see also Supplemental Material) showed three horizontal acquisition segments in which the signals coming from the recordings via channel 1 and 2 (in mV) and the stimulus signal (in V) in channel 3 were displayed (Fig. 4, A and B). The acquisition time for each run was 20 ms. In channels 1 and 2, the y-scaling was chosen according to the expected signal amplitude of 20–100 μV (5, 39) and in channel 3 according to the stimulus amplitude applied.
At the beginning of the first experiment, the stimulator was set to 0.1 ms, which is roughly double the chronaxie value (see below). The threshold is then reached with an already close to minimal stimulation amplitude (rheobase, see below) of ∼1 V. Students were instructed to increase the stimulus amplitude from a starting value of 0.5 V, a usually subthreshold stimulus amplitude (Fig. 4, A and C). By increasing the stimulus amplitude in a stepwise fashion in intervals of ∼0.1 V, the threshold was reached, and an AP was generated in the MGF (Fig. 4B). AP generation could be inferred from similar but time-displaced biphasic waveforms in channels 1 and 2, which were highly reproducible (Fig. 4B and Supplemental Material). The threshold stimulus amplitudes at a duration of 0.1 ms determined in the practical course ranged between 0.6 and 4 V (Fig. 4C) with a mean value of 1.49 ± 0.08 V (n = 74; Table 1). Usually, in both recording channels, the first biphasic AP was followed by a second AP, presumably fired by a motor neuron and, frequently, a third voltage deflection indicated summed muscle potentials (29).
Table 1.
Comparision of rheobase and chronaxie values obtained by students in the practical course compared with the corresponding values analyzed based on Weiss formalism
| Practical Course | Weiss | P Value (by Student's paired t-test) | |
|---|---|---|---|
| Threshold stimulus at 0.1-ms stimulus duration, V | 1.49 ± 0.08 | ||
| n | 74 | ||
| Conduction velocity, m/s (room temperature) | 30.2 ± 0.7 | ||
| n | 74 | ||
| Rheobase, V | 1.012 ± 0.052 | 0.976 ± 0.046 | 0.0161 |
| n | 64 | 64 | |
| Chronaxie, ms | 0.060 ± 0.005 | 0.066 ± 0.007 | 0.1821 |
| n | 64 | 64 |
Shown is a summary table of mean values for the threshold stimulus amplitude at 0.1-ms stimulus duration and conduction velocity as well as rheobase and chronaxie values graphically analyzed according to Lapicque's method (student-derived data). The rheobase and chronaxie values obtained by students in the practical course were compared with the corresponding values obtained by reanalyzing the data based on the Weiss formalism.
The latency difference between the first AP recordings in channels 1 and 2 (generated by the MGF) was determined (Fig. 4B and Supplemental Material). If a worm was long enough to cover an alternative recording site (as can be checked by inspection from the bottom of the chamber; Fig. 3E and Supplemental Material), this procedure was repeated with the modified setting. In the practical course, the calculated values for the conduction velocity (see materials and methods) ranged between as low as 10.3 m/s and as high as 54.8 m/s (Fig. 4D) with a mean value of 30.2 ± 0.7 m/s (n = 74; Table 1).
To determine the amplitude-duration relationship of threshold stimulation, the initial stimulator settings were 1 ms and 0.5 V. The threshold stimulus amplitude was now determined for this extended stimulus duration. Once the threshold had been found, the stimulus duration was decreased in predefined steps. The threshold was determined for each tested stimulus duration down to 0.01 ms, and the results were plotted in a diagram for further analysis (Fig. 5A; see materials and methods). The rheobase values determined by students from the amplitude-duration curves in the practical course ranged between 0.4 and 2.5 V (Fig. 5B) with a mean value of 1.012 ± 0.052 V (n = 64; Table 1). According to Lapicque's method, the chronaxie was determined by students from the curves as the threshold stimulus duration at two times rheobase. The chronaxie values, graphically obtained in this way in the practical course, ranged between 0.02 and 0.3 ms (Fig. 5C) with a mean value of 0.060 ± 0.005 ms (n = 64; Table 1).
Contents of Class Discussion
In this activity, students experimentally demonstrated the all-or-none nature of AP generation. In a concluding discussion we addressed the underlying mechanism: electrical stimulation leads to electrotonic membrane depolarization, which, at a certain stimulus strength, is large enough to activate some voltage-gated Na+ channels. Na+ channel activation leads to Na+ influx, which leads to further depolarization and to the activation of even more, and eventually all, available Na+ channels. This positive feedback mechanism (1, 20, 27) is often called the “Hodgkin cycle.”
Extracellular differential recording, as performed in this activity, yields biphasic AP waveforms that do not reflect the time course of membrane potential change. Rather, the loss of positive charge on the extracellular side at the site of AP generation leads to a signal in one direction when the AP passes the first and in the opposite direction when the AP passes the second of a pair of recording electrodes (1). To demonstrate this basic principle of extracellular differential recording, the students have switched the cable connections at one of the recording sites, which resulted in an inversion of the recorded biphasic signal (see Supplemental Material). Students can recognize that the polarity of an extracellularly recorded AP waveform is just a result of the difference between the signals obtained at the two electrodes. As a consequence, the shape of the recorded signal depends on both the distance between the electrodes and the conduction velocity.
Conduction velocity was measured by the students in this activity. The parameters that determine conduction velocity were discussed: first of all, conduction velocity is higher in myelinated fibers than in nonmyelinated fibers, due to saltatory instead of continuous AP propagation, respectively. Furthermore, conduction velocity is positively correlated to fiber diameter, and it depends on temperature. In this context, we pointed out that the values obtained by the students would be much higher if the experiments had been done at 37°C.
Apart from teaching the basic principles of neuronal excitation and signal conduction, one of our greatest concerns was to draw the students' attention to the clinical relevance of the experiments they have performed in this activity. This included therapeutic as well as diagnostic aspects of electrostimulation, and the contents were adapted to the level of education.
Contents for first-year medical students.
The concept of rheobase and chronaxie is important for various technical aspects of electrostimulation in clinical practice, because stimulation is intended to occur reliably but with a minimal energy and/or high selectivity. Especially for cardiac defibrillation and pacing, the two clinically most relevant forms of nonneuronal electrostimulation, a precise knowledge of chronaxie values is desirable, since the energy necessary for and provided by an electrical stimulus is minimal under chronaxie conditions (Fig. 1C) (14). This energy minimum under chronaxie conditions is of major importance for the appropriate use of cardiac pacemakers, both to protect heart cells from damage and to save battery power. A pulse duration longer than the chronaxie is not preferable because current consumption is increased without decreasing the threshold significantly (22). Modern pacemaker devices are sometimes able to perform a ventricular pacing threshold search with chronaxie determination occurring in predefined time intervals (“threshold tracking”) (9). Chronaxie values may differ among cell types or depending on the cellular compartment stimulated. Thus, amplitude-duration analysis based on intraoperative microstimulation of the human thalamus may also help to identify the neuronal elements, either local cells or axons of passage, involved in deep brain stimulation (16, 28), used for tremor stoppage in Parkinson's disease and related disorders (34).
Additional contents and scientific background for higher-level medical students.
Electrodiagnostic testing represents the basis for the detection and appropriate treatment of diseases of the peripheral nervous system and muscles (6). The standard testing procedure uses a needle electrode electromyograph examination to detect nerve fiber loss or degeneration and a nerve conduction measurement to detect segmental demyelination (6). In addition to these conventional tests, which explore fiber integrity and conduction velocity, threshold testings are increasingly used to examine excitability, i.e., axonal (nodal) membrane properties (2). Application of the amplitude-duration relationship to the generation of a defined fractional amplitude of the maximal compound AP in whole nerves represents one method of threshold testing, and chronaxie is usually referred to as the “strength-duration time constant” (SDTC) in these tests (2). Ectopic axonal hyperexcitability in motor neurons, which is responsible for the frequent fasciculations in amyotrophic lateral sclerosis, is reflected by a lower rheobase and an increased SDTC in the patients (33), and SDTC values are inversely correlated with amyotrophic lateral sclerosis survival rates (26). In carpal tunnel syndrome patients, focal demyelination and slowing of conduction is accompanied by increased rheobase values, whereas SDTC values are not significantly different from SDTC values measured from healthy subjects (32). In accordance with central nervous system demyelination and only mild involvement of the peripheral nervous system, no abnormalities have been observed with peripheral nerve threshold testing in patients with multiple sclerosis (15). However, diphtheria toxin-induced demyelination in single nerve fiber preparations of the rat resulted in an increase of SDTC values (3, 4).
Merits of the Described Activity
It has been recognized early on that using the common earthworm allows one to record noninvasively from a single nerve fiber in a living organism (5, 39). There has been a strong urge to adapt the earthworm experiment for classroom teaching, and different methods for both noninvasive and invasive giant fiber recording have been described in this context (7, 19, 29, 40, 41). However, these methods suffer from several drawbacks, if the acquisition of genuine data with high reproducibility has priority: they are designed for freely moving or anesthetized animals. We used the Heinzel recording method (19, 29) for several years, but with nonanesthetized earthworms. Our experiments were frequently unsuccessful (∼30% failure rate) due to one main problem: when forced into a narrow enclosure on the recording platform, the worm may vigorously twist and turn, and usually it is hard to get the worm in position and restrain it underneath an acrylic glass cover. Also, due to the applied force, the worm may not adopt an optimal position for stimulation and recording once restrained (i.e., often the ventral side of the worm may not face the electrodes). Thus, our experiences confirmed the recommendation to use anesthetized worms when performing electrical stimulations with this recording platform (19, 29). However, due to a number of reasons (as discussed below), we insisted upon performing our experiments with nonanesthetized worms. Therefore, we improved the experimental setup and used a specially designed and easy to handle stimulation and recording chamber. It allows effective restraint of the worm, which enters the chamber freely.
Although not evaluated during the practical course, we had the impression that using nonanesthetized worms, which enter the chamber freely, made the animal experiment ethically and esthetically tolerable among the majority of our medical students. Still, the willingness among medical students to perform animal experiments cannot be taken for granted. Therefore, we offered demonstration material on the computers in our teaching laboratories (see Supplemental Material) including video sequences as a quick online reference as well as previously acquired raw data to be analyzed by students who refused to perform the earthworm experiment. This virtual experiment option was favored by some students. However, from a total of 88 groups, only 7 groups made exclusively use of the previously acquired data, and 12 groups performed both the real animal experiment and the virtual experiment for comparison.
If our method, which works completely without anesthesia and without the use of damaging force to the worm, indeed resulted in a higher acceptance of the animal experiment among students, this represents a great advancement for teaching. Reportedly, students benefit from real hands-on laboratory experience as opposed to purely visual learning based on demonstration material (36). More recently, cost-effective computer-based interactive learning has often been used instead of animal experiments. However, surveys and detailed evaluation have provided evidence that many students acquire a more thorough understanding through the advanced and more challenging first-hand experience of an animal experiment (35). Still, computer-based material should be offered, because, as seen with 12 of 88 groups in our practical course, it may serve as a bridge (35).
Using nonanesthetized worms that enter the chamber on their own also represents a technical advantage: the spontaneously moving worm is in its “upright” position and will direct the ventral nerve cord to the bottom of the chamber where the electrodes are positioned. Thus, it will be in an optimal position for stimulation and recording, which, in our practical course, resulted in a high success rate within <10 min from the time point that the worm started to enter the chamber. From the seven groups (of 88 groups) that chose to exclusively perform the virtual experiment, we do not know whether their choice was made due to technical problems with the experimental setup. Thus, the success rate of the experiments in the described activity was between 92% and 100%.
It should be noted that performing the earthworm experiment without the use of anesthesia also avoids unwanted side effects. Since it is difficult to find the right dosage of anesthesia, it may happen that the worm is so strongly anesthetized that no responses are observed (29, 40, 41). Substances used for anesthesia often act as local anesthetics (29), which can target also MGF Na+ channels. It is known that local anesthetics reduce conduction velocity in a concentration-dependent manner before inducing a complete conduction block (10, 12, 13, 37). In addition, anesthesia may indirectly influence also the functional anatomy of the giant nerve fibers: anesthetized earthworms adopt a thin and extended shape due to the relaxation of the body wall musculature (unpublished observations). As a consequence, the MGF may be stretched and its diameter decreased (5), leading to increased fiber series resistance and reduced conduction velocity. Heinzel and coworkers (29) reported a mean conduction velocity of 16 m/s for the MGF at room temperature, much lower than the one determined in our practical course (Fig. 4D and Table 1). On the other hand, the conduction velocities obtained with nonanesthetized worms in our practical course coincide well with conduction velocities found previously by Drewes and coworkers [mean value: 32.2 m/s (8)] and by Bullock [15–45 m/s or higher (5)], who also reported that conduction velocity critically depends on the state of contraction or extension of the worm.
Chronaxie measurements are influenced by technical factors, so that, even for a given type of tissue or cell type, the variability among reported values may be quite large. The factors that can critically influence chronaxie measurements are stimulus waveform, electrode properties, stimulator output impedance, tissue inhomogeneity, and temperature (14). A relevant reference for a comparison of data within the context of the present report is the work by Kladt and cowokers (29). The chronaxie value reported by these authors (129 μs) is about twice as large as ours, although both sets of experiments were done at room temperature, and in both sets of experiments rectangular stimulus pulses were used. However, Kladt and coworkers (29) used a constant-current generator as the stimulation device and, accordingly, did their chronaxie calculations based on current-duration data rather than voltage-duration data as in our case. This difference offers a plausible explanation for the discrepancy of the obtained chronaxie values. In fact, Holsheimer and coworkers (21) reported that their chronaxie values calculated from voltage-duration data lay 30–40% below those obtained based on current-duration data.
Taken together, the use of our experimental setup has several advantages: first, we have the impression that with our earthworm chamber, the animal experiment has a higher acceptance among students than with our previously used experimental setup; second, the success rate is higher; and third, the parameters of interest, particularly conduction velocity, can be determined with a high reliability, due to the absence of anesthesia. We believe that using an experimental setup, which yields genuine scientifically useful data, motivates students. In addition, our earthworm chamber saves time, since the students do not have to bother at all with technical problems trying to get the worm in position and the experiment to work. This allows a thorough discussion of both the basic principles behind the experimental results and relevant clinical aspects.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B. and C.K.B. analyzed data; R.B. and C.K.B. interpreted results of experiments; R.B. and C.K.B. prepared figures; R.B. drafted manuscript; R.B. and C.K.B. edited and revised manuscript; R.B. and C.K.B. approved final version of manuscript; C.K.B. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all students who attended the practical course and all student assistants who directly helped the course participants with technical questions. In particular, the authors thank Thorsten Homfeldt, Gregor Thurau, Charlotte Wallach, Jonas Thiele, and Peter Bassalay for technical support with the experimental setup. Mirco Paske and Alexander P. Schwoerer were cosupervisors during the practical course and were involved in both teaching and data collection. The idea of designing an earthworm chamber like the one used in this activity was initially developed by Michael Gewecke and coworkers at the Zoology Institute of the University of Hamburg.
Appendix: CRITICAL EXAMINATION OF STUDENT-DERIVED DATA
With the experimental setup used in this activity (Fig. 3A), both stimulus amplitude and stimulus duration were read by students from the crude scale given on the stimulator, a method that certainly involves some inaccuracy. Also, rheobase and chronaxie were determined graphically based on the method proclaimed by Lapicque (see materials and methods), which avoided the use of sophisticated analysis software but represented another source of inaccuracy. To objectively judge the quality of the student-derived data and their graphic analysis, we chose to draw comparisons to analysis results obtained with a mathematical formalism.
Lapicque's definition that chronaxie (c) is the threshold stimulus duration at a stimulus amplitude of two times rheobase (r) (30) can be directly derived, if one uses a formula in which the threshold stimulus expressed as the stimulus amplitude (a) is a hyperbolic function of the stimulus duration (t), as follows:
In this formula, r × c/t describes the hyperbola, and r is the positive offset relative to the x-axis (see also Fig. 1A). If one sets a = 2r, it is obvious that r × c/t has to equal r, which can only be obtained if t = c, i.e., stimulus duration equals chronaxie. Lapicque's approach was based on the law of Weiss (43), which says that the threshold stimulus, expressed as the quantity (q) of electricity applied (the integral of a stimulus pulse over time), is a linear function of t, as follows:
In this formula, r is the slope of the linear relationship and r × c defines the intersection point with the y-axis.
Despite the different approaches taken by Lapicque and Weiss, r and c have identical meanings in the two formulas, which can be derived from one another by multiplying or deviding, respectively, by t (since q = a × t; see also Fig. 1, A and B). The law of Weiss offers a reliable mathematical formalism to judge the quality of the students' data and their analysis (see below).
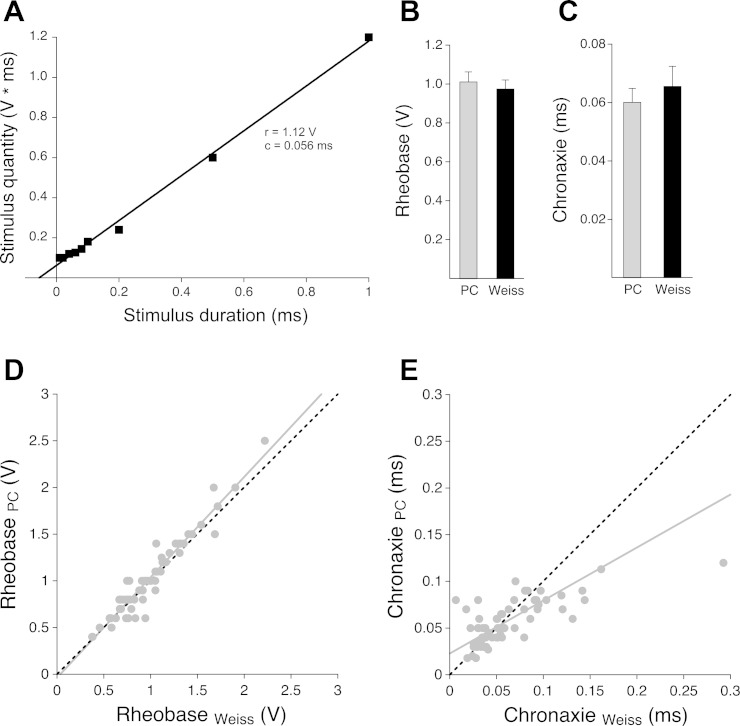
In Fig. 6A, the data of one experiment (the same as shown in Fig. 5A) have been transformed according to Weiss formalism and plotted on a linear scale. The linear dependence of q on t makes the Weiss analysis extremely informative, and, theoretically, only two good measurements would be necessary to determine r and c (31). If more data points have been acquired (as in this activity) and they all come to lie reasonably well on the linear fit (Fig. 6A), then it is safe to assume that the acquired data are of good quality. The transformation of a into q and the linear fitting according to the Weiss formalism was performed for all experimental data acquired in our practical course. In the vast majority of data, application of Weiss formalism gave excellent fits (R2 = 0.9872, SD = 0.0161, n = 64). Mean r and c values derived from the Weiss analysis were 0.976 ± 0.046 V and 0.066 ± 0.007 ms, respectively (n = 64). Figure 6, B and C, shows that these values are in a very good agreement with the results obtained by students. However, although very small, the difference between r values obtained with the two different analysis methods turned out to be statistically significant (P = 0.0161; Fig. 6B and Table 1). The data suggest that students slightly overestimated r with the graphic analysis. No significant difference was detected between mean c values obtained with the two analysis methods (P = 0.1821; Fig. 6C and Table 1).
Fig. 6.
Critical data examination with the law of Weiss. A: stimulus quantity (integral obtained by multiplying stimulus amplitude by stimulus duration) plotted against stimulus duration (x-axis linear scale). Data are from the same experiment as shown in Fig. 5A. According to the law of Weiss, the integral data were fitted by a linear function of the form q = r × (t + c), derived from q = r × t + r × c (see Fig. 1B), where the slope of the fit is the rheobase (r). Rheobase and chronaxie obtained with the Weiss analysis for the experiment shown are 1.12 V and 0.056 ms, respectively. B and C: comparison of mean rheobase (B) and chronaxie (C) values obtained by students in the practical course (PC) with graphic analysis based on the Lapicque method and from the Weiss analysis (Weiss), respectively; the small difference between the mean rheobase values proved to be significant (P = 0.0161), whereas the mean chronaxie values were not significantly different. D: direct correlation of all individual rheobase values obtained in the practical course (RheobasePC) and the corresponding values obtained with the Weiss analysis (RheobaseWeiss). The continuous gray line represents a linear regression fit to the data points; the dashed black line is a regression assuming 100% correlation (slope of 1 and axis intersept at 0). E: same correlation analysis as in D but for chronaxie values (ChronaxiePC and ChronaxieWeiss).
Data were subjected to a closer inspection by performing a 1:1 correlation analysis: for each experiment, and for both r and c, the analysis result obtained by students was plotted against the corresponding result from the Weiss analysis, and a linear regression was performed (Fig. 6, D and E). In accordance with the small but significant difference visible in Fig. 6B, the regression line for r analysis slightly deviated from the line representing a perfect (100%) equivalence of the two analysis results (Fig. 6D). On the other hand, and in accordance with the slightly lower mean c value obtained by students (Fig. 6C), the corresponding regression line was shallower than the 100% equivalence correlation (Fig. 6E). This may be a direct consequence of the aforementioned r overestimation, which automatically leads to c underestimation (see Figs. 1A and 5A).
It should be noted that direct application of a hyperbolic function (see above) to the students' data yielded slightly worse fits (R2 = 0.9434 ± 0.0077; not shown) than the Weiss analysis (R2 = 0.9872 ± 0.0020, n = 64, P < 0.0001). References in support of the notion that experimental amplitude-duration data may deviate from a purely hyperbolic function, while being well fitted by the Weiss equation, can be found in the literature (3, 23, 31). These findings speak for the use of the Weiss analysis for a critical data examination.
Additional Resources
For additional resources, use internet links to technical equipment companies and scientific institutions, which often provide useful background information, either online or in downloadable form, for example:
Footnotes
Supplemental Material for this article is available at the Advances in Physiology Education website.
REFERENCES
- 1. Bear MF, Connors BW, Paradiso MA. Neuroscience. Baltimore, MD: Lippincott, Williams & Wilkins, 2007. [Google Scholar]
- 2. Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21: 137–158, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Bostock H, Sears TA, Sherratt RM. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol 341: 41–58, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brismar T. Electrical properties of isolated demyelinated rat nerve fibres. Acta Physiol Scand 113: 161–166, 1981. [DOI] [PubMed] [Google Scholar]
- 5. Bullock TH. Functional organization of the giant fiber system of lumbricus. J Neurophysiol 8: 55–71, 1945. [Google Scholar]
- 6. Chemali KR, Tsao B. Electrodiagnostic testing of nerves and muscles: when, why, and how to order. Cleve Clin J Med 72: 37–48, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Drewes CD. Non-invasive recording of giant nerve fiber action potentials from freely moving oligochates. In: Tested Studies for Laboratory Teaching, edited by Karcher SJ. Chapel Hill, NC: Proceedings of the 20th Workshop/Conference of the Association for Biology Laboratory Education, 1999, vol. 20, chapt. 2, p. 45–62. [Google Scholar]
- 8. Drewes CD, Landa KB, McFall JL. Giant nerve fibre activity in intact, freely moving earthworms. J Exp Biol 72: 217–227, 1978. [DOI] [PubMed] [Google Scholar]
- 9. Duru F, Bauersfeld U, Schuller H, Candinas R. Threshold tracking pacing based on beat by beat evoked response detection: clinical benefits and potential problems. J Interv Card Electrophysiol 4: 511–522, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Fink BR, Cairns AM. Differential slowing and block of conduction by lidocaine in individual afferent myelinated and unmyelinated axons. Anesthesiology 60: 111–120, 1984. [DOI] [PubMed] [Google Scholar]
- 11. Franz DN, Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol 199: 319–345, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franz DN, Perry RS. Mechanisms for differential block among single myelinated and non-myelinated axons by procaine. J Physiol 236: 193–210, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasser HS, Erlanger J. The role of fiber size in the establishment of a nerve block by pressure or cocaine. Am J Physiol 88: 581–591, 1929. [Google Scholar]
- 14. Geddes LA. Accuracy limitations of chronaxie values. IEEE Trans Biomed Eng 51: 176–181, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Genç G, Bek S, Kasikci T, Ulas UH, Demirkaya S, Odabasi Z. Strength-duration time constant in peripheral nerve: no abnormality in multiple sclerosis. Mult Scler Int 2012: 1–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grill WM, Simmons AM, Cooper SE, Miocinovic S, Montgomery EB, Baker KB, Rezai AR. Temporal excitation properties of paresthesias evoked by thalamic microstimulation. Clin Neurophysiol 116: 1227–1234, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Günther J. Impulse conduction in the myelinated giant fibers of the earthworm. Structure and function of the dorsal nodes in the median giant fiber. J Comp Neurol 168: 505–531, 1976. [DOI] [PubMed] [Google Scholar]
- 18. Hagiwara S, Morita H. Electrotonic transmission between two nerve cells in leech ganglion. J Neurophysiol 25: 721–731, 1962. [DOI] [PubMed] [Google Scholar]
- 19. Heinzel HG. Das Experiment: Neurophysiologische Versuche am intakten Regenwurm. In: Biologie in unserer Zeit. Weinheim: Wiley-Blackwell, 1990, p. 308–313. [Google Scholar]
- 20. Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- 21. Holsheimer J, Dijkstra EA, Demeulemeester H, Nuttin B. Chronaxie calculated from current-duration and voltage-duration data. J Neurosci Meth 97: 45–50, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Irnich W. The chronaxie time and its practical importance. Pacing Clin Electrophysiol 3: 292–301, 1980. [DOI] [PubMed] [Google Scholar]
- 23. Irnich W. The fundamental law of electrostimulation and its application to defibrillation. Pacing Clin Electrophysiol 13: 1433–1447, 1990. [DOI] [PubMed] [Google Scholar]
- 24. Irnich W. Georges Weiss' fundamental law of electrostimulation is 100 years old. Pacing Clin Electrophysiol 25: 245–248, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Irnich W. The terms “chronaxie” and “rheobase” are 100 years old. Pacing Clin Electrophysiol 33: 491–496, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Kanai K, Shibuya K, Sato Y, Misawa S, Nasu S, Sekiguchi Y, Mitsuma S, Isose S, Fujimaki Y, Ohmori S, Koga S, Kuwabara S. Motor axonal excitability properties are strong predictors for survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 83: 734–738, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. New York: McGraw-Hill, 2000. [Google Scholar]
- 28. Kiss ZH, Anderson T, Hansen T, Kirstein D, Suchowersky O, Hu B. Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur J Neurosci 18: 728–732, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Kladt N, Hanslik U, Heinzel HG. Teaching basic neurophysiology using intact earthworms. J Undergrad Neurosci Educ 9: A20–A35, 2010. [PMC free article] [PubMed] [Google Scholar]
- 30. Lapicque L. Définition expérimentale de l'excitabilité. Comptes Rendus Soc Biol 77: 280–283, 1909. [Google Scholar]
- 31. Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of human peripheral nerve. Brain 119: 439–447, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of sensory and motor axons in carpal tunnel syndrome. Muscle Nerve 20: 508–510, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Mogyoros I, Kiernan MC, Burke D, Bostock H. Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain 121: 851–859, 1998. [DOI] [PubMed] [Google Scholar]
- 34. Putzke JD, Wharen RE, Jr, Wszolek ZK, Turk MF, Strongosky AJ, Uitti RJ. Thalamic deep brain stimulation for tremor-predominant parkinson's disease. Parkinsonism Relat Disord 10: 81–88, 2003. [DOI] [PubMed] [Google Scholar]
- 35. Ra'anan AW. The evolving role of animal laboratories in physiology instruction. Adv Physiol Educ 29: 144–150, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Randall WC, Burkholder T. Hands-on laboratory experience in teaching-learning physiology. Adv Physiol Educ 4: 4–7, 1990. [DOI] [PubMed] [Google Scholar]
- 37. Raymond SA. Subblocking concentrations of local anesthetics: effects on impulse generation and conduction in single myelinated sciatic nerve axons in frog. Anesth Analg 75: 906–921, 1992. [DOI] [PubMed] [Google Scholar]
- 38. Roots BI, Lane NJ. Myelinating glia of earthworm giant axons: thermally induced intramembranous changes. Tissue Cell 15: 695–709, 1983. [DOI] [PubMed] [Google Scholar]
- 39. Rushton WA, Barlow HB. Single-fibre response from an intact animal. Nature 152: 597–598, 1943. [Google Scholar]
- 40. Shannon KM, Gage GJ, Jankovic A, Wilson WJ, Marzullo TC. Portable conduction velocity experiments using earthworms for the college and high school neuroscience teaching laboratory. Adv Physiol Educ 38: 62–70, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silver WL. Recording action potentials extracellularly from earthworm giant axons. In: Laboratory Manual for Physiology, edited by Silverthorn DU, Johnson BR, Mills AC. San Francisco, CA: Pearson/Benjamin Cummings, 2005, p. 773–784. [Google Scholar]
- 42. Stough HW. Giant nerve fibers of the earthworm. J Comp Neurol 40: 409–463, 1926. [Google Scholar]
- 43. Weiss G. Sur la possibilité de rendre comparable entre eux les appareils cervant à l'excitation électrique. Arch Ital Biol 35: 413–446, 1901. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.